Abstract

Infrared ion spectroscopy (IRIS), a mass-spectrometry-based technique exploiting resonant infrared multiple photon dissociation (IRMPD), has been applied for the identification of novel psychoactive substances (NPS). Identification of the precise isomeric forms of NPS is of significant forensic relevance since legal controls are dependent on even minor molecular differences such as a single ring-substituent position. Using three isomers of fluoroamphetamine and two ring-isomers of both MDA and MDMA, we demonstrate the ability of IRIS to distinguish closely related NPS. Computationally predicted infrared (IR) spectra are shown to correspond with experimental spectra and could explain the molecular origins of their distinctive IR absorption bands. IRIS was then used to investigate a confiscated street sample containing two unknown substances. One substance could easily be identified by comparison to the IR spectra of reference standards. For the other substance, however, this approach proved inconclusive due to incomplete mass spectral databases as well as a lack of available reference compounds, two common analytical limitations resulting from the rapid development of NPS. Most excitingly, the second unknown substance could nevertheless be identified by using computationally predicted IR spectra of several potential candidate structures instead of their experimental reference spectra.
For several years, the global synthetic drug market has been rapidly expanding to include many new drug substances having chemical structures closely related to traditional drugs such as amphetamine and methylenedioxymethamphetamine (MDMA). These so-called novel psychoactive substances (NPS) often only differ from their traditional drug analogues through a single functional group substitution or minor structural rearrangement. While such minor variations in molecular structure can change their legal status, NPS commonly continue to interact on the same receptors as their traditional drug counterparts and induce similar psychoactive effects. However, their short-, medium-, and long-term health effects are usually not investigated. Hence, NPS consumers—often vulnerable populations such as youngsters—are exposed to “legal” drugs having unknown and potentially serious effects on health, including death.1 The United Nations Office on Drugs and Crime reported in 2019 a clear trend of “innovation” with the emergence of 892 new NPS between 2009 and 2018.2,3
Legal control of NPS drugs differs from country to country. Dating back from the United Nations convention on drugs, many countries, including the United States, Canada, and China and most of the European Union, use an approach with fixed lists of prohibited substances.3,4 These lists are continuously reviewed and updated for new substances; however, such updates are typically time-consuming as several judicial and public health risk assessment procedures need to be conducted. This leads to the situation where judicial control is often lagging behind NPS development, and cases in which one positional isomeric form of a drug is controlled, while the others are not, are frequently encountered. For example, in The Netherlands, 4-fluoroamphetamine (4-FA) and 3,4-methylenedioxymethamphetamine (3,4-MDMA) are controlled substances, while their positional isomers, 2- and 3-fluoroamphetamine (2-FA, 3-FA) and 2,3-methylenedioxymethamphetamine (2,3-MDMA), are currently uncontrolled.5 This produces serious analytical challenges for forensic laboratories as these isomeric compounds have very similar detector responses when analyzed by common analytical technologies including GC-MS(MS), LC-UV, and LC-MS(MS).
Currently, common approaches for isomeric drug identifications are mostly based on GC-FTIR, NMR, multivariate statistics on mass spectra, or chemical derivatization prior to chromatographic strategies.6−10 Also, GC-vacuum ultraviolet detection (GC-VUV) has been recently demonstrated for isomer differentiation.11−14 However, chromatography-hyphenated spectroscopic techniques such as VUV and FTIR are limited in terms of their sensitivity, and chemical derivatization reduces the robustness and ease-of-use. Additionally, reference compounds—or spectral libraries thereof—are required for the identification of unknowns by chromatography, mass spectrometry, and VUV. The applicability of these techniques is problematic when reference material is unavailable due to the novelty of the substance or legal complexity of international import between countries having varying legislation. When chromatography and mass spectrometry are insufficient for NPS identification, NMR is often used for their analysis.15−17 NMR is generally a feasible approach for confiscated samples that are “pure” and when the drug is in high concentration. However, impurities and additives including unreacted reagents, byproducts, tablet fillers, colorants, and adulterants are often troublesome. Furthermore, NMR has insufficient sensitivity for trace analysis of drugs and their metabolites in forensic samples such as hair, clothing, wastewater, urine, and blood.18 Thus, the development of new approaches that can deal with the limited availability of reference samples, sample impurities or mixtures, and trace analysis would be highly beneficial for the forensic field.
