Abstract
Objective
To determine the effect of Medicaid expansion on the use of opioid agonist treatment for opioid use disorder (OUD) and to examine heterogeneous effects by provider supply and Medicaid acceptance rates.
Data Sources
Yearly state‐level data on methadone dispensed from opioid treatment programs (OTPs), buprenorphine dispensed from OTPs and pharmacies, number of OTPs and buprenorphine‐waivered providers, and percent of OTPs and physicians accepting Medicaid.
Study Design
This study used difference‐in‐differences models to examine the effect of Medicaid expansion on the amount of methadone and buprenorphine dispensed in states between 2006 and 2017. Interaction terms were used to estimate heterogeneous effects. Sensitivity analyses included testing the association of outcomes with Medicaid enrollment and state insurance rates.
Principal Findings
The estimated effects of Medicaid expansion on buprenorphine and methadone dispensed were positive but imprecise, meaning we could not rule out negative or null effects of expansion. The estimated associations between state insurance rates and dispensed methadone and buprenorphine were centered near zero, suggesting that improvements in health coverage may not have increased OUD treatment use. The effect of Medicaid expansion was larger in the states with the most waivered providers compared to states with the fewest waivered providers. In the states with the most waivered providers, the average estimated effect of expansion on buprenorphine dispensed was 12 kg/y, enough to treat about 7500 individuals. We did not find evidence that the effect of expansion was consistently modified by OTP concentration, OTP Medicaid acceptance, or physician Medicaid acceptance.
Conclusions
Gains in health coverage may not be sufficient to increase OUD treatment, even in the context of high treatment need. Provider capacity likely limited Medicaid expansion's effect on buprenorphine dispensed. Policies to increase buprenorphine providers, such as ending the waiver requirement, may be needed to ensure coverage gains translate to treatment access.
Keywords: buprenorphine, Medicaid expansion, methadone, opioid use disorder
What This Study Adds.
Prior research suggests Medicaid expansion increased the proportion of substance use disorder (SUD) treatment paid by Medicaid but did not increase the percent of people with SUDs receiving treatment.
Buprenorphine and methadone are effective medications for the treatment for opioid use disorder (OUD), but it remains unknown whether Medicaid expansion increased use of these treatments.
We did not find evidence that Medicaid expansion consistently increased the amount of buprenorphine or methadone dispensed in states or that changes in state uninsurance rates were associated with the amount of these medications dispensed.
Medicaid expansion's effect on buprenorphine dispensed was larger in states with the most buprenorphine prescribers compared to the states with the fewest prescribers, pointing to constrained provider supply as one reason expansion did not increase use.
1. INTRODUCTION
The drug overdose crisis in the United States continues to worsen. In 2017, there were over 70 000 deaths from drug overdoses, a 9 percent increase over the year before.1 More than two‐thirds of these overdose deaths involved opioids, pointing to the importance of reducing harm from opioid use in addressing the overdose crisis. Opioid agonist treatment (OAT) with buprenorphine or methadone is effective at reducing illicit opioid use among people with opioid use disorder (OUD).2 Nevertheless, these medications are vastly underutilized.3 In an analysis of Medicaid claims data, 63 percent of new treatment episodes for OUD did not involve OAT.4
The Affordable Care Act (ACA) was expected to increase use of substance use disorder (SUD) treatment services, in part through Medicaid expansion.5, 6, 7 However, the available evidence to date suggests that the ACA Medicaid expansion did not increase self‐reported treatment rates for SUD.8 The apparent lack of effect of Medicaid expansion on SUD treatment rates is puzzling given research findings that the ACA has resulted in higher rates of insurance among people with SUD,8, 9 that the ACA resulted in increased SUD benefits under Medicaid plans,10 and that Medicaid expansion increased other forms of health care utilization.11
The effect of Medicaid expansion on overall OAT utilization remains unknown. Recent studies have found that Medicaid expansion increased Medicaid‐funded buprenorphine by at least 70 percent.12, 13, 14 However, Medicaid expansion may not have increased overall use of OAT if individuals newly accessing OAT through Medicaid were previously accessing OAT through other sources of payment, such as private insurance, block grants, or self‐pay. Indeed, there is evidence that the number of privately insured individuals using buprenorphine plateaued between 2013 and 2015,15 which could be explained by individuals switching to other payer sources.
A possible reason that Medicaid expansion has not increased SUD treatment rates is that there are not enough SUD treatment providers or enough providers who accept Medicaid to meet the demand for treatment. When it comes to OAT, treatment can be provided through opioid treatment programs (OTPs) or office‐based providers.16 OTPs are strictly regulated programs permitted to dispense methadone and buprenorphine for treatment of OUD.16 Buprenorphine, but not methadone, can also be prescribed by office‐based providers who obtain a waiver of DEA restrictions to prescribe buprenorphine.16 Evidence suggests nearly all states lack enough OTPs and waivered physicians to provide OAT to all individuals in need.17 This shortage is exacerbated by the fact that only approximately half of buprenorphine prescribers report accepting Medicaid for office visits.18
Two studies have provided a partial picture of the effect of Medicaid expansion on overall OAT use. Meinhofer and Witman13 found that Medicaid expansion increased OAT use from OTPs by about 30 percent in states where Medicaid covered buprenorphine and methadone. While this finding is encouraging, OTPs account for a minority of OAT treatment. As of 2012, the treatment capacity of office‐based buprenorphine providers was 3.5 times larger than the number of people receiving methadone in OTPs.17 Saloner and colleagues found in a sample of five states that states that expanded Medicaid had higher rates of buprenorphine prescription fills per person after expansion relative to nonexpansion states controlling for the insurance rate in states.19 However, the authors also found that expansion did not affect the number of days with buprenorphine fills per person. No study has examined the effect of Medicaid expansion on overall OAT across payers in all states.
