Abstract
Over the last decade, the interest in zirconium-89 (89Zr) as a positron-emitting radionuclide increased considerably because of its standardized production and its physical half-life (78.41 h), which matches the biological half-life of antibodies and its successful use in preclinical and clinical applications. So far, desferrioxamine (DFO), a commercially available chelator, has been mainly used as a bifunctional chelating system. However, there are some concerns regarding the in vivo stability of the [89Zr]Zr-DFO complex. In this study, we report the synthesis of an acyclic N-hydroxy-N-methyl succinamide-based chelator (4HMS) with 8 coordination sites and our first investigations into the use of this new chelator for 89Zr complexation. In vitro and in vivo comparative studies with [89Zr]Zr-4HMS and [89Zr]Zr-DFO are presented. The 4HMS chelator was synthesized in four steps starting with an excellent overall yield. Both chelators were quantitatively labeled with 89Zr within 5–10 min at pH 7 and room temperature; the molar activity of [89Zr]Zr-4HMS exceeded (>3 times) that of [89Zr]Zr-DFO. [89Zr]Zr-4HMS remained stable against transmetalation and transchelation and cleared from most tissues within 24 h. The kidney, liver, bone, and spleen uptakes were significantly low for this 89Zr-complex. Positron emission tomography images were in accordance with the results of the biodistribution in healthy mice. Based on DFT calculations, a rationale is provided for the high stability of 89Zr-4HMS. This makes 4HMS a promising chelator for future development of 89Zr-radiopharmaceuticals.
Introduction
Recent years have seen an increased interest in the therapeutic potential of high affinity monoclonal antibodies (mAb) and consequently an increased number of clinical trials.1 Most mAbs have long biological half-lives that can be advantageously used for the treatment of tumors if rapid clearance from non-target tissues can be reached. Labeling of mAb with 89Zr in conjunction with positron emission tomography (PET) imaging is an important step in the planning of immunotherapy and radio-immunotherapy.2,3 With its long half-life (78.41 h) that closely matches the circulation half-live of mAbs, 89Zr is preferred over other positron emitters for mAb-based PET imaging.
Until now, radiolabeling of mAbs with 89Zr was mostly achieved using desferrioxamine (DFO), a bacterial siderophore with six coordination sites (Figure 1A).4 To allow conjugation to biomolecules, a DFO amine function has to be first functionalized.2,5−8 DFO has permitted the development of many 89Zr mAb conjugates, which were successfully used in preclinical research, with a few of them having translated to the clinic.9−16 Despite being the most used chelator for mAb labeling with 89Zr, DFO remains suboptimal in terms of in vivo stability.17,18 A potential explanation is that DFO does not saturate the coordination sphere of 89Zr. It has been proposed that octacoordinate zirconium complexes can form thermodynamically more stable chelates.17 Although significant efforts have been made to design better 89Zr-chelators such as desferrichrome (DFC), four 1-hydroxypyridin-2-one groups appended to a linear tetraamine (HOPO), L1–4, L5, FSC derivatives, TAFC, FOXE, CP256, YM103, DFO-star (DFO*), and oxoDFO*,19−31 there is still a need for new chelators that firmly bind 89Zr in order to prevent its release, which may lead to unwanted radiation dose to the bone marrow.9,12,32
Figure 1.

(A) DFO chelator bearing three hydroxamate groups and (B) 4HMS chelator bearing four hydroxamate arms.
In our previous work, we reported the synthesis of N-methylhydroxamates derived from tetraaza- and triazamacrocycles, namely, DOTHA2 and NOTHA2.33 In the light of these promising results, we were interested in whether spermine, a natural tetraamine, could be advantageously used as a template for preparing an acyclic chelator with four N-hydroxy-N-methyl succinamide pendant arms. The new acyclic chelator, 4HMS, has a branched structure by contrast to DFO that displays a linear scaffold for metal complexation (Figure 1). In this study, we present the synthesis and the characterization of 4HMS. The radiolabeling, the stability, and in vivo behavior of the [89Zr]Zr-4HMS complex were examined and compared with[89Zr]Zr-DFO analogue. Density functional theory (DFT) calculations were also performed to investigate the Zr-4HMS structure and binding energies.
Results and Discussion
The synthesis of the hydroxamate pendant arms was straightforward starting from the commercially available N-methyl-O-benzylhydroxylamine (Scheme 1). The partially protected hydroxylamine was treated with succinic anhydride in tetrahydrofuran at reflux to give the carboxylic acid 1. The latter was quantitatively converted into the corresponding N-hydroxysuccinimide ester 2 following activation with N-hydroxysuccinimide (NHS)/ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) in dimethylformamide (DMF). The NHS-activated ester was used for direct coupling with spermine in dichloromethane. By carefully controlling the temperature, the fully protected chelator was obtained in a good yield (78%) after purification by flash chromatography. The final deprotection was performed with Pd/C-catalyzed hydrogenation affording the new chelator 4HMS 3 in a quantitative yield. The total synthesis resulted in an overall yield of 72%. The product was characterized by high performance liquid chromatography (HPLC), nuclear magnetic resonance (NMR), and mass spectroscopy (Figures S12–S15). The 4HMS chelator shows a high flexibility in solution, which resulted in many overlapping multiplets observed in the proton NMR spectrum because of the presence of several conformers (Figure S13). The product results a single peak by UPLC analysis (Figure S12).
Scheme 1. Synthesis of the New Branched-Chelator 4HMS 3.
First insights into the characterization for 4HMS as a complexing moiety were carried out in the presence of nonradioactive zirconium (natZr). The natZr-4HMS complex was formed by mixing the 4HMS ligand with a slight excess (1.5 equiv) of Zr(IV) acetylacetonate ([Zr(acac)4]) in MeOH for 5 min at room temperature. The natZr-4HMS complex was purified by precipitation from ether and analyzed by UPLC, NMR, and mass spectroscopy (Figures S16–S18). The participation of the hydroxamate groups in the complexation with Zr(IV) was also confirmed by 1H NMR (Figure S17). The 1H NMR of the natZr-4HMS complex still presents overlapping multiplets as seen with 4HMS alone but with substantial changes in the proton signals associated with the metal. A 1:1 metal-to-chelator ratio was confirmed by mass spectrometry, which displays the expected mass signal, Figure S18.
To get a deeper understanding into the binding aspect and the stability of Zr-4HMS, an accurate picture of the 3D arrangement of its four chelating arms, and the way they hold the Zr(IV) metal, we performed density functional theory (DFT) calculations using the B3LYP functional with the SBKJC basis set,34−36 as implemented in GAMESS.37
Although the high level B3LYP/SBKJC DFT method is well suited for transition elements34,38 and particularly zirconium,35,36 we first tested its accuracy with the known 1:1 Zr–HOPO complex,23 a related but much more rigid system, whose structure had been energy minimized with the B3LYP/CEP-121G DFT method. The RMSD between the input (B3LYP/CEP-121G DFT)23 and output (minimum obtained after 53 iterations with B3LYP/SBKJC DFT) structures was 0.032 Å, indicating a close match between the two methods. From its crystal structures with DMSO and water,39,40 it had been demonstrated that Zr(IV) is eight-coordinate in a square antiprismatic configuration, with average Zr–O distances of 2.19 Åin both cases (Figure S19A), D4d point group symmetry). When bidentate hydroxamates replace monodentate water or DMSO molecules, the perfect square antiprism described by the eight oxygen atoms is only marginally disturbed.
