Abstract
Background:
Health care workers (HCWs) are at risk for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection.
Purpose:
To examine the burden of SARS-CoV-2, SARS-CoV-1, and Middle Eastern respiratory syndrome (MERS)-CoV on HCWs and risk factors for infection, using rapid and living review methods.
Data Sources:
Multiple electronic databases including the WHO Database of Publications on Coronavirus Disease and medRxiv preprint server (2003 through 27 March 2020, with ongoing surveillance through 24 April 2020), and reference lists.
Study Selection:
Studies published in any language reporting incidence of or outcomes associated with coronavirus infections in HCWs and studies on the association between risk factors (demographic characteristics, role, exposures, environmental and administrative factors, and personal protective equipment [PPE] use) and HCW infections. New evidence will be incorporated on an ongoing basis by using living review methods.
Data Extraction:
One reviewer abstracted data and assessed methodological limitations; verification was done by a second reviewer.
Data Synthesis:
64 studies met inclusion criteria; 43 studies addressed burden of HCW infections (15 on SARS-CoV-2), and 34 studies addressed risk factors (3 on SARS-CoV-2). Health care workers accounted for a significant proportion of coronavirus infections and may experience particularly high infection incidence after unprotected exposures. Illness severity was lower than in non-HCWs. Depression, anxiety, and psychological distress were common in HCWs during the coronavirus disease 2019 outbreak. The strongest evidence on risk factors was on PPE use and decreased infection risk. The association was most consistent for masks but was also observed for gloves, gowns, eye protection, and handwashing; evidence suggested a dose–response relationship. No study evaluated PPE reuse. Certain exposures (such as involvement in intubations, direct patient contact, or contact with bodily secretions) were associated with increased infection risk. Infection control training was associated with decreased risk.
Limitation:
There were few studies on risk factors for SARS-CoV-2, the studies had methodological limitations, and streamlined rapid review methods were used.
Conclusion:
Health care workers experience significant burdens from coronavirus infections, including SARS-CoV-2. Use of PPE and infection control training are associated with decreased infection risk, and certain exposures are associated with increased risk.
Primary Funding Source:
World Health Organization.
This rapid and living review examines the epidemiology and risk factors for coronavirus infection in health care workers.
A cluster of pneumonia cases in Wuhan, China, was first reported to the World Health Organization (WHO) on 31 December 2019 (1). The cause was identified as the novel coronavirus SARS-CoV-2 (2–4), and the disease was named “coronavirus disease 2019” (COVID-2019) (5).
Health care workers (HCWs) are at risk for SARS-CoV-2 infection (6), and reports have described COVID-19 cases in HCWs since early in the outbreak (7). Preventing HCW infections is important for reducing morbidity and potential mortality, maintaining health system capacity, and reducing secondary transmission (8, 9).
This rapid review summarizes the evidence on the burden of and risk factors for SARS-CoV-2 infections in HCWs. The report will be used by WHO to inform the development of evidence-based guidance. Because evidence is limited on SARS-CoV-2, this review also includes 2 coronaviruses associated with earlier pneumonia outbreaks: SARS-CoV-1 (causing severe acute respiratory syndrome [SARS-1]) and MERS-CoV (causing Middle East respiratory syndrome [MERS]).
Methods
Detailed methods are available in the full report (10). The key questions were developed by WHO with input from the review authors.
Key Question 1. What is the burden of SARS-CoV-2, SARS-CoV-1, and MERS-CoV on HCWs and how do burdens vary according to age, sex, and presence of comorbidities?
Key Question 2. What are the risk factors for HCW infections with SARS-CoV-2, SARS-CoV-1, and MERS-CoV?
Key Question 3. What are the risk factors for household transmission of SARS-CoV-2, SARS-CoV-1, and MERS-CoV from HCWs?
Because of the urgent and ongoing need to support WHO's pandemic response, a rapid, living review approach was used (11). Rapid reviews utilize streamlined systematic review processes. For this review, modified methods included 1) protocol not posted to a systematic review registry; 2) a gray literature search limited to 1 website; 3) dual review of 25% of abstracts; 4) critical appraisal not conducted using a formal instrument; and 5) single-reviewer assessment of study limitations and data abstraction, with second reviewer verification. Living reviews use methods for continual updating, as new evidence becomes available.(12)
Data Sources and Searches
A medical librarian searched PubMed, MEDLINE, and Elsevier Embase (from 2003 through 27 March 2020). Searches had no language restrictions. Search strategies are shown in Appendix Table 1 (all Appendix Tables are available at Annals.org). We also searched the WHO Database on Coronavirus Disease (13) and the medRxiv preprint server (14) and reviewed reference lists. Daily MEDLINE surveillance and weekly surveillance on EMBASE, the WHO Database on Coronavirus Disease, and the medRxiv server is ongoing; this article includes surveillance through 24 April 2020.
Study Selection
Studies were selected by using predefined criteria (Appendix Table 2, available at Annals.org). The population was HCWs at risk for or with SARS-CoV-2, SARS-CoV-1, or MERS-CoV infection. For key question 1, for SARS-CoV-2, we included cohort studies and case series on incidence and severity of infection, mortality, morbidity (including mental health outcomes), and effects on family members and contacts. For SARS-CoV-1 and MERS-CoV, inclusion was restricted to cohort studies on incidence, infection severity, and mortality. For key question 2, potential risk factors were demographic characteristics, exposure history, administrative factors, health care setting/environmental factors, HCW health, and infection control and prevention factors. We included studies that reported risk estimates or infection incidence stratified by risk factor.
One investigator reviewed each citation for potential full-text review. A second investigator reviewed a 25% random sample of citations; disagreements were resolved through consensus. One investigator reviewed each full-text article for inclusion, and a second verified exclusion decisions. We included non–peer-reviewed articles for SARS-CoV-2 because the peer-reviewed literature was sparse. Chinese-language articles were translated by a review team member who was a native speaker.
Data Extraction
One investigator extracted study data into standardized tables and a second verified data: study author, year, setting (country, health care setting, dates), population characteristics (sample size, age, sex, HCW role/position, number of cases), and results. For key question 2, odds ratios were calculated if necessary and the data were available.
Quality Assessment
We did not perform formal risk for bias assessment. Instead, we noted key limitations of each study, such as potential recall, selection, or participation bias; issues regarding evaluation of outcomes and analytic methods; and confounding (15, 16).
Data Synthesis and Analysis
Results were synthesized narratively. For key question 2, unadjusted and adjusted risk estimates were presented. Quantitative synthesis was not possible owing to methodological limitations; study design variability; and heterogeneity in populations, comparisons, and analytic methods.
Living Review
Surveillance for new studies is ongoing, and study selection and quality assessment will follow the same processes described. New evidence that does not substantively change review conclusions will be briefly summarized on a monthly basis; a major update will be performed when new evidence changes the nature or strength of the conclusions.
Role of the Funding Source
The study was funded by the WHO. Staff at the WHO developed the key questions and review scope but did not have any role in the selection, assessment, or synthesis of evidence. The WHO was not involved in the decision to submit this article for publication.
Results
Sixty-four studies met inclusion criteria (17–48-49–80). The Appendix Figure summarizes the study selection process and number of included studies, by key question and coronavirus type.
Appendix Figure.
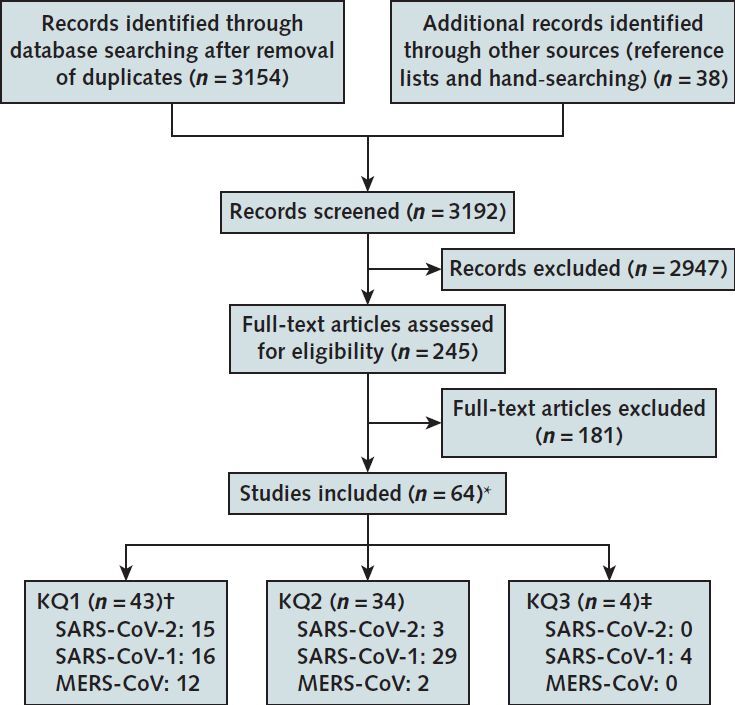
Literature search and selection.
