Abstract
Decapping scavenger enzymes (DcpSs) are important players in mRNA degradation machinery and conserved in eukaryotes. Importantly, human DcpS is the recognized target for spinal muscular atrophy (SMA) and acute myeloid leukemia (AML) therapy, and has recently been connected to development of intellectual disability. Most recombinant DcpSs used in biochemical and biophysical studies are prepared as tagged proteins, with polyhistidine (His-tag) at the N-terminus or C-terminus. Our work is the first report on the parallel characterization of three versions of DcpSs (native and N- or C-terminally tagged) of three species (humans, Caenorhabditis elegans, and Ascaris suum). The native forms of all three enzymes were prepared by N-(His)10 tag cleavage. Protein thermal stability, measured by differential scanning fluorimetry (DSF), was unaffected in the case of native and tagged versions of human and A. suum DcpS; however, the melting temperature (Tm) of C. elagans DcpS of was significantly influenced by the presence of the additional N- or C-tag. To investigate the impact of the tag positioning on the catalytic properties of DcpS, we tested the hydrolytic activity of native DcpS and their His-tagged counterparts toward cap dinucleotides (m7GpppG and m32,2,7GpppG) and m7GDP. The kinetic data indicate that dinucleotide substrates are hydrolyzed with comparable efficiency by native human and A. suum DcpS and their His-tagged forms. In contrast, both His-tagged C. elegans DcpSs exhibited higher activity toward m7GpppG than the native enzyme. m7GDP is resistant to enzymatic cleavage by all three forms of human and nematode DcpS.
Introduction
Hydrolysis of the cap structure catalyzed by decapping scavenger enzymes (DcpSs) is a critical step in eukaryotic mRNA turnover. There are two major pathways of mRNA degradation in eukaryotic cells initiated by poly (A) tail deadenylation: the 5′ to 3′ pathway, where the cap structure is removed in a Dcp2-dependent manner, and then, the decapping product is degraded by 5′ exoribonuclease activity, and the 3′ to 5′ decay pathway.1 DcpS catalyzes the hydrolysis of cap dinucleotides m7GpppN and short-capped oligonucleotides resulting from exosome-mediated 3′ to 5′ digestion of deadenylated transcripts.2,3 Decapping scavengers have been identified in eukaryotes of varying complexities, including yeast, nematodes, and humans.4−6 These enzymes utilize an evolutionary conserved histidine triad (HIT) motif to bind substrates and cleave the 5′,5′-triphosphate bridge within the cap, releasing m7GMP and ppN or diphosphate-terminated oligo-mRNA containing less than 10 nucleotides.7 Taking into account the estimated mRNA transcripts per cell (around 50,000 to 300,000),8 and their average half-lives (from around 3 min to over 90 min),9,10 hundreds of molecules of m7GpppN and m7GDP can be generated every minute in yeast and mammalian cells as a result of mRNA decay. m7GDP was reported to be either a substrate11,12 or a high-affinity inhibitor of DcpS.5,13 Further reports revealed that m7GDP is converted into m7GMP in a two-step process: via enzymatic transformation into m7GTP, which is then hydrolyzed to m7GMP by DcpS.14 This path appears to be conserved in yeast and human cells. Beyond the DcpS function in mRNA decay, it is involved in nuclear pre-mRNA splicing and miRNAs turnover.15,16 The importance of DcpS is further evidenced by the identification of humans with mutations that disturb DcpS enzymatic activity, which in turn is connected with intellectual disability.17,18 It has also been recognized as a potential target in spinal muscular atrophy (SMA)19 and very recently in acute myeloid leukemia (AML) treatment.20
Most recombinant DcpSs used in a variety of biochemical and biophysical studies were prepared as N-terminal or C-terminal His-tagged proteins.5−7,13,21−24 Native (untagged) decapping scavengers have been previously obtained for crystallographic analysis of catalytically inactive mutants of human DcpS (hDcpSH277N)25 and yeast Dcs1p (Dcs1pH268N).26 His-tag is widely used for the affinity purification of recombinant proteins.27,28 It is usually left attached to the proteins, with the assumption that its addition does not alter the protein structure and properties. However, there are an increasing number of reports showing that affinity tags may affect the structure or activity of some proteins.29−33 To investigate the impact of the tag positioning on the specificity and catalytic properties of decapping scavengers of human and two nematode species (Caenorhabditis elegans and Ascaris suum), their native and two His-tagged versions were purified, analyzed in terms of protein thermal stability, and used in comparable studies and kinetic characterization. So far, no direct comparison of tagged and native DcpS properties has been made. Our work is the first attempt to show some differences between the three forms of this enzyme.
Results and Discussion
Recombinant DcpSs (human hDcpS Q96C86, C. elegans CeDcpS G5EFS4, and A. suum AsDcpS D3K0N9) were obtained as His-tagged proteins, either with N-terminal-(His)10 or C-terminal-(His)6 extensions (Figure S1). Native forms of DcpS of all three species were prepared via site-specific proteolytic cleavage of the N-terminal tag with Factor Xa (Materials and Methods). The homogeneity of the purified proteins was analyzed by electrospray ionization mass spectrometry (ESI-MS), Figure 1, and their molecular weights (MW) were experimentally determined and compared to theoretical values (ProtParam tool, ExPasy Server). Single ESI-MS peaks were obtained for native and C-tagged versions of DcpS of all three species. However, the experimental MW values were consistent with the theoretical values only for native DcpS proteins and C-tagged CeDcpS (Table 1).
