Abstract

Understanding the interactions of surfactants and wettability alteration of surfaces is important for many fields, including oil and gas recovery. This work utilizes the quartz crystal microbalance with dissipation to study the interaction of stabilized linear and branched alkylbenzene sulfonates (ABSs), among the most cost-efficient industrial surfactants, with water- and oil-wet calcite surfaces under high-salinity and high-temperature conditions. Confocal laser scanning microscopy is also used to study the effect of the type of ABS on their interaction with oil-wet calcite surfaces. Experiments demonstrate that vesicles made of linear and branched ABSs interact differently with both water- and oil-wet surfaces. Therefore, surfactant formulations made of ABSs for high-salinity applications can further be improved for advantageous wettability properties by varying the hydrophobic chain of the surfactants. When interacting with a water-wet surface, both types of vesicles adsorb onto the surface as is. Upon dilution, however, vesicles made of linear ABS stay adsorbed as is, and vesicles made of branched ABSs disassemble and produce a layered structure with altered wettability. Linear ABSs show greater efficiency in desorbing oil from the oil-wet calcite. The results of this study demonstrate an improved method for studying and understanding the interaction of surfactant formulations with water- and oil-wet surfaces. This approach could significantly benefit applications in which wettability alteration of surfaces is of great interest and facilitate the implementation of low-cost surfactants based on petroleum sulfonates.
Introduction
Crude oil is an important resource for the chemical and energy industries. Producing it efficiently from porous structures of the rock located deep underground is a complicated and extensively researched topic.1,2 Improving recovery of oil can be achieved using various methods depending on the cost of technology and the properties of rock and oil. Surfactant, polymer, steam, alkali, and supercritical-CO2 (sc-CO2) injections alone or in combination are among the most successfully applied tertiary recovery methods.3−7 Surfactant injection is mainly used to decrease oil–water interfacial tension and to alter the wettability of the reservoir rock from oil-wet toward water-wet facilitating the release of oil from rock pores.5,8,9 Polymers allow for changing the viscosity of the water flood to better control the sweep and recover bypassed oil regions.6 The use of steam and sc-CO2 can reduce the viscosity of the oil, while alkali can reduce interfacial tension between oil and water and can passivate certain reservoir rock types to reduce retention of surfactants and polymers.7,10−12
Interactions of injected chemicals with the reservoir rock have been extensively studied for many decades.9,13−15 Recently, researchers have been focusing on reducing retention, favorably altering surface wettability, and better understanding mechanisms of oil recovery from a variety of rock surfaces.5,13 Advancements in analytical and characterization techniques introduced over the past few decades can provide a better understanding of the mechanisms of interaction of chemicals with various surfaces. In particular, the quartz crystal microbalance with dissipation (QCM-D) has been applied to great advantage at the interface of surface science and biology to investigate nanoscale material–surface interactions.16,17
QCM-D is a high-resolution acoustic mass sensing technique based on the inverse piezoelectric effect in AT-cut quartz.16 The piezoelectric effect is the property of certain materials both to become electrically charged (i.e., a voltage develops across the material) when a mechanical stress is applied and to become mechanically deformed when an electric voltage is applied. The instrument measures two main variables: (1) the change in piezoelectric crystal resonance frequency associated with the mass adsorbed and (2) the change in acoustic dissipation energy associated with the rigidity/softness of the adsorbed film (i.e., viscoelastic properties). For rigid films where the mass adsorbed is only a function of resonance frequency, the Sauerbrey equation can be used as expressed in eq 1(18)
| 1 |
where Δf is the crystal resonance frequency (Hz), Δm is the elastic mass change (g), A is the electrode area (cm2), f is the intrinsic crystal frequency (Hz), ρq is the density of quartz (g/cm3), and μ is the shear modulus (dyn/cm2). For viscoelastic films, more complicated models are used such as the Voigt and Maxwell models.18
In the QSence instrument used in the current study, frequency and dissipation are measured at seven different frequency overtone numbers (1, 3, 5, 7, 9, 11, and 13). The overtone frequency is related to the penetration depth (δ) of an acoustic wave, which is given by eq 2(19)
| 2 |
where n is the overtone number, ρf is the fluid density (kg/m3), and ηf is the shear viscosity (kg/(m s)). The higher overtones (e.g., 11th and 13th) are associated with properties of the film near the crystal surface, while lower overtones (e.g., 3rd and 5th) are associated with properties of the film far from the sensor surface (i.e., deeper inside the film).
QCM-D was successfully used to characterize solvate–surface interactions in many fields, especially in biological and biophysical research.17,20,21 One of the early works by Keller and Kasemo demonstrated that adsorption kinetics of vesicles could be surface-specific and result in either intact vesicle adsorption or bilayer formation.22 Similar surface observations for rupture/transformation of the adsorbed vesicles into lipid bilayers were also demonstrated for applications in biology to produce desired planar bilayers on a solid surface.23,24
Recently, QCM-D has been used in petroleum-related research as it has been applied in other fields. Multiple research efforts have successfully investigated the effect of low- and high-salinity brines on desorption of oil from both silica and calcium carbonate surfaces.25−29 Another study by Chen and Akbulut used QCM-D and atomic force microscopy (AFM) to understand the nanoscale dynamics of heavy oil recovery via a model nonionic surfactant.30 Because most experiments related to conventional oil recovery are time-consuming, expensive, and do not provide a nanoscale mechanism of oil desorption or interaction of chemicals with the rock surface, QCM-D could offer insight to better characterize and understand interactions of chemicals with a rock surface.
