Abstract
A novel series of imidazo[2,1-b]thiazole-sulfonyl piperazine conjugates (9aa-ee) has been synthesized and evaluated for carbonic anhydrase (CA, EC 4.2.1.1) inhibitory potency against four isoforms: The cytosolic isozyme hCA I, II and trans-membrane tumor-associated isoform hCA IX and hCA XII, taking acetazolamide (AAZ) as standard drug, using a stopped flow CO2 hydrase assay. The results revealed that most of the compounds showed selective activity against hCA II whereas none of them were active against hCA I, IX, XII (Ki > 100 µM). The physiologically dominant cytosolic isoform hCA II was inhibited by these molecules with inhibition constants in the range of 57.7–98.2 µM. This new derivative, thus, selectively inhibits hCA II over the hCA I, IX, XII isoforms, which may be used for further understanding the physiological roles of some of these isoforms in various pathologies.
Keywords: carbonic anhydrase; hCA I; hCA II; hCA IX; hCA XII; imidazo[2,1-b]thiazole; sulfonyl piperazine
1. Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are a group of zinc containing metalloenzymes that effectively catalyze the reversible hydration of CO2 to bicarbonate and proton, an important reaction for many physiological processes. The human carbonic anhydrases (hCAs) belong to theα- class of carbonic anhydrases having sixteen different form of isoforms. CA isoenzymes play an important role in various cellular processes, such as tumorigenicity, respiration, regulation of pH, electrolytes secretion and many other processes. hCA I and hCA II act as targets for diseases, such as glaucoma and epilepsy, while hCA IX and hCA XII are targets for imaging of tumors and are found to over-express in many cancers. [1,2,3]. Therefore, it is important to develop an effective approach that can selectively inhibit these isoforms involved in different diseases. Nowadays, the major concern of the researchers working in this field is to develop new concept for designing the compounds that are capable of blocking specifically the process catalyzed by one or two isoform ms of these enzymes.
N and S containing fused heterocyclic systems have gained considerable attraction in the field of medicinal chemistry due to their broad spectrum of pharmacological activities [4]. Imidazo[2,1-b]thiazole-guanylhydrazone is a promising lead with potent anticancer activity against a number of cancer cell lines [5]. These fused heterocycles exhibit potent anticancer activity through multiple mechanisms [6]. However, a number of imidazo[2,1-b][1,3,4]thiadiazole derivatives have been reported to exhibit antitumor activity [7]. Sharma and Supuran et al. have reported a series of benzenesulfonamide bearing imidazothiadiazole and thiazolotriazole hybrids as carbonic anhydrases IX and XII inhibitors [8]. The amide derivatives of probenecid have been reported as selective inhibitors of carbonic anhydrase IX and XII by Carradori et al. [9,10] (Figure 1).
Figure 1.
Representative examples of imidazothiazole, imidazothiadiazole, sulfonamides as CAIs and rationale for designed hybrids.
Based on the literature, sulfonamides were the most investigated class of CA inhibitors [11,12]. Acetazolamide (AZA), methazolamide (MZA), ethoxzolamide (EZA), dorzolamide (DZA) (Figure 1) are few of the many sulfonamide-based carbonic anhydrase inhibitors in clinical use [13,14,15]. Pazopanib has been clinically used for several years now whereas indisulam and celecoxib are currently under clinical investigations in various types of tumors (Figure 1) [16]. Supuran et al. have reported various classes of piperazine derivatives as activators of human carbonic anhydrase I, II, IV and VII [17]. Thus, design of novel heterocyclic/aromatic scaffolds with sulfonamide moiety is crucial in developing selective CA inhibitors (CAIs) with potent activity.
Propelled by these findings and continuing our interest in the design of diversified classes of heterocyclic based CAIs, we endeavor to synthesize a new series of imidazo[2,1-b]thiazole-sulfonyl piperazine conjugates (Figure 1), which have not been reported earlier. Thus, a novel imidazo[2,1-b]thiazole-sulfonyl piperazine hybrids (9aa-ee) were synthesized and demonstrated CA inhibition potential against I, II, IX and XII isoforms of CA using acetazolamide (AAZ) as standard.
2. Results and Discussion
2.1. Chemistry
The designed imidazo[2,1-b]thiazole-sulfonyl piperazine conjugates (9aa-ee) were prepared according to pathways outlined in Scheme 1. Ethyl-2-aminothiazole-4-carboxylate (3) was synthesized by the condensation of ethyl bromopyruvate (2) and thiourea (1) in ethanol under reflux for 4 h. Cyclization of intermediate (3) with various phenacyl bromides (4a-e) in ethanol at reflux temperature resulted in the formation of intermediates (5a–e), which upon ester hydrolysis in the presence of lithium hydroxide monohydrate (LiOH·H2O) produced carboxylic acids (6a–e) [18]. Aryl sulfonyl piperazine intermediates (8a-e) were synthesized based on previous reported procedures [19]. Finally, the key intermediate 6a-e with different substitutions were coupled with 8a-e using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI) and 1-hydroxybenzotriazole (HOBt) in dry dimethylformamide (DMF) conditions to afford the corresponding target compounds in good yields (9aa-ee).
Scheme 1.
Synthesis of imidazo[2,1-b]thiazole-sulfonyl piperazine hybrids (9aa-ee).
All synthesized compounds (9aa-ee) were characterized by spectral techniques viz. 1H NMR, 13C NMR and high resolution mass spectrometry (HRMS). The 1H NMR spectrum of 9ab displayed characteristic protons of piperazine around δ2.8–3.8 ppm. Methyl and Methoxy group of phenyl ring was found to be around δ 2.40 ppm and 3.5–4.0 ppm. All the remaining protons appeared in the range of δ 6.8–8.20 ppm. 13C NMR spectrum of 9ab showed the characteristic carbonyl and methyl group’s at δ 158.61, and δ 21.02 ppm, respectively and all the remaining carbons appeared in the range of δ 108.97–158.56 ppm. The similar pattern was observed for the remaining compounds (9aa-ee).
2.2. Carbonic Anhydrase Inhibition
The newly synthesized imidazo[2,1-b]thiazole-sulfonyl piperazines (9aa-ee) were screened for their CA inhibition activity against cytosolic (hCA I, hCA II) and the tumor associated (hCA IX, hCAXII) isoforms by a stopped flow CO2 hydrase assay [20]. Acetazolamide (AAZ), a clinically used reference standard and the obtained results are represented as Ki (µM) are summarized in Table 1. The following structure-activity relationship (SAR) was figured out from the inhibition data of 9aa-ee as shown in Table 1:
-
i.
The cytosolic isoforms hCA I and tumor associated isoform hCA IX, hCAXII were not inhibited by the compounds 9aa-ee (Ki > 100 µM).
-
ii.
The compounds 9aa-ee showed varied inhibitory profiles against cytosolic isoform hCA II. Compound 9ae, 9bb, 9ca, 9cc-ce and 9da elicited inhibitory potencies (Ki) of 57.7–67.9 µM. Potent compounds (9ca, 9cc-ce) having chlorine (R) at aromatic ring of imidazo thiazole whereas other compounds are ineffective. It is assumed that these compounds, i.e., 9ae (Ki = 57.7 µM), 9bb (Ki = 76.4 µM), 9ca (Ki =79.9 µM), 9cc (Ki = 57.8 µM), 9cd (Ki = 71.2 µM), 9ce (Ki = 62.1 µM), 9da (Ki = 67.9 µM) were showing inhibition probably due to the presence of 4-OCH3, 4-CH3, 4-Cl and 4-F as R group and 4-t-butyl, 4-CH3, 4-OCH3, 4-Cl and H as R1 group.
-
iii.
It is evident from the Ki values (Table 1), compound 9ae bearing that R-group (imidazothiazole part) substituted with 4-methoxy and R1 substituted with 4-t-butyl (sulfonyl piperazine part) showed promising inhibitory activity.
-
iv.
Among all the congeners, some of the potent compounds exhibited selective inhibition of hCA II as compared to hCA IX and hCAXII in micromolar range.
Table 1.
Inhibition of hCA isoforms I, II, IX and XII with target compounds (9aa-ee) and acetazolamide (AAZ) as a standard drug (Ki-µM) a.
| Compound | Structure | hCA I | hCA II | hCA IX | hCA XII |
|---|---|---|---|---|---|
| 9aa |

