Abstract
Chemotherapy while revolutionizing cancer management by improving survival and quality of life; is also associated with several adverse effects. Lung is the most common organ affected in chemotherapy-related complications, due to either drug toxicity or more commonly due to infections caused by immunosuppression and less commonly due to immune-mediated injury. Radiology, when used in combination with clinical and lab data, can help reach the specific diagnosis or narrow down the differentials. The common radiological patterns of drug toxicity include pulmonary interstitial and airway infiltrates, diffuse alveolar damage, nonspecific interstitial pneumonia, eosinophilic pneumonia, cryptogenic organizing pneumonia, pulmonary hemorrhage, edema and hypertension. Cancer patients are immunosuppressed due to the underlying malignancy itself or due to therapy and are prone to a gamut of opportunistic infections including viral, bacterial, fungal and mycobacterial pathogens. Immune reconstitution inflammatory syndrome (IRIS), a well-known complication in HIV, is now being increasingly recognized in non-HIV patients with immunosuppression. Engraftment syndrome is specifically seen following hematopoietic stem cell transplant during neutrophil recovery phase. Pulmonary involvement is frequent, causing a radiological picture of noncardiogenic pulmonary edema. Thus, radiology in combination with clinical background and lab parameters helps in detecting and differentiating various causes of pulmonary complications. This approach can help alter potentially toxic treatment and initiate early treatment depending on the diagnosis.
Keywords: Chemotherapy, engraftment syndrome, pulmonary drug toxicity, pulmonary infections
Introduction
Treatment in cancer patients with chemotherapeutic agents is an integral part of management. These drugs are also used as conditioning regimen during hematopoietic stem-cell transplantation. Though these agents have brought many revolutions in the management of patients with cancer by improving the survival and quality of life, these are also associated with several adverse effects and may lead to significant morbidity.[1,2] Among all the organs, lung is the most commonly affected with 75% patients suffering from some form of pulmonary infections[3] due to direct toxic effects on lungs or because of secondary complications due to immunosuppression leading to opportunistic infections. Less commonly, immune recovery induced by dose or regimen alterations may cause immune-mediated lung injury.[4,5] Chemotherapy-induced lung toxicity may manifest as both acute as well as chronic lung disease.[6] Common cytotoxic drugs implicated in lung toxicity are bleomycin, methotrexate, taxanes, cyclophosphamide, gemcitabine and newer targeted agents such as bevacizumab.[7,8]
The cytotoxic drugs act on the rapidly dividing cells, while many of the newer targeted-agents have specific molecular targets, and this understandably follows different mechanisms of drug toxicities.[9,10] Clinically, patients with drug toxicity may have nonspecific findings varying from being asymptomatic to progressive dyspnea. Clinicoradiological findings may be difficult to differentiate from infection, which is also common in these patients. Chest radiographs and HRCT chest are imaging modalities used to assess pulmonary complications.
Radiological features of pulmonary drug toxicity are often nonspecific. Thus, correlation with clinical details, including treatment history with the imaging findings is essential to reach the closest differential diagnosis. Recognition of the pattern of lung involvement would help narrow down the diagnosis and also help in follow up to look for resolution. Often bronchoalveolar lavage (BAL) and cytology or lung biopsy needs to be performed to establish a definite diagnosis. Still, several cases may remain undiagnosed and empirical treatment may be considered based on a consensus between the treating clinician and radiologist.
In this review, we aim to illustrate CT features of various chemotherapy-induced pulmonary complications which help in reaching to close differentials.
Radiological Manifestations
The pathogenesis of drug induced lung injury involves either cytotoxicity or immune-mediated injury. Cytotoxicity involves direct injury to the pneumocytes or to the vascular endothelium. The injury may be mediated by the generation of reactive oxygen species and cytokines or by defective deactivation of drug metabolites. Immune-mediated lung injury involves drug hypersensitivity reaction of any of the four types according to Gell and Coomb.[7]
Pulmonary drug toxicity
The common radiological patterns of drug toxicity include interstitial infiltrates, diffuse alveolar damage (DAD), nonspecific interstitial pneumonia (NSIP), eosinophilic pneumonia, cryptogenic organizing pneumonia (COP), pulmonary hemorrhage, edema and hypertension [Table 1].
Table 1.