Selecting appropriate chemicals to successfully apply in a chemical enhanced oil recovery (CEOR) campaign is challenging.5,31 The chemicals should have certain properties and be soluble in certain salinity brines at high temperatures. Additionally, the cost of the chemicals should be minimal. Alkylbenzene sulfonate (ABS) surfactants, in the form of petroleum sulfonates, are among the most cost-efficient surfactants available in the quantities required for CEOR. Much work has been done to evaluate ABSs for oil field applications and to understand their interactions with various surfaces.15,32−34 Very little information is broadly available on the interaction of ABSs with calcium carbonate surfaces under relevant reservoir conditions of high salinity and high temperature.35,36 Developing ABSs that are stable and effective as CEOR agents will require studies, which fill this gap in our understanding, by examining the nanoscale interactions with various carbonate surfaces under relevant reservoir conditions.
In our previous work, we successfully demonstrated stabilization of one of the lowest cost ABS surfactants in high-salinity and high-temperature brine.37 In the present work, we focus on the understanding of nanoscale interactions of stabilized formulations of linear and branched ABSs with water- and oil-wet surfaces of calcium carbonate using QCM-D. Confocal laser scanning microscopy (CLSM) was also used to visualize and estimate the oil desorption results obtained from QCM-D. Results suggest that surfactant formulations made of encapsulated ABSs37,38 can be specifically modified to achieve improved wettability alterations in challenging high-salinity and high-temperature conditions. Studying the interaction of stabilized ABSs for applications in harsh carbonate reservoir conditions is of great importance because these types of surfactants are available in large quantities and have the potential to be successfully applied in the oil field for various applications.
Results and Discussion
This work investigates the difference between linear and branched ABSs when interacting with a proxy for CaCO3 reservoir rock surfaces using CaCO3-coated quartz crystals. Figure 1 summarizes the experimental procedures performed where surfactant formulations are first studied with water-wet and then with oil-wet CaCO3 surfaces. It is important to emphasize that, as described in previous work,37 ABS surfactants are stabilized by encapsulation into oil-filled vesicles (oil-swollen micelles), which are mainly surrounded by the zwitterionic cocamidopropyl hydroxysultaine surfactant. Therefore, these vesicles would not be similar in structure to micelles made only from ABS surfactants. Previous transmission electron microscope (TEM) and dynamic light scattering (DLS) studies showed that the sizes of these vesicles range from 10 to 60 nm.37
Figure 1.
Depiction of the experimental procedure used to study the interactions of stabilized linear and branched ABS surfactants with (a) water-wet and (b) oil-wet CaCO3 surfaces in high-salinity and high-temperature brines.
Interaction of Surfactants with the Water-Wet CaCO3 Surface
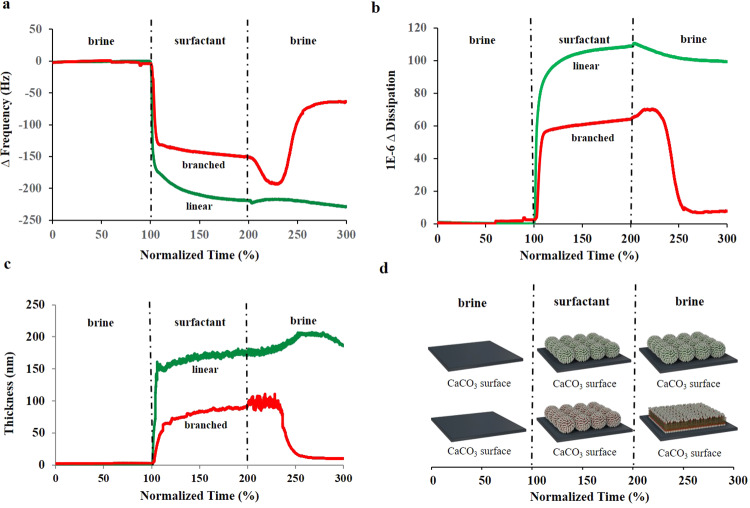
Experimental data obtained from the interaction of stabilized ABSs with a water-wet CaCO3 surface at 80 °C are provided in Figure 2 and Figures S1–S4. Figure 2a,b provides the QCM-D measurements for frequency and dissipation changes of the 3rd overtone, respectively. Other overtones for duplicate measurements are presented in Figures S1 and S3. Negative frequency changes can be related to mass adsorbed to the surface and to the liquid loading effects. In this work, mass adsorbed would correspond to the mass of vesicle composites and water between the molecules. The liquid loading effect could occur when switching from high-salinity water to surfactant solution and can be neglected when comparing retention of linear and branched ABSs since at the end of the experiment surfactant solution is changed back to high-salinity water only. Positive changes in dissipation result from adsorbed entities producing a soft layer, while the absence or decrease of dissipation is a sign that the layer has become more rigid.
Figure 2.
3rd overtone (a) resonance frequency change and (b) dissipation change for both linear (green)- and branched (red)-surfactant-containing vesicles on the water-wet CaCO3 surface. (c) The total thickness of the adsorbed surfactant layer on the water-wet CaCO3 surface obtained by modeling the QCM-D experimental data using the two-layer Voigt model. Plots for individual layers are provided in Figures S2c and S4c. The proposed mechanism (d) of linear (green)- and branched (red)-surfactant-containing vesicle adsorption on the water-wet CaCO3 surface. Meanwhile, vesicles containing the linear surfactant adsorb as is and mostly preserve their shape, and vesicles with the branched surfactant adsorb as vesicles and eventually reorganize into the bilayer form.