|
>100 | >100 | >100 | >100 |
| 9ab |

|
>100 | >100 | >100 | >100 |
| 9ac |

|
>100 | >100 | >100 | >100 |
| 9ad |

|
>100 | >100 | >100 | >100 |
| 9ae |
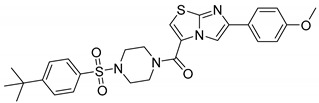
|
>100 | 57.7 | >100 | >100 |
| 9ba |

|
>100 | >100 | >100 | >100 |
| 9bb |

|
>100 | 76.4 | >100 | >100 |
| 9bc |

|
>100 | >100 | >100 | >100 |
| 9bd |

|
nd | nd | nd | nd |
| 9ca |

|
>100 | 79.9 | >100 | >100 |
| 9cb |

|
>100 | >100 | >100 | >100 |
| 9cc |

|
>100 | 57.8 | >100 | >100 |
| 9cd |

|
>100 | 71.2 | >100 | >100 |
| 9ce |

|
>100 | 62.1 | >100 | >100 |
| 9da |
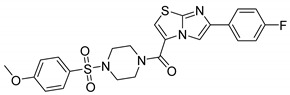
|
>100 | 67.9 | >100 | >100 |
| 9db |

|
>100 | >100 | >100 | >100 |
| 9dc |

|
>100 | 98.2 | >100 | >100 |
| 9dd |

|
>100 | >100 | >100 | >100 |
| 9de |

|
>100 | >100 | >100 | >100 |
| 9ea |

|
>100 | >100 | >100 | >100 |
| 9eb |

|
>100 | >100 | >100 | >100 |
| 9ec |

|
>100 | >100 | >100 | >100 |
| 9ed |

|
>100 | >100 | >100 | >100 |
| 9ee |

|
>100 | >100 | >100 | >100 |
| AAZ |

|
0.25 | 0.012 | 0.026 | 0.006 |
a Mean from 3 different assays, by a stopped flow technique (errors were in the range of ±5–10% of the reported values).
3. Conclusions
In conclusion, we have reported the synthesis of imidazo[2,1-b]thiazole-sulfonyl piperazine conjugates (9aa-ee), which are designed to target CA isoforms. The synthesized compounds (9aa-ee) were screened against the cytosolic isoforms hCA I and II, as well as the transmembrane tumor associated isoforms, hCA IX and XII. Few of the compounds (9ae, 9bb, 9ca, 9cc-ce, 9da and 9dc) exhibited acceptable inhibitory activity against cytosolic isoform hCA II, Ki value in the range of 57.7–67.9 µM, while some compounds were found to be weaker inhibitors of tumor associated isoform hCA IX and hCA XII. Hence, further modifications of these newly designed imidazo[2,1-b]thiazole-sulfonyl piperazine derivatives (9aa-ee) have the potential to emerge as isoform II selective CAIs. Notably, as for all secondary/tertiary sulfonamides reported to date as CAIs, the inhibition mechanism with these derivatives reported here is for the moment unknown [21,22,23].
4. Experimental Section
4.1. Materials and Methods
All Chemicals and reagents were purchased from the commercial suppliers Alfa Aesar, Sigma Aldrich and used without further purification. The reaction progress was monitored by Thin layer chromatography (TLC) was performed using pre-coated silica gel 60 F254 MERCK. TLC plates were visualized and analyzed by exposure to UV light or iodine vapors and aqueous solution of ninhydrin. Column chromatography was performed with Merck flash silica gel with 60–120 mesh size. Melting points were determined on an Electro thermal melting point apparatus and are uncorrected. Nuclear magnetic resonance spectra for 1H NMR were obtained on Avance 300, 400 and 500 MHz and analyzed using Mestrenova software and the chemical shifts are reported in ppm from tetramethylsilane (0 ppm) or the solvent resonance as the internal standard (CDCl3 7.26 ppm, DMSO-d6 2.49 ppm) and for 13C NMR the chemical shifts are reported in ppm from the solvent resonance as the internal standard (CDCl3 77 ppm, DMSO-d6 39.3 ppm). HRMS was performed on a Varian ESI- QTOF instrument Spectral data for all compounds are available in the Supplementary Materials.
4.1.1. General Synthetic Procedure for the Preparation of Compound 3
Compound 3 was synthesized according to the procedures described in the literature [18].
4.1.2. General Synthetic Procedure for the Preparation of Compound 5a-e
Compound 5a-e was synthesized based on previous synthetic procedures [18].
4.1.3. General Synthetic Procedure for the Preparation of Compound 6a-e
Compound 6a-e was synthesized based on previous synthetic procedures [18].
4.1.4. General Synthetic Procedure for the Preparation of Compound 8a-e
Substituted phenyl sulfonyl piperazines 8a-e was synthesized by adding triethylamine (31.5 mmol) slowly to a solution of piperazine in CH2Cl2 at 0 °C, and then commercially available benzenesulfonyl chloride (7a-e, 11.4 mmol) was added and stirred for 30 min. After the completion of reaction, which was confirmed by TLC (petroleum ether and ethyl acetate (2:1)), the reaction was quenched with water and extracted with CH2Cl2. The organic layer was dried over anhydrous Na2SO4 and concentrated on reduced pressure to give 8a-e [19].
