Abstract
Background:
The purpose of this study was to quantify the association of late lower cranial neuropathy (late LCNP) with swallowing-related quality of life (QOL) and functional status among long-term oropharyngeal cancer (OPC) survivors.
Methods:
Eight hundred eighty-nine OPC survivors (median survival time: 7 years) who received primary treatment at a single institution between January, 2000 – December, 2013 completed a cross-sectional survey (56% response rate) that included the MD Anderson Dysphagia Inventory (MDADI) and self-report of functional status. Late LCNP events ≥3-months after cancer therapy were abstracted from medical records. Multivariate models regressed MDADI scores on late LCNP status adjusting for clinical covariates.
Results:
Overall, 4.0% (n=36) of respondents developed late LCNP with median time to onset of 5.25 years post-treatment. LCNP cases reported significantly worse mean composite MDADI (LCNP: 68.0 vs. no LCNP: 80.2, p<0.001). Late LCNP independently associated with worse mean composite MDADI (β= −6.7, p=0.015, 95%CI: −12.0, −1.3) as well as all MDADI domains after multivariate adjustment. LCNP cases were more likely to have a feeding tube at time of survey (OR= 20.5; 95%CI, 8.6 to 48.9), history of aspiration pneumonia (OR= 23.5; 95%CI, 9.6 to 57.6), and tracheostomy (OR= 26.9; 95%CI, 6.0 to 121.7).
Conclusions:
In this large survey study, OPC survivors with late LCNP reported significantly poorer swallowing-related QOL and had significantly higher likelihood of poor functional status. Further efforts are necessary to optimize swallowing outcomes to improve QOL in this subgroup of survivors.
Keywords: Oropharyngeal cancer, lower cranial neuropathy, radiotherapy, dysphagia, survivorship
INTRODUCTION
Swallowing is a complex and multifaceted neuromuscular process that involves 5 cranial nerves and almost 30 muscles in the upper aero-digestive tract. Patients with oropharyngeal cancer (OPC) receive local treatments, radiotherapy (RT), and/or surgery, to this functionally critical region that can cause chronic dysphagia with adverse impact on swallowing-related quality of life (QOL).1–6 Dysphagia is one of the most impactful and prevalent functional toxicities reported in approximately 30–50% of survivors.7–10 Prior analysis of this OPC survivorship found that, among 22 symptoms queried, the severity of dysphagia symptoms most strongly associated with decisional regret about cancer treatment.11 The rising incidence of highly curable HPV-associated OPC leads to greater numbers of OPC survivors at risk of dysphagia with great impetus to understand factors that associate with poor swallowing outcomes and adversely impact QOL in this growing population. Dysphagia also leads to excessive morbidity, negatively impacting functional status and health of OPC survivors. Impaired airway protection can lead to aspiration pneumonia, and inefficient bolus clearance may result in low food intake, extended gastrostomy tube dependence, weight loss, and malnutrition.12 Patients with dysphagia often modify their diet, need extended meal times, feel self-conscious to eat in social settings, and thereby experience social isolation and diminished QOL.12
Radiation-associated dysphagia is typically linked with soft tissue injuries including inflammation, edema, fibrosis, and stricture.13 Acute tissue injury results from cell depletion and inflammation that contribute to edema, erythema, and mucositis of the oropharyngeal region.13,14 Late RT injury is defined classically as 3 months or more after cancer treatment, and may represent persistence of early injury (i.e., “consequential late effects”) or new damage linked to excessive collagen accumulation, microvascular damage, and overproduction of pro-fibrotic growth factors β (TGF-β1) resulting in fibrosis and atrophy.14,15 The superior pharyngeal constrictor (SPC) region comprises minor nerve tracts and the constrictor and longitudinal pharyngeal muscles, which are important for pharyngeal shortening and constriction during swallowing for safe and efficient bolus propulsion into the esophagus.16 Irradiation to this region, specifically the mean SPC region dose, has been reported in numerous studies to be associated with chronic and late radiation associated dysphagia (late-RAD).16–19 Thereby dysphagia may occur as consequence of reduced base of tongue retraction and elevation of larynx, inadequate retroflexion of epiglottis, pharyngeal transit delay, and inadequate swallowing muscle action.14
Surgical treatment for OPC including tongue resection involving geniohyoid or mylohyoid muscles, mandibulotomy-related genioglossus injury and loss of occlusion, lateral soft palate resection may also cause muscle and nerve injury and contribute to dysphagia.13 Site and extent of tumor resection thereby contribute to severity of dysphagia.13 Reports also suggest that head and neck (HNC) patients treated with surgery followed by post-operative RT may experience cumulative effects and more accelerated effects of RT.6, 13, 20 This may contribute to additional decline in swallowing function due to diminished oropharyngeal swallow efficiency.6, 13, 20
Lower cranial neuropathies (LCNP) are a rare, but permanent late effect of HNC treatment that injures the glossopharyngeal (IX), vagus, (X), accessory (XI), and/or hypoglossal (XII) nerves.1, 21–24 These nerves (except XI) play a pivotal role in the oropharyngeal swallowing mechanism and thereby their damage can contribute to profound functional impairment in terms of dysphagia often with co-existing problems in speech and voice and shoulder impairment.1, 16, 21–25 A previous study among 59 OPC survivors treated with intensity modulated radiotherapy (IMRT) reported a 5% incidence rate of late LCNP at median follow-up of 5.7 years (range: 4.6–7.6 years).1 Among LCNP cases, onset of neuropathy preceded quantifiable, clinically significant decline in both patient-reported (per MD Anderson Dysphagia Inventory; MDADI) and clinician-rated (per Modified Barium Swallow Study; MBS) swallowing function.1 Likewise, the investigators recently published a large survey of 889 long-term OPC survivors in which LCNP was significantly associated with excess symptom burden and had the greatest impact on swallowing/ chewing and voice/speech symptoms among the 22 symptom items rated using the MD Anderson Symptom Inventory Head and Neck Cancer Module (MDASI-HN), a validated multi-symptom survey instrument.26
Previous literature also specifically implicates LCNP as a major contributor to late radiation associated dysphagia (late-RAD).21, 22 Patients with late RAD often have clinically detectable LCNP with unilateral paralysis, muscle wasting leading to atrophy of lingual and pharyngeal musculature with clinical series supporting a prominent role of nerve injury in the functional decline experienced by these patients.25 A series of 29 HNC survivors with late-RAD reported that 48% of cases had clinically-detectable cranial neuropathies, and cranial nerve XII and X palsies were most common.25 Several small published series and case reports consistently describe severe problems in swallowing, eating, and extreme functional impairment in pharyngeal phase of swallowing among survivors with late LCNP, with associated swallowing inefficiency, pharyngeal residue, and silent aspiration.1, 16, 21–25 Consequently, about 85% of OPC survivors with late-RAD develop pneumonia and more than 60% require long-term gastrostomy tube placement highlighting the possible extreme functional relevance of late LCNP if it indeed is a driver of late dysphagia.16, 22
The previous literature and prior analysis of symptom burden suggests a strong association between late LCNP and the severity of dysphagia, however the nature of this association has not been comprehensively evaluated or quantified in a large population of survivors. Few studies have addressed late LCNP among OPC survivors, as most of the published literature on LCNP has been comprised of case reports or studies primarily conducted among nasopharyngeal cancer (NPC) survivors.27, 28 Studies suggest that risk of cranial nerve damage increases over time 1, 22, 28 and as survival probabilities improve for OPC, there is an ever-growing pool of OPC survivors who have received surgery and/or curative doses of radiotherapy sufficient to induce LCNP. Therefore, there is urgent need to understand to our fullest ability the functional impact of this disabling late effect of therapy. Thus, the purpose of this analysis was to quantify the association of late LCNP with swallowing-related QOL using the MDADI and functional status metrics. We hypothesized that late LCNP among OPC survivors would be associated with significantly worse swallowing-related QOL (per MDADI survey scores) and LCNP status would relate to differences in functional status metrics.
