Abstract
Surgical resection remains the mainstay treatment for solid cancers, especially for localized disease. However, the postoperative immunosuppression provides a window for cancer cell proliferation and awakening dormant cancer cells, leading to rapid recurrences or metastases. This immunosuppressive status after surgery is associated with the severity of surgical trauma since immunosuppression induced by minimally invasive surgery is less than that of an extensive open surgery. The systemic response to tissue damages caused by surgical operations and the subsequent wound healing induced a cascade alteration in cellular immunity. After surgery, patients have a high level of circulating damage‐associated molecular patterns (DAMPs), triggering a local and systemic inflammation. The inflammatory metrics in the immediate postoperative period was associated with the prognosis of cancer patients. Neutrophils provide the first response to surgical trauma, and the production of neutrophil extracellular traps (NETs) promotes cancer progression. Activated macrophage during wound healing presents a tumor‐associated phenotype that cancers can exploit for their survival advantage. In addition, the amplification and activation of myeloid‐derived suppressor cells (MDSCs), regulatory T cells (Tregs) or the elevated programmed death ligand‐1 and vascular endothelial growth factor expression under surgical trauma, exacerbate the immunosuppression and favor of the formation of the premetastatic niche. Therapeutic strategies to reduce the cellular immunity impairment after surgery include anti‐DAMPs, anti‐postoperative inflammation or inflammatory/pyroptosis signal, combined immunotherapy with surgery, antiangiogenesis and targeted therapies for neutrophils, macrophages, MDSCs, and Tregs. Further, the application of enhanced recovery after surgery also has a feasible outcome for postoperative immunity restoration. Overall, current therapies to improve the cellular immunity under the special condition after surgery are relatively lacking. Further understanding the underlying mechanisms of surgical trauma‐related immunity dysfunction, phenotyping the immunosuppressive cells, and developing the related therapeutic intervention should be explored.
Keywords: damage‐associated molecular pattern, postoperative immunosuppression, solid cancers, surgical trauma
Abbreviations
- ARG1
Arginase‐1
- CCLs
C‐C motif chemokines
- COX‐1 or COX‐2
cyclooxygenase 1 or 2
- CRP
C‐reactive protein
- CSF1
colony‐stimulating factor‐1
- CSF‐1R
colony‐stimulating factor‐1 receptor
- CXCLs
C‐X‐C motif chemokines
- DAMPs,damage‐associated
damage‐associated molecular patterns
- ERAS,enhanced recovery after
enhanced recovery after surgery
- GITR
glucocorticoid‐induced TNFR‐related protein
- G‐MDSC
granulocytic‐MDSC
- HMGB1
high bobility group Box 1
- HSP70
heat shock protein 70
- HVEM
herpes virus entry mediator
- IFN‐γ
interferon‐γ
- IGF I and II
insulin‐like growth factor
- IL‐6
interleukin‐6
- IL‐8
interleukin‐8
- MDSCs
myeloid‐derived suppressor cells
- M‐MDSCs
monocytic‐MDSCs
- mtDNA
mitochondrial DNA
- NETs
neutrophil extracellular traps
- NF‐κB
nuclear factor kappa‐B
- NK cells
nature kill cells
- NLRP3
NOD‐like receptor family, pyrin domain containing 3
- NLRs
NOD‐like receptors
- NSAIDs
nonsteroidal anti‐inflammatory drugs
- PD‐1
programmed death‐1
- PDGF
platelet derived growth factor
- PD‐L1
programmed death ligand‐1
- PGE‐2
prostaglandin E2
- PI3Kδ
phosphatidylinositol 3‐kinase δ
- PRR
pattern recognition receptors
- TGF‐α
transforming growth factor
- TGF‐β
transforming growth factor
- TLRs
Toll‐like receptors
- TNF‐α
tumor necrosis factor alpha
- Tregs
regulatory T cells
- VEGF
vascular endothelial growth factor
1. BACKGROUND
Surgical resection is currently the mainstay treatment for solid cancers. However, even received curative resection, postoperative recurrence remains high at 20–66%. 1 , 2 And metastases are the cause of most cancer‐related mortalities after resection. 3 These clinical evidences indicated surgical resection tend to correlate with the metastatic seeding or proliferation of tumor cells. Surgical operations on solid tumors disseminate circulating tumor cells, 4 which the sterile inflammation after surgery captures thereby supporting their survival and metastatic growth. 5 Besides this, the cellular immunity is proved to be impaired under surgical stress that promotes the proliferation of tumor cells. The secretion of interferon‐γ (IFN‐γ), a cytokine that is integral in controlling metastases, is suppressed following colorectal cancer surgery. 6 In addition, the total number of CD8+ T cells reduced after surgery, which finally promotes the outgrowth of implanted tumors. 7 Up to now, the postoperative immunosuppression state has been clinically confirmed in surgeries involving thoracic cancers, 8 , 9 renal cell carcinoma, 10 gastric cancer, 11 colorectal cancer, 6 pancreas adenocarcinoma, 12 breast cancer, 13 ovarian cancer, 14 prostate cancer, 15 and sarcoma 16 (Table 1).
TABLE 1.
Clinical studies about the immunity dysfunction after surgeries for solid cancers
| Cancer types | Surgical strategies | The immunity dysfunction after surgery | The prognostic value | Reference |
|---|---|---|---|---|
| Lung cancer | Video‐assisted thoracoscopic surgery (VATS) versus open resection | VATS was associated with less effect on circulating CD4+ T cells at 2 days, on NK lymphocytes at 7 days postsurgery, lymphocyte oxidation suppression at 2 days. | ‐ | 8 |
| Colorectal cancer | Open and Laparoscopic | NK cell IFN‐γ secretion is significantly suppressed for up to 2 months following surgery. | ‐ | 6 |
| Prostate cancer | Radical prostatectomy | CD14−HLA‐DR−CD33+CD11b+ cells wereincreased. | ‐ | 15 |
| Breast cancer | Radical mastectomy | Peripheral FOXP3 mRNA level and Treg frequencies were elevated on postoperative day 7. | ‐ | 13 |
| Ovarian cancer | Debulking surgery | The levels of IL‐10 decreased after surgery. | Gal‐1 and CCL2 are independent prognostic factors for progression‐free survival and overall survival. | 14 |
| Gastric cancer | Minimally invasive surgery and Roux‐en‐Y gastric bypass | Differences within the open group were seen for T lymphocytes, NK cells, T‐helper lymphocytes, and CD4/CD8 subsets, significant decreases were found in cytotoxicity on day 1 and 2. | ‐ | 11 |
| Esophagus cancer | Esophagectomy | A thoracoscopic approach was a significant factor in attenuating IL‐6 and IL‐8 levels on postoperative day 1, and a longer operative time was a significant factor in increasing these levels. | ‐ | 9 |
| Pancreas adenocarcinoma | Curative pancreatectomy | The immunologic statusdeteriorated within 3 to 4 days after the operation and recovered after that. | Elevated neutrophil‐to‐lymphocyte ratio at postoperative 1 and 6 months and decreased total lymphocyte count at postoperative 1 month were significant prognosis predictors. | 12 |
| Osteosarcoma | Wide excision | The serum levels of VEGF and endostatin decreased after removal of the tumor. | The postoperative levels of VEGF, VEGF/platelets, and endostatin significantly higher in the recurrence group than the no‐recurrence group. | 16 |
| Renal cancer | Radical nephrectomy or nephron‐sparing surgery | Naïve T‐cells, memory T‐cells, CD16+ NK and total circulating dendritic cells worsened after 12 and 24 h from surgery. | ‐ | 10 |
Surgery‐related factors such as sympathetic nervous system activation, blood transfusion, and anesthetic agents induce a postoperative immunosuppression. Clinical evidence indicated that the immunosuppressive effect after surgery seems to be associated with the invasiveness of surgery. 17 , 18 , 19 Surgical intervention for solid cancers accompanied by unintended soft tissue damages have previously been reported. 20 And open organ surgical operations accompanied by extensive tissue dissection produced a cellular immunity suppression, which was not observed in less invasive procedures. 21 In colorectal cancer, open resection resulted in a greater immunity impairment that was associated with shorter disease‐free intervals and time‐to‐recurrence compared with laparoscopic resection. 22 Reasons underling the relation of cellular immunity loss and the invasiveness of surgery are not clear. But these evidences put the surgical trauma on the central role of the possible reasons.
The immunity suppressive state after surgery may last from several days to 6 months. The surgical intervention disrupts the homeostatic balance of the body, and the plasma levels of cytokine IFN‐γ begin to drop just 1 hour after surgery. 23 In addition, the impairment of T cells proliferation and the activity of nature killer (NK) cells span for about 2 weeks, with each peaking on the fifth to– seventh day after surgery. 24 The suppressed cellular immunity may also stay up to several months after surgery. For instance, a recent clinical study revealed that the decrease in total‐lymphocyte count and elevation of neutrophil‐to‐lymphocyte ratiopersisted for up to 6 months, in pancreatic adenocarcinoma patients who underwent curative pancreatectomy. 25 The 6‐month postoperative period provides clinical physicians an immunological “window of opportunity” to kill cancer cells (Figure 1). Thus, in this review, we sought to outline the advances of surgical trauma and the subsequent biological responses contributing to immunosuppression. We will also summarize the possible therapeutic strategies based on surgical trauma‐related immunity dysfunction to reduce the postoperative tumor progression.
FIGURE 1.
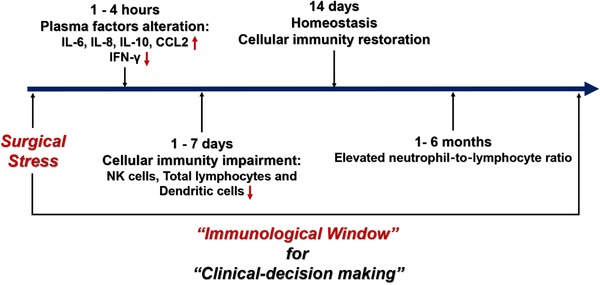
The timeline of the immunity dysfunction after surgical stress. Surgical stress disrupted the innate and adoptive immunity. The levels of proinflammation factors like IL‐6, IL‐8, IL‐10, CCL2, etc increased, and the level of cytokine IFN‐γ, which is secreted by NK cells and T lymphocytes, decreased in several hours after surgery. The activity of NK cells and the count of total lymphocytes and dendritic cells get impaired, and this immunosuppressive status commonly last 1 week after surgery. Due to the homeostasis of our body, the cellular immunity will get restored within 14 days after surgery. Recent clinical evidence revealed that the impairment of cellular immunity may last up to 6 months
2. DAMPs, INFLAMMATION, NEUTROPHILS, AND MACROPHAGES IN SURGICAL TRAUMA‐INDUCED IMMUNOSUPPRESSION
Surgical trauma caused tissue damages that induced a large number of DAMPs released into circulation. These DAMPs initiated the innate immune defense and triggered a systemic and local organ inflammation. Under the inflammatory circumstances, the immunity dysfunction occurred. In addition, the activation of inflammasome and pyroptosis triggered by DAMPs produced chemokines to recruit the immunosuppressive cells included myeloid‐derived suppressor cells (MDSCs), M2‐macrophages, or regulatory T cells (Tregs) (Figure 2). In addition, the activated macrophage during DAMPs clearance or wound healing can be exploited by cancers for their metastasis.
FIGURE 2.
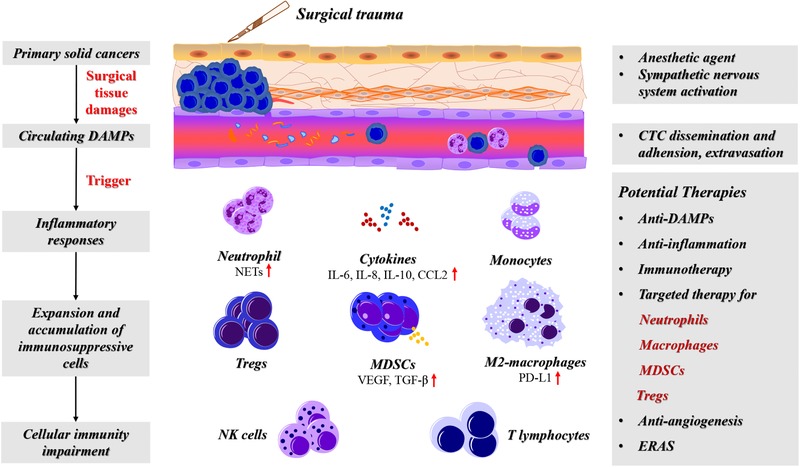
The schematic diagram of surgical trauma and the cellular immunity dysfunction. Tissue damages after surgery caused numerous DAMPs releasing into circulation. These DAMPs triggered a systemic and local organ inflammation response that disrupted the innate and adaptive immunity. Inflammatory metrics after surgery associated with the prognosis of cancer patients. The immunosuppressive cells including MDSCs, Tregs, and tumor‐associated macrophages are expanded under the surgical‐induced inflammation. Potential therapies to reduce the surgical trauma‐induced immunosuppression include anti‐DAMPs, anti‐inflammation therapies, combined immunotherapy with surgery, antiangiogenesis. Also, targeted therapies for MDSCs, Tregs, neutrophils, and macrophages may have surprising efficacy under the special condition in postoperative period. In addition, application of ERAS for patients with solid caners could accelerate the restoration of cellular immunity after surgery
2.1. DAMPs and the inflammatory pathways after surgical trauma
Following trauma, numerous mediators known as DAMPs are immediately released into circulation. 26 The “alarmins” DAMPs may be nuclear or cytosolic proteins, extracellular matrix, or metabolic products 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 (Table 2). Plasma levels of DAPMs are associated with cellular immunity impairment in trauma patients. 45 As intrinsic danger signals, DAMPs are sensitized and recognized via pattern recognition receptors (PRR) that mediated innate immune responses. 46 , 47 Toll‐like receptors (TLRs) are the main PRR for detection of DAMPs and TLR activation could predict the pathological conditions in trauma patients. 48 , 49 An important inflammatory signal TLR‐MyD88 was activated by DAPMs triggered a cascade signal response that increase the transcription activity of nuclear factor kappa‐B (NF‐κB). 44
TABLE 2.