Several input structures were created from two minimized Zr(IV)·(hydroxamate)4 geometries (with relative energies of 0.05 and 2.93 kcal·mol–1 in Figures S19 and 2) considered to be suitable templates capable of holding the rest of the ligand with minimal tension. B3LYP/SBKJC DFT calculations were carried out in the gas phase and produced a structure significantly more stable than all others. It is worth noting that this lowest energy structure corresponds to the stabilized Zr(IV)·(hydroxamate)4 geometry of highest energy (Figure 2) and that it was also obtained from input files generated from the other Zr(IV)·(hydroxamate)4 template, obviously through relaxation of strain. Overall, its geometry is rather simple: the symmetry of the ligand is partially transferred to the complex, which mostly consists in two pseudo-C2-symmetric regions linked by a tetramethylene bridge. The hydroxamate arms are held rigid through complexation with the Zr ion and by intramolecular H-bonds between the NH and CO groups from secondary and tertiary amides, respectively (Figure 2). The chelating N–O– and the C=O parts of the hydroxamates occupy opposite hemispheres of the Zr metal. The longer and the shorter arms bearing the chelating hydroxamates act like complementary claws reaching the outmost and the innermost regions of the complex, respectively.
Figure 2.

Zr(IV)·(hydroxamate)4 templates A and B from which were built the input files leading to the preferred geometry of Zr(IV) complex C after B3LYP/SBKJC DFT calculations.
The preparation of [89Zr]Zr-4HMS was performed by incubating 7–8 nmol of the chelator with 30 MBq of neutralized [89Zr]Zr-oxalate41 within 5–10 min at room temperature at pH 7. The quantitative radiolabeling yield (>99%) for [89Zr]Zr-4HMS was confirmed by radio-thin layer chromatography (radio-TLC) and radio-HPLC (Figures S20 and S21). Apparent molar activity (AMA) was determined for both 4HMS and DFO by chelator titration with [89Zr]Zr-oxalate. AMA of [89Zr]Zr-4HMS (170 GBq/μmol) was approximately 3 fold higher than the value calculated for [89Zr]Zr-DFO (57 GBq/μmol) (Figure S22).
The stability of [89Zr]Zr-4HMS was extensively challenged in the presence of a large excess of diethylenetriaminepentaacetic acid (DTPA), biologically relevant metal ions, and serum proteins. [89Zr]Zr-4HMS demonstrated a remarkable resistance to transchelation and remained stable when incubated with 100 equiv of DTPA over 7 days for the entire range of pH values tested (Table S1). In contrast, some transchelation was observed at day 7 with [89Zr]Zr-DFO. This difference in stability between the two chelators is more pronounced with a 1000-fold excess of DTPA. [89Zr]Zr-4HMS also showed a good stability upon transmetalation with a wide range of metal cations. In fact, when tested with 10-fold metal excess, it showed no demetallation as a result of the enhanced coordination of the metal center by the octadentate ligand. [89Zr]Zr-4HMS remained stable in the presence of FeCl3 for over 7 days (97%), while only 88% of the [89Zr]Zr-DFO complex was still intact at day 7 (Table S2). The experiments were repeated using a 100-fold excess of FeCl3. In these conditions, [89Zr]Zr-4HMS was the most stable of the two complexes with 62% of 89Zr still bound at day 7 compared to 34% for [89Zr]Zr-DFO. However, both chelators remained stable in the presence of all other metals (Table S2).
[89Zr]Zr-4HMS remained relatively stable to transferrin. In fact, only 13% of the complex was transchelated following 7 days of incubation in the presence of a 10-fold excess of transferrin, the major protein in the plasma known to form strong complexes with various metals (Table S3).42 When incubated in mouse plasma for 7 days, [89Zr]Zr-4HMS remained intact, while a decrease to 78% intact complex was observed for [89Zr]Zr-DFO (Table S4). Nevertheless, both [89Zr]Zr-4HMS and [89Zr]Zr-DFO complexes remained stable in human plasma over 7 days. Finally, it is worth mentioning that both [89Zr]Zr-4HMS and [89Zr]Zr-DFO are stable for a week when formulated in phosphate buffer or saline (Table S5).
Biodistribution studies were performed in healthy mice injected via the caudal vein with either [89Zr]Zr-4HMS or [89Zr]Zr-DFO. The animals were euthanized at 1, 4, and 24 h post injection. Blood and various organs were removed to measure tissue-specific activity. For [89Zr]Zr-DFO and [89Zr]Zr-4HMS, the radioactivity was mostly concentrated in kidneys (5.52 ± 0.37 and 6.93 ± 1.66% ID/g, respectively) at 1h post injection suggesting renal elimination (Figure 3 and Table S6). At 4 h post injection, higher retention of radioactivity was seen for [89Zr]Zr-DFO in the pancreas (0.83 ± 1.38% ID/g), liver (1.72 ± 1.32% ID/g), heart (0.04 ± 0.02% ID/g), lungs (0.14 ± 0.09% ID/g), and bone (0.04 ± 0.01% ID/g) as compared to [89Zr]Zr-4HMS (0.02 ± 0.00, 0.08 ± 0.01, 0.02 ± 0.00, 0.05 ± 0.01, and 0.02 ± 0.00% ID/g, respectively, p < 0.05). At 24 h after injection, [89Zr]Zr-4HMS was almost cleared from all tissues and the amount of this 89Zr-chelator remaining in the kidneys (0.91 ± 0.10% ID/g) had dropped by a factor of 7.5 as compared to 1 h post injection and by about half for [89Zr]Zr-DFO (3.02 ± 1.98% ID/g) at the same time points. Even at 24 h after injection, a fair amount of [89Zr]Zr-DFO was still seen in the kidneys (3.02 ± 1.98% ID/g), spleen (0.12 ± 0.01% ID/g), liver (0.22 ± 0.07% ID/g), and bone (0.17 ± 0.13% ID/g), Figure 3 and Table S6. It is to be noted that the bone uptake for [89Zr]Zr-4HMS at 24 h post injection was very low (0.02 ± 0.0% ID/g) as predicted by the in vitro stability results and expected from a stable chelator.
Figure 3.
Biodistribution of [89Zr]Zr-DFO and [89Zr]Zr-4HMS in healthy Balb/c mice.