MATERIALS AND METHODS:
Study Design, Eligibility and Consent
This cross-sectional survey was conducted in 2015 among a cohort of OPC survivors who received primary cancer treatment at MD Anderson Cancer Center (MDACC) between January, 2000 and December, 2013. An institutional review board-approved patient-reported outcome (PRO) survey was administered to eligible OPC survivors in the cohort who were ≥ 18 years of age at diagnosis, completed their treatment at least 1 year prior to survey administration, and consented to the study. Exclusion criteria were: patients who were deceased, those with second primary malignancy (SPM) or recurrent head and neck cancer tumors preceding survey, and those whose primary spoken language was not English. For this analysis, patients diagnosed with LCNP or with clinical signs of LCNP prior to initiation of OPC treatment were excluded. The survey items included in this analysis were the MDADI, a patient-reported adaptation of the Performance Status Scale for Head and Neck cancer (PSS-HN) with questions on normalcy of diet and public eating, as well as self-report of aspiration pneumonia, current feeding tube status, and current weight. A previous publication provides details of survey administration and response.7
MD Anderson Dysphagia Inventory (MDADI)
The MD Anderson Dysphagia Inventory (MDADI) is a 20-item validated patient reported outcomes (PRO) instrument that quantifies perceived limitations in swallowing ability and their impact on day to day activities.29 MDADI provides subscale scores which are comprised of emotional (6 questions), physical (8 questions), and functional components (5 questions). It also estimates a global summary score (based on 1 question- “My swallowing limits my day to day activities”) and a composite score (based on 19 questions excluding the global item).29–32
Scoring of MDADI: The questions related to swallowing function are Likert scaled with the options of ‘strongly agree’, ‘agree’, ‘no opinion’, ‘disagree’, or ‘strongly disagree’, scored on a scale of 1–5, respectively, with the exception of two questions (E7 and F2) for which reverse scoring is calculated. After summation of response scores, mean is estimated and multiplied by 20 to estimate total score.33 Total scores range from 20–100 with higher scores reflecting higher perceived swallowing-related QOL.12, 29, 32, 33 MDADI scores can be analyzed as continuous or categorical variables with scores classified in the following categories: ≥80 as optimal, 60–79 as adequate and <60 as poor.10 MDADI was validated among HNC patients and has internal consistency scored by Cronbach’s alpha of 0.96 and was documented to have test-rest reliability correlations ranging from 0.69 to 0.88.29
Performance Status Scale for Head and Neck (PSS-HN) Adaptation
An adapted version of the PSS-HN, a validated, clinician-rated interview-based measure of performance status among HNC patients was included in the survey instrument.1 The scale was adapted for patient-reported administration and comprised of questions pertaining to the survivor’s diet level and public eating experience.1 Normalcy of diet options included the following: full diet no restriction, full diet with liquid assist, solid food but avoid some hard to eat foods, soft chewable foods, non-chewable or pureed foods, drink warm and cold liquids only, or nothing orally only use a feeding tube. Public eating was coded as the following: no restriction of place, food, or companion, no restriction of place, restrict diet in public, eat only in the presence of selected person in selected places, only eat at home with selected persons, or always eat alone.
Primary and Secondary Outcomes
The primary outcome for this study was mean composite MDADI score which serves as an estimate of overall swallowing-related quality of life.29–33 The secondary outcomes for analysis included the emotional, physical and functional subscale and the global MDADI scores as well as self-reported functional status metrics including current feeding tube status, normalcy of diet, public eating, history of aspiration pneumonia, current weight, understandability of speech, and current tracheostomy. Chart abstracted functional data included baseline weight to calculate percent change in weight between weight at time of survey and pre-treatment weight, and history of dilations due to presence of stricture. Current feeding tube status, aspiration pneumonia history, and current tracheostomy were coded as binary variables. Change in weight was calculated as baseline weight minus current weight and percent change in weight was calculated as change in weight divided by baseline weight. Survey questions on functional status metrics have been listed in Appendix 2.
Primary Exposure
Late LCNP was the primary exposure for this analysis. Late LCNP case status was ascertained by detailed review of medical records of survivors as previously described.26 For this study late LCNP was defined as clinical evidence of neuropathy of at least one of the glossopharyngeal (IX), vagus (X), and hypoglossal (XII) nerves ≥ 3 months after the end of cancer treatment.26 The time period was defined considering the NCI-Common Toxicity Manual’s definition of late radiation effects as occurring 90 days and onwards after RT therapy initiation.34
Clinical and Demographic Variables
Demographic variables including age at diagnosis, sex, race, and education, and clinical variables including primary tumor subsite, tumor and nodal staging (AJCC version VII), treatment modality, chemotherapy, surgery, neck dissection, RT dose, fractionation, and modality were abstracted from the electronic medical records. Pre-treatment diet (ability to eat solid foods) was also collected as a surrogate variable for presence of baseline dysphagia. Survival time for this population was estimated as the difference between age of diagnosis and age at the time of the survey. History of pharyngoesophageal dilation was used as a surrogate variable for stricture which can contribute to dysphagia and act as a confounder in our analysis.
Statistical Analysis
Demographic, clinical, and treatment variables and distribution of MDADI scores by these variables were summarized using descriptive statistics and univariate analysis. With a rare event leading to small case numbers for our primary exposure (LCNP), imputation of MDADI scores was conducted to minimize loss of statistical power due to skipped or missing MDADI items. Imputation used the mean of responses to MDADI items among those patients who responded to that specific item (mean score among non-missing on that item).35 Post-hoc sensitivity analysis was conducted to assess the impact of imputed, missing MDADI responses on study results.
Multiple linear regression was used to investigate the association between late LCNP and MDADI scores controlling for confounders following model building strategies using the purposeful variable selection method.36 Age, subsite, T-stage, treatment modality and smoking based on previous literature were defined a priori as clinically important variables and retained for adjustment in all models. Variance inflation factor was used to assess collinearity among variables. Biologically plausible interaction terms were also assessed using the likelihood ratio tests and were considered statistically significant when p-values were < 0.05. Adequacy and fit of model were assessed using R squares, adjusted R squares and Chi-square goodness of fit tests. Coefficients (univariate and multivariate adjusted) for impact of late LCNP on MDADI scores and their 95% confidence intervals (CI) were estimated. As secondary analyses, the relationships between late LCNP and functional status metrics were assessed according to their distributions using the Fisher’s exact test, Wilcoxon rank-sum test, and Kruskal Wallis test. All reported p-values are two-sided and considered statistically significant at p-value of ≤ 0.05. Statistical analysis was conducted using the STATA software, version 14.0 (StataCorp LP, College Station, TX).
RESULTS
Sample Characteristics
A total of 889 eligible OPC survivors with a median survival time 7.0 (range, 1–16) years were included in the analysis. Table 1 displays the distribution of demographic, tumor, and treatment-related characteristics in the study population. The patient characteristics of this study population have been described fully in an earlier publication.19 Briefly, 84.7% were male, 92.4% were white, 71.7% were educated beyond high school, 76.4% had been treated for T1-T2 tumors, 98.9% could eat a normal solid-food diet prior to treatment, 99.1% were treated with RT of which 76.6% were treated with intensity-modulated radiotherapy split-field technique (IMRT-SF) and median radiation dose was 70 Gy (range, 40–73 Gy). Definitive surgery was rare (2.7%).
