Abstract
Background
Activated eosinophils have been deemed to affect carcinogenesis and tumor progression via various mechanisms in tumor microenvironment. However, the prognostic role of tumor-associated tissue eosinophilia (TATE) in human cancers remains controversial. Therefore, we conducted this meta-analysis to better comprehend the association between TATE and clinical outcomes of patients.
Methods
We searched PubMed, Embase and EBSCO to determine the researches assessing the association between TATE and overall survival (OS) and/or disease-free survival (DFS) in patients with cancer, then combined relevant data into hazard ratios (HRs) or odds ratio (OR) for OS, DFS and clinicopathological features including lymph node metastasis etc. with STATA 12.0.
Results
Twenty six researches with 6384 patients were included in this meta-analysis. We found that the presence of TATE was significantly associated with improved OS, but not with DFS in all types of cancers. In stratified analyses based on cancer types, pooled results manifested that the infiltration of eosinophils was remarkably associated with better OS in esophageal carcinoma and colorectal cancer. In addition, TATE significantly inversely correlated with lymph node metastasis, tumor stage and lymphatic invasion of cancer.
Conclusion
TATE promotes survival in cancer patients, suggesting that it is a valuable prognostic biomarker and clinical application of biological response modifiers or agonists promoting TATE may be the novel therapeutic strategy for patients.
Keywords: Tumor-associated tissue eosinophilia, Favorable outcome, Human solid tumor, Meta-analysis
Background
Tumor microenvironment (TME) linked closely with the initiation, promotion, and progression of cancer [1]. Innate and adaptive immunocytes such as mast cells, macrophages and memory T lymphocytes etc. are the vital components of TME [2]. Multitudinous studies have demonstrated that these immune cells were significantly associated with survival in solid tumors [3, 4]. However, it is essential to distinguish among different types of immune cells as they may play differential roles in the TME. Eosinophils, as the important component of innate immune cells, have proven to play significant roles in a multitude of solid tumors.
Eosinophils are granulocytic leukocytes that are associated with multitudinous pathologic conditions including allergic reactions, parasitic and bacterial infections etc. [5] These cells secrete massive proteins and cytokines upon activation and are involved in a variety of other functions including inducing tissue remodeling and promoting antigen presentation [6]. In the last decade, activated eosinophils have been deemed to affect carcinogenesis and tumor progression via various mechanisms including modulating innate and adaptive immune responses in TME [7]. Eosinophils infiltrating into tumor is also called tumor-associated tissue eosinophilia (TATE) [8]. Recent researches have investigated the TATE in tumor progression and survival, but their results were inconsistent even contradictory [9]. Hence, it needs further evaluation. In addition, the potential of TATE as prognostic biomarker and therapeutic strategy is also required to be investigated.
Herein, we carried out this meta-analysis to expound the relation between TATE and clinical outcomes including overall survival (OS) and disease-free survival (DFS) in patients with cancer.
Methods
Search strategy
This meta-analysis was guided by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) Statement issued in 2009 (Checklist S1). PubMed, Embase and EBSCO were searched for researches from 1980 to May 15th 2019. The keywords applied for search were: (eosinophil [Title/Abstract] OR eosinophilia [Title/Abstract]) AND (neoplasms [Title/Abstract] OR tumor [Title/Abstract] OR cancer [Title/Abstract] OR carcinoma [Title/Abstract]).
Inclusion and exclusion criteria
Researches included in this meta-analysis should meet the following inclusion criteria: (1) been published as original articles; (2) investigated human subjects; (3) examined eosinophils in primary tumor tissues; (4) reported hazard ratios (HRs) with 95% confidence interval (CI), or Kaplan – Meier curves of eosinophil infiltration with clinical outcomes.
The exclusion criteria were as follows: researches (1) were not published as research articles or full texts including commentaries, case reports, letters to the editors and meeting abstracts; (2) didn’t offer ample data to obtain HRs; (3) investigated eosinophils in metastases or not in tumor tissues.
Endpoints
In this study, OS and DFS were regarded as the primary and second endpoint respectively.
Data extraction
GM.H. and SM.W. reviewed and recorded data including number of patients, method to quantify eosinophils, cutoff value to determine TATE and time of follow-up etc. independently. OS, DFS and clinicopathological features such as tumor, node, metastasis (TNM) stage and lymphatic invasion were extracted from the text, tables, or Kaplan – Meier curves.
Quality assessment
Two authors independently assessed the quality of included cohort researches with Newcastle–Ottawa Scale (NOS), [10] and achieved consensus for each item under the help of third or more authors. Research scored 6 or above was regarded as high quality.
