Abstract
The mechanisms of cellular absorption and transport underlying the differences between flavonoid aglycones and glycosides and the effect of the structural feature are not well established. In this study, aglycone, mono-, and diglycosides of quercetin and cyanidin were selected to examine the effects of the structural feature on the bioavailability of flavonoids using hexose transporters SGLT1 and GLUT2 in a Caco-2 BBe1 cell model. Cellular uptake and transport of all glycosides were significantly different. The glycosides also significantly inhibited cellular uptake of d-glucose, indicating the involvement of the two hexose transporters SGLT1 and GLUT2 in the absorption, and the potential of the glycosides in lowering the blood glucose level. The in silico prediction model also supported these observations. The absorption of glycosides, especially diglycosides but not the aglycones, was significantly blocked by SGLT1 and GLUT2 inhibitors (phloridzin and phloretin) and further validated in SGLT1 knockdown Caco-2 BBe1 cells.
Introduction
Sufficient evidence exists that long-term intake of fruits, vegetables, and whole-grains is beneficial to human health and holds great potential for reducing incidences of modern chronic diseases, for example, cardiovascular and neurodegenerative diseases, diabetes, and cancer, owing to the bioactive phytochemicals especially phenolic compounds.1,2 Phenolics are the most common and diverse phytochemical group of food origin and possess a wide spectrum of health-enhancing capabilities including antioxidant and anti-inflammatory effects, the abilities in the regulation/transduction of cellular signaling pathways, and restoring the immune homeostasis, all of which can lead to reduced risks of degenerative diseases and metabolic syndromes in humans.3−5 Flavonoids are the largest class of polyphenols that can be further categorized into several subgroups including flavonols and anthocyanins, both of which are naturally distributed in plant foods as glycosides containing single or multiple sugar moieties. Except in fungi and algae, the most common flavonols of plants, for example, kaempferol, quercetin, and myricetin are predominantly in glycosidic forms.6 Similarly, anthocyanidins, for example, pelargonidin, cyanidin, delphinidin, peonidin petunidin, and malvidin occur almost exclusively in glycosidic forms. Moreover, both flavonols and anthocyanidins are considered as organic pigments that provide colorant features to plant products. For example, rutin is a quercetin disaccharide with a pale yellow color that is commonly found in a wide variety of citrus fruits and onions.7 Anthocyanins are abundant in highly pigmented fruits (berries and grapes), vegetables (red cabbage and purple carrots), and cereals such as black rice and purple wheat. Cyanidin-3-O-glucoside is perhaps the most commonly detected anthocyanin in plants.8 Phenolics or polyphenols are not readily bioavailable in vivo despite the relatively high bioaccessibility. Flavonoid aglycones are generally more bioavailable than their respective glycosides, while their glycosides are rapidly removed from the circulating blood.9 However, anthocyanins have been reported to be quickly absorbed in human blood, suggesting these compounds may have different absorption and uptake mechanisms than other flavonoids.10 The fate of flavonoid glycosides throughout the human digestive tract and the further action of the gut microbiome can all affect the absorption and metabolism of these compounds. The intestinal epithelial environment is a key part of the gastrointestinal tract (GIT) for absorption, uptake, and metabolism, and it provides great means for studying the molecular mechanisms underlying flavonoid absorption and metabolism.
A number of in vitro and in vivo studies have revealed that enzymes and transporters are involved in the absorption, metabolism, and excretion of flavonoids within the GIT.9 Lactase-phloridzin hydrolase (LPH) and cystollic β-glucosidase (CBG) distributed within the small intestine epithelial cells in the brush border are both capable of cleaving polar glucosides and releasing flavonoid aglycones that permeate into the intestinal submucosal layer through passive diffusion.9 However, LPH is not evenly expressed and distributed along the GIT of mammals, primarily due to region specificity and the postweaning decline, and in the lower gut, deglycosylation of flavonoids may be through the action of CBG secreted by the gut microbiota or microbial hydrolases instead of that by the colonic epithelium because LPH and CBG expression in the latter is low and insignificant.11,12 Phase II enzymes can then convert the aglycones into glucuronides, sulphates, and methyl-ester forms that are consequently excreted into blood or effluxed back to the lumen.11 It is well-known that aglycones of flavonols such as quercetin are more readily absorbed because of their relatively higher lipophilicity compared to their glycoside counterparts, where the absorption in vivo is large via passive diffusion.9 Likewise, flavonol glycosides including quercetin-3 glucoside and rutin have been found in the basolateral side of the epithelial membrane monolayer in vitro,13,14 detected and identified in plasma in in vivo studies.15−18 Reports also indicate that all forms of polyphenols including intact aglycones and their original glycosides and their metabolites coexist in fecal samples in the colon.19,20 For these reasons, the mechanisms of absorption in the GIT and how flavonoids, especially various forms of flavonoids, contribute to intestinal health must be revisited.
Both sodium-glucose-linked cotransporter (SGLT1) and glucose transporter (GLUT2) are widely distributed along the intestinal epithelium and responsible for the uptake and efflux of hexoses into the blood stream and have been reported to be involved in sensing and uptaking many intact flavonoid glycosides derived from food matrices.21−24 Apparently, the glycosylation pattern of flavonoids can have significant impact on the bioavailability of these compounds, thus can subsequently affect the metabolism, biological effects, and ultimately health benefits. It is therefore of foremost importance to understand the mechanism of cellular uptake and absorption of flavonoids with different glycosylation patterns. While SGLT1 and GLUT2 are known to be involved in the transport of flavonoid glycosides, there still lacks a close-up investigation into how different forms of flavonoids, that is, aglycones and glycosides pass into the systemic circulation in one study using the same model system. In the present study, two well-known flavonoid aglycones, quercetin, cyanidin, and their respective mono- and diglycosides were used as model molecules in an elaborate intestinal epithelial Caco-2 BBe1 trans-membrane cell model to evaluate flavonoid permeability and cellular uptake. Although Caco-2 cells are low in LPH compared with ex vivo intestinal samples, they have similar physiological properties to human intestinal epithelium particularly that of colon.25 Caco-2 cells have been adopted as an ideal model for investigating flavonoid uptake and relevant molecular mechanisms, especially due to their ability in expressing many intestinal enzymes and transporters.26,27 Caco-2 BBe1, a well-known subclone of Caco-2 cells is characteristic for its enhanced intestinal brush border protein expression.28,29 The main focus of this study is not on the bioavailability of aglycones, as it is already well-established, but on how their glycosides are taken up and transported by the epithelial cells. The interference/interactions of the six flavonoids (two aglycones and their mono- and diglycosides) with intestinal epithelial hexose transporters were also evaluated to confirm their roles in the uptake of the studied flavonoid glycosides and to understand the mechanisms of absorption and metabolism inside the GIT.
