Abstract
Additive manufacturing, known as three-dimensional (3D) printing technologies, has revolutionized production in all domains of science and technology. Although 3D printing has a high impact on research and development, its capacity to implement low-cost, flexible, and robust sample handling automation has not been exploited in full. To this end, we have created a low-cost, robust, and easy-to-utilize kit to transform an off-the-shelf fused deposition modeling 3D printer to a thin layer chromatography (TLC) sample application device. Our technology solution improves TLC convenience when higher throughput of the established method is required. The developed dual-needle sprayer allows simple and exceptionally robust automatic sample application. The device is especially well-suited for high-performance TLC-assisted method selection in counter-current chromatography. A step-by-step guide and list of required parts, including 3D printable files with instruction, can be obtained from the Supporting Information for research usage and open development.
Introduction
Minimal sample preparation, flexibility, and cost efficiency of thin layer chromatography (TLC) render the method attractive to initial proof of concept research and (bio)chemical reaction monitoring.1 High-performance TLC (HPTLC) remains attractive because of its simplicity and yet astonishing analytical power. Fully featured systems for low-cost open access development of TLC methods have been published recently.2 Especially, Morlock et al. have contributed to the community with many open source solutions ranging from piezo-driven InkJet printer utilization to 3D printable TLC plates.2−6 We contribute to the open-access minimal effort transformation of popular RepRap 3D printing platforms, which can be realized in less than a week for less than ∼0.5 k $. For a proof of concept, the device was used for the application of exemplary microalgae crude extracts. Furthermore, we were able to utilize the liquid handling system to estimate sample distributions in standard counter-current chromatography two-phasic solvent systems in reference to the empirical GUESS method (see Figure 3B/C).7,8
Figure 3.
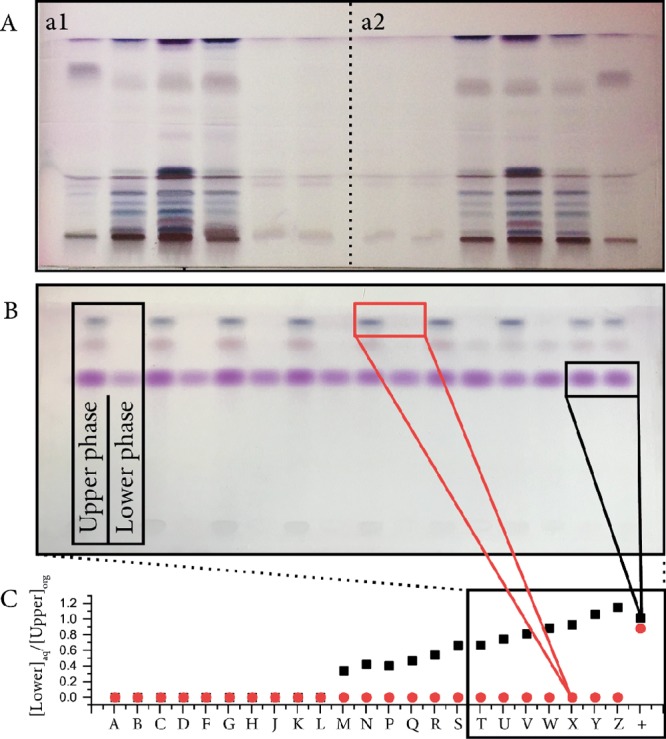
Application of the automated TLC sample-handling device. [A] Separation of exemplary micro-algae extracts, where A1 and A2 are mirrored applications, showing the reproducibility. [B] Separation of the defined samples of two phasic solvent mixtures containing ß-carotene (top, blue band) and ß-ionone. Upper (U) and lower (L) phase samples from two-phasic Arizona solvent systems applied are shown. [C] Distribution of the estimated partitioning coefficients by densitometry was determined using a Gel-Doc imager (Biorad, Hercules, CA, USA) and the ImageLab software.
Results and Discussion
To generate exemplary samples, micro-algal biomass Arthrospira maxima SAG 49.88, Dunaliella sp., and Synechococcus elongatus UTEX 2973 were cultivated according to a previously published cultivation method.9 The sample extracts were prepared by mixing biomass (50 mg) in analytical-grade ethanol (1 mL). Subsequently, the extract was evaporated under nitrogen and solved in (1 mL) analytical-grade n-hexane. To enrich colored carotenoids, the samples were filtered over polyamide solid-phase extraction cartridges (SPE PA6, Macherey und Nagel, Düren, Germany) ensuring chlorophyll depletion.10 Thereafter, ten (10) percent (vol%) of the samples was applied by the constructed device on an HPTLC analytical plate (Figure 2). HPTLC analytical plates were developed with an acetone/hexane/formic acid mobile phase (75:25:1, v/v) and subsequently stained (110 °C for 90 s) with Eckert reagent.11 The developed plates resulted in visually reproducible carotenoid sample chromatograms, as shown in Figure 3A1, and the mirrored application, as shown in Figure 3A2.
