Abstract

Various types of seaweed are potential functional foods as they contain multiple bioactive compounds. N-Methyltaurine (NMT) is a taurine derivative metabolite found in a type of red algae. The functional actions of NMT in mammalian animals have not been investigated, but the parent compound, taurine, possesses a variety of cellular actions. To explore the beneficial role of NMT in animals, the present study analyzed the effect of NMT against glucocorticoid-induced skeletal muscle atrophy. Glucocorticoids are one of the major causes of pathological muscle atrophy. Initially, we assessed the bioavailability of ingested NMT by determining its concentration in mouse blood. The bioavailability of orally administered NMT was found to be 96.1% that of intravenously administered NMT. Mice maintained on water containing 0.5% NMT for several days lead to the distribution of the taurine derivative to various tissues, including skeletal muscles. Like taurine, the delivery of NMT to skeletal muscles or myoblast cells is cytoprotective. The treatment with NMT prevents dexamethasone-induced atrophy of myotubes derived from C2C12 cells. Similarly, the addition of 0.5% NMT to drinking water attenuates dexamethasone-mediated reduction in muscle mass of the treated mice. The present study supports the hypothesis that orally administered NMT partially reverses skeletal muscle atrophy.
Introduction
Seaweed is recognized as a functional food since it is rich in bioactive compounds, such as polysaccharides, polyphenols, and amino acids.1 Taurine, one of the amino acids, is a component of seaweed, being especially abundant in red algae.2 In addition to taurine, red algae Gelidium cartilagineum contains various taurine-derived compounds, including N-methyltaurines (N-methyltaurine (NMT, Figure 1), dimethyltaurine, and trimethyltaurine).3 A variety of other taurine derivatives, including d-glyceryltaurine, rhodoic acid, d-cysteinolic acid, and cysteamide, have been identified in various types of seaweed.4−7 NMT has been identified in some red algae, such as Liagora distenta, Pterocladia pinnata, Gelidium amansii, and Laurencia intermedia.8−10 While some of these algae are used to produce edible products, NMT is present in amounts of 12.6 mmol (1.7 g)/kg dry weight. More recently, NMT was found as the most abundant organic osmolyte in tube worms living deep in the sea.11 The actions of these taurine-related compounds in mammalian animals remain to be elucidated. Moreover, it is also unclear if these compounds are absorbed by mammalian animals after oral intake.
Figure 1.

(A, B) Structures of (A) taurine and (B) N-methyltaurine (NMT).
While taurine is one of the most abundant free amino acids in mammals, the high concentration of taurine in the human body is maintained through both diet and hepatic biosynthesis.12 Taurine possesses a variety of biological actions in cells, such as regulation of cellular osmolality, ion movement, neuromodulation, conjugation of bile acid, antioxidation, and regulation of energy metabolism.13−15 Taurine is also important in skeletal muscles for modulation of intracellular calcium concentration and excitation–contraction coupling.16−18 Taurine treatment improves several muscle disorders, such as muscle soreness, muscle cramp, dystrophy, and muscle weakness in mitochondrial disease.19−22 The studies from taurine-deficient mice showed that taurine loss causes several skeletal muscle disorders and accelerates muscle aging,23−25 suggesting an antiaging role for taurine.
Skeletal muscle atrophy is induced by various physical conditions and diseases, such as disuse, aging, cachexia, sepsis, diabetes, chronic kidney disease, and mitochondrial disease. Some of these conditions are associated with an increase in blood glucocorticoid levels, which may associate with muscle atrophy induced in these conditions.26 While synthetic glucocorticoids are used as anti-inflammatory and immunosuppressive drugs, muscle atrophy is one of the adverse reactions caused by glucocorticoid use.27 Muscle atrophy occurs when protein degradation exceeds protein synthesis. We have previously demonstrated that taurine attenuates muscle atrophy caused by in vitro exposure to the synthetic glucocorticoid, dexamethasone (Dex).28 Moreover, taurine deficiency of skeletal muscles leads to skeletal muscle atrophy in taurine transporter knockout mice, suggesting that the richness of taurine in skeletal muscles is necessary to control the balance between protein synthesis and breakdown.
We hypothesized that taurine-derived compounds also have beneficial actions similar to those of taurine, a property likely related to similar chemical structures. In the present study, we investigated the pharmacokinetics and bioavailability of NMT. Following oral administration, NMT is nearly completely absorbed by the mouse, where it is distributed among other tissues. We further tested the effect of NMT against steroid-induced muscle atrophy in mice.