The biological function of DAMPs released after solid cancer surgeries
| Name | Category | Type | The PPR | Immunity‐related biological function | Reference |
|---|---|---|---|---|---|
| HMGB1 | Proteins | Nonhistone chromatin nuclear peptide | TLRs 2, 4, 9 and RAGE | The increase in HMGB1 levels after surgery related with the decrease in HLA‐DR expression. | 27 |
| Interleukin‐1 | Cytokines (IL‐1α and IL‐1β) | IL‐1R | Induce signaling cascades in target cells via MAPK or NF‐κB pathways. | 28 | |
| Interleukin‐33 | Nuclear alarmin | IL‐1RL1 | Initiating the potential signaling pathway via NF‐κB and MyD88. | 29 | |
| S100A proteins | Low molecular weight calcium‐binding homodimeric proteins | RAGE, TLR4 | Activated p38 MAPK, ERK1/2, and transcription factor NF‐κB. | 30 | |
| Histones | Epigenetic regulator | TLR2, TLR4 or TLR9 | Extracellular histones induce multiple organ injury via direct endothelia disruption, and the subsequent release of other DAMPs. | 31, 32 | |
| Complement factors | Effector arm of humoral immunity | Complement receptor | Activation of complement in trauma patients correlate with disease severity. | 33, 34, 35 | |
| Heat shock proteins | Molecular chaperones | TLR2 and 4 | HSP70 can stimulate monocytes/macrophages, and dendritic cells via TLR 2‐ and 4‐ pathways. | 27 | |
| Nucleic acids | Nonproteins | Nuclear DNA, RNA | TLR3, TLR7, TLR8, TLR9, RAGE | Significantly increased in the immediate posttrauma period. | 36, 37 |
| Adenosine triphosphate | Metabolic DAMPs | Purinergic receptor P2 × 7 | Contributing to the induction of inflammation by activation and recruitment macrophages, neutrophils, and dendritic cells. | 38 | |
| Extracellular vesicles | Proteins, mRNAs, miRNAs, lipids | Cell‐to‐cell communicators | A significant increase in plasma extracellular vesicles after traumatic injury had proinflammatory effects that may influence outcomes. | 39 | |
| Purine metabolites | Uric acid | Uric acid crystals act via inflammasomes, resulting in the production of active proinflammatory cytokines IL‐1β and IL‐18 and neutrophilic influx. | 40 | ||
| Biglycan and Hyaluronic acid | Extracellular matrix | TLR2, TLR4, NLRP3 | Induce the secretion of TNF‐α, MIP‐1, and IL‐1β. | 41 | |
| Succinate | Mitochondrial contents | Succinate receptor | Triggers pro inflammatory differentiation of lymphocytes. | 42 | |
| Formyl peptides | Nucleoproteins | FP | Extracellular formyl peptides act as neutrophil attractants via FP receptors. | 43 | |
| mtDNA | TLR9 | Promote NLRP3 inflammasome activation, acute pulmonary inflammation, and injury through TLR9, p38 MAPK, and NF‐κB pathways. | 44 | ||
| TFAM | Mitochondrial transcription factor A | RAGE | Guides the TFAM‐mtDNA complexes to the endosomal pathways. | 42 |
High mobility group Box 1 (HMGB1) and extracellular DNA are the two well‐studied DAMPs. HMGB1, a nonhistone chromatin nuclear peptide, acts as a DNA chaperon. 50 The release of HMGB1 initiate an adaptive immune response that promotes malignant progression. 51 Trauma resulted in significant elevation in circulation HMGB1 as well as an increase of plasma‐activated TLR activation. 52 Postoperative circulating DNA is associated with surgical invasiveness and was considered as a biomarker of the postoperative complications. 53 , 54 In severely injured patients, plasma mitochondrial DNA (mtDNA) DAMPs are associated with the evolution of system inflammatory reaction syndrome and mortality. 55 In the tumor microenvironment, extracellular DNA promoted the cancer cells survival through induction of autophagy via TLR‐9 signaling. 56
Some trauma‐induced DAMPs are also recognized by NOD‐like receptors (NLRs) that lead to an assembly of intracellular multiprotein complexes known as inflammasomes. NLRP3 inflammasome activation is one of the initial steps in an inflammatory cascade against DAMPs. NLRP3 activation promotes the translocation of the NF‐κB transcription factor to upregulate the expression of pro‐IL‐18 and pro‐IL‐1β. Caspase‐1 cleaved these frontiers, producing active IL‐1β and IL‐18. Through this process, inflammation was aggravated via recruitment of MDSCs, macrophages, and the promotion of pyroptosis (Figure 3). IL‐1β secretion is a marker of NLRP3 activation, which linked the tissue damages with tumor‐promoting inflammation. 57 In breast cancer, fibroblasts sense DAMPs and activate the NLRP3 inflammasome, resulting in proinflammatory signaling upregulation and IL‐1β secretion. This signal facilitated tumor progression, which was attenuated when NLRP3 or IL‐1β was inhibited. 58 Overall, the DAMPs released into circulation are considered main mediators of surgical trauma, and the subsequent inflammatory response, as well as immunity impairment, which is responsible for the outgrowth of circulating or dormant cancer cells.
FIGURE 3.
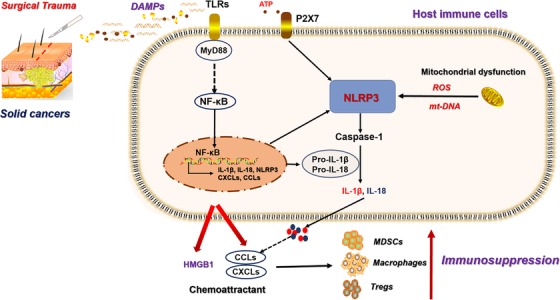
The mechanisms of surgical‐induced DAMPs and immunosuppressive cells accumulation. Surgical resection on solid cancers lead to tissue damages that releasing large amount of DAMPs into circulation. These DAPMs were recognized by PRRs on host immune cells that activate the NF‐κB transcriptional function. This process activates NLRP3 signal and the production of pro‐IL‐1β and pro‐IL‐18. Caspase‐1 cleaved the pro‐IL‐1β and pro‐IL‐18, increasing the secretion of IL‐1β and IL‐18, which promoted the secretion of CXCLs and CCLs. In addition, NF‐κB also upregulates the expression of CCLs and CXCLs expression, as well as the chemoattractant HMGB1
2.2. Postoperative inflammation can predict the prognosis of solid cancers
Surgical interventions triggered a systemic and local organ inflammation that created an ideal environment for premetastatic niche formation and awaken dormant cancer cells. Open surgery, compared to laparoscopic surgery, has greater impact on inflammatory mediator production. 59 Clinical data revealed that the inflammatory metrics like C‐reactive protein (CRP) and systemic inflammation score after surgery can be used to predict a poor clinical outcome in patients with solid cancers 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 (Table 3). The negative correlation between postoperative inflammation and prognosis highlights the perspective efficacy of anti‐inflammation after surgery to improve the long‐term survival of solid cancers. Although inflammation is the normal defensive response to injury and infection, cancer cells hijack the process for their own survival. 75 For example, one day after the injury, the inflammatory factors TNF‐α, IL‐6, and IL‐8 production capacity was significantly higher in patients who developed inflammatory complications compared with patients who did not, after a surgical intervention. 76 In addition, the acute inflammatory response to surgical trauma favors the capture of tumor cells in foreign locations hence promoting metastasis. For example, proinflammatory cytokines such as IL‐1 and TNF‐α stimulated the adhesion of the circulating cancer cells. 77 Under the inflammatory environment after surgery, dormant cancer cells can escape the surveillance of the immune system and manifest an outgrowth pattern. However, perioperative anti‐inflammatory treatment has been shown to significantly reduce the tumor outgrowth and restore the immunoresponses under such circumstances. 7
TABLE 3.
Recent clinical evidence about the correlation between postoperative inflammation and prognosis in solid cancers
| Cancers | Surgery | Inflammatory index | Prognosis | References |
|---|---|---|---|---|
| Esophageal cancer | Transthoracic esophagectomy | C‐reactive protein (CRP) | Patients with intense postoperative inflammatory response showed a significantly shorter overall survival | 60 |
| Clear‐cell renal cell carcinoma | Nephrectomy | Systemic inflammation score (SIS) | A high SIS served as an independent prognostic factor of reduced overall survival | 61 |
| Colorectal cancer | Resection | Systemic inflammation score, postoperative Glasgow prognostic score (poGPS) | poGPS was associated with an incremental increase in the postoperative infective complication rates and a reduction in survival. | 63 |
| Colorectal cancer | Curative surgery | CRP | Complication severity, and postoperative day 4 CRP were associated with disease‐specific survival. | 63 |
| Lung cancer | Curative surgery | Complication and CRP | Postoperative white blood cell counts and CRP levels were significantly higher in those with postoperative respiratory complications than in those without. The incidence of postoperative respiratory complications was a significant predictor of cancer recurrence | 64 |
| Esophageal cancer | Radical esophagectomy | CRP | CRP value on postoperative day 4 may be useful for predicting serious infectious complications | 65 |
| Advanced gastric cancer | Curative resection | Hyperthermia and leukocytosis | Overall survival and relapse‐free survival were significantly worse in the prolonged hyperthermia group. The prolonged leukocytosis group showed significantly worse overall survival and relapse‐free survival | 66 |
| Locally recurrent rectal cancer | Radical surgery | Intraabdominal/pelvic inflammation | Intraabdominal/pelvic inflammation after radical surgery for locally recurrent rectal cancer is associated with poor prognosis | 67 |
| Colorectal cancer | Curative resection | Pre‐ and postoperative CRP | Combination of pre‐ and postoperative CRP levels was an independent prognostic indicator | 68 |
| Gastric adenocarcinoma | Curative surgery | Postoperative ratio (post‐CLR) of the maximum CRP value to the minimum peripheral lymphocyte count | Post‐CLR was an independent prognostic indicator for both the overall survival and disease‐specific survival | 69 |
| Lung adenocarcinoma or squamous cell carcinoma | Lobectomy | Postoperative blood monocyte count | Elevated early postoperative peripheral monocyte count was an independent prognostic factor of poor prognosis and inferior clinicopathological features | 70 |
| Resected colorectal cancer | Tumor resection | Postoperative netrophil‐to‐lymphocyet ratio (NLR), neutrophil, and monocyte to lymphocyte ratio (NMLR), platelet to lymphocyte ratio (PLR), and systemic immune inflammation index (SII) | Higher postoperative NLR, NMLR, PLR, and SII were associated with shorter progression‐free survival. Postoperative inflammation indexes and their dynamic changes, particularly for NMLR and SII are promising prognostic predictors | 71 |
| Stage I gastric cancer | Gastrectomy | CRP | The serum CRP level during the early postoperative period predicts the long‐term outcomes in stage I gastric cancer | 72 |
| Invasive bladder cancer | Radical cystectomy | NLR, monocyte‐to‐lymphocyte ratio (MLR), hemoglobin to platelet ratio (HPR) and CRP | A postoperative NLR at 3 months > 4.68 and a postoperative HPR at 3 months < 0.029 were associated with a significant reduction in cancer specific survival and overall survival | 73 |
| Thoracic esophageal squamous cell carcinoma (Stage I) | Subtotal esophagectomy | Maximum serum CRP level (CRPmax) and white blood cell count (WBCmax) | A high WBCmax in the early postoperative phase was an independent prognostic factor for poor overall survival | 74 |
TABLE 4.