4.1.5. General Synthetic Procedure for the Preparation of (5-(aryl)imidazo[2,1-b]thiazol-2-yl)(4-((aryl)sulfonyl)piperazin-1-yl)methanone (9aa-ee)
Above obtained carboxylic acid (6a-e, 1.0 mmol) was dissolved in dry dimethyl formamide (10 mL), cooled to 0 °C (ice bath). EDCI (1.2 mmol), HOBt (1.2 mmol), amine intermediate (8a-e, 1.0 mmol) and triethylamine (3 mmol) were added slowly. After 10 min removed the ice bath and the mixture was stirred at room temperature for 12 h until the starting materials were consumed. Then ice cold water was added to the reaction mixture and extracted with dichloro methane (DCM). The combined organic extracts were washed with aq NaHCO3 and dried with Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography by using ethyl acetate and hexane solvent system to afford the title compounds (9aa-ee).
(5-(4-Methoxyphenyl)imidazo[2,1-b]thiazol-2-yl)(4-((4-methoxyphenyl)sulfonyl)piperazin-1-yl)methanone (9aa)
White solid: 191mg; 90% yield; mp: 202–204 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.03 (s, 1H), 7.75 (d, J = 8.6 Hz, 2H), 7.68 (d, J = 8.8 Hz, 2H), 7.65 (s, 1H), 7.17 (d, J = 8.8 Hz, 2H), 6.94 (d, J = 8.7 Hz, 2H), 3.85 (s, 3H), 3.77 (s, 7H), 3.00 (s, 4H); 13C NMR (126 MHz, DMSO-d6) δ: 162.9, 158.6, 158.5, 147.5, 146.2, 129.8, 126.6, 126.3, 126.0, 124.0, 116.4, 114.6, 114.0, 108.9, 55.7, 55.0, 45.7; MS (ESI): m/z 513 [M+H]+; HRMS (ESI): m/z calcd for C24H25N4O5S2: 513.12609; found: 513.12467 [M+H]+.
(5-(4-Methoxyphenyl)imidazo[2,1-b]thiazol-2-yl)(4-tosylpiperazin-1-yl)methanone (9ab)
White solid: 186 mg; 88% yield; mp: 216–218 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.03 (s, 1H), 7.76 (d, J = 8.6 Hz, 2H), 7.65 (s, 2H), 7.62 (s, 1H), 7.47 (d, J = 8.0 Hz, 2H), 6.94 (d, J = 8.6 Hz, 2H), 3.77 (s, 7H), 3.01 (s, 4H), 2.41 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 158.6, 158.5, 147.5, 146.2, 143.8, 132.0, 129.9, 127.5, 126.6, 126.0, 124.0, 116.4, 114.0, 108.9, 55.0, 45.6, 21.0; MS (ESI): m/z 497 [M+H]+; HRMS (ESI): m/z calcd for C24H25N4O4S2: 497.13117; found: 497.12979 [M+H]+.
(1-(4-Methoxyphenyl)-9H-pyrido[3,4-b]indol-3-yl)(4-tosylpiperazin-1-yl)methanone (9ac)
White solid: 185 mg; 85% yield; mp: 243–245 °C; 1H NMR (300 MHz, DMSO-d6) δ: 7.98 (s, 1H), 7.77 (s, 1H), 7.74 (s, 2H), 7.70 (s, 2H), 7.67 (s, 1H), 7.61 (s, 1H), 6.91 (d, J = 8.4 Hz, 2H), 3.77 (s, 7H), 3.07 (s, 4H); 13C NMR (75 MHz, CDCl3 +DMSO-d6) δ: 158.5, 147.4, 146.2, 138.4, 133.8, 129.4, 129.2, 126.5, 125.9, 123.8, 116.3, 113.8, 108.7, 54.9, 45.4; MS (ESI): m/z 517 [M+H]+; HRMS (ESI): m/z calcd for C23H22ClN4O4S2: 517.07655; found: 517.07532 [M+H]+.
(5-(4-Methoxyphenyl)imidazo[2,1-b]thiazol-2-yl)(4-(phenylsulfonyl)piperazin-1-yl)methanone (9ad)
White solid: 176 mg; 78% yield; mp: 192–194 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.03 (s, 1H), 7.76 (d, J = 2.1 Hz, 2H), 7.74 (d, J = 2.2 Hz, 3H), 7.68 (d, J = 7.2 Hz, 2H), 7.65 (s, 1H), 6.94 (d, J = 8.8 Hz, 2H), 3.77 (s, 7H), 3.04 (s, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 159.2, 159.1, 148.1, 146.8, 135.6, 134.0, 130.1, 128.1, 127.2, 126.7, 124.6, 117.0, 114.6, 109.5, 55.7, 46.3; MS (ESI): m/z 483 [M+H]+; HRMS (ESI): m/z calcd for C23H23N4O4S2: 483.11552; found: 483.11409 [M+H]+.
(4-((4-(Tert-butyl)phenyl)sulfonyl)piperazin-1-yl)(5-(4-methoxyphenyl)imidazo[2,1-b]thiazol-2-yl)methanone (9ae)
White solid: 180 mg; 80% yield; mp: 216–218 °C; 1H NMR (300 MHz, CDCl3) δ: 8.04 (s, 1H), 7.75 (d, J = 8.5 Hz, 2H), 7.67 (d, J = 5.0 Hz, 5H), 6.93 (d, J = 8.6 Hz, 2H), 3.76 (s, 7H), 3.03 (s, 4H), 1.31 (s, 9H); 13C NMR (101 MHz, DMSO-d6) δ: 158.6, 158.5, 156.4, 147.5, 146.2, 132.2, 127.5, 126.6, 126.4, 126.1, 124.0, 116.5, 114.0, 109.0, 55.1, 45.6, 34.9, 30.7; MS (ESI): m/z 539 [M+H]+; HRMS (ESI): m/z calcd for C27H31N4O4S2: 539.17812; found: 539.17666 [M+H]+.
(4-((4-Methoxyphenyl)sulfonyl)piperazin-1-yl)(5-(p-tolyl)imidazo[2,1-b]thiazol-2-yl)methanone (9ba)