Infrared ion spectroscopy (IRIS) is a powerful technique for molecular structure elucidation in mass spectrometry, and its applications in small molecule identification have recently been reviewed.19,20 IRIS exploits the sensitivity and mass selectivity of mass spectrometry in combination with the structural selectivity of infrared (IR) spectroscopy.21 In IRIS, molecular ions are m/z isolated and held directly inside the ion trapping region of a mass spectrometer (for example, a quadrupole ion trap22 or FT-ICR MS, etc.). A tunable infrared laser is used to induce infrared wavenumber-specific photofragmentation by infrared multiple photon dissociation (IRMPD).19,23 In contrast to conventional absorption IR spectroscopy, IRIS benefits from precursor mass selection prior to the IR measurements, eliminating extensive sample preparation protocols and lengthy chromatographic separations. Furthermore, as an MS-based technique, IRIS operates at MS sensitivity and is thus suitable for the identification of analytes at very low concentrations (>nM).19,24 Finally, molecular vibrations are in most cases reliably predictable for molecular structures using routine quantum chemistry protocols.19,24−26 In many cases, this bypasses the requirement for reference standard materials as experimental results can instead be compared to computational reference spectra for (tentative) molecular structure assignment of unknowns. Alternative approaches of ion spectroscopy used for identification are IR tagging- and UV-cold ion spectroscopy;27−30 however, the technical simplicity of IRIS makes it the method of choice for many practical applications.
In the present work, we demonstrate IRIS as a valuable tool for the identification of NPS. First, we show the determination of ring-substituent positions (ortho-, meta-, and para-) of fluoroamphetamines and give an in-depth analysis of both their experimental and theoretical IR spectra. Additionally, we demonstrate that IRIS can readily distinguish several MDMA-type isomeric NPS. We show, for the first time, IRIS as a tool for identification of NPS in a confiscated street sample where both GC-MS and FTIR proved previously inconclusive. This sample contained two unidentified substances, of which one is identified by IRIS in comparison to the IR spectra of reference standards. For the second unknown substance, however, no reference standards were available, and its successful identification is based on comparison with in silico predicted IR spectra of potential structural candidates.
Experimental Section
Materials
Methanol (UHPLC grade) and water (LC-MS grade) were obtained from Merck (Darmstadt, Germany). Formic acid (LC-MS grade) was purchased from VWR (Leuven, Belgium). The standards 2-fluoroamphetamine (2-FA); 3-fluoroamphetamine (3-FA); 4-fluoroamphetamine (4-FA); 2,3-methylenedioxymethamphetamine (2,3-MDMA); 2,3-methylenedioxyamphetamine (2,3-MDA); and 2-methylmethcathinone (2-MMC) were obtained as >98% purity HCl salts from Cayman Chemical Company (Ann Arbor, MI). Reference standards 3,4-methylenedioxy-methamphetamine (3,4-MDMA); 3,4-methylenedioxy-amphetamine (3,4-MDA); 3-methylmethcathinone (3-MMC); and 4-methylmethcathinone (4-MMC), as well as the case sample, were provided by the Amsterdam Police Laboratory. The case sample originated from a seizure of a suspected drug material by the Amsterdam Police. All molecular structures and legal status of selected synthetic drugs in The Netherlands are shown in Figure 1. From all reference materials, individual 10 μM solutions in MeOH:water (1:1) with 0.1% (v/v) formic acid were prepared. Case samples were dissolved in—and diluted to 5 μg/mL by—the same solvents as the reference standards.
Figure 1.
Molecular structures of the synthetic drugs studied in this work. The underlined (red) compounds are controlled substances in The Netherlands.
Infrared Ion Spectroscopy
IRIS measurements were performed using a modified Bruker (Bremen, Germany) AmaZon ion trap mass spectrometer. The modifications to the mass spectrometer and full details of the experimental setup are reported elsewhere.22 In short, two windows were placed above the ring electrode in the vacuum housing of the ion trap, and 3 mm holes were drilled in both the top and bottom of the ring electrode to guide the laser beam through the ion trap. Two mirror mounts were installed below the ring electrode to guide the laser beam out of the vacuum housing. The infrared laser light, generated by the FELIX free-electron laser,31,32 can interact with the trapped and mass-selected ion cloud inside the mass spectrometer to enable wavenumber-specific IRMPD experiments. Extensive hardware and software modifications are further implemented to fully integrate the operation of the mass spectrometer with the wavelength tuning of the laser.
Sample solutions were infused at a ∼3 μL/min flow rate and ionized by +ESI. Following m/z isolation in the ion trapping region, the ions were irradiated by the IR laser in order to induce resonant photofragmentation when the IR frequency matches that of a molecular vibration, generating frequency-specific fragments for MS detection. For the experiments described here, FELIX was operated with a pulse energy of approximately 100 mJ in the frequency range from 1850 cm–1 to 600 cm–1 and scanned with a 3 cm–1 step size. For each selected wavenumber, MS data from 5 measurements of consecutive trap-cycles were recorded and averaged. IR spectra were constructed from detected MS intensities of both precursor and fragment ions using the ratio of the total (sum) fragment ion intensity and the total (fragment and precursor) ion intensity as the IR photodissociation yield. Full details on spectrum reconstruction from MS signals have been reported previously.24,32 For the measured compounds, selected precursor ions and corresponding fragment ions are presented in Table S1. The IR frequency was calibrated online during measurements using a grating spectrometer, and the IR photodissociation yield was linearly corrected for frequency-dependent variations in the IR pulse energy.