Results and Discussion
Oral Bioavailability of NMT
To study the pharmacokinetic parameters and the oral bioavailability of NMT, we tested the serum concentration of NMT after p.o. or i.v. injection of NMT (Figure 2). Biological fluid was sampled following the injection of NMT. The pharmacokinetic data were calculated by a noncompartmental model, which was used previously to evaluate pharmacokinetic parameters of taurine in rats.29 Oral bioavailability is defined as the fraction of the dose that reaches the systemic circulation. Blood samples were collected at 10 to 180 min after NMT administration and then examined by HPLC. After i.v. administration of NMT, the area under the time–NMT concentration curve (AUC) was 54.0 ± 3.6 min·μg/mL. After NMT p.o. administration of 0.5 and 5 mg/kg body weight, AUC values were 51.9 ± 4.1 and 315 ± 27.9 min·μg/mL, respectively (Table 1). The oral bioavailability values of NMT at 0.5 and 5 mg/kg body weight were 96 and 58%, respectively.
Figure 2.
Plasma NMT–time curves after administration of NMT in mice. (A–C) Plasma NMT level was monitored after (A) intravenous (i.v., 0.5 mg/kg) or (B, C) oral (p.o., 0.5 or 5 mg/kg) administration of NMT. Data are presented as means ± SD of measurements from three mice per dosing group.
Table 1. AUC and Bioavailability of Intravenously (i.v.) or Orally (p.o.) Injected NMTa.
| route of administration | AUC0–180min (min·μg/mL) | bioavailability (%) |
|---|---|---|
| i.v. (0.5 mg/kg BW) | 54.0 ± 3.6 | |
| p.o. (0.5 mg/kg BW) | 51.9 ± 4.1 | 96.1 |
| p.o. (5 mg/kg BW) | 315 ± 27.9 | 58.0 |
Data are presented as means ± standard errors of measurements from three mice per dosing group.
The bioavailability of oral NMT was almost 100%, indicating that the absorption of NMT from the gut into the blood is efficient. Previous pharmacokinetic studies for taurine demonstrated that oral bioavailability is very similar to 1 (100%) in rats.29−31 The taurine transporter (SLC6A6) is responsible for the absorption of taurine from the intestine as well as the uptake of taurine into cells in other tissues.32−35 Importantly, it has been reported that NMT inhibits taurine uptake in brain slices,36 suggesting that the taurine transporter may have an affinity for NMT and may contribute to the absorption of NMT from the intestine. Meanwhile, the oral bioavailability of 5 mg/kg body weight NMT is almost half of that of 0.5 mg/kg body weight NMT. In the case of the taurine transport system in the human intestine cells, the Km value is 4.8 μM, and the transport activity is almost saturated at 50 μM.35 Therefore, it is assumable that the administration of high concentration around 5 mg/kg body weight of NMT causes the saturation of the transport system.
Distribution Study
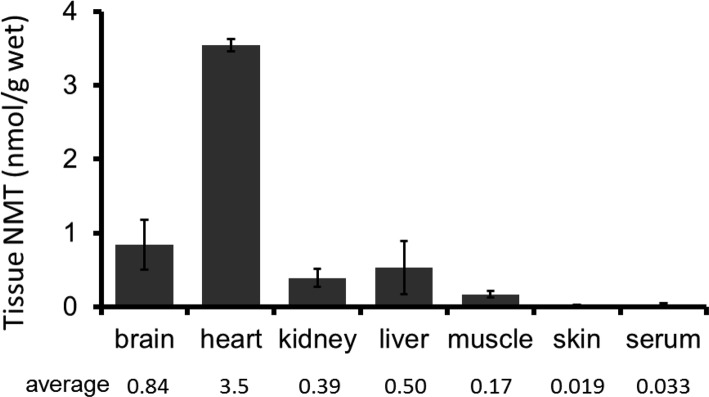
To evaluate the tissue distribution of NMT in mice after administration in the drinking water, the concentration of NMT was measured in tissues and serum 4 days after the original administration (Figure 3). The tissue concentration of NMT was analyzed by HPLC. NMT was widely distributed in the tissues of mice, including in brain, liver, kidney, heart, and muscles. The tissue concentration of NMT was much higher than that of serum concentration, with one exception, the skin (Figure 3).
Figure 3.
Long-term NMT treatment on tissue concentration. Tissue NMT concentration after drinking water containing 0.5% NMT for 4 days. NMT was detected in all tested tissues. Data are presented as means ± SD of measurements from three mice per dosing group.