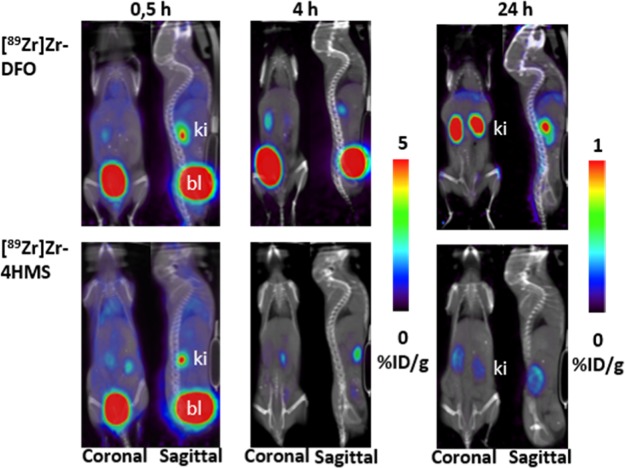
PET/CT images of [89Zr]Zr-4HMS were compared to that of [89Zr]Zr-DFO at 0.5, 4, and 24 h postinjection (Figure 4). The higher uptake of radioactivity in the kidneys and bladder at all time-points on PET images of [89Zr]Zr-DFO and [89Zr]Zr-4HMS was consistent with the results of the biodistribution. However, kidneys and bladder uptake dropped rapidly in the mice receiving [89Zr]Zr-4HMS, suggesting a better elimination profile from these tissues for our chelator. In addition, the stability of [89Zr]Zr-4HMS was proven with the static images at 24h postinjection. Mice injected with [89Zr]Zr-4HMS had less accumulation of radioactivity in the bones compared to the ones that received [89Zr]Zr-DFO in (Figure 4). One should note that the intensity scale has been modified for the 24h postinjection PET/CT images to underline the differences in bone uptake between 89Zr-4HMS and 89Zr-DFO. Time activity curves (TACs) obtained from the dynamic studies showed that both [89Zr]Zr-4HMS and [89Zr]Zr-DFO have similar pharmacokinetics in heart, muscle, liver, kidneys, and bladder at early time point post injection (Figure S23).
Figure 4.
Coronal and sagittal PET/CT images of [89Zr]Zr-DFO and [89Zr]Zr-4HMS in healthy Balb/c mice at 0.5, 4, and 24 h post injection. [89Zr]Zr-4HMS uptake decreased rapidly in kidneys (ki) and bladder (bl).
Based on these encouraging results, it seemed reasonable to propose a comparison with acyclic and macrocyclic 89Zr-chelators recently developed. A direct comparison cannot be done between [89Zr]Zr-4HMS and those 89Zr-chelators because of variability in the study designs. Ma et al. have developed tripodal tris(hydroxypyridinone) acyclic chelators, CP256, and its bifunctional version YM103.30 The authors have stated that both 89Zr-chelators are not optimal in terms of kinetic stability. Indeed, [89Zr]Zr-CP256 was less stable than [89Zr]Zr-DFO in the presence of Fe3+. Similarly, the bifunctional analogue conjugate to an antibody, [89Zr]Zr-YM103-trastuzumab, has presented higher accumulation in bone and joints by PET imaging than the 89Zr-DFO-conjugate in C57Bl/6j mice.30 The macrocyclic [89Zr]Zr-TAFC developed by Zhai et al. and bearing three hydroxamate groups has resulted in higher in-vitro and in-vivo stability than [89Zr]Zr-DFO.22 Summer et al. have published a comparative study between the FSC and DFO conjugated to ZEGFR:2377, an affibody-based probe for EGFR expression in tumor.21 At 24h post injection, [89Zr]Zr-FSC-ZEGFR:2377 has presented a similar bone uptake than [89Zr]Zr-DFO-ZEGFR:2377, but the 89Zr-FSC-affibody has shown a significantly higher accumulation in tumor than the 89Zr-DFO counterpart. A series of macrocyclic chelators (L1-L5) bearing three hydroxamate groups have been synthesized by Boros et al.29 All these 89Zr-chelators have resulted in low in vivo stability when compared to [89Zr]Zr-DFO and [89Zr]Zr-DFO conjugated to trastuzumab.
More recently, Pandya et al. showed that [89Zr]Zr-DOTA was superior to [89Zr]Zr-DFO.43 Although the in vitro stability of 89Zr-DOTA seems higher to that of [89Zr]Zr-4HMS, biodistribution data for both chelators were quite similar. The elevated temperature needed for [89Zr]Zr-DOTA complexation may be a potential limitation for immune-PET application.43 Our in vivo data suggest that [89Zr]Zr-4HMS is slightly more stable in the presence of biologically relevant metal ions than the acyclic chelator HOPO23 and has a comparable in vivo stability than the recently developed tetrahydroxamic DFO* and DFOcyclo*derivatives.44,45 Although being limited, this comparative study suggested that 4HMS is a promising 89Zr-chelator.
Conclusions
This report is the first to depict the structural characterization of Zr-4HMS using DFT calculations and the use of 4HSM as 89Zr-chelator. The intramolecular H-bonds between the NH and CO amide groups seen in the preferred geometry of Zr(IV) complex maintain the 4HMS in a pseudo-cage structure that may explain the great in vitro and in vivo stability of [89Zr]Zr-4HMS. Finally, the very mild 89Zr complexation conditions are the another key factor in favor of the bifunctionalization of the 4HMS to explore its full potential for immunoPET applications.
Materials and Methods
General
The 89Y target (0.25 mm thick, 99.9%) was purchased from Alfa Aesar (Ward Hill, MA, USA/VWR International, CA). Hydrochloric acid (99.999%), oxalic acid (99.999%), Na2CO3 (99.999%) trace metal basis, sodium hydroxide pellets (≥98.0%), Apo-transferrin (≥98.0%), DTPA (≥99%), and DFO mesylate salt (≥92.5%) were obtained from Sigma-Aldrich (Saint-Louis, MO, USA). Acetonitrile (HPLC grade, 99.9%) and High-purity water (Optima LC/MS, ultra-high-performance liquid chromatography ultraviolet grade, 0.03 mm filtered) were purchased from Fisher Scientific (Ottawa, ON, CA). 1H and 13C NMR spectra were recorded in deuterated solvents on a Brucker Ascend 400 NMR instrument. The residual solvent peaks have been used as internal references. The peak multiplicities are described as follows: s (singlet), d (doublet), t (triplet), q (quartet), quin (quintet), m (multiplet), and br (broad). High-resolution mass spectrum (HRMS) were recorded on a Triple TOF 5600, ABSciex mass spectrometer. Analytical HPLC was performed on an Agilent 1200 system (Agilent Technologies, Mississauga, Ontario, Canada) equipped with a Zorbax Eclipse XDB C18 reversed-phase column (4.6 × 250 mm, 5 μ) and Agilent 1200 series diode array UV–vis (Agilent Technologies) using a linear gradient of 0–76% acetonitrile (TFA: 0.025%) in H2O (TFA: 0.05%) over 23 min, 76–100% acetonitrile in water over 1 min, and 100–0% acetonitrile in water over 6 min with a flow rate of 1 mL·min. Alburex-25 (Human plasma, USP) was supplied by Grifols Canada Ltd. (formerly Talecris), CSL Behring. Instant thin-layer chromatography paper (ITLC-SA) was acquired from Agilent Technology (Santa Clara, CA). All glassware were cleaned with chromic sulfuric acid (Fisher Scientific). The labeling efficiency of [89Zr]Zr-4HMS and [89Zr]Zr-DFO was assessed using ITLC-SG with 100 mM DTPA (pH = 7) solution as eluent. The radio-TLC plates were scanned using an Instant Imager scanner (Bioscan, DC, U.S.A.). Radioactivity measurements were performed in an ionization chamber (CRC-25PET; Capintec) on the 89Zr setting (calibration factor: 465) to control process efficiency.