Results and Discussion
Uptake and Transportation of Flavonoids in the Intestinal Epithelial Cell Models
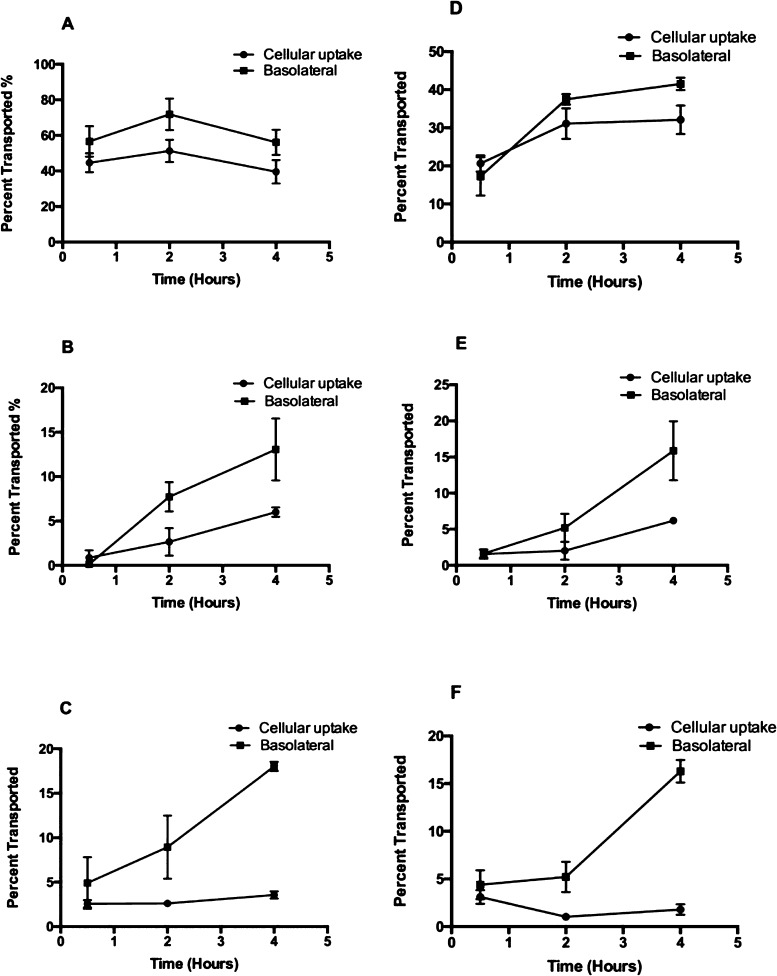
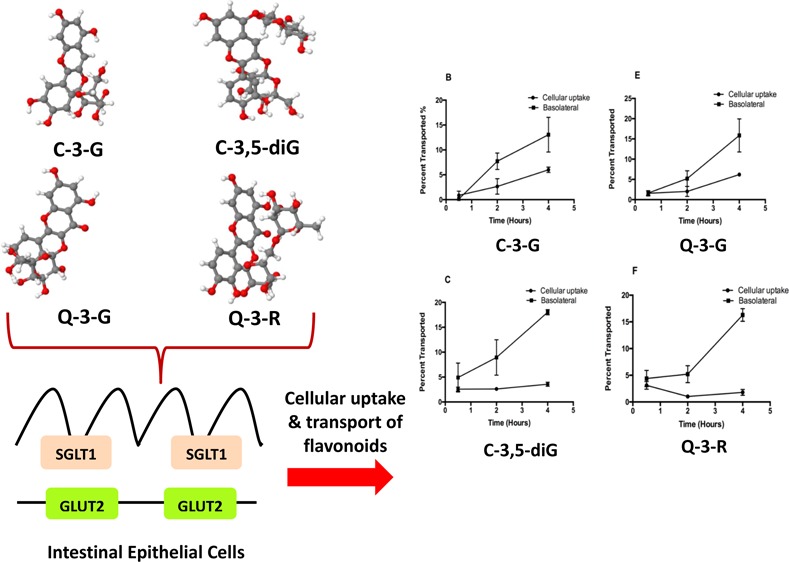
The bioaccessibility and bioavailability of food flavonoids depend on many factors, of which chemical structure, food matrices, and metabolism by gut microbes are most important.30 The present study focuses on the effect of the chemical structure, that is, the glycosylation pattern on the absorption and metabolism of flavonoids in a Caco-2 BBe1 cell model. The permeability curves of the two sets of flavonoids (quercetin and its mono- and diglycosides and cyanidin and its mono- and diglycosides), as shown in Figure 1, suggest that the metabolic kinetics of the aglycones were significantly different from their respective 3-glucoside or diglycoside. Both quercetin and cyanidin (aglycones) readily permeated into the intestinal mucosa within 2 h (Figure 1A,D). Higher percentages of the aglycones in the cells and the basolateral compartment indicate higher cellular uptake and transport efficiencies compared to their respective glycosides. This result along with the reported data collectively supports the hypothesis that aglycone flavonoids can pass into the intestinal lumen and systemic circulation using a mechanism of passive diffusion,9,31 whereas flavonoid glycosides may cross the intestinal epithelial layer using a different mode such as an active transport mechanism.9 Flavonoid glycosides were detected in both cellular lysates and the basolateral side. Introduction of glycosyl groups (i.e. the hexose groups) into the aglycone backbones significantly lowered both cellular uptake and transport of flavonoid glycosides compared with the aglycones (Figure 1). Cellular uptake was particularly low for diglycosides (Figure 1E,F).
Figure 1.
Cellular uptake and trans-membrane transport kinetics of flavonoid aglycones and respective mono- and diglycosides across Caco-2 BBe1 monolayers. The permeability efficiency was assessed by HPLC analysis of the content of flavonoids collected from the cell lysate or basolateral compartment normalized by concentrations in the apical compartment after incubated for 0.5, 2, and 4 h. Cellular uptake and transport of cyanidin (A), C-3-G (B), cyanin (C), quercetin (D), Q-3-G (E), and rutin (F) are shown as percent transported (%). Cellular uptake: filled circles; trans-membrane transport: filled squares. Data are mean ± SEM of at least three independent experiments.
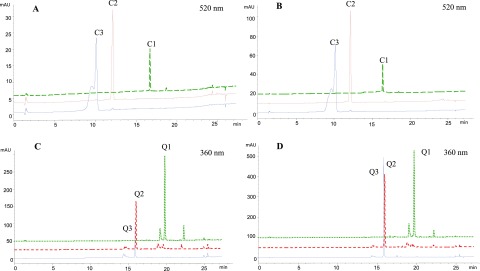
Kinetics study showed that all compounds of the two sets of flavonoids (i.e. quercetin and its mono- and diglucosides and cyanidin and its mono- and diglycosides) were detected in both the cellular lysates (at different time intervals) and the basolateral compartments except a trace amount of C-3-G was detected from cells treated with cyanin possibly as a result of hydrolysis of 5-glucoside (Figure 2). Conventional belief is that flavonoid glucosides cross the intestinal epithelium by SGLT1 and then are released in the aglycone form by CBG and LPH.32 However, the Caco-2 BBe1 cell monolayer treated with flavonoid glycosides in the apical side had no aglycones detected in both cellular lysates and the basolateral side in the present study (Figure 2), suggesting that CBG and LPH may not be a crucial active factor involved in the deglycosylation process. This was validated by the low level of LPH expression in the Caco-2 cells of the present study (Figure S1). We also evaluated the LPH expression levels in mouse jejunum, ileum, and colon and confirmed that LPH was high in the small intestine but low in the colon (Figure S1), a result also supported by previous findings.25 The low LPH level and lack of deglycosylation in the mouse colon and Caco-2 cells suggests that quercetin and cyanidin glycosides detected in the cells and basolateral compartments may be directly transported by the hexose transporters which have been reported to play a key role in the delivery of intact flavonoid glycosides into the enterocytes.24 Carrier-mediated mechanisms might be necessary for the uptake and transport of intact flavonoid glycosides as such mechanisms are known to aid polar compounds to across intestinal epithelial cells.33
Figure 2.
HPLC chromatograms of flavonoid aglycones and respective mono- and diglycosides in cell lysates and basolateral compartment. (A) cyanidin (C1), C-3-G (C2), and cyanin (C3) in cells; (B) cyanidin (C1), C-3-G (C2),and cyanin (C3) in the basolateral compartment; (C) quercetin (Q1), Q-3-G (Q2), and rutin (Q3) in cells; and (D) and quercetin (Q1), Q-3-G (Q2), and rutin (Q3) in the basolateral compartment.
In terms of the aglycones, a noticeable decrease was found for cyanidin in the Caco-2 BBe1 cell lysates and basolateral compartment as the incubation period reached to 4 h (Figure 1A), but for quercetin, both concentrations continued to increase (Figure 1D). The decline in cyanidin (aglycones) in both cells and the basolateral medium may be due to its instability in neutral pH and relatively long incubation time. It is well-known that anthocyanins are generally more stable at acidic pH.34 In general, the cellular uptake and transport rates of cyanidin were faster, and its concentrations were higher than that of quercetin. This supports and may also explain the significantly higher elimination rates of anthocyanins in urinary excretion than quercetin in vivo(35) and that absorption of anthocyanins can occur in the stomach, an acidic environment that favors the stability thus transportation of anthocyanins.36 In addition to pH, glycosylation has been found to increase the stability of anthocyanins throughout the entire digestive process owing to the heterogeneity of the attached sugar moieties.37 This further helps explain our observation that the transport efficiency of the glycosides, that is, C-3-G and cyanin continued to increase as the incubation period extends from 30 min to 4 h (Figure 1B,C), whereas that of the aglycone, that is, cyanidin started to drop after 2 h of incubation (Figure 1A). Similar kinetics were observed for Q-3-G and rutin (Figure 1E,F), but different from cyanidin, quercetin continues to be transported throughout the incubation period (Figure 1D). As noted in recent studies, deglucosylation of conjugated flavonoids may have a counteractive effect on their absorption rates.38,39 The differences in absorption kinetics between the mono- and di-glycosides were not as distinct as between the glycosides and aglycones. There was a significant increase in transport efficiency between C-3-G and cyanin, but that between Q-3-G and rutin was not significant (Figure 1B vs 1C, and 1E vs 1F). It must be pointed out that the two diglycosides of the present study are technically different as the second sugar moiety is attached differently to the flavonoid backbone; therefore to better explain the different kinetics of the two diglycosides, further investigations are necessary when commercial standards are available. Nevertheless, introduction of the second sugar moiety led to enhanced heterogeneity and increased resistance to degradation, which may result in higher bioavailability as the flavonoids pass through the GIT. The effect of the structural/chemical feature of conjugated flavonoids on bioavailability, especially how this is related to the hexose transporters, has recently captured the attention of researchers as this can help understand molecular mechanisms underlying the health benefits observed for flavonoids.9
Molecular Basis of Intestinal Absorption/Uptake of Flavonoids
In our previous study, we demonstrated that hexose transporters mediated the active transport of intact glycosides of cyanidin and petunidin in Caco-2 cells.24 In the present study, we took a computational modeling approach to assess the ability of SGLT1 and GLUT2, two hexose transporters, to interact with and bind to the two sets of flavonoids (Figure S2). The molecular docking calculations showed that the glycosides (i.e. Q-3-G, rutin, C-3-G, and cyanin) had more intense interactions with both SGLT1 and GLUT2 than their respective aglycones (i.e. quercetin and cyanidin) in the parameters tested (Table 1). The geometric score, geometric shape complementarity (topographic area) and the calculated atomic contact energy (ACE) as listed in Table 1 all increased in the order of aglycone, mono- and diglycosides for both sets of flavonoids. Conformational features of the tested flavonoids significantly impacted on ACE, the free energy required for rearranging water molecules within the protein’s interior and for the protein-substrate interactions to proceed, thus on the molecules’ sensing and ligand binding ability of SGLT1 and GLUT2.24
Table 1. Geometric Score, Interface Area Size, and Desolvation Energy (ACE) of GLUT2/SGLT1 Interactions with the Studied Aglycone Flavonoids and Their Glycosidic Forms.