We confirmed that orally administered NMT is distributed to several tissues, including in liver, kidney, muscles, heart, and brain. The taurine transporter is widely distributed among tissues and plays a main role in the uptake of taurine into cells.32−35 Importantly, the distribution of NMT into various tissues is about 10–100 times higher than the serum concentration of NMT, although skin is an exception. Similarly, tissue taurine concentration is about 100 times higher than blood taurine concentration, a situation that is influenced by the activity and Km of the taurine transporter.37,38 These findings also suggest that the taurine transporter contributes to NMT uptake into tissues. Inconsistently, the concentration of NMT in skin is low, while skin might averagely express the taurine transporter gene according to the database for gene expression (https://www.ncbi.nlm.nih.gov/gene/). However, based on the immunohistochemical study, the taurine transporter is highly expressed in epidermis, but not dermis, among the skin layers,39,40 indicating that the location of the taurine transporter may be involved.
Effect of NMT in Dex-Induced Myotube Atrophy in C2C12 Cells
To explore the beneficial effect of NMT against skeletal muscle atrophy in mice, we investigated its protective effect using cultures of C2C12 myotubes. Since the concentration of taurine to treat cultured cells is generally 10 to 20 mM and we expected that NMT would have a similar effect as taurine against muscle atrophy, we used 20 mM NMT in the present experiment. Treatment with Dex for 24 h reduced the width of the myotube, but simultaneous exposure to the medium containing Dex and NMT (20 mM) prevented the decline in myotube width (Figure 4A–C).
Figure 4.

Effect of NMT on Dex-induced myotube atrophy in C2C12 cells. Myotubes differentiated from C2C12 myoblasts were treated with Dex or Dex + NMT (10 mM) for 24 h. (A) Representative images for control, Dex, and Dex + NMT groups (bars = 200 μm). (B) Frequency and (C) average values of myotube diameters were calculated from more than 100 myotubes. (C) Data are presented as means ± SD of measurements from more than 100 myotubes per group. Similar results were obtained from three independent experiments. *p < 0.001 vs control, $p < 0.001 vs Dex alone. (D) Gene expression of Atrogin-1 and MuRF1 in C2C12 myotubes treated with Dex and NMT for indicated time points. The expression level is normalized by the GAPDH mRNA level. Data are presented as means ± SD of four replicates in four independent experiments. *p < 0.05; **p < 0.01 vs control.
Previous reports showed that muscle-specific E3 ubiquitin ligases, Atrogin-1 and MuRF1, are increased by Dex and may play a critical role in muscle atrophy (Figure 4D). We tested whether these ubiquitin ligases are involved in the suppressive effect of NMT against muscle atrophy in the C2C12 myotube. NMT did not act by downregulating those genes.
Effect of NMT in Skeletal Muscle Atrophy in Mice
Next, to investigate the effect of NMT on skeletal muscle atrophy, a mouse atrophy model induced by Dex was employed41 (Figure 5). Mice were administered with Dex daily (i.p., 10 mg/kg body weight) and maintained on drinking water either with no addition or containing 0.5% NMT. Dex treatment caused a continuous loss of body weight. Administration of 0.5% NMT attenuated body weight loss induced by Dex. Furthermore, NMT attenuated Dex-induced loss in wet weight of both tibial anterior muscle and gastrocnemius muscle.
Figure 5.
Effect of NMT on Dex-induced muscle atrophy in ICR mice. (A) Body weight was monitored after daily Dex injection (i.p., 10 mg/kg BW). Statistical significance was confirmed by two-way repeated-measures ANOVA (days, p < 0.001; treatment (Dex vs Dex + NMT), p < 0.001; interaction, p > 0.05). (B, C) Weight of (B) tibial anterior muscle and (C) gastrocnemius muscle 10 days after starting Dex injections. Data are presented as means ± SD of measurements from three mice per dosing group. *p < 0.01 vs control; $p < 0.01 vs Dex.
Muscle atrophy is caused by the activation of proteolytic pathways. Muscle-specific E3 ubiquitin ligases, Atrogin-1 and MuRF1, are increased under atrophy-related conditions, including the glucocorticoids, and they are mainly involved in atrophy.42 In the present study, we found that NMT did not attenuate the increase in ubiquitin ligase activity mediated by Dex, suggesting that NMT may control other mechanisms. We previously reported similar effects of taurine in Dex-induced muscle atrophy in the C2C12 myotube despite no effect on gene expression of ubiquitin ligase.28 Compatible osmolytes, including taurine, contribute not only to the regulation of cell volume but also to the thermal stability of proteins.43−46 Taurine excludes bulk water from proteins to weaken the water–protein interaction as well as directly interact with the proteins to stabilize their folding.44,46,47 Importantly, muscle taurine depletion results in muscle atrophy accompanied by the activation of the unfolded protein response,24 suggesting the importance of osmolytes in preventing atrophy. While taurine can weaken the structure of water around proteins, SO3– is involved in weakening the water structure.44 Therefore, it is possible that NMT can play a similar role in cells. It is also possible that taurine mediates these actions by improving the mitochondrial function. Taurine forms a conjugate with tRNALeu(UUR), which ensures normal mitochondrial protein synthesis and viability of the electron transport chain.48 Mutations that interfere with the formation of the taurine conjugate lead to the development of severe myopathy characterized by muscle wasting. Thus, impaired energy metabolism and the formation of reactive oxygen species by the mitochondria may also contribute to the development of muscle atrophy, as seen in the mitochondrial disease MELAS.