N-Methyl-N(benzyloxy)succinamide (1)
N-Methyl O-benzylhydroxylamine (3.0 g, 22 mmol), dimethylaminopyridine (DMAP; cat) and succinic anhydride (2.2 g, 22 mmol) were dissolved in dry THF (50 mL) and refluxed overnight. The solvent was removed under vacuum, and the resulting slurry was dissolved in ethyl acetate and precipitated with ether to provide 86% of the desired compound 1 as a white solid. Product purity was confirmed to be 96% by analytical reversed phase HPLC: retention time of 14.4 min. MS (LC-ESI-MS) m/z: calcd, (237.1); found, 239.0 (M + 2), 498.0 (2M + 1 + Na). HRMS (ESI-Q-Tof, m/z): calcd for [C12H15NO4], 260.0893 [M + Na]+; found, 260.0901 [M + Na]+ (−3.1 ppm). 1H NMR (400 MHz, CDCl3): δ 2.56 (m, 4H), 3.18 (s, 3H), 4.82 (s, 2H), 7.38 (s, 5H), 10.78 (br, 1H, COOH). 13C NMR (CDCl3): 26.1, 27.4, 27.7, 75.3, 127.8, 128.1, 128.3, 133.3, 169.7, 176.8.
Succinimido Ester of N-Methyl-N(benzyloxy)succinamic Acid (2)
N-Methyl-N(benzyloxy)succinamide 1 (4.0 g, 17 mmol) was dissolved in dry DMF (5 mL) to which N-hydroxy-succinimide (2,0 g, 18.4 mmol) and EDC-HCl (3.5 g, 18.4 mmol) were added at 0 °C. The mixture was stirred at 0 °C for 30 min and then at room temperature overnight. The reaction was diluted with ethyl acetate, washed with 10% of citric acid, saturated NaHCO3 and water. The organic layer was dried over sodium sulfate and evaporated under vacuum. The crude was dissolved in ethyl acetate and precipitated with ether to give 88% of compound 2 as a white solid. MS (LC-ESI-MS) m/z: calcd, (335.1); found, 220.8 (M – ONHS), 336.0 (M + 1), 670.1 (2M + 1). 1H NMR (400 MHz, CDCl3): δ 2.92–2.64 (m, 8H), 3.18 (s, 3H), 4.80 (s, 2H), 7.38 (s, 5H). 13C NMR (CDCl3): 25.6, 25.9, 27.1, 33.7, 76.3, 128.8, 129.2, 129.4, 134.3, 168.4, 169.0, 172.2.
Preparation of Benzyl-Protected Tetrahydroxamate of 4HMS
A solution of succinimido ester of N-Methyl-N(benzyloxy)succinamic acid 2 (1.6 g, 5 mmol) in dry methylene chloride (5 mL) was added dropwise to a stirred solution of spermine (0.2 g, 1 mmol), triethylamine (1.4 mL, 10 mmol), and a catalytic amount of DMAP (5% w/w) in 15 mL of dry methylene chloride at 0 °C. The reaction mixture was warmed to room temperature and stirred for 24 h. The crude was washed with 10% NaHCO3 solution followed by water. The organic phase was dried over sodium sulfate, and the solvent was removed under vacuum. The crude product was purified by flash chromatography to yield to 78% of protected 4HMS as a fluffy beige solid. Product purity was confirmed to be 100% by analytical reversed phase HPLC: retention time of 22.17 min. MS (LC-ESI-MS) m/z: calcd, (1079.28); found, 1080.7 (M + 1), 541.2 (M/2 + 1). HRMS (ESI-Q-Tof, m/z): calcd for [C58H78N8O12], 1101.5631 [M + Na]+; found, 1101.5641 [M + Na]+ (−0.9 ppm). 1H NMR (400 MHz, CDCl3): δ 1.52–1.85 (m, 8H), 2.40–2.80 (m, 16H), 3.10–3.4 (m, 24H), 4.82–5.10 (m, 8H), 6.90 (m, 1H, NH), 7.15 (m, 1H, NH), 7.3–7.50 (m, 20H). 13C NMR (CDCl3): (mixture of rotamers), 25.0, 26.0, 26.2, 27.1, 27.2, 27.3, 27.4, 27.5, 27.6, 27.7, 27.7, 27.7, 27.8, 28.0, 30.5, 30.5, 30.6, 33.6, 33.6, 33.7, 33.7, 33.8, 36.2, 36.2, 36.8, 36.9, 42.6, 42.8, 44.7, 44.9, 47.3, 76.3, 128.7, 128.9, 128.9, 129.0, 129.2, 129.3, 134.4, 134.6, 171.7, 172.2, 172.2, 172.5, 174.2, 174.2, 174.2, 174.2.
Preparation of 4HMS (3)
A solution of protected 4HMS and 10% Pd/C (20% w/w) was suspended in methanol. The reaction mixture was purged with hydrogen gas at room temperature overnight. The crude was filtered and washed with methanol. The solvent was evaporated to give quantitatively 4HMS 3 as a beige fluffy solid. The compound is very hygroscopic and must be kept under nitrogen at −20 °C. The HPLC chromatogram revealed a mixture of three conformational isomers or conformers in variable compositions. MS (LC-ESI-MS) m/z: calcd, (718.38); found, 720.3 (M + 2), 761.2 (M/2 + 1). HRMS (ESI-Q-Tof, m/z): calcd for [C30H54N8O12], 741.3753 [M + Na]+; found, 741.3761 [M + Na]+ (−1.1 ppm). 1H NMR (400 MHz, DMSO): δ 1.22–1.55 (m, 8H), 2.30–2.80 (m, 16H), 2.90–3.44 (m, 23H), 7.75 (m, 1H, NH), 7.80 (m, 1H), 9.75 (m, 4H). 13C NMR (DMSO): (mixture of rotamers), 25.1, 25.1, 25.2, 26.2, 26.3, 27.2, 27.2, 27.2, 27.2, 27.3, 27.7, 27.7, 27.8, 28.1, 29.0, 30.3, 30.3, 30.3, 36.2, 36.2, 36.6, 36.8, 43.5, 43.8, 44.8, 45.1,45.1, 45.1, 45.2, 45.2, 45.2, 47.3, 47.3, 47.4, 49.1, 55.4, 171.3, 172.5, 172.7, 172.0, 172.7, 172.8, 172.8, 47.38, 49.06, 55.37, 171.32, 172.50, 172.74, 172.00, 172.67, 172.84, 172.85.