During the first ∼10 min of flowing high-salinity water only, there was no change in frequency and dissipation observed (Figure 2a,b), suggesting that no retention is happening and that the measurement is stable. Exposing the sensor’s surface to surfactant solutions, however, caused a significant decrease in frequency. Changes, around −130 Hz for branched and around −220 Hz for linear-ABS-containing vesicles, can be observed. The larger change in frequency for linear ABS samples suggests that more mass has been retained compared to the branched ABS surfactants. As Figure 2b depicts, when high-salinity water was switched to the surfactant, an immediate increase in dissipation occurred for both types of ABS, with a change close to 60 × 10–6 for branched and 105 × 10–6 for linear-ABS-containing vesicles. The above dissipation and frequency results suggest that both types of vesicles adsorb to the CaCO3 surface and produce a highly viscoelastic layer. Samples containing linear ABS adsorb more and result in a softer layer than vesicles with branched ABS. This could be attributed to the more negative surface charges of the linear-ABS-containing vesicles due to larger numbers of closely packed linear surfactant molecules, whereas branched surfactants are not well aligned at the interface.39 For the linear sample, as can be observed in Figure 2a,b, the dissipation and frequency graphs during surfactant flooding required more time to equilibrate when compared to the branched sample. This suggests that close packing of the more negatively charged vesicles could take longer. While magnitudes for frequency and dissipation changes are different when linear and branched samples are compared, the shape of the graphs is still relatively similar.
After surfactant flooding for 25–30 min, the solution was switched back to high-salinity water only. From Figure 2a,b, it is clear that frequency and dissipation changes for linear and branched ABSs are significantly different. Frequency changes (Figure 2a) for the linear ABS are almost negligible with only a small difference that could be a contribution from liquid loading and negligible desorption. On the other hand, the frequency graph for the branched ABS (Figure 2a) initially changes from −150 to −200 Hz and later increases to around −60 Hz. Similar trends are observed with the dissipation results (Figure 2b). No significant changes are present for the sample containing linear ABS, only a slight decrease that could be caused by changes in the viscoelasticity of the vesicle-containing layer and factors mentioned earlier. For the branched ABS sample, however, dissipation increases from around 60 × 10–6 to 70 × 10–6 and then significantly decreases to less than 10 × 10–6. Similar behaviors in frequency and dissipation as observed for the branched sample were studied previously for multiple systems.17,22−24 This is typically understood to be the result of vesicle adsorption to the surface with further rupture/reorganization into a mainly bilayer structure, and we believe this is what is occuring in our study as well.
To estimate the thickness of the adsorbed species (Figure 2c, Figures S2c and S4c), a two-layer Voigt model was used to regenerate the above experimental QCM-D data using Q-tools software. The frequency and dissipation graphs generated by this model and the parameters used are presented in Figures S2 and S4 and Tables S1 and S2. The total thickness of the adsorbed layer on the CaCO3 surface was calculated based on the model and is presented in Figure 2c. The total thickness of layers made using vesicles containing linear surfactants during surfactant flooding is 150 nm and varies from 150 to 200 nm when the solution is switched back to high-salinity water. This slight variation in thickness could be the result of vesicle migration on the surface and/or the formation of a layer of vesicles not perfectly aligned through the surface with cases where vesicles could be stacked together in three axes. For the results of vesicles with branched ABS (Figure 2c, Figure S4c), the combined thickness of the adsorbed layer is initially in the range of 80–100 nm, and after switching to high-salinity water, it decreases to less than 10 nm. This confirms the previous statement that vesicles made of branched ABS surfactants break and rearrange into much thinner bilayer structures after high-salinity water flooding.
From the QCM-D measurements, it can be concluded that vesicular structures made of linear and branched ABSs form significantly different adsorbed layers on the water-wet CaCO3 surface. As depicted in Figure 2d, both types of vesicles during surfactant flooding adsorb onto the surface as is, but during high-salinity water flooding, they demonstrate significant differences. Vesicles containing linear ABS mainly preserve a spherical shape, and vesicles made using branched ABS rupture and rearrange themselves into single and/or double layers, possibly due to less efficient packing of the branched surfactants.
In addition to QCM-D measurements, the surface of sensors was also evaluated for wettability alterations. Results provided in Table 1 are for contact angle measurements of water droplets on the CaCO3 surface both before and after QCM-D experiments. For linear ABS formulation, contact angles changed from 53 ± 1 to 40 ± 9°, suggesting that the surface became more water-wet. For the case with branched ABS, values changed from 51 ± 4 to 59 ± 6°, indicating that the surface became slightly less water-wet. This can be explained by the results observed from the QCM-D and suggests that the more water-wet surface after linear ABS formulation experiments is due to the surface coverage with the intact vesicles. The outer surface of the vesicles is composed of ABS and cocamidopropyl hydroxysultaine surfactants with hydrophilic parts facing out. This causes the water droplets to attach more strongly to the vesicles covering the surface and thus reducing the contact angle. Surface wettability alteration after branched ABS experiments toward less water-wet is due to the rearrangement of vesicles on the surface into single and/or bilayer structures. When vesicles rearrange, the first layer of surfactants reorients with hydrophilic parts toward the CaCO3 surface and hydrophobic tails facing out of the sensor plane. The second layer would be forming with hydrophilic surfactant heads out of the sensor plane. Formation of the less water-wet CaCO3 surface could suggest that, for the bilayer, the second layer of surfactant molecules does not completely cover the tails of surfactants located on the first layer. This would suggest that the adsorbed layer is not a complete bilayer. The presence of mineral oil within the vesicles, which have rearranged, also contributes to the wettability alteration of the surface.
Table 1. Contact Angle Measurements of a Water Droplet on the Surface before and after QCM-D Experiments Using Water-Wet CaCO3 Sensors.
| contact angle of water on the surface |
||
|---|---|---|
| formulation | new crystal | after QCM-D |
| linear ABS | 53 ± 1° | 40 ± 9° |
| branched ABS | 51 ± 4° | 59 ± 6° |
Interaction of Surfactants with the Oil-Wet CaCO3 Surface
To study the interaction of stabilized ABSs with an oil-wet CaCO3 surface, QCM-D crystals were first coated with the components of crude oil by spin-coating as described in the Materials and Methods section. Different studies used alternative methods to convert QCM-D sensors to oil-wet. Among the methods used are drop casting to deposit the asphalt film30 and crude oil flooding during the QCM-D experiment to adsorb to the clean surface first, and then the excess liquid crude oil can be cleaned with toluene prior to oil desorption studies.25−28 In this study, an oil-wet surface was achieved by spin-coating and not by oil flooding QCM-D measurement. This allowed further analysis of the coated surface for wettability alteration and for changes in the fluorescence of the crude oil after the QCM-D experiment.