Conclusions
NMT attenuates glucocorticoid-induced muscle atrophy both in vitro and in vivo. While skeletal muscle atrophy is induced by various pathological and physiological conditions, glucocorticoids must interact with these factors to cause skeletal muscle atrophy.26 Therefore, it is possible that NMT is effective against muscle atrophy caused by various factors. We used a high dose of NMT for both in vivo and in vitro experiments as taurine is abundant in skeletal muscles and is commonly used in high concentrations in many studies.28 It is because we expected NMT to function in muscle cells via a similar mechanism as taurine, and high concentrations were employed. Nonetheless, we should determine how much NMT is necessary to prevent muscle atrophy in the future. NMT intake from seaweed diet could be a potential strategy to attenuate muscle atrophy. There are some other natural taurine derivatives besides NMT in red algae. Further studies are necessary to clarify the nutritional roles of taurine derivatives.
Experimental Section
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Fukui Prefectural University. Three-month-old male ICR mice were used for this study. To test the bioavailability of NMT in mice, NMT solution (FUJIFILM Wako Pure Chemical, Osaka, Japan, 10 mg/10 mL in saline) was administered either intravenously (10 mg/kg body weight) or orally (10 or 100 mg/kg body weight). Blood samples were collected from the facial vein (for i.v. injection) or the tail vein (for p.o. injection) at 10, 20, 30, 60, 120, and 180 min after NMT administration.
To measure NMT in the serum and tissues after long-term treatment with NMT, NMT dissolved in the drinking water (0.5%) was given to mice for 4 days. After that, mice were anesthetized by medetomidine (0.3 mg/kg body weight; FUJIFILM Wako Pure Chemical), butorphanol (5 mg/kg body weight; FUJIFILM Wako Pure Chemical), and midazolam (4 mg/kg body weight; FUJIFILM Wako Pure Chemical), and then blood and tissues were collected. Tissues were kept in −80 °C until use.
Measurement of NMT and Taurine by HPLC
Plasma samples were mixed with an equal volume of 5% sulfosalicylic acid (SSA) containing the internal standard chemical sarcosine (0.1 mM). Tissues in 10 volumes of 5% SSA containing an internal control were homogenized using a Polytron homogenizer. Samples were centrifuged, and the supernatant was filtered with 0.45 μm filters and then neutralized with NaHCO3.
NMT was measured by HPLC after derivatization with 9-fluorenylmethyloxycarbonyl chloride (FMOC-Cl) according to a previous report with brief modification.49 First, equal volumes of o-phthalaldehyde (OPA) reagent (5 mM OPA, 23 mM 3-mercaptopropionic acid, 10 mM boric acid, pH 10.4) was added to acid-extracted samples and incubated at room temperature for 1 min for the derivatization of the primary amine. Then, 1 volume of FMOC-Cl reagent (3 mM FMOC-Cl in acetone) was added to the mixture and incubated for additional 1 min for the derivatization of residual secondary amine. After dilution with HPLC mobile phase buffer A (10 mM potassium phosphate, pH 7.3), samples were subjected to HPLC analysis performed on an UltiMate 3000 rapid separation binary system (Thermo Fisher, USA) or Prominence HPLC system (Shimadzu, Japan). Samples were injected into a ZORBAX Eclipse Plus C18 column (Agilent, USA). Mobile phases A (10 mM potassium phosphate, pH 7.3) and B (acetonitrile) were used. The column oven temperature was set at 40 °C. The flow rate of the mobile phase was 1.5 mL/min. The gradient of the mobile phase was increased from 2 to 88% buffer B (from 0.84 to 11.9 min) and was kept at 100% buffer B for 3 min followed by 2% for 15 min. N-Methyltaurine derivatized by FMOC-Cl was monitored by a fluorescence detector (excitation: 266 nm, emission: 305 nm) and diode array detector (Shimadzu).