Figure 1.
ESI Mass Spectra of Native and His-tagged forms of (A) Human, (B) C. elegans, and (C) A. suum DcpSs. Two major peaks observed for the N-tagged enzymes (left panel) are due to the post-translational processing of the N-terminal methionine or glycine (see the main text). Other minor peaks seen here for N-tagged proteins presumably correspond to different modifications of the N-tag amino acid sequence (e.g., N-6-phosphoglyconylation)38 as they are missing after its enzymatic cleavage (central panel).
Table 1. Theoretical and Experimental MWs of Recombinant DcpS Proteins.
| theoretical MW (Da) | experimental MW (Da) | ||
|---|---|---|---|
| human DcpS | N-tagged | 41129.46 | 41164.50 |
| 40987.00 | |||
| (Δ 177.4) | |||
| nativea | 38745.94 | 38741.50 | |
| C-tagged | 39673.92 | 39532.00 | |
| (−141.9) | |||
| C. elegans DcpS | N-tagged | 38997.17 | 39042.50 |
| 38865.50 | |||
| (Δ 177.0) | |||
| nativea | 36613.65 | 36609.50 | |
| C-tagged | 38210.42 | 38204.00 | |
| A. suum DcpS | N-tagged | 37494.56 | 37363.00 |
| 37541.00 | |||
| (Δ 178.0) | |||
| nativea | 35111.04 | 35109.50 | |
| C-tagged | 36252.25 | 36113.50 | |
| (−138.75) |
MW of the native forms of DcpS containing one additional histidine as a result of His-tag cleavage at their N-terminus.
In brackets, the difference between two ESI-MS peaks for N-tagged proteins (Δ177 Da) or between theoretical and experimental MWs for C-tagged hDcpS and AsDcpS is indicated.
With respect to C-tagged hDcpS and AsDcpS, their experimental MWs differ from the theoretical value by about 140 Da, which suggests the lack of N-terminal methionine. Indeed, examination of the preferred substrate sequences for E. coli methionine aminopeptidase (MetAP1), which is involved in the removal of the initiator methionine,34 revealed such a sequence in the N-terminus of hDcpS (MetADAA). E. coliMetAP1 was shown to have a much higher specificity for the amino acid type at the first position adjacent to the removed methionine, with alanine as the most preferred. The AsDcpS N-terminal sequence (MetVDEV) is also a substrate for MetAP1; however, the presence of valine next to the removed methionine causes reduction in cleavage efficiency.35 In the case of CeDcpS, the presence of lysine after initiator methionine (MetKRIA) is connected to its resistance to methionine amiopeptidase cleveage.35 (Figure S1).
For N-tagged DcpSs of all three species, two major peaks were observed in the ESI-MS spectra, which differ in MW by 177 Da (Figure 1). The molecular weights of neither of these peaks correspond to the theoretical MWs of the N-tagged proteins (Table 1). In a report by Yan et al.,36 the pET16b vector was used to produce a His-tagged protein, and exactly the same MW differences were observed in the two detected peaks in the MS spectra (between the two peaks as well as between the predicted and theoretical protein MWs) as we found here for the N-tagged hDcpS and nematode DcpS. The occurrence of these two peaks is consistent with the post-translational processing of the N-terminal methionine of the used type of His-tag extension: the lower molecular weight peaks correspond to a protein that lacks the N-terminal methionine, and the larger MW peaks correspond to the protein with a gluconylated N-terminal glycine of the His-tag sequence, exposed after methionine deletion.36−38
Differential scanning fluorimetry (DSF)48 was used to analyze the potential influence of His-tag present on the thermal stability of all tested DcpS enzymes. As shown in Figure 2, all three forms of C. elegans DcpS (untagged and His-tagged) exhibit the lowest calculated melting temperature (Tm) values (between 37 and 47 °C) in comparison to the Tm values for AsDcpS and hDcpS forms (between 58 and 62 °C), Table 2. In addition, a clear difference in thermal stability between N-tagged, C-tagged, and untagged CeDcpS is seen (Table 2), where the Tm value for C-tagged CeDcpS is around 5 °C lower in comparison to the untagged version (Tm of untagged CeDcpS is equal to 47.9 °C), and N-tagged DcpS shows two distinct melting temperatures (36.9 and 51 °C). In the case of human hDcpS, there is no significant difference in the Tm values between His-tagged and untagged protein versions, and in the case of AsDcpS, the C-tagged form seems to be only slightly less stable than untagged AsDcpS (Table 2). Analysis on the thermal stability of the tested DcpS enzymes in the presence of m7GDP revealed that it stabilizes DcpS forms to the same Tm value (67 °C for hDcpS, 56 °C for CeDcpS and 75 °C for AsDcpS) regardless of the presence or absence of His-tag in the sequence (Table 2, Figure S3). The increased thermal stability of CeDcpS formed upon m7GDP addition to the same Tm value, despite differences in measured melting temperatures for His-tagged and untagged CeDcpS, indicates that ligand binding is unaffected by the presence of additional His-tag sequences.