CoV = coronavirus; KQ = key question; MERS = Middle East respiratory syndrome; SARS = severe acute respiratory syndrome.
* Some studies were included for multiple KQs; includes 6 studies that were not peer-reviewed (28, 39, 46, 47, 59, 79) and 3 Chinese-language studies translated into English (48, 52, 78).
† Data from 2 World Health Organization websites on the incidence of SARS-1 (81) and MERS (82) were also included.
‡ Included in the full evidence review (10).
Key Question 1: Burden of Coronavirus Infections on HCWs
SARS-CoV-2
One cohort study (61), 9 cross-sectional studies (28, 36, 39, 40, 46, 51, 59, 79, 80) and 5 case series (47, 48, 53, 67, 68) reported on the burden of SARS-CoV-2 in HCWs (Appendix Table 3).
Two non–peer-reviewed, retrospective cohort studies reported the proportion of exposed HCWs with polymerase chain reaction (PCR)–confirmed SARS-CoV-2 infection (39, 61). One study evaluated 1353 HCWs in the Netherlands with recent fever or mild respiratory symptoms. Infection with SARS-CoV-2 was present in 6.4% (86 of 1353) of the HCWs; 91.9% (79 of 86) of infections met the COVID-19 case definition. Two HCWs (3.7% [2 of 86]) were hospitalized, with no critical cases or deaths. A second, smaller study of 72 exposed HCWs with acute symptoms in Wuhan, China, reported a COVID-19 incidence of 38.9% (61).
Health care workers accounted for 3.8% (1716 cases) of 44 672 cases of COVID-19 (PCR-confirmed) diagnosed in China through 11 February 2020 (67). The proportion of HCW cases classified as severe or critical was 15% (247 of 1608), and the case-fatality rate was 0.3% (5 of 1716). Health care workers accounted for a higher proportion of cases from 11 to 20 January (5.7%), early in the outbreak when case numbers were increasing sharply. The proportion of cases that were severe or critical was highest from 1 to 10 January (45% [9 of 20]) and lowest after 1 February (8.7% [28 of 322]).
Another non–peer-reviewed study evaluated a large series of 25 961 patients with PCR-confirmed COVID-19 diagnosed in Wuhan, China, through 18 February 2020 (68). Health care workers accounted for 5.1% (1316 of 25 961) of cases. The overall estimated COVID-19 incidence, using epidemiologic data for denominators, was higher in HCWs than the general population (144.7 [95% CI, 137.0 to 152.8] vs. 41.7 [CI 41.2 to 42.2] per 106 people) (Appendix Table 3).
Three case series reported outcomes of COVID-19 infections in HCWs (47, 48, 53). Two separate series (50 and 64 HCWs) reported on infected HCWs in Wuhan, China (47, 48). The average age (35 years) and proportion female (~65%) were similar. In one study, one third of cases were physicians and two thirds were nurses; this was reversed in the other study. There were no deaths. In one study, 1.6% (1 of 64) of HCWs had severe illness not requiring mechanical ventilation (47). In the other study, 13.3% (4 of 30) met criteria for severe pneumonia and received noninvasive ventilation or nasal high-flow oxygen (48). A limitation of the studies is that 20% and 47% of cases remained hospitalized at outcome assessment. In addition, in 1 study, few cases (25% [7 of 30]) were PCR-confirmed (48). The third study found that 29% (50 of 167) of cases in a U.S. long-term care facility were HCWs (53). The median age was 43.5 years, and 76% were female. Six percent (3 of 50) of HCWs were hospitalized, with no deaths.
Seven cross-sectional studies (16 630 HCWs) evaluated the mental health or sleep quality of HCWs in China during the COVID-2019 outbreak (28, 36, 40, 46, 51, 59, 80). The proportion of HCWs meeting clinically relevant (that is, moderate or severe) thresholds was 14% to 15% for depression (40, 80), 12% to 24% for anxiety (40, 46, 80), 30% to 39% for psychological distress (28, 40, 80), 8% to 60% for sleep issues (40, 59), and 29% (36) for a composite mental health outcome. Female sex (28, 40, 80) and direct contact with cases (40, 46, 51, 80) were associated with increased likelihood of mental health issues; effect of HCW role on risk was inconsistent (28, 36, 80). Methodological limitations included no baseline symptom information, no non-HCW comparison groups, and not controlling for work exposures. One cross-sectional study (843 persons) found a high prevalence of anxiety (34%) and psychological distress (29%) in family members of HCWs (79).
No study reported the social or economic effects of SARS-CoV-2 infection in HCWs or the incidence of HCW transmission to close contacts.
SARS-CoV-1
Fourteen cohort studies (25, 30, 32–35, 43, 45, 50, 57, 60, 64, 69, 74), 1 cross-sectional study (27), and 1 case series (44) reported on the burden of SARS-CoV-1 in HCWs (Appendix Table 3). We also included WHO data (81).
The prevalence of SARS-CoV-1 seropositivity in exposed or potentially exposed HCWs ranged from 0.3% to 40% in 6 studies (25, 27, 33, 57, 60, 69), and SARS-1 incidence ranged from 1.2% to 29.4% in 14 studies (25, 30, 32–35, 43, 45, 50, 57, 60, 64, 69, 74). The highest SARS-1 incidence (29.4%) occurred in a large outbreak in Vietnam in a hospital without an isolation ward (57). In addition, infection control measures were not initiated owing to unawareness of the index SARS-1 case. Another study reporting high incidence focused on critical care nurses in Canada who cared for patients with SARS-1 with unstandardized PPE use, often before knowing patients' infection status (50).
Health care workers accounted for 21% (1706 of 8096) of all SARS-1 cases reported to WHO (Appendix Table 4). Among countries with at least 50 cases, HCWs accounted for 19% (China) to 57% (Vietnam). Among all (n = 1755) SARS-1 cases from Hong Kong, the case-fatality rate in HCWs was 2.0% (8 of 405), compared with 21.8% (294 of 1350) in non-HCWs (adjusted OR, 0.3 [CI, 0.1 to 0.7]) (Appendix Table 3) (44).
MERS-CoV
Seven cohort studies (18, 19, 21, 37, 38, 63, 71), 4 case series (17, 20, 22, 29), and 1 cross-sectional study (54) reported on the burden of MERS in HCWs (Appendix Table 3). We also utilized WHO data (82).
In 3 studies with at least 500 HCWs (3311 HCWs in total), the proportion with MERS-CoV infection ranged from 1.12% to 2.0% (21, 37, 54). In 5 smaller studies (9 to 283 HCWs), the proportion ranged from 0% to 7.1% (18, 19, 38, 63, 71).
As of December 2019, HCWs accounted for 19.1% (402 of 2106) of laboratory-confirmed cases of MERS in Saudi Arabia, which accounts for 84% of cases (Appendix Table 4) (82). Globally, among the 651 MERS cases diagnosed in July to December, 14% to 18% were HCWs in 2014 and 2015 and 0 to 4% in 2018 and 2019.
An analysis of all cases of MERS in HCWs reported to WHO found an overall case-fatality rate of 5.8% (24 of 415); excluding primary cases, mortality was slightly lower (4.7%) (29). These figures are lower than the overall MERS case-fatality rate (34.4%) (82). Two smaller case series (166 and 105 HCWs) reported HCW case-fatality rates of 3.0% and 16% (17, 20). Studies that directly compared MERS mortality in HCWs versus non-HCWs also reported lower mortality risk in HCWs (17, 20, 22). In the largest analysis (2260 HCWs), the adjusted OR was 0.07 (CI, 0.001 to 0.35) (22). Factors associated with increased mortality risk in HCWs are older age and presence of comorbid conditions (22, 29).