Data Analysis for Pharmacokinetic Parameters
Pharmacokinetic parameters were calculated by using R (Package “PK”, ver. 1.3-4).
Cell Culture
C2C12 mouse myoblasts were purchased from ATCC (American Type Culture Collection) and were cultured in DMEM containing 10% fetal bovine serum. Cell lines were not tested for mycoplasma contamination in the present study. To induce differentiation of the myotubes, cells were cultured in DMEM containing 2% fetal bovine serum for 1 week. After differentiation, cells were treated with Dex (final concentration, 50 μM) with or without NMT.28 Myotube diameter was measured from microscopic images taken after treatment with Dex.
Dexamethasone-Induced Atrophy Model
To induce muscle atrophy in ICR mice, Dex was suspended in saline (10 mg/mL), and the mixture was intraperitoneally (i.p.) injected at 10 mg/kg body weight once a day for 10 days.41 Body weight was monitored daily. Then, mice were euthanized by cervical dislocation, and muscles were isolated from the mice. The isolated tissue was immediately froze in liquid nitrogen and stored at −80 °C.
mRNA Measurement
Total RNA was isolated from C2C12 cells using Sepasol Super G (Nacalai Tesque, Kyoto, Japan), and cDNA was generated by using ReverTra Ace (Toyobo, Osaka, Japan). Quantitative RT-PCR analysis was performed using Applied Biosystems StepOne (Applied Biosystems) with THUNDERBIRD SYBR qPCR Mix (Toyobo). The primers used are as follows: Atrogin-1 forward: 5′-TTCAG CAGCC TGAAC TACGA-3′, reverse: 5′-AGTAT CCATG GCGCT CCTTC-3′. MuRF1 forward: 5′-GCGTG ACCAC AGAGG GTAAA-3′, reverse: 5′-CTCTG CGTCC AGAGC GTG-3′. GAPDH forward: 5′-GCCGG TGCTG AGTAT GTCGT-3′, reverse: 5′-CCCTT TTGGC TCCAC CCTT-3′.
Statistics
Student’s t test and the Tukey–Kramer test (for multiple comparisons) were used to determine statistical significance between groups. Two-way repeated-measures ANOVA performed with EZR software was used to monitor body weight. Differences were considered statistically significant when the calculated p value was less than 0.05.
Acknowledgments
This work is supported by the grant for Scientific Research from Fukui Prefectural University, Lotte foundation (to T.I.) and the JSPS KAKENHI (grant number 18K11050, to S.M.).
Author Contributions
§ K.H.N. and S.I. equally contributed to this work.
The authors declare no competing financial interest.
References
- Holdt S. L.; Kraan S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. 10.1007/s10811-010-9632-5. [DOI] [Google Scholar]
- Kawasaki A.; Ono A.; Mizuta S.; Kamiya M.; Takenaga T.; Murakami S. The Taurine Content of Japanese Seaweed. Adv. Exp. Med. Biol. 2017, 9s75, 1105–1112. 10.1007/978-94-024-1079-2_88. [DOI] [PubMed] [Google Scholar]
- Lindberg B.; Bjerrum J.; Lundén R.; Prydz H. Methylated Taurines and Choline Sulphate in Red Algae. Acta Chem. Scand. 1955, 9, 1323–1326. 10.3891/acta.chem.scand.09-1323. [DOI] [Google Scholar]
- Wickberg B.; Thamsen J.; Pokras L.; Sillén L. G.; Thorell B. Isolation of N-[D-2,3-Dihydroxy-n-Propyl]-Taurine from Gigartina Leptorhynchos. Acta Chem. Scand. 1956, 10, 1097–1099. 10.3891/acta.chem.scand.10-1097. [DOI] [Google Scholar]
- Sato M.; Takeuchi M.; Kanno N.; Nagahisa E.; Sato Y.. Purification and Properties of Tauropine Dehydrogenase from a Red Alga, Rhodoglossum Japonicum. In Fourteenth International Seaweed Symposium; Springer: Dordrecht, 1993, 673–678, 10.1007/978-94-011-1998-6_91. [DOI] [Google Scholar]
- Wickberg B.; Larsson E. N. V.; Pettersson G. Isolation of 2-L-Amino-3-Hydroxy-1-Propane Sulphonic Acid from Polysiphonia Fastigiata. Acta Chem. Scand. 1957, 11, 506–511. 10.3891/acta.chem.scand.11-0506. [DOI] [Google Scholar]