Pulmonary drug toxicity: Radiopathological finding and the commonly implicated drugs
| Radiopathological finding | Commonly implicated drugs |
|---|---|
| Interstitial infiltrates | bleomycin, methotrexate, taxanes, platins, rituximab, gemcitabine, bortezomib, everolimus, temsorilimus, and gefitinib |
| Diffuse alveolar damage | bleomycin, busulfan, carmustine, melphalan, mitomycin, cyclophosphamide |
| Nonspecific interstitial pneumonia | methotrexate, bleomycin, carmustine, or chlorambucil |
| pulmonary hemorrhage | high-dose cyclophosphamide, cytarabine (ara-C), mitomycin, bevacizumab, platins |
| capillary leak syndrome | gemcitabine and immune-mediated therapies such as interleukin 2 and interferon |
| Eosinophilic pneumonia | methotrexate, bleomycin |
| Cryptogenic organizing pneumonia | Bleomycin, cyclophosphamide, everolimus, and methotrexate |
| Hypersensitivity pneumonitis | methotrexate |
Interstitial infiltrates
Pulmonary airspace or interstitial infiltrates are manifestation of accumulation of blood, pus, fluid, cells or proteins within the lung parenchyma[11] due to various drug-induced mechanisms. These infiltrates are often nonspecific but yet most often the earliest findings of drug toxicity and suspicion needs to be raised even in asymptomatic cases[12] [Figure 1].
Figure 1(A-D).
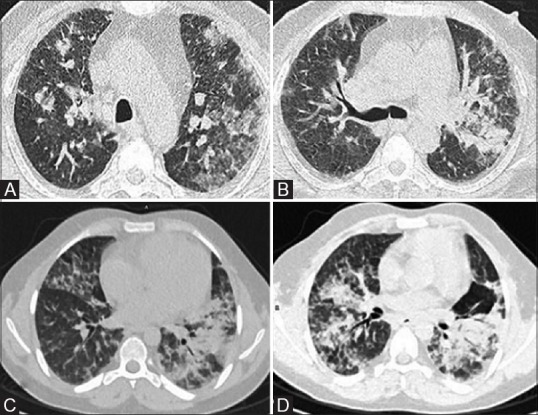
Drug-induced toxicity—interstitial and airspace infiltrates. (A and B) In a 57-year-old male with multiple myeloma, post two cycles of bortezomib with fever and breathlessness on Day 22, HRCT lung window sections show bilateral interstitial infiltrates with ground glass opacities. Patient deteriorated despite being put on antimicrobials (including antifungal) which lead to suspicion of bortezomib-induced drug toxicity. (C and D) In other patient of Hodgkin's lymphoma, post seven cycles of ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) who developed fever, dyspnea and cough, CT done on Day 5 shows multifocal airspace opacities with interstitial thickening in peribronchovascular distribution, consolidation in both upper lobes and subtle ground glass opacities in both lung fields suggesting bleomycin-induced toxicity
Diffuse alveolar damage (DAD)
DAD is caused by injury to the type-2 pneumocytes and endothelial cells in the alveoli. The early findings include homogeneous or inhomogeneous ground glass opacities on radiographs and HRCT.[13] The late phase is characterized by fibrosis, seen as early as a week later. Eventually, these may progress to architectural destruction indicating irreversible lung injury.[7] Most patients with ATRA toxicity show radiological features of pulmonary edema on imaging[14] [Figure 2].
Figure 2(A and B).
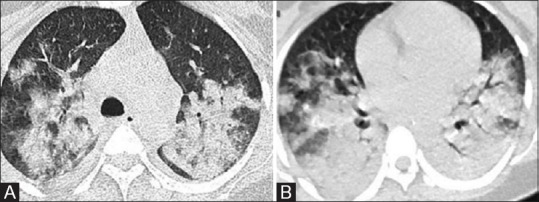
ATRA (All-trans retinoic acid) toxicity. Axial HRCT images of a young patient with acute promyelocytic leukemia developed dyspnea on Day 3 of starting ATRA illustrate confluent airspace opacities in perihilar distribution with bilateral mild pleural effusion suggesting acute respiratory distress syndrome features. ATRA was discontinued; however, patient succumbed to death due to acute renal injury and pulmonary hemorrhage on Day 9
Nonspecific interstitial pneumonia (NSIP)
Unlike the other types of interstitial pneumonias, there is interstitial inflammation with homogeneous but scattered areas of infiltration of mononuclear cells causing interstitial fibrosis and reactive hyperplasia of the type-2 pneumocytes.[15] Patients may present with mild fever, non- productive cough and malaise, usually within few months of initiating chemotherapy. Radiological manifestations typically include scattered areas of ground glass opacities in early stages with later stages showing areas of fibrosis and traction bronchiectasis, predominantly in a basal distribution.[16,17]
Diffuse alveolar hemorrhage
Diffuse alveolar hemorrhage (DAH) occurs rarely with chemotherapy but causes significant morbidity and mortality. It is caused by damage to the alveolar capillaries and is associated with capillaritis. Bevacizumab, an angiogenesis inhibitor used to treat non-small cell lung cancer, is known to cause fatal hemoptysis in 5% of treated individuals due to DAH.[18]
Bilateral heterogeneous and homogeneous opacities are seen on radiographs and HRCT shows bilateral areas of ground-glass opacity, which may be scattered or diffuse. Early recognition of this potentially fatal complication is imperative and radiology with the appropriate clinical setting and drug usage may help suggest the diagnosis [Figure 3].