The potential therapeutic strategies to overcome surgical trauma related cancer progression
| Therapeutic strategies | Targets | Drugs | Mechanisms | References |
|---|---|---|---|---|
| Anti‐inflammation | NSAIDs | Aspirin, corticosteroids | Inhibit formation of fibrin and platelet clots | 146, 147, 148, 149 |
| COXIBs | Celecoxib, Parecoxib | Exhibits relative selectivity for COX‐2 over COX‐1 | 145, 150, 151, 152 | |
| IFN‐α | Infliximab | Activated the host immune response | 153 | |
| IL‐6 | Siltuximab | Blocking the IL‐6–induced expression of proteins responsible for acute inflammation | 154 | |
| DAMPs | Cell‐free DNA clearance | Nucleic acid scavenging microfiber meshes | Reduce the nuclei DNA induced inflammation | 129 |
| Complement therapeutics | C1 esterase, C5a | Reduce tissue inflammation without blocking the complement cascade systemically | 130, 131, 132 | |
| HBMG‐1 | Glycyrrhizin | Reverse and prevent activation of innate immunity and significantly attenuate damage in models of sterile‐induced threat | . 127 , 128 | |
| TLR2 | OPN‐305 | Blocks the activation of TLR 2‐mediated innate immunity signaling | . 136 | |
| TLR4 | MD2 inhibition (GLA‐SE) | Promotes strong Th1 and balanced IgG1/IgG2 responses to protein vaccine antigens | 137 | |
| TLR9 | CpG‐C oligodeoxynucleotides | Senses CpG DNA in endosomes and induces the IFN response | 133 | |
| CMP‐001 | 134 | |||
| RAGE | FPS‐ZM1 | Blocks the binding of amyloid β (Aβ) protein to RAGE and inhibits Aβ40‐ and Aβ42‐induced cellular stress in RAGE‐expressing cells | 135 | |
| NF‐κB | BAY11‐7082 | Suppress NF‐κB activation and reducing the production of chemokines | 140 | |
| IL‐1β | Anakinra | Reduce the secretion of CCL2, CCL5, and CXCL5 | 110 | |
| NLRP3 | MCC950, CY‐09, OLT1177, Tranilast, and Oridonin | Directly target NLRP3 to downregulate the inflammatory and pyroptosis signal | 139 | |
| Immunotherapy | Anti‐PD‐1 | Pembrolizumab | Ameliorated T‐cell proliferation and partially reversed the T‐cell apoptosis induced by surgical trauma | 124, 161 |
| Adoptive cell transfer | NKTT | Supplement the reduced number of NK cells after surgical stress | 160 | |
| Replicating viruses | Oncolytic viruses | Engage and mature conventional dendritic cells, which in turn activate NK‐ and T‐cells | 157 | |
| Prestimulation of immunity | Influenza vaccine | Administration 1 day before surgery, enhancing NK‐cell function through IFN‐α | 158, 159 | |
| Neutrophil‐based therapy | Anti‐NET | DNAse I | Eliminating the NETs that format under surgical stress | 166 |
| CXCR2 | AZD5069, MK‐7123 | Block the chemotaxis of neutrophil in acute inflammation | 164 | |
| Macrophage‐based therapy |
Minor groove of DNA Caspase 8 |
Trabectedin | Reducing the number of TAMs and the production of inflammatory cytokines and chemokines | 182, 183, 184 |
| CSF‐1R | RG7155 | Reduce the recruitment of macrophage and induced the apoptosis of activated macrophage | 171 | |
| TAM (TYRO3, AXL, MER) | RXDX‐106 | Increased intratumoral CD8+ T cells and T cell function as indicated by both IFN‐γ production and LCK phosphorylation | 185 | |
| CD40 | CP‐870,893 | Reprogrammed TAMs create a proinflammatory environment that elicits effective T cell responses | 172, 173, 174 | |
| TLR9 | IMO‐2055 | Reprogramming protumoral macrophage to tumoricidal macrophage | 175, 176, 177 | |
| TLR7 | Imiquimod | Phenotypic switch of TAMs to tumoricidal macrophages | 178, 179, 180 | |
| CD47 | Hu5F9‐G4 | A humanized, IgG4 isotype, CD47‐blocking monoclonal antibody, enables killing and phagocytosis of tumor cells by macrophages | 181 | |
| CCR2 | PF04136309 | Reduced the activated macrophage recruitment and regulated inflammation in wound healing | 170 | |
| CCL2 | Carlumab | CCL2 increased after surgical wound, reduced the activated macrophage recruitment | 167, 168, 169 | |
| MDSCs | Epigenetic therapy | 5‐Azacytidine and entinostat | Downregulation of CCR2 and CXCR2 and promote MDSC differentiation into a more‐interstitial macrophage‐like phenotype | 186 |
| PDE‐5 | Sildenafil, Tadalafil | Downregulating ARG1 and nitric oxide expression | 187, 188 | |
| CXCR1/2 | Reparixin, MK7123 | Inhibit CXCR2+ G‐MDSC trafficking | 190, 191 | |
| ATRA | ‐ | Vitamin A derivative with antiproliferative properties | 193, 194 | |
| Vitamin D3 | ‐ | Induce myeloid cell differentiation and enhance antitumor activity | 195 | |
| Gemcitabine/5‐FU | ‐ | Eliminate MDSC through induction of apoptosis | 196 | |
| Treg based therapy | CD25 | Daclizumab | Deplete CD4+CD25+ Treg cells and subsequently reduced Treg cell‐ mediated suppression of effector T cell function | 206, 207 |
| CCR4 | Mogamulizumab | Augmented the induction of cancer‐testis antigen (NY‐ ESO‐1)‐specific CD4+ and CD8+ T cells | 208, 209, 210 | |
| GITR | MEDI1873 | Activation of antigen‐specific CD4+ effector T cells and selective depletion of Treg cells | 211 | |
| PI3Kδ | Parsaclisib | Treg cell maintenance and function are dependent on PI3Kδ signaling and inactivation of PI3Kδ in Treg cells resulted in increased activity of CD8+ T cells | 212, 213 | |
| Antiangiogenesis | Monoantibody | Bevacizumab | A recombinant humanized monoclonal IgG1 antibody that binds to and inhibits the biologic activity of human VEGF | 204 |
| Endostatin | Endostar | As postoperative complementary chemotherapy, due to the decrease of endostatin after surgery | 203 |
2.3. Neutrophils are the first to respond surgical trauma induced‐inflammation
Being the most abundant white cells in circulation, neutrophils are the first to respond during inflammation. 78 The roles of neutrophils in cancer immunotherapy and tumor progression have previously been implied. For instance, a high circulating neutrophil‐to‐lymphocyte ratio is a biomarker of poor clinical outcome in cancers. 79 After abdomen surgery, the number of low‐density neutrophils with an immature phenotype is increased in peripheral blood in early postoperative period, which may promote the tumor recurrence in patients. 80 Neutrophils undergo a process known as chemotaxis via the amoeboid movement, which enables them to migrate toward the sites of injury. Proinflammatory cytokines, including IL‐6, and LTB4, or CXCR2 chemoattractants pathways, are responsible for this biological behavior. 81 Chemokine‐dependent neutrophil migration patterns result in enhanced tumor cell extravasation rates. 82 In breast cancer, cell‐cell junction and cytokine‐receptor pairs define circulating tumor cells‐neutrophil clusters, highlighting key vulnerabilities of the metastatic process. 83 Since the neutrophils aid movement and survival of the circulating cancer cells, inhibiting the proextravasation and prometastatic effect of inflamed neutrophils is necessary to restrict metastasis of the cancer.
Neutrophil extracellular traps (NETs) compromise a web of fibers composed of chromatin and serine proteases that trap and kill extracellular microbes. 84 Surgical trauma induces the formation of NETs that promote tumor progression and lead to a poor prognosis. In a cohort of patients undergoing curative liver resection for metastatic colorectal cancer, an increase in postoperative NET formation was associated with a > fourfold reduction in disease‐free survival. Furthermore, an increase in NET formation correlated with an accelerated development and progression of metastatic disease, in a murine model of surgical stress. 85 The metastatic dormancy is complicated in breast cancer, and NETs formatted after surgery have been shown to awaken the dormant cancer cells in mice under an inflammatory condition. 86 Apart from awakening the dormant cancer cells, neutrophils also release NETs under the inflammatory condition which can capture circulating cancer cells via β1‐integrin‐mediated interactions, hence support the development of metastatic disease. 87 Thus, treatments aimed to block the postoperative NET formation may benefit the patients undergoing resection of solid cancers.
DAMPs released after surgeries also induce the formation of NET via inflammatory signals. HMGB1 and histones induce NET formation via TLR4‐ and TLR9‐MyD88 signals. After depleting neutrophil, the adoptive transfer of TLR4 knockout or TLR9 knockout neutrophils conferred significant protection from liver ischemia/reperfusion injury with a significant decrease in NET formation. 88 On the other hand, the components of NETs have been recognized as DAMPs, which trigger inflammatory signals to induce pyroptosis and inflammation. NETs induced macrophages producing IL‑1β via the caspase‑1 and caspase‑8 pathways (Figure 4). The secretion of IL‑1β upregulated the production of chemokines that recruit MDSCs or M2‐macrophages. 89 Some studies have revealed that the main content of NETs formed after surgery are mtDNA, with no detectable nuclear DNA component. 90 In an acute peripheral tissue trauma model, the release of mtDNA induced the formation of NETs and sterile inflammation via cGAS‐STING and the TLR9 pathways. 91 These results indicate that clearance of the postoperative DAMPs and blockade of the related inflammatory pathways could reduce the formation of NETs, thus decreasing the early recurrence or metastasis of cancers.
FIGURE 4.
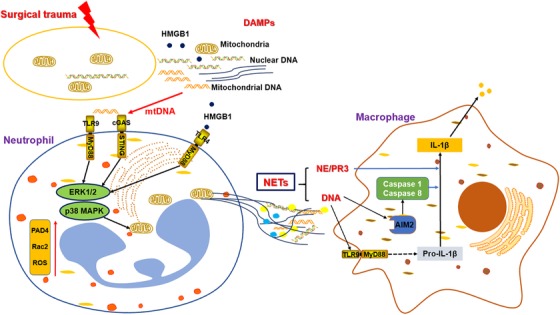
NETs formation induced by surgical trauma and the roles in immunosuppression. DAMPs releasing after surgical trauma included HMGB1 and mtDNA promote the NETs formation via TLR4‐ and TLR9‐MyD88, cGAS‐STING pathways. The main content of NET was mtDNA and could be regarded as DAMPs. NETs stimulate macrophages to secret chemoattractant via IL‐1β signals. NE: neutrophil elastase; PR3: Protease 3
2.4. Cancers exploit the activated macrophages during wound healing for their metastasis
Cancer has often been described as a “wound that does not heal,” 92 since the two share some common features. In melanoma, a strong correlation exists between neutrophil at sites of melanoma ulceration and cell proliferation, which is associated with poor prognosis. 93 Most of the growth factors, chemokines, and cytokines detected during wound healing promote tumor growth, invasion, or angiogenesis. For example, factors such as transforming growth factor‐α (TGF‐α) and transforming growth factor‐β (TGF‐β), insulin‐like growth factor I and II (IGF I and II), and platelet‐derived growth factor have been proved to play a role in the different stages of wound healing. Some of these factors were involved in the outgrowth of tumors. 94 In metastatic mouse breast cancer‐bearing mice, injection of wound fluid close to the tumor site containing cytokines or growth factors accelerated the tumor growth. 95 , 96 Wounding after surgery increased ovarian tumor mass and decreased perioperative cisplatin efficacy in a separate study. 97 Besides promoting the growth of cancer cells, most of the signaling molecules and cells involved in wound healing have also play a role in escape from tumor dormancy and the development of metastases. 98
Macrophages circulate as monocytes, until activation when they migrate to the injury site and become macrophage. 99 The first responder neutrophils dominate the early stages of inflammation setting the stage for the repair of tissue damages by recruited macrophages. 100 HMGB1 induced hepatocytes to produce PC3‐secreted microprotein, which is a potentially attractive therapeutic target for decreasing macrophage infiltration after surgery. 101 The phenotypes of wound‐healing macrophages tend to be similar with tumor‐associated macrophages that be exploited by cancer cells to their advantage. For example, tumor‐associated macrophages expressing fibroblast activation protein alpha and hemoxygenase‐1 have similar phenotype to that observed during the wound‐healing response. The expression of a wound‐like cytokine response within the tumor is clinically associated with poor prognosis in certain cancers. Some tumors exploited this response to facilitate metastasis. 102 However, the macrophage phenotype from animal models cannot represent the characteristic of human macrophages. In a clinical study on human wound‐healing response, M‐1 macrophages had perivascular distribution, while M‐2 macrophages were diffusely distributed in the dermis. In addition, M‐1 macrophages were the main source of TNF‐α and IL‐6 in wound, while arginine (ARG1) correlated with M‐2 macrophage density. 103 Even though these evidences exist, completely deleting macrophages to prevent cancer progression remains a controversial subject. A balance should however be struck between wound healing and how to limit cancer cells from taking advantage of these immune cells.
3. THE EXPANSION OF MDSCS, Tregs, AND ELEVATED PD‐L1 EXPRESSION AFTER SURGERY
3.1. Surgical trauma enhances the expansion and recruitment of MDSCs
MDSCs promote tumor metastasis by inducing the formation of a premetastatic niche, strengthening the angiogenesis and tumor cell invasion. However, surgical stress triggers several changes in phenotype and function, including enhanced the expansion and recruitment of MDSCs. Besides revealing that an increased frequency of postoperative CD14+HLA‐DR−/low MDSC was correlated with early recurrence, a clinical study also predicted the prognosis of patients with hepatocellular carcinoma patients undergoing curative resection. 104 Vascular endothelial growth factor (VEGF) is an important cytokine secreted by MDSCs that contribute to the angiogenesis, and its concentration in serum of lung cancer is often elevated. Such increased concentration has been correlated with the concentration of MDSCs 1 week after surgery. 19 The elevation of the circulating VEGF level after surgery implies that antiangiogenesis can be used to inhibit the outgrowth of cancer metastasis.
Following the MDSCs expansion and recruitment after surgery, the function of NK cells which is critical for killing the circulating cancer cells is impaired. Therefore, perioperative treatments aimed at enhancing the function of NK cells could lower the metastatic recurrence and improve the survival of surgical cancer patients. 105 In addition, the impairment of T cells was also associated with the expansion of MDSCs after surgery. Besides, in a mouse model of cancer resection, MDSC population numbers and functional impairment of tumor‐associated antigen‐specific CD8+ T‐cells were altered under surgical stress. 106 Although arginine is an essential amino acid for normal T‐cell function, its availability decreases after a physical injury, which may inhibit the function of T‐cells after injury. 107
Besides promoting the expansion of MDSCs, proinflammatory cytokines released after tissue damages including IL‐6, TGF‐β, and IL‐10 activate inflammatory signaling cascades including MyD88 and the transcriptional factor NF‐κB. 108 , 109 The following activated IL‐1β associated with increased secretion of CCLs or CXCLs that responsible for monocytic‐MDSCs (M‐MDSCs) and granulocytic‐MDSCs (G‐MDSC) accumulation, respectively. 110 In addition, prostaglandin E2 (PGE‐2) 111 , 112 and TNF signal 113 also involved in the process of MDSCs accumulation. However, in colon cancer, surgical trauma promoted tumor progression by downregulating CXCL4 and inducing the MDSC recruitment, which lead to immunity‐suppressive environment. 114
Some DAMPs can function as chemoattractant that directly recruit MDSCs. Expression of S100A8 in the lungs promotes MDSC accumulation, which suppresses a localized immune response. 115 DAMPs such as S100A8 and S100A9, released after surgery, are potent chemoattractants for MDSC. 116 S100A8/A9 induced serum amyloid A3 that directly recruited MDSC in lungs and facilitated metastasis. 117 HMGB1 was released in large amounts after abdominal surgery, which recruits numerous MDSCs to promote peritoneal metastasis of colon cancer. 118 In addition, HMGB1 contributed to tumor progression though increasing the viability of MDSCs by driving them into a proautophagic state. 119 These evidences show that the mechanisms of the MDSCs expansion under surgical stress may be complex. Therefore, phenotyping the recruited MDSCs responsible for premetastatic niche formation after surgery could provide insights for the related targeted therapy.
3.2. The number of Tregs increases under surgical stress
Treg is a highly immunosuppressive CD4+ T cell that migrate into inflammatory sites and suppress effector lymphocytes such as CD4+ T helper cells and CD8+ T lymphocytes. 120 Tregs serve to maintain the immune homeostasis, which get expanded under surgical stress. Surgical trauma promoted colon cancer progression by increasing CCL18 expression thereby promoting the recruitment of Treg. 121 In patients who received a radical mastectomy, the peripheral Treg levels were markedly elevated on the seventh day postoperation. 13 After gastric surgery, the percentage of Treg cells increased, accompanied by elevated expression of Foxp3 and TGF‐β1, and decreased T helper cells. In addition, the expression of programmed death‐1 (PD‐1) and programmed death ligand‐1 (PD‐L1) increased and the polarization of Tregs was only inhibited after anti‐PD‐1 treatment. 122 Since patients with early stage I/II tumors showed decrease Treg population, while those with late stage III/IV tumors presented greater amounts, 123 accumulation of Treg cells after surgery may be dependent on tumor stage. Tregs exert their immunosuppressive activity through various cellular and humoral mechanisms. Therefore, identifying the immunity phenotype of Treg expanded under the surgical stress and developing the related targeted therapies may have promising prognosis under the special condition after surgery.