- Ito K. Distribution of D-cystenolic acid in marine algae. Bull. Jpn. Soc. Sci. Fish. 1963, 29, 771–775. 10.2331/suisan.29.771. [DOI] [Google Scholar]
- Impellizzeri G.; Mangiafico S.; Oriente G.; Piattelli M.; Sciuto S.; Fattorusso E.; Magno S.; Santacroce C.; Sica D. Amino Acids and Low-Molecular-Weight Carbohydrates of Some Marine Red Algae. Phytochemistry 1975, 14, 1549–1557. 10.1016/0031-9422(75)85349-0. [DOI] [Google Scholar]
- Ito K.; Miyazawa K.; Matsumoto F. Amino Acid Composition of the Ethanolic Extractives from 31 Species of Marine Red Algae. J. Fac. Fish. Anim. Husb., Hiroshima Univ. 1977, 16, 77–90. 10.15027/41276. [DOI] [Google Scholar]
- Miyazawa K. 5. Amino Acids and Peptides in Marine Algae. Nippon Suisan Gakkaishi 1971, 37, 788–794. 10.2331/suisan.37.788. [DOI] [Google Scholar]
- Yin M.; Palmer H. R.; Fyfe-Johnson A. L.; Bedford J. J.; Smith R. A. J.; Yancey P. H. Hypotaurine, N-Methyltaurine, Taurine, and Glycine Betaine as Dominant Osmolytes of Vestimentiferan Tubeworms from Hydrothermal Vents and Cold Seeps. Physiol. Biochem. Zool. 2000, 73, 629–637. 10.1086/317749. [DOI] [PubMed] [Google Scholar]
- Hayes K. C. Taurine Nutrition. Nutr. Res. Rev. 1988, 1, 99–113. 10.1079/NRR19880009. [DOI] [PubMed] [Google Scholar]
- Schaffer S.; Takahashi K.; Azuma J. Role of Osmoregulation in the Actions of Taurine. Amino Acids 2000, 19, 527–546. 10.1007/s007260070004. [DOI] [PubMed] [Google Scholar]
- Ito T.; Schaffer S.; Azuma J. The Effect of Taurine on Chronic Heart Failure: Actions of Taurine against Catecholamine and Angiotensin II. Amino Acids 2014, 46, 111–119. 10.1007/s00726-013-1507-z. [DOI] [PubMed] [Google Scholar]
- Ito T.; Schaffer S. W.; Azuma J. The Potential Usefulness of Taurine on Diabetes Mellitus and Its Complications. Amino Acids 2012, 42, 1529–1539. 10.1007/s00726-011-0883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerino D. C.; Tricarico D.; Pierno S.; Desaphy J.-F.; Liantonio A.; Pusch M.; Burdi R.; Camerino C.; Fraysse B.; De Luca A. Taurine and Skeletal Muscle Disorders. Neurochem. Res. 2004, 29, 135–142. 10.1023/B:NERE.0000010442.89826.9c. [DOI] [PubMed] [Google Scholar]
- De Luca A.; Pierno S.; Camerino D. C. Taurine: The Appeal of a Safe Amino Acid for Skeletal Muscle Disorders. J. Transl. Med. 2015, 13, 243. 10.1186/s12967-015-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicchitano B. M.; Sica G. The Beneficial Effects of Taurine to Counteract Sarcopenia. Curr. Protein Pept. Sci. 2018, 19, 673–680. 10.2174/1389203718666161122113609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrill J. R.; Pinniger G. J.; Graves J. A.; Grounds M. D.; Arthur P. G. Increasing Taurine Intake and Taurine Synthesis Improves Skeletal Muscle Function in the Mdx Mouse Model for Duchenne Muscular Dystrophy. J. Physiol. 2016, 594, 3095–3110. 10.1113/JP271418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeay Y.; Stannard S.; Barnes M. The Effect of Taurine on the Recovery from Eccentric Exercise-Induced Muscle Damage in Males. Antioxidants 2017, 6, 79. 10.3390/antiox6040079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ra S.-G.; Miyazaki T.; Ishikura K.; Nagayama H.; Komine S.; Nakata Y.; Maeda S.; Matsuzaki Y.; Ohmori H. Combined Effect of Branched-Chain Amino Acids and Taurine Supplementation on Delayed Onset Muscle Soreness and Muscle Damage in High-Intensity Eccentric Exercise. J. Int. Soc. Sports Nutr. 2013, 10, 51. 10.1186/1550-2783-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki Y.; Tanaka N.; Yamaguchi T.; Chuganji Y.; Nishi M.; Chiba T.; Osuga T.; Komatsu Y. Effect of Taurine Administration for Muscle Cramp in Chronic Liver Disease. A Case of Decompensated Liver Cirrhosis in Which Muscle Cramp Disappeared after Oral Administration of Taurine. Kanzo 1990, 31, 1464–1469. 10.2957/kanzo.31.1464. [DOI] [Google Scholar]