Figure 3(A-D).
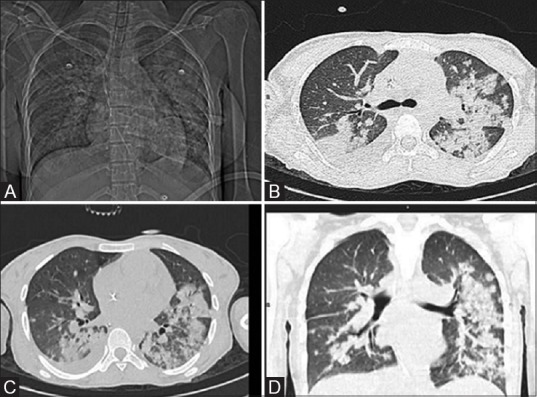
Drug-induced diffuse alveolar hemorrhage. A patient of AML developed febrile neutropenia, cough and hemoptysis on Day 10 of induction (cytarabine and daunorubicin). Patient was intubated with blood suctioned from endotracheal tube. CT Topogram (A) shows diffuse bilateral air-space opacities with relative peripheral sparing. The axial (B and C) and coronal (D) CT lung window sections depict smooth peri-bronchovascular thickening, areas of consolidation and ground glass opacities showing relative peripheral sparing and bilateral pleural effusion
Capillary leak syndrome
Capillary leak syndrome involves an increase in vascular permeability, leading to fluid and protein extravasations from the capillary vessels causing interstitial edema. It may evolve into noncardiogenic pulmonary edema or ARDS.[19]
Radiograph or CT shows diffuse air space disease with ground glass opacities. Several of the newer chemotherapy agents such as gemcitabine and cytokines such as interleukin-2 (in high doses) and interferon have been associated with capillary leak syndrome.[20] Early identification of capillary leak syndrome is crucial as addition of steroids along with diuretics therapy is required.[21]
Eosinophilic pneumonia
Eosinophilic pneumonia is characterized by infiltration of the alveolar septae with eosinophils, lymphocytes and macrophages leading to thickening of the alveolar walls and edema. It is usually associated with peripheral eosinophilia and raised IgE levels.[22] It can be seen in toxicity due to methotrexate and bleomycin.[23]
Radiologically, it is characterized peripheral areas of consolidation giving an appearance of “reverse pulmonary edema”. The reverse halo sign or the atoll sign is also considered highly characteristic of eosinophilic pneumonia, although seen in only 20% of the cases.[24]
Organizing pneumonia
This pattern has infiltration of the distal bronchioles, respiratory bronchioles, and alveoli by polypoid granulation inflammatory tissue[25] and is characterized by areas of consolidation predominantly in the peripheral, subpleural and/or peri-bronchial distribution on imaging. Nodules may also be noted in a similar distribution. Ground glass opacities or mosaic attenuation and areas of bronchial dilatation may also be seen in some cases.[26] Bleomycin, cyclophosphamide, methotrexate, thalidomide, lenalidomide, pomalidomide and everolimus (an mTOR inhibitor) are the most commonly implicated drugs. Bleomycin can show other patterns of lung involvement as well[14] [Figure 4].
Figure 4(A and B).