Figure 2.
Analysis of Thermal Stability of Studied DcpS by Differential Scanning Fluorimetry (DSF). (A) Representative DSF melting curves of differentially tagged variants of hDcpS (solid lines), CeDcpS (dashed lines), and AsDcpS (dotted lines). RFU relative fluorescence units of SYPRO Orange. (B) Plotted curves of the first negative derivative of melting curves shown in (A). Tm values, summarized in Table 2, were calculated by determining the minima of the first negative derivative of melting curves.
Table 2. Melting Temperature Values (Tm) of Studied DcpS Proteins in the apo Form and in the Presence of their Ligand m7GDP Determined by DSFa.
| H. sapiens DcpS | C. elegans DcpS | A. suum DcpS | ||||
|---|---|---|---|---|---|---|
| Tm, °C apo | Tm, °C+m7GDP | Tm, °C apo | Tm, °C+m7GDP | Tm, °C apo | Tm, °C+m7GDP | |
| WT(untagged) | 58.2 ± 0.2 | 67.0 ± 0.1 | 47.9 ± 0.7 | 56.8 ± 0.3 | 61.8 ± 0.2 | 74.8 |
| N = 3 | N = 2 | N = 4 | N = 4 | N = 2 | N = 1 | |
| N-tagged | 59.5 ± 0.5 | 68.0 | 36.9 ± 0.1 and51.0 ± 0.2 | 55.7 ± 0.6 | 62.3 ± 0.4 | 75.8 ± 0.3 |
| N = 2 | N = 1 | N = 7 | N = 4 | N = 6 | N = 2 | |
| C-tagged | 59.5 ± 0.5 | 67.0 | 42.3 ± 0.3 | 56.6 ± 0.3 | 59.3 ± 0.2 | 75.0 |
| N = 4 | N = 1 | N = 4 | N = 3 | N = 4 | N = 1 | |
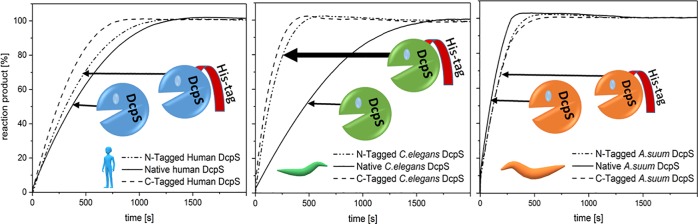
Next, we compared the enzymatic activity of native DcpS enzymes and their His-tagged counterparts toward natural cap dinucleotides (m7GpppG and m32,2,7GpppG, Figure S5). The enzymatic assays were conducted using a previously described fluorometric method.39 The obtained reaction progress curves are shown in Figure 3, and the calculated reaction rate constant40 values are provided in Table 3. As indicated, m7GpppG is hydrolyzed at a comparable rate by native and N-tagged hDcpS and at a slightly higher rate (1.5×) by its C-tagged form (Table 3). Comparable reaction rates were also obtained for native and His-tagged AsDcpS forms and both its substrates. However, His-tagged CeDcpS forms exhibit around 3 to 4 times higher activity toward m7GpppG than the native enzyme. Similar tendency was observed for the hydrolysis of the second CeDcpS substrate, m32,2,7GpppG. Interestingly, in contrast to AsDcpS, both human and C. elegans native enzymes showed almost identical reaction rate values in the case of m7GpppG hydrolysis. Moreover, the rate constants for the His-tagged forms of nematode DcpS were approximately three to five times higher in relation to those of human enzymes. Upon comparison of the kinetic data for the two nematode DcpS substrates (m7GpppG and m32,2,7GpppG), the trimethylated cap analog was the more preferred substrate of all three versions of the CeDcpS enzyme, which was consistent with our previous results obtained for N-tagged CeDcpS,39 and such a preference, although less pronounced, was seen for all AsDcpS forms (Table 3).
Figure 3.
Kinetics of Cap Hydrolysis Catalyzed by Human and Nematode DcpS. (A) Reaction progress curves obtained for the 10 μM m7GpppG substrate in the presence of 30 nM indicated the DcpS enzyme. (B) Reaction progress curves obtained for the 10 μM m32,2,7GpppG substrate in the presence of 5 nM nematode DcpS enzymes. The detailed reaction conditions are found in the Materials and Methods section. Representative reaction progress curves of three independent experiments are shown.