Table 1:
Patient Characteristics (N=889), late LCNP rate, and mean composite MDADI scores
| Composite MDADI Score ± Standard Deviation) | ||||
|---|---|---|---|---|
| Variables | All Patients (n=889) | Patients with LCNP (n=36) | All patients (n=889) | P-valuea, b |
| Continuous Variables | P-valuea | |||
| Age at diagnosis, median (range) | 56 (32–84) | 57 (42–72) | rho = −0.034 | 0.306 |
| Survival time, median (range) | 7 (1–16) | 10.5 (2–16) | rho = −0.076 | 0.023 |
| Radiation Dose, Gy. median (range) | 70 (40–73) | 70 (60–72) | rho = −0.201 | < 0.001 |
| Categorical Variables | All Patients n (%) | n (%) Patients with LCNP | All patients (n=889) | P-valueb |
| Sex | 0.443 | |||
| Female | 136 (15.3) | 5(3.7) | 78.3 ±17.5 | |
| Male | 753 (84.7) | 31(4.1) | 79.9 ±16.3 | |
| Education | < 0.001 | |||
| ≤Highschool | 168(18.9) | 8(4.8) | 75.6 ±16.7 | |
| >Highschool | 637(71.7) | 27(4.2) | 80.9 ±15.9 | |
| Missing | 84(9.4) | 1(1.2) | 78.6 ±18.9 | |
| Race | 0.983 | |||
| Others | 59(6.6) | 3(5.0) | 78.5 ±20.0 | |
| White | 821(92.4) | 32(3.9) | 79.8 ±16.2 | |
| Missing | 9(1.0) | 1(11.1) | 78.4 ±19.3 | |
| Primary Site | 0.200 | |||
| Tonsil | 438(49.3) | 17(3.8) | 80.3 ±16.4 | |
| Base of Tongue | 451(50.7) | 19(4.2) | 79.1 ±16.6 | |
| T classification | < 0.001 | |||
| 1 | 334(37.6) | 8(2.4) | 82.6 ±15.2 | |
| 2 | 345(38.8) | 13(3.8) | 80.8 ±15.7 | |
| 3 | 131(14.7) | 8(6.1) | 75.8 ±17.0 | |
| 4 | 79(8.9) | 7(8.9) | 68.7 ±18.9 | |
| N classification | 0.007 | |||
| N0 | 81(9.1) | 3(3.7) | 79.9 ±16.1 | |
| N1+2a | 236(26.5) | 7(2.9) | 81.8 ±14.7 | |
| 2b+3 | 429(48.3) | 19(4.4) | 80.1 ±16.4 | |
| 2c | 143(16.1) | 7(4.9) | 74.7 ±18.9 | |
| HPV status | 0.033 | |||
| Negative | 56(6.3) | 2(3.6) | 80.9 ±16.8 | |
| Positive | 429(48.3) | 9(2.1) | 81.0 ±15.9 | |
| Unknown | 404(45.4) | 25(6.2) | 78.1 ±17.0 | |
| Smoking | < 0.001 | |||
| Former | 422(47.5) | 17(4.0) | 79.0 ±16.3 | |
| Current | 58(6.5) | 3(5.2) | 72.5 ±17.9 | |
| Solid Food pre-Tx | 0.846 | |||
| Yes | 879(98.9) | 35(4.0) | 79.9 ±14.0 | |
| No | 10(1.1) | 1(10.0) | 79.7 ±16.5 | |
| Treatment Group | < 0.001 | |||
| Single Modality | 278(31.3) | 11(4.0) | 83.2 ±14.3 | |
| Multimodality | 611(68.7) | 25(4.1) | 78.1 ±17.2 | |
| Treatment Group | 0.001 | |||
| RT alone | 270(30.4) | 11(4.1) | 83.0 ±14.4 | |
| Surgery alone | 8(0.9) | 0 | 89.9 ±9.4 | |
| RT plus systemic | 596(67.0) | 23(3.9) | 78.1 ±17.3 | |
| Surgery plus adjuvant | 15(1.7) | 2(13.3) | 78.4 ±14.2 | |
| Radiotherapy | 0.068 | |||
| No | 8(0.9) | 0 | 89.9 ±9.4 | |
| Yes | 881(99.1) | 36(4.1) | 79.6±16.5 | |
| Chemotherapy | < 0.001 | |||
| No | 284(32.0) | 11(3.9) | 83.0 ±14.3 | |
| Yes | 605(68.0) | 25(4.1) | 78.1 ±17.2 | |
| Surgery | 0.403 | |||
| No | 865(97.3) | 34(3.9) | 79.6 ±16.6 | |
| Yes | 24(2.7) | 2(8.3) | 83.0 ±13.8 | |
| Neck Dissection | 0.431 | |||
| No | 665(74.8) | 27(4.1) | 79.9 ±16.5 | |
| Yes | 224(25.2) | 9(4.0) | 79.0 ±16.5 | |
| RT Schedule | 0.002 | |||
| Standard Fractionation | 778(88.3) | 21(2.7) | 80.3 ±16.1 | |
| Accelerated | 95(10.8) | 15(15.8) | 73.5 ±18.3 | |
| Other | 8(0.9) | 0 | 78.3 ±24.3 | |
| RT Type | < 0.001 | |||
| 3d Conformal | 50(5.7) | 9(18.0) | 67.8 ±20.4 | |
| IMRT-SF | 675(76.6) | 23(3.4) | 79.6 ±16.1 | |
| IMRT-WF | 33(3.8) | 1(3.0) | 74.7 ±17.8 | |
| Proton | 23(2.6) | 1(4.4) | 87.5 ±11.3 | |
| IMRT Ipsilateral | 100(11.3) | 2(2.0) | 84.9 ±14.3 | |
| Dilation/Stricture | < 0.001 | |||
| No | 873 (98.2) | 31(3.6) | 80.0 ± 16.3 | |
| Yes | 16 (1.8) | 5(31.3) | 61.0 ± 14.6 | |
Abbreviations: T, tumor; RT, radiotherapy; MDADI, MD Anderson Dysphagia Inventory (MDADI); rho, Spearman rho; pre-Tx, pre-treatment; 3d Conformal, Three Dimensional (3D) Conformal Radiation Therapy; IMRT-SF, Intensity-modulated radiation therapy with split field technique; IMRT-WF, Intensity-modulated radiation therapy with whole field technique.
P-value for Continuous Variables and Composite scores calculated using Spearman Test.
P-value for Categorical Variables and Composite scores calculated using Kruskal Wallis Test.
Late Lower Cranial Neuropathy
Overall, 36 (4.0%) OPC survivors were diagnosed with late LCNP with median time to LCNP onset after treatment of 5.3 (range, 0.3–12.3) years. Among them, 21 (58.3%) of LCNP cases had been treated for T1-T2 tumors, 35 (97.2%) reported eating a normal solid-food diet prior to treatment, all 36 of them received RT, 23 (63.9%) were treated with RT in combination with systemic treatment, 2 (5.6%) had surgery to the primary OPC tumor, 9 (25.0%) had neck dissection, and 23 (63.9%) were treated with IMRT-SF.
MDADI composite scores
The MDADI composite scores reported by OPC survivors are summarized in Table 1. Lowest (worse) scores were reported by patients with T4 tumors (68.7 ± 18.9) and those treated with 3-dimensional conformal RT technique (67.8 ± 20.4), whereas the highest (better) scores were reported by patients who did not receive RT (89.9 ± 9.4) and those treated with proton therapy (87.5 ± 11.3). Unadjusted univariate analyses demonstrated that survival time, education, T-classification, smoking, therapeutic modality, chemotherapy, RT dose, fractionation, and modality, and stricture had significant associations (p<0.25) with composite MDADI scores. Composite MDADI scores were also significantly different based on patient-reported diet levels at the time of survey (p< 0.001).