Statistical analysis
We combined extracted data using STATA 12.0 analysis software, and estimated statistical heterogeneity with the chi-squared based Q-test or I2 (25% was considered low-level heterogeneity, 25–50% moderate-level heterogeneity, and 50% high-level heterogeneity) [11]. Data were pooled based on the random-effect model in the presence of heterogeneity, [12] otherwise, the fixed-effect model was applied [13]. In addition, stratified analyses were conducted based on tumor types; sensitivity analysis, Begg’s funnel plot and Egger’s test [14] were employed to explore the impact of each research on the overall result and potential publication bias respectively. All P values were two-sided and below 0.05 was treated as statistical significance.
Results
Search results and description of studies
Flow chart diagram of research selection was displayed in Fig. S1. Twenty six researches with 6384 patients were ultimately included in this meta-analysis [15–40]. And all the researches were scored 6 or above after careful evaluation with the Newcastle–Ottawa Scale (NOS); Characteristics of those researches being in the light of the inclusion criteria and suitable for data incorporation were exhibited in Table 1 and Table S1.
Table 1.
Main characteristics of the included studies
| Study | Year | Tumor type | No. of Patients | Male/Female | median age (range) (year) | Staining | TATE: Present / absent | Tumor stage | median follow-up date (months) | Survival | Quality Score (NOS) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peurala, E. etal [15] | 2018 | Oral cancer | 99 | 55/44 | 65.3 | H&E | 51/47 | I - III | 40.7 | OS | 8 |
| Oliveira, D. T. etal [16] | 2012 | Oral cancer | 71 | 55/16 | 59 (35, 77) | H&E | 35/36 | I - II | NR | DFS | 7 |
| Tostes Oliveira, D. etal [19] | 2009 | Oral cancer | 43 | 27/16 | 55.79 (28, 83) | H&E | 21/22 | I - IV | (3, 229) | OS | 7 |
| Dorta, R. G. etal [17] | 2002 | Oral cancer | 125 | 105/20 | 58 (30, 95) | H&E | 57/68 | II - III | 88.2 (0, 287.4) | OS, DFS | 7 |
| Dante, P. etal [40] | 2019 | Tongue Carcinoma | 259 | 223/36 | 53.0 ± 12.2 | H&E | NR | I - IV | NR | OS, DFS | 8 |
| Alrawi, S. J. etal [18] | 2005 | Head and neck carcinoma | 87 | NR | (41, 76) | H&E | 13/7 | II - IV | 36 (6, 216) | OS, DFS | 7 |
| Ercan, I. etal [20] | 2005 | Laryngeal carcinoma | 78 | 78/0 | 55.9 (35, 80) | H&E | 25/53 | NR | 41.91 | OS | 7 |
| Sassler, A. M. etal [21] | 1995 | Laryngeal carcinoma | 248 | NR | NR | H&E | 56/192 | III - IV | 48 | OS, DFS | 6 |
