Abstract
Background: There are interesting studies on glioma therapy with modulated electrohyperthermia (mEHT), which combines heat therapy with an electric field. Clinical researchers not only found the mEHT method feasible for palliation but also reported evidence of therapeutic response. Purpose: To study the efficacy and safety of mEHT for the treatment of relapsed malignant glioma and astrocytoma versus best supportive care (BSC). Methods: We collected data retrospectively on 149 patients affected by malignant glioma and astrocytoma. Inclusion criteria were informed consent signed; >18 years old; histological diagnosis of malignant glioma or astrocytoma; relapsed after surgery, adjuvant temozolomide-based chemotherapy, and radiotherapy; and indication for treatment with mEHT in palliative setting. mEHT was performed with capacitive coupling technique keeping the skin surface at 26°C and the tumor temperature at 40°C to 42.5°C for > 90% of treatment duration (20-60 minutes). The applied power was 40 to 150 W using a step-up heating protocol. Results from patients treated with mEHT were compared with those treated with BSC. Results: A total of 149 consecutive patients were enrolled in the study, 111 (74%) had glioblastoma multiforme (GBM), and 38 (26%) had astrocytoma (AST). mEHT was performed for 28 (25%) of GBM and 24 (63%) of AST patients. Tumor response at the 3-month follow-up was observed in 29% and 48% of GBM and AST patients after mEHT, and in 4% and 10% of GBM and AST patients after BSC, respectively. The survival rate at first and second year in the mEHT group was 77.3% and 40.9% for AST, and 61% and 29% for GBM, respectively. The 5-year overall survival of AST was 83% after mEHT versus 25% after BSC and 3.5% after mEHT versus 1.2% after BSC for GBM. The median overall survival of mEHT was 14 months (range 2-108 months) for GBM and 16.5 months (range 3-156 months) for the AST group. We observed 4 long-term survivors in the AST and 2 in the GBM group. Two of the long survivors in AST and 1 in GBM group were treated by mEHT. Conclusions: mEHT in integrative therapy may have a promising role in the treatment and palliation of relapsed GBM and AST.
Keywords: relapsed malignant glioma, modulated electrohyperthermia, survival, tumor response, astrocytoma
Introduction
Hyperthermia has been applied since the 1970s when heat was shown to kill tumor cells because they are more sensitive to heat than healthy cells. In the past decade, hyperthermia has been applied to several types of cancers with encouraging results in both tumor response and safety.1 The most frequently used methods of hyperthermia for cancer treatment are magnetic nanoparticles (mNPs), external radiofrequency, hyperthermic perfusion, modulated electrohyperthermia (mEHT), frequency enhancers associated with a magnetic field, and catheter-mediated hyperthermia.2-5
The combination of traditional hyperthermia (41°C to 43°C) to radiotherapy (RT) or systemic chemotherapy improves and prolongs the clinical benefits of the single methods.6,7 This synergistic effect is due to apoptosis induction, angiogenesis inhibition, chemo- and radiosensitivity activation, high drug concentration induction inside the lesion, and an increase in the tumor sensitivity to immunotherapy.8,9 Gliomas form the majority of brain tumors (80%), and glioblastoma multiforme (GBM) represents 65% of these tumors.10 The prognosis is rather poor due to the infiltration of the surrounding brain tissues and the resistance to chemotherapy and ET.11 Astrocytoma tumors (AST) have a better prognosis than GBM12; however, the incidence is lower than GMB.
Surgery, adjuvant temozolomide (TMZ) and RT is the first treatment option for gliomas, and RT combined with chemotherapy and TMZ is the standard therapy when surgery is not indicated.13,14 Adjuvant therapy with RT in combination with TMZ is effective in prolonging survival13; however, the disease recurrence rate is very high (37%).14
There is no standard treatment option for recurrent gliomas. Choosing the therapy after progression is very challenging and options include combinations of surgery, re-irradiation (re-RT), chemotherapy, anti-angiogenic agents, and combination therapies of hyperthermia with chemotherapy or RT.15-18 In this case, surgery can be performed only for young and fit patients with small-size relapses.
There are interesting studies on glioma therapy with mEHT, which combines the heat therapy with an electric field.21-24 and the literature shows that radiofrequency combined with conventional hyperthermia is an effective treatment for brain tumors.19,20 Brain tumor therapy with an electric field is approved by the US Food and Drug Administration (FDA).23 Clinical researchers have not only found the mEHT method feasible for palliation but have also reported evidence of therapeutic response.16,25,26,27 Literature reports good tolerability with rare adverse events and improvement of survival and quality of life.24-26 We have previously reported beneficial effects from mEHT treatment in our previous study on palliation, with evidence of tumor response and a slight increase of overall survival.16,25 In this article, we describe the efficacy and safety of mEHT on relapsed malignant GBM and AST in a larger number of patients, and compare the results with results obtained from patients treated with conventional palliation as best supportive care (BSC).
Materials and Methods
Patient Selection
This was a retrospective observational multicenter cohort-controlled study, including 4 Italian hospitals: Pesaro, Carrara, Empoli, and L’Aquila, 3 of which had mEHT devices (Pesaro, Carrara, and Empoli). Inclusion criteria were diagnosis of GBM or AST relapsed after surgery, adjuvant RT and TMZ therapy and no second line therapy performed; >18 years old; informed consent signed; Eastern Cooperative Oncology Group (ECOG) performance status 0 to 3; and normal values of standard hematological parameters.
From April 2003 to January 2018, 187 patients with GBM and AST were sent to our centers, 31 of these were lost to follow-up and were not evaluable for tumor response and survival. Seven were not refractory to treatment and were still in remission, and mEHT was used to delay tumor progression. Finally, 149 patients were included in the study: 111 (74%) had GBM and 38 (26%) had AST. The mEHT was administered as palliative care to 28 (25%) GBM patients and 22 (58%) AST patients (Tables 1 and 2). The remaining patients (83 GBM and 14 AST) received BSC, including dexamethasone, 18% glycerol infusion, mannitol, holistic therapy, and psychosocial support. Nitrosoureas or cisplatin-based chemotherapies were allowed in the BSC protocol. The BSC group included 28 (30%) patients (GBM) that received chemotherapy: 12 rechallenge of TMZ, 8 nitrosurea, and 8 cisplatin-based chemotherapies. Chemotherapy was not used for mEHT group. The patient’s intention to treat was the selection basis for the mEHT group.