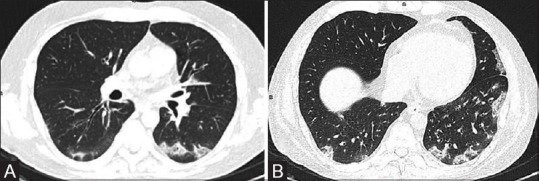
Pomalidomide-induced drug toxicity (Cryptogenic organizing pneumonitis). Patient of relapsed/refractory multiple myeloma; post-autologous stem cell transplant was put on pomalidomide drug regimen. On Day 15, patient developed fever and breathlessness; HRCT chest images show multiple peripheral foci of consolidations and ground glass opacities suggesting cryptogenic organizing pneumonitis likely induced by pomalidomide (A and B). The drug was discontinued and oral prednisolone was administered, following which patient improved clinically
Hypersensitivity pneumonitis
Hypersensitivity pneumonitis commonly occurs as an occupational pulmonary disease but can also be seen as an immune-mediated response to chemotherapy. The acute stage is characterized by predominance of bilateral symmetrical ground glass opacities involving predominantly middle and lower portions of the lung with or without peri-bronchovascular distribution. The subacute stage is characterized by a mixture of ground glass opacities and air-space nodules. More chronic stages of disease lead to fibrosis which has upper lobe predominance.[27]
Pulmonary infections
Pulmonary infections are an important cause of respiratory symptoms, morbidity and mortality in cancer patients on chemotherapy. These patients are immunosuppressed due to the underlying malignancy itself (such as lymphoma or leukemia) or due to the therapy (chemotherapy or immunotherapy). The type of disease and drug administered can provide some clues as to the expected pathogen, but more often than not, there is a mixture of infection in these patients. A composite work-up including clinical, radiological and lab parameters are needed in these patients. The pattern of lung involvement due to various infectious agents is summarized.
Bacterial pneumonia
Bacterial pneumonia is common in the setting of immunosuppression and manifests with air-space consolidations usually involving a particular lobe with or without lymphadenopathy [Figure 5a]. Pleural effusion, particularly asymmetric or unilateral, may be seen in association with bacterial infection. Neutropenic patients may be more susceptible to Staphylococci and aerobic gram-negative bacilli.[28]
Figure 5(A-D).
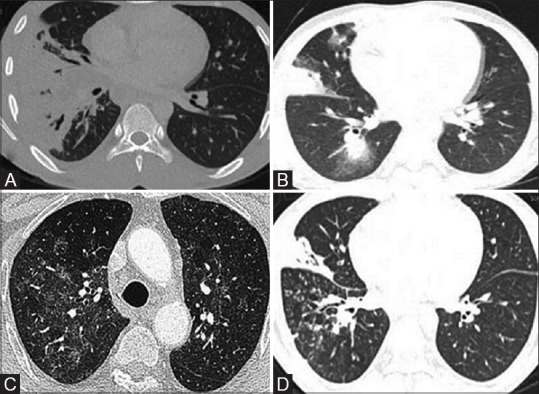
Spectrum of pulmonary infections in patients on chemotherapy. (A) CT image of a 12-year-old male with B-cell acute lymphocytic leukemia who developed fever on Day 29 of induction showing large consolidation in the right lower lobe and right middle lobe, suggesting lobar pneumonia. (B) Multiple foci of airspace opacities with surrounding ground glass densities in the same patient after 4 months of the previous imaging, suggesting invasive fungal infection. Patient improved on antifungal treatment. (C) CT image of a different patient on maintenance therapy for acute lymphocytic leukemia, who developed fever and nonproductive cough, shows bilateral patchy ground-glass opacities, with no pleural effusion and suspicion of Pneumocystis pneumonia was raised. The patient showed improvement with trimethoprim/sulfamethoxazole. (D) Febrile neutropenia in a patient of acute myeloid leukemia on Day 28 of chemotherapy, Chest CT shows consolidation with cavitatory changes and centrilobular nodules in tree-in-bud pattern suggesting endobronchial infection. Sputum analysis revealed multidrug-resistant tuberculosis
Viral pneumonia
Viral pneumonia has varied and nonspecific imaging features. Viral pneumonia can be suspected when there is a predominance of interstitial pattern.[29] It may manifest as air-space nodules, ground glass opacities, reticular or reticulonodular pattern. Early stages of pulmonary edema may also be mistaken for viral pneumonia due to the similar appearance of the interstitial pattern.
