Abstract
Objectives: To assess the clinical evidence for integrative herbal medicine therapy in the management of chemotherapy-induced peripheral neuropathy (CIPN) and hand-foot syndrome (HFS) resulting from treatments for colorectal cancer (CRC). Design: Randomized controlled trials (RCTs) were identified from major English and Chinese databases. Participants had been diagnosed with CRC by pathology and had received or were undergoing chemotherapy. Interventions included herbal medicines administered orally or topically. Controls were placebo, supportive care or conventional chemotherapy for CRC. Methods followed the Cochrane handbook. Meta-analyses were grouped by study design, outcome measure, severity, and chemotherapy. Random-effects models with 95% confidence intervals were used. Heterogeneity was assessed as I2. Results: Sixty-three RCTs (4286 participants) were included. Five used a placebo in the control groups. Fifty-eight studies tested oral herbal medicine, and 5 tested topical herbal medicine. Data were available for CIPN (60 studies) and HFS (12 studies). Fifty-seven studies combined orally administered herbal medicine with chemotherapy compared with the same chemotherapy. For CIPN, 33 studies used World Health Organization (WHO) criteria, 7 used Levi’s criteria, and 10 used the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE). These were analyzed separately. For grades III + IV CIPN, there was a significant reduction in the integrative groups for WHO (relative risk [RR] 0.42 [0.23, 0.77], I2 = 0%) and Levi’s (RR 0.28 [0.11, 0.69], I2 = 0%) but not NCI-CTCAE (RR 0.65 [0.37, 1.13], I2 = 26.4%). Hand and foot baths showed no differences for Levi’s grades III + IV CIPN but a significant reduction in all grades (RR 0.69 [0.50, 0.95], I2 = 68.8%). For HFS (all grades) there was a significant reduction in the integrative groups for WHO (RR 0.62 [0.41, 0.96], I2 = 22%) but not for NCI-CTCAE (RR 0.93 [0.55, 1.55], I2 = 75.7%). Sensitivity analyses explored sources of heterogeneity. Conclusions: Integrative herbal therapy appeared to reduce CIPN and HFS in people receiving chemotherapy for CRC. However, the strength of the evidence was limited by lack of blinding in most studies, potential for bias, and relatively short study durations.
Keywords: colorectal cancer, integrative medicine, herbal medicine, Chinese herbal medicine, chemotherapy-induced peripheral neuropathy, hand and foot syndrome, systematic review, meta-analysis
Introduction
Based on the international GLOBOCAN survey 2012, colorectal cancer (CRC) was the third most common cancer in men and the second most common in women. The age standardized rate (ASR), as cases per 100 000 of population, showed higher incidence in Europe (37.3 males; 23.6 females) and northern America (30.1 males; 22.7 females) compared with eastern Asia (22.4 males; 14.6 females).1 In China, CRC was the fifth most common cancer in men and the fourth most common in women.2 CRC incidence increased from an ASR of 12.8 (14.1 males; 11.5 females) in 2003 to 16.8 per 100 000 (19.7 males; 14.0 females) in 2011 and overall mortality rose from 5.9 to 7.8 per 100 000.3
Chemotherapy-induced peripheral neuropathy (CIPN) is a side effect of a number of chemotherapeutic agents including oxaliplatin, cisplatin, vincristine, and taxanes. CIPN mainly affects the hands and feet and the typical symptoms include numbness, parathesia, pain, and hypersensitivity to mechanical and/or cold stimuli.4 The overall prevalence of CIPN, based on a review of 31 clinical trials, was 68.1% within the first month following chemotherapy declining to 30% at 6 months or longer, however, prevalence varied considerably with the type of chemotherapy.5 In CRC, oxaliplatin-based chemotherapies are a principal cause of CIPN. This involves an acute phase soon after infusion, which typically resolves, and a chronic type which is related to the accumulated dose of oxaliplatin and can persist for years. This type can significantly impair quality of life and may be a reason for ceasing adjuvant or palliative treatment for CRC.4,6 In a study of FOLFOX4 for metastatic CRC, there was neurosensory toxicity in 68% of patients, 16.3% of patients had cumulative paresthesia that interfered with function, and 3.8% ceased treatment after 4 months or more due to sensory neuropathy.7 A study of FOLFOX4 and XELOX for metastatic CRC reported that grade III/IV neurosensory toxicity was about 17% in each regimen, whereas grade III hand-foot syndrome was 6% for XELOX versus 1% for FOLFOX4, and was 30% versus 10% for all grades.8
Hand-foot syndrome (HFS), also known as palmar-plantar erythrodysesthesia, involves tingling, numbness and/or pain of the palms and soles complicated by edema, cracking, blistering and desquamation and hyperpigmentation. Of the drugs used in CRC, capecitabine and 5-fluorouracil (5-FU) can produce HFS, which, when severe, can lead to cessation of therapy.9,10
A number of interventions for the prevention or treatment of CIPN have been tested in clinical trials. The combinations of calcium gluconate and magnesium sulfate, vitamin E, and glutathione have all shown initial promise but no convincing effects in controlled clinical trials; however, duloxetine was found to improve symptoms due to oxaliplatin or paclitaxel.11-13 Monosialotetrahexosylganglioside sodium (GM-1) injection reduced CIPN due to oxaliplatin in a retrospective study and a prospective study.14,15 A number of other systemic and topical therapies are currently undergoing clinical trials.12 The current management of HFS is mainly symptomatic with emollients, wound care, analgesics, and topical steroids for less severe reactions. A number of treatments have been trialed with promising results for turmeric16 and silymarin.17 However, effective therapies for higher grade HFS have yet to be confirmed.9,18
Previous reviews of CIPN have investigated the effects of (a) various Chinese herbs for oxaliplatin-induced CIPN in various cancers,19 (b) the herbal formula Goshajinkigan in the prevention of CIPN in various cancers,20-22 (c) and lifestyle factors, including the use of herbal medicines and supplements in the management of CRC.23
The present systematic review and meta-analysis focuses on randomized controlled trials (RCTs) that tested herbal medicines used in traditional medicine in China, Korea, and Japan in the integrative management of CIPN resulting from treatment for CRC. It aims to assess the effects of the herbal medicines on the prevention and treatment of CIPN and identify any promising directions for future research.
Methods
Searches were conducted of (a) major English language biomedical databases—PubMed, Embase, CINAHL, AHMED, and Cochrane Library; (b) major Chinese language biomedical databases—Chinese BioMedical Literature Database (CBM), VIP Database for Chinese Technical Periodicals (CQVIP), China National Knowledge Infrastructure (CNKI), and Wanfang Data from their respective inceptions to February 2018; and (c) reference lists in studies and reviews (Supplementary 1 PubMed search strategy). Only prospective RCTs were included.
Included participants had been diagnosed with colorectal, colon, or rectal cancer by pathology; had received chemotherapy; and were aged 18 years or older. Studies that included participants with other cancers or other diseases were excluded.
The test interventions were herbal medicines used in traditional medicine in China, Korea, and/or Japan. These could be administered orally and/or topically. Injections were excluded. Studies in which the details of the herbal therapy were unclear were excluded.
The control interventions were placebo for the herbal therapy, conventional chemotherapy or no additional intervention. Co-interventions were conventional chemotherapy for CRC plus usual care. The conventional therapies were required to be the same in each group. The study setting could be a hospital or clinic.
Studies that reported numerical data for an outcome directly related to CIPN and/or HFS due to chemotherapy for CRC were included.
Search results were screened by 2 reviewers and full-text articles were obtained for any paper considered a potential inclusion. These were assessed against the inclusion and exclusion criteria. Data were extracted to a predesigned spread-sheet for citation details (year, country; study design, duration, setting); methodological aspects; participant characteristics (number, age, gender, cancer type); details of interventions (herbal therapy, type of chemotherapy, type of conventional care); details of outcome measures; data for included outcome measures; safety, dropouts, and adverse events in each group. If there were any disagreements between reviewers, a third reviewer was consulted. In the case of discrepancies in the published data it was planned to contact authors but this was not required. Risk of bias was assessed using the Cochrane tool by 2 reviewers independently with a third reviewer available for consultation to resolve any issues.24
Assessments of effect sizes were based on published data and conducted in Stata 12 or RevMan 5.3. Meta-analyses were conducted when studies were comparable and used the same outcome measures. Random-effects models with 95% confidence interval (CI) were applied. Heterogeneity was quantified as I2. Publication bias was assessed using a funnel plot and Egger’s test for asymmetry when ten or more studies were available. Subgroup analyses were planned based on participant characteristics such as cancer type (colorectal, rectal, colon); type of herbal intervention; type of chemotherapy; type of conventional care; and methodological quality. Sensitivity analyses were planned to explore sources of heterogeneity and any effects of the use of Chinese medicine syndrome differentiation.
Results
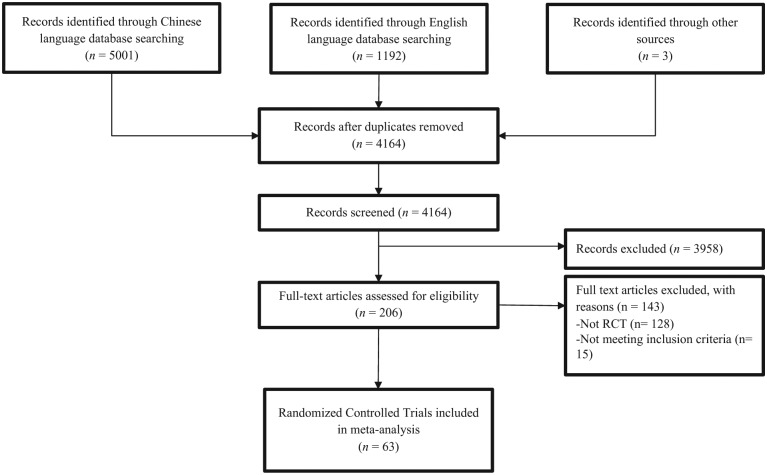
Sixty-three RCTs of herbal therapy for CIPN and/or HFS associated with treatment for CRC were identified (Figure 1). Three studies were conducted in Japan, none in Korea, and 60 in various locations in mainland China.25-87
Figure 1.
Flow diagram of the search, screening, and inclusion process.
The studies enrolled 4286 participants ranging in age from 18 to 88 years. The mean ages ranged from 40 to 72.7 years (not all studies reported mean age). Fifty-two participants dropped out in total, so 4234 participants completed. These included 2509 males and 1738 females (not all studies reported gender). All participants were diagnosed with CRC in 52 studies, with colon cancer in 10 studies, and rectal cancer in 1 study. Twenty-two studies reported Chinese medicine syndrome differentiation (Table l). The most common feature of the syndromes was “spleen deficiency” Pi xu 脾虚 (Supplementary Table S4).
Table 1.
Characteristics of Included Studies of Integrative Herbal Medicine for Chemotherapy-Induced Peripheral Neuropathy and Hand-Foot Syndrome in Colorectal Cancer.
