Abstract
Background
A high multiple sclerosis activity while on alemtuzumab is rather uncommon compared to moderate-efficacy drugs. The purpose of this case report is to present a case of a 37-year-old female patient with bronchial asthma and no other medical history, whose disease activity required switching from dimethyl fumarate to fingolimod, then to alemtuzumab and finally to ocrelizumab.
Case presentation
In our patient, two severe attacks were observed and treated after administration of the first pulse of alemtuzumab. After six months of therapy, patient’s immunological profile showed the expected decrease in CD4+ and CD8+ T-cells and, markedly increased values of CD19+ B-cells. Surprisingly memory B-cells, which typically repopulate very slowly following alemtuzumab treatment, were above baseline levels. Regular administration of ocrelizumab based on a standardised scheme, after the alemtuzumab therapy failure, resulted in the stabilisation of the patient’s condition both clinically and radiologically.
Conclusion
Thus, when the alemtuzumab treatment is unsuccessful, the authors recommend testing T- and B-cell levels and proceeding with an early switch to ocrelizumab if high B-cell counts are found.
Keywords: Multiple sclerosis, Case report, Relapse, Disease progression, Alemtuzumab, Treatment failure, Lymphocytes, Ocrelizumab
Background
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system, characterised by a very broad heterogeneity in terms of clinical features, genetics, pathogenesis and responsiveness to treatments. Treatment of MS includes symptomatic therapies, management of acute relapses (corticosteroids or plasmapheresis) and therapy with disease-modifying drugs (DMDs) which reduces relapse rate, delays accumulation of disability and has beneficial effects on magnetic resonance imaging measures affecting the volume of lesions, number of active lesions, and slowing down the progression of brain atrophy. As confirmed by research and clinical trial results, early diagnosis and treatment in the initial stages of MS can significantly slow disease progression, preserve performance status in the long run, and prevent permanent damage to nerve structures [1]. Therapy of a clinically isolated syndrome and relapsing-remitting forms of MS is usually commenced using the first-line drugs, that include injections of glatiramer acetate (GA), interferons (beta-1a and beta-1b), and oral dimethyl fumarate and teriflunomide. If the response is inadequate or the treatment is poorly tolerated, the patient is switched to another drug within the same line or the therapy is escalated by utilizing intravenous natalizumab, alemtuzumab, ocrelizumab, or oral fingolimod or cladribine.
Case presentation
A 37-year-old female was diagnosed with relapsing-remitting MS (RRMS) in 2005 after two episodes of left optic neuritis. Her baseline Expanded Disability Status Scale (EDSS) was 2.0 and DMD therapy was initiated with glatiramer acetate (GA) 20 mg/daily. In 2014 GA was switched to dimethyl fumarate (DMF) due to persistent local injection site reactions. Patient experienced relapse of left sensory hemisyndrome in February 2017 and protracted attack of quadrupyramidal syndrome requiring repeated intravenous methylprednisolone (IVMP) infusions, with EDSS progression to 4.0 in April 2017. Consequently, the treatment was escalated to fingolimod in June 2017, considering John Cunningham virus (JCV) seropositivity (fingolimod and natalizumab were available at that time). A severe brainstem attack was treated in May 2018 by high-dose IVMP and series of plasma exchange. EDSS worsened to 4.5 and performed magnetic resonance imaging (MRI) revealed noticeable disease progression (Fig. 1).
Fig. 1.
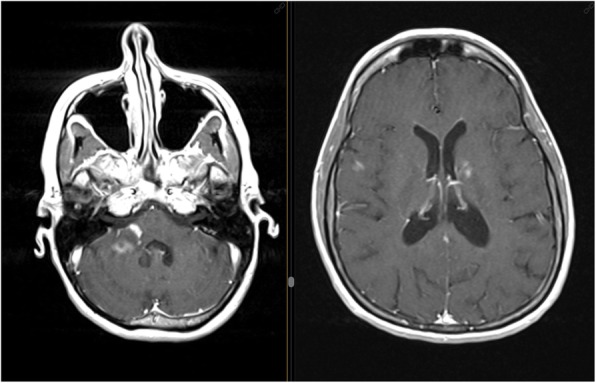
T1 Gd sequences
Given the failure of fingolimod therapy and the persistent presence of positive anti-JCV antibodies (3.22), treatment with alemtuzumab was initiated in October 2018. Although the disease course was favorable during the next 4 months and EDSS decreased to 3.5, patient had rather serious brainstem relapse in February 2019, with EDSS progression to 5.5. Follow-up MRI displayed seven new lesions, one with post-contrast opacification. Another attack clinically presented by central right hemiparesis and expressive aphasia was treated in April 2019, with additional EDSS worsening to 6.5. MRI showed further disease progression in terms of enlarged MS lesions as well as activity of multiple new plaques, both supra- and infratentorial (Fig. 2).