Table 1.
Description of AST Patients Group.
| Parameters (AST) | n | % |
|---|---|---|
| Males | 20 | 52.6 |
| Females | 18 | 47.4 |
| mEHT treated | 24 | 63.2 |
| MGMT methylated | 11 | 28.9 |
| MGMT nonmethylated | 11 | 28.9 |
| MGMT ND | 16 | 42.2 |
| IDH1 mutated | 13 | 34.2 |
| IDH1 wild type | 11 | 28.9 |
| IDH1 ND | 14 | 36.9 |
| Events | 15 | 39.5 |
| Age, years (range) | 22-80 | — |
| Survival, months (range) | 3-156 | — |
Abbreviations: AST, astrocytoma; mEHT, modulated electrohyperthermia; MGMT, O6-methylguanine-DNA methyltransferase; IDH1, isocitrate dehydrogenase 1; ND, non detected.
Table 2.
Description of GBM Patients Group.
| Parameters (GBM) | n | % |
|---|---|---|
| Males | 68 | 61.3 |
| Females | 43 | 38.7 |
| mEHT treated | 28 | 25.2 |
| MGMT methylated | 25 | 22.5 |
| MGMT nonmethylated | 27 | 24.3 |
| MGMT ND | 59 | 53.2 |
| IDH1 mutated | 13 | 11.7 |
| IDH1 wild type | 19 | 17.1 |
| IDH1 ND | 79 | 71.2 |
| Events | 86 | 77.5 |
| Age, years, range | 27-86 | — |
| Survival, months, range | 2-108 | — |
Abbreviations: GBM, glioblastoma multiforme; mEHT, modulated electrohyperthermia; MGMT, O6-methylguanine-DNA methyltransferase; IDH1, isocitrate dehydrogenase 1; ND, non detected.
Device Description and mEHT Protocol
The study was performed with modulated electro-hyperthermia (mEHT) (EHY-2000 Plus device; CE0123, Oncotherm, Troisdorf, Germany). mEHT was used to obtain predefined heating with an electric field,27,28 increasing the field power in an energy-controlled manner.16,17,24,25 The therapeutic temperature used in the mEHT treatment was estimated to range from 40°C to 42.5°C as previously reported.16,17,24-27 The mEHT technique operates with amplitude-modulated short-wave radiofrequency at a carrier frequency of 13.56 MHz. The technique combines impedance coupling with the technique of capacitive coupling. The device has 2 electric capacitive plates (electrodes): one under the patient in the treatment bed, and one movable electrode, which is positioned over the patient’s target anatomical area to be treated. The electrodes have a water bolus to make the best transfer of energy, to distribute the electric current inside the tissue following the actual form of the patient’s body, and to keep the skin temperature safely cooled by cooling the surface with the bolus. The preset skin temperature was 26°C and it did not exceed 39°C at the point of skin contact during the treatment. The electrode was 20 cm in diameter and round in shape.
Preprocedural medication administered to all patients before each mEHT to avoid brain edema was 250 mL of glycerol 18% and dexamethasone 12 mg. The selected area was treated 3 times per week for 8 weeks. At 3 months, the tumor response was assessed on computed tomography (CT) or magnetic resonance imaging (MRI) scans and if the response was positive (complete response [CR], partial response [PR], or stable disease [SD]) then the 8-week course of treatment was repeated. The treatment time and the applied power were increased in each session according to the patient’s tolerance to heat. The first treatment was always performed applying 40 W for 20 minutes (48 kJ energy). Time was gradually raised from 20 to 60 minutes and the power was increased according to a step-up power protocol from 40 up to 150 W (540 kJ) over 2 weeks.25
This protocol achieved an estimated average temperature inside the tumor volume of >40°C for more than 90% of the mEHT sessions, which was indicated by the device and controlled by the phantom measurement. The median number of mEHT treatments/patient were 22 (range 11-62). Summary of the technical mEHT parameters of the treatments is shown in Table 3.
Table 3.
Treatment Parameters.
| Practical Parameters | Value |
|---|---|
| Step-up power, W (from-to) | 40-150 |
| Average energy dose, kJ | 540 |
| Therapeutic temperature, °C | 40-42.5 |
| Treatment time/session, minutes | 60 |
| Treatment frequency (weekly) | 3 |
| Treatment cycle, weeks | 8 |
| Follow-up time, months | 16 |
Outcome Measures
MRI or CT scans were performed every 3 months to study the efficacy of the treatments. Tumor response was assessed with the response evaluation criteria in solid tumors (RECIST, v.1.1). The ECOG performance status scale was used to evaluate the functional recovery. Overall survival (OS) was computed from the date of initial diagnosis to last follow-up or death of the patient. Adverse events were recorded indicating intensity and duration, using the Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Data on O6-methylguanine-DNA-methyltransferase (MGMT) methylation28 and isocitrate dehydrogenase 1 (IDH1) mutational status29 were collected when available (Table 3). The median total follow-up time was 16 months (range 1-156 months).
Statistical Analysis
We used descriptive and Kaplan-Meier nonparametric statistical estimates. The statistical significance was measured by Student’s t test, z test for proportions, and log-rank test for Kaplan-Meier curves; P ≤ .05 indicated statistically significant difference between samples.
Results
Sample Characteristics
Median age was 60 years for GBM (range 33-86 years) and 50 years (25-71 years) for AST group. Both subgroups (GBM and AST) followed the normal distribution and the gender ratio had no significant differences, so the statistical evaluation had an optimal basis. Data for MGMT methylation and IDH1 mutational status were not available for many patients. A summary of the demographic data is shown in Tables 1 and 2.