| transporter | ligand | score | area | ACE | transformation |
|---|---|---|---|---|---|
| Glut2 | quercetin | 4134 | 471.80 | –234.23 | –0.38, −0.00, −2.39, 585.05, −43.81, 179.96 |
| Q-3-G | 5422 | 572.80 | –80.81 | –1.47, 0.60, −1.19, 586.61, −29.97, 205.93 | |
| rutin | 5536 | 629.60 | –14.59 | 3.02, 0.48, −1.11, 577.45, −10.29, 204.91 | |
| cyanidin | 4168 | 439.20 | –69.75 | –1.02, 1.32, −1.26, 586.64, −32.38, 204.32 | |
| C-3-G | 5558 | 595.30 | –109.12 | 1.44, 0.98, −1.07, 586.41, −30.42, 205.51 | |
| cyanin | 6646 | 778.80 | –166.44 | 1.58, −1.10, −1.17, 585.79, −30.41, 205.72 | |
| SGLT1 | quercetin | 4030 | 432.10 | –142.25 | –1.59, 0.95, −1.41, 7.26, −27.48, −44.76 |
| Q-3-G | 5450 | 639.90 | –321.94 | –0.28, −0.84, −0.87, 8.89, −23.03, −64.14 | |
| rutin | 5760 | 795.20 | –472.46 | 2.37, −0.61, −0.69, 9.21, −23.50, −64.64 | |
| cyanidin | 4140 | 449.10 | –106.15 | –1.13, −0.51, −2.78, 1.97, −39.68, −50.14 | |
| C-3-G | 5520 | 677.80 | –311.17 | 0.23, 0.81, −3.04, 10.66, −23.79, −66.20 | |
| cyanin | 6442 | 817.80 | –367.19 | –0.96, 0.89, −2.55, 9.18, −23.61, −62.92 |
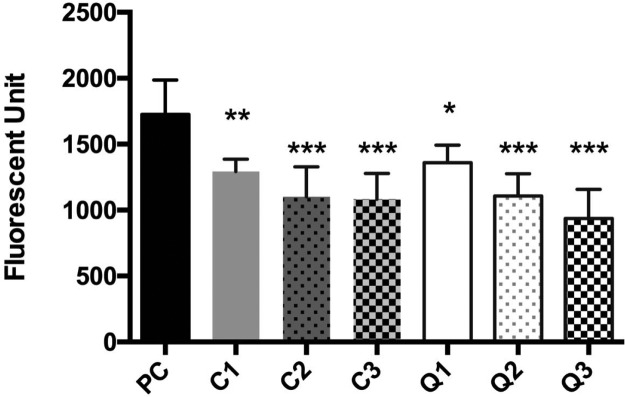
Recent studies have shown that flavonoids inhibit glucose uptake through competitive binding with the hexose transporters distributed in the intestinal brush border.40 Caco-2 cells, representing small-intestinal enterocytes, express both SGLT1 and GLUT2, while only GLUT2 is dominantly distributed in the basolateral membrane.41,42 We previously demonstrated that the purple carrot- and potato-derived anthocyanin glycosides inhibited the glucose uptake in Caco2 cells.24 In the present study, we further demonstrate that the aglycones quercetin (P < 0.05) and cyanidin (P < 0.01) also significantly blocked the glucose uptake (Figure 3). All glycosides (both mono- and diglycosides) in both sets of flavonoids showed even more significant inhibition of glucose uptake (P < 0.001) (Figure 3). Although there was no significant difference in blocking glucose uptake between the mono- and diglycosides, there was a numeric increase in the inhibition by the diglycosides, especially by rutin, suggesting a potential role of glycosylation (Figure 3). These data suggests that glycosylation pattern and steric feature may interfere with glucose binding to the hexose transporters. Our finding supports results of a previous study that rutin but not quercetin inhibited glucose uptake in Caco-2 cells in a SGLT1-depended manner.43 The difference in glucose uptake may also be related to the two distinctly different binding sites for rutin and quercetin within the core of SGLT1 as demonstrated in the molecular docking studies (Figure S3). Collectively, results from the present in vitro study indicate that glucose transporters are possibly responsible for the uptake and transport of flavonoid glycosides in Caco-2 cells and that different flavonoid glycosides may act to block the intestinal glucose uptake in a competitive manner, likely because of the steric hindrance by the different glycosidic moieties. The different binding capacity with the glucose transporters also suggest that the glycosylation patterns of flavonoids are crucial for the active transporters to sense and deliver them across intestinal epithelial cells, leading to different degrees of bioavailability and metabolic fates (Table 1).
Figure 3.
Inhibition of glucose uptake in Caco-2 BBe1 cells by flavonoid aglycones and respective mono- and diglycosides. Cells were pretreated with 50 μM of tested compounds for 2 h. Cellular uptake of glucose is shown as fluorescent units in Caco-2 BBe1 cells from the group of the positive control (PC) and groups treated with cyanidin (C1), C-3-G (C2), cyanin (C3), quercetin (Q1), Q-3-G (Q2), and rutin (Q3). Data are presented as mean ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 versus PC using the one-way ANOVA with Tukey’s post hoc-test.