Fungal pneumonia
Fungal pneumonia is an important cause of infection in neutropenic patients with neutrophil counts less than 1,000 × 109 cells/L. Common offending agents in this setting include Aspergillus and Candida. Fungal pneumonia, particularly invasive Aspergillus, shows areas of consolidation with a “halo” of ground glass opacities on CT.[30] Fungal nodules on treatment undergo cavitation which suggests response to treatment[31] [Figure 5b].
Pneumocystis
Pneumocystis jirovecii (previously P. carinii), a yeast-like fungus, is an important cause of opportunistic infection. It should be suspected when immunocompromised patients present with respiratory symptoms and chest CT reveals areas of ground glass predominance particularly in the peri-bronchovascular locations in the early stages, progressing to nodules, consolidations and cystic changes[32] [Figure 5c]. Lymphadenopathy and pleural effusion are unusual and should prompt a search for an alternate diagnosis.
Mycobacterial infection
Mycobacterial infection can present with acute or chronic infection and may be primary infection or reactivation in a previously exposed individual. The radiological manifestations include centrilobular nodules in a “tree-in-bud” pattern due to the endobronchial spread of infection, areas of consolidation or cavitation particularly with upper lobe predominance, necrotic lymph nodes with rim-enhancement and pleural thickening or effusion[29] [Figure 5d].
Immune-mediated lung injury
Immune reconstitution inflammatory syndrome
Immune reconstitution inflammatory syndrome (IRIS) is a well-known complication in HIV patients receiving highly active antiretroviral therapy (HAART), due to a proinflammatory state following immune reconstitution.[33] However, it is now being increasingly recognized in non-HIV patients with immune suppression, who have immune recovery[5] following the removal of cause of immunosuppression, for example, reduction or withdrawal of immunosuppressive drugs, rapid neutrophil recovery in a formerly neutropenic patient, reversal of pathogen-associated immunosuppression with antimicrobial treatment.
The diagnosis of IRIS is basically by exclusion and it needs to be differentiated from infectious complications as management requires steroids which may flare up underlying infection.[34] IRIS associated lesions may affect the lungs, pleura, brain, retina, liver, and lymph nodes. The pulmonary system is the most frequently and most prominently affected in IRIS. It should be suspected when an immunosuppressed patient with infection on antimicrobial shows worsening of clinical and radiographic picture even when the microbial titers are falling. The radiological picture is usually that of diffuse air-space opacities, pulmonary edema with or without effusion[35] [Figure 6]. These features, although nonspecific, may be helpful in raising suspicion, especially when serial imaging is performed and no new infection is being suspected in the patient.
Figure 6(A-D).
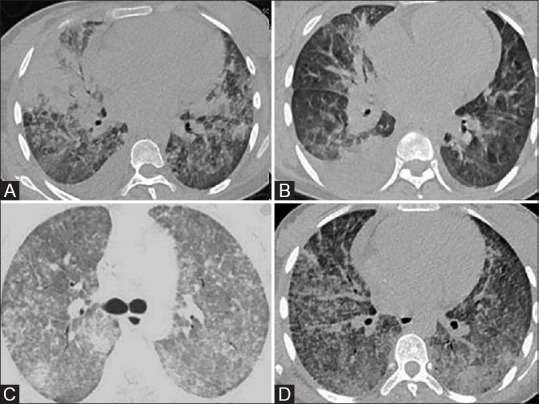
Immune-reconstitution inflammatory syndrome. 22-year-old patient of acute myeloid leukemia on induction therapy with daunorubicin and cytarabine developed febrile neutropenia with breathlessness. Initial scan (A and B) shows multifocal confluent air-space consolidations and bilateral pleural effusion. Patient initially showed clinical improvement with antibiotics and empirical antifungal therapy but subsequently became oxygen dependent with the development of renal failure. Peripheral smear done on Day 20 showed improved counts and galactomannan normalized but patient clinically worsened. Repeat CT (C and D) shows progression of parenchymal findings with bilateral diffuse smooth interlobular septal thickening in addition to the air-space opacities, suggesting immune-reconstitution inflammatory syndrome
Engraftment syndrome
Engraftment syndrome is specifically seen following hematopoietic stem cell transplant (HSCT) during the neutrophil recovery phase. The signs and symptoms include fever, rash and noncardiogenic pulmonary edema. It has been described in both autologous and allogenic stem cell transplant.[36]
The immune injury is attributed to cellular and cytokine interactions associated with recovery in neutrophil count in patients who were neutropenic.[37] Hence, it is also considered an early form of graft versus host disease. In the most extreme form, it can lead to multiorgan failure. Multiorgan failure can also occur due to diffuse endothelial injury, inflammation and thrombosis induced by the chemotherapy, which can be sometimes initiated or aggravated by the neutrophil recovery following engraftment.[38] Although several organ systems may be involved, pulmonary involvement is one of the most frequent causing a radiological picture of noncardiogenic pulmonary edema [Figure 7]. An attempt has been made by Spitzer to develop a uniform definition of ES, which include three major and four minor criteria[36] and diagnosis is made when either all three major or two major with one or more minor criteria are fulfilled within 96 h of the start of neutrophil recovery [Table 2].