The objective of this study was to assess the effect of Medicaid expansion on OAT use across payers and treatment sources using data from all states where Medicaid covered these treatments prior to expansion. We hypothesized that Medicaid expansion would increase OAT use by lowering the cost of OAT for people with OUD newly eligible for Medicaid. An additional objective was to examine whether there was variation in the effect of Medicaid expansion on OAT use by provider concentration and the percent of providers who accept Medicaid. We hypothesized that OAT use would not increase in expansion states with the lowest concentration of providers and percent of providers accepting Medicaid.
2. METHODS
2.1. Measures of OAT utilizations
We conducted a retrospective panel data study using a difference‐in‐differences (DID) approach to examine the causal effect of Medicaid expansion on OAT utilization. The dependent variables in our analyses were the kilograms of methadone and buprenorphine dispensed in each state annually. At a typical dose of 100 mg a day, an additional kilogram of methadone dispensed can treat about 27 individuals for a year. At a typical dose of 16 mg a day, an additional kilogram of buprenorphine can treat about 625 people for a year. We obtained yearly data from the Drug Enforcement Administration's Automation of Reports and Consolidated Orders System (ARCOS) for 2006‐2017. ARCOS contains data on opioids dispensed from all sources and across payers. We included only methadone dispensed from OTPs. We included buprenorphine dispensed from OTPs and pharmacies.
As of 2017, 33 states had adopted ACA Medicaid expansion. In 26 of these states, the ACA Medicaid expansion became effective in January 2014,20 though five of these states began gradual expansions prior to 2014.21 In the remaining seven states, two expanded later in 2014, three expanded in 2015, and two expanded in 2016. We included in our main methadone analysis 28 states whose Medicaid programs reported covering methadone as of 2007.22 Of these 28 states, 19 expanded Medicaid. We similarly included in our main analyses of buprenorphine 45 states whose Medicaid programs reported covering buprenorphine as of 2007.22 Of these 45, 29 expanded Medicaid. In sensitivity analyses, we include states who covered methadone (31 states) or buprenorphine (50 states) by 2013.
The ARCOS data do not allow us to distinguish between buprenorphine formulations approved for OUD treatment and those approved for pain treatment. Following Wen and colleagues' approach,12 we used the Medicaid State Drug Utilization Data to find that more than 99.6 percent of Medicaid‐funded buprenorphine units were for OUD rather than pain between 2010 and 2017. We therefore believe that buprenorphine formulations for pain account for a very small percentage of buprenorphine and are unlikely to significantly bias our findings.
2.2. Measures of Medicaid expansion
The main independent variable in our analyses is an indicator variable of whether states had expanded Medicaid at any time during the year.20 We conducted sensitivity analyses that excluded states that partially expanded Medicaid before 2014 (leaving 23 methadone and 40 buprenorphine states),21 excluded states that expanded Medicaid after 2014 (leaving 27 methadone and 41 buprenorphine states), and excluded states that expanded Medicaid through 1115 waivers (leaving 25 methadone and 38 buprenorphine states). The effect of expansion on OAT use in these states may have differed because of more gradual or limited increases in Medicaid enrollment resulting from early, late, or partial expansion. We also conducted sensitivity analyses controlling for enactment and mandated use of prescription drug monitoring programs (PDMPs) following the approach of Meinhofer and Witman.13
The primary mechanism by which Medicaid expansion may have increased OAT use is by increasing Medicaid enrollment particularly among previously uninsured individuals. However, the extent to which Medicaid expansion increased Medicaid enrollment levels and overall insurance rates varied between states.23 Even states that did not expand Medicaid saw increases in Medicaid enrollment and increases in insurance rates after 2014 because of the woodwork effect and other ACA provisions.23 As a check on our main results, then, we also considered models where the independent variables were the number of people enrolled in Medicaid24, 25 or the annual health insurance rate in each state for those under 65, including public and private coverage sources.26 These models provide estimates of the overall association between our outcomes and changes in Medicaid enrollment and the health insurance rate in our study period. We do not include an indicator for Medicaid expansion in these models, since they are directly estimating an association between enrollment and insurance rate over time.
2.3. Measures of capacity
We used measures of OAT provider capacity from years prior to 2014 because we were interested in the effect of expansion on OAT use based on states' supply of OAT prior to expansion. We used the concentration of OTPs per population in each state in 2013 as a measure of the methadone capacity in states. We obtained the number of OTPs from the National Survey of Substance Abuse Treatment Services (NSSATS).27 We found that the number of OTPs in a state had a Pearson correlation of 0.97 with the number of outpatients receiving methadone reported in NSSATS, suggesting that this is a good measure of treatment capacity.
We used the concentration of buprenorphine‐waivered providers per population in states in 2013 as the measure of buprenorphine capacity. We obtained the yearly number of new buprenorphine waivers by state from the Substance Abuse and Mental Health Services Administration through a Freedom of Information Act request. The number of active waivers in each state in each year was not available. We summed the number of new waivers in each year prior to 2013 to obtain an estimate of the number of active waivers in each state in 2013. This approach likely produced an overestimate of the number of waivers in a state. However, since we used this measure simply to separate states into those with more and fewer waivers, the overestimate likely did not bias our results. As an additional sensitivity analysis, we used the concentration of buprenorphine providers with 100 and 275 patient waivers in states in 2013 as the measure of buprenorphine capacity. This approach may better capture waivered providers who are actively prescribing buprenorphine.