Hexose Transporters Mediate the Active Transport and Explain the Absorption Mechanism of Flavonoids
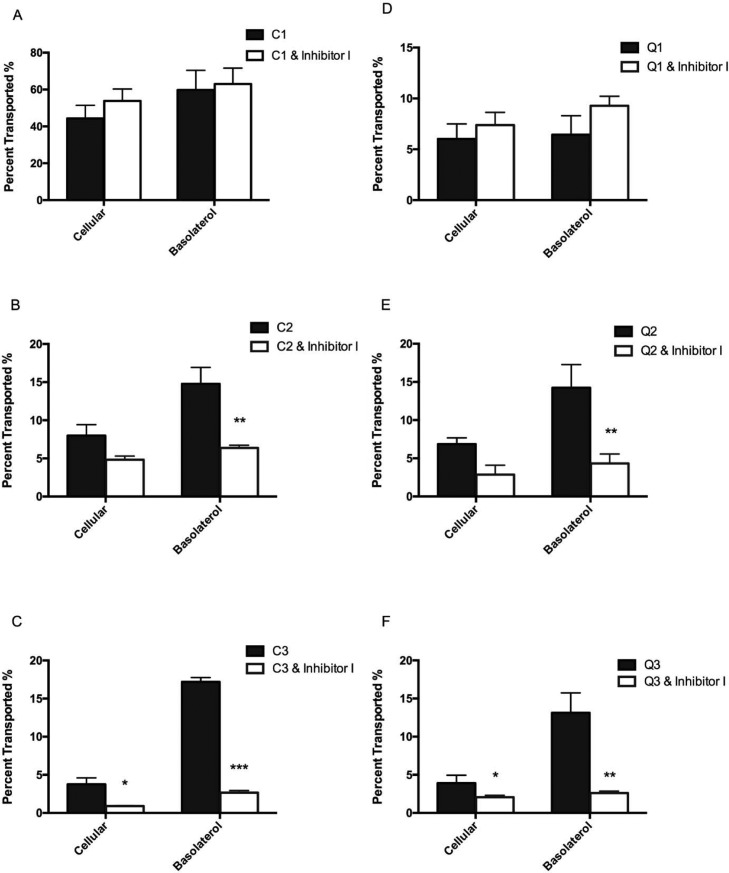
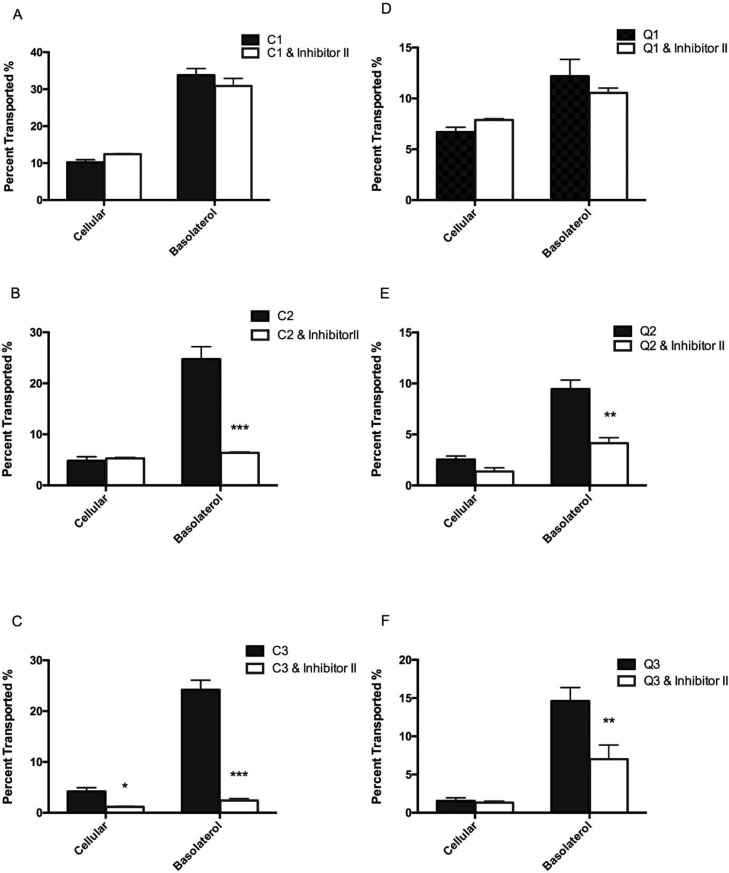
The above-mentioned results in computational docking and glucose uptake of enterocytes demonstrate that delivery and transport of intact flavonoid glycosides through the epithelial layer by SGLT1 and GLUT2 largely depend on the presence of sugar moieties (Table 1, Figure 3). Particular inhibitors for hexose transporters such as phloridzin and small interfering RNA (siRNA) known to specifically target SGLT1 can therefore be used to further verify the involvement of the transporters in the active transport mechanism of flavonoids.44,45 The present study showed that pretreatment with phloridzin indeed significantly reduced the amount of cellular and transported Q-3-G, rutin, C-3-G, and cyanin after 4 h of incubation, whereas it had no effect on the uptake and transport of the aglycones (i.e. cyanidin and quercetin) (Figure 4). The phloridzin (inhibitor I)-mediated reduction of glycoside absorption and transport was in general significantly more drastic for the diglycosides compared to their respective monoglycosides. For cyanidin and its glycosides, cellular uptake of cyanin (i.e. the cyanidin diglycoside) was significantly inhibited by phloridzin (P < 0.05) and that by C-3-G (monoglycoside) was less significant (Figure 4B,C). A similar result was found for rutin and Q-3-G (Figure 4B,C). In terms of phloridzin-mediated inhibition of glycoside transport, the effect was more significant (P < 0.01) for all mono- and diglycosides, that is, 2.31- and 5.77-fold change for Q-3-G and rutin (P < 0.01), respectively (Figure 4B,C) and 3.29- and 6.45-fold change for C-3-G (P < 0.01) and cyanin (P < 0.001), respectively (Figure 4B,C). At the P < 0.001 level, the effect on cyanin transport by phloridzin was distinctly separated from that on Q-3-G, rutin, and C-3-G transport, further suggesting the influence by the glycosylation pattern (Figure 4C). This observation confirms our in silico prediction in which Q-3-G, rutin, and C-3-G had similar geometric scores (5450, 5520, and 5760) but not cyanin (6442) for interactions/binding with SGLT1 (Table 1). Compared to other glycosides, cyanin also had the highest interface area size of 817.8 (Table 1). The significant reduction in flavonoid glycosides by SGLT1 inhibitors such as phloridzin as exhibited in the present study clearly demonstrates that SGLT1 is involved in the cross-epithelial transportation of these compounds. To further verify this finding, SGLT1 knockdown Caco-2 BBe1 cells were used next in this study. As expected, similar reductions in glycosides transported to the basolateral side were observed in SGLT1 knockdown Caco-2 BBe1 cells after 4 h of incubation (P < 0.05), and no significant reduction of aglycones was observed (Figure 5). The efficiency of SGLT1 knockdown was around 50% in the present study (Figure 5A). It was also noted that the fold changes as expressed in transport rate reduction between scramble and SGLT1-knockdown Caco-2 BBe1 cells were more significant for the pair of C-3-G and cyanin (6.35 and 6.45, respectively) than for Q-3-G and rutin (1.92 and 2.6, respectively), although statistically they were all at P < 0.05 (Figure 5B), suggesting that the hexose transporter SGLT1 may play a much more significant role in the transportation of anthocyanidin glycosides than that of flavonol glycosides (Figure 5). These observations collectively suggest that additional factors other than SGLT1 may be involved in the intestinal epithelial transport of quercetin glycosides. Further studies on other possible transport mechanisms of flavonol glycosides are thus warranted. Meanwhile, although data of the present study strongly support the mechanism of active transportation of flavonoid glycosides by SGLT1 in intestinal epithelial cells and there was significant difference between the transport rates of mono- and diglycosides (Figure 5), the latter, that is, the two diglycosides rutin and cyanin had different glycosylation patterns; rutin is a diglycoside with a biose (rutinose) moiety attached to C-3, and the cyanin is a diglycoside with two individual glucose moieties separately attached to the C-3 and C-5 of the flavonoid backbone. Strictly speaking, the steric features of the two diglycosides of the present study are different (Figure S4), and that may affect the binding to the hexose transporters differently. This can be validated in future studies using cyanidin-3-rutinose and quercetin 3,5-diglucoside along with cyanin and rutin when those two compounds are made available.
Figure 4.
Phloridzin (inhibitor I) blocks SGLT1-mediated absorption of flavonoid aglycones and respective mono- and diglycosides in Caco-2 BBe1 cells. Cells were pretreated with or without 1 μM of phloridzin (inhibitor I) and incubated for 30 min, followed by incubation with 50 μM of cyanidin (C1), C-3-G (C2), cyanin (C3), quercetin (Q1), Q-3-G (Q2), and rutin (Q3) for 4 h. Panels (A–C): C1, C2, and C3 with and without inhibitor I, respectively; Panels (D–F), Q1, Q2, and Q3 with and without inhibitor I, respectively. Intact flavonoids were identified in collected cell lysates and basolateral compartments and calculated as transport rate (%) over the concentration of compounds added in the apical compartment. Data are presented as mean ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 versus treatment without the inhibitor using the unpaired Student’s t-test.
Figure 5.