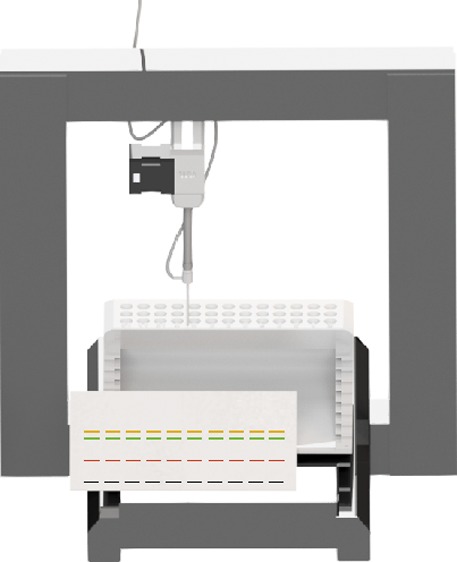
Figure 2.
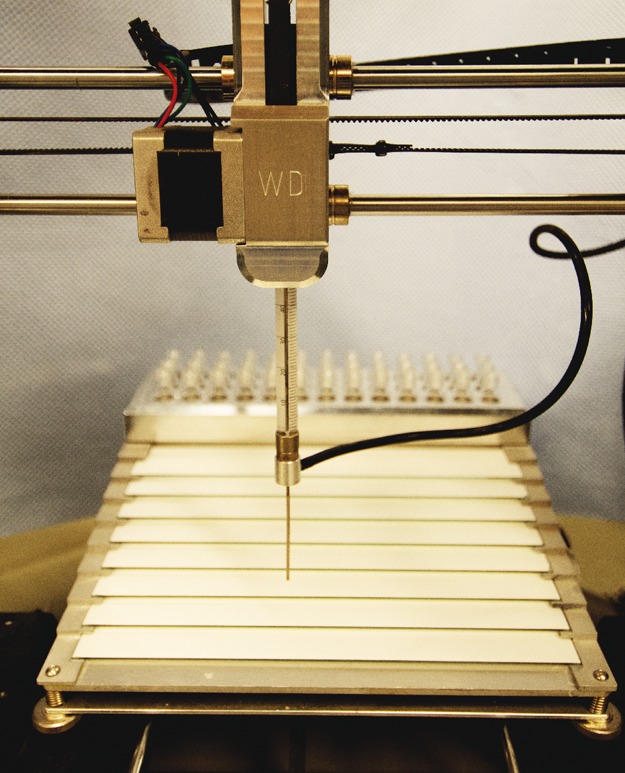
Image of the printing bed filled with eight HPTLC plates and the retrofitted 3D printer. In the background, the sample-holding device is depicted. The atomizer is shown in the foreground attached to the sample containing syringe.
To evaluate the applicability of the device toward liquid–liquid chromatography solvent system selection, we generated a less complex sample set with ß-carotene (50 μg/mL, Sigma Aldrich 22040, Merck KGaA, Darmstadt, Germany) and its potential cleavage product ß-ionone (10 μg/mL, Sigma Aldrich I12603, Merck KGaA, Darmstadt, Germany). This defined sample was dissolved in two-phase solvent systems and four (4) percent (vol%) (1/24, v/v) applied by the automation device, as shown in Figure 3B. The ratio of band intensities, that is, partitioning of compounds in the two-phase solvent systems, was analyzed by densitometry using a Bio-Rad GelDoc device (Bio-Rad, Hercules, CA, USA). Empirically, appropriate partitioning coefficients as described by the so-called GUESS method could be identified. The resulting coefficients could be successfully utilized in further experimental setups [counter-current chromatography (CCC) chromatograms not shown].8,12
Based on the low-cost access and high reproducibility of automated TLC (Figure 3), we were able to select an appropriate two-phase solvent system for counter-current chromatography separation of the potential carotenoid cleavage product ß-ionone. The presented technique enables the use of typical HPTLC performance at reduced cost, for a broader community. In particular, the automation device can help various researchers, which need higher TLC throughput or precise liquid handling at minimal cost.