The effect of Medicaid expansion may rely not only on there being enough OAT providers in states but also enough OAT providers who accept Medicaid. Therefore, we also divided states into thirds by the percent of OAT providers accepting Medicaid. We obtained the percent of OTPs that reported accepting Medicaid in each state in 2013 from the National Survey of Substance Abuse Treatment Services.27 There is no data source for how many buprenorphine‐waivered providers accept Medicaid in each state. As a proxy, we used the overall percentage of physicians who report accepting Medicaid in each state from the 2011 National Ambulatory Medical Care Survey Electronic Medical Records Supplement.28 The percent of buprenorphine prescribers who accept Medicaid likely differs from the overall percent of physicians accepting Medicaid in a state. However, as long the terciles of physician Medicaid acceptance are the same as the terciles of buprenorphine prescriber Medicaid acceptance, our analysis approach will be valid. Capacity measures are summarized in Table S3.
2.4. Statistical analyses
We employed a DID approach using two‐way fixed‐effects models. We used year fixed effects to nonparametrically account for trends in methadone and buprenorphine dispensed in states. We used state fixed effects to account for time‐invariant differences between states. To account for within‐state confounding, we controlled for yearly state unemployment29 and state poverty rates.26 We also included in our models annual state population. This approach more flexibly controls for the effect of population compared to using population as the denominator of our outcomes. We present ordinary least squares (OLS) coefficients with standard errors clustered at the state level.
We tested the DID parallel trend assumption by running models on pre‐2014 data (prior to Medicaid expansion) and checking whether expansion states had a different time slope in OAT use than nonexpansion states by interacting the overall time trend with an expansion indicator. The interaction term was not statistically significantly different from zero for methadone (−0.93, 95% CI: −11 to 8.7) or buprenorphine (1.2, 95% CI: −1.6 to 4.0). Therefore, we were unable to reject the hypothesis that there was no difference in the pre‐2014 trends between expansion and nonexpansions, supporting the use of the DID approach.
To determine whether the effect of expansion differed in states by provider capacity, we divided states into thirds by measures of provider capacity, creating indicators for which tercile each state was in. We then ran models where we interacted these indicators with the Medicaid expansion indicator. These models produced an estimate of the effect of Medicaid expansion in the lowest tercile states, and estimates of interaction terms test the difference in the effect of Medicaid expansion from the lowest tercile states with the top two tercile states. If provider capacity limited the effect of Medicaid expansion, states with fewer OAT providers would not experience increases in buprenorphine and methadone after expansion. On the other hand, if states were able to expand their supply of OAT providers to meet the increased demand that resulted from Medicaid expansion, we would expect to see a positive effect of expansion even in states that had low levels of OAT providers prior to expansion.
One possibility is that provider capacity prior to expansion was correlated with treatment need. That is, states with more OTPs and waivered providers may also be the ones with higher OUD rates and overdose deaths rates. We found that the Pearson correlation between OTP per capita and opioid overdose mortality in 2013 was 0.47, and the Pearson correlation between buprenorphine waivers per capita and overdose mortality in 2013 was 0.37, suggesting a moderate level of correlation between treatment capacity and opioid overdose deaths. We conducted a sensitivity analysis examining differences in the effect of Medicaid expansion by the tercile of opioid overdose death in 2013.
As described above, we conducted sensitivity analyses of our main models that involved excluding states that expanded Medicaid before 2014 (early expansion), expanded Medicaid after 2014 (late expansion), and expanded Medicaid through 1115 waivers. We also conducted sensitivity analyses where we included states whose Medicaid programs covered methadone and buprenorphine as of 2013 rather than 2007. In addition, we tested controlling for the implementation and mandated use of PDMPs. Finally, we conducted a sensitivity analysis where we used the concentration of buprenorphine providers with 100 and 275 patient waivers in states in 2013 as the measure of buprenorphine capacity.
3. RESULTS
3.1. Effect of expansion on methadone dispensed
Methadone kilograms dispensed per capita increase in expansion and nonexpansion states between 2006 and 2017 (Figure 1). In adjusted analyses, the average estimated effect of Medicaid expansion on kilograms of methadone dispensed was 25.0 (95% CI −41.7,91.7) (Table 1—column 1). A 25‐kilogram increase in methadone dispensed would represent a 14 percent increase over the average methadone dispensed in a state‐year. However, because of the wide estimated confidence intervals, we cannot rule out that Medicaid expansion had no effect or negative effects.
Figure 1.