| ID No. | Author(s), Year [Location]a | N Participants (Baseline); N Groups; Male/Female | Cancer Type | CHM Group Interventions; Dosage and Duration | Control Group Intervention(s) |
|---|---|---|---|---|---|
| 1 | Bao YJ et al, 2014 [1] | 60b; 2; 26/34 | Stage II/III CRC after radical surgery | Jianpibushen formula 健脾补肾方 + FOLFOX4; 1 packet per day in 2 doses for 6 months | FOLFOX4 |
| 2 | Cai ZB 2016 [1] | 50; 2; 33/17 | Stage II-IV CRC | Wenjinghuoxue formula 温经活血方 + XELOX; boil until 1000 mL water remains, cool to 35°C to 40°C, soak the hand and feet once a day for 30 minutes, start with chemotherapy for 7 days, 2 × 3-week cycles | XELOX |
| 3 | Cao B 2011 [1] | 85b; 2; 26/34 | Stage II-IV CRC after surgery | NS + FOLFOX4; 1 packet per day in 2 doses, started at 5 days before chemotherapy, until 5 days after chemotherapy, continue for 6 months | FOLFOX4 |
| 4 | Chen CG 2005 [1] | 44b; 2; 27/17 | Stage III/IV CRC | Fuzhengyiliu decoction 扶正抑癌汤 + FOLFOX4; 1 packet per day in 2 doses for 8 weeks | FOLFOX4 |
| 5 | Chen XJ 2010 [1] | 36b; 2; 19/17 | Stage IV CRC | Jiangpihuashiquyu formula 健脾化湿祛瘀方 + XELOX; 1 packet per day in 2 doses for 6 weeks | XELOX |
| 6 | Chen Y 2014 [1] | 40b; 2; 23/17 | Stage III/IV CRC | Weitiaosanhao formula 微调三号方 + SOX; 200 mL, oral, twice a day for 12 weeks | SOX |
| 7 | Cheng XL et al, 2017 [1] | 82; 2; 42/30 | CRC no prior chemo | Huangqiguizhiwuwu decoction 黄芪桂枝五物汤 + FOLFOX series; twice a day for a total of 54 g crude drug/day, for 8 weeks | Placebo + FOLFOX series |
| 8 | Fang ZH et al, 2009 [1] | 62; 2; 40/22 | Advanced CRC | Jianpikangai formula 健脾抗癌方 + FOLFOX; 1 packet per day in 2 doses for 2 months | FOLFOX |
| 9 | Feng YQ 2011 [1] | 40; 2; 27/13 | Colon cancer after surgery | Modified Buyanghuanwu decoction 补阳还五汤 + mFOLFOX6; boil until 500 mL water remains, cool to 38°C to 42°C, soak the hand and feet twice a day for 40 minutes, at 1 day before chemotherapy, then continue for 5 days, 6 × 2-week cycles | mFOLFOX6 |
| 10 | Gai L et al, 2010 [1] | 49; 2; 28/21 | Advanced CRC first-time treatment | Shenyi capsule 参一胶囊 + XELOX; 20 mg, oral, twice a day for 9 weeks. | XELOX |
| 11 | Gao J et al, 2015 [1] | 120; 2; 70/50 | CRC first-time chemo after surgery | Chinese medicine bath formula 中药泡洗方 + FOLFOX4; boil until 2000 mL water remains, cool to 40°C, soak the hand and feet once a day for 30 minutes, from chemotherapy days 1-5, 3 × 2-week cycles | FOLFOX4 |
| 12 | Gao XM 2015 [1] | 60; 2; 29/26 | Stage IIA-IIIC colon cancer after radical surgery, first-time received FOLFOX4 | Modified Bazhen decoction 八珍汤 + FOLFOX4; 1 packet per day in 2 doses for 4 weeks | FOLFOX4 |
| 13 | He JP and Qu JH 2013 [1] | 60; 2; 27/30 | Advanced/recurrences rectal cancer | NS + FOLFOX4; 1 packet per day for 6 weeks | FOLFOX4 |
| 14 | He ZF 2006 [1] | 30b; 2; 17/13 | CRC after radical surgery | Yiqijianpi and huayujiedu formula 益气健脾, 化瘀解毒方 + FOLFOX4; 1 packet per day in 2 doses for 8 weeks | FOLFOX4 |
| 15 | Hou ZB 2014 [1] | 46b; 2; 24/17 | Stage III/IV CRC after radical surgery | Modified Banxiaxiexin decoction 半夏泻心汤 + XELOX; 1 packet per day in 2 doses for 6 weeks | XELOX |
| 16 | Hu B et al, 2015 [1] | 62; 2; 43/19 | Advanced CRC | Tenglongbuzhong decoction 藤龙补中汤 + XELOX; 1 packet per day in 2 doses for 6 weeks | XELOX |
| 17 | Hu FS et al, 2007 [1] | 78; 2; 42/36 | Stage III/IV CRC | Gubenyiliu capsule 固本抑瘤胶囊 + FOLFOX4; 1.6 g, oral, twice a day for 8 weeks | FOLFOX4 |
| 18 | Hu QQ 2013 [1] | 53b; 2; 24/29 | Stage IV CRC | NS + XELOX; formula 1 taken during the first week of chemotherapy, formula 2 taken in weeks 2 and 3; formula 3 taken in the fourth week of chemotherapy, all 1 packet per day in 2 doses | XELOX |
| 19 | Huang L and Guo JH 2014 [1] | 60; 2; 33/27 | Advanced/recurrence colon cancer | Yiqihuatansanjie formula 益气化痰散结方 + XELOX; 1 packet per day in 2 doses for 6 weeks | XELOX |
| 20 | Jiang ZM et al, 2014 [1] | 70; 2; 46/24 | Advanced CRC | Sijunzi decoction 四君子汤 + FOLFOX6; 1 packet per day in 2 doses for 8 weeks | FOLFOX6 |
| 21 | Jiao SJ et al, 2016 [1] | 135b; 3; 72/63 | Stage IV CRC stable after first-line chemotherapy | T1: Zibu decoction滋补汤 T2: Zibu decoction 滋补汤 + XELODA; 1 packet per day in 2 doses for 2 months |
XELODA |
| 22 | Ke SW et al, 2015 [1] | 97; 2; 51/46 | Advanced/recurrence, stage IV CRC | Kangaiyiliu formula 抗癌抑瘤方 + XELOX; 1 packet per day in 2 doses for 2 weeks, 2 × 3-week cycles | XELOX |
| 23 | Kono T et al, 2013 [2] | 93; 2; 48/41 | Advanced/recurrent CRC | Goshajinkigan 牛车肾气丸 + FOLFOX4/mFOLFOX6; 7.5 g, oral, 3 times per day for 26 weeks | Placebo + FOLFOX4/mFOLFOX6 |
| 24 | Lai YQ et al, 2009 [1] | 57; 2; 44/13 | CRC after radical surgery or stage IV CRC | NS + FOLFOX6; 1 packet per day in 2 doses, at 2 days before chemotherapy, then continue for 10 days, 6 × 2-week cycles | FOLFOX6 |
| 25 | Li J 2011 [1] | 40b; 2; 22/18 | Stage IV CRC | Fuzhenghuayujiedusanjie formula 扶正化瘀解毒散结方 + XELOX; 1 packet per day in 2 doses for 2 months | XELOX |
| 26 | Li L 2007 [1] | 30b; 2; 19/11 | Stage IV retreatment CRC | Jianpihuashiquyu formula 健脾化湿祛瘀方 + XELIRI; 1 packet per day in 2 doses, at 1 week before chemotherapy, then continue for 2 months | XELIRI |
| 27 | Li LC 2009 [1] | 30; 2; 20/10 | Dukes B/C CRC after radical surgery | Fufangchangtai 复方肠泰 + FOLFOX4; 1 packet per day in 2 doses for 4 weeks | FOLFOX4 |
| 28 | Li N 2012 [1] | 40b; 2; 26/14 | Stage II/III CRC after radical surgery, first-time treatment, adenocarcinoma | NS + XELOX; 1 packet per day in 2 doses for 6 weeks | XELOX |
| 29 | Li YJ et al, 2007 [1] | 39; 2; 22/16 | Stage III/IV CRC | Wenshenjianpi formula 温肾健脾方 + FOLFOX4; 1 packet per day in 2 doses for 6 weeks | FOLFOX4 |
| 30 | Liang XS et al, 2012 [1] | 84; 2; 59/25 | Stage II-IV CRC after surgery | Yiqilixueyufeng decoction 益气理血愈风汤 + mFOLFOX6; 1 packet per day in 2 doses, at 1 day before chemotherapy, then continue for 4 weeks | mFOLFOX6 |
| 31 | Liang XS et al, 2015 [1] | 135; 2; 91/44 | Stage II-IV CRC after surgery | Lixuequfeng decoction 理血祛风汤 + mFOLFOX6; 1 packet per day in 2 doses, at 1 day before chemotherapy, then continue for 4 weeks | mFOLFOX6 |
| 32 | Liu JP et al, 2017 [1] | 96; 2; 46/50 | Advanced colon cancer | Huachansu capsule 华蟾素胶囊 + XELOX; 0.5 g, oral, 3 times per day, start with chemotherapy for 2 weeks, 2 × 3-week cycles | XELOX |
| 33 | Liu P et al, 2007 [1] | 94; 2; 62/32 | Dukes B/C colon adenocarcinoma after surgery | Boerning capsule 博尔宁胶囊 + FOLFOX4; 4 pills, oral, 3 times per day, used in interval between chemotherapy, 4 × 2-week cycles | FOLFOX4 |
| 34 | Liu SC 2011 [1] | 30; 2; 17/13 | Stage III/IV CRC | NS + FOLFOX6; 1 packet per day in 2 doses for 4 weeks | Placebo+ FOLFOX6 |
| 35 | Liu YF et al, 2013 [1] | 120; 2; 83/37 | CRC | Guilongtongluofang 桂龙通络方; 1 packet per day in 2 doses, at 3 days before chemotherapy, then continue for 10 days, 6 × 2-week cycles | Placebo + FOLFOX4 |
| 36 | Ma J et al, 2015 [1] | 40; 2; 18/21 | Stage IV CRC | Jianpixiaoliu formula 健脾消瘤方 + FOLFOX4; 1 packet per day in 2 doses for 2 months | FOLFOX4 |
| 37 | Mao WD et al, 2011 [1] | 134; 2; 68/66 | Stage II/III CRC after radical surgery | Jianpixiaoaiyin 健脾消癌饮 + FOLFOX4; 1 packet per day in 2 doses for 8 weeks | FOLFOX4 |
| 38 | Mao ZJ et al, 2017 [1] | 84; 2; 45/39 | Stage II/III colon cancer after radical surgery | Jianpi formula 健脾方 + mFOLFOX6; 1 packet per day in 2 doses for 24 weeks | mFOLFOX6 |
| 39 | Nishioka M et al, 2011 [2] | 45; 2; 22/23 | Nonresectable or recurrent CRC | Goshajinkigan 牛车肾气丸 + mFOLFOX6 (or + bevacizumab) median cycles T 13/C 12; 7.5 g/day divided into 2-3 doses, oral | mFOLFOX6 (or + bevacizumab) |
| 40 | Oki E et al, 2015 [2] | 186; 2; 99/83 | Stage III colon adenocarcinoma after radical surgery | Goshajinkigan 牛车肾气丸 + mFOLFOX6; 7.5 g/day, oral, for 24 weeks | Placebo + mFOLFOX6 |
| 41 | Pan RR 2017 [1] | 40b; 2; 24/16 | Stage II-IV CRC after surgery | Erlingyiren decoction 二苓苡仁汤 + XELOX; 1 packet per day in 2 doses for 12 weeks | XELOX |
| 42 | Pu QH 2012 [1] | 45b; 2; 30/15 | Stage IV CRC with liver metastases | Weitiaosanhao formula 微调三号方 + FOLFOX4; 100 mL, oral, twice a day for 8 weeks | FOLFOX4 |
| 43 | Qin CY 2014 [1] | 41b; 2; 32/9 | Stage III/IV CRC | NS + mFOLFOX6; 1 packet per day in 2 doses for 4 weeks | mFOLFOX6 |
| 44 | Shi RQ 2017 [1] | 50b; 2; 27/23 | Stage II-IV CRC after surgery | Yiqijianpi formula 益气健脾方 + XELOX; 1 packet per day in 2 doses for 6 weeks | XELOX |
| 45 | Shu JH et al, 2011 [1] | 90b; 2; 49/41 | Advanced CRC | Yiqijiedu decoction 益气解毒汤 + XELOX; 1 packet per day in 2 doses for 6 weeks | XELOX |
| 46 | Wang H 2008 [1] | 68b; 2; 42/26 | Rectal cancer after radical surgery | Yiqihuoxuebuchang decoction 益气活血补肠汤 + mFOLFOX6; 1 packet per day in 2 doses for 3 months | mFOLFOX6 |
| 47 | Wang JM 2016 [1] | 40b; 2; 22/18 | Stage IV CRC | Jianpiyangyinqushijiedu formula 健脾养阴祛湿解毒方 + XELOX; 1 packet per day in 2 doses for 8 weeks | XELOX |
| 48 | Wang JZ et al, 2011 [1] | 60b; 2; 39/21 | Advanced CRC | Yichangning formula 宜肠宁方 + FOLFOX4; 1 packet per day in 2 doses for 2 months | FOLFOX4 |
| 49 | Wang Q 2015 [1] | 120; 4; 59/61 | CRC after radical surgery | T1: Huangqiguizhiwuwu decoction 黄芪桂枝五物汤 +
calcium gluconate and magnesium sulfate T2: Huangqiguizhiwuwu decoction 黄芪桂枝五物汤 + mFOLFOX6; boil until 200 mL water remains, add warm water to 1000 mL, cool to 35°C to 40°C, soak the hand and feet once a day for 30 minutes for 16 weeks |
C1: calcium gluconate and magnesium sulfate +
mFOLFOX6 C2: mFOLFOX6 |
| 50 | Wang QY et al, 2015 [1] | 75; 2; 44/31 | Advanced colon cancer | NS + mFOLFOX6; for 12 weeks | mFOLFOX6 |
| 51 | Wang SW 2012 [1] | 40b; 2; 22/18 | Stage IV CRC | Tongtai decoction 通泰合剂 + XELOX; 100 mL, oral, twice a day for 6 weeks | XELOX |
| 52 | Xie W 2010 [1] | 60; 2; 42/18 | Stage II/III CRC after radical surgery | Xiaoliu decoction 消瘤汤 + HIPEC; 1 packet per day, start when passing flatus and first defecation occurred, until the 4 cycles of HIPEC finished | HIPEC |
| 53 | Xu C et al, 2012 [1] | 70; 2; 39/31 | Stage III/IV colon cancer | Modified Xiangshaliujunzi decoction 加味香砂六君子汤 + FOLFIRI; 1 packet per day in 2 doses for 8 weeks | FOLFIRI |
| 54 | Xu XQ and Qi YF 2014 [1] | 64; 2; 39/25 | CRC, all participants had CIPN due to oxaliplatin | NS + GM-1 injection; for 4 weeks | GM-1 injection |
| 55 | Yang CD 2015 [1] | 44; 2; 31/13 | Colon adenocarcinoma after surgery | Guizhixixin formula 桂枝细辛方 + FOLFOX4; cool to 38°C to 42°C, soak the hand and feet twice a day for 20 minutes, at 1 day before chemotherapy, then continue for 5 days, 4 × 2-week cycles | FOLFOX4 |
| 56 | Ye HQ et al, 2016 [1] | 59b; 2; 35/24 | Stage IV CRC | Qifulongkui decoction 芪附龙葵汤 + XELOX; 1 packet per day for 8 weeks | XELOX |
| 57 | Zeng JQ et al, 2008 [1] | 60; 2; 37/23 | Advanced CRC had received surgery and chemotherapy included 5-FU, but no oxaliplatin | NS + FOLFOX4; 1 packet per day for 4 weeks | FOLFOX4 |
| 58 | Zeng JY et al, 2010 [1] | 104; 2; 78/26 | Stage II/III CRC after surgery | Xiaoliu decoction 消瘤汤 + HIPEC; 1 packet per day in 3 doses, start when passing flatus and first defecation occurred, then continue for 8 weeks | HIPEC |
| 59 | Zhang C and Han ZG 2015 [1] | 60; 2; 37/23 | CRC radical surgery | NS + mFOLFOX6; 1 packet per day in 2 doses, at 1 week before chemotherapy, until 1 week after chemotherapy | mFOLFOX6 |
| 60 | Zhang Q et al, 2010 [1] | 120; 2; 68/52 | Stage III/IV advanced CRC | Gubenxiaoliu capsule 固本消瘤胶囊 + FOLFOX4; 1.6 g, oral, twice a day for 8 weeks | FOLFOX4 |
| 61 | Zhang WW et al, 2013 [1] | 60; 2; 31/23 | Stage IV CRC | Jiangpijiedu formula 健脾解毒方 + XELODA; 1 packet per day in 2 doses for 6 weeks | XELODA |
| 62 | Zhong MW et al, 2016 [1] | 66; 2; 37/29 | Stage III/IV CRC | Qifulongkui decoction 芪附龙葵汤 + SOX; 1 packet per day in 2 doses for 10 days, then 5 days break, continue for 2 months | SOX |
| 63 | Zhu FY et al, 2016 [1] | 54; 2; 36/18 | Advanced CRC | Jianpiyiqijiedu formula 健脾益气解毒方 + mFOLFOX6; 1 packet per day in 2 doses for more than 8 weeks | mFOLFOX6 |
Abbreviations: CHM, Chinese herbal medicine; CRC, colorectal cancer; NS, no specific formula name; HIPEC, hyperthermic intraperitoneal chemotherapy; CIPN, chemotherapy-induced peripheral neuropathy; GM-1 injection, monosialotetrahexosylganglioside sodium injection.