Table 3. Comparison of Reaction Rate Constants of DcpS-Mediated Cap Hydrolysisa.
| N-tagged | nativeb | C-tagged | |
|---|---|---|---|
| Human DcpS | |||
| m7GpppG | 0.39 s–1 ± 0.05 | 0.37 s–1 ± 0.04 | 0.56 s–1 ± 0.03 |
| (1.5×)c | |||
| C. elegans DcpS | |||
| m7GpppG | 1.13 s–1 ± 0.07 | 0.38 s–1 ± 0.04 | 1.49 s–1 ± 0.06 |
| (2.9×) | (3.9×) | ||
| m32,2,7GpppG | 4.02 s–1 ± 0.18 | 2.34 s–1 ± 0.32 | 6.98 s–1 ± 0.56 |
| (1.7×) | (3.0×) | ||
| A. suum DcpS | |||
| m7GpppG | 1.55 s–1 ± 0.01 | 1.85 s–1 ± 0.06 | 1.39 s–1 ± 0.06 |
| (0.80×) | (0.75×) | ||
| m32,2,7GpppG | 1.84 s–1 ± 0.06 | 2.38 s–1 ± 0.06 | 2.12 s–1 ± 0.01 |
| (0.77×) | (0.89×) | ||
Determined reaction rate constants correspond to the arithmetic mean of three independent experiments (± SE).
Native forms of DcpS were obtained via N-terminal tag cleavage.
In brackets, the relative ratio of reaction rate values of tagged to untagged DcpS is shown.
We examined also whether the native untagged form of DcpS exhibits hydrolytic activity toward m7GDP. The experiments with m7GDP were performed under the same conditions as for m7GpppG but with a 10-times higher enzyme concentration. The HPLC measurements of the postreaction mixture showed that m7GDP does not undergo hydrolysis with untagged enzymes (Figure S2). The same procedure applied to test the N- and C-tagged forms of DcpS clearly demonstrated that m7GDP was resistant to hydrolysis catalyzed by native and both tagged forms of human and nematode DcpSs.
The pivotal point of our study was comparison of the hydrolytic activity of native DcpS enzymes (human, C. elegans, and A. suum) with that of their His-tagged forms. Our work is the first on direct characterization of the kinetic properties of the three versions of DcpSs. In addition, we examined also the thermal stability of tagged and native DcpSs. In light of our results, it is clear that the His-tag introduced either at the N-terminus or C-terminus of hDcpS and AsDcpS does not affect substantially their hydrolytic activity and melting temperature values. Interestingly, it was reported that deletion of the first 33 N-terminal amino acids of hDcpS did not change its hydrolytic activity, and a similar effect was observed when 65 residues from the C-terminus of the yeast homolog of DcpS were deleted.41 This may suggest that, at least to some extent, the terminal residues of hDcpS and AsDcpS do not influence their structure and activity, and additional amino acid tags would have also no detrimental effect on hydrolytic properties.
The His-tag had a more noticeable effect on CeDcpS. In this case, there was about a two- to four-fold increase in the activity of both His-tagged species in comparison to the native form. This result is rather surprising, as only the C-terminal domain of DcpS contains the HIT motif involved in substrate binding and efficient hydrolysis. However, a previous study also indicated that the N-terminal domain facilitates cap binding for hDcpS.41 As C. elegans and human DcpSs share 65% similarity and 37% identity,23 this observation may suggest that in the case of CeDcpS, an additional extension at the N- or C-terminal end affects the interaction with the cap structure. In addition, as the protein thermal melting Tm value is decreased by 5 °C for C-tagged CeDcpS in comparison to the untagged one and by around 10 °C for N-tagged DcpS (with the second Tm value being higher by around 3 °C), we cannot exclude the hypothesis that at least to some extent, the protein stability/flexibility affects the kinetic activity of C. elegans DcpS—in general, the higher flexibility can be related to the higher catalytic activity and the lower stability.49 Such tendency can be seen here for CeDcpS versions toward both tested substrates. In the cases of hDcpS and AsDcpS, virtually the same thermal stability of their N- and C-tagged versions reflects the negligible or minimal effect on their enzymatic activity. As the structure of CeDcpS has not been solved experimentally yet, but has already been modeled based on the hDcpS crystallographic data,23 it is difficult to propose an explanation for why the presence of the tag has a negligible effect on hDcpS activity and affects CeDcpS activity.
In spite of the apparent differences of the His-tag effect on DcpS enzyme kinetics shown here, it provides a highly effective means for isolating all three proteins. Both N- and C-tagged forms of DcpS can be used in subsequent biochemical analyses and initial inhibitor screens, as they maintain the specificity of the untagged native DcpS. However, it should be noted that the N- and C-tagged forms of purified DcpS could differ with respect to their homogeneity, as indicated by ESI-MS analysis. Such heterogeneity found here for N-terminally His-tagged proteins, which resulted from gluconylation, could affect other downstream experiments such as the protein crystallization process.42 Also, in screening tests selecting ligands that effectively interact with a particular protein, the presence or absence of a tag may need to be considered, as it may alter the protein’s activity and screening methods,43 or influence the in vitro drug metabolism kinetic data analysis.44
Materials and Methods
Cap Analogs
Cap analogs investigated in this work (m7GpppG, m32,2,7GpppG, and m7GDP) were synthesized according to the methods described previously.45,46 The concentrations of all compounds were determined on the basis of their absorption coefficients.13
Recombinant Protein Production and Purification
Human hDcpS or nematode DcpS was expressed in the Rosetta2 (DE3) E. coli strain from their respective pET vectors (listed in Table S1) according to the procedure described previously.47
Tag Cleavage
The native form of DcpS that lacks the N-terminal histidine tag was obtained using the protease Factor Xa (Factor Xa Cleavage Capture Kit, Merck Millipore). The working concentration of Factor Xa was experimentally set to a final concentration of 4 ng/μL to minimize the exposure of secondary cleavage sites (Figure S4). Cleavage reactions (250 μL final volume, His-tagged DcpS final concentration 0.2 μg/μL) were performed at 21 °C for 16 h. Subsequently, Factor Xa was removed from the reaction mixture by capturing using Xarrest Agarose and spin filters (according to the supplier’s protocol), and the pooled samples of Xa-cleaved DcpS were incubated with Ni-NTA agarose resin (HIS-Select Nickel Affinity Gel, Sigma Aldrich) by mixing using an up-down mixer at 5 °C for 3 h in order to bind uncleaved proteins. Finally, the Ni-NTA agarose was removed using a spin Ultrafree-MC HV Centrifugal Filter (Merck Millipore), and the cleaved DcpS samples were subsequently concentrated using an Amicon Ultra-4 Centrifugal filter, 10,000 NMWL (Merck Millipore).