Computational Methods
Molecular structures of drugs and their positional isomers were optimized at the B3LYP/6-31++G(d,p) level of theory using the Gaussian 16 software package.33 Harmonic vibrational frequencies were computed for each optimized molecular geometry and were scaled by 0.975 and broadened using a Gaussian line shape with a full-width at half-maximum (fwhm) of 25 cm–1 to facilitate comparison with the experimental spectra. All calculations were carried out at the Cartesius cluster of SurfSARA in Amsterdam.
GC-MS and FTIR Experiments on Confiscated Case Samples
For reference GC-MS experiments at the police laboratory, the same method as described in previous work was used.11 GC-MS spectral match scores were returned by the software on a scale from 0 to 1000 where the latter indicates a perfect match. FTIR experiments were performed on a PerkinElmer (Waltham, MA) Spectrum Two instrument with ATR option using a scan range from 400 to 1400 cm–1. FTIR match scores range from 0.000 to 1.000.
Results and Discussion
Infrared Ion Spectroscopy for the Determination of Ring-Substituent Positions of Fluoroamphetamines
We first demonstrate the applicability of IRIS for the determination of ring-substituent positions in isomeric drug molecules using 2-, 3-, and 4-FA reference standards (all m/z 154 for [M + H]+). IRIS spectra for protonated 2-, 3-, and 4-FA are presented in Figure 2. While some of the prominent IR features (numbered 1 and 2 in Figure 2A–C) are located at common frequencies for all three isomers, the spectra also show vibrational bands (numbered 3–8 in Figure 2A–C) that are unique to each isomer. As these molecules only differ in the position of one ring substituent, these unique isomer-specific vibrations must be related to this aspect of their molecular structures. To better understand the correlation between IR spectral differences and variations in molecular structure (in this case ring-substituent position), IR spectra were computed for each of the three protonated fluoroamphetamine isomers. Experimental and theoretical spectra were overlaid to evaluate the predictive abilities of theoretical spectra for the experimental spectra of these (and potentially other) NPS structures, as presented in Figure S1. The overall agreement between the experimental and calculated spectra is good if one takes into account that the weak vibrational bands are missing in the experimental spectra. At these weak absorptions, more laser power is required to reach the IR dissociation threshold.22,32,34,35 Although this is the first study on NPS, the quality of agreement between experimental and computational spectra is in line with earlier findings on other classes of molecules.19,24,25,28,36−38
Figure 2.
IR spectra for the protonated fluoroamphetamine isomers 2-FA (A), 3-FA (B), and 4-FA (C). Prominent spectral features are labeled in red. The corresponding normal mode vibrations are displayed in Figure S2.
The bands numbered 3–5 and 6–8 both show specific molecular ring vibrations that are dependent on the position of the fluorine atom on the aromatic ring (computed vibrations are shown in Figure S2C–E for 3–5 and Figure S2F–H for 6–8). Most notably, the 3–5 bands (in red) all originate from vibrations caused by the stretching of carbon–carbon bonds in the aromatic ring and shift to higher frequencies when the fluorine atom changes from ortho- to meta- to para-position.39 The nonspecific bands numbered 1 and 2 were found to correspond to amine scissoring and amine bending vibrations (Figure S2A,B) and are observed in all three spectra as a similar amine moiety is present within these three structures. However, an intensity difference is present for absorption band 2, which has a much lower intensity for 2-FA than for 3- and 4-FA. The computations show that this is a result of the contributions from unresolved ring vibrations adding to the intensity of the absorption band 2 for both 3- and 4-FA (Figure S2I,J).
MDMA-Type Isomer Differentiation with Infrared Ion Spectroscopy
Another, different, class of isomeric compounds that have a bicyclic ring structure connected at different positions, the MDMA- and MDA-type drugs, were also investigated. The 3,4-isomers of both MDMA (known as “ecstasy”) and MDA are frequently occurring stimulants that are controlled substances in The Netherlands. However, their 2,3-isomers are both NPS that are not controlled and are nearly indistinguishable from their corresponding 3,4-isomers on the basis of GC-MS, being the method of choice in most forensic laboratories.11 Using IRIS, however, clearly distinctive IR spectra for each isomer could be generated which enables simple identification of these NPS from their controlled counterparts, as presented in Figure 3 (see Figure S3 for a comparison to computationally predicted IR spectra).