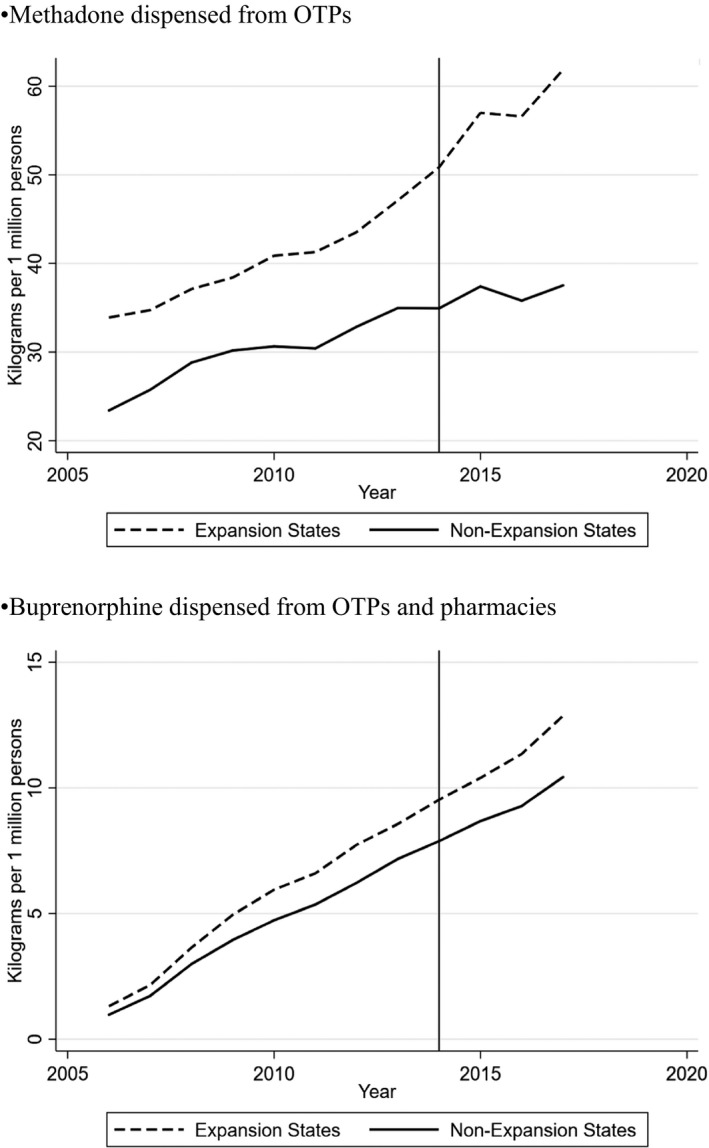
Trends in methadone and buprenorphine dispensed in Medicaid expansion and nonexpansion states per capita. The data presented are from the Drug Enforcement Administration's Automation of Reports and Consolidated Orders System (ARCOS) for 2006‐2017. ARCOS contains data on opioids dispensed from all sources and across payers. We included only methadone dispensed from OTPs in 28 states where Medicaid covered methadone as of 2007. We included buprenorphine dispensed from OTPs and pharmacies in 45 states where Medicaid covered buprenorphine as of 2007
Table 1.
Effect of Medicaid expansion on methadone kilograms dispensed in states by OTP concentration and Medicaid acceptance
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Main model | OTP concentration interacted modela | OTP Medicaid acceptance interacted modelb | Medicaid enrollment sensitivity model | Percent insured sensitivity | |
| Medicaid expansion (average effect) | 25.0 [−41.7,91.7] | ||||
| Medicaid expansion (bottom third) | 14.9 [−42.1,71.8] | 27.4 [−33.6,88.5] | |||
| Expansion × middle third | 34.5 [−43.9,112.9] | 17.8 [−34.1,69.7] | |||
| Expansion × top third | 1.87 [−60.0,63.7] | −25.1 [−87.4,37.1] | |||
| Unemployment (%) | 1.00 [−15.8,17.8] | 1.90 [−14.9,18.7] | 1.56 [−14.8,17.9] | 4.88 [−14.5,24.3] | 0.065 [−19.1,19.3] |
| Population (10 000s) | 0.066 [−1.03,1.16] | 0.0025 [−1.10,1.11] | −0.0063 [−1.10,1.09] | −0.54 [−2.03,0.95] | 0.037 [−1.19,1.27] |
| Poverty rate (%) | −2.52 [−21.4,16.4] | −4.30 [−25.9,17.3] | −1.09 [−19.0,16.9] | −2.83 [−20.1,14.4] | −0.33 [−16.0,15.4] |
| Medicaid enrollment (100 000s) | 4.48 [−2.51,11.5] | ||||
| Insurance rate (%) | −0.13 [−8.85,8.58] | ||||
| State‐years | 336 | 336 | 336 | 336 | 336 |
95% confidence intervals in brackets.
States are divided into thirds by the number of OTPs per 100 000 persons in 2013. Bottom third states (mean 0.24 OTPs per 100 000 persons, six expansion states, four nonexpansion states): FL, HI, MI, MN, MO, OH, OR, VA, WA, and WI. Middle third states (mean 0.45 OTPs per 100 000 persons, five expansion states, four nonexpansion states): AL, AZ, CA, GA, NC, NJ, NV, PA, and UT. Top third states (mean 0.93 OTPs per 100 000 persons, eight expansion states, one nonexpansion state): CT, DE, MA, MD, ME, NH, NY, RI, and VT.
States are divided into thirds by the percent of OTPs in the states accepting Medicaid in 2013. Bottom third states (41 percent OTPs accept Medicaid, four expansion states, six nonexpansion states): AL, AZ, DE, FL, GA, MI, MN, MO, NC, and VA. Middle third states (79 percent OTPs accept Medicaid, seven expansion states, two nonexpansion states): CA, MA, ME, NJ, OH, OR, PA, UT, and WA. Top third states (98 percent OTPs accept Medicaid, eight expansion states, one nonexpansion state): CT, HI, MD, NH, NV, NY, RI, VT, and WI.
P < .05, **P < .01, ***P < .001.
Estimates of the differences in the effect of Medicaid expansion by OTP concentration or OTP Medicaid acceptance among states had similarly wide confidence intervals (Table 1—columns 2‐3). That said, point estimates of the effect of Medicaid expansion were largest for the middle tercile of states by OTP concentration and OTP Medicaid acceptance. We also did not find evidence of a positive association between methadone dispensed and Medicaid enrollment or percent insured in states throughout the study period (Table 1—columns 4‐5), lending support to the finding of no consistent positive effect of expansion on methadone dispensed. While the estimated association of Medicaid enrollment and methadone dispensed was positive at 4.48 (95% CI −2.51,11.5), the estimated association between insurance rates and methadone dispensed was centered close to zero at −0.13 (95% CI −8.85,8.58).