Wettability alteration of the crude oil coated sensors toward oil-wet was assessed by measuring the contact angle of the water droplet with the surface before and after coating. The change in the contact angle from ∼50° for the clean surface to ∼95° for the coated (Table 2) is a clear indication that the surface was successfully coated and modified to oil-wet. Fluorescence images of the clean and oil-coated sensors presented in Figure S5 also confirm the presence of an oil layer.
Table 2. Contact Angle Measurements of a Water Droplet on a Clean Surface of CaCO3 Sensors, Crude Oil Coated Crystals, and Crystals after Surfactant Flooding Experimentsa.
| contact
angle of water on the surface |
|||||
|---|---|---|---|---|---|
| formulation | new crystal | after coating | after QCM-D | estimated oil desorption rate based on QCM-D (g/m2) | estimated oil desorption based on CLSM |
| linear ABS | 52 ± 1° | 95 ± 2° | 59 ± 6° | 0.176 ± 0.009 | 63 ± 2% |
| branched ABS | 53 ± 3° | 96 ± 1° | 79 ± 3° | 0.113 ± 0.00005 | 32 ± 7%° |
Estimated crude oil desorption based on fitted QCM-D experimental data and fluorescence images captured before and after QCM-D experiments.
Similar QCM-D experiments performed on water-wet sensors were repeated in duplicate for linear- and branched-ABS-containing formulations on oil-wet sensors. Figure 3 and Figures S6 and S7 present data for frequency and dissipation changes during QCM-D measurements. As it can be observed, during high-salinity water flooding, frequency and dissipation values remain relatively stable indicating that no significant changes occur on the sensor surface. Small negative changes in frequency and positive changes in dissipation are observable for the sensor used in the experiment with linear-ABS-containing formulation (Figure 3, Figure S6a,b). These observations are due to relatively small swelling and desorption of oil from the sensor.
Figure 3.

3rd overtone (a) resonance frequency change and (b) the dissipation change for both linear (green)- and branched (red)-surfactant-containing formulations on the oil-wet CaCO3 surface. (c) Modeled mass of oil desorbed from the surface during the QCM-D experiment.
After continuous high-salinity water injection, surfactant solutions were introduced. For the frequency changes for both surfactants in Figure 3a, it can be observed that values first change to around −70 Hz and then increase to ∼500 Hz for the sample with linear ABS and to ∼300 Hz for the sample with branched-ABS-containing formulations. The initial decrease in the frequency change is indicative of the mass increase due to surfactant sorption and water entrapment in the oil caused by swelling. The later increase is related to mass reduction and is due to oil desorption from the CaCO3 surface. Larger increases in the frequency change observed for the formulation with linear ABS when compared to branched ABS indicate that it has removed more oil. In addition to the difference in frequency amplitudes, the slope of the graph for the linear sample is much steeper than that for branched, indicating that the rate of oil desorption is higher in the case of linear ABS formulations. The difference in oil removal efficiency during surfactant flooding is also observed when changes in dissipation for both formulations are compared (Figure 3b). The initial dissipation change from ∼0 to ∼120 × 10–6 is an indication that the adsorbed layer is much more viscoelastic/soft than before. This is mainly due to swelling of the oil layer and sorption of surfactants. The difference in slopes is an indication of that layer becoming viscoelastic faster with linear ABS formulation than with branched ABS. Later, dissipation changes to ∼40 × 10–6 for linear ABS and to ∼100 × 10–6 for branched. This is in line with results observed for changes in frequency and suggests that formulations with linear surfactants desorbed larger mass of oil and left a less viscoelastic layer than the formulation with branched ABS.
After surfactant flooding, flow was changed again to high-salinity water only. Neither frequency changes nor dissipation exhibits large differences for either system (Figure 3a,b). Continuous and small downward frequency changes for sensors previously exposed to linear ABS formulation could be due to insignificant swelling of the remaining oil layer, which caused an increase in mass due to water entrapment. This is also confirmed by a continuous small increase in dissipation from ∼40 × 10–6 to ∼55 × 10–6 (Figure 3b). For the sensor used with the branched ABS formulation, the frequency curve also has a small negative slope suggesting that mass on the sensor is slightly increasing, possibly due to swelling of the remaining oil. Dissipation initially shows a slight increase from ∼100 × 10–6 to ∼105 × 10–6 and later decreases back to ∼100 × 10–6. Earlier, it was discussed that vesicles containing branched formulations tend to rapture/restructure into thinner layered-structures when diluted with high-salinity water on the water-wet CaCO3 surface. Therefore, the slight changes in dissipation observed for branched-ABS-containing vesicles on the oil-wet surface could be associated with structural changes of the adsorbed surfactant layer on the oil and oil layer itself.
To calculate the amount of oil desorbed after surfactant flooding, a two-layer Voight viscoelastic model was used to fit QCM-D experimental data and then to calculate the amount of mass desorbed as presented in Figure 3c and Figure S8. Linear-ABS-containing formulation desorbed ≈0.176 g/m2, while branched ABS desorbed ≈0.113 g/m2.
Contact angle measurement studies performed on oil-coated sensors before and after QCM-D experiments are presented in Table 2. For experiments with the linear ABS formulation, contact angles changed from 95 ± 2 to 59 ± 6°, while with branched ABS formulation, they changed from 96 ± 1 to 79 ± 3°. This indicates that sensors flooded with the linear ABS formulation had a surface change to more water-wet when compared to the surface after using the branched ABS formulation.