Late LCNP cases reported significantly worse composite MDADI scores compared to those without LCNP (LCNP: 68.0 ± 17.4, 95%CI, 62.1 to 73.9 vs. no LCNP: 80.2 ± 16.3, 95%CI, 79.1 to 81.3, p< 0.001). Multiple linear regression identified that late LCNP was significantly associated with lower (worse) composite MDADI scores (coefficient, −6.7; 95%CI, −12.0 to −1.3; p value = 0.015; adjusted R2, 0.13) after adjusting for age, survival time, sex, education, subsite, T-stage, smoking, therapeutic modality, RT modality, solid food diet prior to treatment, and stricture. These results have been summarized in Table 2. When MDADI composite scores were categorized, 38.9% (14/36) LCNP cases had poor swallowing scores (MDADI<60) in comparison to 12.9% (110/853) patients without LCNP (OR= 4.3; 95%CI, 2.2 to 8.6).
Table 2.
Univariate and Multivariate Regression: Composite MDADIa (N=889)
| Variables | Univariate Analysis Coefficient (95%CI) | P value | Multivariate Analysis Coefficient (95%CI) | P value |
|---|---|---|---|---|
| Late LCNP | ||||
| No | Reference | Reference | ||
| Yes | −12.2 (−17.6, −6.7) | < 0.001 | −6.6 (−12.0, −1.3) | 0.015 |
| Age at diagnosis | −0.1 (−0.2, 0.1) | 0.328 | −0.1 (−0.2, 0.1) | 0.275 |
| Survival Time | −0.4 (−0.7, −0.1) | 0.009 | −0.2 (−0.6, 0.1) | 0.151 |
| Radiation Dose | −1.1 (−1.5, −0.7) | < 0.001 | ||
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 1.6 (−1.4, 4.6) | 0.305 | 2.3 (−0.6, 5.2) | 0.119 |
| Education | ||||
| ≤Highschool | Reference | Reference | ||
| >Highschool | 5.3 (2.5, 8.1) | < 0.001 | 4.2 (1.5, 6.9) | 0.002 |
| Missing | 3.0 (−1.3, 7.3) | 0.167 | 2.8 (−1.4, 7.0) | 0.196 |
| Race | ||||
| Others | Reference | |||
| White | 1.3 (−3.1, 5.7) | 0.556 | ||
| Missing | −0.1 (−11.7, 11.5) | 0.987 | ||
| Primary Site | ||||
| Tonsil, soft palate, & | Reference | Reference | ||
| pharyngeal wall | ||||
| Base of tongue & GPS | −1.2(−3.4, 1.0) | 0.282 | −1.1 (−3.4, 1.2) | 0.334 |
| T classification | ||||
| 1 | Reference | Reference | ||
| 2 | −1.8 (−4.2, 0.6) | 0.139 | −1.1 (−3.6, 1.5) | 0.407 |
| 3 | −6.9 (−10.1, −3.6) | < 0.001 | −3.3 (−6.8, 0.3) | 0.069 |
| 4 | −14.0 (−17.9, −10.0) | < 0.001 | −9.9 (−14.1, −5.8) | < 0.001 |
| Smoking | ||||
| Never | Reference | Reference | ||
| Former | −2.4 (−4.6, −0.1) | 0.039 | −1.6 (−3.8, 0.5) | 0.141 |
| Current | −8.9 (−13.4, −4.3) | < 0.001 | −7.0 (−11.4, −2.7) | 0.001 |
| Solid Food pre-Tx | ||||
| Yes | Reference | Reference | ||
| No | −0.2 (−10.5, 10.1) | 0.965 | −2.1 (−12.0, 7.8) | 0.675 |
| Treatment Group | ||||
| Single modality Tx. | Reference | Reference | ||
| Multimodality Tx. | −5.1 (−7.4, −2.8) | < 0.001 | −2.7 (−5.4, −0.1) | 0.046 |
| Radiotherapy | ||||
| No | Reference | |||
| Yes | −10.4 (−21.9, 1.1) | 0.077 | ||
| Chemotherapy | ||||
| No | Reference | |||
| Yes | −4.9 (−7.2, −2.6) | < 0.001 | ||
| Surgery | ||||
| No | Reference | |||
| Yes, | 3.5 (−3.2, 10.1) | 0.310 | ||
| Neck Dissection | ||||
| No | Reference | |||
| Yes | −0.9 (−3.4, 1.6) | 0.497 | ||
| RT Schedule | ||||
| Standard Fractionation | Reference | |||
| Accelerated | −6.9 (−10.4, −3.4) | < 0.001 | ||
| Missing | −2.0 (−13.5, 9.4) | 0.731 | ||
| RT Type | ||||
| 3d Conformal | Reference | Reference | ||
| IMRT-SF | 11.8 (7.2, 16.4) | < 0.001 | 8.1 (3.1, 13.1) | 0.002 |
| IMRT-WF | 6.9 (−0.2, 14.0) | 0.057 | 5.9 (−1.3, 13.0) | 0.107 |
| Proton | 19.7 (11.7, 27.7) | < 0.001 | 14.4 (6.0, 22.9) | 0.001 |
| IMRT-Ipsilateral | 17.1 (11.6, 22.5) | < 0.001 | 9.9 (3.8, 16.0) | 0.002 |
| Stricture/Dilation | ||||
| No | Reference | |||
| Yes | −19.0 (−27.1, −10.9) | < 0.001 | −13.1 (−21.1, −5.2) | 0.001 |
Abbreviations: T, tumor; RT, radiotherapy; MDADI, MD Anderson Dysphagia Inventory (MDADI); rho, Spearman rho; pre-Tx, pre-treatment; 3d Conformal, Three Dimensional (3D) Conformal Radiation Therapy; IMRT-SF, Intensity-modulated radiation therapy with split field technique; IMRT-WF, Intensity-modulated radiation therapy with whole field technique. Statistical significance p value < 0.25 after Univariate Analysis. Statistical significance p value < 0.05 after Multivariate Analysis.
Missing values imputed.
MDADI Subscale Scores
Late LCNP cases reported significantly lower (worse) scores on all MDADI subscales and on global MDADI scores. The associations remained significant in multiple linear regression models after adjusting for significant covariates. These results are summarized in Table 3. Additionally, global MDADI scores were also highly correlated with composite MDADI scores (Spearman’s rho = 0.8, p<0.001).
Table 3:
MDADI Scores by late LCNP Status (N=889)
| Mean ± SD (95%CI) | Analysis Coefficient (95%CI) | |||||
|---|---|---|---|---|---|---|
| MDADI SCORESa | Patients with LCNP (n=36) | Patients without LCNP (n=853) | P value | Univariate (95%CI) | Multivariate (95%CI) | P value |
| Composite | 68.0 ± 17.4 (62.1 to 73.9) | 80.2 ±16.3 (79.1 to 81.3) | < 0.001 | −12.2 (−17.6 to −6.7) | −6.7 (−12.0 to −1.3) | 0.015 |
| Global | 65.1 ± 28.9 (55.3 to 74.8) | 81.3 ± 23.2 (79.8 to 82.9) | < 0.001 | −16.3 (−24.1 to −8.4) | −9.1 (−17.0 to −1.3) | 0.023 |
| Emotional | 70.1±19.2 (63.6 to 76.5) | 81.0±16.4 (79.9 to 82.1) | < 0.001 | −10.9 (−16.5 to −5.4) | −5.9 (−11.4 to −0.3) | 0.038 |
| Physical | 62.5±18.0 (56.4 to 68.6) | 75.9±19.0 (74.6 to 77.2) | < 0.001 | −13.5 (−19.8 to −7.1) | −7.7 (−14.0 to −1.3) | 0.018 |
| Functional | 74.4±20.7 (67.4 to 81.4) | 86.0±16.1 (84.9 to 87.1) | < 0.001 | −11.6 (−17.1 to −6.1) | −6.0 (−11.4 to −0.6) | 0.028 |
Abbreviations: MDADI, MD Anderson Dysphagia Inventory (MDADI); LCNP, lower cranial neuropathy. Multiple linear regression models adjusted covariates including, age, survival time, sex, education, subsite, T-stage, smoking, therapeutic modality, RT modality, solid food diet prior to treatment, and stricture. The regression model for global scores adjusted for an additional variable, neck dissection.