Figure 3.
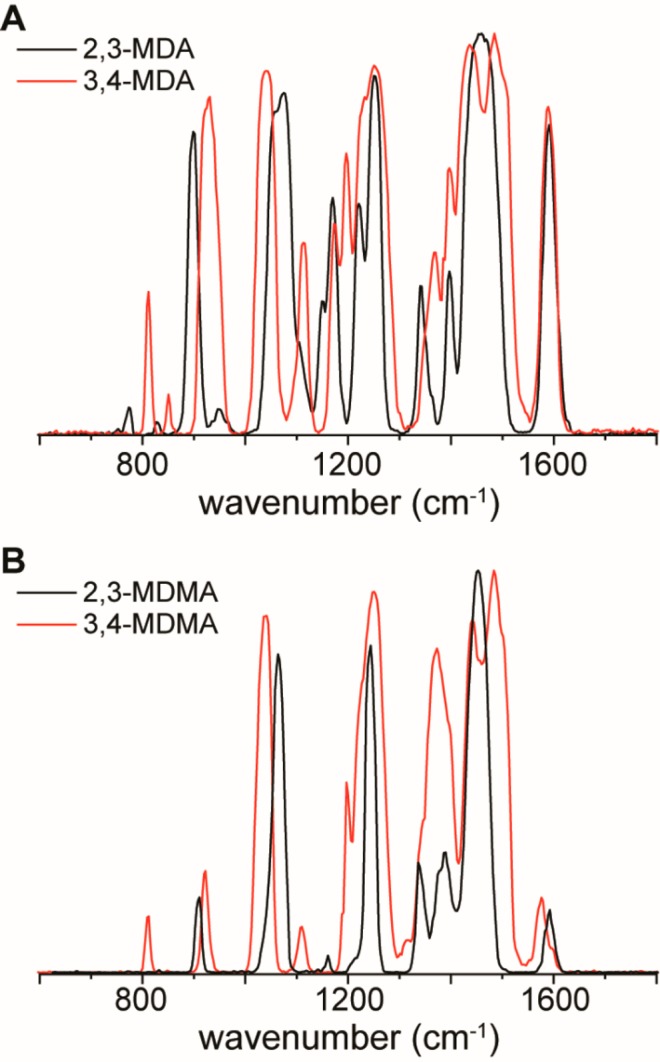
IR spectra recorded for both sets of protonated MDMA and MDA isomers. (A) Overlay of 2,3-MDA and 3,4-MDA. (B) Overlay of 2,3-MDMA and 3,4-MDMA.
Confiscated Street Sample: Reference and Reference-Free Identification of NPS
In order to explore the practicality of using IRIS for the identification of NPS from real-world samples (that are often mixtures), we analyzed a confiscated street sample containing several unknown substances. The selected sample was a powder seized by the Amsterdam Police in December 2018, and preliminary analysis revealed that this sample consisted of a mixture of caffeine and at least two different NPS (shown in Figure S4), whose molecular structures could not be identified by GC-MS or FTIR. GC-MS library searches were inconclusive for the two unidentified chromatographic peaks at retention times 5.02 and 5.51 min. Also, FTIR analysis could not identify these compounds due to overlapping IR signals as a result of the mixing of components in the sample (Figure S5).