Synthesis of natZr-4HMS
To a solution of 3 (2.0 mg, 2.8 μmol) was added Zr(acac)4 (1.6 mg, 3.4 μmol) in 1 mL of MeOH. The resulting solution was stirred for 5 min at room temperature. Then, the mixture was evaporated to half solution and 1 mL of cold ether was added to give a white solid. The complex has a very low solubility; however, enough compound remained in solution to allow some analysis. MS (LC-ESI-MS) m/z: calcd, (805.2); found, 806.6 (M + 1), 404.4 (M/2 + 1). 1H NMR in DMSO remained difficult because of the solubility as well as the overlapping signals of 4HMS ligand itself (Figure S17) but we can see the disappearance of the proton in N–OH bond.
[89Zr]Zr-Oxalate Production and Purification
Irradiation was performed on a TR19 and TR24 cyclotron (H–: 16.1 MeV) (ACSI, Richmond, BC, CA) equipped with a straight 90° target holder (ACSI). 89Zr was produced via 89Y(p,n)89Zr transmutation reaction using a solid 89Y-foil target mounted on custom-made aluminum coin target holders described previously.41
Chelation Chemistry
[89Zr]Zr-oxalate solution was neutralized with sodium carbonate 1 M to pH = 7–7.5. 4HMS and DFO were prepared, respectively, in ethanol and water (1.1 and 1 mg/mL). An aliquot of 4HMS or DFO (5 μL, 7.65 nmol) was radiolabeled with neutralized [89Zr]Zr-oxalate (30 MBq, 200 μL) in either water or 0.9% NaCl at room temperature for 5–10 min with a total volume 205 μL. The radiolabeling was monitored by radio-TLC using Varian ITLC-SG strips and 100 mM DTPA (pH 7) as the mobile phase. In this system, free 89Zr forms a complex with DTPA and eluted with the solvent front, while the 89Zr-ligand complex remained at the origin (Figure S20). The identity of the [89Zr]Zr-4HMS radioactive complex was further confirmed by comparing its radio-HPLC elution profile to the ultraviolet-HPLC spectrum of natZr-4HMS (Figure S21).
Determination of AMA
AMA of 89Zr was determined experimentally via titration of the purified [89Zr]Zr-oxalate with DFO and 4HMS. Briefly, stock solution of DFO and 4HMS (1.5 mM) were prepared in water and ethanol, respectively. Nine reactions were prepared in 1.5 mL microcentrifuge tube via 1:10 and 1:2 serial dilution to give final DFO and 4HMS amounts in the range of (0.0001–0.08 nmol). [89Zr]Zr-oxalate in 1.0 M oxalic acid was neutralized with an appropriate volume of 1.0 M Na2CO3 and diluted with water. Aliquots of the [89Zr]Zr-oxalate (50 μL, 1–1.2 MBq) were added to each solution, and the reactions were vortexed for 15 s and incubated at room temperature for 1 h. The solutions were then quenched with 30 μL of DTPA (50 mM) pH = 5–6 and incubated for another 15 min. Aliquots were withdrawn and analyzed by ITLC using DTPA (100 mM, pH 7) as a mobile phase solvent. Free 89Zr forms a complex with DTPA and eluted with the solvent front, while [89Zr]Zr-DFO and [89Zr]Zr-4HMS remained at the origin. The binding percentages were plotted on a curve (Figure S22). The minimum ligand concentration of DFO and 4HMS for which 100% labeling occurred was assumed to be equal to the concentration of 89Zr present, and the AMA of 89Zr in both ligands in GBq/μmol of zirconium was calculated by correcting for the total activity.
Transchelation Studies Using DTPA
Both 4HMS and DFO (100 μL, 100 μM) were labeled with neutralized [89Zr]Zr-oxalate (15 MBq) at room temperature for 10 min and then incubated with 100- and 1000-fold excess of DTPA (200 μL of 5 and 50 mM), respectively, at a range of pH values (5, 7, and 8.5) at 37 °C for a period of 7 days. To maintain the pH, 100 μL of sodium acetate buffer (0.5 M, pH ≈ 5.5) was added to the solutions. Each solution was prepared in triplicate, and the quality control was performed, as described above for different time points (Table S1).
Transmetalation Studies
Both 4HMS and DFO (1 mL, 200 μM) were labeled with 100 MBq of neutralized [89Zr]Zr-oxalate diluted in 1 mL of water at room temperature for 10 min for a total volume of 2 mL. Each ligand was then incubated with 10-fold excess of various metal salts at pH = 7–7.2. In the case of FeCl3, 100 equiv were also tested. Each metal solution (200 μL) (1 mM, 10 mM for 100 equiv FeCl3) was added to 200 μL of each 89Zr-ligand, and the solutions were incubated for 7 days at 37 °C. To maintain the pH, sodium acetate buffer (0.1 M, pH ≈ 5.5) was added to the solutions. The samples were monitored for 7 days by radio-TLC to determine the percentage of the intact 89Zr-ligand complex. All the studies were done in triplicate (Table S2).
Human Apo-Transferrin Challenging Study
Apo-transferrin (77 kDa) was diluted with 10 mM of sodium carbonate (pH 6.5–7) to reach a final concentration of 10 mg/mL. Apo-transferrin (10 equiv) (820 μL, ∼1.3 × 105 nM) was added to diluted [89Zr]Zr-4HMS (820 μL, ∼40 MBq). The solution was divided into four aliquots that were incubated at 37 °C for 1, 3, 5, and 7 days. An aliquot was transferred to Amicon Ultra 0.5 mL 50 kDa filter (Merck KGaA, Darmstadt, Germany) and centrifuged at 7400G for 12 min. In this system, [89Zr]Zr-4HMS will pass through the filter, while Apo-transferrin and [89Zr]Zr-apo-transferrin will remain on the filter. The radioactivity in each fraction was measured using a dose calibrator. The percentage of transchelation was then calculated. The study was performed in triplicate (Table S3A).
Human Plasma Binding Assay
To measure the in vitro binding of [89Zr]Zr-4HMS to plasma, 205 μL of radiolabeled [89Zr]Zr-4HMS (30 MBq) was added to 1 mL of human plasma in 200 μL of phosphate buffer (pH 7). After gentle mixing, the solution was divided into 4 microcentrifuge tubes (1.5 mL) that were incubated at 37 °C. Protein binding was measured at day 1, 3, 5, and 7 days. Acetonitrile was added into the microcentrifuge tube to precipitate the plasma protein (1/1, acetonitrile/plasma). The sample was then centrifuged for 6 min at 9000 rpm, and the supernatant was recovered. This process was repeated three times with the same volume of acetonitrile, and the supernatants were pooled. The radioactivity content of the supernatant and the precipitated protein were measured in a dose calibrator to obtain the protein binding percentage. The study was performed in triplicate (Table S3–B).