Tumor Response
At the 3-month follow-up period, the AST patients of mEHT group showed 2 (9%) CR, 8 (36%) PR, and 6 (27%) SD. SD was also considered a positive response due to the relapsed nature of the disease in this study. For this reason, the overall positive response of AST (CR + PR + SD) was 72% after mEHT (Table 4, Figure 3A) and is significantly higher than the 37% observed after BSC (PR = 6% and SD = 31%) (P < .005). Also, the objective response (CR + PR) (45% vs 6%, P > .005) was significantly higher than that of the BSC group. Progressive disease (PD) was observed in 4 (18%) patients in mEHT and in 9 (56%) of BSC. At the 3-month follow-up period, the GBM showed 1 (4%) CR, 6 (21%) PR, and SD 8 (29%), with an overall positive response of 54% in the mEHT group, and 13 PD (46%) (Table 5, Figure 3B). The GBM treated with BSC had 2 (2%) CR, 2 (2%) PR, and 12 (14%) SD; whereas PD was observed in 62 (75%). Overall positive response of GBM treated with BCS was 19% significantly lower (P < .05) than that of mEHT group.
Table 4.
Tumor Response and Survival of AST Group.
| Results (AST) | mEHT (n) | mEHT (%) | BSC (n) | BSC (%) |
|---|---|---|---|---|
| CR | 2 | 8 | 0 | 0 |
| PR | 8 | 33 | 1 | 7 |
| SD | 6 | 25 | 5 | 36 |
| PD | 4 | 17 | 9 | 64 |
| ND | 2 | 8 | 1 | 7 |
| OS, months median (range) | 16 (3-156) | — | 16.5 (3-120) | — |
Abbreviations: AST, astrocytoma; mEHT, modulated electrohyperthermia; BSC, best supportive care; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ND, non detected; OS, overall survival.
Figure 3.
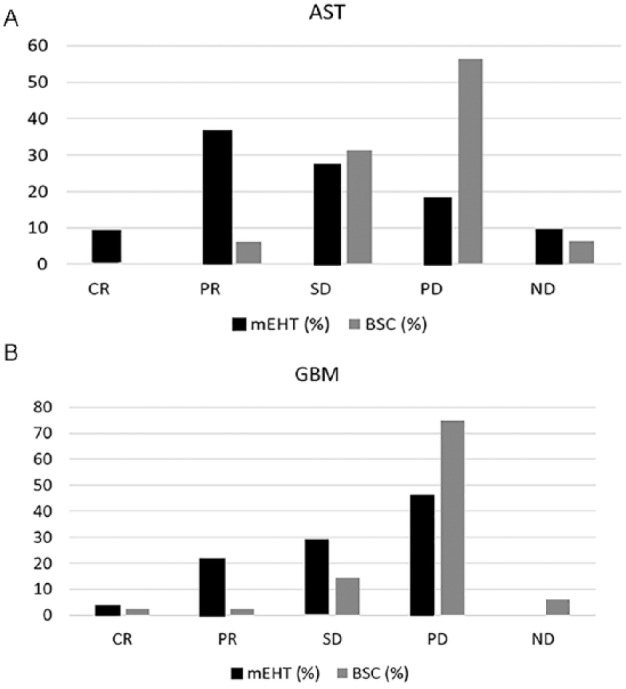
Response rates of (A) astrocytoma (AST) and (B) glioblastoma multiforme (GBM). mEHT, modulated electrohyperthermia; BSC, best supportive care; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ND, non detected
Table 5.
Tumor Response and Survival of GBM Group.