Missing values imputed.
Functional status metrics
LCNP status also significantly associated with (p ≤0.001) worse functional outcomes and health metrics reported by the patient or chart abstracted at the time of survey as detailed in Table 4. LCNP cases were more likely to have a current feeding tube (OR= 20.5; 95%CI, 8.6 to 48.9), history of aspiration pneumonia (OR= 23.5; 95%CI, 9.6 to 57.6), tracheostomy (OR= 26.9; 95%CI, 6.0 to 121.7), and were more likely to have undergone dilation for stricture (OR= 12.3; 95%CI, 4.2 to 36.3) than patients without LCNP. LCNP cases were also more likely to report restricted oral diets at the time of survey (LCNP: OR= 3.5; 95%CI, 1.5 to 8.3). Mean percentage of reported weight loss from baseline weight to weight at time of survey was also significantly higher among LCNP cases than patients without LCNP (LCNP: mean 11.7% vs. no LCNP: 6.0%, p=0.002).
Table 4:
Functional Status Metrics by late LCNP status (n=889)
| Variables | Patients with LCNP n (%) | Patients without LCNP n (%) | P-value | Crude OR (95%CI) |
|---|---|---|---|---|
| Current Feeding Tube | < 0.001 | |||
| No | 25 (71.4) | 819 (98.1) | Reference | |
| Yes | 10 (28.6) | 16 (1.9) | 20.5 (8.6 to 48.9) | |
| Normalcy Diet | < 0.001 | |||
| Full Diet no restrictions | 6 (18.2) | 357 (43.7) | Reference | |
| Full Diet with liquid assist | 8 (24.2) | 315 (38.5) | 3.5 (1.5 to 8.3) | |
| Solid food but avoid some hard to eat foods | 10 (30.3) | 96 (11.7) | ||
| Soft chewable foods | 2 (6.1) | 33 (4.0) | ||
| Non-chewable or pureed foods | 1 (3.0) | 3 (0.4) | ||
| Warm and cold liquids | 2 (6.1) | 10 (1.2) | ||
| Not eat or drink anything by mouth | 4 (12.1) | 4 (0.5) | ||
| Public Eating | < 0.001 | |||
| No restriction of place/food/companion | 8 (25.8) | 582 (70.3) | Reference | |
| No restriction of place, but restrict diet in public | 14 (45.2) | 191 (23.1) | 6.8 (3.1 to 15.1) | |
| In presence of selected person in selected places | 7 (22.6) | 36 (4.3) | ||
| Only eat at home with selected persons | 1 (3.2) | 14 (1.7) | ||
| Always eat alone | 1 (3.2) | 5 (0.6) | ||
| Aspiration Pneumonia | < 0.001 | |||
| No | 21 (67.7) | 741 (98.0) | Reference | |
| Yes | 10 (32.3) | 15 (2.0) | 23.5 (9.6 to 57.6) | |
| Weight loss | 0.050 | |||
| No | 4 (11.4) | 202 (24.4) | Reference | |
| Yes | 31 (88.6) | 626 (75.6) | 2.5 (0.9 to 6.9) | |
| Change in Weight; mean, median (range)a | 22.9, 16.8(14.2,87.8) | 13.3, 9.4(103.1,164.6) | 0.005 | |
| % Change in Weight; mean ± SD, median, (range)b | 11.7±10.4, 9.9(−7.9,33.4) | 6.0 ±10.7, 5.1(−96.4, 43.4) | 0.002 | |
| Understandability of Speech | < 0.001 | |||
| Always understandable | 6 (17.6) | 528 (63.3) | Reference | |
| Understandable most of the time | 16 (47.1) | 269 (32.3) | 8.1 (3.4 to 19.2) | |
| Usually understandable | 3 (8.8) | 19 (2.3) | ||
| Difficult to understand | 8 (23.5) | 17 (2.0) | ||
| Never understandable | 1 (2.9) | 1 (0.1) | ||
| Tracheostomy | 0.001 | |||
| No | 31 (91.2) | 834 (99.6) | Reference | |
| Yes | 3 (8.8) | 3 (0.4) | 26.9(6.0 to 121.7) | |
| Dilation/ Stricture | < 0.001 | |||
| No | 31 (86.11) | 842 (98.71) | Reference | |
| Yes | 5 (13.89) | 11 (1.29) | 12.3 (4.2 to 36.3) |
P values estimated by Fishers Exact Test.
P values estimated by Wilcoxon Rank-Sum Test. Odds Ratio for normalcy of diet calculated with full diet no restrictions as reference category and all other categories collapsed. Odds Ratio for public eating calculated with no restriction of place/food/companion as reference category and all other categories collapsed. Odds Ratio for understandability of speech calculated with always understandable as reference category and all other categories collapsed.
DISCUSSION
Late LCNP is rare with reports of incidence ranging from 3.7% to 25.6%. However, our previous report confirmed high symptom burden among OPC survivors who developed LCNP, with largest effect sizes (coefficient, 2.3 of 10) on swallowing-related symptoms.26 This phenomenon is also clinically recognized, but previous work has failed to quantify the impact of LCNP on individual swallowing domains and functional metrics. This large single-center cross-sectional survivorship survey study among OPC survivors provides a comprehensive evaluation and found significant associations with moderate effect size between late LCNP and overall swallowing-related quality of life, domain-specific swallowing function, as well as functional status metrics related to swallowing.
Overall, swallowing-related quality of life among all 889 OPC respondents suggested most survivors perceived acceptable levels of functioning (as per composite MDADI means of 79.7 ± 16 and 55.2% of survivors reported composite scores ≥80), but the small group of survivors (n=36) with late LCNP reported a clinically meaningful reduction of > 10 points difference relative to survivors without LCNP in univariate analyses.37 This meaningful reduction was observed for all summary and domain-specific MDADI scores. After multivariate adjustment for clinical covariates, on an average, composite MDADI scores were 6.7 points lower (worse) among late LCNP cases versus those without late LCNP. The adjusted R2 demonstrated that late LCNP explained 13% of the variation in composite MDADI scores after accounting for the effect of other covariates, which according to Cohen’s criteria is a moderate effect.38 This moderate effect size is consistent with effect estimate for the impact of LCNP on patient-reported MDASI-HN swallowing/chewing symptoms (coefficient, 2.3 of 10) reported in an earlier study and may in part reflect the subjective nature of PROs that likely vary with individuals’ overall contentment and satisfaction with life and functional abilities.12, 13, 39
Late LCNP was also significantly associated with all domain-specific MDADI subscale scores. Late LCNP cases experienced the greatest deterioration of physical subscale scores which represent patient perception of swallowing ability; LCNP explained 10% of the variation in this domain controlling for important confounders. Previous studies have also reported lowest MDADI scores on the physical subscale among HNC patients.10, 37 Further, among late LCNP cases, the least impact of nerve injury was on the emotional subscale scores. Emotional subscale scores reflect psychological response to diminished swallowing ability and functional subscale scores reflect the impact of swallowing impairment on daily functioning and activities.32 Previous studies among HNC patients have reported the highest subscale scores in the functional domain and substantial recovery of emotional MDADI scores over time.10, 40 This may be indicative of adjustment and adaptation to a decline in swallowing function overtime.40
It is generally believed that PRO instruments may underestimate the prevalence of dysphagia.40, 41 For this reason, we also explored the relationship between LCNP with other functional status measures of swallowing ability. As expected, late LCNP status was also significantly associated with worse functional status metrics including current feeding tube status, normalcy of diet, public eating, self-reported history of aspiration pneumonia, weight loss since diagnosis, understandability of speech, tracheostomy, and esophageal dilations due to presence of stricture. Thereby late LCNP was consistently associated with substantial functional morbidity among OPC survivors. These results are not surprising given the degree of swallowing dysfunction previously reported among long-term OPC survivors in earlier case reports that suggested that treatment-related LCNP may play a major role in late RAD, and precipitate delayed but extreme oropharyngeal impairment as recorded by MBS studies.1, 21, 22
Approximately one-third (28.6%) of late LCNP patients in our study, reported having a feeding tube at the time of survey. High rates of gastrostomy dependence among LCNP cases again support a high prevalence of dysphagia in this population. In an earlier study among OPC patients with advanced stage treated with concurrent RT and chemotherapy, feeding tube use had the maximum impact on QOL (−30 points compared to controls) evaluated by SF36 and HNQOL.42 Late LCNP cases also had significantly higher rates of aspiration pneumonia (32.3%), which support association with high dysphagia-related morbidity. Similarly, a study using SEER data among HNC patients treated with chemoradiation reported 23.8% five-year rates of aspiration pneumonia.43 Additionally, as late LCNP occurs many years after treatment with a tendency for silent aspiration, symptoms of LCNP may be missed due to lack of adequate surveillance among OPC survivors. This may further enhance risk of aspiration pneumonia and contribute to debilitating functional morbidity with increased feeding tube dependence, hospitalization, weight loss, and life-threatening complications.