We found analogous results in our sensitivity analyses, which excluded states by expansion timing, 1115 waiver use, Medicaid OAT coverage, and controlled for PDMPs laws (Table S1). While all sensitivity analyses of the effect of Medicaid expansion had confidence intervals spanning zero, point estimates of the effect of Medicaid expansion when removing early expansion states were notably smaller than estimates from other models.
3.2. Effect of Medicaid expansion on buprenorphine dispensed
Buprenorphine kilograms dispensed per capita increase in expansion and nonexpansion states between 2006 and 2017 (Figure 1). The average estimated effect of Medicaid expansion on kilograms of buprenorphine dispensed was 1.42 (95% −12.2,15.1). A 1.42‐kilogram increase in buprenorphine dispensed would represent a 4.0 percent increase over the average buprenorphine dispensed in a state‐year. However, as was the case with our methadone findings, the wide estimated confidence intervals suggest that we cannot rule out that Medicaid expansion had no effect or negative effects.
We also did not find evidence of a positive association between buprenorphine dispensed and Medicaid enrollment or percent insured among all states (Table 2—columns 4‐5), lending support to the finding that Medicaid expansion did not consistently increase buprenorphine dispensed. As was the case with methadone, the association between buprenorphine dispensed and Medicaid enrollment was positive at 1.86 (95% CI −0.80,4.51), while the association between buprenorphine dispensed and the insurance rate was centered near zero at −0.49 (−2.51,1.53).
Table 2.
Effect of Medicaid expansion on buprenorphine kilograms dispensed in states by waiver concentration and physician medicaid acceptance
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Main model | Waiver concentration interacted modela | Physician Medicaid acceptance interacted modelb | Medicaid enrollment sensitivity model | Percent insured sensitivity model | |
| Medicaid expansion (average effect) | 1.42 [−12.2,15.1] | ||||
| Medicaid expansion (bottom third) | −10.9 [−22.4,0.73] | 16.6 [−8.90,42.1] | |||
| Expansion × middle third | 8.77 [−19.8,37.3] | −17.5 [−48.5,13.5] | |||
| Expansion × top third | 22.7* [4.19,41.1] | −25.6 [−53.8,2.66] | |||
| Unemployment (%) | −2.30 [−5.66,1.06] | −1.97 [−5.14,1.21] | −1.47 [−5.15,2.20] | −0.67 [−3.93,2.59] | −2.44 [−5.77,0.89] |
| Population (10 000s) | 0.21** [0.079,0.35] | 0.22** [0.066,0.36] | 0.19** [0.055,0.32] | 0.058 [−0.13,0.25] | 0.22** [0.077,0.36] |
| Poverty rate (%) | 4.60 [−0.53,9.74] | 3.45 [−1.24,8.14] | 3.41 [−1.85,8.66] | 2.62 [−2.34,7.57] | 4.67 [−0.52,9.86] |
| Medicaid enrollments (100 000s) | 1.86 [−0.80,4.52] | ||||
| Insurance rate (%) | −0.49 [−2.51,1.53] | ||||
| State‐years | 540 | 540 | 540 | 540 | 540 |
95% confidence intervals in brackets.
States are divided into thirds by the number of buprenorphine waivers per 100 000 people in 2013. Bottom third states (12 waivers per 100 000 persons, eight expansion states, seven nonexpansion states): AR, IA, IL, IN, KS, MN, MO, MT, NC, ND, NE, NH, OK, TX, and WY. Middle third states (23 waivers per 100 000 persons, eight expansion states, seven nonexpansion states): AL, AZ, CA, CO, DE, FL, GA, HI, NV, OH, SC, TN, VA, WI, and WV. Top third states (52 waivers per 100 000 persons, 13 expansion states, two nonexpansion states): AK, CT, MA, MD, ME, MI, NJ, NM, NY, OR, PA, RI, UT, VT, and WA.
States are divided into thirds by the percent of physicians accepting Medicaid in 2011. Bottom third states (63 percent of physicians accept Medicaid, eight expansion states, seven nonexpansion states): AL, CA, CO, CT, FL, GA, IL KS, MD, MO, NJ, NY, OK, PA, and TN. Middle third states (75 percent of physicians accept Medicaid, four expansion states, 11 nonexpansion states): AZ, DE, HI, IN, MA, ME, NC, NV, OH, OR, RI, TX, VA, VT, and WA. Top third states (88 percent of physicians accept Medicaid, five expansion states, 10 nonexpansion states): AK, AR, IA, MI, MN, MT, ND, NE, NH, NM, SC, UT, WI, WV, and WY.
P < .05, **P < .01, ***P < .001.
We did find evidence that the effect of Medicaid expansion differed in the states with the most waivered providers compared to the states with the fewest waivered providers (Table 2—column 2). In states with the most waivered providers, Medicaid expansion led to an estimated average yearly increase of 12 kg of buprenorphine dispensed. This increase is equivalent to a 33 percent increase in buprenorphine dispensed in a state‐year and is enough to treat 7500 patients at a daily dose of 16 mg. The estimated differences in expansion effect by physician Medicaid acceptance had wide confidence intervals spanning zero, but the largest point estimates were for the states with lowest physician Medicaid acceptance (Table 2 columns 3). Our sensitivity analyses' results were consistent with our main models' results (Table S2).