Location at which the study was conducted: 1, China; 2, Japan.
Mentioned that syndrome differentiation was used.
One study45 included 3 groups so it was included in 2 comparisons: herbal medicine versus XELOX and herbal medicine plus XELOX versus XELOX. Another study73 involved 4 groups (n = 30 per group): (1) herbal medicine plus mFOLFOX6, (2) mFOLFOX6, (3) herbal medicine plus calcium gluconate and magnesium sulphate plus mFOLFOX6, and (4) calcium gluconate and magnesium sulfate plus mFOLFOX6. This study was included in 2 comparisons (groups 1 vs 2; and 3 vs 4).
Test interventions included: orally administered herbal medicine (58 studies) and topical herbal medicine (5 studies). All groups used a form of usual care. The chemotherapy regimens included: FOLFOX type in 37 studies (FOLFOX4, 19 studies; FOLFOX6, 2 studies; mFOLFOX6, 13 studies; FOLFOX, 1 study; FOLFOX series, 1 study; FOLFOX4/FOLFOX6, 1 study); XELOX, 17 studies; XELODA, 2 studies; XELIRI, 1 study; FOLFIRI, 1 study; SOX (S1 + oxaliplatin), 2 studies; and hyperthermic intraperitoneal chemotherapy (HIPEC), 2 studies. Five studies used a placebo for the herbal medicine. Study durations ranged from 4 weeks to 6 months but in some studies, treatment was discontinuous. Outcome data were available for CIPN (60 studies), and HFS (12 studies), with 9 studies reporting both outcomes.
The most frequently used herbal formula was Goshajinkigan (Niu che shen qi wan) 牛车肾气丸, which was tested in 3 studies (Table 1). Another 3 formulae were tested in 2 studies each: Qi fu long kui tang 芪附龙葵汤,80,86 Wei tiao san hao fang 微调3号方,30,66 and Xiao liu tang 消瘤汤.76,82 Some other formulae had the same names but different ingredients, so these were considered to be different formulae.
The herbs most frequently used in the oral formulae were the following: Astragalus membranaceus (n = 40), Atractylodes macrocephala (n = 38), Poria cocos (n = 36), Coix lacryma-jobi (n = 29), Glycyrrhiza uralensis (n = 28), Codonopsis pilosula (n = 25), Paeonia lactiflora (n = 21), Hedyotis diffusa (n = 18), Scutellaria barbata (n = 15), and Citrus reticulata (n = 14). The herbs most frequently used in the 5 hand and foot bath formulae were Carthamus tinctorius (n = 4), Astragalus membranaceus (n = 4), Cinnamomum cassia (n = 3), and Prunus persica (n = 3) (see Supplementary Tables S1 and S2 for Chinese names).
Risk of Bias
Of the 63 studies, 31 were judged as low risk for sequence generation since a proper method was described, three were judged high risk since a standard method appears not to have been used, and the remainder were unclear due to lack of information. Allocation concealment was described properly in 8 studies that were judged low risk, whereas the others did not mention this and were judged as unclear risk. For blinding, 5 studies blinded participants while 4 of these also blinded personnel and outcome assessors. These were judged low risk for these domains while the other studies which did not mention blinding were judged high risk. For incomplete outcome data, most studies had few if any dropouts and were judged as low risk, but in 2 studies some inconsistencies in dropout reporting led to a judgement of unclear risk. For selective outcome reporting, the majority did not have a protocol but reported on all outcomes mentioned in their methods, so these were judged unclear risk. The 2 studies for which protocols were available were judged low risk (Table 2).
Table 2.
| Study ID | Author(s), Year | SG | AC | BPt | BPn | BOA | IOD | SOR |
|---|---|---|---|---|---|---|---|---|
| 1 | Bao YJ et al, 2014 | L | L | H | H | U | L | U |
| 2 | Cai ZB, 2016 | U | U | H | H | U | L | U |
| 3 | Cao B, 2011 | L | U | H | H | U | L | U |
| 4 | Chen CG, 2005 | U | U | H | H | U | L | U |
| 5 | Chen XJ, 2010 | U | U | H | H | U | L | U |
| 6 | Chen Y, 2014 | L | U | H | H | U | L | U |
| 7 | Cheng XL et al, 2017 | L | L | L | L | L | L | U |
| 8 | Fang ZH et al, 2009 | U | U | H | H | U | L | U |
| 9 | Feng YQ, 2011 | L | U | H | H | U | L | U |
| 10 | Gai L et al, 2010 | U | U | H | H | U | L | U |
| 11 | Gao J et al, 2015 | U | U | H | H | U | L | U |
| 12 | Gao XM 2015 | L | U | H | H | U | L | U |
| 13 | He JP and Qu JH, 2013 | U | U | H | H | U | L | U |
| 14 | He ZF, 2006 | U | U | H | H | U | L | U |
| 15 | Hou ZB, 2014 | L | U | H | H | U | U | U |
| 16 | Hu B et al, 2015 | U | U | H | H | U | L | U |
| 17 | Hu FS et al, 2007 | L | U | H | H | U | L | U |
| 18 | Hu QQ, 2013 | L | U | H | H | U | L | U |
| 19 | Huang L and Guo JH, 2014 | U | U | H | H | U | L | U |
| 20 | Jiang ZM et al, 2014 | H | U | H | H | U | L | U |
| 21 | Jiao SJ et al, 2016 | U | U | H | H | U | L | U |
| 22 | Ke SW et al, 2015 | L | U | H | H | U | L | U |
| 23 | Kono T et al, 2013 | L | L | L | L | L | L | L |
| 24 | Lai YQ et al, 2009 | U | U | H | H | U | L | U |
| 25 | Li J, 2011 | U | U | H | H | U | L | U |
| 26 | Li L, 2007 | U | U | H | H | U | L | U |
| 27 | Li LC, 2009 | L | U | H | H | U | L | U |
| 28 | Li N, 2012 | U | U | H | H | U | L | U |
| 29 | Li YJ et al, 2007 | L | L | H | H | U | L | U |
| 30 | Liang XS et al, 2012 | L | U | H | H | U | L | U |
| 31 | Liang XS et al, 2015 | L | U | H | H | U | L | U |
| 32 | Liu JP et al, 2017 | L | U | H | H | U | L | U |
| 33 | Liu P et al, 2007 | U | U | H | H | U | L | U |
| 34 | Liu SC, 2011 | L | L | L | H | U | L | U |
| 35 | Liu YF et al, 2013 | L | L | L | L | L | L | U |
| 36 | Ma J et al, 2015 | U | U | H | H | U | L | U |
| 37 | Mao WD et al, 2011 | U | U | H | H | U | L | U |
| 38 | Mao ZJ et al, 2017 | U | U | H | H | U | L | U |
| 39 | Nishioka M et al, 2011 | U | L | H | H | H | L | U |
| 40 | Oki E et al, 2015 | L | L | L | L | L | L | L |
| 41 | Pan RR, 2017 | L | U | H | H | U | L | U |
| 42 | Pu QH, 2012 | L | U | H | H | U | L | U |
| 43 | Qin CY, 2014 | L | U | H | H | H | L | U |
| 44 | Shi RQ, 2017 | L | U | H | H | U | L | U |
| 45 | Shu JH et al, 2011 | U | U | H | H | U | L | U |
| 46 | Wang H, 2008 | U | U | H | H | U | L | U |
| 47 | Wang JM, 2016 | U | U | H | H | U | L | U |
| 48 | Wang JZ et al, 2011 | L | U | H | H | U | L | U |
| 49 | Wang Q, 2015 | L | U | H | H | U | L | U |
| 50 | Wang QY et al, 2015 | U | U | H | H | U | L | U |
| 51 | Wang SW, 2012 | U | U | H | H | U | L | U |
| 52 | Xie W, 2010 | L | U | H | H | U | L | U |
| 53 | Xu C et al, 2012 | U | U | H | H | U | U | U |
| 54 | Xu XQ and Qi YF, 2014 | H | U | H | H | U | L | U |
| 55 | Yang CD, 2015 | L | U | H | H | U | L | U |
| 56 | Ye HQ et al, 2016 | L | U | H | H | U | L | U |
| 57 | Zeng JQ et al, 2008 | U | U | H | H | U | L | U |
| 58 | Zeng JY et al, 2010 | L | U | H | H | U | L | U |
| 59 | Zhang C and Han ZG, 2015 | U | U | H | H | U | L | U |
| 60 | Zhang Q et al, 2010 | L | U | H | H | U | L | U |
| 61 | Zhang WW et al, 2013 | U | U | H | H | U | L | U |
| 62 | Zhong MW et al, 2016 | L | U | H | H | U | L | U |
| 63 | Zhu FY et al, 2016 | H | U | H | H | U | L | U |
| Totals | 31 L, 3 H | 8 L, 0 H | 5 L, 58 H | 4 L, 59 H | 4 L, 2 H | 61 L, 0 H | 2 L, 0 H |
Risk of bias categories: SG, sequence generation; AC, allocation concealment; BPt, blinding of participants; BPn, blinding of personnel; BOA, blinding of outcome assessment; IOD, incomplete outcome data; SOR, selective outcome reporting.
Risk of bias judgments: L, low risk; U, unclear risk or no information specified; H, high risk.
The difference in dropout rates between treatment and control groups did not exceed 20% in any study. Oki 2015 and Kono 2013 had an available protocol. Nishioka 2011 and Cheng 2017 had trial registration numbers but the protocols could not be accessed.
Studies of Orally Administered Herbal Medicine
The studies included 1 study of oral herbal medicine plus GM-1 injection versus GM-1 injection alone for CIPN,78 1 study of herbal medicine versus chemotherapy,45 52 studies of herbal medicine plus chemotherapy versus the same chemotherapy, and 5 studies of herbal medicine plus chemotherapy versus placebo plus the same chemotherapy.
Herbal Medicine Plus GM-1 Injection for CIPN
In 1 study (No. 54) all participants had CIPN due to previous oxaliplatin-based chemotherapy for CRC.78 All received GM-1 injection for the CIPN and the test group also received the herbal medicine. Based on modified World Health Organization (WHO) criteria,88 there was no significant difference in the incidence of grade III plus IV CIPN (relative risk [RR] 0.50 [0.05, 5.24]) which was very low in both groups, or in all grades of CIPN (RR 0.83 [0.66, 1.04], n = 64).
Herbal Medicine Versus Chemotherapy for Hand-Foot Syndrome
One study (No. 21) compared herbal medicine without chemotherapy with XELODA and reported data for HFS.45 It included 3 groups (45 participants per group). All participants had advanced CRC and had previously received first-line chemotherapy. In this study, they were receiving maintenance treatment. The control group received XELODA as two 3-week cycles. There were no dropouts. For incidence of HFS after 2 months treatment there was no statistical difference between groups (RR 0.33 [0.04, 3.09], n = 90). The results for the herbal medicine plus XELODA arm are reported below.
Herbal Medicine Plus Chemotherapy Versus Chemotherapy
In 57 studies, an orally administered herbal medicine was combined with chemotherapy and compared to the same chemotherapy. In 5 of these studies a placebo for the herbal medicine was used in the control group.31,47,59,64
Chemotherapy-Induced Peripheral Neurotoxicity
Fifty-four studies reported on CIPN. Thirty-three studies used the WHO criteria, 7 studies used Levi’s modified WHO criteria,88 10 studies used the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) criteria, 2 studies63,64 used the Neurotoxicity Criteria of Debiopharm (DEB-NTC), and 3 studies did not specify the criteria. Meta-analysis results were reported separately for each of these outcome measures.