Fig. 2.
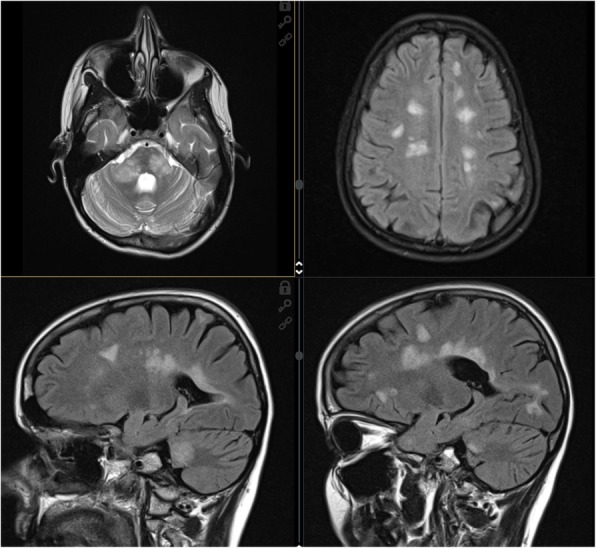
4/2019 – T2 and FLAIR images. Multiple MS lesions of supratentorial and infratentorial white matter
Given the insufficient response to alemtuzumab and ongoing disease activity, the patient was switched to ocrelizumab in June 2019. The therapy has been beneficial so far and patient remains clinico-radiologically stable, with no other attacks, EDSS has stabilized at 4.5, and the latest MRI scan from December 2019 demonstrated significant improvement of the finding (Fig. 3).
Fig. 3.
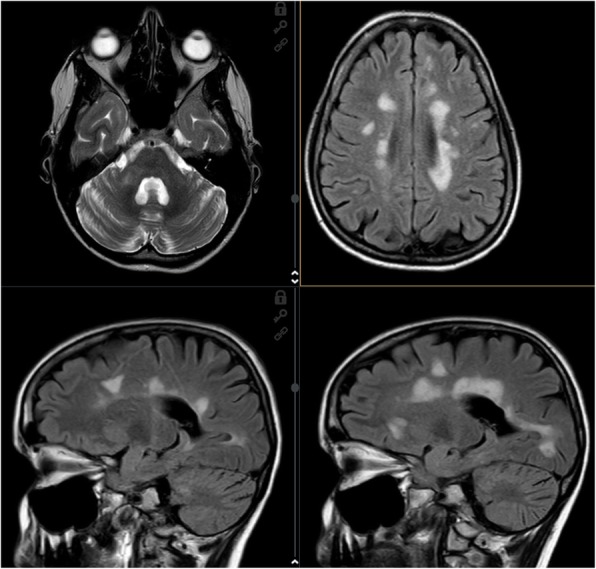
12/2019 T2 and FLAIR images. MRI after switch to ocrelizumab. Almost complete regression of inratentorial RS lesions. Supratentorial lesions without progression
Discussion and conclusion
The present case report describes the 15-year period of a female patient with aggressive RRMS who received GA, DMF, fingolimod, alemtuzumab and ocrelizumab, respectively. Switching from DMF to fingolimod and from fingolimod to alemtuzumab is not unusual in common clinical practice. However, high MS activity while on alemtuzumab is rather uncommon compared to moderate-efficacy drugs. Alemtuzumab depletes circulating T- and B-lymphocytes with the lowest cell counts observed at the first post-baseline assessment. B-cell counts normalise within 3 months of administration while T-cell counts recover over a longer period of time [2]. After 6 months of therapy, patient’s immunological profile showed the expected decrease in CD4+ and CD8+ T-cells and, markedly increased values of CD19+ B-cells (3.4 times the upper reference limit of the laboratory). As expected, an increase in naïve B-lymphocytes was recorded (4.3 times the upper limit of the laboratory). However, high values of class-switched memory B-cells (3.5 times the upper limit of the laboratory) were detected. These results are unexpected when compared to the results of Baker et al. [3], where after 6 months of treatment absolute CD19+ B lymphocytes were + 5% vs. baseline and memory B-cells – 80% vs. baseline. Previous works demonstrated that memory B-cells typically repopulate very slowly [3–5] and according to Akgün et al. [5] this process took years. Therefore, it is of interest that depletion of cells within this subset and controlling their repopulation have been associated with treatment efficacy [4, 6]. Thus, the treatment was switched to ocrelizumab, which selectively targets the CD20-positive B-lymphocytes resulting in B-cell depletion whereas natural immunity and total T-cell count remain unaffected [7, 8]. Whilst, a lack of CD4+ T-cell decrease in alemtuzumab-treated patients, or their rapid repopulation, has been suggested to lead to persistent relapses [5, 9, 10], this has remains controversial [11, 12]. Nevertheless, this study is consistent with disease breakthrough relating to B-cell activity [6]. Therefore, we recommend testing B-lymphocytes in case of failure of alemtuzumab and considering early change of treatment to ocrelizumab.