Experimental Section
Hardware
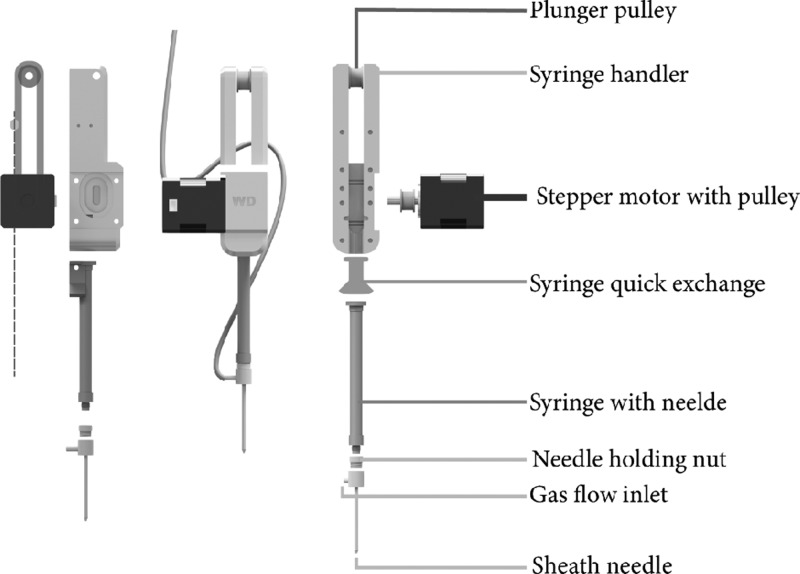
A commercial RepRap Prusa i3-based 3D printing platform (Wanhao i3, Monoprice, CA, USA) was retrofitted with the developed sample application device. The fused deposition modeling printing head tool was removed and disassembled, and the sample application device was connected, as shown in Figure 1. The developed part comprises the following components and arrangements: an interchangeable microvolume syringe with a removable needle, specifically a cone tip SGE 25–100 μL P/N: 003050, 004050, and 005050 (Trajan Scientific and Medical, Melbourne, Australia) with an elongated plunger. In order to disperse samples on a TLC plate, a sheath needle sprayer was developed, with new geometry, as shown in Figure 3, with the syringe needle placed into a secondary outer spray needle, whose tip is functioning as an atomization nozzle. Thus, robust and mechanically maintenance-free sample atomization is possible. In order to connect the secondary needle (outer diameter: 1.7 mm) to the syringe, a swivel nut adapter with a gas inlet port was build, which is attached to the SGE cover nut after adding a corresponding fine thread with a lathe. Alternatively, a downloadable 3D printable part can be utilized for a mechanically less effortful solution. To these, a 4 mm hose is connected in line with a solenoid that controls the gas flow via the 3D printers Melzi board. The gas flow disperses the sample approximately 1 mm above the HPTLC plate during sample application, depending on the used solvent and nozzle adjustment. The solenoid-driven micro gas valve for sample dispersion is connected to the power supply of the formerly 3D printing filament cooling fan or any other 5V controllable output. The JetCat microsolenoid is obtainable from M. Zipperer GmbH (Ballrechten-Dottingen, Germany).
Figure 1.
Illustration of the sample application kit for 3D printers, showing the assembly of the syringe handler and sample atomizer.
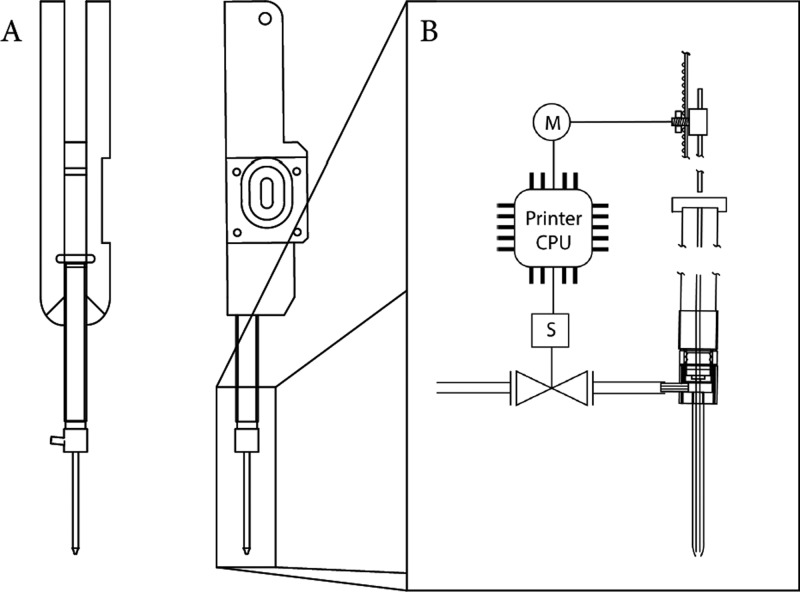
The syringe and atomizer assembly (Figure 4) is connected to a syringe-handler part (Figure 5A) and slides with the back flange of the syringe into a designed quick-release cavity. The following components and their assembly are described in more detail in the Supporting Information by a step-by-step guide. The required plunger actuation is realized by installing a GT2 6 mm closed drive belt connected to the syringe plunger by a clamp (Figure 5B). The belt is driven by the 3D printer’s extruder motor which was exchanged to a NEMA 14 stepper motor with a GT2 gear (14HS13-0804S) attached. A further pivoted GT 2 pulley is used to hold the drive belt in place (Hebben&Binke GbR, Muenster, Germany). The stepper, drive belt, and syringe assembly is attached to the printable syringe-holding part using four