| Thompson, A. C. etal [22] | 1994 | Laryngeal carcinoma | 104 | 85/19 | 64.6 (39, 91) | H&E | 31/73 | NR | ≥ 60 | OS | 6 |
| Fujii, M. etal [23] | 2002 | Nasopharyngeal carcinoma | 53 | 40/13 | 49.4 (15, 81) | H&E | 26/27 | I - IV | 90.5 (35.3, 199.9) | DFS | 7 |
| Leighton, S. E. etal [24] | 1996 | Nasopharyngeal carcinoma | 96 | 68/28 | NR | H&E | 65/31 | NR | 57 | OS, DFS | 6 |
| Harbaum, L. etal [25] | 2015 | Colorectal cancer | 381 | 166/215 | 68.5 | H&E | 101/280 | I - IV | 45 (1, 182) | OS | 8 |
| Fernandez-Acenero, M. J. etal [26] | 2000 | Colorectal cancer | 126 | 70/56 | 67.35 (32, 87) | H&E | 29/97 | Duke’s A-C | ≥ 60 | OS, DFS | 8 |
| Nielsen, H.J. etal [27] | 1999 | Colorectal cancer | 584 | 240/344 | 61 (49, 75) | H&E | 150/115 | Duke’s A-D | 61 (49, 75) | OS | 7 |
| Prizment, A. E etal [28] | 2016 | Colorectal cancer | 441 | 0/441 | (55, 69) | H&E; EPX | 197 /244 | NR | 60 | OS | 8 |
| Zhang, Y. etal [29] | 2014 | Esophageal carcinoma | 36 | 25/11 | 59 (45, 77) | H&E | 18/18 | I - IV | 22 (2, 143) | OS | 7 |
| Ishibashi, S. etal [30] | 2006 | Esophageal carcinoma | 97 | 82/15 | 62.7 ± 8.9 | H&E | 30/31 | NR | 61.7 (5.3, 165.4) | OS | 7 |
| Hollander, P. etal [31] | 2018 | Hodgkin’s lymphoma | 459 | 242/217 | < 45: 68%; ≥45: 32% | H&E | NR | I - IV | 154.8 | OS | 8 |
| Kereszres, K. etal [32] | 2007 | Hodgkin’s lymphoma | 104 | 54/50 | 33 (12, 72) | H&E | 64/40 | I - IV | 110 (24, 214) | OS, DFS | 7 |
| von Wasielewski, R. etal [33] | 2000 | Hodgkin’s lymphoma | 1511 | 745/766 | (15, 75) | H&E | 510/823 | I - IV | 120 | OS | 8 |
| Enblad, G.etal [34] | 1993 | Hodgkin’s lymphoma | 140 | NR | 45 (11, 94) | H&E | 26/114 | I - IV | 48 (20, 85) | DFS | 6 |
| van Driel, W.J. etal [35] | 1996 | Cervical cancer | 83 | 0/83 | 42.1 | H&E | NR | I - IIA | 44.6 (5, 108) | OS, DFS | 7 |
| Bethwaite, P. B. etal [36] | 1993 | Cervical cancer | 67 | 0/67 | 43.7 (25, 76) | H&E | 28/39 | IB | 62.4 (1, 93) | OS | 7 |
| Flamm, J. etal [37] | 1992 | Bladder cancer | 428 | 289/139 | 70.2 (29, 91) | H&E | 99/329 | NR | 84 | OS | 7 |
| Iwasaki, K. etal [38] | 1986 | Gastric cancer | 647 | 364/283 | (22, 84) | H&E | 157/490 | I - IV | (8, 92) | OS | 7 |
| Ono, Y. etal [39] | 2002 | Penile cancer | 17 | 17/0 | 68 (36, 84) | H&E | 9/8 | I - IV | NR | OS | 6 |
H&E haematoxilyn and eosin, EPX eosinophil peroxide, NR not reported
Meta-analyses
Overall survival (OS)
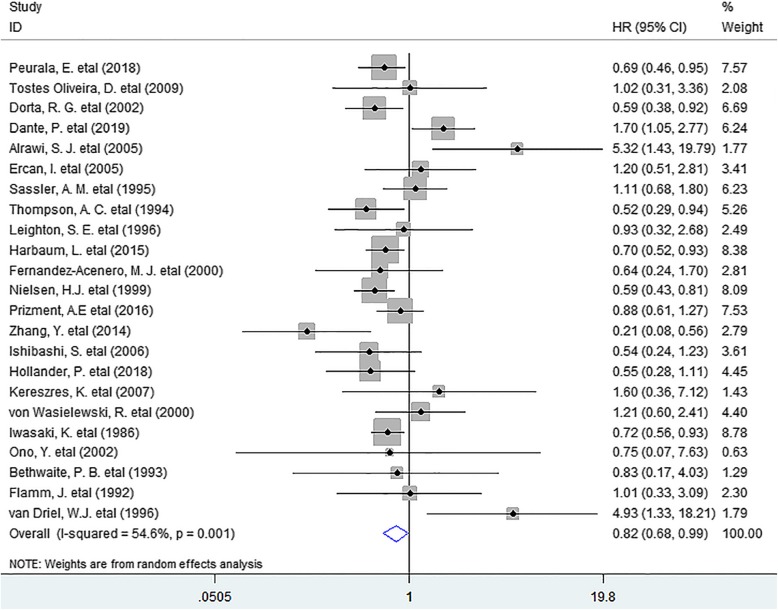
In this meta-analysis, we discovered that the presence of TATE was notably associated with improved OS (HR = 0.82, 95% CI 0.68 to 0.99, P = 0.041) in patients with solid tumor. (Fig. 1).
Fig. 1.