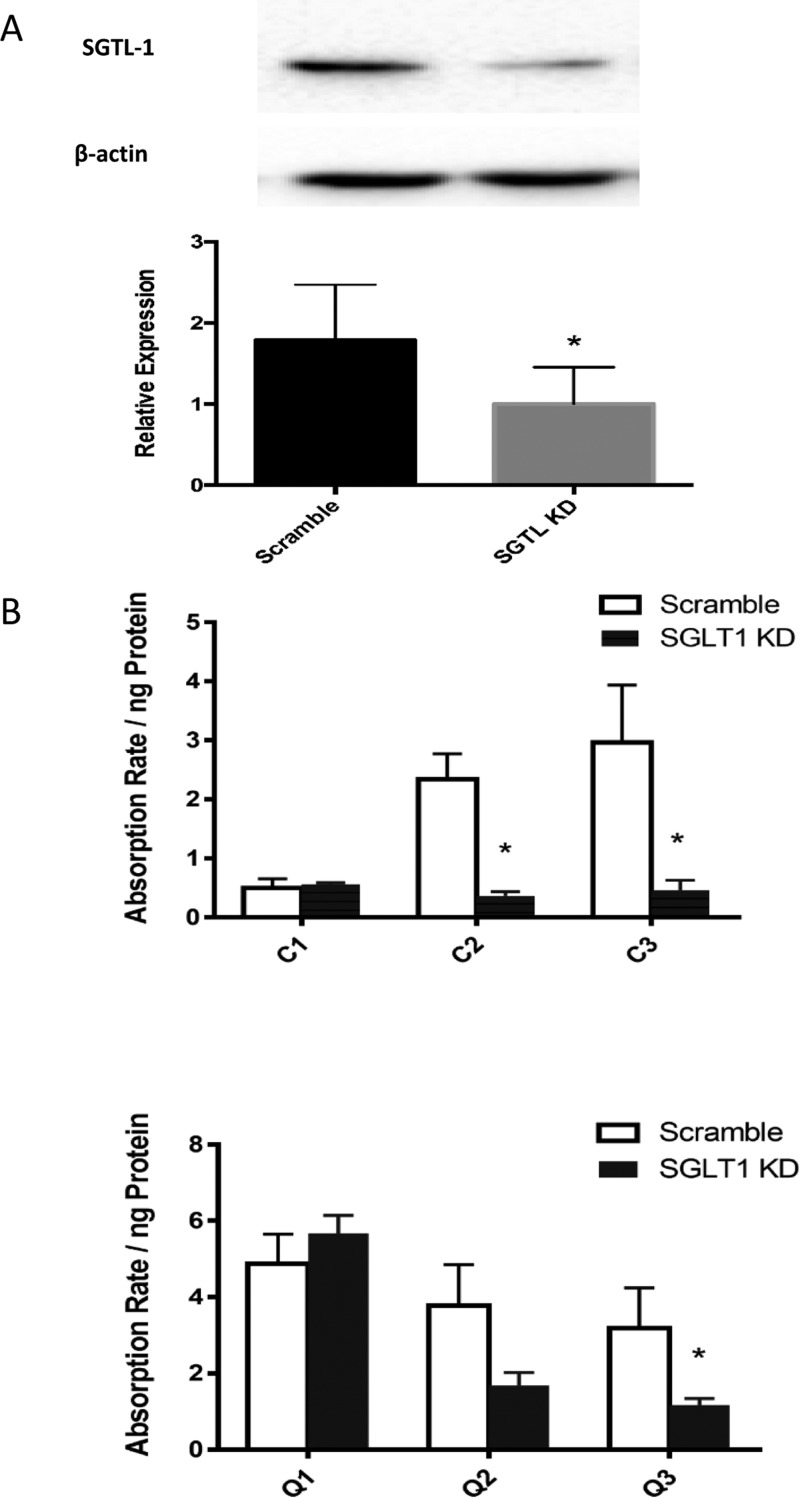
Confirmation of SGLT1 in transport and intestinal absorption of flavonoid aglycones and respective mono- and diglycosides using SGLT1 knockdown Caco-2 BBe1 cells. Cells were transiently transfected with control siRNA (Scramble) or specific SGLT1 siRNA to generate SGLT1 deficient cells (SGLT1 KD). SGLT1 KD or control (scramble) Caco-2 BBE1 cells were treated with 50 μM of flavonoid aglycones and respective mono- and diglycosides for 4 h. (A) SGLT1 knockdown was confirmed by Western blotting, using β-actin as the loading control. (B) Cellular uptake of cyanidin (C1), C-3-G (C2), cyanin (C3), quercetin (Q1), Q-3-G (Q2), and rutin (Q3) were evaluated in the cellular lysate and expressed as absorption rate/ng protein. Data are presented as mean ± SEM of at least three independent experiments. *P < 0.05 versus treatment without inhibitors using the unpaired Student’s t-test.
The other hexose transporter, GLUT2, which is mainly distributed along the intestinal epithelial basolateral membrane, is believed to play a primary role in the excretion of intact flavonoids into the lamina propria.21 Phloretin, a specific inhibitor of GLUT2,46 was applied in the present study to block GLUT2-mediated transportation of the flavonoids. Similar to the effects with the SGLT1, phloretin (inhibitor II) had no effect on GLUT2 in both cellular uptake and transport of the aglycones but significantly inhibited cellular uptake of cyanin (P < 0.05) and very significantly inhibited the transmembrane transport of all glycosides, that is, Q-3-G, rutin, C-3-G, and cyanin, to the basolateral compartment (P < 0.01) (Figure 6). Similar to SGLT1, GLUT2 tends to favor more of transporting cyanidin glycosides (C-3G and cyanin) (P < 0.001), as shown in fold changes of the transport rate that were affected by phloretin (Figure 6B,C). These data also confirm results from the molecular docking experiments, where cyanidin glycosides, particularly cyanin had shown higher affinity for GLUT2 than those of quercetin; they had the highest affinity score (6646), the topographic area (778.8), and a low ACE (−166.44) that are required to rearrange water molecules within the GLUT2 pocket, allowing for transporter/ligand interactions (Table 1). The drastic difference in ACE between rutin and cyanin may explain the significant differences between the two diglycosides in the phloretin inhibition experiment (Figure 6). Our data also suggest that rutin, which had the highest ACE requirement, may possibly have a different mechanism(s) of absorption. The high ACE value of rutin suggests that it could possibly act as a competitive inhibitor of GLUT2 in a different mechanism of conventional inhibitors of GLUT2 (such as phloretin) as rutin occupies a different binding site in comparison to phloretin (Figure 7D). Finally, a similar approach was taken to use commercially available siRNA to generate GLUT2 knockdown cells; however because of the short time span of the effect of siRNA (24–72 h) and the fact that Caco-2 BBe1 cells needed at least 9 days to differentiate and polarize,47 we were unable to conduct the experiment for further validation of the above-proposed mechanism. In spite of this, it can be concluded that absorption of glycosidic flavonoids (i.e. Q-3-G, rutin, C-3-G, and cyanin) was significantly inhibited by knocking down SGLT1 and by using known inhibitors phloridzin and phloretin for SGLT1 and GLUT2, respectively. These findings suggest that absorption of glycosidic flavonoids in the GIT is primarily mediated by both SGLT1 and GLUT2. Because the sugar moiety is an essential ligand targeted by intestinal SGLT1 and GLUT2, it is therefore considered a major determinant for flavonoid glycoside absorption. Our finding also supports that an increasing number of sugar moieties enhance absorption of flavonoid glycosides mediated by intestinal hexose transporters and provides complementary knowledge to the known fact that flavonol aglycones such as quercetin are absorbed by passive diffusion.
Figure 6.
Phloretin (inhibitor II) blocks GLUT2 mediated transport of flavonoid aglycones and respective mono- and diglycosides in Caco-2 BBe1 cells. Cells were pretreated with or without 1 μM of phloretin (inhibitor II) and incubated for 30 min, followed by incubation with 50 μM of cyanidin (C1), C-3-G (C2), cyanin (C3), quercetin (Q1), Q-3-G (Q2), and rutin (Q3) for 4 h. Panels (A–C): C1, C2, and C3 with and without inhibitor II, respectively; Panels( D–F), Q1, Q2, and Q3 with and without inhibitor II, respectively. Intact flavonoids were identified in collected cell lysates and basolateral compartments and calculated as a transport rate (%) over the concentration of compounds added in the apical compartment. Data are presented as mean ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 versus treatment without inhibitors using the unpaired Student’s t-test.
Figure 7.
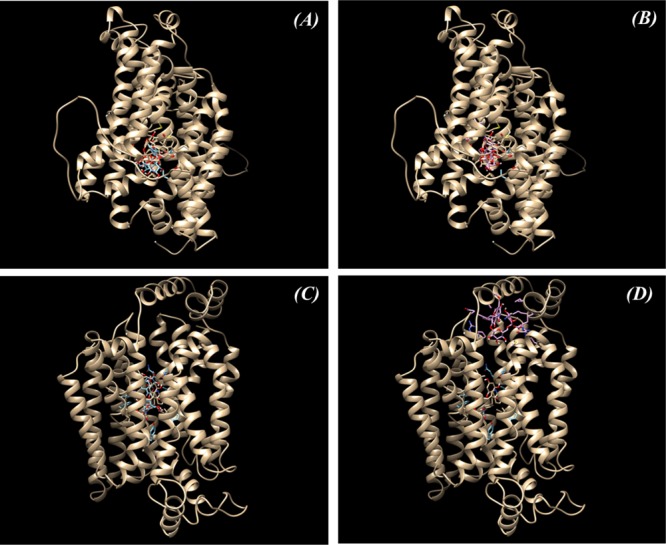
Molecular docking of SGLT1 and GLUT2 with inhibitors and cyanin/rutin: (A) Predicted binding sites in SGLT1 with phloridzin (inhibitor I) and cyanin; (B) predicted binding sites in SGLT1 with phloridzin (inhibitor I) and rutin; (C) predicted binding sites in GLUT2 with phloretin (inhibitor II) and cyanin; and (D) predicted binding sites in GLUT2 with phloretin (inhibitor II) and rutin.