Figure 7(A and B).
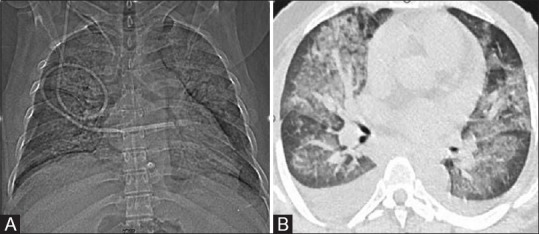
Engraftment Syndrome. Relapsed Hodgkin's lymphoma with chronic liver disease, post autologous stem cell transplant had on and off spikes of fever from Day 5. On Day 16, the patient developed fever and sudden onset of dyspnea with petechiae, hematuria, and hemorrhage from endotracheal tube. CT Topogram (A) and axial lung window sections (B) show confluent opacities in perihilar distribution with ground glass opacities and bilateral pleural effusion. With clinical corroboration, possibility of pulmonary hemorrhage and noncardiogenic pulmonary edema with suspicion of engraftment syndrome was given. Patient succumbed to death on Day 19 due to disseminated intravascular coagulation
Table 2.
Major and minor criteria for the diagnosis of engraftment syndrome
| Major criteria | Minor criteria |
|---|---|
| a) Temperature >38.3°C with no identifiable infections b) Erythrodermatous rash involving more than one-fourth of body surface area, not attributable to a medication c) Noncardiogenic pulmonary edema, radiologically manifesting as diffuse pulmonary infiltrates consistent with the diagnosis and hypoxia |
a) Hepatic dysfunction with either total bilirubin >2 mg/dl or transaminase levels >two times normal b) Renal insufficiency (serum creatinine >twice the baseline) c) Weight gain >2.5% of baseline body weight d) Transient encephalopathy unexplained by any other etiology. |
Conclusion
The developments and utilization of chemotherapy in the oncology have led to an increase in survival but have also led to a parallel increase in the complications due to direct or indirect effects of the therapy. While different organ systems may be involved, the pulmonary system is of prime importance owing to its frequency of involvement and the significant morbidity and mortality associated with it. Imaging plays a key role in detecting and differentiating the various causes of pulmonary complications of chemotherapy when used in combination with the clinical background and lab parameters. Using an algorithmic approach can reduce the morbidity by early recognition of complications which can help alter potentially toxic treatment and initiate early addition of antimicrobials or corticosteroids depending on the diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Martin L, Schilder RJ. Novel non-cytotoxic therapy in ovarian cancer: Current status and future prospects. J Natl Compr Cancer Netw. 2006;4:955–66. doi: 10.6004/jnccn.2006.0079. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulou I, Bamias A, Lyberopoulos P, Dimopoulos MA. Pulmonary toxicity from novel antineoplastic agents. Ann Oncol Off J Eur Soc Med Oncol. 2006;17:372–9. doi: 10.1093/annonc/mdj057. [DOI] [PubMed] [Google Scholar]
- 3.Oh YW, Effmann EL, Godwin JD. Pulmonary infections in immunocompromised hosts: Importance of correlating the conventional radiologic appearance with the clinical setting. Radiology. 2000;217:647–56. doi: 10.1148/radiology.217.3.r00dc35647. [DOI] [PubMed] [Google Scholar]
- 4.Stover DE, Kaner RJ. Pulmonary complications in cancer patients. CA Cancer J Clin. 1996;46:303–20. doi: 10.3322/canjclin.46.5.303. [DOI] [PubMed] [Google Scholar]
- 5.Sun H-Y, Singh N. Immune reconstitution inflammatory syndrome in non-HIV immunocompromised patients. Curr Opin Infect Dis. 2009;22:394–402. doi: 10.1097/QCO.0b013e32832d7aff. [DOI] [PubMed] [Google Scholar]
- 6.Cooper JA, White DA, Matthay RA. Drug-induced pulmonary disease. Part 1: Cytotoxic drugs. Am Rev Respir Dis. 1986;133:321–40. doi: 10.1164/arrd.1986.133.2.321. [DOI] [PubMed] [Google Scholar]