Key Question 2: Risk Factors for Coronavirus Infection in HCWs
SARS-CoV-2
Three retrospective cohort studies evaluated risk factors for COVID-19 in exposed HCWs (Appendix Table 5) (55, 61, 70). One study evaluated risk factors for COVID-19 in 72 exposed HCWs (clinicians and nurses) in Wuhan, China, who had acute symptoms (61). The median age was 31 years, and 69% of HCWs were female; PCR-confirmed COVID-19 occurred in 38.9% (28 of 72 HCWs). Risk factors were working in a high risk versus general department (relative risk [RR], 2.13 [CI, 1.45 to 3.95]), suboptimal handwashing before or after patient contact (RR, 3.10 [CI, 1.43 to 6.73] and 2.82 [CI, 1.11 to 7.18], respectively), longer work hours (log-rank P = 0.02), and improper PPE use (RR, 2.82 [CI, 1.11 to 7.18]). Such procedures as endotracheal tube removal, cardiopulmonary resuscitation, fiberoptic bronchoscopy, and sputum suction were not associated with increased risk. Having a diagnosed family member was associated with increased risk (RR, 2.76 [CI 2.02 to 3.77]), suggesting that some HCW infections may have been acquired outside the hospital. The study was susceptible to recall bias, it was unclear whether risk estimates were adjusted, and some estimates were imprecise.
Another study evaluated 41 HCWs exposed to a patient with COVID-19 and an aerosol-generating procedure for 10 or more minutes at a distance of 2 meters or less (55). Eighty-five percent of HCWs used a surgical mask, and 15% used an N95 respirator. No COVID-19 cases occurred; therefore, it was not possible to draw conclusions about effects of mask type. One other study reported a strong association between N95 respirator use and decreased COVID-19 risk, but had serious limitations (70). Mask use was based on the department worked (not on individual use), departments varied in other infection control measures (such as handwashing), and estimates were very imprecise.
SARS-CoV-1
Seventeen cohort studies (23, 25, 30, 32–35, 43, 45, 50, 57, 60, 64, 69, 72, 75, 77), 11 case–control studies (26, 41, 49, 52, 56, 58, 62, 65, 66, 76), and one cross-sectional study (27) evaluated risk factors for SARS-CoV-1 infection in HCWs (Appendix Table 5). Seven studies evaluated risk for SARS-CoV-1 seropositivity, not necessarily meeting the SARS-1 case definition (25–27, 33, 60, 69, 72). The remainder evaluated risk for SARS-1 meeting the case definition, usually with laboratory confirmation. Ten studies reported adjusted risk estimates from multivariate models (26, 41, 49, 52, 57, 58, 60, 66, 76, 78). Of these, 2 studies evaluated correlations between risk factors (for example, between use of different types of PPE) to inform variable selection for model building (49, 76). All studies except for 1 (32) were retrospective. The studies were limited in their ability to measure and control for the amount and intensity of exposures.
Age and Sex.
Six studies indicated no association between sex and risk for SARS-CoV-1 infection in HCWs (Appendix Table 6) (27, 56, 60, 66, 69). One study found no association between age and risk for SARS-CoV-1 infections after controlling for other factors (adjusted OR, 0.97 [CI, 0.90 to 1.03]) (57). Five other studies that did not control for confounders also found no association between age and risk for SARS-CoV-1 infection (27, 56, 60, 66).
Professional Profile.
Twelve studies reported SARS-CoV-2 infection incidence by HCW role (Appendix Table 6) (25, 27, 30, 32, 34, 43, 52, 56, 57, 60, 69). Infections occurred in HCWs across various clinical and nonclinical (including nonpatient contact) roles. There was no consistent difference in risk between nurses and physicians, the most commonly evaluated HCW roles, based on 12 studies (25, 27, 30, 32, 34, 43, 45, 52, 56, 57, 60, 69). There were too few studies and cases to determine risks for other HCW roles relative to nurses and physicians.
Exposure History.
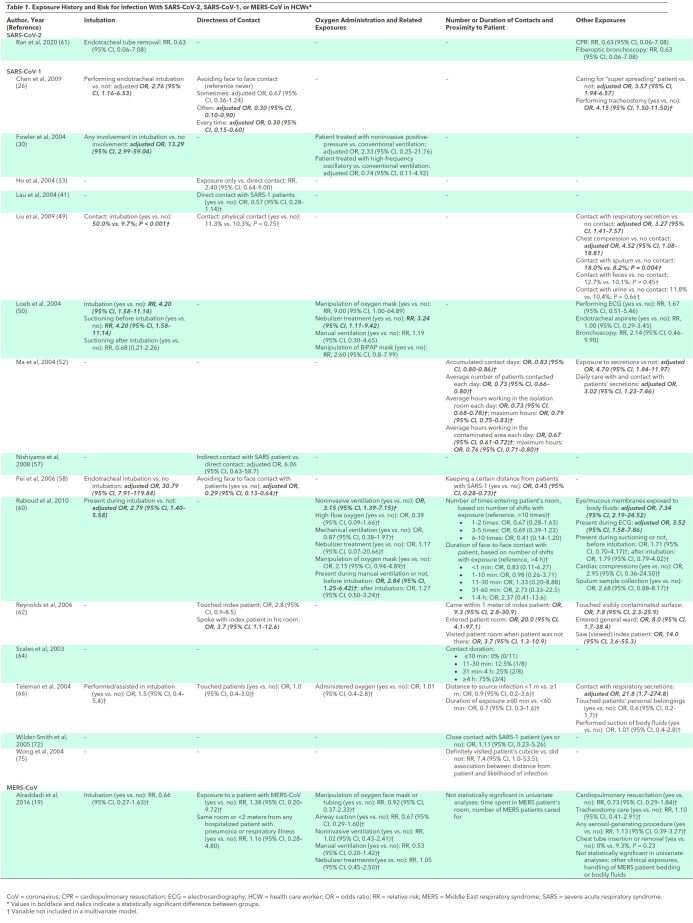
Exposure during endotracheal intubation was strongly and consistently associated with increased risk for HCW SARS-CoV-1 infections in 6 studies (Table 1) (26, 30, 49, 50, 58, 60). Of these, 4 studies found exposure during endotracheal intubation to be independently associated with risk (26, 30, 58, 60). One study (50) found oxygen mask manipulation to be associated with increased risk for infection in a univariate analysis, but 2 other studies (60, 66) found that oxygen mask manipulation or oxygen administration were not independent predictors. Few studies evaluated risks associated with other procedures involving oxygen administration, such as noninvasive positive-pressure ventilation (30, 50, 60), high-frequency oscillatory ventilation (30), nebulizer treatment (50, 60), manual ventilation (50), high-flow oxygen (60), or mechanical ventilation (60), and estimates were often imprecise. Other procedures associated with increased risk but only evaluated in 1 or 2 studies each were electrocardiography (50, 60), chest compressions (49, 60), and suctioning before intubation (50). In most studies, direct patient contact was associated with increased risk compared with less direct contact, though some inconsistency was present (26, 33, 41, 49, 57, 58, 62, 66, 72). Other exposures associated with increased risk for infections in HCWs were exposure of eyes or mucous membranes to patient bodily fluids (60, 64), contact with more severely ill patients (60), contact with a “super spreading” patient (26), closer proximity to infected patients (58, 62, 64, 75), and contact with respiratory secretions (49, 52). Evidence on the association between duration of contact with patients and risk for infection was inconsistent (52, 60, 64, 66).
Table 1. Exposure History and Risk for Infection With SARS-CoV-2, SARS-CoV-1, or MERS-CoV in HCWs*.
Administrative Factors.
One study found administrative measures (having a crisis response team, exclusion of visitors, or provision of administrative support) and PPE use policies (requiring N95 respirator in the emergency department, within certain hospital zones, or on entering the hospital) were not associated with risk for HCW infections (Appendix Table 7) (76). Another study (with the same lead author) found a lower incidence of HCW infections in a hospital in which an integrated infection control strategy was implemented compared with 86 control hospitals, but did not control for use of infection control measures or degree of SARS-1 exposure (77).
Health Care Setting and Environmental Factors.
One study of hospitals found installation of a fever screen station outside of the emergency department and alcohol dispensers for hand sanitation to be associated with decreased likelihood of HCW SARS-1 infections (adjusted OR, 0.05 [CI, 0.004 to 0.692] and 0.043 [CI, 0.003 to 0.63], respectively) (Appendix Table 7) (76). One study found a higher risk for infections in the emergency department compared with hospital wards (69), and 1 study reported HCW infections in multiple hospital departments (27). Natural air ventilation was associated with decreased risk for SARS-CoV-1 infection versus artificial ventilation in 1 study (adjusted OR, 0.40 [CI, 0.18 to 0.88]) (26); another study found a well-ventilated office to be associated with a non–statistically significant decreased risk (adjusted OR, 0.32 [CI, 0.09 to 1.15]) (58). One study attempted to assess physical aspects of the hospital ward and risk for SARS-1 infection in HCWs, but only evaluated 4 wards, with many confounding factors (35).