White solid: 185 mg; 84% yield; mp: 209–211 °C; 1H NMR (500 MHz, DMSO-d6) δ: 8.08 (s, 1H), 7.72 (d, J = 8.1 Hz, 2H), 7.70–7.67 (m, 2H), 7.66 (s, 1H), 7.18 (d, J = 2.8 Hz, 2H), 7.17 (d, J = 3.7 Hz, 2H), 3.85 (s, 3H), 3.76 (s, 4H), 3.00 (s, 4H), 2.30 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 162.9, 158.5, 147.6, 146.3, 136.3, 131.1, 129.8, 129.2, 126.3, 124.7, 124.0, 116.7, 114.6, 109.6, 55.7, 45.7, 20.8; MS (ESI): m/z 497 [M+H]+; HRMS (ESI): m/z calcd for C24H25N4O4S2: 497.13117; found: 497.13004 [M+H]+.
(5-(p-Tolyl)imidazo[2,1-b]thiazol-2-yl)(4-tosylpiperazin-1-yl)methanone (9bb)
White solid: 187 mg; 86% yield; mp: 214–216 °C; 1H NMR (500 MHz, DMSO-d6) δ: 8.08 (s, 1H), 7.72 (d, J = 8.1 Hz, 2H), 7.66 (s, 1H), 7.64 (d, J = 8.2 Hz, 2H), 7.46 (d, J = 8.1 Hz, 2H), 7.18 (d, J = 8.0 Hz, 2H), 3.76 (s, 4H), 3.01 (s, 4H), 2.41 (s, 3H), 2.30 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 158.6, 147.6, 146.3, 143.9, 136.3, 132.0, 131.2, 130.0, 129.2, 127.6, 124.7, 124.0, 116.7, 109.6, 45.6, 21.0, 20.8; MS (ESI): m/z 481 [M+H]+; HRMS (ESI): m/z calcd for C24H25N4O3S2: 481.13626; found: 481.13497 [M+H]+.
(4-((4-Chlorophenyl)sulfonyl)piperazin-1-yl)(5-(p-tolyl)imidazo[2,1-b]thiazol-2-yl)methanone (9bc)
White solid: 178 mg; 80% yield; mp: 195–197 °C; 1H NMR (500 MHz, DMSO-d6) δ: 8.09 (s, 1H), 7.76 (d, J = 4.8 Hz, 3H), 7.72 (d, J = 8.0 Hz, 2H), 7.70 (d, J = 1.7 Hz, 1H), 7.65 (s, 1H), 7.18 (d, J = 7.9 Hz, 2H), 3.76 (s, 4H), 3.07 (s, 4H), 2.30 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 158.5, 147.6, 146.3, 138.4, 136.3, 133.8, 131.1, 129.7, 129.4, 129.1, 124.7, 124.0, 116.6, 109.5, 45.5, 20.7; MS (ESI): m/z 501 [M+H]+; HRMS (ESI): m/z calcd for C23H22ClN4O3S2: 501.08164; found: 501.08059 [M+H]+.
(4-((4-(Tert-butyl)phenyl)sulfonyl)piperazin-1-yl)(5-(p-tolyl)imidazo[2,1-b]thiazol-2-yl)methanone (9bd)
White solid: 182 mg; 82% yield; mp: 222–224 °C; 1H NMR (500 MHz, DMSO-d6) δ: 8.09 (s, 1H), 7.71 (d, J = 8.1 Hz, 2H), 7.68 (s, 4H), 7.67 (s, 1H), 7.17 (d, J = 7.9 Hz, 2H), 3.77 (s, 4H), 3.03 (s, 4H), 2.30 (s, 3H), 1.31 (s, 9H); 13C NMR (101 MHz, DMSO-d6) δ: 158.6, 156.4, 147.5, 146.3, 136.3, 132.2, 131.1, 129.1, 127.4, 126.3, 124.7, 124.0, 116.7, 109.6, 45.6, 34.9, 30.7, 20.7; MS (ESI): m/z 523 [M+H]+; HRMS (ESI): m/z calcd for C27H31N4O3S2: 523.18321; found: 523.18172 [M+H]+.
(5-(4-Chlorophenyl)imidazo[2,1-b]thiazol-2-yl)(4-((4-methoxyphenyl)sulfonyl)piperazin-1-yl)methanone (9ca)
Off White solid: 184 mg; 85% yield; mp: 216–218 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.21 (s, 1H), 7.86 (d, J = 8.5 Hz, 2H), 7.69 (s, 2H), 7.67 (s, 1H), 7.42 (d, J = 8.5 Hz, 2H), 7.17 (d, J = 8.8 Hz, 2H), 3.85 (s, 3H), 3.76 (s, 4H), 3.00 (s, 4H); 13C NMR (75 MHz, DMSO-d6) δ: 162.9, 158.4, 144.9, 132.8, 131.4, 129.7, 128.6, 126.4, 124.0, 117.1, 114.6, 110.5, 55.7, 45.6; MS (ESI): m/z 517 [M+H]+; HRMS (ESI): m/z calcd for C23H22ClN4O4S2: 517.07655; found: 517.07509 [M+H]+.
(5-(4-Chlorophenyl)imidazo[2,1-b]thiazol-2-yl)(4-tosylpiperazin-1-yl)methanone (9cb)
Brown solid: 182 mg; 84% yield; mp: 228–230 °C; 1H NMR (500 MHz, DMSO-d6) δ: 8.21 (s, 1H), 7.86 (d, J = 8.6 Hz, 2H), 7.69 (s, 1H), 7.64 (d, J = 8.2 Hz, 2H), 7.47 (d, J = 8.1 Hz, 2H), 7.42 (d, J = 8.6 Hz, 2H), 3.76 (s, 4H), 3.01 (s, 4H), 2.41 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 158.5, 148.0, 145.0, 143.9, 132.8, 132.0, 131.5, 130.0, 128.6, 127.6, 126.5, 124.0, 117.2, 110.6, 45.7, 21.0; MS (ESI): m/z 501 [M+H]+; HRMS (ESI): m/z calcd for C23H22ClN4O3S2: 501.08164; found: 501.08056 [M+H]+.
(5-(4-Chlorophenyl)imidazo[2,1-b]thiazol-2-yl)(4-((4-chlorophenyl)sulfonyl)piperazin-1-yl)methanone (9cc)
Brown solid: 190 mg; 90% yield; mp: 226–228 °C; 1H NMR (500 MHz, DMSO-d6) δ: 8.22 (s, 1H), 7.86 (d, J = 6.9 Hz, 2H), 7.76 (s, 3H), 7.69 (d, J = 5.6 Hz, 2H), 7.43 (d, J = 7.0 Hz, 2H), 3.76 (s, 4H), 3.06 (s, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 158.5, 148.0, 145.0, 138.5, 133.8, 132.8, 131.5, 129.7, 129.5, 128.6, 126.4, 124.0, 117.1, 110.6, 45.6; MS (ESI): m/z 521 [M+H]+; HRMS (ESI): m/z calcd for C22H19Cl2N4O3S2: 521.02701; found: 521.02595 [M+H]+.
(5-(4-Chlorophenyl)imidazo[2,1-b]thiazol-2-yl)(4-(phenylsulfonyl)piperazin-1-yl)methanone (9cd)
Off White solid: 179 mg; 80% yield; mp: 229–231 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.20 (s, 1H), 7.85 (d, J = 8.1 Hz, 2H), 7.74 (s, 3H), 7.68 (d, J = 6.0 Hz, 3H), 7.42 (d, J = 8.0 Hz, 2H), 3.76 (s, 4H), 3.03 (s, 4H); 13C NMR (126 MHz, DMSO-d6) δ: 158.5, 148.0, 145.0, 134.9, 133.5, 132.8, 131.5, 129.6, 128.6, 127.5, 126.4, 124.0, 117.2, 110.6, 45.7, 45.0; MS (ESI): m/z 487 [M+H]+; HRMS (ESI): m/z calcd for C22H20ClN4O3S2: 487.06599; found: 487.06481[M+H]+.