Forest plots describing HR of the association between TATE and OS in human solid tumors
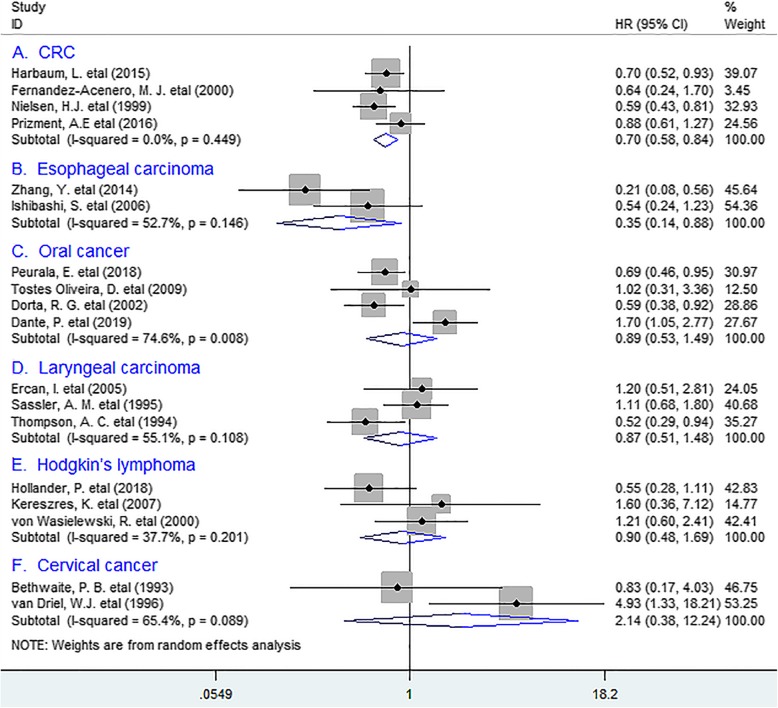
In stratified analyses according to tumor types, the combined results manifested that TATE was markedly associated with better OS in colorectal cancer (CRC) (HR = 0.70, 95% CI 0.58 to 0.84, P = 0.000), with no heterogeneity detected (I2 = 0%, P = 0.449). Similar data was obtained between TATE and OS in esophageal carcinoma (EC) (HR = 0.35, 95% CI 0.14 to 0.88, P = 0.026); Whereas no distinct relation existed between eosinophil infiltration and OS in oral cancer (OC) (HR = 0.89, 95% CI 0.53 to 1.49, P = 0.657), laryngeal carcinoma (HR = 0.87, 95% CI 0.51 to 1.48, P = 0.599), Hodgkin’s lymphoma (HR = 0.90, 95% CI 0.48 to 1.69, P = 0.741) or cervical cancer (HR = 2.14, 95% CI 0.38 to 12.24, P = 0.391). (Fig. 2).
Fig. 2.
Stratified analyses describing HRs of the association between TATE and OS
Disease-free survival (DFS)
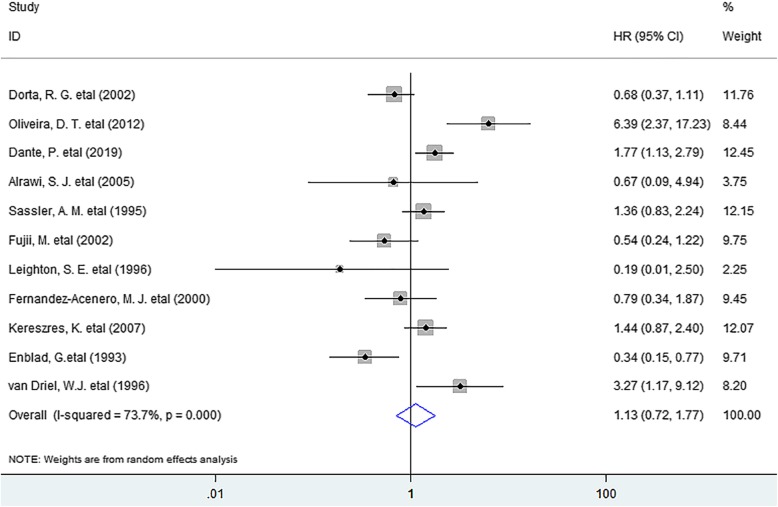
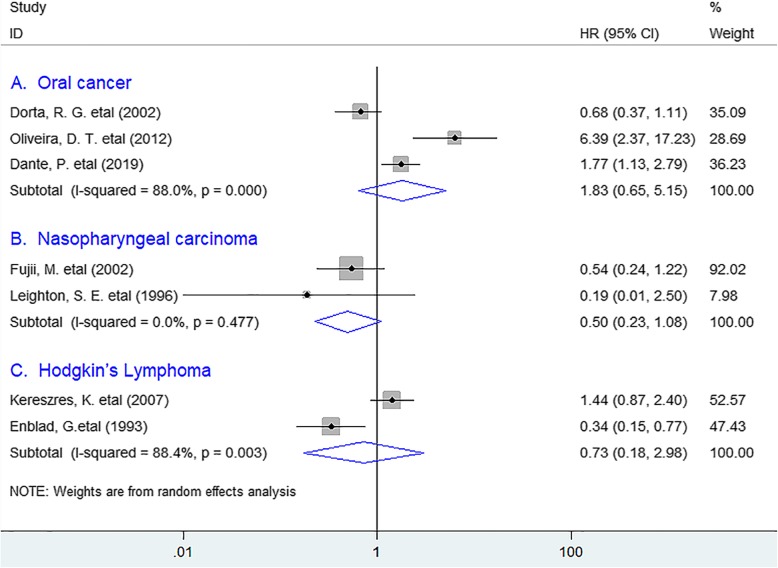
As for DFS, the meta-analysis indicated that no noticeable association existed between eosinophil infiltration and DFS (HR = 1.13, 95% CI 0.72 to 1.77, P = 0.598) in solid tumors. (Fig. 3) In the stratified analyses, the incorporated results revealed that TATE was not significantly associated with improved DFS in oral cancer (HR = 1.83, 95% CI 0.65 to 5.15, P = 0.253), nasopharyngeal carcinoma (HR = 0,50, 95% CI 0.23 to 1.08, P = 0.079) or Hodgkin’s lymphoma (HR = 0.73, 95% CI 0.18 to 2.98, P = 0.657). (Fig. 4).