For WHO grades III plus IV CIPN, 17 studies had zero events in both groups so 14 studies were included in the meta-analysis (Table 3). For the pool of seven studies of FOLFOX4, there was no difference between groups (RR 0.41 [0.16, 1.04], I2 = 0%), but there was a significant reduction in the single study of mFOLFOX6 and there was a significant reduction in CIPN in the integrative therapy groups in the pool of 9 studies that used similar chemotherapies (RR 0.33 [0.16, 0.70], I2 = 0%). There was no difference between groups in the pooled result for 3 studies of XELOX or any of the other subgroups but the total result for 14 studies showed a significant reduction in the incidence of grade III/IV CIPN in the integrative groups (RR 0.42 [0.23, 0.77], I2 = 0%).
Table 3.
Integrative Herbal Medicine: Chemotherapy-Induced Peripheral Neurotoxicity (WHO Grades III + IV).a
| Chemotherapy Regimen: No. of Studies (No. of Participants); No. of Studies With 0 Events in Both Groups | No. of Studies (No. of Participants) in Meta-Analysis | Effect Size RR [95% CI] I2 | Study ID (RCTs With 0 Events in Both Groups) |
|---|---|---|---|
| FOLFOX4: 13 (836); 6 | 7 (532) | 0.41 [0.16, 1.04] 0% | 13, 17, 29, 37, 42, 48, 60 (1, 3, 4, 12, 14, 27) |
| FOLFOX6: 1 (70); 1 | 0 (0) | Both groups = 0 events | (20) |
| mFOLFOX6: 2 (129); 1 | 1 (75) | 0.18 [0.04, 0.75]b | 50 (63) |
| FOLFOX: 1 (62); 0 | 1 (62) | 0.50 [0.05, 5.23] | 8 |
| Pool for similar chemotherapyc: 17 (1097); 8 | 9 (669) | 0.33 [0.16, 0.70]b 0% | 8, 13, 17, 29, 37, 42, 48, 50, 60 (1, 3, 4, 12, 14, 20, 27, 63) |
| XELOX: 10 (490);74 | 3 (180) | 0.66 [0.18, 2.47] 0% | 10, 15, 45 (19, 25, 28, 51) |
| SOX: 2 (106); 0 | 2 (106) | 0.67 [0.11, 3.92] 0% | 6, 62 |
| XELIRI: 2 (66); 2 | 0 (0) | Both groups = 0 events | (5, 26) |
| Total pool: 31 (1759); 17 | 14 (955) | 0.42 [0.23, 0.77]b 0% | All above |
Abbreviations: WHO, World Health Organization; CI, confidence interval; RR, relative risk; RCT, randomized controlled trial.
All studies used oral herbal medicines combined with chemotherapy versus the same chemotherapy.
Statistically significant.
These chemotherapy regimens all used oxaliplatin, 5-fluorouracil (5-FU) plus leucovorin (LV).
For all grades of CIPN (WHO criteria), 33 studies reported data, but 1 study had no events in both groups, so 32 studies were included in the meta-analysis (Table 4, Supplementary Figure S1). There was a significant reduction in CIPN in the pooled result for all 14 studies that used FOLFOX4 (RR 0.77 [0.66, 0.89], I2 = 0%) but there was no significant difference between groups in the subgroup of FOLFOX4 as adjuvant chemotherapy after radical surgery. In the pool of 18 studies of similar chemotherapies, there was a significant difference between groups (RR 0.70 [0.52, 0.96], I2 = 88%) but the heterogeneity was considerable. This was due to 1 study,74 which reported that all participants had CIPN at baseline. When this study was removed from the pool, the heterogeneity was reduced to zero and the significant reduction in CIPN in the integrative therapy groups remained (RR 0.75 [0.66, 0.86], I2 = 0%, n = 1116). In the 10 studies of XELOX, the pooled result showed no difference between groups and there was no difference for SOX or XELIRI. In the total pool of 32 studies, there was a significant reduction in CIPN in the integrative therapy groups, but the heterogeneity was considerable (RR 0.78 [0.66, 0.91], I2 = 71%). When the study in which all participants had CIPN74 was excluded the result was similar but without heterogeneity (RR 0.83 [0.76, 0.91] I2 = 0%). Seventeen studies employed syndrome differentiation. These found a similar result to the total pool but without heterogeneity.
Table 4.
Integrative Herbal Medicine: Chemotherapy-Induced Peripheral Neurotoxicity (WHO All Grades).a
| Chemotherapy Regimen: No. of Studies (No. of Participants); No. of Studies With 0 Events in Both Groups | No. of Studies (No. of Participants) in Meta-Analysis | Effect Size RR [95% CI] I2 | Study ID (RCTs With 0 Events in Both Groups) |
|---|---|---|---|
| FOLFOX4 (all): 14 (930); 0 | 14 (930) | 0.77 [0.66, 0.89]b 0% | 1, 3, 4, 12, 13, 14, 17, 27, 29, 33, 37, 42, 48, 60 |
| FOLFOX4 (adjuvant, after radical surgery): 5 (309); 0 | 5 (309) | 0.77 [0.44, 1.33] 0% | 1, 12, 14, 27, 37 |
| FOLFOX6: 1 (70); 0 | 1 (70) | 0.81 [0.58, 1.13] | 20 |
| mFOLFOX6: 2 (129); 0 | 2 (129) | 0.63 [0.03, 12.41] 99% | 50, 63 |
| FOLFOX: 1 (62); 0 | 1 (62) | 0.75 [0.29, 1.91] | 8 |
| Pool for similar chemotherapyc: 18 (1191); 0 | 18 (1191) | 0.70 [0.52, 0.96]b 88% | 1, 3, 4, 8, 12, 13, 14, 17, 20, 27, 29, 33, 37, 42, 48, 50, 60, 63 |
| XELOX (all): 10 (490); 0 | 10 (490) | 0.92 [0.81, 1.05] 0% | 10, 15, 19, 25, 28, 41, 44, 45, 47, 51 |
| XELOX (after surgery): 4 (171); 0 | 4 (171) | 0.95 [0.80, 1.12] 0% | 15, 28, 41, 44 |
| XELOX (without surgery): 6 (319); 0 | 6 (319) | 0.88 [0.72, 1.09] 0% | 10, 19, 25, 45, 47, 51 |
| SOX: 2 (106); 0 | 2 (106) | 0.91 [0.57, 1.45] 0% | 6, 62 |
| XELODA: 1 (60); 1 | 0 (0) | Both groups = 0 events | (61) |
| XELIRI: 2 (66); 0 | 2 (66) | 0.57 [0.28, 1.16] 0% | 5, 26 |
| Total pool: 33 (1913); 1 | 32 (1853) | 0.78 [0.66, 0.91]b 71% | All above |
| Sensitivity | 31 (1778) | 0.83 [0.76, 0.91]b 0% | Exclude 50d |
| Sensitivity (all syndromes) | 17 (811) | 0.86 [0.76, 0.97]b 0% | 1, 3, 4, 5, 6, 14, 15, 25, 26, 28, 41, 42, 44, 45, 47, 48, 51 |
| Sensitivity (Pi xu 脾虚 syndrome) | 13 (611) | 0.86 [0.75, 0.98]b 0%, | 1, 3, 4, 5, 6, 15, 26, 28, 41, 42, 44, 47, 48 |
Abbreviations: WHO, World Health Organization; CI, confidence interval; RR, relative risk; RC, randomized controlled trial.
All studies used oral herbal medicines combined with chemotherapy versus the same chemotherapy.
Statistically significant.
These chemotherapy regimens all used oxaliplatin, 5-fluorouracil (5-FU) plus leucovorin (LV).
Since all participants had chemotherapy-induced peripheral neuropathy at baseline.
Seven studies used Levi’s criteria88 (Table 5, Supplementary Figure S3). For grades III plus IV CIPN, the pooled result for 6 studies found there was a significant reduction in the integrative therapy groups (RR 0.28 [0.11, 0.69], I2 = 0%). For all grades, the pooled result for 8 studies showed a significant reduction in CIPN in the integrative groups (RR 0.54 [0.38, 0.76], I2 = 82.3%) but heterogeneity was considerable. This was due to 2 studies of FOLFOX regimens that reported high incidences of CIPN in both groups (70%-80%).62,81 When excluded in the sensitivity analysis, the pooled result of 5 studies remained significant without heterogeneity.
Table 5.
Integrative Herbal Medicine: Chemotherapy-Induced Peripheral Neurotoxicity (Levi 1992 Criteria).a
| Chemotherapy Regimen: No. of Studies (No. of Participants); No. of Studies With 0 Events in Both Groups | No. of Studies (No. of Participants) in Meta-Analysis | Effect Size RR [95% CI] I2 | Study ID (RCTs With 0 Events in Both Groups) |
|---|---|---|---|
| Grades III + IV | |||
| FOLFOX4: 1 (60); 1 | 0 (0) | Both groups = 0 events | (57) |
| FOLFOX6: 1 (57); 0 | 1 (57) | 0.10 [0.01, 1.78] | 24 |
| mFOLFOX6: 3 (303); 0 | 3 (303) | 0.23 [0.05, 1.15] 34.7% | 30, 31, 38 |
| FOLFOX series 1 (72); 0 | 1 (72) | 0.14 [0.01, 2.67] | 7 |
| Pool for similar chemotherapyc: 6 (492): 1 | 5 (432) | 0.25 [0.09, 0.68]b 0% | 7, 24, 30, 31, 38 (57) |
| XELOX: 1 (53); 0 | 1 (53) | 0.48 [0.05, 5.00] | 18 |
| Total pool: 7 (545); 6 | 6 (485) | 0.28 [0.11, 0.69]b 0% | All above |
| All grades | |||
| FOLFOX4: 1 (60); 0 | 1 (60) | 0.88 [0.67, 1.15] | 57 |
| FOLFOX6: 1 (57); 0 | 1 (57) | 0.30 [0.13, 0.72]b | 24 |
| mFOLFOX6: 3 (303); 0 | 3 (303) | 0.62 [0.40, 0.95]b 85% | 30, 31, 38 |
| FOLFOX series 1 (72); 0 | 1 (72) | 0.39 [0.21, 0.73]b | 7 |
| Pool for similar chemotherapyc: 6 (492); 0 | 6 (492) | 0.58 [0.42, 0.82]b 81.4% | 7, 24, 30, 31, 38, 57 |
| XELOX: 1 (53); 0 | 1 (53) | 0.32 [0.17, 0.62] | 18 |
| Total pool: 7 (545); 0 | 7 (545) | 0.54 [0.38, 0.76]b 82.3% | All above |
| Sensitivity | 5 (401) | 0.46 [0.37, 0.56]b 0% | Exclude 38, 57d |
Abbreviations: CI, confidence interval; RR, relative risk; RCT, randomized controlled trial.
All studies used oral herbal medicines combined with chemotherapy versus the same chemotherapy.
Statistically significant.
These chemotherapy regimens all used oxaliplatin, 5-fluorouracil (5-FU) plus leucovorin (LV).
Studies ID 38 and 57 showed very high incidences of chemotherapy-induced peripheral neurotoxicity in both groups (70%-80%), both used FOLFOX regimens.
Ten studies used the NCI-CTCAE criteria for CIPN (Table 6, Supplementary Figure S4). Nine studies reported data for grades III plus IV but there were zero events in both groups in 2 studies, so 7 studies were pooled in the meta-analysis. The pooled result found no significant difference between groups (RR 0.65 [0.37, 1.13], I2 = 26.4%). For all grades, the pooled result for 9 studies showed there was a significant difference between the integrative therapy groups and the chemotherapy alone groups (RR 0.74 [0.58, 0.94], I2 = 13.5%). The pooled result for the 2 studies that used syndrome differentiation found a similar result without heterogeneity.
Table 6.
Integrative Herbal Medicine: Chemotherapy-Induced Peripheral Neurotoxicity (NCI-CTCAE).a
| Chemotherapy regimen: No. studies (no. participants); No. studies with 0 events in both groups | No. studies (no. participants) in meta-analysis | Effect Size RR [95% CI] I2 | Study ID (RCTs with 0 events in both groups) |
|---|---|---|---|
| Grades III + IV | |||
| FOLFOX4: 2 (160); 0 | 2 (160) | 0.38 [0.18, 0.80]b 0% | 35, 36 |
| FOLFOX6: 1 (30); 0 | 1 (30) | 0.33 [0.04, 2.85] | 34 |
| mFOLFOX6: 2 (223); 0 | 2 (223) | 1.51 [0.74, 3.11] 0% | 40, 43 |
| FOLOX4/FOLFOX6: 1 (89); 0 | 1 (89) | 0.55 [0.24, 1.25] | 23 |
| Pool for similar chemotherapyc: 6 (502); 0 | 6 (502) | 0.66 [0.36, 1.21] 37% | 23, 34, 35, 36, 40, 43 |
| XELOX: 3 (252); 2 | 1 (59) | 0.32 [0.01, 7.61] | 56 (22, 32) |
| Total pool: 9 (754); 2 | 7 (561) | 0.65 [0.37, 1.13] 26.4% | 23, 34, 35, 36, 40, 43, 56 (22, 32) |
| All grades | |||
| FOLFOX4: 2 (160); 0 | 2 (160) | 0.71 [0.54, 0.94]b 0% | 35, 36 |
| FOLFOX6: 1 (30); 0 | 1 (30) | 0.44 [1.17, 1.13] | 34 |
| mFOLFOX6: 2 (223); 0 | 2 (223) | 1.10 [0.46, 2.61] 43.4% | 40, 43 |
| Pool for similar chemotherapyc: 5 (413); 0 | 5 (413) | 0.66 [0.36, 1.21] 32.7% | 34, 35, 36, 40, 43 |
| XELOX: 4 (314); 0 | 4 (314) | 0.74 [0.50, 1.09] 9.2% | 16, 22, 32, 56 |
| Total pool: 9 (727); 0 | 9 (727) | 0.74 [0.58, 0.94]b 13.5% | All above |
| Sensitivity (syndrome) | 2 (100) | 0.57 [0.33, 0.98]b 0% | 43, 56 |
Abbreviations: NCI-CTCAE; National Cancer Institute Common Terminology Criteria for Adverse Events; CI, confidence interval; RR, relative risk; RCT, randomized controlled trial.