The concentration of purified DcpS was estimated spectrophotometrically using a theoretical excitation coefficient (ProtParam tool, ExPasy): ε280 = 30,495 M–1 cm–1 for human DcpS, ε280 = 44,350 M–1 cm–1 for A. suum DcpS, and ε280 = 38,975 M–1 cm–1 for C. elegans DcpS. The purity of the proteins was examined by SDS-PAGE electrophoresis, and the protein identity was confirmed by molecular mass determination (ESI-MS, Laboratory of Mass Spectrometry, IBB PAS, Warsaw).
Thermal Stability of DcpS Proteins
Analysis of the thermal stability of His-tagged and untagged DcpS proteins was performed by differential scanning fluorimetry.48 The assay sample (25 μL) contained 2× SYPRO Orange (Sigma Aldrich) and 3 μM particular DcpS form (final concentrations) in 50 mM HEPES and 150 mM NaCl (pH 7.2). m7GDP was added to the analyzed samples at a final concentration of 100 μM. A CFX96 Real-Time PCR (Bio-Rad) was used to increase the temperature starting from 25 °C and increased to 95 °C by 0.5 °C increments, and the fluorescence intensity (FRET channel) was measured at each step. The melting temperature (Tm) was calculated using CFX Manager Software (Bio-Rad) as the minimum of the first negative derivative of the DSF melting curves.
Hydrolysis Kinetics
Enzymatic hydrolysis of dinucleotide cap analogs was performed using DcpS at a final concentration of 30 nM (in experiments with m7GpppG) and at a final concentration of 5 nM (in experiments with m32,2,7GpppG). The substrate concentration in the reaction mixture was 10 μM. The reactions were conducted at 20 °C in 50 mM Tris–HCl buffer containing 150 mM NaCl, pH 7.2. The fluorometric method, where the increase in fluorescence due to DcpS-mediated cap dinucleotide hydrolysis is recorded, was used to measure the progress of cap analog hydrolysis.22 The reactions were monitored for 30–60 min by recording the time-dependent increase of the fluorescence intensity, which is caused by removal of intramolecular stacking as a result of enzymatic cleavage of the dinucleotide cap analogs. On the basis of fluorometric data, the fraction of the generated product was plotted against time, and the pseudo-first order enzymatic decapping rate constants were calculated.40 Fluorescence experiments were performed using a LS-50B spectrofluorometer (Perkin-Elmer Co., Norwalk, CT, USA).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00304.
Supplementary methods, list of vectors, and primers (Table S1), DcpS sequence alignment (Figure S1), HPLC profiles (Figure S2), DSF thermal stability analysis in the presence of m7GDP (Figure S3), N-tag cleavage optimization (Figure S4), and scheme of DcpS-mediated hydrolysis of cap dinucleotides (Figure S5) (PDF)
This study was supported by grants from the Polish National Science Centre (UMO/2013/08/A/NZ1/00866 and UMO/2017/27/B/NZ1/01859) and the National Centre of Research and Development (STRATEGMED 1/235773/19/NCBR/2016).
The authors declare no competing financial interest.