Conclusions
In conclusion, the present study elucidated the different absorption mechanisms of aglycone flavonoids and their glycosides using a human intestinal epithelial cells model. Using pharmacological inhibitors (phloridzin and phloretin) and siRNA-generated SGLT1 deficient Caco-2 BBe1 cells, two intestinal hexose transporters, SGLT1 and GLUT2, were found to play a key role in the absorption of flavonoid glycosides in the GIT. Both SGLT1 and GLUT2 were more important for the cross-membrane transport of diglycosides than monoglycosides of quercetin and cyanidin. Our data also suggest that absorption of flavonoid aglycones is not by these transporters but is likely mediated by other mechanisms such as passive diffusion. The information collected through the in silico modeling/docking of transporters/ligands coupled with data obtained by blocking the activity of involved hexose transporters clearly demonstrates that the sugar moieties of the flavonoid glycosides are an essential part of the hexose transporter mechanism. The difference in absorption between the two sets of flavonoids also suggests that the structural feature (i.e. flavonols vs anthocyanidins) and the number and type of the sugar moieties attached to the flavonoid backbone can also have noticeable impact on the absorption, metabolism, and ultimately in the functionality of these compounds. By using novel techniques, we successfully demonstrated the critical and decisive role of intestinal hexose transporters in mediating the cellular uptake and transmembrane transport of flavonoid glycosides, hence their cellular bioavailability. This explains why and how flavonoid glycosides are also found in the plasma and other tissues in vivo. Also important is the finding that flavonoid glycosides are potent competitive inhibitors of glucose uptake initiated by hexose transporters. This provides a strong and promising support for dietary intervention for hyperglycemia and diabetes mellitus with glycosidic flavonoids. Consumption of flavonoid-rich diets, especially those rich in anthocyanins (glycosides) such as purple fruits, vegetables, and grains can contribute to the maintenance of healthy cellular glucose hemostasis, thereby reducing the risks of developing metabolic disorders. Considering that these flavonoids also possess strong antioxidant and anti-inflammatory activities, flavonoid-rich foods can be a significant part of the managing strategy for many chronic diseases.
Materials and Methods
Chemicals and Reagents
All materials including cyanidin, cyanidin 3-O-glucoside (C-3-G), cyanin (cyanidin 3,5-diglucoside), quercetin, quercetin 3-β-D-glucoside (Q-3-G), and rutin (quercetin 3-rutinoside) were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Sodium acetate, ferric chloride hexahydrate, sodium phosphate monobasic, sodium phosphate dibasic, and HPLC-grade solvents, including methanol, formic acid (FA), and hydrochloric acid (HCl) were all purchased from EMD Chemicals (Gibbstown, NJ, USA), VWR (Westchester, PA, USA) and Caledon Laboratories Ltd. (Georgetown, ON, Canada) unless otherwise specified. Western blotting was carried out using the following antibodies: anti-SGLT1 (ab14686) (Abcam Inc., Toronto, ON, Canada) and CST-β-actin (13 × 105) (Cell Signaling Technology, Inc., Danvers, MA).
Cell Culture
Caco-2 BBe1 cells were obtained from the American Type Culture Collection (ATCC). Cells were seeded between the 50th to 60th passages onto the polyester (PET) membrane permeable support inserts (6.5 mm, 0.4 μM pore size, Corning Inc., Corning, NY, USA) at a cell density of 3 × 104 cells/well and grown in the DMEM high glucose medium (Thermo Fisher Scientific, Mississauga, ON, Canada) supplemented with 10% (v/v) FBS, 50 U/mL penicillin–streptomycin, 25 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES; Thermo Fisher Scientific), 1 mM sodium pyruvate with nonessential amino acids (Thermo Fisher Scientific), and 0.01 μg/ml Human Transferrin (Sigma-Aldrich) at 37 °C in a humidity-controlled 5% CO2 cell culture incubator. The culture medium was changed every second day until the cells became confluent monolayer/polarized. Only monolayers displaying transepithelial electrical resistance (TEER) values greater than 400 Ω cm2 on the 9th postseeding day were incorporated in actual experiments. TEER values were measured after equilibrating the cells with HBSS for 30 min at 37 °C using a Millicell-ERS Volt-Ohm Meter (Millipore, Bedford, MA).
Flavonoids Transport and Absorption
The transportation and absorption experiments were carried out by following a previously described procedure.24 After washing the polarized and differentiated cells with warm HBSS, the flavonoids (50 μM equilibrated into 0.5 mL of HBSS, pH 6.4 for 30 min at 37 °C) were applied to the apical compartment of each well in which Caco-2 BBe1 cells were cultured and incubated up to 4 h. One mL of HBSS solution (containing 10 mM HEPES, pH 7.4) was added to the basolateral compartment. The transepithelial transport of flavonoids was subsequently assessed at 30 min, 2, and 4 h time intervals in order to determine the metabolic kinetics of glycosylated and aglycone flavonoids. An equal volume of methanol containing 2% FA was added to the collected solutions obtained from the basolateral compartment. This mixture was then applied to the equilibrium Strata-X polymeric solid-phase extraction (SPE) cartridges (30 mg, Phenomenex, Torrance, CA, USA) and subsequently collected the methanol containing 1% FA-eluted purified samples according to the manufacturer’s instructions after washing with water. The eluted samples were later evaporated to dryness for subsequent HPLC analysis (described below). After removing the cell culture media from each individual insert, 500 μL of cold PBS was added to each insert before extracting and tracking the cellular uptake of flavonoids. The detached cells were transferred to a 1.5 mL tube(s) and homogenized for 1 min by brief sonication (Qsonica Sonicator Q500, Fisher Scientific). A short centrifugation at 2000g for 5 min followed by the addition of 500 μL of methanol (2% FA) was conducted in order to completely extract intracellular flavonoids. The supernatant was collected and cleaned up in a similar fashion to the procedures used for the transepithelial samples before subjecting to HPLC analysis. The transport efficiency was calculated as the percentage of flavonoids detected in the basolateral side/compartment of the cells over the original amount added to the apical side of the cells/inserts.
RNA Interference and Inhibition of Flavonoid Uptake and Transport
After inducing the RNA interference treatment for 48 h (see Section siRNA Transfections), transfected Caco-BBe1 cells were treated with the above-mentioned flavonoids to identify the involvement of SGLT1 in flavonoid uptake. After a 4 h incubation period, the flavonoids that were added previously to the apical buffer were removed from each well. Cold PBS was carefully and gently added into each well to wash cells. After washing, cell membranes were detached using 0.5 mL PBS and were collected into a 1.5 mL tube to extract intracellular flavonoids. The extraction was carried out exactly as described above. Inhibition studies were also carried out using the inhibitor phloridzin in order to investigate the involvement of hexose transporters in the transportation of flavonoids in Caco-2 BBe1 cells. One μM of phloridzin was used to pretreat Caco-2 BBe1 cells, which were incubated for at least 30 min before adding any of the flavonoids.
High Performance Liquid Chromatography (HPLC) Analyses of Transported and Intracellular Flavonoids
The flavonoids transported by intestinal epithelial cells were analyzed by an Agilent 1100 series HPLC system consisting of an autosampler, a degasser, a quaternary pump, and a diode array detector (DAD) as previously described.24 The flavonoids were separated on a Phenomenex Luna phenyl-hexyl column (5 μm, 250 × 4.6 mm) (Phenomenex Inc., Torrance, CA, USA). A binary mobile phase consisting of 5% FA in water (v/v) (solvent A) and 95% methanol mixed with 5% acetonitrile (v/v) (solvent B) was used. The solvent gradient was 0–20 min, 0–60% B; 20–25 min, 60–100% B; 25–27 min, 100% B; and 27–33.5 min, 100–0% B. Peaks were monitored at 360 and 520 nm. Quantification of flavonoid compounds was performed with external standards of cyanidin, cyanidin 3-O-glucoside, cyanin, quercetin, Q-3-G, and rutin, using linear curves generated from solutions containing predefined concentrations (between 0 and 50 μM). Data analysis was conducted using the provided Agilent ChemStation software.
Cellular Glucose Uptake Assays
Glucose uptake was measured using the commercial glucose uptake cell-based assay (Cayman, Ann Arbor, MI, USA) according to the manufacturer’s instructions. Caco-2 BBe1 cells were seeded at 1 × 105 cells per well in 96-well black/clear flat bottom plates (Corning, Costar) and grown for overnight. After washing with PBS, cells were pretreated with 50 μM of each of the above-mentioned flavonoids (individually) in 100 μL of glucose-free DMEM and incubated for 2 h before adding 100 μL of glucose-free DMEM containing 300 μg/mL 2-deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-D-glucose) (2-NBDG), which was incubated for another 2 h. After the cells were washed with PBS, the fluorescence intensity was measured using a fluorescence spectrophotometer PLX800 (Bio-Tek Inc., Winooski, VT, USA) at an excitation wavelength of 485 nm and an emission wavelength of 535 nm.