Stability Assay
The stability assay of both [89Zr]Zr-DFO and [89Zr]Zr-4HMS was evaluated in fresh mouse and human plasma. A volume of 30 μL (4 MBq) of the 89Zr-ligands was incubated over 7 days at 37 °C in human plasma (0.5 mL) and mouse plasma (0.2 mL) in either 0.5 mL of phosphate buffer (pH 7) or 0.5 mL of 0.9% NaCl. The samples were analyzed by ITLC-SG, intact 89Zr-ligand remained at the origin of the ITLC strip, while free 89Zr was either bound by serum protein or complexed to DTPA in the mobile phase and migrated along the ITLC strip. Free 89Zr incubated in serum appeared to be as large broad peaks on radio-TLC, and this is probably due to different components in plasma. In order to confirm the stability results, Amicon Ultra 0.5 mL 50 kDa filter (Merck KGaA, Darmstadt, Germany) was used and the [89Zr]Zr-DFO and [89Zr]Zr-4HMS ligands were centrifuged at 9000 rpm for 15 min. The liquid collected at different time points (1, 3, 5, and 7 days) post incubation were spotted directly on ITLC-SG plates using DTPA (100 mM, pH 7) as a developing solvent.
Animal Studies
Animal experiments were performed in adult female BALB/c mice, in accordance with the guidelines of the Canadian Council on animal Care and of the in-house Animal Experiment Ethic Committee.
Biodistribution Studies
Under isoflurane anaesthesia (Induction 2–2.5%, maintained 1–1.5% oxygen flow 1–1.5 L/min) the mice (n = 3/group) were injected via the caudal vein with either [89Zr]Zr-DFO or [89Zr]Zr-4HMS (∼15 MBq, 0.2 mL, ∼3.8 nmol of ligand). At 1, 4 and 24 h post injection, the mice were euthanized by CO2 inhalation under isoflurane anaesthesia and the organs of interest were collected, washed, blotted dry, weighted, and counted in a γ-counter (Cobra II auto-gamma counter, Packard). The results were expressed in terms of percentage of injected dose per gram (% ID/g).
PET Imaging Studies
PET imaging was done under isoflurane anaesthesia (induction 2–2.5%, maintained 1–1.5% oxygen flow 1–1.5 L/min); a catheter was installed in the caudal vein for the administration of the radiotracer. The mouse was positioned in the field of view of the PET scanner (LabPET-8, Gamma Medica-IDEAS Inc., Sherbrooke, Quebec, Canada), and a 60 min dynamic acquisition was acquired in the list mode followed immediately by the administration of either [89Zr]Zr-DFO or [89Zr]Zr-4HMS (∼15 MBq, 0.2 mL, ∼3.8 nmol of ligand). The dynamic acquisition was followed by 30 min static images at 4 and 24 h. The images were reconstructed using the three-dimensional maximum likelihood estimation method algorithm, and analysis was performed using AMIDE software. To quantify the radiotracer uptake, regions of interest (ROIs) were drawn around organs. These ROIs were then applied to all frames to obtain TACs for each organ. The ROI activity was expressed as percent injected dose per gram of tissue (% ID/g) with the whole body radioactivity measured by PET.
Statistics
Triplicate data were performed and collected for all experiments. Accordingly, mean and % of standard deviation were calculated. In addition, p-value was calculated using t-test formula with two tails method for biodistribution study data.
Acknowledgments
BG is a member of the CRCHUS funded by the Fonds de recherche du Québec - Santé (FRQS) and holder of the Jeanne and J.-Louis Lévesque Chair in Radiobiology at Université de Sherbrooke. The work was financially supported by the Natural Sciences and Engineering Research Council of Canada (NSERC, grant no. RGPIN-2014-04354). A.H.A. received a scholarship from the King Fahad Specialist Hospital-Dammam, Kingdom of Saudi Arabia. We thank cyclotron operators for their help with the production of 89Zr.
Glossary
Abbreviations
- CP256
tris(hydroxypyridinone) ligand
- CPM
counts per minute
- DFC
desferrichrome
- DFO
desferrioxamine
- DFO*
DFO-star
- DOTA
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
- DTPA
diethylenetriaminepentaacetic acid
- EDC-HCl
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide-HCl
- FOXA
desferrioxamineA
- FOXB
desferrioxamine B
- FOXE
desferrioxamine E
- FSC
fusarinine C
- h
hour
- HEPES
4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid
- HOPO
four 1-hydroxypyridin-2-one groups appended to a linear tetraamine
- HPLC
high performance liquid chromatography
- HRMS
high resolution mass spectrum
- 4HMS
N-hydroxy-N-methyl succinamide-based chelator
- L1-4
four azamacrocycle-based hydroxamate chelators
- mAb
monoclonal antibody
- MeOH
methanol
- NMR
nuclear magnetic resonance
- RT
retention time
- Rf
retention factor
- ITLC
instant thin layer chromatography
- ITLC-SG
ITLC-silica gel
- kBq
kilobecquerel
- LC/MS
liquid chromatography/mass spectroscopy
- MBq
megabecquerel
- Na2CO3
sodium carbonate
- PET
positron emission tomography
- % ID/g
percent injected dose per gram of tissue
- ROI
region of interest
- TAC
time activity curve
- TAFC
desferritriacetylfusarinine C
- TFA
trifluoroacetic acid
- TLC
thin layer chromatography
- tz
trastuzumab
- UPLC
ultra-performance liquid chromatography
- UV-HPLC
ultraviolet-HPLC
- 89Y
yttrium-89
- YM103
tris(hydroxypyridinone) ligand with a maleimide group
- 89Zr
zirconium-89
- Zr(acac)4
zirconium(IV) acetylacetonate
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00207.