Fluorescence images of the oil-wet sensors were captured by CLSM before and after QCM-D experiments and used (i) to calculate the crude oil intensity difference for quantitative analysis on oil desorption, which was then compared to modeled QCM-D results, and (ii) to qualitatively observe desorption of the oil from the surface by each surfactant. Figure 4a presents images of oil fluorescence before and after linear-ABS-formulation flooding, whereas Figure 5a shows the oil-wet surface used with branched ABS formulation. Histograms in Figures 4b and 5b are based on the fluorescence intensity of the corresponding images when converted to grayscale. The difference in fluorescence intensity is correlated to the amount of oil present on the surface. When visually comparing images in Figures 4a and 5a before and after QCM-D experiments, it can be observed that, for both cases, the intensity of oil fluorescence has decreased after surfactant flooding, confirming that the studied formulations desorb oil from the surface as it was discussed in the QCM-D section. The oil fluorescence intensity change was calculated from the generated histograms to relatively quantify the amount of oil desorbed after surfactant flooding, and the results are presented in Table 2. After linear-ABS-formulation flooding, the estimated amount of oil desorbed is ∼63%, and after branched ABS, it is ∼32%. The difference between surfactants’ oil desorption efficiencies is differentiable and suggests that linear-ABS-containing vesicles have recovered more crude oil from the CaCO3 surface compared to branched. Although desorption estimates would not translate into a potential amount of oil recovery from an underground oil reservoir, fluorescence images help to support the results of QCM-D and contact angle measurements and the conclusion that linear-ABS-containing vesicles have desorbed more oil from the CaCO3 surface when compared to branched.
Figure 4.
(a) Fluorescence images before and after linear-surfactant-containing formulation flooding taken with confocal microscopy. (b) Histograms with intensity for corresponding fluorescence images when converted to a grayscale image using ImageJ software.
Figure 5.
(a) Fluorescence images before and after branched-surfactant-containing formulation flooding taken with confocal microscopy. (b) Histograms with intensity for corresponding fluorescence images when converted to a grayscale image using ImageJ software.
Additionally, fluorescence images reveal that the remaining oil on the surface is not in a continuous phase but rather remains as patches scattered across the surface (Figures 4a, 5a). Images suggest that the same mechanism of oil desorption, as described in earlier studies for oil recovery from a silica substrate using a nonionic surfactant30 and from chalk using an anionic surfactant,40 is also taking place here. The mechanism involves sorption/diffusion of surfactant entities onto the surface of the oil layer, swelling of the oil layer, and formation of water channels.
Conclusions
In this work, QCM-D and CLSM methods are applied to study the interactions of water- and oil-wet CaCO3 surfaces with linear and branched alkylbenzene sulfonate (ABS) surfactants that were stabilized into vesicular structures under high-salinity (≈6 wt % TDS) and high-temperature (80 °C) conditions. Results suggest that both types of vesicles adsorb onto the water-wet CaCO3 surface as is but that significant changes occur upon dilution with high-salinity water. While vesicles containing linear ABS surfactants remained adsorbed as is and did not undergo structural changes, vesicles with branched ABS surfactants rearranged to produce a layered structure on the surface. Flooding of these vesicles slightly altered the CaCO3 surface wettability, with the linear ABS surfactants causing the surface to be more water-wet and the branched ABS surfactants causing it to be less water-wet.
Interaction of vesicles with oil-wet surfaces reveals that vesicles containing linear ABS surfactants resulted in more oil desorbed and greater alteration of surface wettability toward water-wet, when compared to branched-ABS-containing vesicles. Fluorescence images of the oil film obtained before and after the QCM-D experiments allowed a visual comparison of oil-desorption efficiency of surfactants and confirmed a previous mechanism proposed for the removal of heavy oil.30 Ultimately, these results can help to better understand the nanoscale interactions of low-cost alkylbenzene sulfonate surfactants with surfaces under high-salinity and high-temperature conditions as well as to improve the screening process of potential surfactant candidates for lab and field implementation.
Materials and Methods
Materials
Bio-Soft S-126 (linear, alkyl (C12.6) benzene sulfonic acid) and Sulfonic 100 (branched, alkyl (C12) benzene sulfonic acid) surfactants used in this study were provided by Stepan Company (Northfield, IL). The ColaTeric CBS cocamidopropyl hydroxysultaine surfactant was obtained from Colonial Chemical (South Pittsburgh, TN), while Puritol TM/MC mineral oil was from Petro-Canada Lubricants (Mississauga, Canada). Crude oil used had a density of 0.8171 g/cm3, and according to SARA (saturate, aromatic, resin, and asphaltene) analysis, it is composed of 33.1% saturates, 47.3% aromatics, 8.7% resins (polars I), and 10.9% asphaltenes (polars II). Other chemicals were obtained from Fisher Scientific (Fair Lawn, USA).
Preparation of Stable Alkylbenzene Sulfonate Solutions
Stabilizing alkylbenzene sulfonates for high-salinity and -temperature applications was achieved as described previously.37 First, synthetic high-salinity water (∼60000 mg/L total dissolved salts (TDS)) was prepared by dissolving NaCl (0.7022 mol), CaCl2·2H2O (0.0162 mol), MgCl2·6H2O (0.0868 mol), Na2SO4 (0.0447 mol), and NaHCO3 (0.002 mol) in 1 L of deionized (DI) water. Then, to prepare 0.2 wt % surfactant solutions used in the experiments during surfactant flooding, 1 mL of 5 wt % mineral oil doped alkylbenzene sulfonates (linear or branched) and 1.5 mL of 4 wt % cocamidopropyl hydroxysultaine were mixed together, and 52 mL of synthetic high-salinity water was added to the premixed solution. 4 wt % active cocamidopropyl hydroxysultaine solution was prepared by dissolving 8.33 g of as-received ColaTeric CBS (48 wt % active) in 92 g of DI water. Mineral oil doped 5 wt % linear and branched alkylbenzene sulfonate solutions were prepared first by thoroughly mixing 0.25 g of Puritol TM/MC mineral oil and 4.75 g of linear or branched ABSs (heating mixtures to 60 °C reduced viscosity for better mixing). Next, the mixture was dissolved in 50 g of DI water and neutralized to ∼pH 7 using aqueous NaOH, and the final mass of solution was adjusted to 95 g by addition of DI water.