| Results (GBM) | mEHT (n) | mEHT (%) | BSC (n) | BSC (%) |
|---|---|---|---|---|
| CR | 1 | 4 | 2 | 2 |
| PR | 6 | 21 | 2 | 2 |
| SD | 8 | 29 | 12 | 14 |
| PD | 13 | 46 | 62 | 75 |
| ND | 0 | 0 | 5 | 6 |
| OS, months, median (range) | 14 (2-108) | — | 9 (2-84) | — |
Abbreviations: GBM, glioblastoma multiforme; mEHT, modulated electrohyperthermia; BSC, best supportive care; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ND, non detected; OS, overall survival.
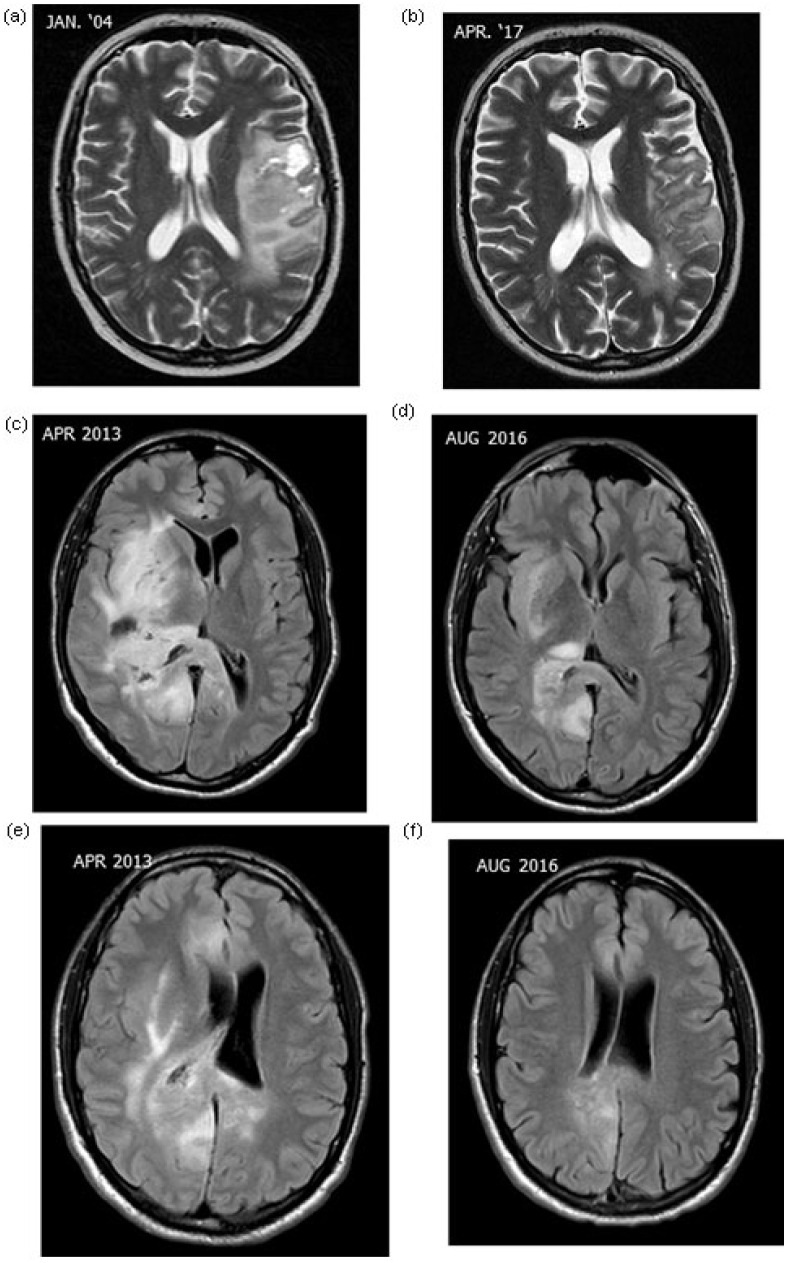
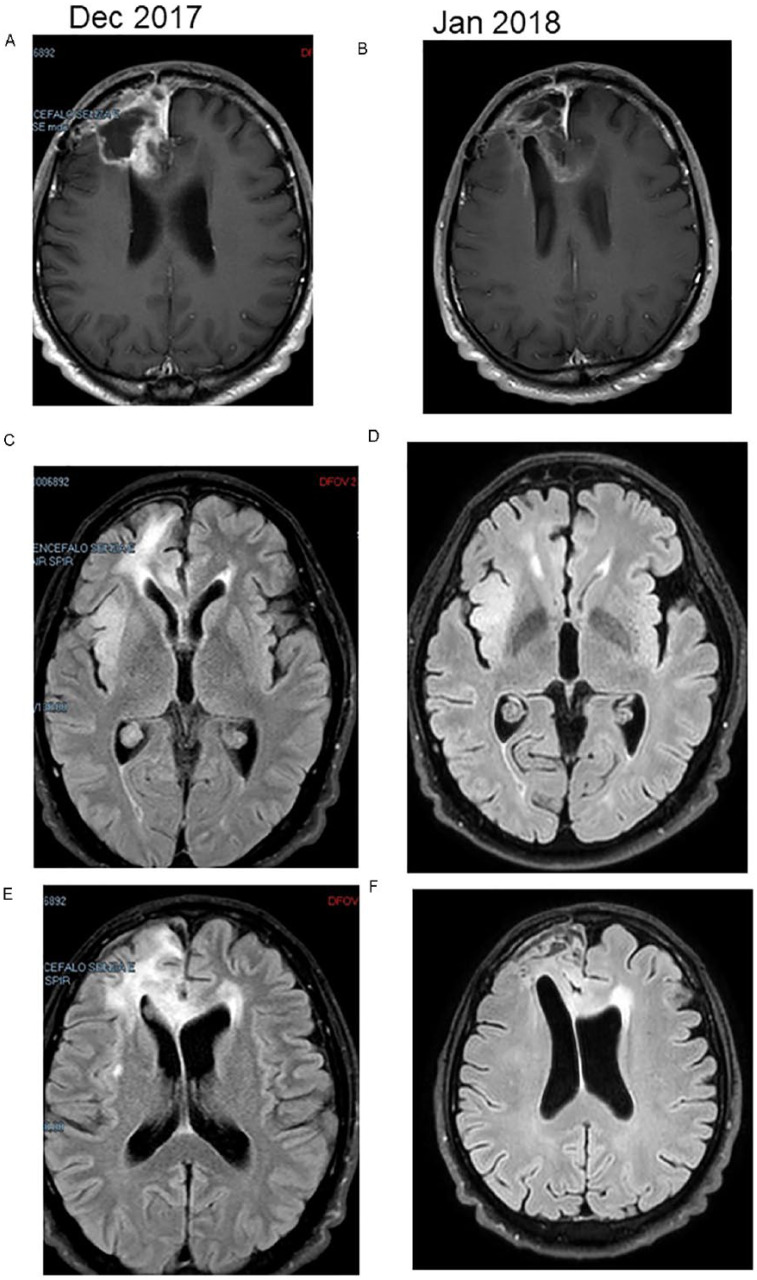
Two characteristic cases of AST and 1 case of GBM treated with mEHT are shown in Figures 1 and 2, respectively. Figure 1A and B shows relapsed AST (grade III) cases in a 24-year-old man and Figure 1C-F shows relapsed AST (grade III) in a 32-year-old man. The tumors in both cases were well controlled locally. Figure 2 shows the MRI of a patient (male, 54 years old) with relapsed GBM (grade IV).
Figure 1.
Magnetic resonance imaging of astrocytomas: case 1 (male 24 years), relapsed astrocytoma grade III (a, b) and case 2 (male, 32 years), relapsed astrocytoma grade III (c, d, e, f).
Figure 2.
Magnetic resonance imaging of glioblastoma. Relapsed glioblastoma that received 20 hyperthermia sessions with partial regression of tumor and edema (B, D, F).
Survival
The response rates of AST and GBM are shown in Figure 3A and B, respectively. The Kaplan-Meier estimates of the treatment survivals are shown for AST and GBM in Figures 4 and 5, respectively. The median OS of AST was 16.5 months (range 3-120 months) and 16 months (range 3-156 months) in the BSC and mEHT groups, respectively (P = .0065) (Figure 6). Figure 6 shows the advantage of mEHT over BSC in terms of survival for AST. Survival rate of AST at the first and second year in the mEHT group was 77.3% and 40.9%, respectively. The 5-year OS of AST was 83% after mEHT versus 25% after BSC.
Figure 4.