Fig. 3.
Forest plots describing HR of the association between TATE and DFS in human solid tumors
Fig. 4.
Stratified analyses describing HRs of the association between eosinophil infiltration and DFS
Clinicopathological features
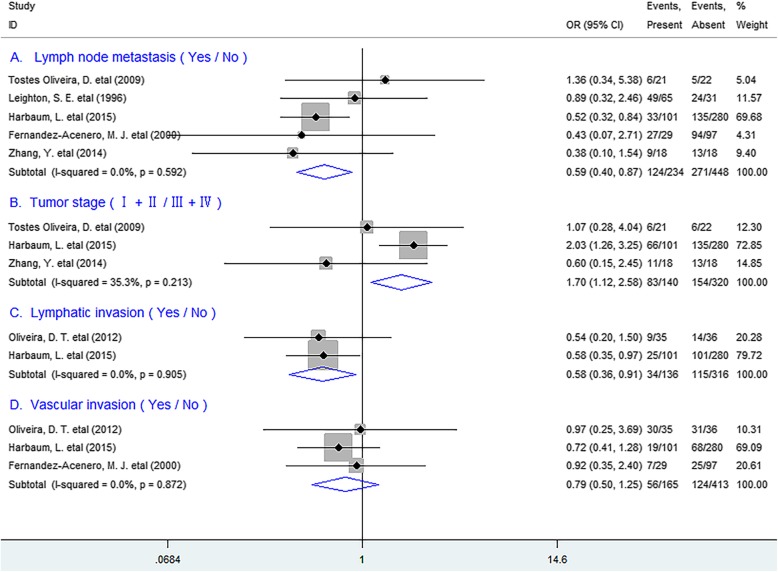
We next tested the relation between TATE and clinicopathological features, and found that TATE was remarkably inversely correlated with lymph node metastasis (OR = 0.59, 95% CI 0.40 to 0.87, P = 0.007), TNM stage (OR = 1.70, 95% CI 1.12 to 2.58, P = 0.013) and lymphatic invasion (OR = 0.58, 95% CI 0.36 to 0.91, P = 0.018), but not with vascular invasion (OR = 0.79, 95% CI 0.50 to 1.25, P = 0.308) of patients. (Fig. 5).
Fig. 5.
Forest plots indicating ORs of the association between eosinophil infiltration and clinicopathological feature
Sensitivity analysis
Sensitivity analysis demonstrated that each included research had no impact on the overall result for OS or DFS. (Fig. S2).
Publication bias
No publication bias existed between TATE and OS (P = 0.152) or DFS (P = 0.876) in patients by Funnel plot (Fig. S3) and Egger’s test.
Discussion
Eosinophilia is commonly associated with allergies, helminth infections and several inflammatory states. Recently, it has also been noted in human solid tumors. The present meta-analysis revealed that TATE had a positive effect in improving survival in human solid tumors, especially in CRC and EC. Moreover, It significantly inversely correlated with lymph node metastasis etc. of tumor. Hence, these data offered important evidence in uncovering the positive prognostic role of TATE in human solid tumors.
The close relation between TATE and better clinical outcome identified in this study possibly attribute to the following reasons: eosinophils in the TME can express same receptors and mediators such as granzyme A etc. as cytotoxic T lymphocytes (CTLs) and be directly involved in anti-tumor response, [41] and they can also secret several chemokines including CCL5, CXCL9 to promote anti-tumor immunity through attracting CD8+ T cells to the tumor site [42]. In addition, eosinophils are capable of regulating immunity, for instance, they can release major basic protein (MBP), a highly cationic protein to stimulate maturation of dendritic cells by increasing cell surface activation markers including MHC-II, CD80 and CD86, [43] which has the potential to overcome immune tolerance and induce anti-tumor immunity with the powerful antigen-presentation ability [44]. Furthermore, they can induce cell death of various cell lines such as colo-205 cell line with some selectivity in their tumoricidal properties, which are dependent on the CD11a/CD18-mediated stable contacts with target cells [45]. Hence, it is rational to conclude that TATE is capable of regulating tissue homeostasis of the TME and inhibiting tumor growth and metastasis thereby improving survival. However, in other tumor types, TATE as a prognostic marker for survival has been a controversial issue. This may be because of differences in methods of counting TATE as well as heterogeneity of material.