All studies used oral herbal medicines combined with chemotherapy versus the same chemotherapy.
Statistically significant.
These chemotherapy regimens all used oxaliplatin, 5-fluorouracil (5-FU) plus leucovorin (LV).
This group included 4 placebo-controlled studies. When these were considered separately, for grades III + IV there was no significant difference between groups (RR 0.64 [0.30, 1.39], I2 62%, n = 421) with the heterogeneity being due to 1 study (No. 40).64 When this was excluded the result was significant (RR 0.44 [0.25, 0.76], I2 0%, n = 239) without heterogeneity. Only 3 of the placebo-controlled trials reported data for all grades, and the pooled result was not significant (RR 0.82 [0.46, 1.47], I2 = 61%, n = 332). The heterogeneity was again due to Oki et al.64 When excluded, the result showed a significant difference (RR 0.70 [0.50, 0.96], I2 = 6%, n = 150).
One of the studies (No. 39) that used DEB-NTC reported data suitable for analysis. It compared the formula Goshajinkigan plus mFOLFOX6 versus mFOLFOX6.63 Some participants also received bevacizumab. The rates of grade III CIPN after 10 cycles was 0% in the integrative therapy group and 12% in the control group. After 20 cycles of chemotherapy, the rates were 33% in the integrative group, and 75% in the chemotherapy alone group, but there were no statistically significant differences between groups with regard to the incidence of grade I or higher and grade II or higher CIPN. The percentage of grade II/III CIPN in each cycle was lower in the integrative group than the control group.
Three studies did not specify the criteria for CIPN.70,82,83 For all grades of CIPN, 1 study of mFOLFOX6 in rectal adenocarcinoma with metastasis after radical surgery found no significant difference between groups (RR 1.00 [0.50, 1.99], n = 68).70 One study of HIPEC for stage II/III CRC after surgery82 also found no significant difference (RR 0.59 [0.34, 1.02], n = 104). In the pooled result for these 2 studies, there was no significant difference between groups in incidence of all grades CIPN (RR 0.74 [0.44, 1.23], I2 = 28%, n = 172). In the single study that only reported on perioral paresthesia associated with mFOLFOX6 after radical surgery for CRC, there was a significant reduction in the herbal medicine plus mFOLFOX6 group (RR 0.17 [0.04, 0.68], n = 60).83
Hand-Foot Syndrome
Of the 12 studies that reported data for chemotherapy-related HFS, 6 used the WHO criteria, 5 used the NCI-CTCAE criteria, and 1 did not specify the criteria.
For grade III (WHO), data were available for 4 studies. Two of these reported zero events in both groups30,43 (Table 7). There were no significant differences between groups in the other two studies and the pooled result found no significant difference (RR 0.42 [0.06, 2.76], I2 = 0%). For all grades, there were no significant differences between groups for the studies of FOLFOX4, mFOLFOX6, XELOX, or SOX but there was a significant difference for HIPEC. The pooled result found no significant difference between groups (RR 0.59 [0.18, 1.92], I2 = 96%) but the heterogeneity was considerable. This was mainly due to 1 study of mFOLFOX6 (No. 50) that reported presence of HFS in all participants.74 When this study was excluded from the meta-analysis pool, the heterogeneity was reduced and there was a significant reduction in HFS (all grades) in the integrative therapy groups (RR 0.62 [0.41, 0.96], I2 = 22%). The pooled result for the 2 studies that used syndrome differentiation found no significant difference between groups without heterogeneity.
Table 7.
Integrative Herbal Medicine: Chemotherapy-Related Hand-Foot Syndrome (WHO).a
| Chemotherapy Regimen: No. of Studies (No. of Participants); No. of Studies With 0 Events in Both Groups | No. of Studies (No. of Participants) in Meta-Analysis | Effect Size RR [95% CI] I2 | Study ID (RCTs With 0 Events in Both Groups) |
|---|---|---|---|
| Grades III + IV | |||
| FOLFOX4: 1 (45); 0 | 1 (45) | 0.32 [0.01, 7.45] | 42 |
| mFOLFOX6: 1 (75); 0 | 1 (75) | 0.49 [0.05, 5.14] | 50 |
| XELOX: 1 (60); 1 | 0 (0) | Both groups = 0 events | (19) |
| SOX: 1 (40); 1 | 0 (0) | Both groups = 0 events | (6) |
| Total pool: 4 (220); 2 | 2 (120) | 0.42 [0.06, 2.76] 0% | 42, 50 (6, 19) |
| All grades | |||
| FOLFOX4: 1 (45); 0 | 1 (45) | 0.82 [0.33, 2.06] | 42 |
| mFOLFOX6: 2 (125); 0 | 2 (125) | 0.38 [0.00, 76.97] | 50, 53 |
| Pool for similar chemotherapyc: 3 (170); 0 | 3 (170) | 0.54 [0.08, 3.83] 93% | 42, 50, 53 |
| XELOX: 1 (60); 0 | 1 (60) | 0.77 [0.40, 1.47] | 19 |
| SOX: 1 (40); 0 | 1 (40) | 0.80 [0.40, 1.60] | 6 |
| HIPEC: 1 (60); 0 | 1 (60) | 0.38 [0.17, 0.83]b | 52 |
| Total pool: 6 (330); 0 | 6 (330) | 0.59 [0.18, 1.92] 96% | All above |
| Sensitivity | 5 (255) | 0.62 [0.41, 0.96]b 22% | Exclude 50d |
| Sensitivity (syndrome) | 2 (100) | 0.81 [0.46, 1.40] 0% | 6, 42 |
Abbreviations: WHO, World Health Organization; CI, confidence interval; RR, relative risk; RCT, randomized controlled trial; HIPEC, hyperthermic intraperitoneal chemotherapy.
All studies used oral herbal medicines combined with chemotherapy versus the same chemotherapy.
Statistically significant.
These chemotherapy regimens all used oxaliplatin, 5-fluorouracil (5-FU) plus leucovorin (LV).
Since all participants had hand-foot syndrome at baseline.
Five studies used the NCI-CTCAE criteria. One reported zero grade III cases in both groups46 (Table 8). There were no significant differences between groups in the other 2 studies and the pooled result also found no significant difference (RR 0.25 [0.03, 2.22] I2 = 0%). For all grades, there was no significant difference between groups in the subgroup results for XELOX or FOLFOX4/FOLFOX6. The pooled result showed no differences between groups in incidence of all grades of HFS (RR 0.93 [0.55, 1.55], I2 = 75.7%) with substantial heterogeneity. No single study or factor was the main contributor so a sensitivity analysis was not feasible.
Table 8.
Integrative Herbal Medicine: Chemotherapy-Related Hand-Foot Syndrome (NCI-CTCAE).a
| Chemotherapy Regimen | Participants: Characteristics (No.) | Effect Size RR [95% CI] I2 | Study ID |
|---|---|---|---|
| Grade III | |||
| XELOX (without surgery) | Advanced or recurrent stage IV CRC (97) | Both groups=0 events | 22 |
| Stage IV CRC (53) | 0.32 [0.01, 7.55] | 18 | |
| Advanced colon cancer (96) | 0.20 [0.01, 4.06] | 32 | |
| Pooled result | 2 (149) | 0.25 [0.03, 2.22] 0% | 18, 32 |
| All grades | |||
| XELOX (without surgery) | Advanced CRC (62) | 0.70 [0.35, 1.43] | 16 |
| Advanced or recurrent stage IV CRC (97) | 0.98 [0.58, 1.65] | 22 | |
| Stage IV CRC (53) | 0.41 [0.23, 0.73]b | 18 | |
| Advanced colon cancer (96) | 1.50 [1.05, 2.15]b | 32 | |
| Pooled result (XELOX) | 4 (308) | 0.83 [0.46, 1.50] 80.4% | 16, 18, 22, 32 |
| FOLFOX4/FOLFOX6 | Advanced or recurrent CRC, placebo in control group (89) | 1.61 [0.69, 3.77] | 23 |
| Pooled result | 5 (397) | 0.93 [0.55, 1.55] 75.7% | All above |
Abbreviations: NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; CI, confidence interval; RR, relative risk; CRC, colorectal cancer.
All studies used oral herbal medicines combined with chemotherapy versus the same chemotherapy.
Statistically significant.
In the single study that did not specify the criteria (No. 21), XELODA was used as a maintenance treatment in stage IV CRC.45 There was no difference between groups in all grades HFS (RR 0.67 [0.12, 3.80], n = 90).
Topical Herbal Medicine Plus Chemotherapy Versus Chemotherapy
Five RCTs employed hand and foot baths for prevention of the symptoms of CIPN due to oxaliplatin-based chemotherapy (Table 1, Supplementary Tables S1 and S4). All used Levi’s criteria.88
For grade III/IV CIPN, there was no significant difference between groups (RR 0.35 [0.10, 1.20] I2 = 0%), but the overall incidence was low (3 vs 13 cases). For all grades, there was a significant reduction in the number of people developing CIPN in the integrative therapy groups compared with the control groups (RR 0.69 [0.50, 0.95], I2 = 68.8%) with substantial heterogeneity (Table 9).
Table 9.
Herbal Hand and Foot Bath Plus Chemotherapy: Chemotherapy-Induced Peripheral Neurotoxicity (CIPN).a
| Characteristics (No. of Participants) | CIPN Grades III + IV; Effect Size RR [95% CI] I2 | CIPN All Grades; Effect Size RR [95% CI] I2 | Study ID |
|---|---|---|---|
| FOLFOX4, CRC, first-time chemo after surgery (120) | 0.20 [0.01, 4.08] | 0.59 [0.36, 0.95]b | 11 |
| FOLFOX4, colon cancer, after surgery (44) | 1.00 [0.07, 15.00] | 0.47 [0.26, 0.86]b | 55 |
| Calcium and magnesium + mFOLFOX6, CRC, adjuvant chemotherapy (60) | 0.33 [0.01, 7.87] | 0.83 [0.61, 1.14] | 49.1 |
| mFOLFOX6, CRC, adjuvant chemotherapy (60) | 0.20 [0.01, 4.00] | 0.96 [0.76, 1.22] | 49.2 |
| mFOLFOX6, colon cancer, after surgery (40) | 0.33 [0.04, 2.94] | 0.47 [0.24, 0.89]b | 9 |
| XELOX, CRC, first time received oxaliplatin treatment (50) | 0.25 [0.03, 2.08] | 0.40 [0.19, 0.86]b | 2 |
| Total pool, 5 RCTs, 6 comparisons (374) | 0.35 [0.10, 1.20] 0% | 0.69 [0.50, 0.95]b 68.8% | All above |
| Sensitivity 1, 5 RCTs, 5 comparisons (314)c | 0.32 [0.11, 0.99]b 0% | 0.58 [0.37, 0.91]b 75% | 2, 9, 11, 49.2, 55 |
| Sensitivity 2, 4 RCTs, 4 comparisons (254)d | 0.35 [0.10, 1.17] 0% | 0.50 [0.37, 0.67]b 0% | 2, 9, 11, 55 |
Abbreviations: CI, confidence interval; RR, relative risk; RCT, randomized controlled trial.
All studies used herbal hand and foot baths combined with chemotherapy versus the same chemotherapy.
Statistically significant.
Sensitivity 1 excluded Study No. 49 group T1 to remove double counting due to this study having 2 comparisons.
Sensitivity 2 excluded Study No. 49 altogether (groups T1 and T2).
One study (No. 49) included 2 comparisons73 and one of these used a complex control (calcium and magnesium plus mFOLFOX6), so this arm was excluded in a sensitivity analysis. This showed a marginally significant result for grades III + IV but the heterogeneity remained for all grades. In a further sensitivity analysis that excluded this study, the result was similar to that for the total pool but without heterogeneity.