(4-((4-(Tert-butyl)phenyl)sulfonyl)piperazin-1-yl)(5-(4-chlorophenyl)imidazo[2,1-b]thiazol-2-yl)methanone (9ce)
Cream color solid: 178 mg; 76% yield; mp: 272–274 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.21 (s, 1H), 7.86 (d, J = 8.5 Hz, 2H), 7.70 (s, 1H), 7.68 (s, 4H), 7.42 (d, J = 8.5 Hz, 2H), 3.78 (s, 4H), 3.03 (s, 4H), 1.30 (s, 9H); 13C NMR (101 MHz, DMSO-d6) δ: 158.5, 156.4, 148.0, 144.9, 132.8, 132.2, 131.5, 128.6, 127.5, 126.4, 126.4, 124.0, 117.2, 110.7, 45.6, 34.9, 30.7; MS (ESI): m/z 543 [M+H]+; HRMS (ESI): m/z calcd for C26H28ClN4O3S2: 543.12859; found: 543.12754 [M+H]+.
(5-(4-Fluorophenyl)imidazo[2,1-b]thiazol-2-yl)(4-((4-methoxyphenyl)sulfonyl)piperazin-1-yl)methanone (9da)
Cream color solid: 188 mg; 88% yield; mp: 212–214 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.15 (s, 1H), 7.92–7.82 (m, 2H), 7.73–7.64 (m, 3H), 7.26–7.12 (m, 5H), 3.85 (s, 3H), 3.76 (s, 4H), 3.00 (s, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 162.8, 158.4, 147.6, 145.1, 130.3, 130.4, 129.6, 126.5 (d, J = 8.25 Hz), 126.2, 123.9, 116.8, 115.3 (d, J = 21.45 Hz), 114.5, 109.9, 55.6, 45.5; MS (ESI): m/z 501 [M+H]+; HRMS (ESI): m/z calcd for C23H22FN4O4S2: 501.10610; found: 501.10522 [M+H]+.
(5-(4-Fluorophenyl)imidazo[2,1-b]thiazol-2-yl)(4-tosylpiperazin-1-yl)methanone (9db)
Cream color solid: 185 mg; 84% yield; mp: 238–240 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.15 (s, 1H), 7.87 (dd, J = 8.6, 5.6 Hz, 2H), 7.68 (s, 1H), 7.64 (d, J = 8.2 Hz, 2H), 7.47 (d, J = 8.1 Hz, 2H), 7.20 (t, J = 8.9 Hz, 2H), 3.76 (s, 4H), 3.01 (s, 4H), 2.41 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 158.4, 147.6, 145.1, 143.7, 131.9, 130.4(d, J= 2.75 Hz), 129.8, 127.4, 126.6 (d, J = 8.25 Hz), 123.9, 116.8, 115.2, 115.4, 109.9, 45.5, 20.9; MS (ESI): m/z 485 [M+H]+; HRMS (ESI): m/z calcd for C23H22FN4O3S2: 485.11119; found: 485.11009 [M+H]+.
(4-((4-Chlorophenyl)sulfonyl)piperazin-1-yl)(5-(4-fluorophenyl)imidazo[2,1-b]thiazol-2-yl)methanone (9dc)
Cream color solid: 180 mg; 82% yield; mp: 219–221 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.16 (s, 1H), 7.87 (dd, J = 8.6, 5.6 Hz, 2H), 7.76 (s, 3H), 7.69 (d, J = 7.5 Hz, 2H), 7.20 (t, J = 8.9 Hz, 2H), 3.76 (s, 4H), 3.06 (s, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 162.6, 160.2, 158.5, 147.8, 145.3, 138.4, 133.8, 130.5, 129.6 (d, J = 24.5 Hz), 126.7 (d, J = 7.9 Hz), 124.0, 116.9, 115.4 (d, J = 21.5 Hz), 110.0, 45.5, 44.9; MS (ESI): m/z 505 [M+H]+; HRMS (ESI): m/z calcd for C22H19ClFN4O3S2: 505.05656; found: 505.05542 [M+H]+.
(5-(4-Fluorophenyl)imidazo[2,1-b]thiazol-2-yl)(4-(phenylsulfonyl)piperazin-1-yl)methanone (9dd)
Brown color solid: 175 mg; 76% yield; mp: >350 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.15 (s, 1H), 7.92–7.82 (m, 2H), 7.75 (s, 3H), 7.69 (d, J = 4.3 Hz, 3H), 7.20 (t, J = 8.6 Hz, 2H), 3.77 (s, 4H), 3.04 (s, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 162.6, 158.5, 147.8, 145.2, 134.9, 133.4, 130.5, 129.5 (d, J = 6.9 Hz), 127.4 (d, J = 20.0 Hz), 126.6 (d, J = 8.0 Hz), 124.0, 116.9, 115.4 (d, J = 21.5 Hz), 110.0, 45.6, 44.9; MS (ESI): m/z 471 [M+H]+; HRMS (ESI): m/z calcd for C22H20FN4O3S2: 471.09554; found: 471.09438 [M+H]+.
(4-((4-(Tert-butyl)phenyl)sulfonyl)piperazin-1-yl)(5-(4-fluorophenyl)imidazo[2,1-b]thiazol-2-yl)methanone (9de)
Cream color solid: 175 mg; 78% yield; mp: 244–246 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.16 (s, 1H), 7.87 (dd, J = 8.5, 5.6 Hz, 2H), 7.68 (d, J = 4.6 Hz, 5H), 7.19 (t, J = 8.8 Hz, 2H), 3.78 (s, 4H), 3.03 (s, 4H), 1.31 (s, 9H); 13C NMR (101 MHz, DMSO-d6) δ: 162.6, 158.5, 156.4, 147.8, 145.2, 132.2, 130.5, 127.5, 126.6 (d, J = 8.0 Hz), 126.3, 124.0, 117.0, 115.4 (d, J = 21.5 Hz), 110.0, 45.6, 34.9, 30.7; MS (ESI): m/z 527 [M+H]+; HRMS (ESI): m/z calcd for C26H28FN4O3S2: 527.15814; found: 527.15693 [M+H]+.
(4-((4-Methoxyphenyl)sulfonyl)piperazin-1-yl)(5-phenylimidazo[2,1-b]thiazol-2-yl)methanone (9ea)