Previous studies have demonstrated that cytokines such as IL-2, IL-4 could recruit eosinophils and lead to eosinophilia and enhanced eosinophil activation, thereby exert potent anti-tumor immune responses [41, 46]. Thus, based on our present result that TATE improving survival in human solid tumors identified in this study and the function of IL-2 and IL-4 stated above, we harbor the idea that clinical application of biological response modifiers (BRM) such as carrier-assisted recombined human IL-2 /or IL-4 may have the potential to treat human solid tumors.
Quite a few limitations should be noted from this study. First, morphometric analyses for TATE adopted in included researches were not exactly consistent. In addition, researches with negative results might not be published, which might result in potential publication bias.
Conclusions
TATE promotes survival in solid tumors especially in CRC and EC, suggesting that it is a valuable prognostic biomarker and clinical application of biological response modifiers or agonists promoting TATE may be a novel therapeutic strategy for patients.
Supplementary information
Additional file 1: Figure S1. Flow chart diagram of study selection. Figure S2. Plots describing the influence of individual studies on the overall HRs for OS (A) and DFS (B) in human cancers. Figure S3. Funnel plots displayed the potential publication bias between TATE and OS (A) or DFS (B) in patients. Table S1. Characteristics of the included studies for OR analysis of clinicopathological features.
Acknowledgements
Not applicable.
Abbreviations
- TATE
Tumor-associated tissue eosinophilia
- OS
Overall survival
- DFS
Disease-free survival
- HR
Hazard ratio
- OR
Odds ratio
- Cl
Confidence interval
- TNM
Tumor, node, metastasis
- OC
Oral cancer
- CRC
Colorectal cancer
- EC
Esophageal carcinoma
- NR
Not reported
- TME
Tumor microenvironment
- BRM
Biological response modifier
Authors’ contributions
GM.H. conceived of the study, participated in its design, extracted data, performed the statistical analysis and drafted the manuscript. SM.W. participated in data extraction; KF.Z., F. X. and LM.H. participated in statistical analysis and manuscript revision. W.C. and P.C. participated in its design and manuscript revision. All authors read and approved the final manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Grant No. 81702803, GMH) and was also partly supported by Shaoxing Science and TechnologyPlanProject (2018C30055, LMH; 2018C30075, KFZ; 2017B70036, FX). We used the funding to perform data collection, analysis and interpretation.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Ethics approval and consent to participate
The ethical approval was unnecessary because this study based on summary and analysis of the results of previous studies.
Consent for publication
Not applicable.
Competing interests
The authors have declared that no competing interests exist.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guoming Hu and Shimin Wang contributed equally to this work.
Contributor Information
Guoming Hu, Email: hgmplj@126.com.
Wei Chen, Email: cwzjsx5018@163.com.
Pu Cheng, Email: drchengpu@zju.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12885-020-06966-3.
References
- 1.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11(10):702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 2.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu G, Wang S, Cheng P. Tumor-infiltrating tryptase(+) mast cells predict unfavorable clinical outcome in solid tumors. Int J Cancer. 2018;142(4):813–821. doi: 10.1002/ijc.31099. [DOI] [PubMed] [Google Scholar]
- 4.Hu G, Wang S. Tumor-infiltrating CD45RO+ Memory T Lymphocytes Predict Favorable Clinical Outcome in Solid Tumors. Sci Rep. 2017:7(1). [DOI] [PMC free article] [PubMed]
- 5.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38(5):709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 6.Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy. 2008;38(8):1254–1263. doi: 10.1111/j.1365-2222.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev. 2011;242(1):161–177. doi: 10.1111/j.1600-065X.2011.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain M, Kasetty S, Sudheendra US, Tijare M, Khan S, Desai A. Assessment of tissue eosinophilia as a prognosticator in oral epithelial dysplasia and oral squamous cell carcinoma-an image analysis study. Pathol Res Int. 2014;2014:507512. doi: 10.1155/2014/507512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marichal T, Tsai M, Galli SJ. Mast cells: potential positive and negative roles in tumor biology. Cancer Immunol Res. 2013;1(5):269–279. doi: 10.1158/2326-6066.CIR-13-0119. [DOI] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuritz SJ, Landis JR, Koch GG. A general overview of mantel-Haenszel methods: applications and recent developments. Annu Rev Public Health. 1988;9:123–160. doi: 10.1146/annurev.pu.09.050188.001011. [DOI] [PubMed] [Google Scholar]