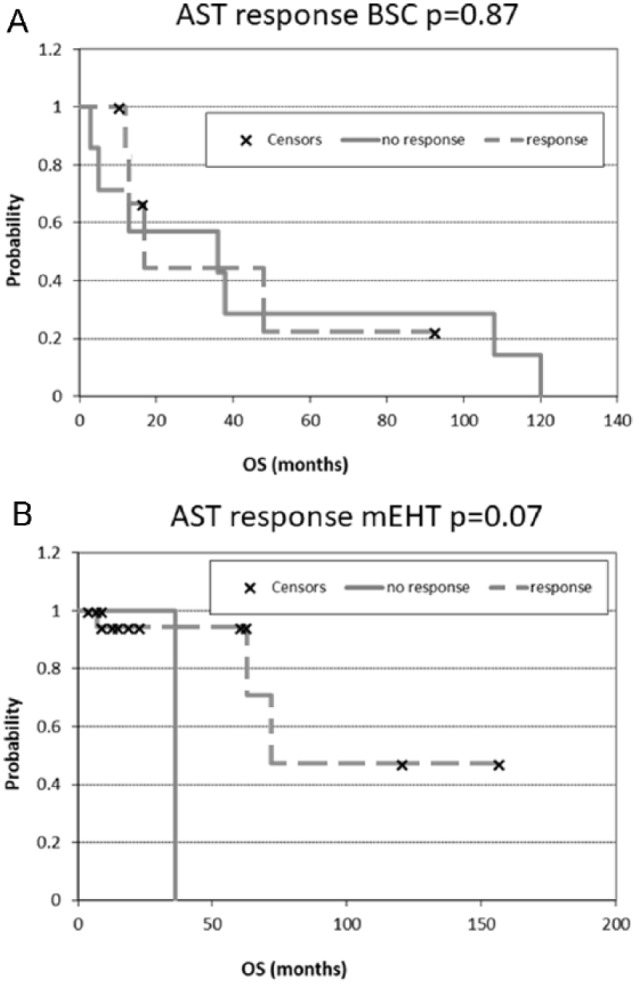
Kaplan-Meier plots for astrocytoma (AST). (A) after best supportive care (BSC) treatment and (B) after modulated electrohyperthermia (mEHT) treatment. OS, overall survival.
Figure 5.
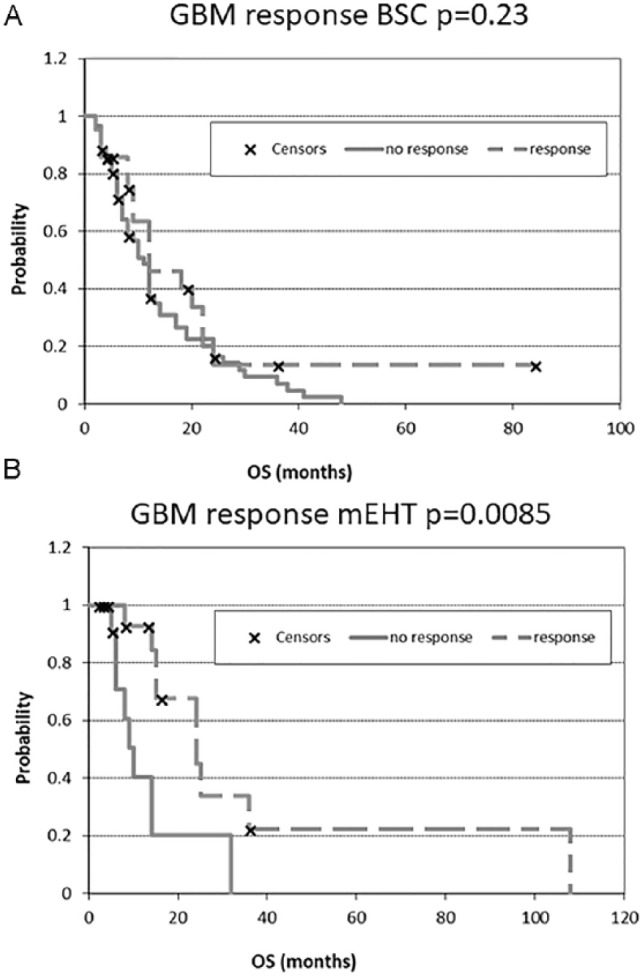
Kaplan-Meier plots for glioblastoma multiforme (GBM). (A) after best supportive care (BSC) treatment and (B) after modulated electrohyperthermia (mEHT) treatment. OS, overall survival.
Figure 6.
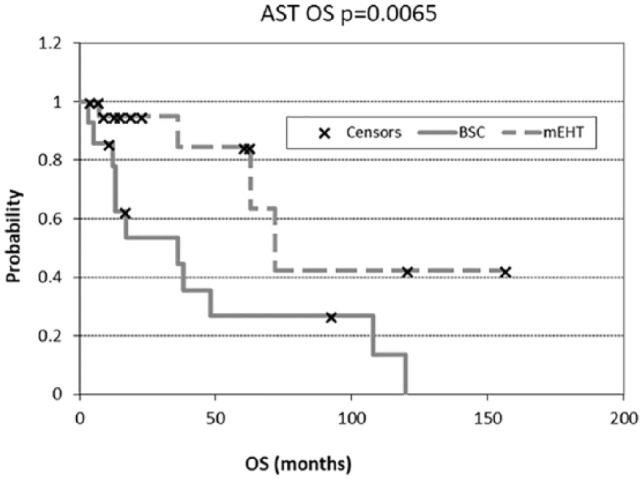
Overall survival (OS) of astrocytoma (AST) in best supportive care (BSC) and modulated electrohyperthermia (mEHT) groups.
The median OS for GBM was 14 months (range 2-108 months) after mEHT and 9 months (range 2-84 months) after BSC (Figure 7) (P = .047).
Figure 7.

Overall survival (OS) of glioblastoma multiforme (GBM) in best supportive care (BSC) and modulated electrohyperthermia (mEHT) groups.
We observed 4 long-term survivors in the AST and 2 in the GBM group. Two of the long survivors in the AST and 1 in the GBM group were treated by mEHT, with an OS of 156, 62, and 108 months, respectively. The long survivor of BSC group had a survival of 84 months.
Most patients reported a better quality of life (evaluated by subjective responses as reported during follow-up visits) after mEHT. Six patients had an objective clinical response and therapeutic benefit by mEHT measured by decreased ECOG values from 3 to 1 in 2 (9%) AST and 2 (7%) GBM patients and from 3 to 0 in 2 (9%) AST patients.