- Warskulat U.; Flögel U.; Jacoby C.; Hartwig H.-G.; Thewissen M.; Merx M. W.; Molojavyi A.; Heller-Stilb B.; Schrader J.; Häussinger D. Taurine Transporter Knockout Depletes Muscle Taurine Levels and Results in Severe Skeletal Muscle Impairment but Leaves Cardiac Function Uncompromised. FASEB J. 2004, 18, 577–579. 10.1096/fj.03-0496fje. [DOI] [PubMed] [Google Scholar]
- Ito T.; Yoshikawa N.; Inui T.; Miyazaki N.; Schaffer S. W.; Azuma J. Tissue Depletion of Taurine Accelerates Skeletal Muscle Senescence and Leads to Early Death in Mice. PLoS One 2014, 9, e107409 10.1371/journal.pone.0107409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T.; Kimura Y.; Uozumi Y.; Takai M.; Muraoka S.; Matsuda T.; Ueki K.; Yoshiyama M.; Ikawa M.; Okabe M.; et al. Taurine Depletion Caused by Knocking out the Taurine Transporter Gene Leads to Cardiomyopathy with Cardiac Atrophy. J. Mol. Cell. Cardiol. 2008, 44, 927–937. 10.1016/j.yjmcc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Schakman O.; Kalista S.; Barbé C.; Loumaye A.; Thissen J. P. Glucocorticoid-Induced Skeletal Muscle Atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. 10.1016/j.biocel.2013.05.036. [DOI] [PubMed] [Google Scholar]
- Pereira R. M. R.; Freire de Carvalho J. Glucocorticoid-Induced Myopathy. Jt., Bone, Spine 2011, 78, 41–44. 10.1016/j.jbspin.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Uozumi Y.; Ito T.; Hoshino Y.; Mohri T.; Maeda M.; Takahashi K.; Fujio Y.; Azuma J. Myogenic Differentiation Induces Taurine Transporter in Association with Taurine-Mediated Cytoprotection in Skeletal Muscles. Biochem. J. 2006, 394, 699–706. 10.1042/BJ20051303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sved D. W.; Godsey J. L.; Ledyard S. L.; Mahoney A. P.; Stetson P. L.; Ho S.; Myers N. R.; Resnis P.; Renwick A. G. Absorption, Tissue Distribution, Metabolism and Elimination of Taurine given Orally to Rats. Amino Acids 2007, 32, 459–466. 10.1007/s00726-007-0494-3. [DOI] [PubMed] [Google Scholar]
- Nielsen C. U.; Bjerg M.; Ulaganathan N.; Holm R. Oral and Intravenous Pharmacokinetics of Taurine in Sprague-Dawley Rats: The Influence of Dose and the Possible Involvement of the Proton-Coupled Amino Acid Transporter, PAT1, in Oral Taurine Absorption. Physiol. Rep. 2017, 5, e13467 10.14814/phy2.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalán-Latorre A.; Nácher A.; Merino V.; Díez O.; Merino Sanjuán M. A Preclinical Study to Model Taurine Pharmokinetics in the Undernourished Rat. J. Geophys. Res. Oceans 2018, 119, 826–835. 10.1017/S0007114518000156. [DOI] [PubMed] [Google Scholar]
- Ito T.; Fujio Y.; Hirata M.; Takatani T.; Matsuda T.; Muraoka S.; Takahashi K.; Azuma J. Expression of Taurine Transporter Is Regulated through the TonE (Tonicity-Responsive Element)/TonEBP (TonE-Binding Protein) Pathway and Contributes to Cytoprotection in HepG2 Cells. Biochem. J. 2004, 382, 177–182. 10.1042/BJ20031838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K.; Azuma M.; Yamada T.; Ohyabu Y.; Takahashi K.; Schaffer S. W.; Azuma J. Taurine Transporter in Primary Cultured Neonatal Rat Heart Cells: A Comparison between Cardiac Myocytes and Nonmyocytes. Biochem. Pharmacol. 2003, 65, 1181–1187. 10.1016/S0006-2952(03)00003-0. [DOI] [PubMed] [Google Scholar]