3.3. Expression of PD‐L1 increases under surgical stress
Surgical trauma increased PD‐1/PD‐L1 expression on immune cells. In in vivo studies, administration of anti‐PD‐1 antibody significantly ameliorated T‐cell proliferation and partially reversed the T‐cell apoptosis induced by surgical trauma. 124 Besides, in a surgical stress mice model, surgical stress reduced the total number of CD8+ T cells and impaired the function of cytotoxic lymphocytes. Conversely, blocking PD‐1 with specific antibody restored the numbers and the secretion ability of CD8+ T cell. In addition, expression of PGE2 was dramatically upregulated, and anti‐PD‐1 plus PGE2 inhibitor restored cytotoxic T lymphocytes dysfunction after surgery. 125 Under systemic inflammation induced by surgical stress, the expression of PD‐L1 on activated macrophages outnumber carcinoma cells, suggesting a prominent role for tumor‐associated macrophage‐associated PD‐L1 in immunosuppression and tumor outgrowth. 7 For instance, gastric cancer resection altered the balance of Th17/Treg cells and increased PD‐1/PD‐L1 expression, while transfection of Ad‐sh‐PD1 in an in vitro study ameliorated Th17/Treg cell imbalance partially by increasing the expression of miR‐21. 126 These lines of evidence suggest that the combination of surgery and immune checkpoint inhibitor may have a synergy that can be exploited to reduce tumor progression after surgery.
4. POTENTIAL THERAPEUTIC STRATEGIES
The postoperative period provides a special immunological opportunity for cancer therapy. Compelling clinical evidence show that postoperative inflammation is common among solid cancer patients. Acute inflammation after surgery is a kind of innate response, and the alterations of immunity in this period suggest that the current therapies which are ineffective in normal conditions may actually be effective during this period after surgery (see Table 4). In addition, treatment options should balance the acute surgery‐induced inflammation, wounding healing, and cancer metastasis. Solid cancers with a large tumor burden may have a relative abundance of circulating tumor cells, or micrometastatic lesions and will take advantage of this special postoperative period for their outgrowth. Many chemokines, growth factors, cytokines and immunity cells like neutrophils and macrophages are also involved in the acute inflammatory response after surgery and during wound healing. Under this condition, some immunosuppressive cells like MDSCs and Tregs are activated and amplificated, then migrate to targeted organs, resulting in the formation of a premetastatic niche. Deletion of neutrophils and macrophages after surgery may however not be the best way to reduce the postoperative cancer progression since these cells are essential for normal immune response to injury. The immune homeostasis limits the period of immunodysfunction within 2 weeks after surgery, even though the impact on the immunity or survival of patients may be longer. Therapeutic strategies aimed at targeting DAMPs released after surgical trauma, anti‐inflammation, clearance of NETs, preventing the activated macrophages migrating to distant organs, and targeted therapy for MDSCs, or Tregs, may have superior efficacy.
4.1. Anti‐DAMPs and the related inflammatory signals
Anti‐DAMPs and the related inflammatory/pyroptosis signal are the overall strategy based on surgical trauma to reduce the postoperative immunosuppression. Strategies for targeted DAMPs therapy included specific DAMP inhibition, blockage of the related PRR pathways such as TLRs or receptor of advanced glycation endproducts (RAGE), and blockage of the NF‐κB, NLRP3, and IL‐1β signal. HMGB1 is involved in many aspects of trauma‐induced immune suppression. Treatment using anti‐HMGB1 monoclonal antibody antibodies was found to ameliorated impairment of the trauma‐induced T cells activity and accumulation of MDSCs, 2 days after injury. 127 In addition, the postoperative immunosuppression was also associated with infectious conditions like sepsis. Anti‐HMGB1 antibodies were reported to attenuate inflammation in murine sepsis models and reduce mortality without potentiating immunosuppression. 128 Clearance of cell‐free DNA improved the clinical outcome in some inflammatory diseases. In fact, nucleic acid scavenging microfiber meshes represent an effective strategy for combating inflammation and thrombosis in trauma. 129 Other therapeutic options included anticomplement system therapies 130 , 131 , 132 and drugs against PRR receptors. For instance, administration of the TLR9 inhibitor ODN2088 was found to inhibit mtDNA‐triggered systemic inflammation. 133 Similarly, CMP‐001, a virus‐like particle that encapsulating an immunostimulatory CpG‐A oligodeoxynucleotide TLR9 agonist, was reported to CMP‐001 induced production of cytokine IFN‐α from plasmacytoid dendritic cells. 134 In addition, RAGE inhibitors, such as FPS‐ZM1, a novel class of RAGE inhibitors screened from 5000 small molecules, have been reported to inhibit cancer progression and metastasis. 135 Furthermore, OPN‐305, a first humanized IgG4 monoclonal antibody against TLR2, was reported to produce full TLR2 receptor blockade on monocytes, with a linear effect on the inhibition of IL‐6 release after TLR2 stimulation. 136 Other inhibition strategies have employed MD2 inhibitor (GLA‐SE) with adjuvant GLA‐SE found to promote expansion in human T follicular helper cells and the emergence of public TCR‐β clonotypes. 137
As the secretion of chemokines related to activated inflammatory or pyroptosis pathways, blockage of NF‐κB, IL‐1β, and NLRP3 is presented as promising therapeutic strategies to reduce the accumulation of postoperative immunosuppressive. In mouse breast cancer, blocking IL‐1β reversed the immunosuppression and synergized with anti‐PD‐1 for tumor abrogation. 138 Anakinra is an IL‐1 receptor antagonist with the potential to inhibit distal metastasis via pyroptosis/IL‐1β pathways blockade. Administration of anakinra reduced the recruitment of M2‐macrohages and MDSCs via downregulation of CCL2, CCL5, and CXCL5 secretion. 110 Inhibitors specific to NLRP3 inflammasome may be the best option for therapy of NLRP3‐related disease. However, even many small molecules have been identified as NLPR3 inflammasome inhibitors, few of them have been clinically studied for human diseases. Among them, MCC950, CY‐09, OLT1177, Tranilast, and Oridonin displayed the good therapeutic properties, as they directly target NLRP3. 139 BAY11‐7082 is a NF‐κB inhibitor and reduced the secretion of CXCL1, CXCL2, and CXCL8 which are essential chemokines for CXCR2+ MDSCs. 140 However, most of the aforementioned drugs have not been used at the postoperative period. In this work, these anti‐DAMPs treatments are presented to highlight their potential and promise as therapies for improving clinical outcomes of patients with solid cancers after surgery.
4.2. Anti‐inflammation therapies
Nonsteroidal anti‐inflammatory drugs (NSAIDs) effectively inhibited systemic inflammation and eliminated suppression of NK cell populations after surgery. 141 , 142 Aspirin presents anticancer properties by suppressing inflammatory cytokines that regulate cell proliferation, angiogenesis, and apoptosis. In patients with bladder cancer, daily aspirin administration was associated with significantly improved survival after radical cystectomy. 143 With a combination therapy comprising aspirin and NSAIDs, after surgery, lowered the risk of early recurrence of breast cancer, and colorectal cancers as well as hepatocellular carcinoma. 144 , 145 , 146 Dexamethasone, a classic anti‐inflammation option and a single intraoperative dose of dexamethasone, was independently associated with improved overall survival rates in patients with pancreatic adenocarcinoma. 147 However, the efficacy of anti‐inflammation therapies in inhibition of postoperative tumor progression remains unclear. For example, Elecoxib, a COX2‐specific inhibitor, did not significantly affect apoptosis in prostate, and breast cancer as well as cervical intraepithelial neoplasia. 142 , 148 , 149 Similarly, intraoperative use of a single dose of parecoxib, another NSAIDs drug, was not associated with decreased cancer recurrence after bladder cancer surgery. 150
As the anti‐inflammatory agents also directly inhibit tumor growth, 151 , 152 in order to explore the efficacy of these drugs that have not been inferred, in a wounded‐mouse prior to breast cancer cells injection that aimed to eliminate the influence of the primary tumor, treatment with meloxicam altered the phenotype of tumor‐associated macrophages. Before meloxicam administration, distant surgical wounding induced upregulation of CD206 on the surface of tumor‐associated macrophages, indicative of a protumor M‐2 polarization that is often implicated in immunosuppressive properties. Administration of wounded mice with meloxicam prevented the increased CD206 expression and led to a decrease in PD‐L1 expression on tumor‐associated macrophages. 7 This result demonstrated the influence of surgical trauma‐induced inflammation on the immunosuppression phenotype of innate immune cells. A combination treatment compromising inhibition the COX and blockage of PD‐1 indicates a synergistic efficacy in inducing eradication of tumors. 152 Inhibition of other proinflammatory factors, like IL‐6, and IFN‐α, in some inflammatory diseases has also been documented under normal conditions. 153 , 154 It is possible that these may have varied efficacies under postoperative condition.
4.3. Combination therapy of immunotherapy and surgery
Combining perioperative immunotherapy with standard‐of‐care surgery has the potential to improve survival in surgical cancer patients. 155 Surgical attack causes a global dysfunction in NK cells. However, perioperative therapies aimed at enhancing NK cell function could reduce metastatic and recurrent rates in patients received solid cancer resection. 156 For example, preoperative administration of replicating viruses, such as novel anticancer oncolytic viruses, was reported to effectively prevent postoperative NK‐cell dysfunction. 157 Similarly, stimulating the immune system using nonreplicating viral vaccines, such as influenza vaccine, prevented postoperative metastases by enhancing NK‐cell function through IFN‐α. However, this strategy requires an accurate timing and is therefore applied 1 day before surgery, to allow for sufficient time for optimal activation of NK cells prior to surgical stress. 158 , 159 NK cell–based transfer therapy is another attractive method for overcoming NK cells dysfunction after surgical stress. However, this strategy has not been tested in the perioperative period, due to the highly cytotoxic nature of NK cells. 160
Upregulation of PD‐L1 expression in immunosuppressive macrophages after surgical stress indicates that blockage of PD‐1 using monoclonal antibodies may be an effective treatment therapy for restoring immunosuppression. In a surgical stress mice model, blockage of PD‐1 using specific antibodies restored CD8+ T cell numbers as well as their secretion ability. In addition, PGE2 expression was dramatically upregulated after surgery, and anti‐PD‐1 plus PGE2 inhibitors restored the dysfunction of cytotoxic T lymphocytes induced by surgery. 161 Herpes virus entry mediator (HVEM) is a new potential mediator of trauma‐induced immunosuppression, with surgical trauma patients exhibiting greater expression levels of HVEM+/CD3+ lymphocytes. 162 The identification of novel surface markers on effective lymphocytes, after surgery, could contribute to development of related immunotherapies.
4.4. Targeted therapies for neutrophils and NETs
Neutrophils function as the main innate defense factors during early stages of injury. Apoptosis of neutrophils is programed cell death and helps to maintain immune homeostasis. However, inflammatory responses due to tissue injury disrupts programmed neutrophil death, leading to a dysfunction of immunity. Doxorubicin‐conjugated protein nanoparticles can be used for selective in situ targeting of inflammatory neutrophils for intracellular delivery of doxorubicin to induce apoptosis. 163 In addition, the CXCR2 signaling pathway is a potential target for modifying neutrophil dynamics in inflammatory diseases. For instance, AZD5069, a CXCR2 antagonist, was reported to selectively reduce absolute neutrophil counts in asthma patients. 164 This treatment may also reduce the infiltration of these inflammatory neutrophils to the premetastatic organ. However, AZD5069's potential as a therapeutic agent for cancer patients is still under investigation. Another CXCR2 antagonist, MK‐7123, is under evaluation with pembrolizumab in patients with selected advanced/metastatic solid tumors (NCT03473925).
Studies targeting neutrophils‐releasing neutrophil elastase in cancer patients have been reported. For instance, administration of sivelestat sodium hydrate, a selective inhibitor of neutrophil elastase after transthoracic esophagectomy was found to improve the condition of systemic inflammation and postoperative clinical courses. 165 There is no direct report about elimination of NETs by DNase I digestion to minimize the postoperative tumor progression. Although several clinical trials are ongoing, no results have been reported regarding targeting NET in patients with cancers. 166 Anti‐NET may have promising clinical benefits for patients under the specific conditions, including the postoperative period.
4.5. Targeted therapies for macrophages
Current strategies focusing on macrophage‐targeted therapy include blocking macrophage recruitment, inducing apoptosis of tumor‐associated macrophages, and their reprogramming to release cancer promotion phenotype. Recruitment of macrophages or inflammatory monocytes mostly depend on the CCL2‐CCR2 chemokine axis. Levels of circulating CCL2 increased immediately after surgical trauma, indicating its potential efficacy in targeted therapy. Carlumab, a human anti‐CCL2 IgG1κ mAb, presents preliminary antitumor activity. 167 , 168 , 169 Similarly, a combination therapy, comprising PF‐04136309, a CCR2 inhibitor with FOLFIRINOX, achieved generated effective tumor response and achieved local tumor control in 97% of patients with pancreatic cancer. 170 In addition, blockade of the IL‐1β/pyroptosis pathway by the IL‐1 receptor inhibitor anakinra reduced the secretion of CCL2 and the following M2‐macrophage recruitment. 110 CSF‐1R binds to CSF1 and IL‑34 to regulate macrophage differentiation, proliferation, and survival. Administration of Emactuzumab, a humanized mAb that binds to CSF‐1R and blocks its dimerization, was found to reduce recruitment and induce apoptosis of tumor‐associated macrophages. A combination therapy involving CSF‐1R and Emactuzumab has also been reported. For instance, combining CSF‐1R inhibition with Emactuzumab in locally advanced diffuse‐type tenosynovial giant cell tumors achieved a response in 24 (86%) out of 28 patients, with two (7%) exhibiting a complete response. 171 Another promising strategy involves rebalancing microenvironment immune infiltration from a protumoral status to effective antitumor in synergy with T cell‐enhancing drugs. Current therapies for reprograming macrophages include the use of anti‑CD40, anti‑CD47, and antibodies as well as TLR agonists. 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181
Trabectedin was reported to reduce the number of tumor‐associated macrophages via G2‐M phase cell cycle arrest and apoptosis. 182 In addition, Trabectedin monotherapy reduced the risk of disease progression in patients with advanced translocation‐related sarcoma. 183 , 184 Pan‐tumor association macrophages small‐molecule kinase inhibitor RXDX‐106 (TYRO3, AXL, MER) activates both innate and adaptive immunity to inhibit the growth and progression of tumors. In addition, RXDX‐106 also potentiated the effects of α‐PD‐1 Ab, leading to enhanced antitumor efficacy and survival. 185 A better understanding of the mechanisms underlying how cancers utilize activated macrophages during wound healing is needed. Preventing activated macrophages from travelling to distant organs and promoting the formation of premetastatic niche, after surgical trauma, would be a key step in the targeted therapies. Furthermore, identifying ideal markers for targeted therapy, as well as phenotyping the macrophages during wound healing are also critical in development of targeted therapies for macrophages.