HCW Health.
Two studies found no association between presence of comorbid conditions in HCWs and SARS-CoV-1 infection risk (60, 66). One study found having an upper respiratory infection in the past 6 months to be associated with decreased risk for SARS-CoV-1 infection (62). Another study found an HCW history of to be diabetes associated with increased univariate risk for infection, but it was not an independent predictor (58).
Infection Prevention and Control Factors.
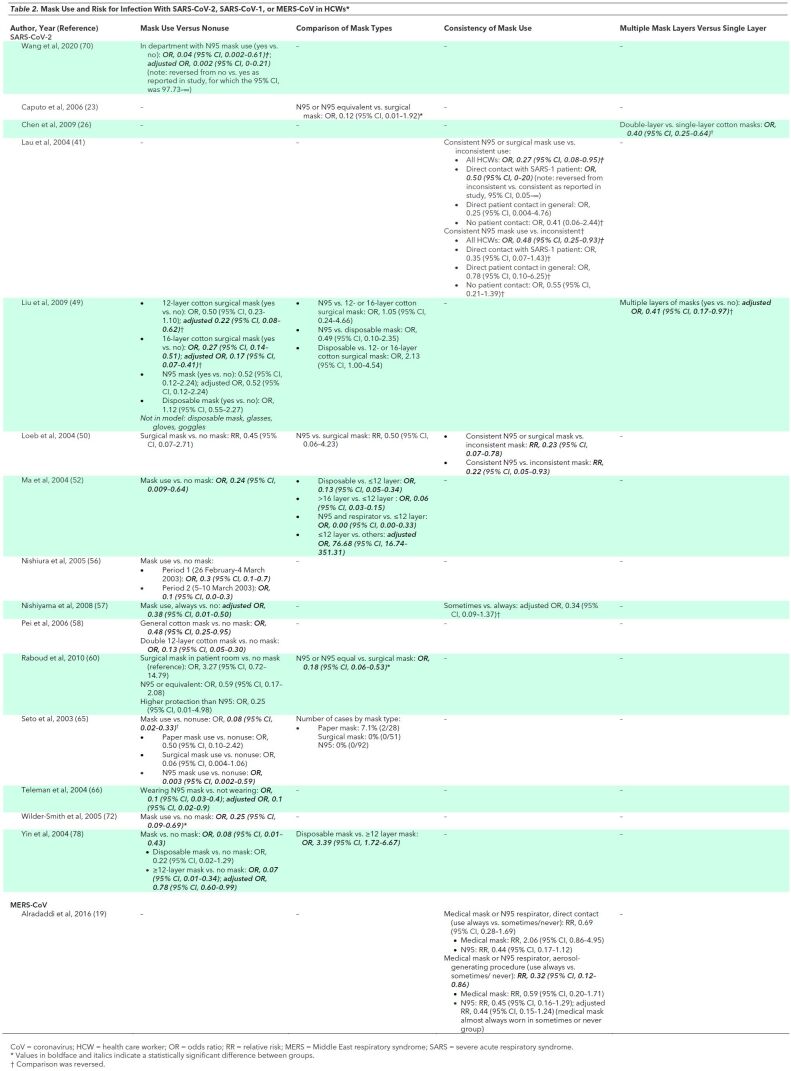
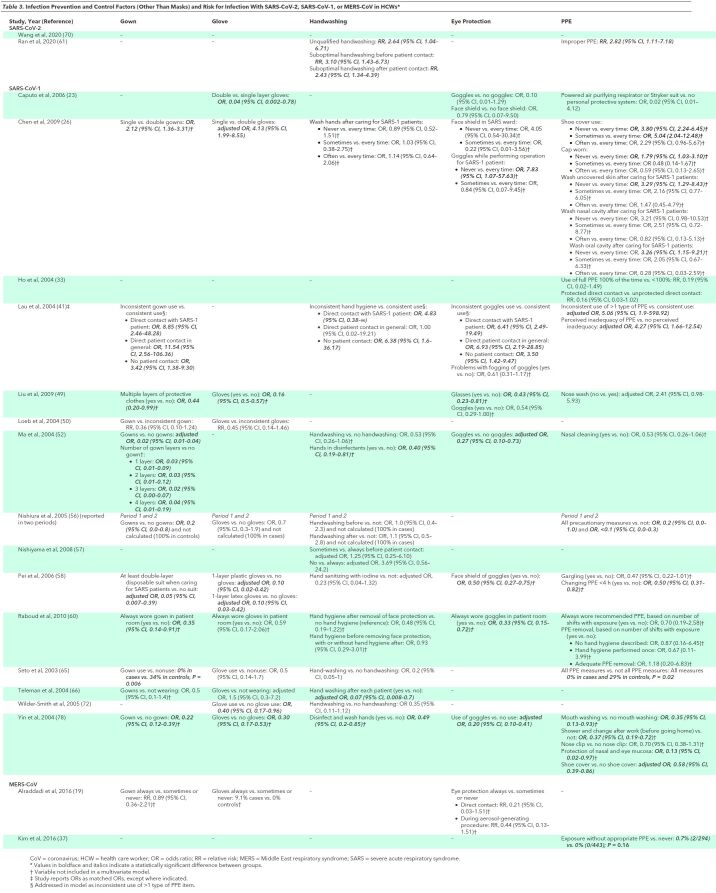
The most consistent and robust evidence on PPE measures was on the association between use of masks and decreased infection risk (Table 2>) (26, 41, 49, 50, 52, 56–58, 60, 65, 66, 72, 78). Four studies found N95 respirators to be associated with decreased risk versus surgical masks in unadjusted analyses (23, 49, 50, 60). Evidence was inconsistent on the effectiveness of multiple masks versus a single mask (26, 49). Most studies found an association between use of gloves (49, 50, 56, 58, 60, 65, 66, 72, 78), gowns (41, 50, 52, 56, 60, 65, 66, 78), eye protection (23, 26, 41, 49, 52, 58, 60, 78), or shoe covers (26, 78) and decreased risk for HCW infections (Table 3). In some studies, individual PPE measures were not included in multivariate models, but information on the degree of correlation between PPE measures was lacking. When evaluated as “inconsistent use of more than one type of PPE,” 1 study found a strong, independent association with increased risk for HCW infection (adjusted OR 5.06, 95% CI 5.06 to 598.92) (41). Studies also found full PPE use (gloves, mask, gown, and eye protection) to be associated with reduced infection risk versus partial PPE (33, 56, 65, 78); some studies found a dose–response relationship between more frequent or consistent PPE use and decreased risk (26, 33, 41, 78). Handwashing was associated with decreased risk for SARS-CoV-1 infection in most studies (41, 52, 56, 57, 65, 66, 72), but there was no association in others (26, 56), and handwashing was not included in some multivariate models (26, 52). Nasal washing was not independently associated with decreased risk for infection in HCWs in 3 studies (26, 49, 52). No study evaluated the association between reuse of PPE and infection risk. One study found perceived inadequacy of PPE supplies associated with increased risk for HCW infections (41). Infection control training and education were consistently associated with decreased infection risk, though this finding was not always retained in multivariate models (Table 3) (26, 41, 49, 57, 58).
Table 2. Mask Use and Risk for Infection With SARS-CoV-2, SARS-CoV-1, or MERS-CoV in HCWs*.
Table 3. Infection Prevention and Control Factors (Other Than Masks) and Risk for Infection With SARS-CoV-2, SARS-CoV-1, or MERS-CoV in HCWs*.
MERS-CoV
One retrospective cohort study of 283 HCWs at a Saudi Arabian hospital found participation in MERS-CoV training to be associated with decreased risk for MERS-CoV seropositivity (adjusted RR, 0.33 [CI 0.12 to 0.90]) (Appendix Table 7) (19). Cases occurred almost exclusively among HCWs with close contact with patients with MERS. Always using an N95 respirator was associated with a non–statistically significant decreased risk compared with some or no use (adjusted RR, 0.44 [CI, 0.15 to 1.24]). Past or current smoking was associated with a nonstatistically increased risk for infection.
Another study evaluated risk factors for MERS-CoV seropositivity in 737 HCWs who had direct contact with a patient with MERS in 31 hospitals in South Korea (37), but only reported 2 cases in HCWs (both of whom had not used appropriate PPE).