Safety of the CHMs
In 49 studies, there was no mention of the safety of the herbal medicines. Ten studies stated that there were no adverse events associated with the herbal medicines. One study (No. 11) that used hand and foot bath mentioned that 1 patient in the test group had mild allergy, which was likely due to the herbal hand and foot bath.35
In another study (No. 53), the adverse events and/or reasons for dropouts were stated for the two groups: in the CHM plus chemotherapy group, 2 refused chemotherapy, 2 had chemotherapy-related adverse reactions, and there were 2 deaths; in the chemotherapy group, 3 refused chemotherapy, 8 had chemotherapy-related adverse reactions and there were 3 deaths.77 In this study, it was unclear whether any of the adverse events were due to the herbal medicines. In the study by Kono et al,47 there were 3 dropouts in the CHM group and 1 dropout in the control group, but these were prior to commencement of treatment. In Nishioka et al,63 more people discontinued chemotherapy in the CHM group (n = 13) than in the control group (n = 11); the same numbers showed progressive disease (9 vs 9); more experienced an allergic reaction to oxaliplatin (4 vs 1); and fewer had persistent grade III oxaliplatin-induced neuropathy (0 vs 1); but it was unclear whether these differences were influenced by the herbal medicine. In Oki et al,64 the most common comorbidities were hypertension and diabetes with no between-group difference in their incidence, but the incidence of grade II or greater CIPN was higher in the integrative group at the 8-month analysis (mean 8-9 cycles) so the study was discontinued. The analysis of oxaliplatin dose showed it was higher in the integrative group, but it was unclear whether this accounted for the increased CIPN rate. Overall, no serious adverse events associated with the herbal medicines were identified but data were incomplete.
Discussion
The majority of the data were for studies that combined an orally administered herbal medicine with a chemotherapy regimen compared to the same chemotherapy regimen with CIPN as an outcome. A further 5 studies had poolable data for hand and foot baths for CIPN and 5 studies assessed orally administered herbal medicine for HFS.
Effects on CIPN
The main outcome measures were the WHO criteria, Levi’s modified WHO criteria, or the NCI-CTCAE criteria. Although these criteria are similar there are some differences, so the results were pooled separately. For each outcome measure, the more severe grades of CIPN (grades III + IV) were reported first. Such severe events are clinically relevant since they are likely to lead to chemotherapy cessation. In general, the incidences of Grade III + IV CIPN were low in both groups and a number of studies had zero events. Studies that had zero events in both groups did not contribute to the pooled results, so the incidences and identity of such studies are recorded in the results tables.
For incidence of grades III + IV CIPN, there was a significant reduction in the integrative therapy groups based on 14 studies that used the WHO criteria (955 participants) without heterogeneity (Table 3). However, the overall incidence at end of treatment was low, with 1.6% in the integrative groups and 4.1% in the chemotherapy alone groups (Supplementary Table S5). It is notable that the reduction was mainly in the subgroup of FOLFOX regimens. This result was similar to a previous meta-analysis of CHM plus FOLFOX4 in advanced CRC, which found slightly higher incidences (2.2% vs 6.0%).89 In the 6 studies (485 participants) that used Levi’s criteria the meta-analysis results and the incidences (1.4% vs 9.2%) were similar (Table 5 and Supplementary Table S5). In the 7 studies (561 participants) that used the NCI-CTCAE criteria there was no significant difference between groups with some heterogeneity, and considerably higher incidence rates (8.5% vs 13.0%) (Table 6 and Supplementary Table S5).
Overall, the incidences of grade III plus IV CIPN in the WHO and Levi groups were much lower than in the trials by de Gramont et al7 and Cassidy et al.8 A likely reason is that most studies were of too short a duration for severe CIPN to develop. Larger proportions were evident in the FOLFOX regimens for NCI-CTCAE criteria which included 4 placebo-controlled studies (4-26 weeks’ duration).47,58,59,64 In this group, the grade III plus IV incidence was 18.9% in the chemotherapy controls (all FOLFOX), which is similar to the 17% reported by Cassidy et al,8 versus 12.9% in the integrative therapy groups.
For all grades, the majority of studies provided nonzero data. For the 32 studies that used the WHO criteria (1853 participants) there was a significant reduction in CIPN in the integrative groups, but the heterogeneity was considerable (Table 4). Since this was due to a single study in which all participants already had CIPN at baseline, it was reasonable to exclude this study from the pooled result to obtain a more accurate estimate. This sensitivity analysis had the effect of eliminating the heterogeneity, while finding a similar result. The resultant incidence rates were 32.7% in the integrative groups and 42.8% in the chemotherapy controls. In the 7 studies that used Levi’s criteria, there was also a significant reduction in all grades CIPN in the integrative groups, but with considerable heterogeneity (Table 5). This was due to 2 studies with very high CIPN incidences in both groups (73%-82%) that found no differences between groups. When excluded, the CIPN incidence rates for the remaining five studies were 32.4% for integrative therapy versus 73.0% for the chemotherapy controls (Supplementary Table S5). For the NCI-CTCAE criteria, the pool of 9 studies (727 participants) showed a significant reduction in the integrative groups (Table 6), with incidence rates of 25.8% versus 33.3% (Supplementary Table S5). Notably, the positive results were associated with the FOLFOX regimens. In comparison, in the review by Seretny et al,5 the mean rates of all grades CIPN were 60% at 3 months and 30% at 6 months or longer.
It was expected that the results of the subgroup of studies that used syndrome differentiation would show less heterogeneity than the overall pools since the participant groups should be less variable due to this additional selection criterion. This was evident for all grades CIPN in a number of pools. Notably, for the WHO criteria, the 17 studies that used syndrome differentiation found a benefit for adding the herbal medicine without heterogeneity, as did the group of 13 studies of Pi xu 脾虚 syndromes (Table 4).
In the studies of hand and foot baths, the pooled result for grades III + IV did not show a significant difference between groups but there were few cases in total (Table 9). For all grades, the incidences in the four studies included in the final sensitivity analysis were 60% in the chemotherapy-alone groups and 30% in the integrative groups indicating the herbal hand and foot baths produced a significant reduction in CIPN.
Effects on Hand-Foot Syndrome
For grade III HFS (WHO criteria) the incidence was very low, at 0.9% in the integrative groups and 2.8% in the chemotherapy control groups. In the studies that used the NCI-CTCAE criteria, there was only 1 case in total (Supplementary Table S5). So, there were insufficient data for a meaningful assessment.
For WHO all grades (Table 7), the pooled result for 6 studies found no significant difference between groups with considerable heterogeneity, which was due to the previously-mentioned study that only enrolled participants with HFS (grade I-II), none of whom resolved in either group. When excluded, there was a significant difference, with 23.3% incidence in the integrative groups versus 41.8% in the chemotherapy alone groups (Supplementary Table S5). In the 5 studies that used the NCI-CTCAE criteria, there was no difference between groups with substantial heterogeneity and rates of 40% in both groups (Table 8 and Supplementary Table S5).
When compared with the results reported in Cassidy et al,8 the low rate of grade III was consistent with most studies using a FOLFOX regimen, which generally do not produce high rates. However, the single study of XELOX reported zero grade III cases in both groups. For all grades, the rates were relatively high in the WHO criteria group, due mainly to the XELOX, SOX, and HIPEC subgroups. Four of the studies in the NCI-CTCAE criteria group used XELOX and this sub-group produced the highest incidence rates (44% vs 48%), as could be expected. Adding a herbal medicine did not have a significant effect in this subgroup. Overall, the herbal medicines appeared to reduce the incidence of HFS, but the results for XELOX were heterogeneous.
Limitations of This Review
This meta-analysis was based on published data mostly from unblinded studies, so the results are likely to be biased in favor of the integrative therapy groups. Asymmetry in the funnel plot (Supplementary Figure S2) suggested bias due to missing data, due either to small studies with nonsignificant results not being published or published studies omitting mention of CIPN. Therefore, caution is required when interpreting the meta-analysis results.
In the 5 placebo-controlled studies, the poolable results were mixed with one study showing higher rates in the integrative group64 whereas the others showed improvements. Such data are difficult to interpret, each study showed distinct characteristics and no clear subgroups were evident.
In the larger meta-analysis pools, it was possible to identify some differences due to the chemotherapy regimen used, but there was no plausible method of taking duration of treatment or accumulated dose of chemotherapy into account due to inconsistencies in how such data were reported. These factors are likely to have influenced the incidences of CIPN and HFS and contributed to heterogeneity.
Another issue is variability in the herbal interventions. Although there were similar ingredients in many of the oral formulae, it was not clear which had been included to counter CIPN or HFS and which had been included for other reasons, since these outcomes were not primary in most studies. Goshajinkigan was the only oral formula that has been studied for reducing CIPN in multiple retrospective90-92 and prospective47,63,64 studies of CRC. Also, it has been reported to reduce the incidence of CIPN in ovarian cancer.93 Recent meta-analyses of Goshajinkigan have shown mixed results when multiple cancer types and comparators were pooled but there was considerable heterogeneity.20,22 Although the retrospective studies of this formula showed benefits in CRC, the results of the 3 prospective studies included in this review were mixed, with 2 studies reporting benefits47,63 and 1 finding possible detriment.64 For Goshajinkigan and the 3 other formulae that were used in 2 studies each, differences between studies in the comparators or outcome measures precluded data pooling for these specific CHMs. Consequently, it was not possible to select a best CHM intervention for CIPN or HFS.
Data on the safety of the herbal medicines were poorly reported in most studies. This was in part due to the overriding effects of the toxicity due to the chemotherapies. It was not possible to assess whether any of the herbal medicines reduced the effectiveness of the chemotherapies since parallel data sets were not available. However, previous meta-analyses that have addressed this issue found no evidence that the addition of herbal medicines to chemotherapy reduced tumor response rates.94,95
Implications for Clinical Practice and Further Research
Based on the pooled results, the evidence for reduction in grades III + IV CIPN is weak due to the small number of cases in the analyses. Larger studies of oxaliplatin regimens that are long enough in duration for severe CIPN to accumulate are needed to explore this question.
For all grades of CIPN, it appeared that addition of orally administered herbal medicines was likely to reduce less severe CIPN. However, since most studies were relatively short, it is unclear whether this effect would translate into overall reduction in incidence or severity of CIPN with longer term oxaliplatin use. It is possible that the herbal medicines delayed the onset of the CIPN but not its ultimate progression. Further study is needed to monitor the progression of CIPN in relation to accumulated oxaliplatin dose. There were very little data for non-oxaliplatin regimens, so it is not possible determine if any herbal medicines were beneficial.
The herbal hand and foot baths appear to have provided some reductions in CIPN. From a clinical perspective, the use of topical herbal medicines should reduce the potential for interactions with a patient’s medications, but it is important to note the potential for allergic reactions and to test each patient’s sensitivity to the formula before undertaking a course of treatment.
For HFS, the results of the studies showed no convincing evidence of a benefit, but there was heterogeneity in the result and the sample size was relatively small. Further studies of oral and topical preparations are needed to determine the role of herbal medicines in this condition.
Conclusions
Data on the effects of herbal medicines for CIPN and/or HFS were available for 63 RCTs. For orally administered herbal formulae combined with chemotherapies, the evidence indicated a reduction in all grades CIPN (WHO toxicity criteria) in the integrative therapy groups based on 31 RCTs with 1778 participants. Similar results were evident for other criteria but based on smaller pools. For grades III + IV severe CIPN, there were also reductions, but the evidence was weaker. The use of the herbal hand and foot baths appeared to reduce all grades of CIPN, but the results were based on a small sample. The oral herbal formulae did not appear to improve HFS. The strength of these conclusions is limited by lack of blinding in the majority of studies and the possibility of reporting bias. Future clinical studies are needed that focus on specific herbal medicines for CIPN and HFS. Experimental studies are required to determine the mechanisms of action of any promising herbs.
Supplemental Material
Supplemental material, IM_for_CIPN_Supplementary_materials_17_10_2018 for Integrative Herbal Medicine for Chemotherapy-Induced Peripheral Neuropathy and Hand-Foot Syndrome in Colorectal Cancer: A Systematic Review and Meta-Analysis by Yihong Liu, MMed, Brian H. May, PhD, Anthony Lin Zhang, PhD, Xinfeng Guo, PhD, Chuanjian Lu, PhD, Charlie Changli Xue, PhD and Haibo Zhang, MD in Integrative Cancer Therapies
Acknowledgments
We wish to thank Dr Meaghan Coyle for her help with searches.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors acknowledge the funding support provided by the China-Australia International Research Centre for Chinese Medicine (CAIRCCM)—a joint initiative of RMIT University, Australia and Guangdong Provincial Academy of Chinese Medical Sciences, China, and the Foundation for Chinese Medicine and Technology Research of Guangdong Provincial Hospital of Chinese Medicine (2017KT1820, 2016KT1571).