- 13.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peurala E, Tuominen M, Loyttyniemi E, Syrjanen S, Rautava J. Eosinophilia is a favorable prognostic marker for oral cavity and lip squamous cell carcinoma. APMIS. 2018;126(3):201–207. doi: 10.1111/apm.12809. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira DT, Biassi TP, Faustino SE, Carvalho AL, Landman G, Kowalski LP. Eosinophils may predict occult lymph node metastasis in early oral cancer. Clin Oral Investig. 2012;16(6):1523–1528. doi: 10.1007/s00784-011-0651-7. [DOI] [PubMed] [Google Scholar]
- 17.Dorta RG, Landman G, Kowalski LP, Lauris JR, Latorre MR, Oliveira DT. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology. 2002;41(2):152–157. doi: 10.1046/j.1365-2559.2002.01437.x. [DOI] [PubMed] [Google Scholar]
- 18.Alrawi SJ, Tan D, Stoler DL, Dayton M, Anderson GR, Mojica P, Douglas W, Hicks W, Jr, Rigual N, Loree T. Tissue eosinophilic infiltration: a useful marker for assessing stromal invasion, survival and locoregional recurrence in head and neck squamous neoplasia. Cancer J. 2005;11(3):217–225. doi: 10.1097/00130404-200505000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Tostes Oliveira D, Tjioe KC, Assao A, Sita Faustino SE, Lopes Carvalho A, Landman G, Kowalski LP. Tissue eosinophilia and its association with tumoral invasion of oral cancer. Int J Surg Pathol. 2009;17(3):244–249. doi: 10.1177/1066896909333778. [DOI] [PubMed] [Google Scholar]
- 20.Ercan I, Cakir B, Basak T, Ozdemir T, Sayin I, Turgut S. Prognostic significance of stromal eosinophilic infiltration in cancer of the larynx. Otolaryngol Head Neck Surg. 2005;132(6):869–873. doi: 10.1016/j.otohns.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 21.Sassler AM, McClatchey KD, Wolf GT, Fisher SG. Eosinophilic infiltration in advanced laryngeal squamous cell carcinoma. Veterans administration laryngeal cooperative study group. Laryngoscope. 1995;105(4 Pt 1):413–416. doi: 10.1288/00005537-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Thompson AC, Bradley PJ, Griffin NR. Tumor-associated tissue eosinophilia and long-term prognosis for carcinoma of the larynx. Am J Surg. 1994;168(5):469–471. doi: 10.1016/s0002-9610(05)80102-3. [DOI] [PubMed] [Google Scholar]
- 23.Fujii M, Yamashita T, Ishiguro R, Tashiro M, Kameyama K. Significance of epidermal growth factor receptor and tumor associated tissue eosinophilia in the prognosis of patients with nasopharyngeal carcinoma. Auris Nasus Larynx. 2002;29(2):175–181. doi: 10.1016/s0385-8146(01)00135-3. [DOI] [PubMed] [Google Scholar]
- 24.Leighton SE, Teo JG, Leung SF, Cheung AY, Lee JC, van Hasselt CA. Prevalence and prognostic significance of tumor-associated tissue eosinophilia in nasopharyngeal carcinoma. Cancer. 1996;77(3):436–440. doi: 10.1002/(SICI)1097-0142(19960201)77:3<436::AID-CNCR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 25.Harbaum L, Pollheimer MJ, Kornprat P, Lindtner RA, Bokemeyer C, Langner C. Peritumoral eosinophils predict recurrence in colorectal cancer. Mod Pathol. 2015;28(3):403–413. doi: 10.1038/modpathol.2014.104. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Acenero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer. 2000;88(7):1544–1548. [PubMed] [Google Scholar]
- 27.Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brunner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol. 1999;189(4):487–495. doi: 10.1002/(SICI)1096-9896(199912)189:4<487::AID-PATH484>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 28.Prizment AE, Vierkant RA, Smyrk TC, Tillmans LS, Lee JJ, Sriramarao P, Nelson HH, Lynch CF, Thibodeau SN, Church TR, Cerhan JR, Anderson KE, Limburg PJ. Tumor eosinophil infiltration and improved survival of colorectal cancer patients: Iowa Women's health study. Mod Pathol. 2016;29(5):516–527. doi: 10.1038/modpathol.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Ren H, Wang L, Ning Z, Zhuang Y, Gan J, Chen S, Zhou D, Zhu H, Tan D, Zhang H. Clinical impact of tumor-infiltrating inflammatory cells in primary small cell esophageal carcinoma. Int J Mol Sci. 2014;15(6):9718–9734. doi: 10.3390/ijms15069718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishibashi S, Ohashi Y, Suzuki T, Miyazaki S, Moriya T, Satomi S, Sasano H. Tumor-associated tissue eosinophilia in human esophageal squamous cell carcinoma. Anticancer Res. 2006;26(2B):1419–1424. [PubMed] [Google Scholar]