- 7.Matsuno O. Drug-induced interstitial lung disease: Mechanisms and best diagnostic approaches. Respir Res. 2012;13:39. doi: 10.1186/1465-9921-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flieder DB, Travis WD. Pathologic characteristics of drug-induced lung disease. Clin Chest Med. 2004;25:37–45. doi: 10.1016/S0272-5231(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 9.Souza FF, Smith A, Araujo C, Jagannathan J, Johnston C, O'Regan K, et al. New targeted molecular therapies for cancer: Radiological response in intrathoracic malignancies and cardiopulmonary toxicity: What the radiologist needs to know. Cancer Imaging. 2014;14:26. doi: 10.1186/1470-7330-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan BA, Hughes BGM. Targeted therapy for non-small cell lung cancer: Current standards and the promise of the future. Transl Lung Cancer Res. 2015;4:36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meadors M, Floyd J, Perry MC. Pulmonary toxicity of chemotherapy. Semin Oncol. 2006;33:98–105. doi: 10.1053/j.seminoncol.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol. 2001;13:242–8. doi: 10.1097/00001622-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Rossi SE, Erasmus JJ, McAdams HP, Sporn TA, Goodman PC. Pulmonary drug toxicity: Radiologic and pathologic manifestations. RadioGraphics. 2000;20:1245–59. doi: 10.1148/radiographics.20.5.g00se081245. [DOI] [PubMed] [Google Scholar]
- 14.Cardinale L, Asteggiano F, Moretti F, Torre F, Ulisciani S, Fava C, et al. Pathophysiology, clinical features and radiological findings of differentiation syndrome/all-trans-retinoic acid syndrome. World J Radiol. 2014;6:583–8. doi: 10.4329/wjr.v6.i8.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Bois R, King TE. Challenges in pulmonary fibrosis 5: The NSIP/UIP debate. Thora×. 2007;62:1008–12. doi: 10.1136/thx.2004.031039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakubovic BD, Donovan A, Webster PM, Shear NH. Methotrexate-induced pulmonary toxicity. Can Respir J Can Thorac Soc. 2013;20:153–5. doi: 10.1155/2013/527912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debnath J, Singh H, George R, Satija L, Chawla N, Sarma Y, et al. Reversible bleomycin toxicity. Med J Armed Forces India. 2010;66:290–1. doi: 10.1016/S0377-1237(10)80071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda S, Sekine A, Kato T, Yoshida M, Ogata R, Baba T, et al. Diffuse alveolar hemorrhage as a fatal adverse effect of bevacizumab: An autopsy case. Jpn J Clin Oncol. 2014;44:497–500. doi: 10.1093/jjco/hyu023. [DOI] [PubMed] [Google Scholar]
- 19.Siddall E, Khatri M, Radhakrishnan J. Capillary leak syndrome: Etiologies, pathophysiology, and management. Kidney Int. 2017;92:37–46. doi: 10.1016/j.kint.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 20.Hamon MD, Prentice HG, Gottlieb DJ, Macdonald ID, Cunningham JM, Smith OP, et al. Immunotherapy with interleukin 2 after ABMT in AML. Bone Marrow Transplant. 1993;11:399–401. [PubMed] [Google Scholar]
- 21.Teutonico A, Chimienti D, Antonelli M, Bruno A, Libutti P, Lisi P, et al. The systemic capillary leak syndrome: A scarcely known nephrological entity. J Nephrol. 2012;25:262–5. doi: 10.5301/jn.5000065. [DOI] [PubMed] [Google Scholar]
- 22.Tazelaar HD, Linz LJ, Colby TV, Myers JL, Limper AH. Acute eosinophilic pneumonia: Histopathologic findings in nine patients. Am J Respir Crit Care Med. 1997;155:296–302. doi: 10.1164/ajrccm.155.1.9001328. [DOI] [PubMed] [Google Scholar]
- 23.From E. Methotrexate pneumonitis in a psoriatic. Br J Dermatol. 1975;93:107–10. doi: 10.1111/j.1365-2133.1975.tb06485.x. [DOI] [PubMed] [Google Scholar]