4.6. Targeted therapies for MDSCs
To date, no agents that specifically target MDSC have been developed, due to the heterogeneous features and a lack of ideal markers for selective targeting. However, there were still some drugs that target MDSC have been developed and are indirectly used for by cancer patients. A recent study revealed that administration of low‐dose DNA methyltransferase and histone deacetylase inhibitors inhibited the trafficking of MDSCs through the downregulation of CCR2 and CXCR2 and promoted MDSC differentiation into a more‐interstitial macrophage‐like phenotype. 186 A preclinical study reported that perioperative phosphodiesterase‐5 inhibition with sildenafil reduced the function of surgery‐derived G‐MDSC. In addition, sildenafil plus perioperative influenza vaccination removed surgery‐derived immunosuppressive mechanisms of MDSCs and reduce postoperative metastasis. 187 , 188 Studies have implicated C‐X‐C motif chemokine pathways in the recruitment of G‐MDSCs. Here, tumor cells secrete CXCL1 via the VEGF pathway that interact with the CXCR2 pathway to recruit G‐MDSCs. Administration of and a combination of Reparixin, an inhibitor of CXCR1/2, with weekly paclitaxel in patients with HER‐2‐negative metastatic breast cancer achieved a 30% response rate. 189 Administration of this drug also attenuated postoperative granulocytosis in patients who received on‐pump coronary artery bypass graft surgery. 190 In addition, administering MK‐7123, a specific CXCR2 inhibitor reduced neutrophil chemotaxis and alleviated airway inflammation in chronic obstructive pulmonary disease (NCT 01006616). 191 Furthermore, other potential therapeutic targets are currently under preclinical evaluation. Snail involving the CXCR2 chemotaxis of MDSCs and snail‐knockdown reduced the expression of CXCR2 ligands, CXCL1, and CXCL2. 192 Other drugs that inhibition of MDSCs in cancer patients included all‐trans retinoic acid, 193 , 194 vitamin D3, 195 and Gemcitabine/5‐FU.196 The expansion and recruitment of MDSCs after surgical trauma forms a premetastatic niche in distant organs that favor the colonization of circulating cancer cells. Preventing expansion of MDSCs after surgery is a much rational strategy to alter the postoperative “fertile soil” for metastasis, with low risk of wound‐healing problems. Even though limited drugs have been used under this condition, future studies are expected to explore the relevant therapies directly used after surgical trauma. Furthermore, it will be important to understand the mechanisms underlying expansion and recruitment of MDSCs after surgery.
4.7. Antiangiogenesis after surgery
Studies have reported an increase in serum VEGF increased after solid tumor resection, indicating that antiangiogenesis is a promising option to slow postoperative tumor progression. 197 , 198 , 199 Functionally, expanded and recruited MDSCs in premetastatic organs after surgery secrete the angiogenetic factor VEGF. 200 Antiangiogenesis drugs have achieved significantly favorable efficacy in some solid cancers, suggesting the potential of these drugs in prevention of postoperative angiogenesis. In patients with colorectal cancer undergoing radical surgery, IL‐2 preoperative immunotherapy partially reduced VEGF increase during the postoperative period. This evidence suggested that presurgical immunotherapy using IL‐2 may counteract surgery‐induced stimulation of the angiogenesis by reducing the increase of the angiogenic factor VEGF. 201 Moreover, Endostatin was found to significantly decrease after primary osteosarcoma removal, while treatment with the antiangiogenic reagent TNP‐470 suppressed postoperative progression of pulmonary metastasis in osteosarcoma. 202 In patients with nonsmall cell lung cancer, postoperative complementary chemotherapy comprising application of recombinant human endostatin (Endostar) achieved a significantly increased average progression‐free survival in a treatment group by 9.8 months, which was significantly different compared to the control group. 203 Bevacizumab is an antineoplastic agent, which acts to prevent angiogenesis thereby preventing or reducing metastatic progression. Perioperative bevacizumab benefits patients who have undergone lung metastasectomy. 204 However, when administered in the perioperative phase in patients with solid cancers, antiangiogenic agents may have drawbacks on biological activity that involve physiological angiogenesis, such as wound healing. Such treatment should, however, be administered with caution in the perioperative period until the therapy is validated using results from large clinical trials. 205
4.8. Targeted therapies for Tregs
Strategies for targeting Tregs included targeting the receptors on Tregs or the intracellular signal. Consequently, studies have focused on evaluating the effects of depletion of Tregs by targeting CD25 with antibodies or IL‐2R with a recombinant protein composed of IL‐2. 206 , 207 CCR4+ Tregs with strong immunosuppressive activity is present in the blood of patients with melanoma. Mogamulizumab, an anti‐CCR4 antibody, is currently being explored for its efficacy in patients with advanced‐stage solid tumors (NCT02281409 and NCT01929486). 208 , 209 , 210 In addition, MEDI1873, a novel, potent GITR agonist has the ability to regulate T‐cell responses. 211 This GITR agonist, individually or in combinations, is currently being investigated for its efficacy in advanced stage solid cancers (NCT02583165 and NCT02628574). In addition to the aforementioned compounds, signals crucial for Tregs survival and functions are also promising targets for Tregs‐related therapies. For instance, a combination treatment of Parsaclisib, a selective phosphatidylinositol 3‐kinase δ (PI3Kδ) inhibitor, is currently being explored during the initial stages of a phase I trial in advanced solid cancers. 212 , 213
4.9. Enhanced recovery after surgery
Surgical stress influences oncological outcomes and survival of cancer patients. Consequently, an enhanced recovery after surgery (ERAS) protocol designed to reduce perioperative stress has subsequently shown potential in reducing postoperative inflammation as well as the related morbidity. In patients with nonmetastatic colorectal cancer undergoing laparoscopic surgery, the ERAS protocol decreases risk of death at 3 years after surgery by 56% in patients compared to the control group. 214 In gastric carcinoma patients, ERAS reduced postoperative inflammation and improved cellular immunity. In addition, patients subjected to ERAS exhibited higher serum albumin and prealbumin levels at day 7 postoperation. With regard to cellular immunity, the total T lymphocytes were higher in patients on whom ERAS was performed on postoperative day 3. 215 Despite these benefits of ERAS on short‐term results in cancer patients, little is known about its impact on long‐term results. In patients with colorectal cancer, application of the ERAS protocol is associated with improved 5‐year survival after surgery, with low CRP levels on postoperative day 1. 216 Therefore, the ERAS protocol improves the postoperative status and quick recovery of the immunity, benefiting patients with solid cancers under severe surgical stress.
5. CONCLUSION AND FUTURE PROSPECTS
Surgical resection remains the main treatment for localized solid cancers. The immediate postoperative period indeed supplies an immunological “window of opportunity” for developing relevant therapies to eliminate the circulating cancer cells or the dormant cancer cells. To achieve this, understanding the postoperative biological changes is critical. Previous studies have described the impact of anesthesia, the alteration of neuroendocrine, circulation system, and sympathetic nervous systems on postoperative cancer progression. Clinical evidence shows that immunosuppressive status correlates with the severity of surgical trauma, while postoperative inflammation is a predictor for the prognosis of cancer patients. Altering inflammation alteration after surgery and the significance of clinical relevance put the tissue damages after surgical manipulation at the center of the postoperative immunosuppression. Surgical patients exhibit high levels of circulating DAMPs, which trigger the local and systemic inflammation, associated with immunity dysfunction. Cancer cells take advantage of the activated or expanded effector immunosuppressive cells such as MDSCs, Tregs, macrophages, and neutrophils to favor their progression. However, the exact mechanisms underlying the immunological alteration of these cells as well as their phenotype remain unclear. Since the related targeted therapies have developed very slowly, it is imperative to evaluate effective therapies for application under special condition during the postoperative period. Most of the anti‐inflammatory drugs have improved the survival of patients after cancer resection. However, the timing for their administration is critical. Other therapies including immunotherapy, targeted therapies based on immunosuppressive cells, antiangiogenesis, as well as enhanced recovery protocol are also effective. Preoperative stimulation of the immune system has generated promising efficacies, although its long‐term benefits remain unclear. In addition, owing to the development of immunoresponse to certain cancers, the response after tumor implantation tends to follow the biological features of the tumors. Therefore, a further understanding of the postoperative immunity changes is needed to enable development of related treatments that improve survival rates of patients with solid cancers. Overall, we have highlighted surgical trauma‐induced immunity dysfunction, which is a critical point for reducing postoperative cancer metastasis, especially for cancer types that require extensive surgical resection during removal of local tumors.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
We would think that Fan Tang was funded by Post‐Doctor Research Project, West China Hospital, Sichuan University (No. 19HXBH052) and China Postdoctoral Science Foundation (No. 2019M663509).
Tang F, Tie Y, Tu C, Wei X. Surgical trauma‐induced immunosuppression in cancer: Recent advances and the potential therapies. Clin Transl Med. 2020;10:199–223. 10.1002/ctm2.24
Contributor Information
Chongqi Tu, Email: tcq_orthoncologist@163.com.
Xiawei Wei, Email: xiaweiwei@scu.edu.cn.
REFERENCES
- 1. Pan H, Gray R, Braybrooke J, et al. 20‐year risks of breast‐cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836‐1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long‐term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090‐1098. [DOI] [PubMed] [Google Scholar]
- 3. Mahvi DA, Liu R, Grinstaff MW, Colson YL, Raut CP. Local cancer recurrence: the realities, challenges, and opportunities for new therapies. CA Cancer J Clin. 2018;68(6):488‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alieva M, van Rheenen J, Broekman MLD. Potential impact of invasive surgical procedures on primary tumor growth and metastasis. Clin Exp Metastasis. 2018;35(4):319‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tohme S, Simmons RL, Tsung A. Surgery for cancer: a trigger for metastases. Cancer Res. 2017;77(7):1548‐1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Angka L, Martel AB, Kilgour M, et al. Natural killer cell IFNgamma secretion is profoundly suppressed following colorectal cancer surgery. Ann Surg Oncol. 2018;25(12):3747‐3754. [DOI] [PubMed] [Google Scholar]
- 7. Krall JA, Reinhardt F, Mercury OA, et al. The systemic response to surgery triggers the outgrowth of distant immune‐controlled tumors in mouse models of dormancy. Sci Transl Med. 2018;10(436). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leaver HA, Craig SR, Yap PL, Walker WS. Lymphocyte responses following open and minimally invasive thoracic surgery. Eur J Clin Invest. 2000;30(3):230‐238. [DOI] [PubMed] [Google Scholar]
- 9. Okamura A, Takeuchi H, Matsuda S, et al. Factors affecting cytokine change after esophagectomy for esophageal cancer. Ann Surg Oncol. 2015;22(9):3130‐3135. [DOI] [PubMed] [Google Scholar]
- 10. Porta C, Bonomi L, Lillaz B, et al. Immunological stress in kidney cancer patients undergoing either open nephrectomy or nephron‐sparing surgery: an immunophenotypic study of lymphocyte subpopulations and circulating dendritic cells. Oncol Rep. 2008;20(6):1511‐1519. [PubMed] [Google Scholar]
- 11. Whitson BA, D'Cunha J, Hoang CD, et al. Minimally invasive versus open Roux‐en‐Y gastric bypass: effect on immune effector cells. Surg Obes Relat Dis. 2009;5(2):181‐193. [DOI] [PubMed] [Google Scholar]
- 12. Kim EY, Hong TH. Changes in total lymphocyte count and neutrophil‐to‐lymphocyte ratio after curative pancreatectomy in patients with pancreas adenocarcinoma and their prognostic role. J Surg Oncol. 2019;120(7):1102‐1111. [DOI] [PubMed] [Google Scholar]
- 13. Zhou L, Li Y, Li X, et al. Propranolol attenuates surgical stress‐induced elevation of the regulatory T cell response in patients undergoing radical mastectomy. J Immunol. 2016;196(8):3460‐3469. [DOI] [PubMed] [Google Scholar]
- 14. Coosemans A, Decoene J, Baert T, et al. Immunosuppressive parameters in serum of ovarian cancer patients change during the disease course. Oncoimmunology. 2015;5(4):e1111505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brusa D, Simone M, Gontero P, et al. Circulating immunosuppressive cells of prostate cancer patients before and after radical prostatectomy: profile comparison. Int J Urol. 2013;20(10):971‐978. [DOI] [PubMed] [Google Scholar]
- 16. Kaya M, Wada T, Nagoya S, Kawaguchi S, Isu K, Yamashita T. Concomitant tumour resistance in patients with osteosarcoma. A clue to a new therapeutic strategy. J Bone Joint Surg Br. 2004;86(1):143‐147. [PubMed] [Google Scholar]
- 17. Zheng C, Liu S, Geng P, et al. Minimally invasive video‐assisted versus conventional open thyroidectomy on immune response: a meta analysis. Int J Clin Exp Med. 2015;8(2):2593‐2599. [PMC free article] [PubMed] [Google Scholar]
- 18. Gryglewski A, Szczepanik M. The effect of surgical stress on postoperative Tαβ and Tγδ cell distribution. Immunol Invest. 2017;46(5):481‐489. [DOI] [PubMed] [Google Scholar]