Brown color solid: 185 mg; 86% yield; mp: 232–234 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.15 (s, 1H), 7.83 (d, J = 7.5 Hz, 2H), 7.69 (d, J = 6.3 Hz, 3H), 7.37 (t, J = 7.5 Hz, 2H), 7.26 (d, J = 7.2 Hz, 1H), 7.17 (d, J = 8.7 Hz, 2H), 3.85 (s, 3H), 3.76 (s, 4H), 3.00 (s, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 162.9, 158.5, 147.8, 146.2, 133.9, 129.8, 128.6, 127.2, 126.3, 124.8, 124.0, 116.9, 114.7, 110.1, 55.7, 45.7; MS (ESI): m/z 483 [M+H]+; HRMS (ESI): m/z calcd for C23H23N4O4S2: 483.11552; found: 483.11424 [M+H]+.
(5-Phenylimidazo[2,1-b]thiazol-2-yl)(4-tosylpiperazin-1-yl)methanone (9eb)
Cream color solid: 182 mg; 84% yield; mp: 250–252 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.15 (s, 1H), 7.83 (d, J = 7.3 Hz, 2H), 7.68 (s, 1H), 7.64 (d, J = 8.1 Hz, 2H), 7.47 (d, J = 8.1 Hz, 2H), 7.37 (t, J = 7.5 Hz, 2H), 7.25 (t, J = 7.3 Hz, 1H), 3.76 (s, 4H), 3.01 (s, 4H), 2.40 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 158.5, 147.8, 146.2, 143.9, 133.9, 132.0, 130.0, 128.6, 127.6, 127.2, 124.7, 124.0, 116.9, 110.1, 45.7, 21.0; MS (ESI): m/z 467 [M+H]+; HRMS (ESI): m/z calcd for C23H23N4O3S2: 467.12067; found: 467.11942 [M+H]+.
(4-((4-Chlorophenyl)sulfonyl)piperazin-1-yl)(5-phenylimidazo[2,1-b]thiazol-2-yl)methanone (9ec)
Cream color solid: 180 mg; 80% yield; mp: 245–247 °C; 1H NMR (300 MHz, CDCl3+DMSO-d6) δ: 8.16 (s, 1H), 7.83 (d, J = 7.3 Hz, 2H), 7.76 (d, J = 1.3 Hz, 3H), 7.68 (d, J = 7.7 Hz, 2H), 7.38 (t, J = 7.6 Hz, 2H), 7.25 (t, J = 7.3 Hz, 1H), 3.76 (s, 4H), 3.07 (s, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 158.5, 147.8, 146.2, 138.5, 133.9, 133.8, 129.7, 129.5, 128.6, 127.2, 124.7, 124.0, 116.9, 110.1, 45.6, 44.9; MS (ESI): m/z 487 [M+H]+; HRMS (ESI): m/z calcd for C22H20ClN4O3S2: 487.06599; found: 487.06491 [M+H]+.
(5-Phenylimidazo[2,1-b]thiazol-2-yl)(4-(phenylsulfonyl)piperazin-1-yl)methanone (9ed)
Brown color solid: 175 mg; 75% yield; mp: >350 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.14 (s, 1H), 7.83 (d, J = 7.5 Hz, 2H), 7.76 (d, J = 7.4 Hz, 3H), 7.68 (d, J = 4.7 Hz, 3H), 7.37 (t, J = 7.5 Hz, 2H), 7.26 (d, J = 7.3 Hz, 1H), 3.77 (s, 4H), 3.04 (s, 4H); 13C NMR (126 MHz, DMSO-d6) δ: 158.6, 147.8, 146.2, 134.9, 133.9, 133.5, 129.6, 128.6, 127.5, 127.3, 127.1, 124.7, 116.9, 110.1, 45.7, 45.0; MS (ESI): m/z 453 [M+H]+; HRMS (ESI): m/z calcd for C22H21N4O3S2: 453.10496; found: 453.10394 [M+H]+.
(4-((4-(Tert-butyl)phenyl)sulfonyl)piperazin-1-yl)(5-phenylimidazo[2,1-b]thiazol-2-yl)methanone (9ee)
Cream color solid: 178 mg; 80% yield; mp: 257–259 °C; 1H NMR (300 MHz, DMSO-d6) δ: 8.15 (s, 1H), 7.83 (d, J = 7.3 Hz, 2H), 7.69 (s, 1H), 7.68 (s, 4H), 7.37 (t, J = 7.5 Hz, 2H), 7.24 (t, J = 7.3 Hz, 1H), 3.78 (s, 4H), 3.03 (s, 4H), 1.30 (s, 9H); 13C NMR (101 MHz, DMSO-d6) δ: 158.6, 156.4, 147.8, 146.1, 133.9, 132.2, 128.6, 127.5, 127.1, 126.4, 124.7, 124.0, 117.0, 110.1, 45.6, 34.9, 30.7; MS (ESI): m/z 509 [M+H]+; HRMS (ESI): m/z calcd for C26H29N4O3S2: 509.16756; found: 509.16640 [M+H]+.
4.2. CA Inhibition
The inhibition assay of selected CA isozymes was performed using SX.18V-R Applied Photophysics (Oxford, UK) stopped flow instrument. 10 mM Hepes (pH 7.4) as a buffer, with Phenol Red (at a concentration of 0.2 mM) as an indicator, 0.1M Na2SO4 or NaClO4 (To maintain the constant ionic strength; these anions are not inhibitory in the used concentration), following the CA-catalyzed CO2 hydration reaction for a period of 5–10 s. Saturated CO2 solutions in water at 25 °C were used as substrate. Stock solutions of inhibitors were prepared at a concentration of 10 mM (in DMSO-water 1:1 v/v) and dilutions up to 0.01 nM done with the assay buffer mentioned above. At least 7 different inhibitor concentrations have been used for measuring the inhibition constant. Inhibitor and enzyme solutions were pre-incubated together for 10 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. Triplicate experiments were done for each inhibitor concentration, and the values reported in this paper are the mean of such results. The inhibition constants were obtained by non-linear least square methods using the Cheng-Prusoff equation, as reported earlier, [24,25] and represent the mean from at least three different determinations. All CA isozymes used here were recombinant proteins obtained as reported earlier by our group [26,27,28,29].
Acknowledgments