- 24.Jeong YJ, Kim K-I, Seo IJ, Lee CH, Lee KN, Kim KN, et al. Eosinophilic lung diseases: A clinical, radiologic, and pathologic overview. Radiographics. 2007;27:617–37. doi: 10.1148/rg.273065051. [DOI] [PubMed] [Google Scholar]
- 25.Niksarlıoǧlu EY, Özkan GZ, Bakan ND, Yurt S, Kılıç L, Çamsarı G. Cryptogenic organizing pneumonia: Clinical and radiological features, treatment outcomes of 17 patients, and review of the literature. Turk J Med Sci. 2016;46:1712–8. doi: 10.3906/sag-1508-114. [DOI] [PubMed] [Google Scholar]
- 26.Nazer L, Alnajjar T, Salah S, Khzouz J, Alfaqeer N, Qandeel M. Fatal case of cryptogenic organizing pneumonia associated with everolimus. Ann Saudi Med. 2014;34:437–9. doi: 10.5144/0256-4947.2014.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glazer CS. Chronic hypersensitivity pneumonitis: Important considerations in the work-up of this fibrotic lung disease. Curr Opin Pulm Med. 2015;21:171–7. doi: 10.1097/MCP.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 28.Sousa D, Justo I, Domínguez A, Manzur A, Izquierdo C, Ruiz L, et al. Community-acquired pneumonia in immunocompromised older patients: Incidence, causative organisms and outcome. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2013;19:187–92. doi: 10.1111/j.1469-0691.2012.03765.x. [DOI] [PubMed] [Google Scholar]
- 29.Diederich S. Chest CT for suspected pulmonary complications of oncologic therapies: How I review and report. Cancer Imaging Off Publ Int Cancer Imaging Soc. 2016;16:7. doi: 10.1186/s40644-016-0066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YR, Choi YW, Lee KJ, Jeon SC, Park CK, Heo JN. CT halo sign: The spectrum of pulmonary diseases. Br J Radiol. 2005;78:862–5. doi: 10.1259/bjr/77712845. [DOI] [PubMed] [Google Scholar]
- 31.Marom EM, Kontoyiannis DP. Imaging studies for diagnosing invasive fungal pneumonia in immunocompromised patients. Curr Opin Infect Dis. 2011;24:309–14. doi: 10.1097/QCO.0b013e328348b2e1. [DOI] [PubMed] [Google Scholar]
- 32.Mu X-D, Jia P, Gao L, Su L, Zhang C, Wang R-G, et al. Relationship between radiological stages and prognoses of pneumocystis pneumonia in non-AIDS immunocompromised patients. Chin Med J (Engl) 2016;129:2020–5. doi: 10.4103/0366-6999.189068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma SK, Soneja M. HIV and immune reconstitution inflammatory syndrome (IRIS) Indian J Med Res. 2011;134:866–77. doi: 10.4103/0971-5916.92632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alawin IA, Karnath BM. Paradoxical immune reconstitution syndrome presenting as acute respiratory distress syndrome in a Leukemia patient during neutrophil recovery? Case Rep Hematol. 2012;2012:670347. doi: 10.1155/2012/670347. doi: 10.1155/2012/670347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajeswaran G, Becker JL, Michailidis C, Pozniak AL, Padley SPG. The radiology of IRIS (immune reconstitution inflammatory syndrome) in patients with mycobacterial tuberculosis and HIV co-infection: Appearances in 11 patients. Clin Radiol. 2006;61:833–43. doi: 10.1016/j.crad.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893–8. doi: 10.1038/sj.bmt.1703015. [DOI] [PubMed] [Google Scholar]
- 37.Deeg HJ. Cytokines in graft-versus-host disease and the graft-versus-Leukemia reaction. Int J Hematol. 2001;74:26. doi: 10.1007/BF02982546. [DOI] [PubMed] [Google Scholar]
- 38.Gorak E, Geller N, Srinivasan R, Espinoza-Delgado I, Donohue T, Barrett AJ, et al. Engraftment syndrome after nonmyeloablative allogeneic hematopoietic stem cell transplantation: Incidence and effects on survival. Biol Blood Marrow Transplant. 2005;11:542–50. doi: 10.1016/j.bbmt.2005.04.009. [DOI] [PubMed] [Google Scholar]