Key Question 3: Risk Factors for Transmission of Coronavirus Infection From HCWs
No study evaluated risk factors for transmission of coronavirus infections from HCWs to household or other close contacts. Four studies (24, 31, 42, 73) that did not evaluate risk factors for HCW transmission but compared SARS-CoV-1 transmission incidence from HCWs versus non-HCWs to household contacts are described in the full report (10).
Discussion
This rapid, living review summarizes the evidence on the burden of and risk factors for HCW coronavirus infections. Health care workers account for a significant proportion of infections in these outbreaks. Exposed HCWs may experience a high incidence of infections, particularly for unprotected and repeated exposures, though they appear to experience less severe illness and mortality than non-HCWs, possibly related to younger age and fewer comorbid conditions. Evidence that depression, anxiety, and psychological distress are common in HCWs in the COVID-19 outbreak is consistent with findings from the SARS-1 outbreak (83–90). Evidence on risk factors for coronavirus infections in HCWs is primarily available for SARS-CoV-1, with the strongest evidence indicating an association between PPE use versus nonuse and decreased risk. The association was most consistent for masks but was also observed for gloves, gowns, and eye protection, as well as handwashing. There was evidence that more consistent and full use of recommended PPE measures was associated with decreased risk for infection, suggesting a dose–response relationship, and evidence that N95 respirators might be associated with decreased risk for infection versus surgical masks. Evidence also indicated an association between certain exposures (such as involvement in intubations, direct contact with infected patients, or contact with bodily secretions) and increased infection risk. Education and training in infection control measures were consistently associated with decreased risk for HCW infections.
Our findings are generally consistent with prior reviews on risk factors for respiratory infections in HCWs, including PPE use (91–96). It differs from prior reviews by including recent evidence on risk factors (including those related to SARS-CoV-2 infections), focusing on coronavirus infections, excluding surrogate markers for transmission risk, evaluating a broader array of potential risk factors, and including a more comprehensive set of relevant studies. In addition, we implemented living review processes to incorporate new evidence on an ongoing basis.
The evidence base has important limitations. The evidence on SARS-CoV-2 infections in HCWs is sparse and has methodological limitations. Many studies on the burden of SARS-CoV-2 infections are case series and epidemiologic evaluations; evaluations of clinical cohorts of exposed HCWs are lacking. Studies on SARS-CoV-2 infections in HCWs that reported mental health or sleep outcomes used a cross-sectional design, did not control for baseline status, and did not include a non-HCW comparison group. Almost all studies on risk factors were retrospective and susceptible to recall bias with regard to PPE use and other factors. Some risk factor studies did not control for confounders, and those that did had limited ability to control for exposure intensity and frequency. Few studies that analyzed risk factors in multivariate models addressed collinearity (97), complicating interpretation for potentially correlated risk factors (for example, use of different types of PPE). Case–control studies did not match cases and controls on such factors as age, sex, or HCW role. Applicability of evidence on SARS-CoV-1 and MERS-CoV infections to SARS-CoV-2 is uncertain, owing to decreased transmission propensity, greater illness severity, or variability in affected populations. Most evidence on SARS-CoV-2 in HCWs is from China; studies from other settings, including those with decreased availability or use of infection prevention and control measures, are needed.
The review process had limitations, in particular the use of streamlined rapid review methods for searching and selecting studies. We did not assess study quality by using a formal instrument, though key methodological limitations were highlighted. We included non–peer-reviewed studies on SARS-CoV-2 infection in HCWs, given the lack of peer-reviewed literature, which may reduce data quality. Meta-analysis was not attempted owing to study limitations and heterogeneity in study designs, comparisons, and analyses.
Studies are needed to better understand the proportion of exposed HCWs who are infected with SARS-CoV-2 and associated outcomes, including economic effects; ability to work; social effects (for example, need for child care); and effects on family members and other close contacts, including transmission. Studies evaluating mental health and other outcomes should control for baseline status, include non-HCW controls, and incorporate longitudinal follow-up. Recovered HCWs require evaluation to understand outcomes over time (such as after return to work). For assessing SARS-CoV-2 infection risk factors, studies that prospectively measure exposures, PPE use, and other factors would increase measurement accuracy, reduce recall bias, and enable analyses that minimize confounding. Multivariate analyses of risk factors should account for potential collinearity. Given current limitations related to PPE supply, research on effects of PPE reuse is a priority (98). Studies are needed on the association between administrative factors, environmental factors, and HCW health and risk for HCW infections.
In conclusion, HCWs experience significant burdens from coronavirus infections, including SARS-CoV-2. Use of PPE and infection control training are associated with decreased infection risk and certain exposures are associated with increased risk. Research is urgently needed on optimal methods for reducing HCW risk for SARS-CoV-2 infections.
Biography
Disclaimer: This article is the work of the authors and does not represent the views or position of the World Health Organization.
Acknowledgment: The authors thanks Susan L. Norris, MD, MPH, for her role in the development of the Key Questions and scope of this rapid review.
Funding: By the World Health Organization.
Disclosures: Drs. Chou, Buckley, Selph, and Totten and Ms. Dana report grants from the World Health Organization during the conduct of the study. Dr. Fu reports grants received by OHSU during the conduct of the study. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M20-1632.
Editors' Disclosures: Christine Laine, MD, MPH, Editor in Chief, reports that her spouse has stock options/holdings with Targeted Diagnostics and Therapeutics. Darren B. Taichman, MD, PhD, Executive Editor, reports that he has no financial relationships or interests to disclose. Cynthia D. Mulrow, MD, MSc, Senior Deputy Editor, reports that she has no relationships or interests to disclose. Eliseo Guallar, MD, MPH, DrPH, Deputy Editor, Statistics, reports that he has no financial relationships or interests to disclose. Jaya K. Rao, MD, MHS, Deputy Editor, reports that she has stock holdings/options in Eli Lilly and Pfizer. Christina C. Wee, MD, MPH, Deputy Editor, reports employment with Beth Israel Deaconess Medical Center. Sankey V. Williams, MD, Deputy Editor, reports that he has no financial relationships or interests to disclose. Yu-Xiao Yang, MD, MSCE, Deputy Editor, reports that he has no financial relationships or interest to disclose.
Corresponding Author: Roger Chou, MD, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Mail Code BICC, Portland, OR 97239; e-mail, chour@ohsu.edu.
Current Author Addresses: Drs. Chou, Buckley, Selph, Fu, and Totten and Ms. Dana: Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Mail Code BICC, Portland, OR 97239.
Author Contributions: Conception and design; R. Chou, T. Dana, D.I. Buckley.
Analysis and interpretation of the data: R. Chou, T. Dana, D.I. Buckley, R.W. Fu, A.M. Totten.
Drafting of the article: R. Chou, T. Dana, A.M. Totten.
Critical revision for important intellectual content: R. Chou, D.I. Buckley, A.M. Totten.
Final approval of the article: R. Chou, T. Dana, D.I. Buckley, S.S. Selph, R.W. Fu, A.M. Totten.
Statistical expertise: R. Chou, R.W. Fu.
Obtaining of funding: R. Chou.
Administrative, technical, or logistic support: R. Chou, T. Dana.
Collection and assembly of data: R. Chou, T. Dana, D.I. Buckley, S.S. Selph, R.W. Fu, A.M. Totten.
Footnotes
This article was published at Annals.org on 5 May 2020.
References
- 1. World Health Organization. Novel coronavirus—China 2020. Accessed at www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ on 30 March 2020.
- 2. doi: 10.1016/S0140-6736(20)30251-8. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565-574. [PMID: 32007145] doi:10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed]
- 3. doi: 10.1038/s41586-020-2012-7. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [PMID: 32015507] doi:10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed]
- 4. doi: 10.1056/NEJMoa2001017. Zhu N, Zhang D, Wang W, et al; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [PMID: 31978945] doi:10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed]
- 5. World Health Organization. Novel coronavirus (2019-nCov)—Situation Report 22 2020. Accessed at www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf?sfvrsn=fb6d49b1_2. on 30 March 2020.
- 6. doi: 10.1093/occmed/kqaa036. Koh D. Occupational risks for COVID-19 infection [Editorial]. Occup Med (Lond). 2020;70:3-5. [PMID: 32107548] doi:10.1093/occmed/kqaa036. [DOI] [PMC free article] [PubMed]
- 7. doi: 10.1001/jama.2020.1585. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020. [PMID: 32031570] doi:10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed]
- 8. doi: 10.1001/jama.2020.3972. Adams JG, Walls RM. Supporting the health care workforce during the COVID-19 global epidemic. JAMA. 2020. [PMID: 32163102] doi:10.1001/jama.2020.3972. [DOI] [PubMed]
- 9. doi: 10.1001/jamanetworkopen.2020.4006. Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:e204006. [PMID: 32202641] doi:10.1001/jamanetworkopen.2020.4006. [DOI] [PubMed]
- 10. Chou R, Dana T, Buckley D, et al. Healthcare workers and coronaviruses: epidemiology and risk factors for infection rapid review. World Health Organization. 2020. [Forthcoming].