Supplementary Material
References
- Meyer S.; Temme C.; Wahle E. Messenger RNA turnover in eukaryotes: pathways and enzymes. Crit. Rev. Biochem. Mol. Biol. 2010, 39, 197–216. 10.1080/10409230490513991. [DOI] [PubMed] [Google Scholar]
- Milac A. L.; Bojarska E.; Wypijewska del Nogal A. Decapping Scavenger (DcpS) enzyme: Advances in its structure, activity and roles in the cap-dependent mRNA metabolism. Biochim. Biophys. Acta 2014, 1839, 452–462. 10.1016/j.bbagrm.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Grudzien-Nogalska E.; Kiledjian M. New insight into decapping enzymes and selective mRNA decay. WIREs RNA 2016, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malys N.; Carroll K.; Miyan J.; Tollervey D.; McCarthy J. E. The “scavenger” m7GpppX pyrophosphatase activity of Dcs1 modulates nutrient-induced responses in yeast. Nucl. Acids Res. 2004, 32, 3590–3600. 10.1093/nar/gkh687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. S.; Mikhli C.; Friedman C.; Jankowska-Anyszka M.; Stepinski J.; Darzynkiewicz E.; Davis R. E. Nematode m7GpppG and m32,2,7GpppG decapping: Activities in Ascaris embryos and characterization of C. elegans scavenger DcpS. RNA 2004, 10, 1609–1624. 10.1261/rna.7690504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougot N.; van Dijk E.; Babajko S.; Séraphin B. Cap-tabolism. Trends Biochem. Sci. 2004, 29, 436–444. 10.1016/j.tibs.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Liu H.; Rodgers N. D.; Jiao X.; Kiledjian M. The scavenger mRNA decapping enzyme DcpS is a member of the HIT family of pyrophosphatases. EMBO J. 2002, 21, 4699–4708. 10.1093/emboj/cdf448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinov G. K.; Williams B. A.; McCue K.; Schroth G. P.; Gertz J.; Myers R. M.; Wold B. J. From single-cell to cell-pool transcriptomes: stochasticity in gene expression and RNA splicing. Genome Res. 2014, 24, 496–510. 10.1101/gr.161034.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Liu C. L.; Storay J. D.; Tibshirani R. J.; Herschlag D.; Brown P. O. Precision and functional specificity in mRNA decay. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 5860–5865. 10.1073/pnas.092538799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E.; van Nimwegan E.; Zavolan M.; Rajewsky N.; Schroeder M.; Magnasco M.; Darnell J. E. Jr. Decay rates of human mRNAs: correlation with functional characteristics and sequence attributes. Genome Res. 2003, 13, 1863–1872. 10.1101/gr.1272403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk E.; Le Hir H.; Seraphin B. DcpS can act in the 5′-3’ mRNA decay pathway in addition to the 3′-5′ pathway. Proc. Natl. Acad. Sci. U. S. A. 2011, 100, 12081–12086. 10.1073/pnas.1635192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malys N.; McCarthy J. E. G. Dcs2, a novel stress-induced modulator of m7GpppX pyrophosphatase activity that locates to P bodies. J. Mol. Biol. 2006, 363, 370–382. 10.1016/j.jmb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Wypijewska A.; Bojarska E.; Lukaszewicz M.; Stepinski J.; Jemielity J.; Davis R. E.; Darzynkiewicz E. 7-methylguanosine diphosphate (m7GDP) is not hydrolyzed, but strongly bound by decapping scavenger (DcpS) enzymes and potently inhibits their activity. Biochemistry 2012, 51, 8003–8013. 10.1021/bi300781g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverniti V.; Seraphin B. Elimination of cap structure generated by mRNA decay involves the new scavenger mRNA decapping enzyme Aph1/FHIT together with DcpS. Nucleic Acids Res. 2015, 43, 482–492. 10.1093/nar/gku1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen V.; Liu H.; Liu S. W.; Jiao X.; Kiledjian M. DcpS scavenger decapping enzyme can modulate pre-mRNA splicing. RNA 2008, 14, 1132–1142. 10.1261/rna.1008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse G. D.; Rueggers S.; Ow M. C.; Vasquez-Rifo A.; Rondeau E. L.; Ambros V. R.; Grosshans H.; Simard M. J. The decapping scavenger enzyme DCS-1 controls microRNA level in Caenorhabditis elegans. Mol. Cell 2013, 50, 281–287. 10.1016/j.molcel.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C. K. L.; Shboul M.; Taverniti V.; Bonnard C.; Lee H.; Eskin A.; Nelson S. F.; Al-Raqad M.; Altawalbeh S.; Séraphin B.; Reversade B. Loss of the scavenger mRNA decapping enzyme DCPS causes syndromic intellectual disability with neuromuscular defects. Hum. Mol. Genet. 2015, 24, 3163–3171. 10.1093/hmg/ddv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed I.; Buchert R.; Zhou M.; Jiao X.; Mittal K.; Sheikh T. I.; Scheller U.; Vasli N.; Rafiq M. A.; Brohi M. Q.; Mikhailov A.; Ayaz M.; Bhatti A.; Sticht H.; Nasr T.; Carter M. T.; Uebe S.; Reis A.; Ayub M.; John P.; Kiledjian M.; Vincent J. B.; Jamra R. A. Mutations in DCPS and EDC3 in autosomal recessive intellectual disability indicate a crucial role for mRNA decapping in neurodevelopment. Hum. Mol. Genet. 