screws only, thereby aligning the syringe, drive belt, and plunger to the Z-axis. The syringe-handling part requires four additional screws to fit the linear motion ball-bearing slide unit, thus making it replaceable in a few minutes. An HPLTC plate slide-in rack was designed and machined to place up to eight (100 mm × 200 mm) analytical plates (Macherey Nagel, Nano adamant) on the printing bed of the RepRap-based 3D printer. The remaining space of the printing bed was used for the allocation of samples and needle wash solutions, which allows us to store and apply multiple samples in one batch. A simple transformation guide has been created in conjunction with open access to design files necessary to replicate the required hardware. The step-by-step guide is shared in the Supporting Information, and multiple design files can be obtained for research purpose from: https://www.department.ch.tum.de/wssb/hptlc/
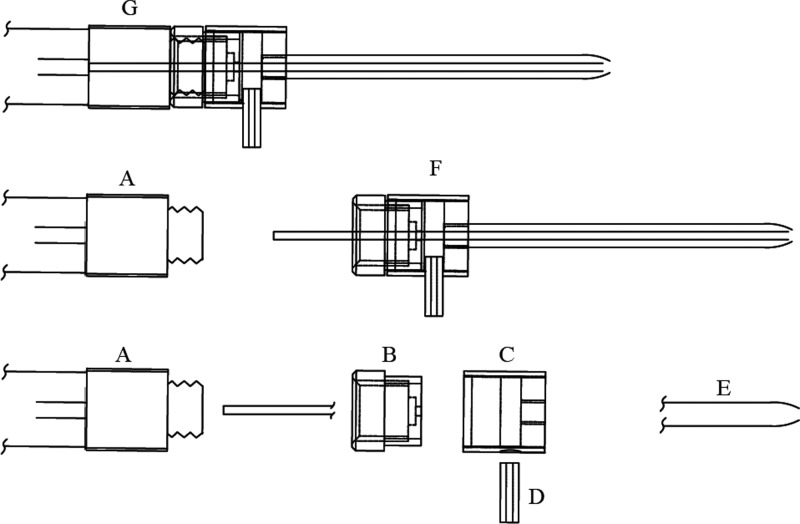
Figure 4.
Technical drawing of the needle assembly (G) required to generate a sheath flow for solenoid-controlled sample atomization. (A) Syringe, (B) swivel nut, (C) cap, (D) hose nipple, (E) dual needle tip, and (F) atomizer. A 3D printable alternative file is available to download, for maximal accessibility (see the Supporting Information, Figure S4b).
Figure 5.
[A] Syringe-handling assembly. [B] Control of the syringe plunger and sheath atomizer by the printers’ Melzi board connected to the extruder stepper motor and an additional solenoid.
Software
The commercial 3D printer (Wanhao Duplicator i3, Monoprice, CA, USA) firmware of the printing platform on a Melzi board needs no further modifications or alterations. Thus, requiring only a text file with a sequential manual position commands in numerical control (NC) language (G-Code/RS-274), allowing flexible low-complexity liquid handling workflow creations.
The syringe piston movement is addressable through the extruder motor GCODE control parameter. X–Y–Z factory-calibrated movements remain unaltered except for the repositioning of the Z-axis end-stop limit switch. Pressurized nitrogen gas coupled to the solenoid allows for control of the sheath gas stream along the sampling needle. The previous filament cooling fan parameter controls the solenoid. Although pulse width modulated, when set to the maximal value, this does not affect the operation of the solenoid. The sequential operating NC script for HPTLC was adapted for sample withdrawal, application, and subsequent wash steps. Visualization of the traveling path can be used to assist workflow creation by the free software Repetier-host GCODE Editor (https://www.repetier.com/, Hot-World GmbH & Co. KG, Willich, Germany). An annotated NC script file containing sample application and syringe wash commands can be obtained from the Supporting Information.