Quartz Crystal Microbalance with Dissipation (QCM-D) Measurements
The interactions of stabilized linear- and branched-ABS-surfactant formulations in high-salinity brine with water- and oil-wet CaCO3 surfaces at 80 °C were studied using QCM-D (Biolin Scientific, Gothenburg, Sweden). The instrument was a QSence High-Temperature Chamber connected to the QSense Analyzer electronic unit. CaCO3-coated quartz crystals/sensors (QSX 999) were used to represent the reservoir rock surface and purchased from Biolin Scientific. Both frequency and dissipation measurements were recorded at several overtones (1st, 3rd, 5th, 7th, 9th, 11th, and 13th). Experiments were performed at 80 °C with a constant fluid flow rate of 100 μL/min. All solutions were filtered before performing QCM-D experiments using a 0.25 μm polytetrafluoroethylene (PTFE) syringe filter and degassed using a vacuum chamber to avoid formation of gas bubbles during measurements. Solutions were kept preheated at 70 °C on a hot plate before they were introduced into a heating loop within the QCM-D chamber.
For the interaction of surfactant formulations with a water-wet CaCO3 surface, new CaCO3-coated crystals were used every time. First, sea water was flooded into the system in order to stabilize the oscillation and obtain a baseline of frequency and dissipation. After high-salinity water, 0.2 wt % linear or branched ABS formulation was introduced, and finally, high-salinity water was flooded. Each flow was maintained until the resonance frequency and dissipation signal stabilized. A similar experiment was repeated for both formulations in duplicate using new CaCO3 sensors.
Interaction of formulations with the oil-wet CaCO3 surface and crude oil desorption experiments were performed on precoated CaCO3 sensors. Oil deposition was achieved using a spin-coater (KW-4A). First, crude oil was diluted with toluene in a ratio of 1:20 (oil:toluene). Dilution was performed to reduce viscosity of the crude oil to achieve a thin and uniform coating. Depositing crude oil as is resulted in a thicker layer, which was significantly impacting sensitivity of the QCM-D crystals. Four drops of diluted crude oil (i.e., approximately 30 μL) were placed on a clean CaCO3 sensor while spinning at 4000 rpm for 5 min. Then, the coated crystals were left to dry in an oven at 100 °C for 5 min. A similar procedure, as outlined above, was followed for the oil-wet QCM-D experiment, with the only difference being that crystals were now coated with oil. Experiments were also repeated in duplicate for both linear- and branched-ABS-containing formulations. Wettability of the CaCO3 sensor surface was determined by measuring contact angles of DI water droplets (∼10 μL) in the air and at room temperature using a goniometer (ramé-hart, Succasunna, USA) and performed for new crystals, oil-coated, and after QCM-D experiments. The contact angle was measured at three different regions on the crystal for each sample, and values were reported as the average of three measurements for each case. Equilibration time before contact angle measurement was ∼1 min.
Confocal Laser Scanning Microscopy (CLSM)
CLSM (Zeiss LSM 780) was used to characterize the surface of the crude oil coated crystal based on fluorescence before and after flowing the surfactant. A 488 nm laser was used in Lambda imaging mode (i.e., multichannel mode) at a resolution of 1980 × 1980, pinhole of 38.9 Airy Units, and gain of 800. Horizontal images were taken for each sample after coating and after surfactant flooding in the QCM-D experiment. The dimension of all images was 849.76 μm × 849.76 μm. Then, the images were processed in ImageJ software where they were converted to grayscale images for easier analysis. Intensity histograms of images were generated in order to estimate the amount of oil desorbed from the surface of CaCO3 sensors.
Acknowledgments
Authors would like to acknowledge Gawain Thomas for help with CLSM, Dr. Martin Poitzsch for fruitful discussions, and Cristina Young for editing the language of the manuscript. Work was funded by Saudi Aramco.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00478.
Resonance frequency change and the dissipation change as a function of the adsorbed species (both in response to the amount and to the distribution or arrangement of the material on the surface) for all the QCM-D experiments. The physical properties and the modeling parameters used for QCM-D data modeling using the Voigt model. Fluorescence images of the QCM-D crystal surfaces. Modeled mass of oil desorbed from the surface during different QCM-D experiments (PDF)
Author Present Address
§ Department of Chemical Engineering, The University of Texas at Austin, Austin, Texas 78712, United States (M.R.K.).
The authors declare no competing financial interest.