- 31.Hollander P, Rostgaard K, Smedby KE, Molin D, Loskog A, de Nully BP, Enblad G, Amini RM, Hjalgrim H, Glimelius I. An anergic immune signature in the tumor microenvironment of classical Hodgkin lymphoma is associated with inferior outcome. Eur J Haematol. 2018;100(1):88–97. doi: 10.1111/ejh.12987. [DOI] [PubMed] [Google Scholar]
- 32.Keresztes K, Szollosi Z, Simon Z, Tarkanyi I, Nemes Z, Illes A. Retrospective analysis of the prognostic role of tissue eosinophil and mast cells in Hodgkin's lymphoma. Pathol Oncol Res. 2007;13(3):237–242. doi: 10.1007/BF02893504. [DOI] [PubMed] [Google Scholar]
- 33.von Wasielewski R, Seth S, Franklin J, Fischer R, Hubner K, Hansmann ML, Diehl V, Georgii A. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin's disease, allowing for known prognostic factors. Blood. 2000;95(4):1207–1213. [PubMed] [Google Scholar]
- 34.Enblad G, Sundstrom C, Glimelius B. Infiltration of eosinophils in Hodgkin's disease involved lymph nodes predicts prognosis. Hematol Oncol. 1993;11(4):187–193. doi: 10.1002/hon.2900110404. [DOI] [PubMed] [Google Scholar]
- 35.van Driel WJ, Hogendoorn PC, Jansen FW, Zwinderman AH, Trimbos JB, Fleuren GJ. Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum Pathol. 1996;27(9):904–911. doi: 10.1016/s0046-8177(96)90216-6. [DOI] [PubMed] [Google Scholar]
- 36.Bethwaite PB, Holloway LJ, Yeong ML, Thornton A. Effect of tumour associated tissue eosinophilia on survival of women with stage IB carcinoma of the uterine cervix. J Clin Pathol. 1993;46(11):1016–1020. doi: 10.1136/jcp.46.11.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flamm J. Tumor-associated tissue inflammatory reaction and eosinophilia in primary superficial bladder cancer. Urology. 1992;40(2):180–185. doi: 10.1016/0090-4295(92)90524-z. [DOI] [PubMed] [Google Scholar]
- 38.Iwasaki K, Torisu M, Fujimura T. Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer. 1986;58(6):1321–1327. doi: 10.1002/1097-0142(19860915)58:6<1321::aid-cncr2820580623>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 39.Ono Y, Ozawa M, Tamura Y, Suzuki T, Suzuki K, Kurokawa K, Fukabori Y, Yamanaka H. Tumor-associated tissue eosinophilia of penile cancer. Int J Urol. 2002;9(2):82–87. doi: 10.1046/j.1442-2042.2002.00424.x. [DOI] [PubMed] [Google Scholar]
- 40.Paz D, Chang KP, Kao HK, Lao WWK, Huang YC, Chang YL, Huang Y. Clinical implications of tumor-associated tissue eosinophilia in tongue squamous cell carcinoma. Laryngoscope. 2018;129(5):1123–1129. doi: 10.1002/lary.27413. [DOI] [PubMed] [Google Scholar]
- 41.Gatault S, Legrand F, Delbeke M, Loiseau S, Capron M. Involvement of eosinophils in the anti-tumor response. Cancer Immunol Immunother. 2012;61(9):1527–1534. doi: 10.1007/s00262-012-1288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16(6):609–617. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 43.Lotfi R, Lotze MT. Eosinophils induce DC maturation, regulating immunity. J Leukoc Biol. 2008;83(3):456–460. doi: 10.1189/jlb.0607366. [DOI] [PubMed] [Google Scholar]
- 44.Sheng KC, Pietersz GA, Wright MD, Apostolopoulos V. Dendritic cells: activation and maturation--applications for cancer immunotherapy. Curr Med Chem. 2005;12(15):1783–1800. doi: 10.2174/0929867054367248. [DOI] [PubMed] [Google Scholar]
- 45.Legrand F, Driss V, Delbeke M, Loiseau S, Hermann E, Dombrowicz D, Capron M. Human eosinophils exert TNF-alpha and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J Immunol. 2010;185(12):7443–7451. doi: 10.4049/jimmunol.1000446. [DOI] [PubMed] [Google Scholar]
- 46.Sosman JA, Bartemes K, Offord KP, Kita H, Fisher SG, Kefer C, Ellis TA, Fisher RI, Higgins TJ, Gleich GJ. Evidence for eosinophil activation in cancer patients receiving recombinant interleukin-4: effects of interleukin-4 alone and following interleukin-2 administration. Clin Cancer Res. 1995;1(8):805–812. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Flow chart diagram of study selection. Figure S2. Plots describing the influence of individual studies on the overall HRs for OS (A) and DFS (B) in human cancers. Figure S3. Funnel plots displayed the potential publication bias between TATE and OS (A) or DFS (B) in patients. Table S1. Characteristics of the included studies for OR analysis of clinicopathological features.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.