HPLC, spectroscopic data, and NMR spectra of new compounds (PDF)
Author Contributions
∥ A.H.A. and S.A.-M. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Zeglis B. M.; Houghton J. L.; Evans M. J.; Viola-villegas N.; Lewis J. S. Underscoring the influence of inorganic chemistry on nuclear imaging with radiometals. Inorg. Chem. 2014, 53, 1880. 10.1021/ic401607z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perk L. R.; Vosjan M. J. W. D.; Visser G. W. M.; Budde M.; Jurek P.; Kiefer G. E.; van Dongen G. A. M. S. p-Isothiocyanatobenzyl-desferrioxamine: a new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 250. 10.1007/s00259-009-1263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura G.; Kühn H.; Sauter M.; Haberkorn U.; Mier W. Radiolabeling Strategies for Tumor-Targeting Proteinaceous Drugs. Molecules 2014, 19, 2135. 10.3390/molecules19022135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijs W. E.; Herscheid J. D. M.; Haisma H. J.; Pinedo H. M. Evaluation of desferal as a bifunctional chelating agent for labeling antibodies with Zr-89. Int. J. Rad. Appl. Instrum. A 1992, 43, 1443. 10.1016/0883-2889(92)90170-j. [DOI] [PubMed] [Google Scholar]
- Verel I.; Visser G. W. M.; Boellaard R.; Stigter-van Walsum M.; Snow G. B.; van Dongen G. A. 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med. 2003, 44, 1271. [PubMed] [Google Scholar]
- Zeglis B. M.; Mohindra P.; Weissmann G. I.; Divilov V.; Hilderbrand S. A.; Weissleder R.; Lewis J. S. Modular strategy for the construction of radiometalated antibodies for positron emission tomography based on inverse electron demand Diels-Alder click chemistry. Bioconjugate Chem. 2011, 22, 2048. 10.1021/bc200288d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijs W. E.; Haisma H. J.; Klok R. P.; van Gog F. B.; Kievit E.; Pinedo H. M.; Herscheid J. D. Zirconium-labeled monoclonal antibodies and their distribution in tumor-bearing nude mice. J. Nucl. Med. 1997, 38, 112. [PubMed] [Google Scholar]
- Tinianow J. N.; Gill H. S.; Ogasawara A.; Flores J. E.; Vanderbilt A. N.; Luis E.; Vandlen R.; Darwish M.; Junutula J. R.; Williams S.-P.; Marik J. Site-specifically 89Zr-labeled monoclonal antibodies for ImmunoPET. Nucl. Med. Biol. 2010, 37, 289. 10.1016/j.nucmedbio.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Verel I.; Visser G. W.; Boellaard R.; Boerman O. C.; van Eerd J.; Snow G. B.; Lammertsma A. A.; van Dongen G. A. Quantitative 89Zr immuno-PET for in vivo scouting of 90Y-labeled monoclonal antibodies in xenograft-bearing nude mice. J. Nucl. Med. 2003, 44, 1663. [PubMed] [Google Scholar]
- Nagengast W. B.; de Vries E. G.; Hospers G. A.; Mulder N. H.; de Jong J. R.; Hollema H.; Brouwers A. H.; van Dongen G. A.; Perk L. R.; Lub-de Hooge M. N. In Vivo VEGF Imaging with Radiolabeled Bevacizumab in a Human Ovarian Tumor Xenograft. J. Nucl. Med. 2007, 48, 1313. 10.2967/jnumed.107.041301. [DOI] [PubMed] [Google Scholar]
- Holland J. P.; Normand G.; Ruggiero A.; Lewis J. S.; Grimm J. Intraoperative imaging of positron emission tomographic radiotracers using Cerenkov luminescence emission. Mol. Imaging 2011, 10, 177. 10.2310/7290.2010.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjesson P. K. E.; Jauw Y. W. S.; de Bree R.; Roos J. C.; Castelijns J. A.; Leemans C. R.; van Dongen G. A. M. S.; Boellaard R. Radiation Dosimetry of 89Zr-Labeled Chimeric Monoclonal Antibody U36 as Used for Immuno-PET in Head and Neck Cancer Patients. J. Nucl. Med. 2009, 50, 1828. 10.2967/jnumed.109.065862. [DOI] [PubMed] [Google Scholar]
- Perk L. R.; Visser O. J.; Stigter-Van Walsum M.; Vosjan M. J. W. D.; Visser G. W. M.; Zijlstra J. M.; Huijgens P. C.; van Dongen G. A. M. S. Preparation and evaluation of 89Zr-Zevalin for monitoring of 90Y-Zevalin biodistribution with positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1337. 10.1007/s00259-006-0160-0. [DOI] [PubMed] [Google Scholar]
- Hong H.; Zhang Y.; Severin G. W.; Yang Y.; Engle J. W.; Niu G.; Nickles R. J.; Chen X.; Leigh B. R.; Barnhart T. E.; Cai W. Multimodality Imaging of Breast Cancer Experimental Lung Metastasis with Bioluminescence and a Monoclonal Antibody Dual-Labeled with 89Zr and IRDye 800CW. Mol. Pharm. 2012, 9, 2339. 10.1021/mp300277f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink T. H.; de Vries E. G. E.; Vedelaar S. R.; Timmer-Bosscha H.; Schröder C. P.; Brouwers A. H.; Lub-de Hooge M. N. Lapatinib and 17AAG reduce 89Zr-trastuzumab-F(ab’)2 uptake in SKBR3 tumor xenografts. Mol. Pharm. 2012, 9, 2995. 10.1021/mp3002182. [DOI] [PubMed] [Google Scholar]
- Fischer G.; Seibold U.; Schirrmacher R.; Wängler B.; Wängler C. 89Zr, a Radiometal Nuclide with High Potential for Molecular Imaging with PET: Chemistry, Applications and Remaining Challenges. Molecules 2013, 18, 6469. 10.3390/molecules18066469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérard F.; Lee Y.-S.; Tripier R.; Szajek L. P.; Deschamps J. R.; Brechbiel M. W. Investigation of Zr(IV) and 89Zr(IV) complexation with hydroxamates: Progress towards designing a better chelator than desferrioxamine B for immuno-PET imaging. Chem. Commun. 2013, 49, 1002. 10.1039/c2cc37549d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. P.; Divilov V.; Bander N. H.; Smith-Jones P. M.; Larson S. M.; Lewis J. S. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med. 2010, 51, 1293. 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heskamp S.; Raavé R.; Boerman O.; Rijpkema M.; Goncalves V.; Denat F. 89Zr-Immuno-Positron Emission Tomography in Oncology: State-of-the-Art 89Zr Radiochemistry. Bioconjugate Chem. 2017, 28, 2211. 10.1021/acs.bioconjchem.7b00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt N.; Pandya D.; Wadas T. Recent Advances in Zirconium-89 Chelator Development. Molecules 2018, 23, 638. 10.3390/molecules23030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summer D.; Garousi J.; Oroujeni M.; Mitran B.; Andersson K. G.; Vorobyeva A.; Löfblom J.; Orlova A.; Tolmachev V.; Decristoforo C. Cyclic versus Noncyclic Chelating Scaffold for 89Zr-Labeled ZEGFR:2377 Affibody Bioconjugates Targeting Epidermal Growth Factor Receptor Overexpression. Mol. Pharm. 2018, 15, 175. 10.1021/acs.molpharmaceut.7b00787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai C.; Summer D.; Rangger C.; Franssen G. M.; Laverman P.; Haas H.; Petrik M.; Haubner R.; Decristoforo C. Novel Bifunctional Cyclic Chelator for 89Zr Labeling-Radiolabeling and Targeting Properties of RGD Conjugates. Mol. Pharm. 2015, 12, 2142. 