For the chromatographic peak at 5.02 min with molecular ion m/z 177, the GC-MS spectra provided a starting point with spectral matches (scores ∼900) for three methylmethcathinone isomers. In general, match scores above 900 are accepted for identification when no other library components show a similar score. However, all cathinone-type ring-isomers yield GC-MS match scores above 900, thus being inconclusive.11 For its identification, IR spectra were obtained of the unidentified substance (m/z 178 for [M + H]+), as well as the 2-MMC, 3-MMC, and 4-MMC (mephedrone) reference standards. Note that precursor ion selection in IRIS provides the required selectivity to acquire a compound-specific IR spectrum of m/z 178 from the sample mixture. Figure 4 presents the obtained spectra of both sample and reference compounds. The black line in each panel is the IR spectrum of the unknown m/z 178 in the street sample, whereas the blue, green, and red shadings represent the IR spectra of 2-MMC, 3-MMC, and 4-MMC reference standards, respectively. Clearly, the IR spectrum of the unidentified m/z 178 in the street sample matches with the IR spectrum of 3-MMC. It can therefore be concluded that the street sample contains 3-MMC (apart from caffeine and one more unidentified substance). The second major unidentified chromatographic peak at 5.51 min (shown in Figure S4) gave inconclusive GC-MS library matches (scores between 885 and 934) for three chloroethcathinone (CEC) isomers and a single chlorodimethcathinone (CdMC) structure. It is important to note that, due to the rapid development of NPS, two possible isomers (2- and 3-) of CdMC are currently not yet available in standard mass spectral libraries, and these alternative structures can obviously not be excluded on the basis of their absence in the database. Additionally, no reference standards are currently available for any of the CEC or CdMC compounds. Therefore, quantum-chemical computations (geometry optimizations and vibrational frequency calculations) were performed on all six potential candidates, the isomeric CEC and CdMC molecular structures (shown in Figure S6), to obtain predicted reference IR spectra for the identification of the unknown compound (m/z 212 in +ESI, [M + H]+). Figure 5 presents the experimental IR spectrum of m/z 212 (black line), overlaid in each panel with one of the predicted reference spectra (blue fill). First, the position of the chlorine atom on the aromatic ring can easily be established as para- by matching the computed band at 1100 cm–1 (highlighted in orange), which is only present for the para-substituted forms. Thereafter, the distinction between ethyl or dimethyl for the two remaining cathinones (Figure 5C,F) could be made by the absorption band at 1560 cm–1 (highlighted in green), which is present in the calculated spectrum of 4-CEC but is absent in the m/z 212 experimental spectrum as well as in the computed spectrum for 4-CdMC. This computed absorption band for 4-CEC originates from an NH2 scissoring vibration (depicted in Figure S7) of its amine moiety and is specific for the ethylated-isomer 4-CEC, as 4-CdMC comprises a tertiary amine that (obviously) cannot bind two hydrogen atoms. Although minor contributions from 4-CEC cannot be excluded, we can identify m/z 212 as protonated 4-CdMC.
Figure 4.
IR spectra for the methylmethcathinone isomer reference standards (blue, green, red) overlaid in each panel with the spectrum of the unknown m/z 178 compound in the street sample (black line). Molecular structures of each of the reference compounds are shown.
Figure 5.
Experimental IR spectrum for the unknown m/z 212 ion in the case sample (black line), overlaid with computed IR spectra for all six CEC and CdMC isomers (blue shading).
From these combined results, we conclude that the confiscated street sample most likely consists of a mixture of three primary substances; 3-MMC, 4-CdMC, and caffeine.
Conclusions
IRIS has been demonstrated to be a powerful analytical tool for drugs-of-abuse and NPS identification. It provides an orthogonal combination of mass spectral and spectroscopic features and was used to identify the molecular structure of isomeric NPS compounds, including ortho-, meta-, or para-positioned ring substituents. Using this technique, we analyzed a real forensic confiscated street sample that contained two substances which could not be identified by routine GC-MS and FT-IR analysis. The first substance was simply identified using IRIS as 3-MMC by comparison with IRIS spectra from reference standards. For the second unknown substance, a reference standard free approach was successful for its identification as 4-CdMC, which was achieved by comparison with computed IR reference spectra for several potential candidates. As the sensitivity of IRIS is that of regular MS/MS measurements, we foresee that IRIS can be used for future research in trace analysis of drugs, NPS, and their metabolites on materials or body fluids such as hair, clothing, wastewater, fingerprints, plasma, and urine.
Acknowledgments
The authors gratefully acknowledge support from Radboud University through an interfaculty collaboration grant, the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) for the support of the FELIX Laboratory [Grants VICI 724.011.002, TTW 15769], and SURFsara for computational resources [NWO Rekentijd Grant 2019.062]. Rianne van Outersterp is acknowledged for stimulating discussions on isomeric compound differentiation. We thank the FELIX staff for their helpful and kind assistance.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.analchem.0c00915.
m/z values, overlays of experimental and theoretical IR spectra, vibrations corresponding with the fluoroamphetamine IRIS spectra, inconclusive GC-MS results from the case sample, MS library match scores, inconclusive FTIR results, FTIR library match scores, molecular structures of chloroethcathinone and chlorodimethcathinone compounds, and a computed vibration band (1567 cm–1) for 4-chloroethcathinone (PDF)
Author Contributions
† R.F.K. and F.A.M.G.v.G. contributed equally to this manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Kraemer M.; Boehmer A.; Madea B.; Maas A. Death Cases Involving Certain New Psychoactive Substances: A Review of the Literature. Forensic Sci. Int. 2019, 298, 186–267. 10.1016/j.forsciint.2019.02.021. [DOI] [PubMed] [Google Scholar]
- United Nations publication . World Drug Report; Sales No. E.19.XI.8.; 2019.
- United Nations Office on Drugs and Crime . Early warning advisory on new psychoactive substances. https://www.unodc.org/LSS/Page/NPS (accessed December 10, 2019).