- Uchida S.; Kwon H. M.; Yamauchi A.; Preston A. S.; Marumo F.; Handler J. S. Molecular Cloning of the CDNA for an MDCK Cell Na+– And Cl--Dependent Taurine Transporter That Is Regulated by Hypertonicity. Proc. Natl. Acad. Sci. U. S. A. 1992, 89, 8230–8234. 10.1073/pnas.89.17.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satsu H.; Watanabe H.; Arai S.; Shimizu M. Characterization and Regulation of Taurine Transport in Caco-2, Human Intestinal Cells. J. Biochem. 1997, 121, 1082–1087. 10.1093/oxfordjournals.jbchem.a021698. [DOI] [PubMed] [Google Scholar]
- Kontro P.; Oja S. S. Hypotaurine Transport in Brain Slices - Comparison with Taurine and GABA. Neurochem. Res. 1981, 6, 1179–1191. 10.1007/BF00966676. [DOI] [PubMed] [Google Scholar]
- Fujihara E.; Takahashi N.; Matsui T.; Hattori Y.; Arai T. Plasma and Tissue Concentrations of Free Taurine and Amino Acids in Fasted, and Hyper- or Hypothyroid Diet Fed Rats. Chem. Pharm. Bull. 1971, 19, 424–428. 10.1248/cpb.19.424. [DOI] [PubMed] [Google Scholar]
- Brand H. S.; Jörning G. G. A.; Chamuleau R. A. F. M. Changes in Urinary Taurine and Hypotaurine Excretion after Two-Thirds Hepatectomy in the Rat. Amino Acids 1998, 15, 373–383. 10.1007/BF01320901. [DOI] [PubMed] [Google Scholar]
- Foster A. R.; el Chami C.; O’Neill C. A.; Watson R. E. B. Osmolyte Transporter Expression Is Reduced in Photoaged Human Skin: Implications for Skin Hydration in Aging. Aging Cell 2020, 19, e13058 10.1111/acel.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeke G.; Siefken W.; Carstensen S.; Springmann G.; Bleck O.; Steinhart H.; Höger P.; Wittern K. P.; Wenck H.; Stäb F.; et al. Role of Taurine Accumulation in Keratinocyte Hydration. J. Invest. Dermatol. 2003, 121, 354–361. 10.1046/j.1523-1747.2003.12366.x. [DOI] [PubMed] [Google Scholar]
- Sun H.; Gong Y.; Qiu J.; Chen Y.; Ding F.; Zhao Q. TRAF6 Inhibition Rescues Dexamethasone-Induced Muscle Atrophy. Int. J. Mol. Sci. 2014, 15, 11126–11141. 10.3390/ijms150611126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine S. C.; Baehr L. M. Skeletal Muscle Atrophy and the E3 Ubiquitin Ligases MuRF1 and MAFbx/Atrogin-1. Am. J. Physiol. - Endocrinol. Metab. 2014, 307, E469–E484. 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P. H. Organic Osmolytes as Compatible, Metabolic and Counteracting Cytoprotectants in High Osmolarity and Other Stresses. J. Exp. Biol. 2005, 208, 2819–2830. 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]
- Bruździak P.; Panuszko A.; Kaczkowska E.; Piotrowski B.; Daghir A.; Demkowicz S.; Stangret J. Taurine as a Water Structure Breaker and Protein Stabilizer. Amino Acids 2018, 50, 125–140. 10.1007/s00726-017-2499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal S.; Poddar N. K.; Singh L. R.; Dar T. A.; Rishi V.; Ahmad F. Relationship between Functional Activity and Protein Stability in the Presence of All Classes of Stabilizing Osmolytes. FEBS J. 2009, 276, 6024–6032. 10.1111/j.1742-4658.2009.07317.x. [DOI] [PubMed] [Google Scholar]
- Abe Y.; Ohkuri T.; Yoshitomi S.; Murakami S.; Ueda T. Role of the Osmolyte Taurine on the Folding of a Model Protein, Hen Egg White Lysozyme, under a Crowding Condition. Amino Acids 2015, 47, 909–915. 10.1007/s00726-015-1918-0. [DOI] [PubMed] [Google Scholar]
- Macchi F.; Eisenkolb M.; Kiefer H.; Otzen D. E. The Effect of Osmolytes on Protein Fibrillation. Int. J. Mol. Sci. 2012, 13, 3801–3819. 10.3390/ijms13033801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer S. W.; Jong C. J.; Ito T.; Azuma J. Role of Taurine in the Pathologies of MELAS and MERRF. Amino Acids 2014, 46, 47–56. 10.1007/s00726-012-1414-8. [DOI] [PubMed] [Google Scholar]
- Cigić I. K.; Vodošek T. V.; Košmerl T.; Strlič M. Amino Acid Quantification in the Presence of Sugars Using HPLC and Pre-Column Derivatization with 3-MPA/OPA and FMOC-Cl. Acta Chim. Slov. 2008, 55, 660–664. [Google Scholar]