Molecular Modeling and Computational Docking
Three dimensional (3D) molecular models of the full-length Homo sapiens glucose transporter 2 (GLUT2) and H. sapiens sodium/glucose cotransporter 1 (SGLT1) were constructed as described previously.24 The optimized 3D structures of cyanidin ((PubChem CID: 128861), cyanidin 3-O-glucoside (PubChem CID: 12303203), cyanin (PubChem CID: 441688), quercetin (PubChem CID: 5280343), Q-3-G (PubChem CID: 25203368), and rutin ((PubChem CID: 5280805) were all obtained from the PubChem (https://pubchem.ncbi.nlm.nih.gov/) database of chemical molecules maintained by the National Center for Biotechnology Information (NCBI). The docking of the above-mentioned flavonoids with the established models of GLUT2 and SGLT1 transporters was conducted and refined by PATCHDOCK48 and was completed through the visual inspection of generated transporter/flavonoids complexes by PyMol,49 Deep View,50 and/or UCSF Chimera 1.12.51
siRNA Transfections
To knockdown SGLT1 (SLC5A1) levels/expression, Caco-2 BBe1 cells were transiently transfected with three different human SGLT1 small interfering RNA oligos (#116833, #8138, and #106056) commercially obtained from Ambion (Thermo Fisher Scientific). Scrambled siRNA (# 1027310) was used as a negative control (QIAGEN, Germantown, MD) in these experiments. Fifty nM of siRNA was dissolved in 200 μL of Opti-MEM and mixed with the Attractene transfection reagent (QIAGEN) at 1:1 (v/v) according to the manufacturer’s instructions. After 20 min incubation at room temperature, the transfection mixture was dispensed into the 24-well plates. An equal volume of Opti-MEM containing 5% FBS and seed cells at 2 × 106 cells/mL was added to each well. After 48 h post-transfection, transfected Caco-2 BBe1 cells were treated with the flavonoids.
RNA Extraction and RT-PCR
Total RNA was extracted from small intestine tissues obtained from C57BL/6 mice (Charles River Laboratories, QC, Canada) or Caco-2 BBe1 cells using the PureLink RNA Mini Kit (Thermo Scientific, Wilmington, DE) according to the manufacturer’s instructions. Extracted RNA samples were quantified using a NanoDrop ND-1000 spectrophotometer (Thermo Scienfitic, Wilmington, DE). RNA (100 ng) was reverse transcribed using a qScript cDNA Synthesis Kit (Quanta Biosciences, Inc., Gaithersburg, MD), and real-time quantitative PCR was carried out using a MyiQ Single Color RT-PCR detection system (Bio-Rad, Laboratories, Inc.). The sequence of primers is listed in Table S1. Relative gene expression was calculated using the 2–ΔΔCt method using GAPDH in cells and 18S in tissues as the reference gene. Results are presented as fold expression change relative to positive control (PC) group.
Western Blotting
Following the siRNA treatment, cells were washed twice with 1 mL of cold PBS and lysed in 100 μL of ice-cold radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher) containing Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher). The Western blot analysis was carried out using the following procedure described previously52 with slight modifications. Cell lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad). Membranes were incubated with 10 mL of 5% bovine serum albumin (BSA) Tris-buffered saline with Tween 20 (TBST) containing primary antibody at 1:1000–1:2000 (v/v) dilution for overnight at 4 °C after blocking with 5% skim milk. Detection was carried out using 10 mL of HRP-conjugated antimouse or antirabbit IgG (Promega, Madison, MI, USA) at a dilution rate of 1:10,000 (v/v) and the ECL Western Blotting Detection Reagent (GE Healthcare, Mississauga, ON, Canada). After visualized using the ChemiDoc system (Bio-Rad Laboratories Ltd., Mississauga, ON, Canada), the density of acquired bands was measured using Image J (Image Processing and Analysis in Java, National Institutes of Health, http://rsbweb.nih.gov/ij/).
Statistical Analysis
Results were expressed as mean ± standard error of the mean (SEM) of at least three measurements unless otherwise specified. Statistical analyses were carried out using GraphPad (San Diego, CA, USA). Significant differences between two independent groups were calculated by the unpaired Student’s t-test. Significant differences between multiple comparisons were determined using the two-way or one-way ANOVA followed by Tukey’s multiple-comparison tests with p < 0.05 set as the threshold of significance.
Acknowledgments
This project was supported by the A-base funding of Agriculture & Agri-Food Canada (Project # J-001322.001.04, #J-002252.001.04 and J-000991.001.33). This work was also partially supported by the Natural Science Foundation of Jilin Province of China (# 20180414028GH).
Glossary
Abbreviations
- GIT
gastrointestinal track
- C-3-G
cyanidin-3-glucoside
- Q-3-G
quercetin-3-glucoside
- HBSS
hank’s balanced salt solution
- FBS
foetal bovine serum
- PBS
phosphate buffer solution
- siRNA
short interfering RNA
- SGLT1
sodium-dependent glucose transporter 1
- GLUT2
glucose transporter 2
- Scr
scramble
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00379.
Expression levels of LPH, 3D structures of flavonoids and SGLT1 and GLUT2, predicted binding/docking sites of flavonoids within SGLT1 (A) and GLUT2 (B), and topographic presentation of the diglycosides (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Tsuda T. Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. 10.1002/mnfr.201100526. [DOI] [PubMed] [Google Scholar]
- Cooke D.; Steward W. P.; Gescher A. J.; Marczylo T. Anthocyans from fruits and vegetables–does bright colour signal cancer chemopreventive activity?. Eur. J. Cancer 2005, 41, 1931–1940. 10.1016/j.ejca.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Hertog M. G. L.; Feskens E. J. M.; Kromhout D.; Hertog M. G. L.; Hollman P. C. H.; Hertog M. G. L.; Katan M. B. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- Hertog M. G. L. Epidemiological evidence on potential health properties of flavonoids. Proc. Nutr. Soc. 1996, 55, 385–397. 10.1079/pns19960037. [DOI] [PubMed] [Google Scholar]
- Hollman P. C. H.; Katan M. B. Dietary flavonoids: intake, health effects and bioavailability. Food Chem. Toxicol. 1999, 37, 937–942. 10.1016/s0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- Vinson J. A.; Dabbagh Y. A.; Serry M. M.; Jang J. Plant flavonoids, especially tea flavonols, are powerful antioxidants using an in vitro oxidation model for heart disease. J. Agric. Food Chem. 1995, 43, 2800–2802. 10.1021/jf00059a005. [DOI] [Google Scholar]
- Ross J. A.; Kasum C. M. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- He J.; Giusti M. M. Anthocyanins: natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- Del Rio D.; Rodriguez-Mateos A.; Spencer J. P. E.; Tognolini M.; Borges G.; Crozier A. Dietary (poly) phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signaling 2013, 18, 1818–1892. 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H.; Inaba H.; Kishi M.; Tominaga S.; Hirayama M.; Tsuda T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J. Agric. Food Chem. 2001, 49, 1546–1551. 10.1021/jf001246q. [DOI] [PubMed] [Google Scholar]
- Gonzales G. B. In vitro bioavailability and cellular bioactivity studies of flavonoids and flavonoid-rich plant extracts: questions, considerations and future perspectives. Proc. Nutr. Soc. 2017, 76, 175–181. 10.1017/s0029665116002858. [DOI] [PubMed] [Google Scholar]
- TROELSEN J. T.; MITCHELMORE C.; Spodsberg N.; Jensen A. M.; Norén O.; Sjöström H. Regulation of lactase–phlorizin hydrolase gene expression by the caudal-related homoeodomain protein Cdx-2. Biochem. J. 1997, 322, 833–838. 10.1042/bj3220833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Song J.; Shi X.; Miao S.; Li Y.; Wen A. Absorption and metabolism characteristics of rutin in Caco-2 cells. Int. J. Sci. World 2013, 2013, 382350. 10.1155/2013/382350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S.; Jiang W.; Yin T.; Hu M. Highly variable contents of phenolics in St. John’s Wort products affect their transport in the human intestinal Caco-2 cell model: pharmaceutical and biopharmaceutical rationale for product standardization. J. Agric. Food Chem. 2010, 58, 6650–6659. 10.1021/jf904459u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andlauer W.; Stumpf C.; Fürst P. Intestinal absorption of rutin in free and conjugated forms11Abbreviations: ESI–, electrospray ionization. Biochem. Pharmacol. 2001, 62, 369–374. 10.1016/s0006-2952(01)00638-4. [DOI] [PubMed] [Google Scholar]