Supplemental Material: The online supplementary material is available at http://journals.sagepub.com/doi/suppl/10.1177/1534735418817833
ORCID iD: Xinfeng Guo  https://orcid.org/0000-0003-2699-9740
https://orcid.org/0000-0003-2699-9740
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [DOI] [PubMed] [Google Scholar]
- 3. Zhu J, Tan Z, Hollis-Hansen K, et al. Epidemiological trends in colorectal cancer in China: an ecological study. Dig Dis Sci. 2017;62:235-243. [DOI] [PubMed] [Google Scholar]
- 4. Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on chemotherapy-induced peripheral neuropathy (CIPN): a narrative review. Br J Anaesth. 2017;119:737-749. [DOI] [PubMed] [Google Scholar]
- 5. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155:2461-2470. [DOI] [PubMed] [Google Scholar]
- 6. Cioroiu C, Weimer LH. Update on chemotherapy-induced peripheral neuropathy. Curr Neurol Neurosci Rep. 2017;17:47. [DOI] [PubMed] [Google Scholar]
- 7. de Gramont A, Bosset JF, Milan C, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol. 1997;15:808-815. [DOI] [PubMed] [Google Scholar]
- 8. Cassidy J, Clarke S, Diaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006-2012. [DOI] [PubMed] [Google Scholar]
- 9. Nikolaou V, Syrigos K, Saif MW. Incidence and implications of chemotherapy related hand-foot syndrome. Expert Opin Drug Saf. 2016;15:1625-1633. [DOI] [PubMed] [Google Scholar]
- 10. Kwakman JJ, Punt CJ. Oral drugs in the treatment of metastatic colorectal cancer. Expert Opin Pharmacother. 2016;17:1351-1361. [DOI] [PubMed] [Google Scholar]
- 11. Avan A, Postma TJ, Ceresa C, et al. Platinum-induced neurotoxicity and preventive strategies: past, present, and future. Oncologist. 2015;20:411-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poupon L, Kerckhove N, Vein J, et al. Minimizing chemotherapy-induced peripheral neuropathy: preclinical and clinical development of new perspectives. Expert Opin Drug Saf. 2015;14:1269-1282. [DOI] [PubMed] [Google Scholar]
- 13. Brewer JR, Morrison G, Dolan ME, Fleming GF. Chemotherapy-induced peripheral neuropathy: current status and progress. Gynecol Oncol. 2016;140:176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen XF, Wang R, Yin YM, et al. The effect of monosialotetrahexosylganglioside (GM1) in prevention of oxaliplatin induced neurotoxicity: a retrospective study. Biomed Pharmacother. 2012;66:279-284. [DOI] [PubMed] [Google Scholar]
- 15. Zhu Y, Yang J, Jiao S, Ji T. Ganglioside-monosialic acid (GM1) prevents oxaliplatin-induced peripheral neurotoxicity in patients with gastrointestinal tumors. World J Surg Oncol. 2013;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scontre VA, Martins JC, de Melo Sette CV, et al. Curcuma longa (turmeric) for prevention of capecitabine-induced hand-foot syndrome: a pilot study. J Diet Suppl. 2018;15:606-612. [DOI] [PubMed] [Google Scholar]
- 17. Elyasi S, Shojaee FSR, Allahyari A, Karimi G. Topical silymarin administration for prevention of capecitabine-induced hand-foot syndrome: a randomized, double-blinded, placebo-controlled clinical trial. Phytother Res. 2017;31:1323-1329. [DOI] [PubMed] [Google Scholar]
- 18. Miller KK, Gorcey L, McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol. 2014;71:787-794. [DOI] [PubMed] [Google Scholar]
- 19. Wei XC, Zhu LQ, Wang H, et al. Efficacy of traditional Chinese medicines in preventing oxaliplatin-induced peripheral neurotoxicity in cancer patients: a network meta-analysis. Chin Herb Med. 2017;9:161-168. [Google Scholar]
- 20. Hoshino N, Ganeko R, Hida K, Sakai Y. Goshajinkigan for reducing chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Int J Clin Oncol. 2018;23:434-442. [DOI] [PubMed] [Google Scholar]
- 21. Cascella M, Muzio MR. Potential application of the Kampo medicine goshajinkigan for prevention of chemotherapy-induced peripheral neuropathy. J Integr Med. 2017;15:77-87. [DOI] [PubMed] [Google Scholar]
- 22. Kuriyama A, Endo K. Goshajinkigan for prevention of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Supportive Care Cancer. 2018;26:1051-1059. [DOI] [PubMed] [Google Scholar]
- 23. Derksen TME, Bours MJL, Mols F, Weijenberg MP. Lifestyle-related factors in the self-management of hemotherapy-induced peripheral neuropathy in colorectal cancer: a systematic review. Evid Based Complement Alternat Med. 2017;2017:7916031. doi: 10.1155/2017/7916031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [Google Scholar]
- 25. Bao YJ, Qiu YY, Hu SJ, et al. The effect of Jianpibushen formula on postoperative adjuvant treatment of colorectal cancer [健脾补肾方对大肠癌术后辅助治疗的疗效]. Shanghai Med J. 2014;37:984-986. [Google Scholar]
- 26. Cai ZB. Clinical Observation of Prevention of Oxaliplatin-Induced Neurotoxicity by Wenjinghuoxue Formula Bath [温经活血方外洗预防奥沙利铂周围神经毒性的临床观察]. Guangzhou, China: Guangzhou University of Chinese Medicine; 2016;12-16. [Google Scholar]
- 27. Cao B. The clinical efficacy of Yiqijianpi decoction combined with FOLFOX4 in postoperative colorectal cancer patients [益气健脾汤联合FOLFOX4方案治疗结直肠癌术后患者的临床疗效]. Cancer Res Prev Treat. 2011;38:820-822. [Google Scholar]
- 28. Chen CG. Clinical Trial of Jianpiyishen and Huayujiedu Therapy Combined With FOLFOX4 for Medium and Advanced Colorectal Cancer [健脾益肾,化瘀解毒法联合FOLFOX-4方案治疗中,晚期大肠癌的临床研究]. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2005;20-31. [Google Scholar]
- 29. Chen XJ. Clinical Trial of Jianpihuashiquyu Formula Combined With Chemotherapy for Advanced Colorectal Cancer [健脾化湿祛瘀方联合化疗治疗晚期大肠癌的临床研究]. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2010:14-20. [Google Scholar]
- 30. Chen Y. Experimental Study of the Influence of Weitiaosanhao Formula on Inflammatory Microenvironment of Colorectal Cancer and Clinical Trial of the Formula Plus SOX in Colorectal Cancer Patients [微调三号方对大肠癌炎性微环境影响的实验研究及其联合SOX方案的临床研究]. Nanjing: Nanjing University of Traditional Chinese Medicine; 2014:28-40. [Google Scholar]
- 31. Cheng XL, Huo JG, Wang DW, et al. Herbal medicine AC591 prevents oxaliplatin-induced peripheral neuropathy in animal model and cancer patients. Front Pharmacol. 2017;8:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fang ZH, Li Y, Chen Y, Chen DL. Clinical observation of Jianpikangai formula combined with chemotherapy for advanced colorectal cancer [健脾抗癌方配合化疗治疗晚期大肠癌31例]. Shanghai J Tradit Chin Med. 2009;43:29-31. [Google Scholar]
- 33. Feng YQ. Clinical observation of prevention and treatment of oxaliplatin-induced neurotoxicity by Chinese medicine hand and foot bath [中药外洗防治奥沙利铂神经毒性的护理观察]. Straits Pharm J. 2011;23:203-204. [Google Scholar]
- 34. Gai L, Shi B, Zhang XB. The effect of Shenyi capsule plus XELOX versus XELOX for colorectal cancer [参一胶囊联合XELOX方案与单纯XELOX方案治疗晚期直肠癌的临床观察]. Mod J Integ Trad Chin West Med. 2010;19:1066-1067. [Google Scholar]
- 35. Gao J, Yan SH, Li DP, et al. Clinical observation of Chinese medicine bath for prevention of oxaliplatin-induced neurotoxicity [中药泡洗预防奥沙利铂所致神经毒性的疗效观察]. Hebei J Trad Chin Med. 2015;37:994-996. [Google Scholar]
- 36. Gao XM. Clinical Trial of Modified Bazhen Decoction on Decreasing the Adjuvant Chemotherapy Side Effects in Postoperative Colon Cancer Patients With Deficiency of Qi and Blood [八珍汤加减对结肠癌术后气血两虚型患者辅助化疗减毒作用的临床研究]. Fuzhou, China: Fujian University of Traditional Chinese Medicine; 2015:3-11. [Google Scholar]
- 37. He JP, Qu JH. Clinical trial of Gubenjiedu therapy plus FOLFOX4 for advanced or recurrent rectal cancer [固本解毒法联合FOLFOX4方案化疗治疗晚期或复发性直肠癌的临床]. J Sichuan Trad Chin Med. 2013;31:76-78. [Google Scholar]
- 38. He ZF. Clinical Trial of Yiqijianpi and Huayujiedu Formula Combined With FOLFOX for Postoperative Colorectal Cancer [益气健脾,化瘀解毒方合FOLFOX方案化疗治疗术后大肠癌的临床研究]]. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2006:8-17. [Google Scholar]
- 39. Hou ZB. The Effect of Modified Banxiaxiexin Decoction for Postoperative Colorectal Cancer and Related Tumor Markers [半夏泻心汤加减对结直肠癌术后的临床疗效及相关血清肿瘤标志物的影响]. Guiyang, China: Guiyang College of Traditional Chinese Medicine; 2014:7-21. [Google Scholar]
- 40. Hu B, Li G, An HM, et al. Clinical trial of Tenglong Buzhong decoction plus chemotherapy for advanced colorectal cancer [藤龙补中汤联合化疗治疗晚期大肠癌临床研究]. Chin Arch Trad Chin Med. 2015;33:37-39. [Google Scholar]
- 41. Hu FS, Zhang Q, Wang XM, et al. Clinical trial of Gubenxiaoliu capsule plus FOLFOX4 for advanced colorectal cancer [固本消瘤胶囊联合FOLFOX4方案治疗晚期大肠癌的临床研究]. Chin J Inf Trad Chin Med. 2007;14:13-14. [Google Scholar]
- 42. Hu QQ. Clinical trial of Chinese medicine three-step cytotherapy plus modified XELOX for stage IV colorectal cancer [中药三步周期疗法联合改良XELOX方案治疗Ⅳ期大肠癌的临床研究]. http://cdmd.cnki.com.cn/Article/CDMD-10315-1014127885.htm. Published 2013. Accessed Novenmber 21, 2018.
- 43. Huang L, Guo JH. Clinical trial of Yiqihuatansanjie formula plus XELOX for advanced recurrent colon cancer [益气化痰散结方联合XELOX化疗方案治疗晚期复发性结肠癌的临床研究]. Chin Arch Trad Chin Med. 2014;32:670-672. [Google Scholar]
- 44. Jiang ZM, Hu LQ, Jiang SS. Clinical observation of modified Sijunzi decoction plus FOLFOX6 for advanced colorectal cancer [加味四君子汤联合FOLFOX6方案治疗晚期结直肠癌临床观察]. Zhejiang Clin Med J. 2014;16:1296-1298. [Google Scholar]
- 45. Jiao SJ, Fan CQ, An GY, et al. Clinical trial of Zibu decoction plus capecitabine as maintenance treatment in advanced colorectal cancer with deficiency of Qi and Blood after first-line chemotherapy [滋补汤联合卡培他滨维持治疗一线化疗后气血两虚证晚期结直肠癌的研究]. Mod J Int Trad Chin West Med. 2016;25:1258-1260,1317. [Google Scholar]
- 46. Ke SW, Huang GD, Zhao J. Clinical trial of Kangaiyiliu formula plus CapeOx for advanced colorectal cancer [抗癌抑瘤方联合CapeOx方案治疗晚期结直肠癌]. Chin Trad Patent Med. 2015;37:49-54. [Google Scholar]
- 47. Kono T, Hata T, Morita S, et al. Goshajinkigan oxaliplatin neurotoxicity evaluation (GONE): a phase 2, multicenter, randomized, double‑blind, placebo‑controlled trial of goshajinkigan to prevent oxaliplatin‑induced neuropathy. Cancer Chemother Pharmacol. 2013;72:1283-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lai YQ, Chen NJ, Wu DH, Chen YY. Clinical observation of prevention of oxaliplatin-induced neurotoxicity by Jianpiyishen therapy in 27 cases [健脾益肾法预防奥沙利铂周围神经毒性27例. 福建中医药]. Fujian J Trad Chin Med. 2009;40:22-23. [Google Scholar]
- 49. Li J. Clinical Observation and Mechanism Research of Fuzhenghuayujiedusanjie Method Plus Chemotherapy for Advanced Colorectal Cancer [扶正化瘀解毒散结配合化疗治疗晚期大肠癌的临床观察及机理研究]. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2011:12-24. [Google Scholar]
- 50. Li L. Clinical trial of Jianpihuashiquyu Formula Plus Caplri for Advanced Colorectal Cancer [健脾化湿祛瘀方联合Caplri方案化疗治疗晚期大肠癌的临床研究]. Nanjing, China:Nanjing University of Traditional Chinese Medicine; 2007:11-20. [Google Scholar]
- 51. Li LC. Clinical and experimental study of compound Changtai in treating colorectal cancer[复方肠泰治疗大肠癌的临床和实验研究]. http://cdmd.cnki.com.cn/Article/CDMD-10315-2009251849.htm. Accessed November 21, 2018.