2015, 24, 3172–3180. 10.1093/hmg/ddv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogliotti R. G.; Cardona H.; Singh J.; Bail S.; Emery C.; Kuntz N.; Jorgensen M.; Durens M.; Xia B.; Barlow C.; Heier C. R.; Plasterer H. L.; Jacques V.; Kiledjian M.; Jarecki J.; Rusche J.; DiDonato C. J. The DcpS inhibitor RG3039 improves survival, function and motor unit pathologies in two SMA mouse models. Hum. Mol. Genet. 2013, 22, 4084–4101. 10.1093/hmg/ddt258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T.; Masuda T.; Canver M. C.; Seiler M.; Semba Y.; Shboul M.; Al-Raqad M.; Maeda M.; Schoonenberg V. A. C.; Cole M. A.; Macias-Trevino C.; Ishikawa Y.; Yao Q.; Nakano M.; Arai F.; Orkin S. H.; Reversade B.; Buonamici S.; Pinello L.; Akashi K.; Bauer D. E.; Maeda T. Genome-wide CRISPR-Cas9 Screen Identifies Leukemia-Specific Dependence on a Pre-mRNA Metabolic Pathway Regulated by DCPS. Cancer Cell 2018, 33, 386–400.e5. 10.1016/j.ccell.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z. M.; Bojarska E.; Stepinski J.; Jemielity J.; Jankowska-Anyszka M.; Davis R. E.; Darzynkiewicz E. Affinity of dinucleotide cap analogs for human decapping scavenger (hDcpS). Nucleosides, Nucleotides and Nucleic Acids 2007, 26, 1349–1352. 10.1080/15257770701533818. [DOI] [PubMed] [Google Scholar]
- Wypijewska A.; Bojarska E.; Stepinski J.; Jankowska-Anyszka M.; Jemielity J.; Davis R. E.; Darzynkiewicz E. Structural requirements for Caenorhabditis elegans DcpS substrates based on fluorescence and HPLC enzyme kinetic studies. FEBS J. 2010, 277, 3003–3013. 10.1111/j.1742-4658.2010.07709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wypijewska del Nogal A.; Surleac M. D.; Kowalska J.; Lukaszewicz M.; Jemielity J.; Bisaillon M.; Darzynkiewicz E.; Milac A. L.; Bojarska E. Analysis of decapping scavenger cap complex using modified cap analogs reveals molecular determinants for efficient cap binding. FEBS J. 2013, 280, 6508–6527. 10.1111/febs.12553. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z. M.; Bojarska E.; Kowalska J.; Lewdorowicz M.; Jemielity J.; Kalek M.; Stepinski J.; Davis R. E.; Darzynkiewicz E. Interaction of human decapping scavenger with 5’mRNA cap analogues: structural requirements for catalytic activity. J Phys Condens Matter 2007, 19, 285217. 10.1088/0953-8984/19/28/285217. [DOI] [Google Scholar]
- Gu M.; Fabrega C.; Liu S.-W.; Liu H.; Kiledjian M.; Lima C. D. Insight into the structure, mechanism, and regulation of scavenger mRNA decapping activity. Mol. Cell 2004, 14, 67–80. 10.1016/S1097-2765(04)00180-7. [DOI] [PubMed] [Google Scholar]
- Neu A.; Neu U.; Fuchs A.-L.; Schlager B.; Sprangers R. An excess of catalytically required motions inhibit the scavenger decapping enzyme. Nat. Chem. Biol. 2015, 11, 697–704. 10.1038/nchembio.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornhorst J. A.; Falke J. J. Purification of proteins using polyhistidine affinity tags. Methods Enzymol. 2000, 326, 245–254. 10.1016/S0076-6879(00)26058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnau J.; Lauritzen C.; Petersen G. E.; Pedersen J. Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Prot Expr Purif 2006, 48, 1–13. 10.1016/j.pep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Woenstenek E. A.; Hammarström M.; van den Berg S.; Härd T.; Berglund H. His tag effect on solubility of human proteins produced in Escherichia coli: a comparison between four expression vectorss. J Struct Funct Genomics 2004, 5, 217–229. 10.1023/B:jsfg.0000031965.37625.0e. [DOI] [PubMed] [Google Scholar]
- Chant A.; Kraemer-Pecore C. M.; Watkin R.; Kneale G. G. Attachment of a histidine tag to the minimal zinc finger protein of the Aspergillus nidulans gene regulatory protein Area causes a conformational change at the DNA-binding site. Protein Expression Purif. 2005, 39, 152–159. 10.1016/j.pep.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Horchani H.; Ouertani S.; Gargouri Y.; Sayari A. The N-terminal His-tag and the recombinant process affects the biochemical properties of Staphylococcus aureus lipase produced in Escherichia coli. J. Mol. Catal. B:.Enzym. 2009, 61, 194–201. 10.1016/j.molcatb.2009.07.002. [DOI] [Google Scholar]