The authors K.L.M., S.P. and A.S. are thankful to National Institute of Pharmaceutical Education and Research (NIPER), Balanagar, Hyderabad & Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Govt. of India. C.T.S. is grateful to the Italian Ministry for University and Research for the grant PRIN: rot. 2017XYBP2R.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-1989/10/4/136/s1, File S1: spectral and chemical data for the new compounds.
Author Contributions
K.L.M. Conceptualization, S.P. and A.S. contributed in the writing-original draft preparation; M.A. (Mallika Alvala) supervision, review and editing; M.A. (Mohammed Arifuddin) visualization; C.T.S. and A.A. Investigation and manuscript editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research receives no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Supuran C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008;7:168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 2.Supuran C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016;473:2023–2032. doi: 10.1042/BCJ20160115. [DOI] [PubMed] [Google Scholar]
- 3.Supuran C.T. Carbonic anhydrases and metabolism. Metabolites. 2018;8:25. doi: 10.3390/metabo8020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karki S.S., Panjamurthy K., Kumar S., Nambiar M., Ramareddy S.A., Chiruvella K.K., Raghavan S.C. Synthesis and biological evaluation of novel 2-aralkyl-5-substituted-6-(4′-fluorophenyl)-imidazo [2, 1-b][1, 3, 4] thiadiazole derivatives as potent anticancer agents. Eur. J. Med. Chem. 2011;46:2109–2116. doi: 10.1016/j.ejmech.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 5.Andreani A., Granaiola M., Leoni A., Locatelli A., Morigi R., Rambaldi M., Lenaz G., Fato R., Bergamini C., Farruggia G. Potential Antitumor Agents. 37. Synthesis and Antitumor Activity of Guanylhydrazones from Imidazo[2,1-b]thiazoles and from the New Heterocyclic System Thiazolo[2′,3′:2,3]imidazo[4,5-c]quinoline. J. Med. Chem. 2005;48:3085–3089. doi: 10.1021/jm040888s. [DOI] [PubMed] [Google Scholar]
- 6.Demirayak S., Zitouni G., Chevallet P., Erol K., Kilic F.S. Synthesis and vasodilatory activity of some thiazolo-triazole derivative. II Farm. 1993;48:707–712. [PubMed] [Google Scholar]
- 7.Ibrahim D.A. Synthesis and biological evaluation of 3,6-disubstituted [1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives as a novel class of potential anti-tumor agents. Eur. J. Med. Chem. 2009;44:2776–2781. doi: 10.1016/j.ejmech.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R., Bua S., Ram S., Prete S.D., Capasso C., Supuran C.T., Sharma P.K. Benzenesulfonamide bearing imidazothiadiazole and thiazolotriazole scaffolds as potent tumor associated human carbonic anhydrase IX and XII inhibitors. Bioorg. Med. Chem. 2017;25:1286–1293. doi: 10.1016/j.bmc.2016.12.047. [DOI] [PubMed] [Google Scholar]
- 9.Nishimori I., Vullo D., Innocenti A., Scozzafava A., Mastrolorenzo A., Supuran C.T. Carbonic Anhydrase Inhibitors. The Mitochondrial Isozyme VB as a New Target for Sulfonamide and Sulfamate Inhibitors. J. Med. Chem. 2005;48:7860–7866. doi: 10.1021/jm050483n. [DOI] [PubMed] [Google Scholar]
- 10.Scozzafava A., Menabuoni L., Mincione F., Supuran C.T. Carbonic Anhydrase Inhibitors. A General Approach for the Preparation of Water-Soluble Sulfonamides Incorporating Polyamino–Polycarboxylate Tails and of Their Metal Complexes Possessing Long-Lasting, Topical Intraocular Pressure-Lowering Properties. J. Med. Chem. 2002;45:1466–1476. doi: 10.1021/jm0108202. [DOI] [PubMed] [Google Scholar]
- 11.Tanpure R.P., Ren B., Peat T.S., Bornaghi L.F., Vullo D., Supuran C.T., Poulsen S.-A. Carbonic Anhydrase Inhibitors with Dual-Tail Moieties to Match the Hydrophobic and Hydrophilic Halves of the Carbonic Anhydrase Active Site. J. Med. Chem. 2015;58:1494–1501. doi: 10.1021/jm501798g. [DOI] [PubMed] [Google Scholar]
- 12.Angapelly S., Ramya P.V.S., Angeli A., Prete S.D., Capasso C., Arifuddin M., Supuran C.T. Development of sulfonamides incorporating phenylacrylamido functionalities as carbonic anhydrase isoforms I, II, IX and XII inhibitors. Bioorg. Med. Chem. 2017;25:5726–5732. doi: 10.1016/j.bmc.2017.08.047. [DOI] [PubMed] [Google Scholar]
- 13.Supuran C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug. Discov. 2017;12:61–88. doi: 10.1080/17460441.2017.1253677. [DOI] [PubMed] [Google Scholar]