- 19. Wang J, Su X, Yang L, et al. The influence of myeloid‐derived suppressor cells on angiogenesis and tumor growth after cancer surgery. Int J Cancer. 2016;138(11):2688‐2699. [DOI] [PubMed] [Google Scholar]
- 20. Menna C, De Falco E, Teodonio L, et al. Surgical wound‐site inflammation: video‐assisted thoracic surgery versus thoracotomy. Interact Cardiovasc Thorac Surg. 2019;28(2):240‐246. [DOI] [PubMed] [Google Scholar]
- 21. Gitzelmann CA, Mendoza‐Sagaon M, Talamini MA, Ahmad SA, Pegoli W Jr, Paidas CN. Cell‐mediated immune response is better preserved by laparoscopy than laparotomy. Surgery. 2000;127(1):65‐71. [DOI] [PubMed] [Google Scholar]
- 22. Lacy AM, García‐Valdecasas JC, Delgado S, et al. Laparoscopy‐assisted colectomy versus open colectomy for treatment of non‐metastatic colon cancer: a randomised trial. Lancet. 2002;359(9325):2224‐2229. [DOI] [PubMed] [Google Scholar]
- 23. Dedej T, Lamaj E, Marku N, Ostreni V, Bilali S. Alterations in homeostasis after open surgery. A prospective randomized study. G Chir. 2013;34(7‐8):202‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mafune K, Tanaka Y. Influence of multimodality therapy on the cellular immunity of patients with esophageal cancer. Ann Surg Oncol. 2000;7(8):609‐616. [DOI] [PubMed] [Google Scholar]
- 25. Kim EY, Hong TH. Changes in total lymphocyte count and neutrophil‐to‐lymphocyte ratio after curative pancreatectomy in patients with pancreas adenocarcinoma and their prognostic role. J Surg Oncol. 2019;120(7):1102‐1111. [DOI] [PubMed] [Google Scholar]
- 26. Harris HE, RaucciA. Alarmin(g) news about danger: workshop on innate danger signals and HMGB1. EMBO Rep. 2006;7(8):774‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Timmermans K, Kox M, Vaneker M, et al. Plasma levels of danger‐associated molecular patterns are associated with immune suppression in trauma patients. Intensive Care Med. 2016;42(4):551‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lefrançais E, Cayrol C. Mechanisms of IL‐33 processing and secretion: differences and similarities between IL‐1 family members. Eur Cytokine Netw. 2012;23(4):120‐127. [DOI] [PubMed] [Google Scholar]
- 29. Lefrançais E, Roga S, Gautier V, et al. IL‐33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. PNAS. 2012;109(5):1673‐1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vogl T, Stratis A, Wixler V, et al. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J Clin Invest. 2018;128(5):1852‐1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kawai C, Kotani H, Miyao M, et al. Circulating extracellular histones are clinically relevant mediators of multiple organ injury. Am J Pathol. 2016;186(4):829‐843. [DOI] [PubMed] [Google Scholar]
- 32. Abrams ST, Zhang N, Manson J, et al. Circulating histones are mediators of trauma‐associated lung injury. Am J Respir Crit Care Med. 2013;187(2):160‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burk A‐M, Martin M, Flierl MA, et al. Early complementopathy after multiple injuries in humans. Shock (Augusta, GA). 2012;37(4):348‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paredes RM, Reyna S, Vernon P, Tadaki DK, Dallelucca JJ, Sheppard F. Generation of complement molecular complex C5b‐9 (C5b‐9) in response to poly‐traumatic hemorrhagic shock and evaluation of C5 cleavage inhibitors in non‐human primates. Int Immunopharmacol. 2018;54:221‐225. [DOI] [PubMed] [Google Scholar]
- 35. Li Y, Yang Z, Chavko M, et al. Complement inhibition ameliorates blast‐induced acute lung injury in rats: potential role of complement in intracellular HMGB1‐mediated inflammation. PLoS One. 2018;13(8):e0202594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cedervall J, Zhang Y, Olsson A‐K. Tumor‐induced NETosis as a risk factor for metastasis and organ failure. Cancer Res. 2016;76(15):4311‐4315. [DOI] [PubMed] [Google Scholar]
- 37. Sandler N, Kaczmarek E, Itagaki K, et al. Mitochondrial DAMPs are released during cardiopulmonary bypass surgery and are associated with postoperative atrial fibrillation. Heart Lung & Circ. 2018;27(1):122‐129. [DOI] [PubMed] [Google Scholar]
- 38. Lotfi R, Steppe L, Hang R, et al. ATP promotes immunosuppressive capacities of mesenchymal stromal cells by enhancing the expression of indoleamine dioxygenase. Immun Inflamm Dis. 2018;6(4):448‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuravi SJ, Yates CM, Foster M, et al. Changes in the pattern of plasma extracellular vesicles after severe trauma. PLoS One. 2017;12(8):e0183640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout‐associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237‐241. [DOI] [PubMed] [Google Scholar]
- 41. Alvarez K, Vasquez G. Damage‐associated molecular patterns and their role as initiators of inflammatory and auto‐immune signals in systemic lupus erythematosus. Int Rev Immunol. 2017;36(5):259‐270. [DOI] [PubMed] [Google Scholar]
- 42. Rodríguez‐Nuevo A, Zorzano A. The sensing of mitochondrial DAMPs by non‐immune cells. Cell Stress. 2019;3(6):195‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu G, Zhu Q, Zeng J, et al. Extracellular mitochondrial DNA promote NLRP3 inflammasome activation and induce acute lung injury through TLR9 and NF‐kappaB. J Thorac Dis. 2019;11(11):4816‐4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Timmermans K, Kox M, Vaneker M, et al. Plasma levels of danger‐associated molecular patterns are associated with immune suppression in trauma patients. Intensive Care Med. 2016;42(4):551‐561. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schindler SM, Frank MG, Annis JL, Maier SF, Klegeris A. Pattern recognition receptors mediate pro‐inflammatory effects of extracellular mitochondrial transcription factor A (TFAM). Mol Cell Neurosci. 2018;89:71‐79. [DOI] [PubMed] [Google Scholar]
- 47. Velloso FJ, Trombetta‐Lima M, Anschau V, Sogayar MC, Correa RG. NOD‐like receptors: major players (and targets) in the interface between innate immunity and cancer. Biosci Rep. 2019;39(4):BSR20181709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Darrabie MD, Cheeseman J, Limkakeng AT, et al. Toll‐like receptor activation as a biomarker in traumatically injured patients. J Surg Res. 2018;231:270‐277. [DOI] [PubMed] [Google Scholar]
- 49. Miyake K, Shibata T, Ohto U, et al. Mechanisms controlling nucleic acid‐sensing Toll‐like receptors. Int Immunol. 2018;30(2):43‐51. [DOI] [PubMed] [Google Scholar]
- 50. Pandolfi F, Altamura S, Frosali S, Conti P. Key role of DAMP in inflammation, cancer, and tissue repair. Clin Ther. 2016;38(5):1017‐1028. [DOI] [PubMed] [Google Scholar]
- 51. He Y, Zha J, Wang Y, Liu W, Yang X, Yu P. Tissue damage–associated “danger signals” influence T‐cell responses that promote the progression of preneoplasia to cancer. Cancer Res. 2013;73(2):629‐639. [DOI] [PubMed] [Google Scholar]
- 52. Darrabie MD, Cheeseman J, Limkakeng AT, et al. Toll‐like receptor activation as a biomarker in traumatically injured patients. J Surg Res. 2018;231:270‐277. [DOI] [PubMed] [Google Scholar]
- 53. McIlroy DJ, Bigland M, White AE, et al. Cell necrosis‐independent sustained mitochondrial and nuclear DNA release following trauma surgery. J Trauma Acute Care Surg. 2015;78(2):282‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Merkle J, Daka A, Deppe AC, Wahlers T, Paunel‐Görgülü A. High levels of cell‐free DNA accurately predict late acute kidney injury in patients after cardiac surgery. PLoS One. 2019;14(6):e0218548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Simmons JD, Lee YL, Mulekar S, et al. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013;258(4):591‐596. Discussion 596–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Anunobi R, Boone BA, Cheh N, et al. Extracellular DNA promotes colorectal tumor cell survival after cytotoxic chemotherapy. J Surg Res. 2018;226:181‐191. [DOI] [PubMed] [Google Scholar]
- 57. Sharma B, McLeland CB, Potter TM, Stern ST, Adiseshaiah PP. Assessing NLRp3 inflammasome activation by nanoparticles. Methods Mol Biol. 2018;1682:135‐147. [DOI] [PubMed] [Google Scholar]
- 58. Ershaid N, Sharon Y, Doron H, et al. NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat Commun. 2019;10(1):4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Siekmann W, Eintrei C, Magnuson A, et al. Surgical and not analgesic technique affects postoperative inflammation following colorectal cancer surgery: a prospective, randomized study. Colorectal Dis. 2017;19(6):O186‐O195. [DOI] [PubMed] [Google Scholar]
- 60. Matsuda S, Takeuchi H, Kawakubo H, et al. Correlation between intense postoperative inflammatory response and survival of esophageal cancer patients who underwent transthoracic esophagectomy. Ann Surg Oncol. 2015;22(13):4453‐4460. [DOI] [PubMed] [Google Scholar]
- 61. Chang Y, An H, Xu L, et al. Systemic inflammation score predicts postoperative prognosis of patients with clear‐cell renal cell carcinoma. Br J Cancer. 2015;113(4):626‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Watt DG, McSorley ST, Park JH, Horgan PG, McMillan DC. A postoperative systemic inflammation score predicts short‐ and long‐term outcomes in patients undergoing surgery for colorectal cancer. Ann Surg Oncol. 2017;24(4):1100‐1109. [DOI] [PubMed] [Google Scholar]
- 63. McSorley ST, Watt DG, Horgan PG, McMillan DC. Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann Surg Oncol. 2016;23(9):2832‐2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nojiri T, Hamasaki T, Inoue M, et al. Long‐term impact of postoperative complications on cancer recurrence following lung cancer surgery. Ann Surg Oncol. 2017;24(4):1135‐1142. [DOI] [PubMed] [Google Scholar]
- 65. Kano K, Aoyama T, Nakajima T, et al. Prediction of postoperative inflammatory complications after esophageal cancer surgery based on early changes in the C‐reactive protein level in patients who received perioperative steroid therapy and enhanced recovery after surgery care: a retrospective analysis. BMC Cancer. 2017;17(1):812‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Okumura Y, Hiki N, Kumagai K, et al. Postoperative prolonged inflammatory response as a poor prognostic factor after curative resection for gastric cancer. World J Surg. 2017;41(10):2611‐2618. [DOI] [PubMed] [Google Scholar]
- 67. Tanaka M, Kanemitsu Y, Shida D, et al. Prognostic impact of intra‐abdominal/pelvic inflammation after radical surgery for locally recurrent rectal cancer. Dis Colon Rectum. 2017;60(8):827‐836. [DOI] [PubMed] [Google Scholar]
- 68. Yamamoto M, Saito H, Uejima C, et al. Prognostic value of the combination of pre‐ and postoperative C‐reactive protein in colorectal cancer patients. Surg Today. 2018;48(11):986‐993. [DOI] [PubMed] [Google Scholar]
- 69. Kono Y, Saito H, Murakami Y, et al. Postoperative ratio of the maximum C‐reactive protein level to the minimum peripheral lymphocyte count as a prognostic indicator for gastric cancer patients. Surg Today. 2019;49(3):206‐213. [DOI] [PubMed] [Google Scholar]
- 70. Hai Y, Chen N, Wu W, et al. High postoperative monocyte indicates inferior clinicopathological characteristics and worse prognosis in lung adenocarcinoma or squamous cell carcinoma after lobectomy. BMC Cancer. 2018;18(1):1011‐1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhou Z‐Q, Pang S, Yu X‐C, et al. Predictive values of postoperative and dynamic changes of inflammation indexes in survival of patients with resected colorectal cancer. Curr Med Sci. 2018;38(5):798‐808. [DOI] [PubMed] [Google Scholar]
- 72. Migita K, Matsumoto S, Wakatsuki K, et al. Postoperative serum c‐reactive protein level predicts long‐term outcomes in stage I gastric cancer. J Surg Res. 2019;242:323‐331. [DOI] [PubMed] [Google Scholar]
- 73. Albisinni S, Moussa I, Aoun F, et al. The impact of postoperative inflammatory biomarkers on oncologic outcomes of bladder cancer. Prog Urol. 2019;29(5):270‐281. [DOI] [PubMed] [Google Scholar]
- 74. Toyokawa T, Tamura T, Sakurai K, et al. Postoperative inflammation is an independent prognostic factor in patients with thoracic esophageal squamous cell carcinoma. Anticancer Res. 2019;39(6):2777‐2784. [DOI] [PubMed] [Google Scholar]
- 75. Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125(9):3347‐3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jia R, Zhou M, Tuttle CSL, Maier AB. Immune capacity determines outcome following surgery or trauma: a systematic review and meta‐analysis. Eur J Trauma Emerg Surg. 2019. 10.1007/s00068-019-01271-6 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. van der Bij GJ, Oosterling SJ, Beelen RHJ, Meijer S, Coffey JC, van Egmond M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg. 2009;249(5):727‐734. [DOI] [PubMed] [Google Scholar]
- 78. Wu L, Saxena S, Awaji M, Singh RK. Tumor‐associated neutrophils in cancer: going pro. Cancers. 2019;11(4):564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shaul ME, Fridlender ZG. Tumour‐associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16(10):601‐620. [DOI] [PubMed] [Google Scholar]
- 80. Kumagai Y, Ohzawa H, Miyato H, et al. Surgical stress increases circulating low‐density neutrophils which may promote tumor recurrence. J Surg Res. 2019;246:52‐61. [DOI] [PubMed] [Google Scholar]
- 81. Petri B, Sanz M‐J. Neutrophil chemotaxis. Cell Tissue Res. 2018;371(3):425‐436. [DOI] [PubMed] [Google Scholar]
- 82. Chen MB, Hajal C, Benjamin DC, et al. Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proc Natl Acad Sci. 2018;115(27):7022‐7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Szczerba BM, Castro‐Giner F, Vetter M, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566(7745):553‐557. [DOI] [PubMed] [Google Scholar]
- 84. Burgener SS, Schroder K. Neutrophil extracellular traps in host defense. Cold Spring Harb Perspect Biol. 2019. 10.1101/cshperspect.a037028. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tohme S, Yazdani HO, Al‐Khafaji AB, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76(6):1367‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Albrengues J, Shields MA, Ng D, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409):eaao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Najmeh S, Cools‐Lartigue J, Rayes RF, et al. Neutrophil extracellular traps sequester circulating tumor cells via β1‐integrin mediated interactions. Int J Cancer. 2017;140(10):2321‐2330. [DOI] [PubMed] [Google Scholar]