- 11. doi: 10.1186/s12961-016-0155-7. Haby MM, Chapman E, Clark R, et al. What are the best methodologies for rapid reviews of the research evidence for evidence-informed decision making in health policy and practice: a rapid review. Health Res Policy Syst. 2016;14:83. [PMID: 27884208] [DOI] [PMC free article] [PubMed]
- 12. doi: 10.1016/j.jclinepi.2017.08.010. Elliott JH, Synnot A, Turner T, et al; Living Systematic Review Network. Living systematic review: 1. Introduction-the why, what, when, and how. J Clin Epidemiol. 2017;91:23-30. [PMID: 28912002] doi:10.1016/j.jclinepi.2017.08.010. [DOI] [PubMed]
- 13. World Health Organization. Database of publications on coronavirus disease (COVID-19) 2020. Accessed at www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov. on 30 March 2020.
- 14. Cold Spring Harbor Laboratory. medRxiv: the preprint server of health sciences. Accessed at www.medrxiv.org/ on 30 March 2020.
- 15. University of Bristol Centre for Research Synthesis and Decision Analysis. The ROBINS-E tool (Risk Of Bias In Non-randomized Studies of Exposures). Accessed at www.bristol.ac.uk/population-health-sciences/centres/cresyda/barr/riskofbias/robins-e. on 30 March 2020.
- 16. Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed at www.ohri.ca/programs/clinical_epidemiology/oxford.asp. on 30 March 2020.
- 17. doi: 10.1016/j.jiph.2018.12.002. Adegboye O, Saffary T, Adegboye M, et al. Individual and network characteristic associated with hospital-acquired Middle East Respiratory Syndrome coronavirus. J Infect Public Health. 2019 May - Jun;12:343-349. [PMID: 30578142] doi:10.1016/j.jiph.2018.12.002. [DOI] [PMC free article] [PubMed]
- 18. doi: 10.1093/cid/ciu359. Al-Abdallat MM, Payne DC, Alqasrawi S, et al; Jordan MERS-CoV Investigation Team. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59:1225-33. [PMID: 24829216] doi:10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed]
- 19. doi: 10.3201/eid2211.160920. Alraddadi BM, Al-Salmi HS, Jacobs-Slifka K, et al. Risk factors for Middle East respiratory syndrome coronavirus infection among healthcare personnel. Emerg Infect Dis. 2016;22:1915-1920. [PMID: 27767011] doi:10.3201/eid2211.160920. [DOI] [PMC free article] [PubMed]
- 20. doi: 10.1016/j.ajic.2019.04.007. Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus in the last two years: health care workers still at risk. Am J Infect Control. 2019;47:1167-1170. [PMID: 31128983] doi:10.1016/j.ajic.2019.04.007. [DOI] [PMC free article] [PubMed]
- 21. doi: 10.1016/j.ijid.2018.04.001. Amer H, Alqahtani AS, Alaklobi F, et al. Healthcare worker exposure to Middle East respiratory syndrome coronavirus (MERS-CoV): revision of screening strategies urgently needed. Int J Infect Dis. 2018;71:113-116. [PMID: 29649550] doi:10.1016/j.ijid.2018.04.001. [DOI] [PMC free article] [PubMed]
- 22. doi: 10.1038/s41598-019-43586-9. Bernard-Stoecklin S, Nikolay B, Assiri A, et al. Comparative analysis of eleven healthcare-associated outbreaks of Middle East respiratory syndrome coronavirus (Mers-cov) from 2015 to 2017. Sci Rep. 2019;9:7385. [PMID: 31089148] doi:10.1038/s41598-019-43586-9. [DOI] [PMC free article] [PubMed]
- 23. doi: 10.1007/BF03021815. Caputo KM, Byrick R, Chapman MG, et al. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth. 2006;53:122-9. [PMID: 16434750] [DOI] [PubMed]
- 24. doi: 10.1016/j.amjmed.2003.11.011. Chan LY, Wong JT, Li PK, et al. Risk of transmission of severe acute respiratory syndrome to household contacts by infected health care workers and patients. Am J Med. 2004;116:559-60. [PMID: 15063819] [DOI] [PMC free article] [PubMed]
- 25. doi: 10.3201/eid1006.030972. Chang WT, Kao CL, Chung MY, et al. SARS exposure and emergency department workers. Emerg Infect Dis. 2004;10:1117-9. [PMID: 15207066] [DOI] [PMC free article] [PubMed]
- 26. doi: 10.1186/1471-2458-9-81. Chen WQ, Ling WH, Lu CY, et al. Which preventive measures might protect health care workers from SARS? BMC Public Health. 2009;9:81. [PMID: 19284644] doi:10.1186/1471-2458-9-81. [DOI] [PMC free article] [PubMed]
- 27. doi: 10.3201/eid1101.040138. Chen WQ, Lu CY, Wong TW, et al. Anti-SARS-CoV immunoglobulin G in healthcare workers, Guangzhou, China. Emerg Infect Dis. 2005;11:89-94. [PMID: 15705328] [DOI] [PMC free article] [PubMed]
- 28. Dai Y, Hu G, Xiong H, et al. Psychological impact of the coronavirus disease 2019 (COVID-19) outbreak on healthcare workers in China. medRxiv. 2020:2020.03.03.20030874. doi: 10.1101/2020.03.03.20030874.
- 29. doi: 10.1016/j.jiph.2019.04.011. Elkholy AA, Grant R, Assiri A, et al. MERS-CoV infection among healthcare workers and risk factors for death: retrospective analysis of all laboratory-confirmed cases reported to WHO from 2012 to 2 June 2018. J Infect Public Health. 2020;13:418-422. [PMID: 31056437] doi:10.1016/j.jiph.2019.04.011. [DOI] [PMC free article] [PubMed]
- 30. doi: 10.1164/rccm.200305-715OC. Fowler RA, Guest CB, Lapinsky SE, et al. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med. 2004;169:1198-202. [PMID: 14990393] [DOI] [PubMed]
- 31. doi: 10.3201/eid1002.030676. Goh DL, Lee BW, Chia KS, et al. Secondary household transmission of SARS, Singapore. Emerg Infect Dis. 2004;10:232-4. [PMID: 15030688] [DOI] [PMC free article] [PubMed]
- 32. doi: 10.7326/0003-4819-139-7-200310070-00008. Ho AS, Sung JJ, Chan-Yeung M. An outbreak of severe acute respiratory syndrome among hospital workers in a community hospital in Hong Kong. Ann Intern Med. 2003;139:564-7. [PMID: 14530227] [DOI] [PubMed]
- 33. doi: 10.1086/381558. Ho KY, Singh KS, Habib AG, et al. Mild illness associated with severe acute respiratory syndrome coronavirus infection: lessons from a prospective seroepidemiologic study of health-care workers in a teaching hospital in Singapore. J Infect Dis. 2004;189:642-7. [PMID: 14767817] [DOI] [PMC free article] [PubMed]
- 34. doi: 10.1086/421019. Ip M, Chan PK, Lee N, et al. Seroprevalence of antibody to severe acute respiratory syndrome (SARS)-associated coronavirus among health care workers in SARS and non-SARS medical wards. Clin Infect Dis. 2004;38:e116-8. [PMID: 15227633] [DOI] [PMC free article] [PubMed]
- 35. Jiang S, Huang L, Chen X, et al. Ventilation of wards and nosocomial outbreak of severe acute respiratory syndrome among healthcare workers. Chin Med J (Engl). 2003;116:1293-7. [PMID: 14527351] [PubMed]
- 36. doi: 10.1016/j.bbi.2020.03.028. Kang L, Ma S, Chen M, et al. Impact on mental health and perceptions of psychological care among medical and nursing staff in Wuhan during the 2019 novel coronavirus disease outbreak: a cross-sectional study. Brain Behav Immun. 2020. [PMID: 32240764] doi:10.1016/j.bbi.2020.03.028. [DOI] [PMC free article] [PubMed]
- 37. doi: 10.1016/j.cmi.2016.07.017. Kim CJ, Choi WS, Jung Y, et al. Surveillance of the Middle East respiratory syndrome (MERS) coronavirus (CoV) infection in healthcare workers after contact with confirmed MERS patients: incidence and risk factors of MERS-CoV seropositivity. Clin Microbiol Infect. 2016;22:880-886. [PMID: 27475739] doi:10.1016/j.cmi.2016.07.017. [DOI] [PMC free article] [PubMed]
- 38. doi: 10.1016/j.cmi.2015.09.007. Kim T, Jung J, Kim SM, et al. Transmission among healthcare worker contacts with a Middle East respiratory syndrome patient in a single Korean centre [Letter]. Clin Microbiol Infect. 2016;22:e11-e13. [PMID: 26384679] doi:10.1016/j.cmi.2015.09.007. [DOI] [PMC free article] [PubMed]
- 39. Kluytmans M, Buiting A, Pas S, et al. SARS-CoV-2 infection in 86 healthcare workers in two Dutch hospitals in March 2020. medRxiv. 2020:2020.03.23.20041913. doi: 10.1101/2020.03.23.20041913.