The states with the most waivered providers were very similar to the set of states with the most providers with 100 and 275 patient waivers, so dividing states by 100 and 275 patient waivers produced nearly identical estimates as stratifying by all waivers (results not presented). We similarly found no evidence of differences in the effect of Medicaid expansion by the tercile of opioid overdose death rate in 2013 (results not presented).
4. DISCUSSION
We did not find evidence that Medicaid expansion consistently increased the amount of methadone dispensed in states that had Medicaid coverage of methadone. Among states where Medicaid covered buprenorphine, we found that Medicaid expansion had a larger effect on buprenorphine dispensed in states with the highest concentrations of providers waivered to prescribe buprenorphine compared to states with the lowest concentrations of waivered providers. The estimated associations between state insurance rates and dispensed buprenorphine and methadone were centered at zero. This result suggests gains in health coverage were likely not sufficient to increase OAT use during the study period despite evidence of high treatment need.
Our results suggest that the supply of providers waivered to prescribe buprenorphine restricted the ability of Medicaid expansions to increase use of OUD treatment at a time of high overdose deaths. Prior research has documented numerous barriers to buprenorphine prescription such as prior authorization policies, low reimbursement, perceived lack of training, perceived lack of community psychosocial services, and more.30, 31, 32, 33, 34 Policies to increase the supply of buprenorphine prescribers may be especially important to improve access to OUD treatment.35 A possible approach to vastly increase the supply of buprenorphine prescribers could be to do away with the waiver requirement altogether so that any licensed prescriber could provide buprenorphine treatment without additional certification. Critics of the waiver requirement have argued that it disrupts adoption of a safe and effective treatment that can be provided within the scope of usual primary care practice.36
Our estimated effect of expansion on buprenorphine dispensed was imprecise, possibly suggesting that the effect of Medicaid expansion was variable among states. Despite the imprecise estimates, our point estimate suggested a modest average effect equivalent to a 4.0 percent increase in the average buprenorphine dispensed in a state‐year. This result stands in contrast with a prior finding that expansion increased the number of Medicaid‐paid buprenorphine prescriptions in expansion states by 70 percent.12 These disparate findings are explainable if Medicaid expansion shifted buprenorphine payment from non‐Medicaid payers to Medicaid without substantially increasing overall buprenorphine use, as research suggests, took place with SUD treatment overall.8 Such a shift may have beneficial effects for individuals, whose out‐of‐pocket costs for treatment may have significantly decreased, providing more available income for other needs such as housing and food.
As in the case of buprenorphine, our estimated average effect of Medicaid expansion on methadone dispensed was imprecise, possibly suggesting variable effects among states. Our point estimates suggested a moderate average effect equivalent to a 14 percent increase in the average methadone dispensed in a state‐year. Our imprecise estimates may result in part from the few states whose Medicaid programs covered methadone, limiting the statistical power of our analyses. On the other hand, our sample does capture the entire relevant “population,” so more precise estimates are not possible without additional years of data. Our results differed from those of Meinhofer and Witman13 who found that Medicaid expansion increased the combined amount of methadone and buprenorphine dispensed from OTPs by about 30 percent. Our models differed from those of Meinhofer and Witman in that we included more years of data and more flexibly controlled for the effects of population changes on methadone dispensed.
A possible explanation for why Medicaid expansion did not increase methadone dispensed is that OTP capacity is highly constrained in all states. There is evidence that the number of persons receiving methadone treatment remained relatively flat between 2003 and 2012, even as opioid overdose deaths were rising dramatically.17 OTP expansion is likely limited by restrictive regulations37 and lack of reimbursement for methadone treatment from private health plans.38 Even if more OTPs opened in response to greater treatment demand, the highly regimented nature of methadone treatment under current regulations, wherein patients must visit clinics daily during working hours, makes methadone an unattractive option for many people with OUD.39 Reforming the methadone regulatory regime to be in line with other high‐income countries, including by allowing office‐based prescription of methadone for OUD, may be necessary for insurance gains to translate to greater methadone treatment access.40
The effect of expansion on methadone dispensed may also be gradual, possibly as OTP capacity slowly increases. Indeed, we found that removing early expansion states from our models decreased the point estimates of expansion's effect on methadone dispensed. This may suggest that early expansion states are inflating the main estimates, possibly because expansion did increase methadone dispensed in these states. That finding could mean more years of data are needed to detect effects of expansion on methadone dispensed in states that expanded in 2014. That said, excluding late expansion states from our models did not substantially increase our point estimates.
Contrary to our hypothesis, we estimated larger point estimates for Medicaid expansion in the states with the lowest physician Medicaid participation. Medicaid expansion may have increased the number of buprenorphine prescribers accepting Medicaid; however, we cannot verify this possibility because of lack of data on Medicaid acceptance among buprenorphine prescribers. More research on how policies affect Medicaid acceptance among SUD treatment providers is needed. Similarly, contrary to our hypothesis, we estimated larger point estimates of the effect of Medicaid expansion in states in the middle tercile of OTP concentration and OTP Medicaid acceptance. It is possible that these states represent the marginal cases where expansion had an effect on OTPs' decision to treat more patients. States with the lowest OTP concentrations and OTP Medicaid acceptance may be states where OTP capacity and Medicaid policies limited treatment access even when after coverage expansion, whereas states with the highest OTP capacity and OTP Medicaid acceptance may have been close to meeting treatment demand before expansion. That said, our imprecise estimates limit our ability to draw conclusions.