- 88. Huang H, Tohme S, Al‐Khafaji AB, et al. Damage‐associated molecular pattern‐activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62(2):600‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hu Z, Murakami T, Tamura H, et al. Neutrophil extracellular traps induce IL‐1beta production by macrophages in combination with lipopolysaccharide. Int J Mol Med. 2017;39(3):549‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. McIlroy DJ, Jarnicki AG, Au GG, et al. Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. J Crit Care. 2014;29(6):1133.e1‐5. [DOI] [PubMed] [Google Scholar]
- 91. Liu L, Mao Y, Xu B, et al. Induction of neutrophil extracellular traps during tissue injury: involvement of STING and Toll‐like receptor 9 pathways. Cell Prolif. 2019;52(3):e12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sundaram GM, Quah S, Sampath P. Cancer: the dark side of wound healing. FEBS J. 2018;285(24):4516‐4534. [DOI] [PubMed] [Google Scholar]
- 93. Antonio N, Bonnelykke‐Behrndtz ML, Ward LC, et al. The wound inflammatory response exacerbates growth of pre‐neoplastic cells and progression to cancer. Embo J. 2015;34(17):2219‐2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hofer SO, Molema G, Hermens RA, Wanebo HJ, Reichner JS, Hoekstra HJ. The effect of surgical wounding on tumour development. Eur J Surg Oncol. 1999;25(3):231‐243. [DOI] [PubMed] [Google Scholar]
- 95. Stuelten CH, Barbul A, Busch JI, et al. Acute wounds accelerate tumorigenesis by a T cell‐dependent mechanism. Cancer Res. 2008;68(18):7278‐7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Murthy SM, Goldschmidt RA, Rao LN, Ammirati M, Buchmann T, Scanlon EF. The influence of surgical trauma on experimental metastasis. Cancer. 1989;64(10):2035‐2044. [DOI] [PubMed] [Google Scholar]
- 97. Lee Y, Kollara A, May T, Brown TJ. Wounding promotes ovarian cancer progression and decreases efficacy of cisplatin in a syngeneic mouse model. J Ovarian Res. 2018;11(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dillekås H, Straume O. The link between wound healing and escape from tumor dormancy. Surg Oncol. 2019;28:50‐56. [DOI] [PubMed] [Google Scholar]
- 99. Yang J, Zhang L, Yu C, Yang X‐F, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2(1):1‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train. 2006;41(4):457‐465. [PMC free article] [PubMed] [Google Scholar]
- 101. She S, Wu X, Zheng D, et al. PSMP/MSMP promotes hepatic fibrosis through CCR2 and represents a novel therapeutic target. J Hepatol. 2020;72(3):506‐518. [DOI] [PubMed] [Google Scholar]
- 102. Muliaditan T, Caron J, Okesola M, et al. Macrophages are exploited from an innate wound healing response to facilitate cancer metastasis. Nat Commun. 2018;9(1):2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chen L, Wang J, Li S, et al. The clinical dynamic changes of macrophage phenotype and function in different stages of human wound healing and hypertrophic scar formation. Int Wound J. 2019;16(2):360‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gao XH, Tian L, Wu J, et al. Circulating CD14(+) HLA‐DR(‐/low) myeloid‐derived suppressor cells predicted early recurrence of hepatocellular carcinoma after surgery. Hepatol Res. 2017;47(10):1061‐1071. [DOI] [PubMed] [Google Scholar]
- 105. Tai LH, de Souza CT, Belanger S, et al. Preventing postoperative metastatic disease by inhibiting surgery‐induced dysfunction in natural killer cells. Cancer Res. 2013;73(1):97‐107. [DOI] [PubMed] [Google Scholar]
- 106. Ananth AA, Tai LH, Lansdell C, et al. Surgical stress abrogates pre‐existing protective T cell mediated anti‐tumor immunity leading to postoperative cancer recurrence. PLoS One. 2016;11(5):e0155947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhu X, Pribis JP, Rodriguez PC, et al. The central role of arginine catabolism in T‐cell dysfunction and increased susceptibility to infection after physical injury. Ann Surg. 2014;259(1):171‐178. [DOI] [PubMed] [Google Scholar]
- 108. Gabrilovich DI, Nagaraj S. Myeloid‐derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liu Y, Xiang X, Zhuang X, et al. Contribution of MyD88 to the tumor exosome‐mediated induction of myeloid derived suppressor cells. Am J Pathol. 2010;176(5):2490‐2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cheng R, Billet S, Liu C, et al. Periodontal inflammation recruits distant metastatic breast cancer cells by increasing myeloid‐derived suppressor cells. Oncogene. 2020;39(7):1543‐1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sinha P, Clements VK, Fulton AM, Ostrand‐Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid‐derived suppressor cells. Cancer Res. 2007;67(9):4507‐4513. [DOI] [PubMed] [Google Scholar]
- 112. Zhang Y, Liu Q, Zhang M, Yu Y, Liu X, Cao X. Fas signal promotes lung cancer growth by recruiting myeloid‐derived suppressor cells via cancer cell‐derived PGE2. J Immunol. 2009;182(6):3801‐3808. [DOI] [PubMed] [Google Scholar]
- 113. Zhao X, Rong L, Zhao X, et al. TNF signaling drives myeloid‐derived suppressor cell accumulation. J Clin Invest. 2012;122(11):4094‐4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Xu P, He H, Gu Y, et al. Surgical trauma contributes to progression of colon cancer by downregulating CXCL4 and recruiting MDSCs. Exp Cell Res. 2018;370(2):692‐698. [DOI] [PubMed] [Google Scholar]
- 115. Vrakas CN, O'Sullivan RM, Evans SE, et al. The measure of DAMPs and a role for S100A8 in recruiting suppressor cells in breast cancer lung metastasis. Immunol Invest. 2015;44(2):174‐188. [DOI] [PubMed] [Google Scholar]
- 116. Sinha P, Okoro C, Foell D, Freeze HH, Ostrand‐Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid‐derived suppressor cells. J Immunol. 2008;181(7):4666‐4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hiratsuka S, Watanabe A, Sakurai Y, et al. The S100A8‐serum amyloid A3‐TLR4 paracrine cascade establishes a pre‐metastatic phase. Nat Cell Biol. 2008;10(11):1349‐1355. [DOI] [PubMed] [Google Scholar]
- 118. Li W, Wu K, Zhao E, et al. HMGB1 recruits myeloid derived suppressor cells to promote peritoneal dissemination of colon cancer after resection. Biochem Biophys Res Commun. 2013;436(2):156‐161. [DOI] [PubMed] [Google Scholar]
- 119. Parker KH, Horn LA, Ostrand‐Rosenberg S. High‐mobility group box protein 1 promotes the survival of myeloid‐derived suppressor cells by inducing autophagy. J Leukoc Biol. 2016;100(3):463‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356‐371. [DOI] [PubMed] [Google Scholar]
- 121. Sun Z, Du C, Xu P, Miao C. Surgical trauma‐induced CCL18 promotes recruitment of regulatory T cells and colon cancer progression. J Cell Physiol. 2019;234(4):4608‐4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dong L, Zheng X, Wang K, Wang G, Zou H. Programmed death 1/programmed cell death‐ligand 1 pathway participates in gastric surgery‐induced imbalance of T‐helper 17/regulatory T cells in mice. J Trauma Acute Care Surg. 2018;85(3):549‐559. [DOI] [PubMed] [Google Scholar]
- 123. Wu M, Chen X, Lou J, et al. Changes in regulatory T cells in patients with ovarian cancer undergoing surgery: preliminary results. Int Immunopharmacol. 2017;47:244‐250. [DOI] [PubMed] [Google Scholar]
- 124. Xu P, Zhang P, Sun Z, Wang Y, Chen J, Miao C. Surgical trauma induces postoperative T‐cell dysfunction in lung cancer patients through the programmed death‐1 pathway. Cancer Immunol Immunother. 2015;64(11):1383‐1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sun Z, Mao A, Wang Y, et al. Treatment with anti‐programmed cell death 1 (PD‐1) antibody restored postoperative CD8+ T cell dysfunction by surgical stress. Biomed Pharmacother. 2017;89:1235‐1241. [DOI] [PubMed] [Google Scholar]
- 126. Zheng X, Dong L, Wang K, et al. MiR‐21 participates in the PD‐1/PD‐L1 pathway‐mediated imbalance of Th17/Treg cells in patients after gastric cancer resection. Ann Surg Oncol. 2019;26(3):884‐893. [DOI] [PubMed] [Google Scholar]
- 127. Ruan X, Darwiche SS, Cai C, Scott MJ, Pape H‐C, Billiar TR. Anti‐HMGB1 monoclonal antibody ameliorates immunosuppression after peripheral tissue trauma: attenuated T‐lymphocyte response and increased splenic CD11b (+) Gr‐1 (+) myeloid‐derived suppressor cells require HMGB1. Mediators Inflamm. 2015;2015:458626‐458626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Stevens NE, Chapman MJ, Fraser CK, Kuchel TR, Hayball JD, Diener KR. Therapeutic targeting of HMGB1 during experimental sepsis modulates the inflammatory cytokine profile to one associated with improved clinical outcomes. Sci Rep. 2017;7(1):5850‐5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lee J, Jackman JG, Kwun J, et al. Nucleic acid scavenging microfiber mesh inhibits trauma‐induced inflammation and thrombosis. Biomaterials. 2017;120:94‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Riedl MA, Grivcheva‐Panovska V, Moldovan D, et al. Recombinant human C1 esterase inhibitor for prophylaxis of hereditary angio‐oedema: a phase 2, multicentre, randomised, double‐blind, placebo‐controlled crossover trial. Lancet. 2017;390(10102):1595‐1602. [DOI] [PubMed] [Google Scholar]
- 131. Jayne DRW, Bruchfeld AN, Harper L, et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA‐associated vasculitis. J Am Soc Nephrol. 2017;28(9):2756‐2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cofiell R, Kukreja A, Bedard K, et al. Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood. 2015;125(21):3253‐3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wei X, Shao B, He Z, et al. Cationic nanocarriers induce cell necrosis through impairment of Na(+)/K(+)‐ATPase and cause subsequent inflammatory response. Cell Res. 2015;25(2):237‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lemke‐Miltner CD, Blackwell SE, Yin C, et al. Antibody opsonization of a TLR9 agonist‐containing virus‐like particle enhances in situ immunization. J Immunol. 2020:204(5):1386‐1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kwak T, Drews‐Elger K, Ergonul A, et al. Targeting of RAGE‐ligand signaling impairs breast cancer cell invasion and metastasis. Oncogene. 2017;36(11):1559‐1572. [DOI] [PubMed] [Google Scholar]
- 136. Reilly M, Miller RM, Thomson MH, et al. Randomized, double‐blind, placebo‐controlled, dose‐escalating phase I, healthy subjects study of intravenous OPN‐305, a humanized anti‐TLR2 antibody. Clin Pharmacol Ther. 2013;94(5):593‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hill DL, Pierson W, Bolland DJ, et al. The adjuvant GLA‐SE promotes human Tfh cell expansion and emergence of public TCRβ clonotypes. J Exp Med. 2019;216(8):1857‐1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kaplanov I, Carmi Y, Kornetsky R, et al. Blocking IL‐1β reverses the immunosuppression in mouse breast cancer and synergizes with anti‐PD‐1 for tumor abrogation. Proc Natl Acad Sci U S A. 2019;116(4):1361‐1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10(2):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Cheng Y, Ma XL, Wei YQ, Wei XW. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer. 2019;1871(2):289‐312. [DOI] [PubMed] [Google Scholar]
- 141. Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15(4):205‐218. [DOI] [PubMed] [Google Scholar]
- 142. Glasner A, Avraham R, Rosenne E, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta‐adrenergic antagonist and a cyclooxygenase‐2 inhibitor. J Immunol. 2010;184(5):2449‐2457. [DOI] [PubMed] [Google Scholar]
- 143. Lyon TD, Frank I, Shah PH, et al. The association of aspirin use with survival following radical cystectomy. J Urol. 2018;200(5):1014‐1021. [DOI] [PubMed] [Google Scholar]
- 144. Huang X‐z, Gao P, Sun J‐X, et al. Aspirin and nonsteroidal anti‐inflammatory drugs after but not before diagnosis are associated with improved breast cancer survival: a meta‐analysis. Cancer Causes & Control. 2015;26(4):589‐600. [DOI] [PubMed] [Google Scholar]
- 145. Sung JJY, Ho JMW, Chan FCH, Tsoi KKF. Low‐dose aspirin can reduce colorectal cancer mortality after surgery: a 10‐year follow‐up of 13 528 colorectal cancer patients. J Gastroenterol Hepatol. 2019;34(6):1027‐1034. [DOI] [PubMed] [Google Scholar]
- 146. Yeh C‐C, Lin J‐T, Jeng L‐B, et al. Nonsteroidal anti‐inflammatory drugs are associated with reduced risk of early hepatocellular carcinoma recurrence after curative liver resection: a nationwide cohort study. Ann Surg. 2015;261(3):521‐526. [DOI] [PubMed] [Google Scholar]
- 147. Sandini M, Ruscic KJ, Ferrone CR, et al. Intraoperative dexamethasone decreases infectious complications after pancreaticoduodenectomy and is associated with long‐term survival in pancreatic cancer. Ann Surg Oncol. 2018;25(13):4020‐4026. [DOI] [PubMed] [Google Scholar]
- 148. Aristarco V, Serrano D, Gandini S, et al. A randomized, placebo‐controlled, phase II, presurgical biomarker trial of celecoxib versus exemestane in postmenopausal breast cancer patients. Cancer Prev Res. 2016;9(5):349‐356. [DOI] [PubMed] [Google Scholar]
- 149. Grabosch SM, Shariff OM, Helm CW. Non‐steroidal anti‐inflammatory agents to induce regression and prevent the progression of cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2018;2(2):CD004121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Mao S, Wu Y, Wang R, et al. Intraoperative use of single dose of nonsteroidal anti‐inflammatory drugs was not associated with cancer recurrence and mortality after bladder cancer surgery: a retrospective study. Ann Palliat Med. 2020;9(1):8‐18. [DOI] [PubMed] [Google Scholar]
- 151. Kurtova AV, Xiao J, Mo Q, et al. Blocking PGE2‐induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517(7533):209‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zelenay S, van der Veen AG, Böttcher JP, et al. Cyclooxygenase‐dependent tumor growth through evasion of immunity. Cell. 2015;162(6):1257‐1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Puchalski T, Prabhakar U, Jiao Q, Berns B, Davis HM. Pharmacokinetic and pharmacodynamic modeling of an anti‐interleukin‐6 chimeric monoclonal antibody (siltuximab) in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2010;16(5):1652‐1661. [DOI] [PubMed] [Google Scholar]
- 154. Ponsioen CY, de Groof EJ, Eshuis EJ, et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn's disease: a randomised controlled, open‐label, multicentre trial. Lancet Gastroenterol Hepatol. 2017;2(11):785‐792. [DOI] [PubMed] [Google Scholar]