Safety
Safety of mEHT was also studied. Previously, it was hypothesized in some experts’ opinions that mEHT could not safely heat tumor cells while keeping normal temperature in non cancerous tissues.
mEHT toxicity was mostly mild (grade 1). We observed in the entire mEHT group (28 GBM and 22 AST) 1 (2%) headache, 1 (2%) scalp burn, and 5 (10%) seizures. All patients who reported seizures had experienced this symptom from the beginning of the disease. Seizure occurred during mEHT treatment in 1 case. The others had a seizure more than 1 hour after the treatment. Three patients had absence seizures and 2 had tonic-clonic spasm for a few minutes (range 1-3). Seizures were resolved with medication, including diazepam 10 mg in 100 mL of saline and levetiracetam in tablets without any further episodes. The small total number of adverse events (5%) in this study supports the strong safety profile of mEHT.
Cardiac evaluation was performed for all patients with electrocardiography and echocardiography before and after the cycle of mEHT. No significant variations were observed.
Discussion
Relapsed glioma has a poor prognosis, and there are currently no standard treatments available. The main limitation of systemic drugs is blockage from reaching their target by the blood-brain barrier.12 The absence of a standard treatment exposes the patients to physician choices, which range from surgery (not always indicated) to second-/third-line chemotherapies, re-irradiation, biodegradable carmustine wafers, gene therapy, and mEHT.12,15-17,19,21,24-26
The method of mEHT is effective in relapsed gliomas because the electric field–induced heating damages the tumor cells30,31 and increases apoptosis.32,33 The basis of the hyperthermia effect is that tumor cells are more sensitive than healthy cells to heat with the synergy of an electric field. Conventional hyperthermia works by applying a uniform, homogeneous overall heating up to 42°C to 43°C to targeted areas, and it can be used in association with chemotherapy or RT.5-7 The mEHT method heats selected malignant cells up to 42°C to 43°C, but the whole average temperature is lower than that obtained with the conventional method. This mild hyperthermia increases blood flow,18-20,34,35 which is necessary to support complementary chemotherapy and RT.
The main advantage of mEHT is the application of high temperature on selected cells, while keeping surrounding cells at moderate temperature. For this reason, mEHT is ideal for intracranial heating, where high temperatures of the brain could be life-threatening. The selective heating of mEHT is safer than the overall homogeneous heating of conventional hyperthermia and increases remission rate, OS, and progression-free survival.16,17,24,25 This study shows that mEHT is safe and well tolerated, rarely resulting in mild pain, burns, or discomfort. The rare adverse events were observed for only 7 patients (14%) out of the whole mEHT group (28 GBM and 22 AST) and were temporary in duration. No signs of damage to healthy tissues during mEHT therapy were observed.
The overall positive response (CR + PR + SD) of AST after mEHT was 72% and is significantly higher than the 37% observed after BSC (P < .005), as is the objective response (CR + PR) (45% vs 6% P > .005). The overall PR of GBM after mEHT is 54% and is significantly higher than that of 18% obtained with BSC (P = .001) as is the objective response (25% vs 4%, P = .016). This may suggest a better effect of mEHT than BSC. Efficacy of mEHT in terms of tumor response and survival are comparable to those obtained in other studies and in our previously reported results of mEHT.16,17,25 Some authors report a response rate 66% for GBM after mEHT; however, they observed only SD or PR and no CR.16,17,24,26
The median duration of relapse-free survival of the mEHT (AST + GBM) group was longer (16 months, range 6-120 months) compared with our previous study, which was 10 months.16 This may be due to the larger number of patients enrolled. Tumor response was associated with an improvement in performance status in 6 (12%) patients of the mEHT group (AST + GBM).
The median OS for GBM is 14 months (range 2-108 months) after mEHT and 9 months (range 2-84 months) after BSC, whereas the OS of AST is 16 months (range 3-156 months) after mEHT and 16.5 months (range 3-120 months) after BSC. These results on the median OS are similar to that of 19.5 months reported in our previous study25 and are higher than the 2.1 to 7.9 months reported by Silva et al36 in their study on magnetic hyperthermia used as a palliative therapy in recurrent gliomas. OS was comparable to results reported by Sneed et al22 (31% at the 2-year follow-up); however, that study used invasive processes, while mEHT treatment is a non invasive therapy. The difference in the 5-year OS of 83% after mEHT versus 25% after BSC in the AST group seems a relevant and important parameter.
The relationship of age to the results is interesting (Radiation Therapy Oncology Group [RTOG] classification37). In the case of BSC treatment for AST patients, age is a crucial parameter. BSC treatments are significantly better in survival for younger patients than for older (P = .04); while the mEHT treatment in AST patients is not affected by age as concerns survival (P = .82) (Figure 8). In the case of GBM patients, neither BSC nor mEHT treatments are influenced by the age of patients (P = .75 and P = .39, respectively) (Figure 9).
Figure 8.
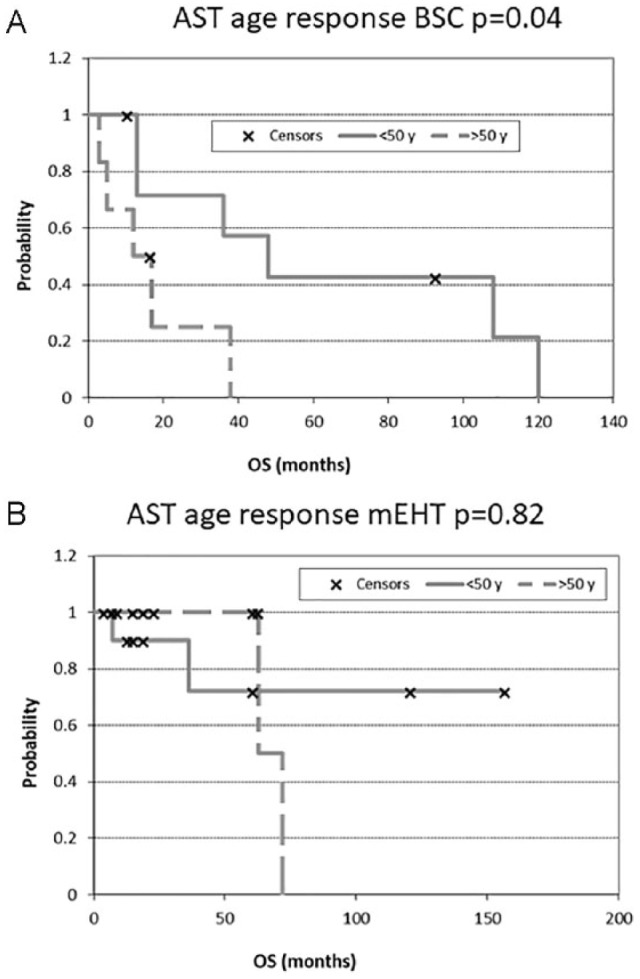
Overall survival (OS) according to astrocytoma (AST) patients’ age: (A) after best supportive care (BSC) treatment and (B) after modulated electrohyperthermia (mEHT) treatment.