Overall, late LCNP with accompanying dysphagia is a clinical condition of great concern as it does not typically respond well to treatment. With progressive long-term functional decline onset with aspiration and recurring aspiration-pneumonia, long-standing feeding tube dependence and elective laryngectomy may be required.1, 16, 21, 22, 44 Therefore, risk-reduction and management of late effects like LCNP, late-RAD and associated functional toxicities need to be prioritized in contemporary HNC treatment and management. This research may help to provide benchmarks for novel interventions and surveillance efforts. Routine PRO administration coupled with instrumental examination using Fiberoptic Endoscopic Evaluation of Swallowing (FEES) and MBS may also help identify patients in need of more intense, targeted therapy.44 Multi-disciplinary supportive treatment including routine swallowing and speech assessment, risk-based treatment planning, swallowing and nutritional therapy, counselling to improve coping skills, and guidance in effective meal preparation may help to attenuate the impact of late LCNP-associated swallowing impairment, diminish life-threatening complications, and enhance swallowing-related QOL.44
This study is the first to quantify the association between late LCNP and swallowing-related quality of life in a study population of almost 900 OPC survivors finding the hypothesized significant associations. However, there are limitations to acknowledge. Complete case analysis was not feasible as 126/889 (14.2%) respondents returned surveys with skipped or missing MDADI items. Thus, complete case analysis would have contributed to attrition of approximately one-third of LCNP cases that would have substantially diminished power in our study that focused on a rare event like LCNP. Therefore, we imputed missing MDADI scores. The validity of our imputed results is supported by sensitivity analyses finding similar effect size estimates using imputed vs non-imputed data (Appendix: Table 1). Since respondents were far more likely not to have LCNP (given the low rate of LCNP) and report higher (better) scores, imputation using the group mean was more likely to bias study effect estimates towards the null. Thereby, our study results may underestimate the impact of late LCNP on MDADI scores. Post-imputation, unadjusted means and accompanying standard deviations of composite, global, emotional, physical, and functional scores were similar to estimates of means and standard deviations of an earlier study among HNC patients.37 Further, consistency of results with previous literature was demonstrated as survivors in our study treated with multimodality treatment versus single modality, those who did not receive chemotherapy versus those who did, those treated with accelerated RT versus standard fractionation, those who received conventional 3D conformal RT versus IMRT/ proton therapy and current smokers versus never smokers reported significantly worse composite scores and those with early stage versus more advanced stages reported significant positive trend for better swallowing scores5, 8–10, 33, 44 These results indicate that our primary outcome variable, composite MDADI variable consistently performed well and showed expected variation across clinical and tumor-related factors. Large and statistically significant differences in functional metrics by LCNP status also support our findings of high functional morbidity among LCNP cases. Our study results also support a previous survey analysis in this study population, which used complete case analysis of MDASI-HN, with low attrition of cases due to missing data and demonstrated a strong impact of LCNP on swallowing, choking, mucus, fatigue and voice symptoms.26 Our study may also be subject to limitations inherent to cross-sectional PRO survey collection including survival bias, which we tried to alleviate by controlling for survival time in all our multivariate models. Finally, our study population has been treated at a single tertiary cancer care institution and its demographic characteristics may limit generalizability to other more varied populations. However, our study population demographics are similar to those expected among OPC patients across the US.
CONCLUSIONS
In this large cross-sectional analysis, OPC survivors with late LCNP had significantly lower (worse) swallow-related QOL as per MDADI scores with significantly higher likelihood of adverse functional status metrics like dietary restrictions, nutritional impairment, weight-loss, decline in public food consumption with possible consequences of social isolation, aspiration pneumonia, long-term feeding tube dependence, and tracheostomy. These data support and quantify the detrimental relationship of late LCNP with swallowing-related measures.
Supplementary Material
Figure 1. Multivariate Adjusted Coefficients for Late LCNP and MDADI Scores.
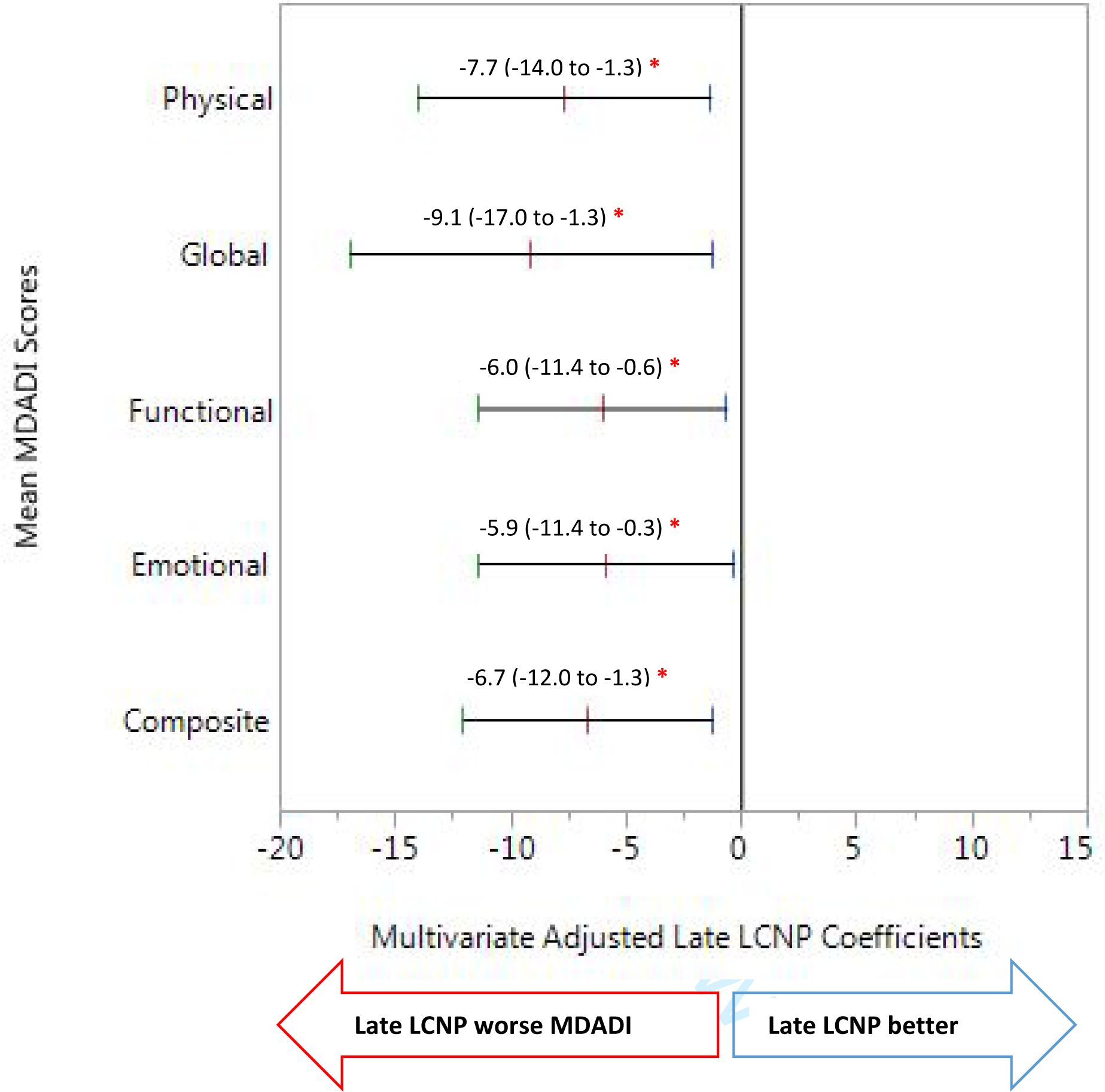
Multiple linear regression models adjusted for age, survival time, sex, education, subsite, T-stage, smoking, therapeutic modality, RT modality, solid food diet prior to treatment, and stricture. The regression model for global scores adjusted for an additional variable, neck dissection.