- 155. Bakos O, Lawson C, Rouleau S, Tai L‐H. Combining surgery and immunotherapy: turning an immunosuppressive effect into a therapeutic opportunity. J Immunother Cancer. 2018;6(1):86‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Tai L‐H, de Souza CT, Bélanger S, et al. Preventing postoperative metastatic disease by inhibiting surgery‐induced dysfunction in natural killer cells. Cancer Res. 2013;73(1):97‐107. [DOI] [PubMed] [Google Scholar]
- 157. Tai LH, Tanese de Souza C, Sahi S, et al. A mouse tumor model of surgical stress to explore the mechanisms of postoperative immunosuppression and evaluate novel perioperative immunotherapies. J Vis Exp. 2014(85). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Tai LH, Zhang J, Auer RC. Preventing surgery‐induced NK cell dysfunction and cancer metastases with influenza vaccination. Oncoimmunology. 2013;2(11):e26618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Tai LH, Zhang J, Scott KJ, et al. Perioperative influenza vaccination reduces postoperative metastatic disease by reversing surgery‐induced dysfunction in natural killer cells. Clin Cancer Res. 2013;19(18):5104‐5115. [DOI] [PubMed] [Google Scholar]
- 160. Cata JP, Conrad C, Rezvani K. Potential use of natural killer cell transfer therapy in the perioperative period to improve oncologic outcomes. Scientifica (Cairo). 2015;2015:732438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Sun Z, Mao A, Wang Y, et al. Treatment with anti‐programmed cell death 1 (PD‐1) antibody restored postoperative CD8+ T cell dysfunction by surgical stress. Biomed Pharmacother. 2017;89:1235‐1241. [DOI] [PubMed] [Google Scholar]
- 162. Wakeley ME, Shubin NJ, Monaghan SF, Gray CC, Ayala A, Heffernan DS. Herpes virus entry mediator (HVEM): a novel potential mediator of trauma‐induced immunosuppression. J Surg Res. 2020;245:610‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Zhang CY, Dong X, Gao J, Lin W, Liu Z, Wang Z. Nanoparticle‐induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Sci Adv. 2019;5(11):eaax7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. O'Byrne PM, Metev H, Puu M, et al. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: a randomised, double‐blind, placebo‐controlled trial. Lancet Respir Med. 2016;4(10):797‐806. [DOI] [PubMed] [Google Scholar]
- 165. Suda K, Kitagawa Y, Ozawa S, et al. Neutrophil elastase inhibitor improves postoperative clinical courses after thoracic esophagectomy. Dis Esophagus. 2007;20(6):478‐486. [DOI] [PubMed] [Google Scholar]
- 166. Park J, Wysocki RW, Amoozgar Z, et al. Cancer cells induce metastasis‐supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8(361):361ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Raghu G, Martinez FJ, Brown KK, et al. CC‐chemokine ligand 2 inhibition in idiopathic pulmonary fibrosis: a phase 2 trial of carlumab. Eur Respir J. 2015;46(6):1740‐1750. [DOI] [PubMed] [Google Scholar]
- 168. Brana I, Calles A, LoRusso PM, et al. Carlumab, an anti‐C‐C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open‐label, multicenter phase 1b study. Targeted Oncol. 2015;10(1):111‐123. [DOI] [PubMed] [Google Scholar]
- 169. Sandhu SK, Papadopoulos K, Fong PC, et al. A first‐in‐human, first‐in‐class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC‐chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71(4):1041‐1050. [DOI] [PubMed] [Google Scholar]
- 170. Nywening TM, Wang‐Gillam A, Sanford DE, et al. Targeting tumour‐associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single‐centre, open‐label, dose‐finding, non‐randomised, phase 1b trial. Lancet Oncol. 2016;17(5):651‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Cassier PA, Italiano A, Gomez‐Roca CA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse‐type tenosynovial giant cell tumours of the soft tissue: a dose‐escalation and dose‐expansion phase 1 study. Lancet Oncol. 2015;16(8):949‐956. [DOI] [PubMed] [Google Scholar]
- 172. Rüter J, Antonia SJ, Burris HA, Huhn RD, Vonderheide RH. Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors. Cancer Biol Ther. 2010;10(10):983‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Beatty GL, Torigian DA, Chiorean EG, et al. A phase I study of an agonist CD40 monoclonal antibody (CP‐870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19(22):6286‐6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Smith DA, Conkling P, Richards DA, et al. Antitumor activity and safety of combination therapy with the Toll‐like receptor 9 agonist IMO‐2055, erlotinib, and bevacizumab in advanced or metastatic non‐small cell lung cancer patients who have progressed following chemotherapy. Cancer Immunol Immunother. 2014;63(8):787‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Machiels J‐P, Kaminsky M‐C, Keller U, et al. Phase Ib trial of the Toll‐like receptor 9 agonist IMO‐2055 in combination with 5‐fluorouracil, cisplatin, and cetuximab as first‐line palliative treatment in patients with recurrent/metastatic squamous cell carcinoma of the head and neck. Invest New Drugs. 2013;31(5):1207‐1216. [DOI] [PubMed] [Google Scholar]
- 177. Chan E, Kwak EL, Hwang J, Heiskala M, de La Bourdonnaye G, Mita M. Open‐label phase 1b study of FOLFIRI plus cetuximab plus IMO‐2055 in patients with colorectal cancer who have progressed following chemotherapy for advanced or metastatic disease. Cancer Chemother Pharmacol. 2015;75(4):701‐709. [DOI] [PubMed] [Google Scholar]
- 178. Donin NM, Chamie K, Lenis AT, et al. A phase 2 study of TMX‐101, intravesical imiquimod, for the treatment of carcinoma in situ bladder cancer. Urol Oncol. 2017;35(2):39.e31‐39.e37. [DOI] [PubMed] [Google Scholar]
- 179. Arits AHMM, Mosterd K, Essers BA, et al. Photodynamic therapy versus topical imiquimod versus topical fluorouracil for treatment of superficial basal‐cell carcinoma: a single blind, non‐inferiority, randomised controlled trial. Lancet Oncol. 2013;14(7):647‐654. [DOI] [PubMed] [Google Scholar]
- 180. Salazar LG, Lu H, Reichow JL, et al. Topical imiquimod plus Nab‐paclitaxel for breast cancer cutaneous metastases: a phase 2 clinical trial. JAMA Oncol. 2017;3(7):969‐973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Advani R, Flinn I, Popplewell L, et al. CD47 blockade by Hu5F9‐G4 and rituximab in non‐Hodgkin's lymphoma. N Engl J Med. 2018;379(18):1711‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Germano G, Frapolli R, Simone M, et al. Antitumor and anti‐inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res. 2010;70(6):2235‐2244. [DOI] [PubMed] [Google Scholar]
- 183. Gadducci A, Grosso F, Scambia G, et al. A phase II randomised (calibrated design) study on the activity of the single‐agent trabectedin in metastatic or locally relapsed uterine leiomyosarcoma. Br J Cancer. 2018;119(5):565‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Kawai A, Araki N, Sugiura H, et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation‐related sarcoma: a randomised, open‐label, phase 2 study. Lancet Oncol. 2015;16(4):406‐416. [DOI] [PubMed] [Google Scholar]
- 185. Yokoyama Y, Lew ED, Seelige R, et al. Immuno‐oncological efficacy of RXDX‐106, a novel TAM (TYRO3, AXL, MER) family small‐molecule kinase inhibitor. Cancer Res. 2019;79(8):1996‐2008. [DOI] [PubMed] [Google Scholar]
- 186. Lu Z, Zou J, Li S, et al. Epigenetic therapy inhibits metastases by disrupting premetastatic niches. Nature. 2020;579(7798):284‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Tai L‐H, Alkayyal AA, Leslie AL, et al. Phosphodiesterase‐5 inhibition reduces postoperative metastatic disease by targeting surgery‐induced myeloid derived suppressor cell‐dependent inhibition of natural killer cell cytotoxicity. Oncoimmunology. 2018;7(6):e1431082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Tobin RP, Davis D, Jordan KR, McCarter MD. The clinical evidence for targeting human myeloid‐derived suppressor cells in cancer patients. J Leukoc Biol. 2017;102(2):381‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Schott AF, Goldstein LJ, Cristofanilli M, et al. Phase Ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER‐2‐negative metastatic breast cancer. Clin Cancer Res. 2017;23(18):5358‐5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Opfermann P, Derhaschnig U, Felli A, et al. A pilot study on reparixin, a CXCR1/2 antagonist, to assess safety and efficacy in attenuating ischaemia‐reperfusion injury and inflammation after on‐pump coronary artery bypass graft surgery. Clin Exp Immunol. 2015;180(1):131‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Rennard SI, Dale DC, Donohue JF, et al. CXCR2 antagonist MK‐7123. A phase 2 proof‐of‐concept trial for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(9):1001‐1011. [DOI] [PubMed] [Google Scholar]
- 192. Taki M, Abiko K, Baba T, et al. Snail promotes ovarian cancer progression by recruiting myeloid‐derived suppressor cells via CXCR2 ligand upregulation. Nat Commun. 2018;9(1):1685‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Mirza N, Fishman M, Fricke I, et al. All‐trans‐retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66(18):9299‐9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI. Mechanism of all‐trans retinoic acid effect on tumor‐associated myeloid‐derived suppressor cells. Cancer Res. 2007;67(22):11021‐11028. [DOI] [PubMed] [Google Scholar]
- 195. Wiers KM, Lathers DM, Wright MA, Young MR. Vitamin D3 treatment to diminish the levels of immune suppressive CD34+ cells increases the effectiveness of adoptive immunotherapy. J Immunother. 2000;23(1):115‐124. [DOI] [PubMed] [Google Scholar]
- 196. Najjar YG, Finke JH. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol. 2013;3:49‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Kadosawa T, Watabe A. The effects of surgery‐induced immunosuppression and angiogenesis on tumour growth. Vet J. 2015;205(2):175‐179. [DOI] [PubMed] [Google Scholar]
- 198. Tagliabue E, Agresti R, Carcangiu ML, et al. Role of HER2 in wound‐induced breast carcinoma proliferation. Lancet. 2003;362(9383):527‐533. [DOI] [PubMed] [Google Scholar]
- 199. Hormbrey E, Han C, Roberts A, McGrouther DA, Harris AL. The relationship of human wound vascular endothelial growth factor (VEGF) after breast cancer surgery to circulating VEGF and angiogenesis. Clin Cancer Res. 2003;9(12):4332‐4339. [PubMed] [Google Scholar]
- 200. Wang J, Su X, Yang L, et al. The influence of myeloid‐derived suppressor cells on angiogenesis and tumor growth after cancer surgery. Int J Cancer. 2016;138(11):2688‐2699. [DOI] [PubMed] [Google Scholar]
- 201. Brivio F, Lissoni P, Rovelli F, et al. Effects of IL‐2 preoperative immunotherapy on surgery‐induced changes in angiogenic regulation and its prevention of VEGF increase and IL‐12 decline. Hepatogastroenterology. 2002;49(44):385‐387. [PubMed] [Google Scholar]
- 202. Tsunemi T, Nagoya S, Kaya M, et al. Postoperative progression of pulmonary metastasis in osteosarcoma. Clin Orthop Relat Res. 2003(407):159‐166. [DOI] [PubMed] [Google Scholar]
- 203. Zhu Q, Zang Q, Jiang Z‐M, et al. Clinical application of recombinant human endostatin in postoperative early complementary therapy on patients with non‐small cell lung cancer in Chinese mainland. Asian Pac J Cancer Prev. 2015;16(9):4013‐4018. [DOI] [PubMed] [Google Scholar]
- 204. Renaud S, Schaeffer M, Falcoz PE, et al. Perioperative bevacizumab improves survival following lung metastasectomy for colorectal cancer in patients harbouring v‐Ki‐ras2 Kirsten rat sarcoma viral oncogene homologue exon 2 codon 12 mutationsdagger. Eur J Cardiothorac Surg. 2017;51(2):255‐262 [DOI] [PubMed] [Google Scholar]
- 205. van der Bilt JDW, Borel Rinkes IHM. Surgery and angiogenesis. Biochim Biophys Acta. 2004;1654(1):95‐104. [DOI] [PubMed] [Google Scholar]
- 206. Kappos L, Wiendl H, Selmaj K, et al. Daclizumab HYP versus interferon beta‐1a in relapsing multiple sclerosis. N Engl J Med. 2015;373(15):1418‐1428. [DOI] [PubMed] [Google Scholar]
- 207. Giovannoni G, Gold R, Selmaj K, et al. Daclizumab high‐yield process in relapsing‐remitting multiple sclerosis (SELECTION): a multicentre, randomised, double‐blind extension trial. Lancet Neurol. 2014;13(5):472‐481. [DOI] [PubMed] [Google Scholar]
- 208. Kim YH, Bagot M, Pinter‐Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T‐cell lymphoma (MAVORIC): an international, open‐label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192‐1204. [DOI] [PubMed] [Google Scholar]
- 209. Duvic M, Pinter‐Brown LC, Foss FM, et al. Phase 1/2 study of mogamulizumab, a defucosylated anti‐CCR4 antibody, in previously treated patients with cutaneous T‐cell lymphoma. Blood. 2015;125(12):1883‐1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Sakamoto Y, Ishida T, Masaki A, et al. CCR4 mutations associated with superior outcome of adult T‐cell leukemia/lymphoma under mogamulizumab treatment. Blood. 2018;132(7):758‐761. [DOI] [PubMed] [Google Scholar]
- 211. Tigue NJ, Bamber L, Andrews J, et al. MEDI1873, a potent, stabilized hexameric agonist of human GITR with regulatory T‐cell targeting potential. Oncoimmunology. 2017;6(3):e1280645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Forero‐Torres A, Ramchandren R, Yacoub A, et al. Parsaclisib, a potent and highly selective PI3Kδ inhibitor, in patients with relapsed or refractory B‐cell malignancies. Blood. 2019;133(16):1742‐1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Yue EW, Li Y‐L, Douty B, et al. INCB050465 (Parsaclisib), a novel next‐generation inhibitor of phosphoinositide 3‐kinase delta (PI3Kδ). ACS Med Chem Lett. 2019;10(11):1554‐1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Pisarska M, Torbicz G, Gajewska N, et al. Compliance with the ERAS protocol and 3‐year survival after laparoscopic surgery for non‐metastatic colorectal cancer. World J Surg. 2019;43(10):2552‐2560. [DOI] [PubMed] [Google Scholar]
- 215. Wang W‐K, Tu C‐Y, Shao C‐X, et al. Impact of enhanced recovery after surgery on postoperative rehabilitation, inflammation, and immunity in gastric carcinoma patients: a randomized clinical trial. Braz J Med Biol Res (Rev Bras Pesqui Med Biol). 2019;52(5):e8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Gustafsson UO, Oppelstrup H, Thorell A, Nygren J, Ljungqvist O. Adherence to the ERAS protocol is associated with 5‐year survival after colorectal cancer surgery: a retrospective cohort study. World J Surg. 2016;40(7):1741‐1747. [DOI] [PubMed] [Google Scholar]