Figure 9.
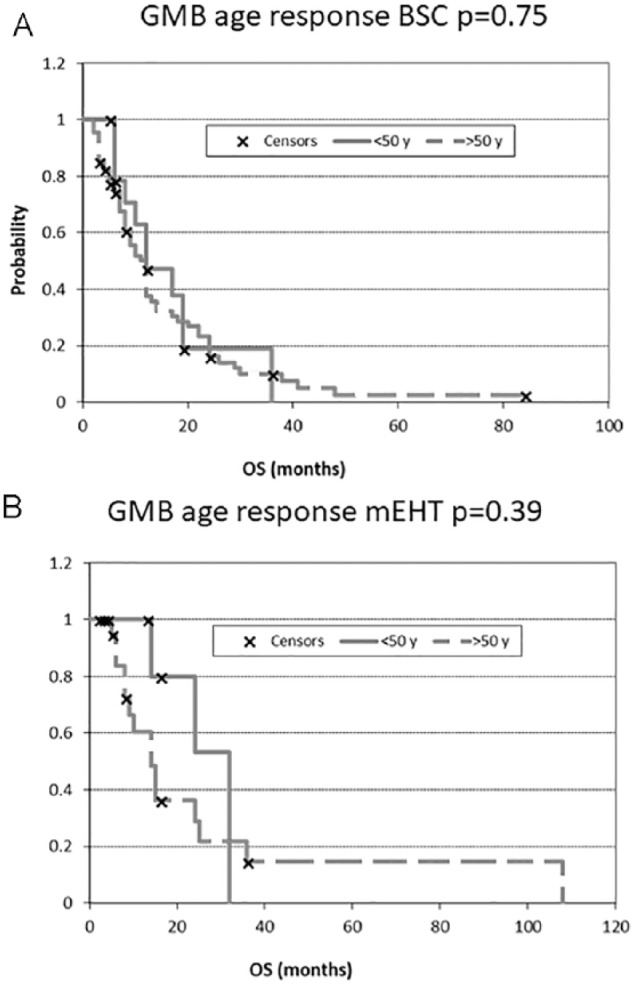
Overall survival (OS) according to glioblastoma multiforme (GBM) patients’ age: (A) after best supportive care (BSC) treatment and (B) after modulated electrohyperthermia (mEHT) treatment.
The main limitations of this study are the absence of randomization and the retrospective collection of data. The general sample of consecutive patients without arbitrary exclusions may be considered as control sample; however, there was no randomization as this was a retrospective observational study. Further multicenter randomized and prospective studies with a greater number of patients are needed to confirm these results.
Conclusions
Recurrent gliomas (GBM and AST) may benefit from mEHT, a safe and effective integrative therapy. Both GBM and AST patients showed higher response rates after mEHT than those who received best supportive care. Together with local control, the OS was also significantly improved by mEHT compared with the conventional BSC palliation in recurrent glioma patients. Only a few mild adverse events were reported. Quality of life is also improved, as assessed by subjective reports of the patients and a decrease in ECOG performance status scores. This study shows that mEHT is safe and well tolerated for the management of recurrent gliomas, rarely resulting in mild pain, burns, or discomfort. Further studies are required to confirm these results.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Mallory M, Gogineni E, Jones GC, Greer L, Simone CB., 2nd Therapeutic hyperthermia: the old, the new, and the upcoming. Crit Rev Oncol Hematol. 2016;97:56-64. [DOI] [PubMed] [Google Scholar]
- 2. Kim KS, Hernandez D, Lee SY. Time-multiplexed two-channel capacitive radiofrequency hyperthermia with nanoparticle mediation. Biomed Eng Online. 2015;14:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Datta NR, Krishnan S, Speiser DE, et al. Magnetic nanoparticle-induced hyperthermia with appropriate payloads: Paul Ehrlich’s “magic (nano) bullet” for cancer theranostics? Cancer Treat Rev. 2016;50:217-227. [DOI] [PubMed] [Google Scholar]
- 4. Solari N, Sucameli F, Gipponi M, De Cian F, Cafiero F. Laparoscopic hyperthermic isolated limb perfusion a new minimally invasive approach for HILP. Int J Hyperthermia. 2017;33:862-866. [DOI] [PubMed] [Google Scholar]
- 5. Ranieri G, Ferrari C, Di Palo A, et al. Bevacizumab-based chemotherapy combined with regional deep capacitive hyperthermia in metastatic cancer patients: a pilot study. Int J Mol Sci. 2017;18:E1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee SY, Lee NR, Cho DH, Kim JS. Treatment outcome analysis of chemotherapy combined with modulated electro-hyperthermia compared with chemotherapy alone for recurrent cervical cancer, following irradiation. Oncol Lett. 2017;14:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brüningk SC, Ijaz J, Rivens I, Nill S, Ter Haar G, Oelfke U. A comprehensive model for heat-induced radio-sensitisation. Int J Hyperthermia. 2018;34:392-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baronzio G, Gramaglia A, Fiorentini G. Hyperthermia and immunity. A brief overview. In Vivo. 2006;20:689-695. [PubMed] [Google Scholar]
- 9. Moy AJ, Tunnell JW. Combinatorial immunotherapy and nanoparticle-mediated hyperthermia. Adv Drug Deliv Rev. 2017;114:175-183. [DOI] [PubMed] [Google Scholar]
- 10. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;(17 suppl 4):iv1-iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garrett MD, Yanagihara TK, Yeh R, et al. Monitoring radiation treatment effects in glioblastoma: FLAIR volume as significant predictor of survival. Tomography. 2017;3:131-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rees JH. Diagnosis and treatment in neuro-oncology: an oncological perspective. Br J Radiol. 2011;84(spec no. 2):S82-S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459-466. [DOI] [PubMed] [Google Scholar]
- 14. Clarke JL, Iwamoto FM, Sul J, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2009;27:3861-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heo J, Kim SH, Oh YT, Chun M, Noh OK. Concurrent hyperthermia and re-irradiation for recurrent high-grade gliomas. Neoplasma. 2017;64:803-808. [DOI] [PubMed] [Google Scholar]
- 16. Fiorentini G, Giovanis P, Rossi S, et al. A phase II clinical study on relapsed malignant gliomas treated with electro-hyperthermia. In Vivo. 2006;20:721-724. [PubMed] [Google Scholar]