- 52. Li N. Clinical Trial of Jianpiyishen Therapy for Postoperative Colorectal Cancer [健脾益肾法治疗术后大肠癌的临床研究]. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2012;10-18. [Google Scholar]
- 53. Li YJ, Chen JZ, Huang WX, Li XF. Clinical observation of Wenshenjianpi formula plus chemotherapy for elderly patients with advanced colorectal cancer [温肾健脾方联合化疗治疗老年进展期大肠癌的临床观察]. Fujian J Trad Chin Med. 2007;38:13-14. [Google Scholar]
- 54. Liang XS, Chen MC, Chen DL, Lin Q. Clinical observation of prevention and treatment of oxaliplatin-induced neurotoxicity by Yiqilixueyufeng decoction in 46 cases [益气理血愈风汤防治奥沙利铂所致外周神经毒性反应46例]. Chin J Trad Med Sci Technol. 2012;19:94. [Google Scholar]
- 55. Liang XS, Yan LL, Lin Q. Clinical observation of prevention and treatment of oxaliplatin-induced neurotoxicity by Lixuequfeng decoction [理血祛风汤防治大肠癌术后奥沙利铂所致外周神经毒性反应74例]. Zhejiang J Tradl Chin Med. 2015;50:522-523. [Google Scholar]
- 56. Liu JP, Jiang J, Wei H, et al. Clinical observation of Huachansu capsule plus XELOX for advanced colon cancer [华蟾素联合XELOX方案治疗晚期结肠癌的临床疗效观察]. Med J West China. 2017;29:1560-1563. [Google Scholar]
- 57. Liu P, Liu JH, Zhu DQ, et al. Clinical observation of Boerning capsule plus FOLFOX4 for colon cancer [博尔宁胶囊联合FOLFOX4方案治疗结肠癌的临床观察]. Chin J Clin Oncol. 2007;34:89-91. [Google Scholar]
- 58. Liu SC. Clinical Observation of Yiqiyangying Method Plus FOLFOX6 for Medium and Advanced Colorectal Cancer [益气养阴中药配合FOLFOX6方案化疗治疗中晚期大肠癌的临床观察]. Beijing, China: Beijing University of Chinese Medicine; 2011:26-35. [Google Scholar]
- 59. Liu YF, Zhu GY, Han L, Liu J, Ma T, Yu H. Clinical study on the prevention of oxaliplatin-induced neurotoxicity with Guilongtongluofang: results of a randomized, double-blind, placebo-controlled trial. Evid Based Complement Alternat Med. 2013;2013:541217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ma J, Lu CW, Cai DF, Xu JM. Clinical observation of Jianpixiaoliu formula plus FOLFOX4 for advanced colon cancer [健脾消瘤方联合FOLFOX4方案治疗晚期结直肠癌临床观察]. J New Chin Med. 2015;47:208-210. [Google Scholar]
- 61. Mao WD, Jiang LX, Zhou JY, Jiang HM. The effect of Chinese medicine plus chemotherapy to prevent the recurrence and metastasis of postoperative colorectal cancer [中药加化疗对大肠癌术后复发转移的影响研究]. Med J China. 2011;8:70-72. [Google Scholar]
- 62. Mao ZJ, Zhu LM, Lu YL, Shen KP. The effect of Jianpi therapy plus FOLFOX on cancer-related fatigue, Th1/Th2 immune response balance and peripheral neuropathy in postoperative colon cancer patients [中医健脾法配合FOLFOX化疗方案对结肠癌术后患者癌因性疲乏, Th1/Th2免疫应答平衡及周围神经病变的影响]. Mod J Int Trad Chin West Med. 2017;26:4027-4030. [Google Scholar]
- 63. Nishioka M, Shimada M, Kurita N, et al. The Kampo medicine, Goshajinkigan, prevents neuropathy in patients treated by FOLFOX regimen. Int J Clin Oncol. 2011;16:322-327. [DOI] [PubMed] [Google Scholar]
- 64. Oki E, Emi Y, Kojima H, et al. Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): a placebo‑controlled, double‑blind, randomized phase III study. Int J Clin Oncol. 2015;20:767-775. [DOI] [PubMed] [Google Scholar]
- 65. Pan RR. Clinical Trial of Erlingyiren Decoction Plus XELOX in Postoperative Colorectal Cancer Patients With Spleen Deficiency, Dampness and Heat Syndrome [二苓苡仁汤联合XELOX方案化疗治疗肠癌术后脾虚湿热证的临床研究]. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2017:11-21. [Google Scholar]
- 66. Pu QH. Experimental Study of the Effect of Weitiaosanhao Formula on VEGF, nm23-H1 and CK20 for Colorectal Cancer With Liver Metastasis and Clinical Trial of the Formula Plus FOLFOX4 in Colorectal Cancer With Liver Metastasis Patients [微调三号方对大肠癌肝转移VEGF, nm23-H1和CK20的影响的实验研研究及其联合FOLFOX4方案的临床研究]. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2012;31-41. [Google Scholar]
- 67. Qin CY. Clinical Observation of Prevention and Treatment of Chemotherapy Side Effects by Jianpiyishen Therapy in Medium and Advanced Colorectal Cancer [健脾益肾治法防治中晚期结直肠癌化疗毒副反应的临床观察]. Beijing, China: Beijing University of Chinese Medicine; 2014;25-35. [Google Scholar]
- 68. Shi RQ. Clinical Trial of Yiqijianpi Formula Plus XELOX for Postoperative Colorectal Cancer [益气健脾方联合XELOX方案治疗大肠癌术后患者的临床研究]. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2017;8-15. [Google Scholar]
- 69. Shu JH, Zhou RY, Zhong Y, Wu LY. Clinical observation of Yiqijiedu decoction plus CapeOX for advanced colorectal cancer [益气解毒汤联合CapeOX方案治疗晚期大肠癌45例]. Shanghai J Trad Chin Med. 2011;45:33-35,45. [Google Scholar]
- 70. Wang H. Clinical observation of Yiqihuoxuebuchang decoction plus chemotherapy for postoperative rectal cancer [益气活血补肠汤合化疗治疗直肠癌术后患者的疗效观察]. J Liaoning Univ Trad Chin Med. 2008;10:81-82. [Google Scholar]
- 71. Wang JM. Clinical Observation of Jianpiyangyingqushijiedu Therapy Plus XELOX for Medium and Advanced Colorectal Cancer [健脾养阴祛湿解毒法联合XELOX方案治疗中晚期结直肠癌的临床疗效观察]. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2016;11-18. [Google Scholar]
- 72. Wang JZ, Ke YH, Liu PC, et al. Clinical trial of Yichangning formula plus FOLFOX4 for advanced colorectal cancer in 30 cases [宜肠宁方联合FOLFOX-4方案治疗晚期结直肠癌30例临床研究]. Fujian J Trad Chin Med. 2011;42:23-24. [Google Scholar]
- 73. Wang Q. Clinical observation of prevention and treatment of oxaliplatin-induced neurotoxicity by Huangqiguizhiwuwu decoction hand and foot bath plus calcium gluconate and magnesium sulphate [黄芪桂枝五物汤手足浴联合钙镁合剂防治奥沙利铂神经毒性的临床观察]. Mod J Integ Trad Chin West Med. 2015;24:318-320. [Google Scholar]
- 74. Wang QY, He W, Lin Q, Li LR. Clinical observation of Chinese medicine plus chemotherapy for advanced colon cancer [中药联合化疗治疗晚期结肠癌的临床疗效观察]. Mod Digestion Intervent. 2015;20:387-389. [Google Scholar]
- 75. Wang SW. Clinical Observation of Tongtai Decoction Plus XELOX for Advanced Colorectal Cancer [通泰合剂联合XELOX方案化疗治疗晚期大肠癌疗效临床观察]. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2012;14-23. [Google Scholar]
- 76. Xie W. Clinical Observation on the Inhibitory Effect of Xiaoliu Decoction for Antiangiogenic VEGF in Colorectal Cancer [消瘤汤对结直肠癌VEGF抑制作用的临床研究]. Nanning, China: Guangxi University of Traditional Chinese Medicine; 2010;6-14. [Google Scholar]
- 77. Xu C, Yu XW, Li M. Clinical observation of modified Xiangshaliujunzi decoction plus FOLFIRI for advanced colon cancer [加味香砂六君子汤联合FOLFIRI方案治疗晚期结肠癌]. Chin J Clin Med. 2012;19:36-37. [Google Scholar]
- 78. Xu XQ, Qi YF. Clinical observation of Yiqihexue therapy for chemotherapy-induced neurotoxicity in colorectal cancer patients [益气和血法治疗大肠癌患者化疗所致神经毒性疗效分析]. World J Integ Trad West Med. 2014;9:1087-1089. [Google Scholar]
- 79. Yang CD. Clinical observation on the effect of Chinese herbal fumigation on the peripheral neurotoxicity of oxaliplatin [中药熏洗治疗奥沙利铂所致周围神经毒性的临床观察]. China Clin Nurs. 2015;7:234-235. [Google Scholar]
- 80. Ye HQ, Zhong MW, Chen XL, Tang RD. Clinical observation of Qifulongkui decoction plus chemotherapy for metastatic colorectal cancer in 30 cases [芪附龙葵汤联合化疗治疗转移性结直肠癌30例临床观察]. J Trad Chin Med. 2016;57:1490-1492,1496. [Google Scholar]
- 81. Zeng JQ, Li ZP, Wang X. Clinical observation of Chinese medicine plus chemotherapy for advanced colorectal cancer in 30 cases [中医辨证施治配合化疗治疗晚期大肠癌30例]. J Jiangxi Univ Trad Chin Med. 2008;20:39-41. [Google Scholar]
- 82. Zeng JY, He J, Wang QJ, et al. The effect of Xiaoliu decoction plus hyperthermic intraperitoneal chemotherapy on postoperative immune function and VEGF for advanced colorectal cancer [消瘤汤联合热灌注化疗对进展期结直肠癌术后免疫功能和血清血管内皮生长因子水平的影响]. J Guangxi Med Univ. 2010;27:415-416. [Google Scholar]
- 83. Zhang C, Han ZG. Clinical observation of Chinese medicine plus chemotherapy for postoperative colorectal cancer [化疗联合中药治疗在结直肠癌术后的临床效果观察]. China Mod Med. 2015;22:135-138,141. [Google Scholar]
- 84. Zhang Q, Wang XM, Yang GW, et al. Clinical trial of Gubenxiaoliu capsule plus FOLFOX4 for advanced colorectal cancer [固本消瘤胶囊联合FOLFOX4化疗方案治疗晚期大肠癌的临床研究]. Beijing J Trad Chin Med. 2010;29:255-257. [Google Scholar]
- 85. Zhang WW, Chen J, Xie GQ, et al. Clinical trial of Jianpijiedu formula plus capecitabine for advanced colorectal cancer [健脾解毒方结合卡培他滨片治疗晚期大肠癌临床研究]. J Shanghai Univ Trad Chin Med. 2013;27:31-34. [Google Scholar]
- 86. Zhong MW, Ye HQ, Li QZ, et al. The effect of Qifulongkui decoction plus chemotherapy on quality of life in the patient with metastatic colorectal cancer [芪附龙葵汤联合化疗对转移性结直肠癌患者生存质量的影响]. Mod J Integ Trad Chin West Med. 2016;25:1980-1982. [Google Scholar]
- 87. Zhu FY, Wang B, Ai Y, et al. Clinical trial of Jianpiyiqijiedu formula plus chemotherapy for advanced colorectal cancer [健脾益气解毒方联合化疗治疗晚期大肠癌临床研究]. Mod J Integ Trad Chin West Med. 2016;25:261-263. [Google Scholar]
- 88. Levi F, Misset JL, Brienza S, et al. A chronopharmacologic phase-II clinical-trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump. High antitumor effectiveness against metastatic colorectal-cancer. Cancer. 1992;69:893-900. [DOI] [PubMed] [Google Scholar]
- 89. Chen M, May BH, Zhou IW, Xue CC, Zhang Al. FOLFOX 4 combined with herbal medicine for advanced colorectal cancer: a systematic review. Phytother Res. 2014;28:976-991. [DOI] [PubMed] [Google Scholar]
- 90. Hosokawa A, Ogawa K, Ando T, et al. Preventive effect of traditional Japanese medicine on neurotoxicity of FOLFOX for metastatic colorectal cancer: a multicenter retrospective study. Anticancer Res. 2012;32:2545-2550. [PubMed] [Google Scholar]
- 91. Kono T, Mamiya N, Chisato N, et al. Efficacy of Goshajinkigan for peripheral neurotoxicity of oxaliplatin in patients with advanced or recurrent colorectal cancer. Evid Based Complement Alternat Med. 2011;2011:418481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yoshida N, Hosokawa T, Ishikawa T, et al. Efficacy of goshajinkigan for oxaliplatin-induced peripheral neuropathy in colorectal cancer patients. J Oncol. 2013;2013:139740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kaku H, Kumagai S, Onoue H, et al. Objective evaluation of the alleviating effects of Goshajinkigan on peripheral neuropathy induced by paclitaxel/carboplatin therapy: a multicenter collaborative study. Exp Ther Med. 2012;3:60-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chen M, May BH, Zhou IW, et al. Meta-analysis of oxaliplatin-based chemotherapy combined with traditional medicines for colorectal cancer: contributions of specific plants to tumor response. Integr Cancer Ther. 2016;15:40-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chen M, May BH, Zhou IW, Sze DM, Xue CC, Zhang AL. Oxaliplatin-based chemotherapy combined with traditional medicines for neutropenia in colorectal cancer: a meta-analysis of the contributions of specific plants. Crit Rev Oncol Hematol. 2016;105:18-34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, IM_for_CIPN_Supplementary_materials_17_10_2018 for Integrative Herbal Medicine for Chemotherapy-Induced Peripheral Neuropathy and Hand-Foot Syndrome in Colorectal Cancer: A Systematic Review and Meta-Analysis by Yihong Liu, MMed, Brian H. May, PhD, Anthony Lin Zhang, PhD, Xinfeng Guo, PhD, Chuanjian Lu, PhD, Charlie Changli Xue, PhD and Haibo Zhang, MD in Integrative Cancer Therapies