Supplementary Material
References
- Alvarado V.; Manrique E. Enhanced Oil Recovery: An Update Review. Energies 2010, 3, 1529–1575. 10.3390/en3091529. [DOI] [Google Scholar]
- Muggeridge A.; Cockin A.; Webb K.; Frampton H.; Collins I.; Moulds T.; Salino P. Recovery Rates, Enhanced Oil Recovery and Technological Limits. Philos. Trans. R. Soc., A 2014, 372, 20120320. 10.1098/rsta.2012.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfarge D.; Wei M.; Bai B.. IOR Methods in Unconventional Reservoirs of North America: Comprehensive Review. In SPE Western Regional Meeting; Society of Petroleum Engineers: 2017. 10.2118/185640-MS. [DOI] [Google Scholar]
- Talebian S. H.; Masoudi R.; Tan I. M.; Zitha P. L. J. Foam Assisted CO2-EOR: A Review of Concept, Challenges, and Future Prospects. J. Pet. Sci. Eng. 2014, 120, 202–215. 10.1016/j.petrol.2014.05.013. [DOI] [Google Scholar]
- Hirasaki G. J.; Miller C. A.; Puerto M.. Recent Advances in Surfactant EOR. In IPTC 2008: International Petroleum Technology Conference; Society of Petroleum Engineers: 2008. 10.2118/115386-MS. [DOI]
- Kamal M. S.; Sultan A. S.; Al-Mubaiyedh U. A.; Hussein I. A. Review on Polymer Flooding: Rheology, Adsorption, Stability, and Field Applications of Various Polymer Systems. Polym. Rev. 2015, 55, 491–530. 10.1080/15583724.2014.982821. [DOI] [Google Scholar]
- Sheng J. J. Critical Review of Alkaline-Polymer Flooding. J. Pet. Explor. Prod. Technol. 2017, 7, 147–153. 10.1007/s13202-016-0239-5. [DOI] [Google Scholar]
- Negin C.; Ali S.; Xie Q. Most Common Surfactants Employed in Chemical Enhanced Oil Recovery. Petroleum 2017, 3, 197–211. 10.1016/j.petlm.2016.11.007. [DOI] [Google Scholar]
- Sheng J. J. Status of Surfactant EOR Technology. Petroleum 2015, 1, 97–105. 10.1016/j.petlm.2015.07.003. [DOI] [Google Scholar]
- Guo H.; Li Y.; Wang F.; Yu Z.; Chen Z.; Wang Y.; Gao X. ASP Flooding: Theory and Practice Progress in China. J. Chem. 2017, 2017, 1–18. 10.1155/2017/8509563. [DOI] [Google Scholar]
- Ghoodjani E.; Kharrat R.; Vossoughi M.; Bolouri S. H. A Review on Thermal Enhanced Heavy Oil Recovery from Fractured Carbonate Reservoirs. J. Pet. Environ. Biotechnol. 2011, 02, 1–7. 10.4172/2157-7463.1000109. [DOI] [Google Scholar]
- Choudhary N.; Narayanan Nair A. K.; Che Ruslan M. F. A.; Sun S. Bulk and Interfacial Properties of Decane in the Presence of Carbon Dioxide, Methane, and Their Mixture. Sci. Rep. 2019, 9, 19784. 10.1038/s41598-019-56378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal M. S.; Hussein I. A.; Sultan A. S. Review on Surfactant Flooding: Phase Behavior, Retention, IFT, and Field Applications. Energy Fuels 2017, 31, 7701–7720. 10.1021/acs.energyfuels.7b00353. [DOI] [Google Scholar]
- Somasundaran P.; Middleton R.; Viswanathan K. V.. Relationship Between Surfactant Structure and Adsorption. In Structure/Performance Relationships in Surfactants; Rosen M. J., Ed.; American Chemical Society: Washington, D. C, 1984; Vol. 253, pp 269–290. [Google Scholar]
- Dick S. G.; Fuerstenau D. W.; Healy T. W. Adsorption of Alkylbenzene Sulfonate (A.B.S.) Surfactants at the Alumina-Water Interface. J. Colloid Interface Sci. 1971, 37, 595–602. 10.1016/0021-9797(71)90337-7. [DOI] [Google Scholar]
- Reviakine I.; Johannsmann D.; Richter R. P. Hearing What You Cannot See and Visualizing What You Hear: Interpreting Quartz Crystal Microbalance Data from Solvated Interfaces. Anal. Chem. 2011, 83, 8838–8848. 10.1021/ac201778h. [DOI] [PubMed] [Google Scholar]
- Kanazawa K.; Cho N.-J. Quartz Crystal Microbalance as a Sensor to Characterize Macromolecular Assembly Dynamics. J. Sens. 2009, 2009, 1–17. 10.1155/2009/824947. [DOI] [Google Scholar]
- Marx K. A. Quartz Crystal Microbalance: A Useful Tool for Studying Thin Polymer Films and Complex Biomolecular Systems at the Solution–Surface Interface. Biomacromolecules 2003, 4, 1099–1120. 10.1021/bm020116i. [DOI] [PubMed] [Google Scholar]
- Voinova M. V.; Rodahl M.; Jonson M.; Kasemo B. Viscoelastic Acoustic Response of Layered Polymer Films at Fluid-Solid Interfaces: Continuum Mechanics Approach. Phys. Scr. 1999, 59, 391–396. 10.1238/Physica.Regular.059a00391. [DOI] [Google Scholar]
- Saad N. A.; Zaaba S. K.; Zakaria A.; Kamarudin L. M.; Wan K.; Shariman A. B.. Quartz Crystal Microbalance for Bacteria Application Review. In 2014 2nd International Conference on Electronic Design (ICED); IEEE: 2014; pp 455–460. 10.1109/ICED.2014.7015849. [DOI] [Google Scholar]
- Ferreira G. N. M.; da-Silva A.-C.; Tomé B. Acoustic Wave Biosensors: Physical Models and Biological Applications of Quartz Crystal Microbalance. Trends Biotechnol. 2009, 27, 689–697. 10.1016/j.tibtech.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Keller C. A.; Kasemo B. Surface Specific Kinetics of Lipid Vesicle Adsorption Measured with a Quartz Crystal Microbalance. Biophys. J. 1998, 75, 1397–1402. 10.1016/S0006-3495(98)74057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N.-J.; Cho S.-J.; Cheong K. H.; Glenn J. S.; Frank C. W. Employing an Amphipathic Viral Peptide to Create a Lipid Bilayer on Au and TiO 2. J. Am. Chem. Soc. 2007, 129, 10050–10051. 10.1021/ja0701412. [DOI] [PubMed] [Google Scholar]