- 17. Roussakow SV. Clinical and economic evaluation of modulated electrohyperthermia concurrent to dose-dense temozolomide 21/28 days regimen in the treatment of recurrent glioblastoma: a retrospective analysis of a two-centre German cohort trial with systematic comparison and effect-to-treatment analysis. BMJ Open. 2017;7:e017387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wust P, Hildebrandt B, Sreenivasa G, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3:487-497. [DOI] [PubMed] [Google Scholar]
- 19. Lee Titsworth W, Murad GJ, Hoh BL, Rahman M. Fighting fire with fire: the revival of thermotherapy for gliomas. Anticancer Res. 2014;34:565-574. [PubMed] [Google Scholar]
- 20. Andocs G, Renner H, Balogh L, Fonyad L, Jakab C, Szasz A. Strong synergy of heat and modulated electro-magnetic field in tumor cell killing, study of HT29 xenograft tumors in a nude mice model. Strahlenther Onkol. 2009;185:120-126. [DOI] [PubMed] [Google Scholar]
- 21. Page RC, Ricca GF, Dohan FC. Hyperthermia for the treatment of brain tumors. In: Bicher HI, McLaren JR, Pigliucci GM. eds. Consensus of Hyperthermia for the 1990s. Part of the “Advances in Experimental Medicine and Biology” Book Series (AEMB, Volume 267). Boston, MA: Springer Verlag; 1990:145-153. [DOI] [PubMed] [Google Scholar]
- 22. Sneed PK, Stauffer PR, McDermott MW, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/− hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40:287-295. [DOI] [PubMed] [Google Scholar]
- 23. Hendrick B. FDA approves new device for brain tumor treatment. Portable device treats brain tumors with electrical fields. WebMD. https://www.webmd.com/cancer/brain-cancer/news/20110418/fda-approves-new-device-brain-tumor-treatment#1. Published April 18, 2011. Accessed April 22, 2018.
- 24. Hager ED, Sahinbas H, Groenemeyer DH, Migeod F. Prospective phase II trial for recurrent high-grade gliomas with capacitive coupled low radiofrequency (LRF) hyperthermia. J Clin Oncol. 2008;26(15 suppl):2047. [Google Scholar]
- 25. Fiorentini G, Sarti D, Milandri C, Dentico P, Mambrini A, Guadagni S. Retrospective observational clinical study on relapsed malignant gliomas treated with electro-hyperthermia. Int J Neurooncol Brain Tumor. 2017;1:9-13. [Google Scholar]
- 26. Wismeth C, Dudel C, Pascher C, et al. Transcranial electro-hyperthermia combined with alkylating chemotherapy in patients with relapsed high-grade gliomas: phase I clinical results. J Neurooncol. 2010;98:395-405. [DOI] [PubMed] [Google Scholar]
- 27. Sahinbas H, Grönemeyer DHW, Böecher E, Szász A. Retrospective clinical study of adjuvant electro-hyperthermia treatment for advanced brain-gliomas. Dtsch Z Onkol. 2007;39:154-160. [Google Scholar]
- 28. Thon N, Kreth S, Kreth FW. Personalized treatment strategies in glioblastoma: MGMT promoter methylation status. Onco Targets Ther. 2013;6:I363-I372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hodges TR, Choi BD, Bigner DD, Yan H, Sampson JH. Isocitrate dehydrogenase 1: what it means to the neurosurgeon: a review. J Neurosurg. 2013;118:1176-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun J, Guo M, Pang H, Qi J, Zhang J, Ge Y. Treatment of malignant glioma using hyperthermia. Neural Regen Res. 2013;8:2775-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andocs G, Meggyeshazi N, Balogh L, et al. Upregulation of heat shock proteins and the promotion of damage-associated molecular pattern signals in a colorectal cancer model by modulated electrohyperthermia. Cell Stress Chaperones. 2015;20:37-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andocs G, Rehman MU, Zhao QL, Tabuchi Y, Kanamori M, Kondo T. Comparison of biological effects of modulated electro-hyperthermia and conventional heat treatment in human lymphoma U937 cell. Cell Death Discov. 2016;2:16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meggyeshazi N, Andocs G, Balogh L, et al. DNA fragmentation and caspase-independent programmed cell death by modulated electrohyperthermia. Strahlenther Onkol. 2014;190:815-822. [DOI] [PubMed] [Google Scholar]
- 34. Lee SY, Kim JH, Han YH, Cho DH. The effect of modulated electro-hyperthermia on temperature and blood flow in human cervical carcinoma. Int J Hyperthermia. 2018;34:953-960. [DOI] [PubMed] [Google Scholar]
- 35. Glaser T, Han I, Wu L, Zeng X. Targeted nanotechnology in glioblastoma multiforme. Front Pharmacol. 2017;8:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Silva AC, Oliveira TR, Mamani JB, et al. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int J Nanomedicine. 2011;6:591-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scott CB, Scarantino C, Urtasun R, et al. Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG 90-06. Int J Radiat Oncol Biol Phys. 1998;40:51-55. [DOI] [PubMed] [Google Scholar]