- Zakalskiy A. E.; Zakalska O. M.; Rzhepetskyy Y. A.; Potocka N.; Stasyk O. V.; Horak D.; Gonchar M. V. Overexpression of (His)6-tagged human arginase I in Saccharomyces cerevisiae and enzyme purification using metal affinity chromatography. Protein Expression Purif. 2012, 81, 63–68. 10.1016/j.pep.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Carson M.; Johnson D. H.; McDonald H.; Brouillette C.; DeLucas L. J. His-tag impact on structure. Acta Crystallogr., Sect. D: Biol.Crystallogr. 2007, 63, 295–301. 10.1107/S0907444906052024. [DOI] [PubMed] [Google Scholar]
- Xiao Q.; Zhang F.; Nacev B. A.; Liu J. O.; Pei D. Protein N-Terminal Processing: Substrate Specificity of Escherichia coli and Human Methionine Aminopeptidases. Biochemistry 2010, 49, 5588–5599. 10.1021/bi1005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frottin F.; Martinez A.; Peynot P.; Mitra S.; Holz R. C.; Giglione C.; Meinnel T. The proteomics of N-terminal methionine cleavage. Mol. Cell. Proteomics 2006, 5, 2336–2349. 10.1074/mcp.M600225-MCP200. [DOI] [PubMed] [Google Scholar]
- Yan Z.; Caldwell G. W.; McDonell P. A.; Jones W. J.; August A.; Masucci J. A. Mass spectrometric determination of a novel modification of the N-terminus of histidine-tagged proteins expressed in bacteria. Biochem. Biophys. Res. Commun. 1999, 259, 271–282. 10.1006/bbrc.1999.0770. [DOI] [PubMed] [Google Scholar]
- Yan Z.; Caldwell G. W.; McDonell P. A. Identification of a gluconic acid derivative attached to the N-terminus of histidine-tagged proteins expressed in bacteria. Biochem. Biophys. Res. Commun. 1999, 262, 793–800. 10.1006/bbrc.1999.1304. [DOI] [PubMed] [Google Scholar]
- Geoghegan K. F.; Dixon H. B.; Rosner P. J.; Hoth L. R.; Lanzetti A. J.; Borzilleri K. A.; Marr E. S.; Pezzullo L. H.; Martin L. B.; LeMotte P. K.; McColl A. S.; Kamath A. V.; Stroh J. G. Spontaneous alpha-N-6-phosphogluconoylation of a ″His tag″ in Escherichia coli: the cause of extra mass of 258 or 178 Da in fusion proteins. Anal. Biochem. 1999, 267, 169–184. 10.1006/abio.1998.2990. [DOI] [PubMed] [Google Scholar]
- Wypijewska A.; Bojarska E.; Stepinski J.; Jankowska-Anyszka M.; Jemielity J.; Davis R. E.; Darzynkiewicz E. Structural requirements for Caenorhabditis elegans DcpS substrates based on fluorescence and HPLC enzyme kinetic studies. FEBS J. 2010, 277, 3003–3013. 10.1111/j.1742-4658.2010.07709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. W.; Rajagopal V.; Patel S. S.; Kiledjian M. Mechanistic and kinetic analysis of the DcpS scavenger decapping enzyme. J. Biol. Chem. 2008, 283, 16427–16436. 10.1074/jbc.M800341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. W.; Jiao X.; Liu H.; Gu M.; Lima C. D.; Kiledjian M. Functional analysis of mRNA scavenger decapping enzymes. RNA 2004, 10, 1412–1422. 10.1261/rna.7660804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. M.; Yi E. C.; Baker D.; Zhang K. Y. J. Post-translational modification of the N-terminal His tag interferes with the crystallization of the wild-type and mutant SH3 domains from chicken src tyrosine kinase. Acta Crystallogr., Sect. D: Biol.Crystallogr. 2001, 57, 759–762. 10.1107/S0907444901002918. [DOI] [PubMed] [Google Scholar]
- Majorek K. A.; Kuhn M. L.; Chruszcz M.; Anderson W. F.; Minor W. Double trouble - Buffer selection and His-tag presence may be responsible for nonreproducibility of biomedical experiments. Protein Sci. 2014, 23, 1359–1368. 10.1002/pro.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy T. S.; Hummel M. A. Modeling kinetic data from in vitro drug metabolism enzyme experiments. Drug Metab. Rev. 2004, 36, 231–242. 10.1081/DMR-120033999. [DOI] [PubMed] [Google Scholar]
- Stepinski J.; Bretner M.; Jankowska M.; Felczak K.; Stolarski R.; Wieczorek Z.; Cai A.-L.; Rhoads R. E.; Temeriusz A.; Haber D.; et al. Synthesis and properties of P1,P2-, P1,P3, and P1,P4-dinucleoside di-, tri- and tetraphosphate mRNA cap analogues. Nucleosides Nucleotides 1995, 14, 717–721. [Google Scholar]
- Jankowska M.; Stepinski J.; Stolarski R.; Wieczorek Z.; Temeriusz A.; Haber D.; Darzynkiewicz E. 1H NMR and fluorescence studies of new mRNA 5′- cap analogues. Coll. Czech. Chem. Commun. 1996, 61, S197. [Google Scholar]
- Pietrow P.; Ferenc-Mrozek A.; Piecyk K.; Bojarska E.; Darzynkiewicz E.; Jankowska-Anyszka M. Decapping Scavenger Enzyme Activity toward N2-Substituted 5′ End mRNA Cap Analogues. ACS Omega 2019, 4, 17576–17580. 10.1021/acsomega.9b02715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesen F. H.; Berglund H.; Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat. Protoc. 2007, 2, 2212–2221. 10.1038/nprot.2007.321. [DOI] [PubMed] [Google Scholar]
- Quezada A. G.; Díaz-Salazar A. J.; Cabrera N.; Pérez-Montfort R.; Piñeiro Á.; Costas M. Interplay between Protein Thermal Flexibility and Kinetic Stability. Structure 2017, 25, 167–179. 10.1016/j.str.2016.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.