Abbreviations: MDADI, MD Anderson Dysphagia Inventory (MDADI); LCNP, lower cranial neuropathy.
Conflicts of Interest and Financial Disclosures:
This work was directly supported by the Charles and Daneen Stiefel Oropharynx Fund. The funding source had no role in the design and conduct of the study; data collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
APPENDIX:
Table 1:
SENSTIVITY ANALYSIS COMPARING IMPUTED VERSUS NON-IMPUTED MDADI SCORES
| IMPUTED MDADI SCORES | NON-IMPUTED MDADI SCORES | |||
|---|---|---|---|---|
| MDADI SCORE | Multivariate Analysis Coefficient (95%CI) | P Value | Multivariate Analysis Coefficient (95%CI) | P Value |
| Composite | −6.7 (−12.0 to −1.3) | 0.015 | −4.8 (−11.3 to 1.6) | 0.142 |
| Global | −9.1 (−17.0 to −1.3) | 0.023 | −10.6 (−18.9 to −2.4) | 0.012 |
| Emotional | −5.9 (−11.4 to −0.3) | 0.038 | −5.6 (−11.7 to 0.6) | 0.077 |
| Physical | −7.7 (−14.0 to −1.3) | 0.018 | −7.8 (−15.0 to −0.6) | 0.033 |
| Functional | −6.0 (−11.4 to −0.6) | 0.028 | −5.3 (−11.1 to 0.5) | 0.073 |
Comment: Other than Composite scores all effect estimates are not very different.
REFERENCES
- 1.Hutcheson K, Yuk M, Hubbard R, Gunn G, Fuller C, Lai S, … Hutcheson K (2017). Delayed lower cranial neuropathy after oropharyngeal intensity-modulated radiotherapy: A cohort analysis and literature review. Head & Neck, 39(8), 1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarrazin JL, Toulgoat F, & Benoudiba F (2013). The lower cranial nerves: IX, X, XI, XII. Diagnostic and interventional imaging, 94(10), 1051–1062. [DOI] [PubMed] [Google Scholar]
- 3. http://teachmeanatomy.info/head/cranial-nerves/summary/
- 4.Tolentino, de Souza Elen, Centurion, Stuchi Bruna, Ferreira, Helena Caetano Lúcia, Souza, Pereira de Andréia, Damante, Humberto José, & Rubira-Bullen, Regina Fischer Izabel. (2011). Oral adverse effects of head and neck radiotherapy: literature review and suggestion of a clinical oral care guideline for irradiated patients. Journal of Applied Oral Science, 19(5), 448–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goepfert RP, Lewin JS, Barrow MP, Gunn GB, Fuller CD, Beadle BM, … & Lai SY(2017). Long-term, prospective performance of the MD Anderson Dysphagia Inventory in “low-intermediate risk” oropharyngeal carcinoma after intensity modulated radiation therapy. International Journal of Radiation Oncology* Biology* Physics, 97(4), 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter KU,MD, Schipper M, PhD, Feng FY,MD, et al. Toxicities Affecting Quality of Life After Chemo-IMRT of Oropharyngeal Cancer: Prospective Study of Patient-Reported, Observer-Rated, and Objective Outcomes. International Journal of Radiation Oncology, Biology, Physics. 2013; 85:935–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutcheson KA, Holsinger FC, Kupferman ME, & Lewin JS (2015). Functional outcomes after TORS for oropharyngeal cancer: a systematic review. European Archives of Oto-Rhino-Laryngology, 272(2), 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutcheson KA, & Lewin JS (2013). Functional Assessment and Rehabilitation – How to Maximize Outcomes. Otolaryngologic Clinics of North America, 46(4), 657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schindler Antonio, Denaro Nerina, Russi Elvio G., Pizzorni Nicole, Bossi Paolo, Merlotti Anna, Massimo Spadola Bissetti Gianmauro Numico, Gava Alessandro, Orlandi Ester, Caspiani Orietta, Buglione Michela, Alterio Daniela, Bacigalupo Almalina, Vitaliana De Sanctis Giovanni Pavanato, Ripamonti Carla, Merlano Marco C., Licitra Lisa, Sanguineti Giuseppe, Langendijk Johannes A., Murphy Barbara, Dysphagia in head and neck cancer patients treated with radiotherapy and systemic therapies: Literature review and consensus, In Critical Reviews in Oncology/Hematology, Volume 96, Issue 2, 2015, Pages 372–384 [DOI] [PubMed] [Google Scholar]
- 10.Wilson JA, Carding PN, Patterson JM. Dysphagia after nonsurgical head and neck cancer treatment: patients’ perspectives. Otolaryngol Head Neck Surg 2011; 145:767–771. [DOI] [PubMed] [Google Scholar]
- 11.Goepfert RP, Fuller CD, Gunn GB, et al. Symptom 13 burden as a driver of decisional regret in long-term 14 oropharyngeal carcinoma survivors. Head Neck. 15 2017;39(11):2151–2158 [DOI] [PubMed] [Google Scholar]
- 12.Pedersen A, Wilson Janet, McColl Elaine, Carding Paul, Patterson Jo, Swallowing outcome measures in head and neck cancer – How do they compare? In Oral Oncology, Volume 52, 2016, Pages 104–108. [DOI] [PubMed] [Google Scholar]
- 13.Russi Elvio G., Renzo Corvò Anna Merlotti, Alterio Daniela, Franco Pierfrancesco, Pergolizzi Stefano, Vitaliana De Sanctis Maria Grazia Ruo Redda, Ricardi Umberto, Paiar Fabiola, Bonomo Pierluigi, Merlano Marco C., Zurlo Valeria, Chiesa Fausto, Sanguineti Giuseppe, Bernier Jacques, Swallowing dysfunction in head and neck cancer patients treated by radiotherapy: Review and recommendations of the supportive task group of the Italian Association of Radiation Oncology, In Cancer Treatment Reviews, Volume 38, Issue 8, 2012, Pages 1033–1049 [DOI] [PubMed] [Google Scholar]
- 14.King SN, Dunlap NE, Tennant PA, Pitts T. Pathophysiology of Radiation-Induced Dysphagia in Head and Neck Cancer. Dysphagia. 2016;31(3):339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutcheson KA, Lewin JS. Functional outcomes after chemoradiotherapy of laryngeal and pharyngeal cancers. Curr Oncol Rep. 2012;14(2):158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awan MJ, Mohamed AS, Lewin JS, Baron CA, Gunn GB, Rosenthal DI, … & Hutcheson KA (2014). Late radiation-associated dysphagia (late-RAD) with lower cranial neuropathy after oropharyngeal radiotherapy: a preliminary dosimetric comparison. Oral oncology, 50(8), 746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiff D, & Wen P (Eds.). (2002). Cancer neurology in clinical practice. Springer Science & Business Media. [Google Scholar]