- European Monitoring Centre for Drugs and Drug Addiction and Europol . EU Drug Markets Report; Publications Office of the European Union: Luxembourg, 2019.
- Dutch Opium Act, status per July 19th 2019. https://wetten.overheid.nl/BWBR0001941/2019-07-19 (accessed October 10, 2019).
- DeRuiter J.; Smith F.; Abiedalla Y.; Neel L.; Clark C. R. GC-MS and GC-IR Analysis of Regioisomeric Cannabinoids Related to 1-(5-Fluoropentyl)-3-(1-Naphthoyl)-Indole. Forensic Chem. 2018, 10, 48–57. 10.1016/j.forc.2018.07.005. [DOI] [Google Scholar]
- Carlsson A.; Sandgren V.; Svensson S.; Konradsson P.; Dunne S.; Josefsson M.; Dahlén J. Prediction of Designer Drugs: Synthesis and Spectroscopic Analysis of Synthetic Cathinone Analogs That May Appear on the Swedish Drug Market. Drug Test. Anal. 2018, 10 (7), 1076–1098. 10.1002/dta.2366. [DOI] [PubMed] [Google Scholar]
- Kranenburg R. F.; Peroni D.; Affourtit S.; Westerhuis J. A.; Smilde A. K.; van Asten A. C. Revealing Hidden Information in GC-MS Spectra from Isomeric Drugs: Chemometrics Based Identification from 15 EV and 70 EV EI Mass Spectra. Forensic Chemistry 2020, 18, 100225. 10.1016/j.forc.2020.100225. [DOI] [Google Scholar]
- Abiedalla Y.; DeRuiter J.; Clark C. R. GC–MS, GC–MS/MS and GC-IR Differentiation of Carbonyl Modified Analogues of MDPV. Forensic Chem. 2017, 3, 58–68. 10.1016/j.forc.2016.11.002. [DOI] [Google Scholar]
- Almalki A. J.; Clark C. R.; DeRuiter J. GC–MS Analysis of Regioisomeric Substituted N-Benzyl-4-Bromo-2,5-Dimethoxyphenethylamines. Forensic Chem. 2019, 14, 100164. 10.1016/j.forc.2019.100164. [DOI] [Google Scholar]
- Kranenburg R. F.; García-Cicourel A. R.; Kukurin C.; Janssen H.-G.; Schoenmakers P. J.; van Asten A. C. Distinguishing Drug Isomers in the Forensic Laboratory: GC-VUV in Addition to GC-MS for Orthogonal Selectivity and the Use of Library Match Scores as a New Source of Information. Forensic Sci. Int. 2019, 302, 109900. 10.1016/j.forsciint.2019.109900. [DOI] [PubMed] [Google Scholar]
- Skultety L.; Frycak P.; Qiu C.; Smuts J.; Shear-Laude L.; Lemr K.; Mao J. X.; Kroll P.; Schug K. A.; Szewczak A.; et al. Resolution of Isomeric New Designer Stimulants Using Gas Chromatography – Vacuum Ultraviolet Spectroscopy and Theoretical Computations. Anal. Chim. Acta 2017, 971, 55–67. 10.1016/j.aca.2017.03.023. [DOI] [PubMed] [Google Scholar]
- Buchalter S.; Marginean I.; Yohannan J.; Lurie I. S. Gas Chromatography with Tandem Cold Electron Ionization Mass Spectrometric Detection and Vacuum Ultraviolet Detection for the Comprehensive Analysis of Fentanyl Analogues. J. Chromatogr. A 2019, 1596, 183–193. 10.1016/j.chroma.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Roberson Z. R.; Goodpaster J. V. Differentiation of Structurally Similar Phenethylamines via Gas Chromatography–Vacuum Ultraviolet Spectroscopy (GC–VUV). Forensic Chem. 2019, 15, 100172. 10.1016/j.forc.2019.100172. [DOI] [Google Scholar]
- McLaughlin G.; Morris N.; Kavanagh P. V.; Power J. D.; Twamley B.; O’Brien J.; Talbot B.; Dowling G.; Brandt S. D. The Synthesis and Characterization of the ‘Research Chemical’ N-(1-Amino-3-Methyl-1-Oxobutan-2-Yl)-1-(Cyclohexylmethyl)-3-(4-Fluorophenyl)-1H-Pyrazole-5-Carboxamide (3,5-AB-CHMFUPPYCA) and Differentiation from Its 5,3-Regioisomer. Drug Test. Anal. 2016, 8 (9), 920–929. 10.1002/dta.1864. [DOI] [PubMed] [Google Scholar]