10.1021/acs.molpharmaceut.5b00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deri M. A.; Ponnala S.; Zeglis B. M.; Pohl G.; Dannenberg J. J.; Lewis J. S.; Francesconi L. C. Alternative chelator for 89Zr radiopharmaceuticals: radiolabeling and evaluation of 3,4,3-(LI-1,2-HOPO). J. Med. Chem. 2014, 57, 4849. 10.1021/jm500389b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deri M. A.; Ponnala S.; Kozlowski P.; Burton-Pye B. P.; Cicek H. T.; Hu C.; Lewis J. S.; Francesconi L. C. A Superior Bifunctional Chelator for 89Zr ImmunoPET. Bioconjugate Chem. 2015, 26, 2579. 10.1021/acs.bioconjchem.5b00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik M.; Zhai C.; Novy Z.; Urbanek L.; Haas H.; Decristoforo C. In Vitro and In Vivo Comparison of Selected Ga-68 and Zr-89 Labelled Siderophores. Mol. Imaging Biol. 2016, 18, 344. 10.1007/s11307-015-0897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams C. J.; Wilson J. J.; Boros E. Multifunctional Desferrichrome Analogues as Versatile89Zr(IV) Chelators for ImmunoPET Probe Development. Mol. Pharm. 2017, 14, 2831. 10.1021/acs.molpharmaceut.7b00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand M.; Aulsebrook M. L.; Mindt T. L.; Gasser G. A Solid Phase-Assisted Approach for the Facile Synthesis of a Highly Water-Soluble Zirconium-89 Chelator for Radiopharmaceutical Development. Dalton Trans. 2017, 46, 16387. 10.1039/c7dt03639f. [DOI] [PubMed] [Google Scholar]
- Patra M.; Bauman A.; Mari C.; Fischer C. A.; Blacque O.; Häussinger D.; Gasser G.; Mindt T. L. An Octadentate Bifunctional Chelating Agent for the Development of Stable Zirconium-89 Based Molecular Imaging Probes. Chem. Commun. 2014, 50, 11523. 10.1039/c4cc05558f. [DOI] [PubMed] [Google Scholar]
- Boros E.; Holland J. P.; Kenton N.; Rotile N.; Caravan P. Macrocycle-Based Hydroxamate Ligands for Complexation and Immunoconjugation of 89Zirconium for Positron Emission Tomography (PET) Imaging. ChemPlusChem 2016, 81, 274. 10.1002/cplu.201600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M. T.; Meszaros L. K.; Paterson B. M.; Berry D. J.; Cooper M. S.; Ma Y.; Hider R. C.; Blower P. J. Tripodal Tris(Hydroxypyridinone) Ligands for Immunoconjugate PET Imaging with 89Zr4+: Comparison with Desferrioxamine-B. Dalton Trans. 2015, 44, 4884. 10.1039/c4dt02978j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai C.; He S.; Ye Y.; Rangger C.; Kaeopookum P.; Summer D.; Haas H.; Kremser L.; Lindner H.; Foster J.; Sosabowski J.; Decristoforo C. Rational Design, Synthesis and Preliminary Evaluation of Novel Fusarinine C-Based Chelators for Radiolabeling with Zirconium-89. Biomolecules 2019, 9, 91. 10.3390/biom9030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verel I.; Visser G. W. M.; Boerman O. C.; van Eerd J. E. M.; Finn R.; Boellaard R.; Vosjan M. J. W. D.; Stigter-van Walsum M.; Snow G. B.; van Dongen G. E. M. Long-lived positron emitters zirconium-89 and iodine-124 for scouting of therapeutic radioimmunoconjugates with PET. Cancer Biother. Radiopharm. 2003, 18, 655. 10.1089/108497803322287745. [DOI] [PubMed] [Google Scholar]
- Ait-Mohand S.; Denis C.; Tremblay G.; Paquette M.; Guérin B. Development of Bifunctional Chelates Bearing Hydroxamate Arms for Highly Efficient 64Cu Radiolabeling. Org. Lett. 2014, 16, 4512. 10.1021/ol5020575. [DOI] [PubMed] [Google Scholar]
- Cundari T. R.; Stevens W. J. Effective core potential methods for the lanthanides. J. Chem. Phys. 1993, 98, 5555. 10.1063/1.464902. [DOI] [Google Scholar]
- Rossi M. L.; Taylor C. D. Atomistic Simulations of Formation of Elementary Zr-I Systems. O. J. Phys. Chem. 2011, 01, 104. 10.4236/ojpc.2011.13014. [DOI] [Google Scholar]
- Messner C. B.; Hofer T. S.; Randolf B. R.; Rode B. M. Structure and dynamics of the Zr4+ ion in water. Phys. Chem. Chem. Phys. 2011, 13, 224. 10.1039/c0cp01330g. [DOI] [PubMed] [Google Scholar]
- Schmidt M. W.; Baldridge K. K.; Boatz J. A.; Elbert S. T.; Gordon M. S.; Jensen J. H.; Koseki S.; Matsunaga N.; Nguyen K. A.; Su S.; Windus T. L.; Dupuis M.; Montgomery J. A. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347. 10.1002/jcc.540141112. [DOI] [Google Scholar]
- Baillargeon P.; Fortin D.; Dory Y. L. Hierarchical Self-Assembly of Lactams into Supramolecular CO-Spiked ″Sea Urchins″ and Then into a Channeled Crystal. Cryst. Growth Des. 2010, 10, 4357. 10.1021/cg1003935. [DOI] [Google Scholar]
- Hagfeldt C.; Kessler V.; Persson I. Structure of the hydrated, hydrolysed and solvated zirconium(iv) and hafnium(iv) ions in water and aprotic oxygen donor solvents. A crystallographic, EXAFS spectroscopic and large angle X-ray scattering study. Dalton Trans. 2004, 2142. 10.1039/b402804j. [DOI] [PubMed] [Google Scholar]
- Persson I. Hydrated metal ions in aqueous solution: How regular are their structures?. Pure Appl. Chem. 2010, 82, 1901. 10.1351/pac-con-09-10-22. [DOI] [Google Scholar]
- Alnahwi A.; Tremblay S.; Guérin B. Comparative Study with 89Y-foil and 89Y-pressed Targets for the Production of 89Zr. Appl. Sci. 2018, 8, 1579. 10.3390/app8091579. [DOI] [Google Scholar]
- Tainer J. A.; Roberts V. A.; Getzoff E. D. Metal-binding sites in proteins. Curr. Opin. Biotechnol. 1991, 2, 582. 10.1016/0958-1669(91)90084-i. [DOI] [PubMed] [Google Scholar]
- Pandya D. N.; Bhatt N.; Yuan H.; Day C. S.; Ehrmann B. M.; Wright M.; Bierbach U.; Wadas T. J.; Wadas T. J. Zirconium tetraazamacrocycle complexes display extraordinary stability and provide a new strategy for zirconium-89-based radiopharmaceutical development. Chem. Sci. 2017, 8, 2309. 10.1039/c6sc04128k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vugts D. J.; Klaver C.; Sewing C.; Poot A. J.; Adamzek K.; Huegli S.; Mari C.; Visser G. W. M.; Valverde I. E.; Gasser G.; Mindt T. L.; van Dongen G. A. M. S. Comparison of the octadentate bifunctional chelator DFO*-pPhe-NCS and the clinically used hexadentate bifunctional chelator DFO-pPhe-NCS for 89Zr-immuno-PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 286. 10.1007/s00259-016-3499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raavé R.; Sandker G.; Adumeau P.; Jacobsen C. B.; Mangin F.; Meyer M.; Moreau M.; Bernhard C.; Da Costa L.; Dubois A.; Goncalves V.; Gustafsson M.; Rijpkema M.; Boerman O.; Chambron J.-C.; Heskamp S.; Denat F. Direct comparison of the in vitro and in vivo stability of DFO, DFO* and DFOcyclo* for 89Zr-immunoPET. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1966. 10.1007/s00259-019-04343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.