- 14.Supuran C.T., Vullo D., Manole G., Casini A., Scozzafava A. Designing of Novel Carbonic Anhydrase Inhibitors and Activators. Curr. Med. Chem. 2004;2:49–68. doi: 10.2174/1568016043477305. [DOI] [PubMed] [Google Scholar]
- 15.Supuran C.T. Exploring the multiple binding modes of inhibitors to carbonic anhydrases for novel drug discovery. Expert Opin. Drug Discov. 2020;25:1–16. doi: 10.1080/17460441.2020.1743676. in press. [DOI] [PubMed] [Google Scholar]
- 16.Supuran C.T. Carbonic anhydrases: From biomedical applications of the inhibitors and activators to biotechnological use for CO2 capture. J. Enzym. Inhib. Med. Chem. 2013;28:229–230. doi: 10.3109/14756366.2013.761876. [DOI] [PubMed] [Google Scholar]
- 17.Angeli A., Chiaramonte N., Manetti D., Romanelli M.N., Supuran C.T. Investigation of piperazines as human carbonic anhydrase I, II, IV and VII activators. J. Enzym. Inhib. Med. Chem. 2017;33:303–308. doi: 10.1080/14756366.2017.1417277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaik S.P., Nayak V.L., Sultana F., Rao A.V.S., Shaik A.B., Babu K.S., Kamal A. Design and synthesis of imidazo [2,1-b] thiazole linked triazole conjugates: Microtubule-destabilizing agents. Eur. J. Med. Chem. 2017;126:36–51. doi: 10.1016/j.ejmech.2016.09.060. [DOI] [PubMed] [Google Scholar]
- 19.Jadala C., Sathish M., Anchi P., Tokala R., Lakshmi U.J., Reddy V.G., Shankaraiah N., Godugu C., Kamal A. Synthesis of Combretastatin-A4 Carboxamidest that Mimic Sulfonyl Piperazines by a Molecular Hybridization Approach: In vitro Cytotoxicity Evaluation and Inhibition of Tubulin Polymerization. Chemmedchem. 2019;14:2052–2060. doi: 10.1002/cmdc.201900541. [DOI] [PubMed] [Google Scholar]
- 20.Khalifah R.J. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971;246:2561–2573. [PubMed] [Google Scholar]
- 21.Maresca A., Temperini C., Vu H., Pham N.B., Poulsen S.A., Scozzatava A., Quinn P.J., Supuran C.T. Non-zinc mediated inhibition of carbonic anhydrases: Coumarins are a new class of suicide inhibitors. J. Am. Chem. Soc. 2009;131:3057–3062. doi: 10.1021/ja809683v. [DOI] [PubMed] [Google Scholar]
- 22.Supuran C.T. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat. 2018;28:709–712. doi: 10.1080/13543776.2018.1523897. [DOI] [PubMed] [Google Scholar]
- 23.Korkmaz N., Obaidi O.A., Senturk M., Astley D., Ekinci D., Supuran C.T. Synthesis and biological activity of novel thiourea derivatives as carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2015;30:75–80. doi: 10.3109/14756366.2013.879656. [DOI] [PubMed] [Google Scholar]
- 24.Supuran C., Nicolae A., Popescu A. Carbonic anhydrase inhibitors. Part 35. Synthesis of Schiff bases derived from sulfanilamide and aromatic aldehydes: The first inhibitors with equally high affinity towards cytosolic and membrane-bound isozymes. Eur. J. Med. Chem. 1996;31:431–438. doi: 10.1016/0223-5234(96)85163-4. [DOI] [Google Scholar]
- 25.Carta F., Aggarwal M., Maresca A., Scozzafava A., McKenna R., Masini E., Supuran C.T. Dithiocarbamates strongly inhibit carbonic anhydrases and show antiglaucoma action in vivo. J. Med. Chem. 2012;55:1721–1730. doi: 10.1021/jm300031j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ceruso M., Bragagni M., AlOthman Z., Osman S.M., Supuran C.T. New series of sulfonamides containing amino acid moiety act as effective and selective inhibitors of tumor-associated carbonic anhydrase XII. J. Enzym. Inhib. Med. Chem. 2015;30:430–434. doi: 10.3109/14756366.2014.942659. [DOI] [PubMed] [Google Scholar]
- 27.Alafeefy A.M., Abdel-Aziz H.A., Carta F., Supuran C.T., Pathak S.K., Prasad O., Sinha L. Exploring QSARs of some benzenesulfonamides incorporating cyanoacrylamide moieties as a carbonic anhydrase inhibitors (specifically against tumorassociated isoforms IX and XII) J. Enzym. Inhib. Med. Chem. 2015;30:519–523. doi: 10.3109/14756366.2014.948435. [DOI] [PubMed] [Google Scholar]
- 28.Nocentini A., Supuran C.T. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin. Drug. Discov. 2019;14:1175–1197. doi: 10.1080/17460441.2019.1651289. [DOI] [PubMed] [Google Scholar]
- 29.Supuran C.T. How many carbonic anhydrase inhibition mechanisms exist? J. Enzym. Inhib. Med. Chem. 2016;31:345–360. doi: 10.3109/14756366.2015.1122001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