- Martek B. A.; Mihelač M.; Gazvoda M.; Virant M.; Urankar D.; Krivec M.; Gostič T.; Nemec B.; Koštrun B.; Janežič M.; et al. 1H–15N HMBC NMR as a Tool for Rapid Identification of Isomeric Azaindoles: The Case of 5F-MDMB-P7AICA. Drug Test. Anal. 2019, 11 (4), 617–625. 10.1002/dta.2573. [DOI] [PubMed] [Google Scholar]
- Bovens M.; Bissig C.; Staeheli S. N.; Poetzsch M.; Pfeiffer B.; Kraemer T. Structural Characterization of the New Synthetic Cannabinoids CUMYL-PINACA, 5F-CUMYL-PINACA, CUMYL-4CN-BINACA, 5F-CUMYL-P7AICA and CUMYL-4CN-B7AICA. Forensic Sci. Int. 2017, 281, 98–105. 10.1016/j.forsciint.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Kyriakou C.; Pellegrini M.; García-Algar O.; Marinelli E.; Zaami S. Recent Trends in Analytical Methods to Determine New Psychoactive Substances in Hair. Curr. Neuropharmacol. 2017, 15 (5), 663–681. 10.2174/1570159X15666161111112545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens J.; van Outersterp R. E.; Vreeken R. J.; Cuyckens F.; Coene K. L. M.; Engelke U. F.; Kluijtmans L. A. J.; Wevers R. A.; Buydens L. M. C.; Redlich B.; Berden G.; Oomens J. Infrared Ion Spectroscopy: New Opportunities for Small-Molecule Identification in Mass Spectrometry - a Tutorial Perspective. Anal. Chim. Acta 2020, 1093, 1–15. 10.1016/j.aca.2019.10.043. [DOI] [PubMed] [Google Scholar]
- Cismesia A. P.; Bell M. R.; Tesler L. F.; Alves M.; Polfer N. C. Infrared Ion Spectroscopy: An Analytical Tool for the Study of Metabolites. Analyst 2018, 143 (7), 1615–1623. 10.1039/C8AN00087E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler J. R. Infrared Multiple Photon Dissociation Spectroscopy of Ions in Penning Traps. Mass Spectrom. Rev. 2009, 28 (3), 448–467. 10.1002/mas.20217. [DOI] [PubMed] [Google Scholar]
- Martens J.; Berden G.; Gebhardt C. R.; Oomens J. Infrared Ion Spectroscopy in a Modified Quadrupole Ion Trap Mass Spectrometer at the FELIX Free Electron Laser Laboratory. Rev. Sci. Instrum. 2016, 87 (10), 103108. 10.1063/1.4964703. [DOI] [PubMed] [Google Scholar]
- Polfer N. C. Infrared Multiple Photon Dissociation Spectroscopy of Trapped Ions. Chem. Soc. Rev. 2011, 40 (5), 2211–2221. 10.1039/c0cs00171f. [DOI] [PubMed] [Google Scholar]
- Martens J.; Berden G.; Bentlage H.; Coene K. L. M.; Engelke U. F.; Wishart D.; van Scherpenzeel M.; Kluijtmans L. A. J.; Wevers R. A.; Oomens J. Unraveling the Unknown Areas of the Human Metabolome: The Role of Infrared Ion Spectroscopy. J. Inherited Metab. Dis. 2018, 41 (3), 367–377. 10.1007/s10545-018-0161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Outersterp R. E.; Houthuijs K. J.; Berden G.; Engelke U. F.; Kluijtmans L. A. J.; Wevers R. A.; Coene K. L. M.; Oomens J.; Martens J. Reference-Standard Free Metabolite Identification Using Infrared Ion Spectroscopy. Int. J. Mass Spectrom. 2019, 443, 77–85. 10.1016/j.ijms.2019.05.015. [DOI] [Google Scholar]
- Bell M. R.; Tesler L. F.; Polfer N. C. Cryogenic Infrared Ion Spectroscopy for the Structural Elucidation of Drug Molecules: MDMA and Its Metabolites. Int. J. Mass Spectrom. 2019, 443, 101–108. 10.1016/j.ijms.2019.06.001. [DOI] [Google Scholar]
- Kopysov V.; Makarov A.; Boyarkin O. V. Identification of Isomeric Ephedrines by Cold Ion UV Spectroscopy: Toward Practical Implementation. Anal. Chem. 2017, 89 (1), 544–547. 10.1021/acs.analchem.6b04182. [DOI] [PubMed] [Google Scholar]
- Gorlova O.; Colvin S. M.; Brathwaite A.; Menges F. S.; Craig S. M.; Miller S. J.; Johnson M. A. Identification and Partial Structural Characterization of Mass Isolated Valsartan and Its Metabolite with Messenger Tagging Vibrational Spectroscopy. J. Am. Soc. Mass Spectrom. 2017, 28 (11), 2414–2422. 10.1007/s13361-017-1767-z. [DOI] [PubMed] [Google Scholar]