- 40. doi: 10.1001/jamanetworkopen.2020.3976. Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3:e203976. [PMID: 32202646] doi:10.1001/jamanetworkopen.2020.3976. [DOI] [PMC free article] [PubMed]
- 41. doi: 10.3201/eid1002.030534. Lau JT, Fung KS, Wong TW, et al. SARS transmission among hospital workers in Hong Kong. Emerg Infect Dis. 2004;10:280-6. [PMID: 15030698] [DOI] [PMC free article] [PubMed]
- 42. doi: 10.3201/eid1002.030626. Lau JT, Lau M, Kim JH, et al. Probable secondary infections in households of SARS patients in Hong Kong. Emerg Infect Dis. 2004;10:235-43. [PMID: 15030689] [DOI] [PMC free article] [PubMed]
- 43. doi: 10.3201/eid1008.040041. Lau JT, Yang X, Leung PC, et al. SARS in three categories of hospital workers, Hong Kong. Emerg Infect Dis. 2004;10:1399-404. [PMID: 15496240] [DOI] [PMC free article] [PubMed]
- 44. doi: 10.7326/0003-4819-141-9-200411020-00006. Leung GM, Hedley AJ, Ho LM, et al. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann Intern Med. 2004;141:662-73. [PMID: 15520422] [DOI] [PubMed]
- 45. doi: 10.1001/jama.290.20.2662. Li L, Cheng S, Gu J. SARS infection among health care workers in Beijing, China [Letter]. JAMA. 2003;290:2662-3. [PMID: 14645305] [DOI] [PubMed]
- 46. doi: 10.1017/S0950268820001107. Liu C, Yang YZ, Zhang XM, et al. The prevalence and influencing factors for anxiety in medical workers fighting COVID-19 in China: a cross-sectional survey. medRxiv. 2020:2020.03.05.20032003. doi: 10.1101/2020.03.05.20032003. [DOI] [PMC free article] [PubMed]
- 47. Liu J, Ouyang L, Guo P, et al. Epidemiological, clinical characteristics and outcome of medical staff infected with COVID-19 in Wuhan, China: a retrospective case series analysis. medRxiv. 2020:2020.03.09.20033118. doi: 10.1101/2020.03.09.20033118.
- 48. doi: 10.3760/cma.j.issn.1001-0939.2020.03.014. Liu M, He P, Liu HG, et al. [Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:209-214. [PMID: 32164090] doi:10.3760/cma.j.issn.1001-0939.2020.03.014. [DOI] [PubMed]
- 49. Liu W, Tang F, Fang LQ, et al. Risk factors for SARS infection among hospital healthcare workers in Beijing: a case control study. Trop Med Int Health. 2009;14(SUPPL. 1):52-9. doi: 10.1111/j.1365-3156.2009.02255.x.
- 50. doi: 10.3201/eid1002.030838. Loeb M, McGeer A, Henry B, et al. SARS among critical care nurses, Toronto. Emerg Infect Dis. 2004;10:251-5. [PMID: 15030692] [DOI] [PMC free article] [PubMed]
- 51. doi: 10.1016/j.psychres.2020.112936. Lu W, Wang H, Lin Y, et al. Psychological status of medical workforce during the COVID-19 pandemic: a cross-sectional study. Psychiatry Res. 2020;288:112936. [PMID: 32276196] doi:10.1016/j.psychres.2020.112936. [DOI] [PMC free article] [PubMed]
- 52. Ma HJ, Wang HW, Fang LQ, et al. [A case-control study on the risk factors of severe acute respiratory syndromes among health care workers]. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:741-4. [PMID: 15555351] [PubMed]
- 53. doi: 10.1056/NEJMoa2005412. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020. [PMID: 32220208] doi:10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed]
- 54. doi: 10.1111/1469-0691.12562. Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Screening for Middle East respiratory syndrome coronavirus infection in hospital patients and their healthcare worker and family contacts: a prospective descriptive study. Clin Microbiol Infect. 2014;20:469-74. [PMID: 24460984] doi:10.1111/1469-0691.12562. [DOI] [PMC free article] [PubMed]
- 55. doi: 10.7326/L20-0175. Ng K, Poon BH, Kiat Puar TH, et al. COVID-19 and the risk to health care workers: a case report. Ann Intern Med. 2020. [PMID: 32176257] doi:10.7326/L20-0175. [DOI] [PMC free article] [PubMed]
- 56. Nishiura H, Kuratsuji T, Quy T, et al. Rapid awareness and transmission of severe acute respiratory syndrome in Hanoi French Hospital, Vietnam. Am J Trop Med Hyg. 2005;73:17-25. [PMID: 16014825] [PubMed]
- 57. Nishiyama A, Wakasugi N, Kirikae T, et al. Risk factors for SARS infection within hospitals in Hanoi, Vietnam. Jpn J Infect Dis. 2008;61:388-90. [PMID: 18806349] [PubMed]
- 58. Pei LY, Gao ZC, Yang Z, et al. Investigation of the influencing factors on severe acute respiratory syndrome among health care workers. Beijing Da Xue Xue Bao Yi Xue Ban. 2006;38:271-5. [PMID: 16778970] [PubMed]
- 59. doi: 10.1016/j.sleep.2020.05.023. Qi J, Xu J, Li B, et al. The evaluation of sleep disturbances for Chinese frontline medical workers under the outbreak of COVID-19. medRxiv. 2020:2020.03.06.20031278. doi: 10.1101/2020.03.06.20031278. [DOI] [PMC free article] [PubMed]
- 60. doi: 10.1371/journal.pone.0010717. Raboud J, Shigayeva A, McGeer A, et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One. 2010;5:e10717. [PMID: 20502660] doi:10.1371/journal.pone.0010717. [DOI] [PMC free article] [PubMed]
- 61. doi: 10.1093/cid/ciaa287. Ran L, Chen X, Wang Y, et al. Risk factors of healthcare workers with corona virus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020. [PMID: 32179890] doi:10.1093/cid/ciaa287. [DOI] [PMC free article] [PubMed]
- 62. doi: 10.1186/1471-2458-6-207. Reynolds MG, Anh BH, Thu VH, et al. Factors associated with nosocomial SARS-CoV transmission among healthcare workers in Hanoi, Vietnam, 2003. BMC Public Health. 2006;6:207. [PMID: 16907978] [DOI] [PMC free article] [PubMed]
- 63. doi: 10.5365/wpsar.2018.9.3.002. Ryu B, Cho SI, Oh MD, et al. Seroprevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) in public health workers responding to a MERS outbreak in Seoul, Republic of Korea, in 2015. Western Pac Surveill Response J. 2019 Apr-Jun;10:46-48. [PMID: 31720054] doi:10.5365/wpsar.2018.9.3.002. [DOI] [PMC free article] [PubMed]
- 64. doi: 10.3201/eid0910.030525. Scales DC, Green K, Chan AK, et al. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerg Infect Dis. 2003;9:1205-10. [PMID: 14609453] [DOI] [PMC free article] [PubMed]
- 65. doi: 10.1016/S0140-6736(03)13168-6. Seto WH, Tsang D, Yung RW, et al; Advisors of Expert SARS group of Hospital Authority. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet. 2003;361:1519-20. [PMID: 12737864] [DOI] [PMC free article] [PubMed]
- 66. doi: 10.1017/s0950268804002766. Teleman MD, Boudville IC, Heng BH, et al. Factors associated with transmission of severe acute respiratory syndrome among health-care workers in Singapore. Epidemiol Infect. 2004;132:797-803. [PMID: 15473141] [DOI] [PMC free article] [PubMed]
- 67. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145-151. [PMID: 32064853] doi:10.3760/cma.j.issn.0254-6450.2020.02.003.