Acknowledgments
This work was partially supported by Ministry of Health, Czech Republic – conceptual development of research organisation (University Hospital Hradec Králové – FN HK, 00179906), by the grant projects of Ministry of Health of the Czech Republic (FN HK, 00179906) and by Charles University in Prague, Czech Republic (PROGRES Q40/15).
Abbreviations
- DMF
dimethyl fumarate
- DMDs
disease-modifying drugs
- EDSS
Expanded Disability Status Scale
- GA
glatiramer acetate
- IVMP
intravenous methylprednisolone
- JCV
John Cunningham virus
- MRI
magnetic resonance imaging
- MS
multiple sclerosis
- RRMS
relapsing-remitting multiple sclerosis
Authors’ contributions
MV, PR, SH, BK, and ZP equally contributed to the drafting, analyses and final version of the whole manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
On request from the head of the Department of Neurology of the University Hospital of Hradec Kralove.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the University Hospital of Hradec Kralove, Czech Republic. The subject gave a written consent to participate in this study.
Consent for publication
The subject gave a written consent for the publication of the data and it is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miller JR. The importance of early diagnosis of multiple sclerosis. J Manag Care Pharm. 2004;10(3 Suppl B):S4–S11. [PubMed] [Google Scholar]
- 2.Kasper LH, Arnold DL, Cohen JA, Coles AJ, Fox EJ, Hartung HP. Lymphocyte subset dynamics following alemtuzumab treatment in CARE-MS II study. Presented at: 29th Congress of the European Committee for Research and Treatment in Multiple Sclerosis, Copenhagen, Denmark, 2–5 October 2013, P531.
- 3.Baker D, Herrod SS, Alvarez-Gonzalez C, Giovannoni G, Schmierer K. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol. 2017;74:961–969. doi: 10.1001/jamaneurol.2017.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker D, Marta M, Pryce G, Giovannoni G, Schmierer K. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine. 2017;16:41–50. doi: 10.1016/j.ebiom.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akgün K, Blankenburg J, Marggraf M, Haase R, Ziemssen T. Event-driven immunoprofiling predicts return of disease activity in alemtuzumab-treated multiple sclerosis. Front Immunol. 2020;11:56. doi: 10.3389/fimmu.2020.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novi G, Fabbri S, Bovis F, Spragia E, Gazzola P, Maietta I, et al. Tailoring B-cells depleting therapy in MS according to memory B-cells monitoring: a pilot study. Presented at: 35th Congress of the European Committee for Research and Treatment in Multiple Sclerosis, Stockholm, Sweden, 11–13 September 2019, P971.
- 7.Lycke J. Monoclonal antibody therapies for the treatment of relapsing-remitting multiple sclerosis: differentiating mechanisms and clinical outcomes. Ther Adv Neurol Disord. 2015;8:274–293. doi: 10.1177/1756285615605429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein C, Lammens A, Schäfer W, Georges G, Schwaiger M, Mössner E, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs. 2013;5:22–33. doi: 10.4161/mabs.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rolla S, De Mercanti SF, Bardina V, Horakova D, Habek M, Adamec I, et al. Lack of CD4+ T cell percent decrease in alemtuzumab-treated multiple sclerosis patients with persistent relapses. J Neuroimmunol. 2017;313:89–91. doi: 10.1016/j.jneuroim.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Cossburn MD, Harding K, Ingram G, El-Shanawany T, Heaps A, Pickersgill TP, et al. Clinical relevance of differential lymphocyte recovery after alemtuzumab therapy for multiple sclerosis. Neurology. 2013;80:55–61. doi: 10.1212/WNL.0b013e31827b5927. [DOI] [PubMed] [Google Scholar]
- 11.Kousin-Ezewu O, Azzopardi L, Parker RA, Tuohy O, Compston A, Coles A, et al. Accelerated lymphocyte recovery after alemtuzumab does not predict multiple sclerosis activity. Neurology. 2014;82:2158–2164. doi: 10.1212/WNL.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiendl H, Carraro M, Comi G, Izquierdo G, Kim HJ, Sharrack B, et al. Lymphocyte pharmacodynamics are not associated with autoimmunity or efficacy after alemtuzumab. Neurol Neuroimmunol Neuroinflamm. 2019;7:e635. doi: 10.1212/NXI.0000000000000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
On request from the head of the Department of Neurology of the University Hospital of Hradec Kralove.