- Cho N.-J.; Kanazawa K. K.; Glenn J. S.; Frank C. W. Employing Two Different Quartz Crystal Microbalance Models To Study Changes in Viscoelastic Behavior upon Transformation of Lipid Vesicles to a Bilayer on a Gold Surface. Anal. Chem. 2007, 79, 7027–7035. 10.1021/ac0709504. [DOI] [PubMed] [Google Scholar]
- Liu X.; Yan W.; Stenby E. H.; Thormann E. Release of Crude Oil from Silica and Calcium Carbonate Surfaces: On the Alternation of Surface and Molecular Forces by High- and Low-Salinity Aqueous Salt Solutions. Energy Fuels 2016, 30, 3986–3993. 10.1021/acs.energyfuels.6b00569. [DOI] [Google Scholar]
- Farooq U.; Asif N.; Tweheyo M. T.; Sjöblom J.; Øye G. Effect of Low-Saline Aqueous Solutions and PH on the Desorption of Crude Oil Fractions from Silica Surfaces. Energy Fuels 2011, 25, 2058–2064. 10.1021/ef1013538. [DOI] [Google Scholar]
- Nourani M.; Tichelkamp T.; Gaweł B.; Øye G. Method for Determining the Amount of Crude Oil Desorbed from Silica and Aluminosilica Surfaces upon Exposure to Combined Low-Salinity Water and Surfactant Solutions. Energy Fuels 2014, 28, 1884–1889. 10.1021/ef402494c. [DOI] [Google Scholar]
- Al-Khafaji A.; Neville A.; Wilson M.; Wen D. Effect of Low Salinity on the Oil Desorption Efficiency from Calcite and Silica Surfaces. Energy Fuels 2017, 31, 11892–11901. 10.1021/acs.energyfuels.7b02182. [DOI] [Google Scholar]
- Nourani M.; Tichelkamp T.; Gaweł B.; Øye G. Desorption of Crude Oil Components from Silica and Aluminosilicate Surfaces upon Exposure to Aqueous Low Salinity and Surfactant Solutions. Fuel 2016, 180, 1–8. 10.1016/j.fuel.2016.04.008. [DOI] [Google Scholar]
- Chen I.-C.; Akbulut M. Nanoscale Dynamics of Heavy Oil Recovery Using Surfactant Floods. Energy Fuels 2012, 26, 7176–7182. 10.1021/ef301241f. [DOI] [Google Scholar]
- Han M.; AlSofi A.; Fuseni A.; Zhou X.; Hassan S.. Development of Chemical EOR Formulations for a High Temperature and High Salinity Carbonate Reservoir. In IPTC 2013: International Petroleum Technology Conference; European Association of Geoscientists & Engineers: 2013. 10.2523/IPTC-17084-MS. [DOI]
- Sandvik E. I.; Gale W. W.; Denekas M. O. Characterization of Petroleum Sulfonates. Soc. Pet. Eng. J. 1977, 17, 184–192. 10.2118/6120-PA. [DOI] [Google Scholar]
- Pachón-Contreras Z. D. P.; Rojas-Ruíz F.-A.; Rondón-Antón M.-J.; Vidal-Prada J.-C.; Pulido-Solano F.-A. Petroleum Sulfonates Preparation and Evaluation for Chemical Enhanced Oil Recovery in Colombian Oil Fields. CTF - Cienc. Tecnol. Futuro 2014, 5, 55–74. 10.29047/01225383.33. [DOI] [Google Scholar]
- Knaggs E. A.; Nussbaum M. L.; Carlson J. B.; Guenzani R. C.. Petroleum Sulfonate Utilization in Enhanced Oil Recovery Systems. In SPE Annual Fall Technical Conference and Exhibition; Society of Petroleum Engineers: 1976. 10.2118/6006-MS. [DOI] [Google Scholar]
- Harbaugh J. W.Chapter 7 Carbonate Oil Reservoir Rocks. In Developments in Sedimentology; Elsevier: 1967; Vol. 9, pp 349–398, 10.1016/S0070-4571(08)71115-4. [DOI] [Google Scholar]
- Akbar M.; Chakravorty S.; Russell S. D.; Al Deeb M. A.; Efnik M. R. S.; Thower R.; Karakhanian H.; Mohamed S. S.; Bushara M. N.. Unconventional Approach to Resolving Primary and Secondary Porosity in Gulf Carbonates from Conventional Logs and Borehole Images. In Abu Dhabi International Petroleum Exhibition and Conference; Society of Petroleum Engineers: 2000. 10.2118/87297-MS. [DOI] [Google Scholar]
- Gizzatov A.; Mashat A.; Kosynkin D.; Al-Hazza N.; Kmetz A.; Eichmann S. L.; Abdel-Fattah A. I. Nanofluid of Petroleum Sulfonate Nanocapsules for Enhanced Oil Recovery in High Temperature and Salinity Reservoirs. Energy Fuels 2019, 33, 11567–11573. 10.1021/acs.energyfuels.9b02609. [DOI] [Google Scholar]
- Chen H.; Gizzatov A.; Abdel-Fattah A. I. Molecular Assembly of Surfactant Mixtures in Oil-Swollen Micelles: Implications for High Salinity Colloidal Stability. J. Phys. Chem. B 2019, 124, 568–576. 10.1021/acs.jpcb.9b09929. [DOI] [PubMed] [Google Scholar]
- Frank C.; Frielinghaus H.; Allgaier J.; Prast H. Nonionic Surfactants with Linear and Branched Hydrocarbon Tails: Compositional Analysis, Phase Behavior, and Film Properties in Bicontinuous Microemulsions. Langmuir 2007, 23, 6526–6535. 10.1021/la0637115. [DOI] [PubMed] [Google Scholar]
- Standnes D. C.; Austad T. Wettability alteration in chalk: 2. Mechanism for wettability alteration from oil-wet to water-wet using surfactants. J. Pet. Sci. Eng. 2000, 28, 123–143. 10.1016/S0920-4105(00)00084-X. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.