Our results regarding provider Medicaid acceptance should not be taken as definitive evidence that Medicaid acceptance is not a barrier to treatment expansion. In the lowest tercile of states by OTP Medicaid acceptance, only 43 percent of OTPs accepted Medicaid in 2013. Even in states where Medicaid covers methadone, Medicaid programs may employ low reimbursement rates and high administrative burdens that discourage OTPs from accepting Medicaid.41 Our use of physician Medicaid acceptance may have been an imperfect proxy for buprenorphine provider Medicaid acceptance. Approximately half of buprenorphine prescribers in a national survey reported accepting Medicaid for office visits,18 far below the reported average rates of physician Medicaid acceptance. Future studies should continue examining the role of Medicaid acceptance on OAT access, including policies to increased Medicaid acceptance. Evidence from Virginia suggests that increased Medicaid reimbursements for SUD services increased the number of buprenorphine prescribers billing Medicaid and the rate of OAT treatment among enrollees.42
Our analyses have limitations. Our data provide an all‐payer source of OAT medication dispensed, but the data do not allow us to observe the number of individuals receiving OAT or examine the extent to which buprenorphine prescriptions were for off‐label uses. We were also unable to account for Medicaid policy changes, such as changes to prior authorization, since comprehensive longitudinal data on these variables are unavailable.
Our results should not be taken to mean that expanding insurance coverage is not important in increasing access to OAT, but that insurance expansion is likely not enough. These results point to the importance of increasing the capacity of OAT providers, particularly for buprenorphine prescribers.
Supporting information
ACKNOWLEDGMENTS
Joint Acknowledgment/Disclosure Statement: Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under award number F30DA044668. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
No Other Disclosures.
Gertner AK, Robertson AG, Jones H, Powell BJ, Silberman P, Domino ME. The effect of Medicaid expansion on use of opioid agonist treatment and the role of provider capacity constraints. Health Serv Res. 2020;55:383–392. 10.1111/1475-6773.13282
REFERENCES
- 1. Ahmad F, Rossen L, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics; Published 2018. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Accessed May 22, 2019. [Google Scholar]
- 2. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence In: Mattick RP, ed. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2014:1‐69. [Google Scholar]
- 3. Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication‐assisted therapies — tackling the opioid‐overdose epidemic. N Engl J Med. 2014;370(22):2063‐2066. [DOI] [PubMed] [Google Scholar]
- 4. Stein BD, Gordon AJ, Sorbero M, Dick AW, Schuster J, Farmer C. The impact of buprenorphine on treatment of opioid dependence in a Medicaid population: Recent service utilization trends in the use of buprenorphine and methadone. Drug Alcohol Depend. 2012;123(1‐3):72‐78. [DOI] [PubMed] [Google Scholar]
- 5. Beronio K, Glied S, Frank R. How the Affordable Care Act and mental health parity and addiction equity act greatly expand coverage of behavioral health care. J Behav Heal Serv Res. 2014;41(4):410‐428. [DOI] [PubMed] [Google Scholar]
- 6. Mechanic D. Seizing opportunities under the affordable care act for transforming the mental and behavioral health system. Health Aff. 2012;31(2):376‐382. [DOI] [PubMed] [Google Scholar]
- 7. Buck JA. The looming expansion and transformation of public substance abuse treatment under the affordable care act. Health Aff. 2011;30(8):1402‐1410. [DOI] [PubMed] [Google Scholar]
- 8. Olfson M, Wall M, Barry CL, Mauro C, Mojtabai R. Impact of Medicaid expansion on coverage and treatment of low‐income adults with substance use disorders. Health Aff. 2018;37(8):1208‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feder KA, Mojtabai R, Krawczyk N, et al. Trends in insurance coverage and treatment among persons with opioid use disorders following the Affordable Care Act. Drug Alcohol Depend. 2017;179:271‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andrews CM, Grogan CM, Smith BT, et al. Medicaid benefits for addiction treatment expanded after implementation of the Affordable Care Act. Health Aff. 2018;37(8):1216‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mazurenko O, Balio CP, Agarwal R, Carroll AE, Menachemi N. The effects of Medicaid expansion under the ACA: a systematic review. Health Aff. 2018;37(6):944‐950. [DOI] [PubMed] [Google Scholar]
- 12. Wen H, Hockenberry JM, Borders TF, Druss BG. Impact of Medicaid expansion on Medicaid‐covered utilization of buprenorphine for opioid use disorder treatment. Med Care. 2017;55(4):336‐341. [DOI] [PubMed] [Google Scholar]
- 13. Meinhofer A, Witman AE. The role of health insurance on treatment for opioid use disorders: evidence from the Affordable Care Act Medicaid expansion. J Health Econ. 2018;60:177‐197. [DOI] [PubMed] [Google Scholar]
- 14. Maclean JC, Saloner B, Solander B.The effect of public insurance expansions on substance use disorder treatment: evidence from the Affordable Care Act. 2017. http://www.nber.org/papers/w23342. Accessed September 25, 2018. [PMC free article] [PubMed]
- 15. Roberts AW, Saloner B, Dusetzina SB. Buprenorphine use and spending for opioid use disorder treatment: trends from 2003 to 2015. Psychiatr Serv. 2018;69(7):832‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kresina TF, Litwin A, Marion I, Lubran R, Clark HW. United States Government oversight and regulation of medication assisted treatment for the treatment of opioid dependence. J Drug Policy Anal. 2009;2(1):1‐23. [Google Scholar]
- 17. Jones CM, Campopiano M, Baldwin G, McCance‐Katz E. National and state treatment need and capacity for opioid agonist medication‐assisted treatment. Am J Public Health. 2015;105(8):e1‐e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knudsen HK, Studts JL. Physicians as mediators of health policy: acceptance of Medicaid in the context of buprenorphine treatment. J Behav Health Serv Res. 2019;46:151‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saloner B, Levin J, Chang H, Jones C, Alexander GC. Changes in buprenorphine‐naloxone and opioid pain reliever prescriptions after the Affordable Care Act Medicaid expansion. JAMA Netw Open. 2018;1(4):e181588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaiser Family Foundation . Status of state action on the Medicaid expansion. Published 2018. http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/. Accessed November 1, 2016.