- 18.Kang MY, Holland JM, & Stevens KR (2000). Cranial neuropathy following curative chemotherapy and radiotherapy for carcinoma of the nasopharynx. The Journal of Laryngology & Otology, 114(4), 308–310. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Sun Y, Liang S-B, Zong J-F, Li W-F, Chen M, Chen L, Mao Y-P, Tang L-L, Guo Y, Lin A-H, Liu M-Z and Ma J (2013), Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China. Cancer, 119: 2230–2238. [DOI] [PubMed] [Google Scholar]
- 20.Gillespie MB, Brodsky MB, Day TA, Lee FS, Martin-Harris B. Swallowing-related quality of life after head and neck cancer treatment. Laryngoscope. 2004; 114:1362–1367. [DOI] [PubMed] [Google Scholar]
- 21.Hutcheson KA, Yuk MM, Holsinger FC, Gunn GB, & Lewin JS (2015). Late radiation-associated dysphagia with lower cranial neuropathy in long-term oropharyngeal cancer survivors: Video case reports. Head & neck, 37(4), E56–E62. [DOI] [PubMed] [Google Scholar]
- 22.Katherine AH, (2013). Late Radiation-Associated Dysphagia (RAD) in Head and Neck Cancer Survivors. Perspect Swal Swal Dis (Dysph), 22(2), 61–72. [Google Scholar]
- 23.Luk YS, Shum JS, Sze HC, Chan LL, Ng WT, & Lee AW (2013). Predictive factors and radiological features of radiation-induced cranial nerve palsy in patients with nasopharyngeal carcinoma following radical radiotherapy. Oral oncology, 49(1), 49–54. [DOI] [PubMed] [Google Scholar]
- 24.Huang AT, Song S, Dominguez LM, Nguyen J, Goldman RA, & Reiter ER (2013). Delayed lower cranial neuropathies following primary radiotherapy for oropharyngeal squamous cell carcinoma. The Laryngoscope, 123(5), 1207–1209. [DOI] [PubMed] [Google Scholar]
- 25.Hutcheson KA, Lewin JS, Barringer DA, Lisec A, Gunn G.Brandon., Moore MWS and Holsinger FC (2012), Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer, 118: 5793–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal P, Zaveri JS, Goepfert RP, et al. Symptom Burden Associated with Late Lower Cranial Neuropathy in Long-term Oropharyngeal Cancer Survivors. JAMA otolaryngology-- head & neck surgery. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YS, Jen YM, & Lin JC (2002). Radiation-related cranial nerve palsy in patients with nasopharyngeal carcinoma. Cancer, 95(2), 404–409. [DOI] [PubMed] [Google Scholar]
- 28.Kong L, Lu JJ, Liss AL, et al. Radiation-induced cranial nerve palsy: a cross-sectional study of nasopharyngeal cancer patients after definitive radiotherapy. Int. J. Radiat. Oncol. Biol. Phys 2011;79(5):1421–1427. [DOI] [PubMed] [Google Scholar]
- 29.Chen AY, et al. , The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg, 2001. 127(7): p. 870–6. [PubMed] [Google Scholar]
- 30.Pedersen A, Wilson Janet, McColl Elaine, Carding Paul, Patterson Jo, Swallowing outcome measures in head and neck cancer – How do they compare? In Oral Oncology, Volume 52, 2016, Pages 104–108. [DOI] [PubMed] [Google Scholar]
- 31.Dwivedi RC, Rose SS, Roe JW, Khan AS, Pepper C, Nutting CM, … & Kazi R 2010). Validation of the Sydney Swallow Questionnaire (SSQ) in a cohort of head and neck er patients. Oral oncology, 46(4), e10–e14. [DOI] [PubMed] [Google Scholar]
- 32.Robertson SM, Yeo JC, Dunnet C, Young D, & MacKenzie K (2012). Voice, swallowing, and quality of life after total laryngectomy—results of the west of Scotland laryngectomy audit. Head & neck, 34(1), 59–65. [DOI] [PubMed] [Google Scholar]
- 33.Dwivedi RC, Chisholm EJ, Khan AS, et al. An exploratory study of the influence of clinico-demographic variables on swallowing and swallowing-related quality of life in a cohort of oral and oropharyngeal cancer patients treated with primary surgery. Eur Arch Otorhinolaryngol 2012; 269:1233–1239. [DOI] [PubMed] [Google Scholar]
- 34. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcmanual_v4_10-499.pdf.
- 35.Gunn GB, Mendoza TR, Fuller CD, Gning I, Frank SJ, Beadle BM, … & Rosenthal DI (2013). High symptom burden prior to radiation therapy for head and neck cancer: a patient-reported outcomes study. Head & neck, 35(10), 1490–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosmer DW Jr, & Lemeshow S (2004). Applied logistic regression. John Wiley & Sons. [Google Scholar]
- 37.Hutcheson KA, Barrow MP, Lisec A, Barringer DA, Gries K, Lewin JS. What is a clinically relevant difference in MDADI scores between groups of head and neck cancer patients? Clinically Meaningful Difference in MDADI Scores. The Laryngoscope. 2016; 126:1108–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen J Set Correlation and Contingency Tables. Applied Psychological Measurement. 1988; 12:425–434 [Google Scholar]
- 39.Browne JD, Butler S, Rees C. Functional outcomes and suitability of the temporalis myofascial flap for palatal and maxillary reconstruction after oncologic resection. Laryngoscope. Jun 2011;121(6):1149–1159 [DOI] [PubMed] [Google Scholar]
- 40.Roe JWG, Drinnan MJ, Carding PN, Harrington KJ, Nutting CM. Patient-reported outcomes following parotid-sparing intensity-modulated radiotherapy for head and neck cancer. How important is dysphagia? Oral Oncology. 2014; 50:1182–1187. [DOI] [PubMed] [Google Scholar]
- 41.Goldsmith TA, Justin W G Roe. Human papilloma virus-related oropharyngeal cancer: opportunities and challenges in dysphagia management Current opinion in otolaryngology & head and neck surgery. United States:2015; 23:185–190. [DOI] [PubMed] [Google Scholar]
- 42.Servagi-Vernat S, Ali D, Roubieu C, Durdux C, Laccourreye O, Giraud P. Dysphagia after radiotherapy: State of the art and prevention. European Annals of Otorhinolaryngology, Head and Neck Diseases. 2014;2015; 132:25–29. [DOI] [PubMed] [Google Scholar]
- 43.Teo PM, Leung SF, Chan AT, et al. Final report of a randomized trial on altered-fractionated radiotherapy in nasopharyngeal carcinoma prematurely terminated by significant increase in neurologic complications. Int J Radiat Oncol Biol Phys. 2000;48(5):1311–1322. [DOI] [PubMed] [Google Scholar]
- 44.Teguh DN,MD, Levendag, Peter C., M.D., Ph.D., Noever I,RTT, et al. Treatment Techniques and Site Considerations Regarding Dysphagia-Related Quality of Life in Cancer of the Oropharynx and Nasopharynx. International Journal of Radiation Oncology, Biology, Physics. 2008; 72:1119–1127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