- Solovyeva E. M.; Kopysov V. N.; Pereverzev A. Y.; Lobas A. A.; Moshkovskii S. A.; Gorshkov M. V.; Boyarkin O. V. Method for Identification of Threonine Isoforms in Peptides by Ultraviolet Photofragmentation of Cold Ions. Anal. Chem. 2019, 91 (10), 6709–6715. 10.1021/acs.analchem.9b00770. [DOI] [PubMed] [Google Scholar]
- Dyukova I.; Carrascosa E.; Pellegrinelli R. P.; Rizzo T. R. Combining Cryogenic Infrared Spectroscopy with Selective Enzymatic Cleavage for Determining Glycan Primary Structure. Anal. Chem. 2020, 92 (2), 1658–1662. 10.1021/acs.analchem.9b04776. [DOI] [PubMed] [Google Scholar]
- Oepts D.; van der Meer A. F. G.; van Amersfoort P. W. The Free-Electron-Laser User Facility FELIX. Infrared Phys. Technol. 1995, 36 (1), 297–308. 10.1016/1350-4495(94)00074-U. [DOI] [Google Scholar]
- Berden G.; Derksen M.; Houthuijs K. J.; Martens J.; Oomens J. An Automatic Variable Laser Attenuator for IRMPD Spectroscopy and Analysis of Power-Dependence in Fragmentation Spectra. Int. J. Mass Spectrom. 2019, 443, 1–8. 10.1016/j.ijms.2019.05.013. [DOI] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A. V.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian H. P.; Ortiz J. V.; Izmaylov A. F.; Sonnenberg J. L.; Williams-Young D.; Ding F.; Lipparini F.; Egidi F.; Goings J.; Peng B.; Petrone A.; Henderson T.; Ranasinghe D.; Zakrzewski V. G.; Gao J.; Rega N.; Zheng G.; Liang W.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E. N.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Millam J. M.; Klene M.; Adamo C.; Cammi R.; Ochterski J. W.; Martin R. L.; Morokuma K.; Farkas O.; Foresman J. B.; Fox D. J.. Gaussian 16; Gaussian, Inc.: Wallingford CT, 2016.
- Munshi M. U.; Martens J.; Berden G.; Oomens J. Gas-Phase Infrared Ion Spectroscopy Characterization of Cu(II/I)Cyclam and Cu(II/I)2,2′-Bipyridine Redox Pairs. J. Phys. Chem. A 2019, 123 (19), 4149–4157. 10.1021/acs.jpca.9b00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi M. U.; Berden G.; Martens J.; Oomens J. Gas-Phase Vibrational Spectroscopy of Triphenylamine: The Effect of Charge on Structure and Spectra. Phys. Chem. Chem. Phys. 2017, 19 (30), 19881–19889. 10.1039/C7CP02638B. [DOI] [PubMed] [Google Scholar]
- Stearns J. A.; Mercier S.; Seaiby C.; Guidi M.; Boyarkin O. V.; Rizzo T. R. Conformation-Specific Spectroscopy and Photodissociation of Cold, Protonated Tyrosine and Phenylalanine. J. Am. Chem. Soc. 2007, 129 (38), 11814–11820. 10.1021/ja0736010. [DOI] [PubMed] [Google Scholar]
- Bush M. F.; O’Brien J. T.; Prell J. S.; Saykally R. J.; Williams E. R. Infrared Spectroscopy of Cationized Arginine in the Gas Phase: Direct Evidence for the Transition from Nonzwitterionic to Zwitterionic Structure. J. Am. Chem. Soc. 2007, 129 (6), 1612–1622. 10.1021/ja066335j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnke S.; Seo J.; Boschmans J.; Sobott F.; Scrivens J. H.; Bleiholder C.; Bowers M. T.; Gewinner S.; Schöllkopf W.; Pagel K.; et al. Protomers of Benzocaine: Solvent and Permittivity Dependence. J. Am. Chem. Soc. 2015, 137 (12), 4236–4242. 10.1021/jacs.5b01338. [DOI] [PubMed] [Google Scholar]
- van Outersterp R. E.; Martens J.; Berden G.; Koppen V.; Cuyckens F.; Oomens J.. Mass Spectrometry-Based Identification of Ortho-, Meta- and Paraisomers Using Infrared Ion Spectroscopy. ChemRxiv, 2020. 10.26434/chemrxiv.11871837.v1. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