- 21. Sommers B, Arntson E, Kenney G, Epstein A. Lessons from early Medicaid expansions under the Affordable Care Act. Health Affairs Blog. https://www.healthaffairs.org/do/10.1377/hblog20130614.032114/full/. Published June 14, 2013. [Google Scholar]
- 22. Burns RM, Pacula RL, Bauhoff S, et al. Policies related to opioid agonist therapy for opioid use disorders: the evolution of state policies from 2004 to 2013. Subst Abus. 2016;37:63‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Medicaid and CHIP Payment and Access Commission . Medicaid enrollment changes following the ACA. Published 2018. https://www.macpac.gov/subtopic/medicaid-enrollment-changes-following-the-aca/. Accessed May 22, 2019.
- 24. Kaiser Family Foundation . Total monthly Medicaid and CHIP enrollment. Published 2018. http://kff.org/health-reform/state-indicator/total-monthly-medicaid-and-chip-enrollment/. Accessed April 19, 2016.
- 25. Snyder L, Rudowitz R, Ellis E, Roberts D. Medicaid enrollment: December 2013 data snapshot. 2014. http://kff.org/medicaid/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/.Enrollment. Accessed August 15, 2018.
- 26. US Census Bureau . Small area health insurance estimates. Published 2018. https://www.census.gov/data-tools/demo/sahie/#/. Accessed May 22, 2019.
- 27. Substance Abuse and Mental Health Services Administration . National survey of substance abuse treatment services. Published 2018. https://wwwdasis.samhsa.gov/dasis2/nssats.htm. Accessed May 22, 2019.
- 28. Decker SL. In 2011 nearly one‐third of physicians said they would not accept new Medicaid patients, but rising fees may help. Health Aff. 2012;31(8):1673‐1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bureau of Labor Statistics . Bureau of Labor Statistics data: unemployment rates. Seasonally adjusted unemployment rate: 2007‐2016. Published 2017. https://www.bls.gov/data/. Accessed May 22, 2019.
- 30. Hutchinson E, Catlin M, Andrilla CHA, Baldwin LM, Rosenblatt RA. Barriers to primary care physicians prescribing buprenorphine. Ann Fam Med. 2014;12(2):128‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kermack A, Flannery M, Tofighi B, McNeely J, Lee JD. Buprenorphine prescribing practice trends and attitudes among New York providers. J Subst Abuse Treat. 2017;74:1‐6. [DOI] [PubMed] [Google Scholar]
- 32. Andrilla CHA, Coulthard C, Larson EH. Barriers rural physicians face prescribing buprenorphine for opioid use disorder. Ann Fam Med. 2017;15(4):359‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas CP, Reif S, Haq S, Wallack SS, Hoyt A, Ritter GA. Use of buprenorphine for addiction treatment: perspectives of addiction specialists and general psychiatrists. Psychiatr Serv. 2008;59(8):909‐916. [DOI] [PubMed] [Google Scholar]
- 34. Barry DT, Irwin KS, Jones ES, et al. Integrating buprenorphine treatment into office‐based practice: a qualitative study. J Gen Intern Med. 2009;24(2):218‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haffajee RL, Bohnert ASB, Lagisetty PA. Policy pathways to address provider workforce barriers to buprenorphine treatment. Am J Prev Med. 2018;54(6):S230‐S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fiscella K, Wakeman SE, Beletsky L. Buprenorphine deregulation and mainstreaming treatment for opioid use disorder. JAMA Psychiatry. 2019;76(3):229. [DOI] [PubMed] [Google Scholar]
- 37. Jaffe J, O'Keeffe C. From morphine clinics to buprenorphine: regulating opioid agonist treatment of addiction in the United States. Drug Alcohol Depend. 2003;70(2):S3‐S11. [DOI] [PubMed] [Google Scholar]
- 38. Reif S, Horgan CM, Hodgkin D, Matteucci A‐MM, Creedon TB, Stewart MT. Access to addiction pharmacotherapy in private health plans. J Subst Abuse Treat. 2016;66:23‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Siegel Z. The hell of getting methadone when you're away from home. Vice. 2018. https://www.vice.com/en_us/article/a3mvgj/the-hell-of-getting-methadone-when-youre-away-from-home. Accessed May 22, 2019.
- 40. Samet JH, Botticelli M, Bharel M. Methadone in primary care — one small step for congress, one giant leap for addiction treatment. N Engl J Med. 2018;379(1):7‐8. [DOI] [PubMed] [Google Scholar]
- 41. Andrews CM, Grogan CM, Westlake MA, et al. Do benefits restrictions limit Medicaid acceptance in addiction treatment? Results from a national study. J Subst Abuse Treat. 2018;87:50‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cunningham P, Barnes A, Tong S, et al.Addiction and recovery treatment services access, utilization, and spending for the period of VCU Evaluation Staff. 2017. https://hbp.vcu.edu/media/hbp/policybriefs/pdfs/VCUARTS5monthreport.1.04.18.pdf. Accessed October 31, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