- 68. Wang C, Liu L, Hao X, et al. Evolving epidemiology and impact of non-pharmaceutical interventions on the outbreak of coronavirus disease 2019 in Wuhan, China. medRxiv. 2020:2020.03.03.20030593. doi: 10.1101/2020.03.03.20030593.
- 69. doi: 10.1080/00365540600951226. Wang FD, Chen YY, Lee YM, et al. Positive rate of serum SARS-CoV immunoglobulin G antibody among healthcare workers. Scand J Infect Dis. 2007;39:152-6. [PMID: 17366033] [DOI] [PubMed]
- 70. doi: 10.1016/j.jhin.2020.02.021. Wang X, Pan Z, Cheng Z. Association between 2019-nCoV transmission and N95 respirator use [Letter]. J Hosp Infect. 2020. [PMID: 32142885] doi:10.1016/j.jhin.2020.02.021. [DOI] [PMC free article] [PubMed]
- 71. doi: 10.1186/s13756-016-0120-9. Wiboonchutikul S, Manosuthi W, Likanonsakul S, et al. Lack of transmission among healthcare workers in contact with a case of Middle East respiratory syndrome coronavirus infection in Thailand. Antimicrob Resist Infect Control. 2016;5:21. [PMID: 27222710] doi:10.1186/s13756-016-0120-9. [DOI] [PMC free article] [PubMed]
- 72. doi: 10.3201/eid1107.041165. Wilder-Smith A, Teleman MD, Heng BH, et al. Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerg Infect Dis. 2005;11:1142-5. [PMID: 16022801] [DOI] [PMC free article] [PubMed]
- 73. doi: 10.1503/cmaj.050876. Wilson-Clark SD, Deeks SL, Gournis E, et al. Household transmission of SARS, 2003. CMAJ. 2006;175:1219-23. [PMID: 17098951] [DOI] [PMC free article] [PubMed]
- 74. doi: 10.1007/s10096-003-1068-2. Wong SF, Chow KM, Shek CC, et al. Measures to prevent healtcare workers from contracting severe acute respiratory syndrome during high-risk surgical procedures. Eur J Clin Microbiol Infect Dis. 2004;23:131-3. [PMID: 14712366] [DOI] [PMC free article] [PubMed]
- 75. doi: 10.3201/eid1002.030452. Wong TW, Lee CK, Tam W, et al; Outbreak Study Group. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg Infect Dis. 2004;10:269-76. [PMID: 15030696] [DOI] [PMC free article] [PubMed]
- 76. doi: 10.1016/j.jhin.2010.12.002. Yen MY, Lin YE, Lee CH, et al. Taiwan's traffic control bundle and the elimination of nosocomial severe acute respiratory syndrome among healthcare workers. J Hosp Infect. 2011;77:332-7. [PMID: 21316802] doi:10.1016/j.jhin.2010.12.002. [DOI] [PMC free article] [PubMed]
- 77. doi: 10.1016/j.jhin.2005.02.011. Yen MY, Lin YE, Su IJ, et al. Using an integrated infection control strategy during outbreak control to minimize nosocomial infection of severe acute respiratory syndrome among healthcare workers. J Hosp Infect. 2006;62:195-9. [PMID: 16153744] [DOI] [PMC free article] [PubMed]
- 78. Yin WW, Gao LD, Lin WS, et al. [Effectiveness of personal protective measures in prevention of nosocomial transmission of severe acute respiratory syndrome]. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:18-22. [PMID: 15061941] [PubMed]
- 79. Ying Y, Kong F, Zhu B, et al. Mental health status among family members of health care workers in Ningbo, China during the coronavirus disease 2019 (COVID-19) outbreak: a cross-sectional study. medRxiv. 2020:2020.03.13.20033290. doi: 10.1101/2020.03.13.20033290.
- 80. Zhu Z, Xu S, Wang H, et al. COVID-19 in Wuhan: immediate psychological impact on 5062 health workers. medRxiv. 2020:2020.02.20.20025338. doi: 10.1101/2020.02.20.20025338.
- 81. World Health Organization. Emergency preparedness response—summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Accessed at www.who.int/csr/sars/country/table2004_04_21/en. on 30 March 2020.
- 82. World Health Organization Regional Office for the Eastern Mediterranean. MERS situation update 2019. Accessed at http://applications.emro.who.int/docs/EMCSR246E.pdf?ua=1. on 30 March 2020.
- 83. doi: 10.1176/appi.ps.55.9.1055. Bai Y, Lin CC, Lin CY, et al. Survey of stress reactions among health care workers involved with the SARS outbreak. Psychiatr Serv. 2004;55:1055-7. [PMID: 15345768] [DOI] [PubMed]
- 84. doi: 10.1177/070674370705200406. McAlonan GM, Lee AM, Cheung V, et al. Immediate and sustained psychological impact of an emerging infectious disease outbreak on health care workers. Can J Psychiatry. 2007;52:241-7. [PMID: 17500305] [DOI] [PubMed]
- 85. doi: 10.1177/070674370404900609. Chua SE, Cheung V, Cheung C, et al. Psychological effects of the SARS outbreak in Hong Kong on high-risk health care workers. Can J Psychiatry. 2004;49:391-3. [PMID: 15283534] [DOI] [PubMed]
- 86. doi: 10.1136/emj.2006.035089. Lin CY, Peng YC, Wu YH, et al. The psychological effect of severe acute respiratory syndrome on emergency department staff. Emerg Med J. 2007;24:12-7. [PMID: 17183035] [DOI] [PMC free article] [PubMed]
- 87. doi: 10.3201/eid1212.060584. Maunder RG, Lancee WJ, Balderson KE, et al. Long-term psychological and occupational effects of providing hospital healthcare during SARS outbreak. Emerg Infect Dis. 2006;12:1924-32. [PMID: 17326946] [DOI] [PMC free article] [PubMed]
- 88. doi: 10.1503/cmaj.1031077. Nickell LA, Crighton EJ, Tracy CS, et al. Psychosocial effects of SARS on hospital staff: survey of a large tertiary care institution. CMAJ. 2004;170:793-8. [PMID: 14993174] [DOI] [PMC free article] [PubMed]
- 89. doi: 10.1177/070674370905400504. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54:302-11. [PMID: 19497162] [DOI] [PMC free article] [PubMed]
- 90. doi: 10.1097/01.mlr.0000167181.36730.cc. Koh D, Lim MK, Chia SE, et al. Risk perception and impact of Severe Acute Respiratory Syndrome (SARS) on work and personal lives of healthcare workers in Singapore: what can we learn? Med Care. 2005;43:676-82. [PMID: 15970782] [DOI] [PubMed]
- 91. doi: 10.1111/irv.12745. Bartoszko JJ, Farooqi MAM, Alhazzani W, et al. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir Viruses. 2020. [PMID: 32246890] doi:10.1111/irv.12745. [DOI] [PMC free article] [PubMed]
- 92. doi: 10.1136/bmj.39393.510347.BE. Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ. 2008;336:77-80. [PMID: 18042961] [DOI] [PMC free article] [PubMed]
- 93. doi: 10.1136/bmj.h694. MacIntyre CR, Chughtai AA. Facemasks for the prevention of infection in healthcare and community settings. BMJ. 2015;350:h694. [PMID: 25858901] doi:10.1136/bmj.h694. [DOI] [PubMed]
- 94. doi: 10.1093/cid/cix681. Offeddu V, Yung CF, Low MSF, et al. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta-analysis. Clin Infect Dis. 2017;65:1934-1942. [PMID: 29140516] doi:10.1093/cid/cix681. [DOI] [PMC free article] [PubMed]
- 95. doi: 10.1371/journal.pone.0035797. Tran K, Cimon K, Severn M, et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7:e35797. [PMID: 22563403] doi:10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed]
- 96. doi: 10.1002/14651858.CD011621.pub4. Verbeek JH, Rajamaki B, Ijaz S, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev. 2020;4:CD011621. [PMID: 32293717] doi:10.1002/14651858.CD011621.pub4. [DOI] [PMC free article] [PubMed]
- 97. Mela CF, Kopalle PK. The impact of collinearity on regression analysis: the asymmetric effect of negative and positive correlations. Applied Economics. 2002;34:667-77. doi: 10.1080/00036840110058482.
- 98. doi: 10.1056/NEJMp2006141. Ranney ML, Griffeth V, Jha AK. Critical supply shortages - the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382:e41. [PMID: 32212516] doi:10.1056/NEJMp2006141. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.