Acknowledgments
This work was supported by the Bavarian Ministry of Economic Affairs, Energy and Technology (20E1507A), the Federal Ministry of Education and Research (033RC012B), and the graduate school of the Technical University of Munich (TUM). The authors acknowledge Franziska Bezold for discussion on computational CCC method development.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01096.
Manual (step-by-step guide) for the assembly and operation of the 3D printer-based TLC autosampler; list of all components, checklist for operation, and GCODE numerical script guide; and required printable or machinable parts available for download from the TUM WSSB online repository: https://www.department.ch.tum.de/wssb/hptlc/, step-by-step assembly guide, and standard operation information (PDF)
Author Contributions
D.V.W. and N.M. wrote the manuscript. D.V.W. designed and constructed the device. D.V.W. and M.H. conducted CCC experiments. T.B.B. supervised and corrected the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Krüger S.; Winheim L.; Morlock G. E. Planar Chromatographic Screening and Quantification of Coumarin in Food, Confirmed by Mass Spectrometry. Food Chem. 2018, 239, 1182–1191. 10.1016/j.foodchem.2017.07.058. [DOI] [PubMed] [Google Scholar]
- Fichou D.; Morlock G. E. Office Chromatography: Miniaturized All-in-One Open-Source System for Planar Chromatography. Anal. Chem. 2018, 90, 12647–12654. 10.1021/acs.analchem.8b02866. [DOI] [PubMed] [Google Scholar]
- Fichou D.; Morlock G. E. Open-Source-Based 3D Printing of Thin Silica Gel Layers in Planar Chromatography. Anal. Chem. 2017, 89, 2116–2122. 10.1021/acs.analchem.6b04813. [DOI] [PubMed] [Google Scholar]
- Fichou D.; Morlock G. E. QuanTLC, an Online Open-Source Solution for Videodensitometric Quantification. J. Chromatogr. A 2018, 1560, 78–81. 10.1016/j.chroma.2018.05.027. [DOI] [PubMed] [Google Scholar]
- Kirchert S.; Schulz M.; Oberle M.; Morlock G. E. Development of a New Particulate 4-Μm Adsorbent Layer for Ultrathin-Layer Chromatography (Miniaturized Chromatogram). J. Chromatogr. A 2019, 1587, 247–255. 10.1016/j.chroma.2018.11.035. [DOI] [PubMed] [Google Scholar]
- Fichou D.; Ristivojević P.; Morlock G. E. Proof-of-Principle of RTLC, an Open-Source Software Developed for Image Evaluation and Multivariate Analysis of Planar Chromatograms. Anal. Chem. 2016, 88, 12494–12501. 10.1021/acs.analchem.6b04017. [DOI] [PubMed] [Google Scholar]
- Berthod A.; Hassoun M.; Ruiz-Angel M. J. Alkane Effect in the Arizona Liquid Systems Used in Countercurrent Chromatography. Anal. Bioanal. Chem. 2005, 383, 327–340. 10.1007/s00216-005-0016-7. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Friesen J. B.; Klein L. L.; McAlpine J. B.; Lankin D. C.; Pauli G. F.; Chen S.-N. The Generally Useful Estimate of Solvent Systems (GUESS) Method Enables the Rapid Purification of Methylpyridoxine Regioisomers by Countercurrent Chromatography. J. Chromatogr. A 2015, 1426, 248–251. 10.1016/j.chroma.2015.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woortman D. V.; Fuchs T.; Striegel L.; Fuchs M.; Weber N.; Brück T. B.; Rychlik M. Microalgae a Superior Source of Folates: Quantification of Folates in Halophile Microalgae by Stable Isotope Dilution Assay. Front. Bioeng. Biotechnol. 2020, 7, 481. 10.3389/fbioe.2019.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tswett M. Physikalisch-Chemische Studien Über Das Chlorophyll. Die Adsorptionen. Ber. Dtsch. Bot. Ges. 1906, 24, 316–323. 10.1111/j.1438-8677.1906.tb06524.x. [DOI] [Google Scholar]
- Thin Layer Chromatography in Phytochemistry; Waksmundzka-Hajnos M.; Sherma J.; Kowalska T., Eds.; CRC Press: Boca Raton, Florida, 2008; pp 1–875. [Google Scholar]
- Brent Friesen J.; Pauli G. F. G.U.E.S.S. - A Generally Useful Estimate of Solvent Systems in CCC. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2777–2280. 10.1080/10826070500225234. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.