- Chen I.-L.; Tsai Y.-J.; Huang C.-M.; Tsai T.-H. Lymphatic absorption of quercetin and rutin in rat and their pharmacokinetics in systemic plasma. J. Agric. Food Chem. 2010, 58, 546–551. 10.1021/jf9026124. [DOI] [PubMed] [Google Scholar]
- Chen M.; Zhang X.; Wang H.; Lin B.; Wang S.; Hu G. Determination of rutin in rat plasma by ultra performance liquid chromatography tandem mass spectrometry and application to pharmacokinetic study. J. Chromatogr. Sci. 2015, 53, 519–525. 10.1093/chromsci/bmu078. [DOI] [PubMed] [Google Scholar]
- Wang H. C.; Bao Y. R.; Wang S.; Li T. J.; Meng X. S. Simultaneous determination of eight bioactive components of Cirsium setosum flavonoids in rat plasma using triple quadrupole LC/MS and its application to a pharmacokinetic study. Biomed. Chromatogr. 2019, 33, e4632 10.1002/bmc.4632. [DOI] [PubMed] [Google Scholar]
- Manach C.; Morand C.; Demigné C.; Texier O.; Régérat F.; Rémésy C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997, 409, 12–16. 10.1016/s0014-5793(97)00467-5. [DOI] [PubMed] [Google Scholar]
- Juśkiewicz J.; Milala J.; Jurgoński A.; Król B.; Zduńczyk Z. Consumption of polyphenol concentrate with dietary fructo-oligosaccharides enhances cecal metabolism of quercetin glycosides in rats. Nutrition 2011, 27, 351–357. 10.1016/j.nut.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Röder P. V.; Geillinger K. E.; Zietek T. S.; Thorens B.; Koepsell H.; Daniel H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One 2014, 9, e89977 10.1371/journal.pone.0089977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano S.; Williamson G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol. Nutr. Food Res. 2010, 54, 1773–1780. 10.1002/mnfr.201000019. [DOI] [PubMed] [Google Scholar]
- Blaschek W. Natural products as lead compounds for sodium glucose cotransporter (SGLT) inhibitors. Planta Med. 2017, 83, 985–993. 10.1055/s-0043-106050. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Hassan Y. I.; Renaud J.; Liu R.; Yang C.; Sun Y.; Tsao R. Bioaccessibility, bioavailability, and anti-inflammatory effects of anthocyanins from purple root vegetables using mono-and co-culture cell models. Mol. Nutr. Food Res. 2017, 61, 1600928. 10.1002/mnfr.201600928. [DOI] [PubMed] [Google Scholar]
- Németh K.; Plumb G. W.; Berrin J.-G.; Juge N.; Jacob R.; Naim H. Y.; Williamson G.; Swallow D. M.; Kroon P. A. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. 10.1007/s00394-003-0397-3. [DOI] [PubMed] [Google Scholar]
- Hur S. J.; Lim B. O.; Decker E. A.; McClements D. J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. 10.1016/j.foodchem.2010.08.036. [DOI] [Google Scholar]
- Delie F.; Rubas W. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: advantages and limitations of the Caco-2 model. Crit. Rev. Ther. Drug Carrier Syst. 1997, 14, 221–286. 10.1615/critrevtherdrugcarriersyst.v14.i3.20. [DOI] [PubMed] [Google Scholar]
- Peterson M. D.; Mooseker M. S. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J. Cell Sci. 1992, 102, 581–600. [DOI] [PubMed] [Google Scholar]
- Gupta V.; Doshi N.; Mitragotri S. Permeation of insulin, calcitonin and exenatide across Caco-2 monolayers: measurement using a rapid, 3-day system. PLoS One 2013, 8, e57136 10.1371/journal.pone.0057136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- Day A. J.; DuPont M. S.; Ridley S.; Rhodes M.; Rhodes M. J. C.; Morgan M. R. A.; Williamson G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver β-glucosidase activity. FEBS Lett. 1998, 436, 71–75. 10.1016/s0014-5793(98)01101-6. [DOI] [PubMed] [Google Scholar]
- Day A. J.; Gee J. M.; DuPont M. S.; Johnson I. T.; Williamson G. Absorption of quercetin-3-glucoside and quercetin-4′-glucoside in the rat small intestine: the role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem. Pharmacol. 2003, 65, 1199–1206. 10.1016/s0006-2952(03)00039-x. [DOI] [PubMed] [Google Scholar]
- Yang N. J.; Hinner M. J., Getting across the cell membrane: an overview for small molecules, peptides, and proteins. Site-Specific Protein Labeling; Springer, 2015; pp 29–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopoldini M.; Russo N.; Toscano M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. 10.1016/j.foodchem.2010.08.012. [DOI] [Google Scholar]
- Manach C.; Scalbert A.; Morand C.; Rémésy C.; Jiménez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Passamonti S.; Vrhovsek U.; Vanzo A.; Mattivi F. The stomach as a site for anthocyanins absorption from food 1. FEBS Lett. 2003, 544, 210–213. 10.1016/s0014-5793(03)00504-0. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Pandey A. K., Chemistry and biological activities of flavonoids: an overview. J. Sci. World 2013,2013. 10.1155/2013/162750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell K. D. R.; Brown N. M.; Desai P.; Zimmer-Nechemias L.; Wolfe B. E.; Brashear W. T.; Kirschner A. S.; Cassidy A.; Heubi J. E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001, 131, 1362S–1375S. 10.1093/jn/131.4.1362s. [DOI] [PubMed] [Google Scholar]
- Saarinen N. M.; Smeds A.; Mäkelä S. I.; Ämmälä J.; Hakala K.; Pihlava J.-M.; Ryhänen E.-L.; Sjöholm R.; Santti R. Structural determinants of plant lignans for the formation of enterolactone in vivo. J. Chromatogr. B 2002, 777, 311–319. 10.1016/s1570-0232(02)00339-2. [DOI] [PubMed] [Google Scholar]
- Cermak R.; Landgraf S.; Wolffram S. Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br. J. Nutr. 2004, 91, 849–855. 10.1079/bjn20041128. [DOI] [PubMed] [Google Scholar]
- Mahraoui L.; Rodolosse A.; Barbat A.; Dussaulx E.; Zweibaum A.; Rousset M.; Brot-Laroche E. Presence and differential expression of SGLT1, GLUT1, GLUT2, GLUT3 and GLUT5 hexose-transporter mRNAs in Caco-2 cell clones in relation to cell growth and glucose consumption. Biochem. J. 1994, 298, 629–633. 10.1042/bj2980629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T.; Fujikura K.; Koyama H.; Matsuzaki T.; Takahashi Y.; Takata K. The apical localization of SGLT1 glucose transporter is determined by the short amino acid sequence in its N-terminal domain. Eur. J. Cell Biol. 2001, 80, 765–774. 10.1078/0171-9335-00204. [DOI] [PubMed] [Google Scholar]
- Johnston K.; Sharp P.; Clifford M.; Morgan L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett. 2005, 579, 1653–1657. 10.1016/j.febslet.2004.12.099. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz J. R. L.; Lewis N. G.; Ronald Kahn C.; Roth J. Phlorizin: a review. Diabetes/Metab. Res. Rev. 2005, 21, 31–38. 10.1002/dmrr.532. [DOI] [PubMed] [Google Scholar]
- Zou T.-B.; Feng D.; Song G.; Li H.-W.; Tang H.-W.; Ling W.-H. The role of sodium-dependent glucose transporter 1 and glucose transporter 2 in the absorption of cyanidin-3-O-β-glucoside in Caco-2 cells. Nutrients 2014, 6, 4165–4177. 10.3390/nu6104165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon O.; Eck P.; Chen S.; Corpe C. P.; Lee J. H.; Kruhlak M.; Levine M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. 10.1096/fj.06-6620com. [DOI] [PubMed] [Google Scholar]
- Lechanteur A.; Almeida A.; Sarmento B. Elucidation of the impact of cell culture conditions of Caco-2 cell monolayer on barrier integrity and intestinal permeability. Eur. J. Pharm. Biopharm. 2017, 119, 137–141. 10.1016/j.ejpb.2017.06.013. [DOI] [PubMed] [Google Scholar]
- Schneidman-Duhovny D.; Inbar Y.; Nussinov R.; Wolfson H. J. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res. 2005, 33, W363–W367. 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger D.; de Groot B. L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput.-Aided Mol. Des. 2010, 24, 417–422. 10.1007/s10822-010-9352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N.; Peitsch M. C. SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Pettersen E. F.; Goddard T. D.; Huang C. C.; Couch G. S.; Greenblatt D. M.; Meng E. C.; Ferrin T. E. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Kovacs-Nolan J.; Kodera T.; Eto Y.; Mine Y. γ-Glutamyl cysteine and γ-glutamyl valine inhibit TNF-α signaling in intestinal epithelial cells and reduce inflammation in a mouse model of colitis via allosteric activation of the calcium-sensing receptor. Biochim. Biophys. Acta, Mol. Basis Dis. 2015, 1852, 792–804. 10.1016/j.bbadis.2014.12.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.