Abstract

The obstruction of traditional chemical activation lies in the addition of excessive catalysts to prepare the highly porous graphitic activated carbon (HPGAC), we propose the hydrothermal pretreatment-assisted chemical activation method to synthesize HPGAC as electrode materials using a small amount of Na-based catalysts (20 wt %). Hydrolysis accompanied by the strong depolymerization and reorganization of the coal framework is beneficial to the removal of different kinds of oxygen-containing structures (including cross-linking bonds, functional groups, and heterocycles) from lignite; thus, the deoxidization effect of hydrothermal carbonization (HTC) on hydrochar gradually strengthens with the increase in pretreatment temperature from 180 to 300 °C, resulting in the formation of a lot of disordered nanostructures and a smooth and compact surface. In the subsequent chemical activation stage, the microstructure of hydrochar is beneficial to the formation of a lot of graphene-like sheets and developed micropores even under a small amount of Na-based catalysts (20 wt %). The as-obtained C-HTC-300 with a highly ordered microstructure and a high specific surface area (SBET) of 1945.33 m2/g has an excellent electrochemical performance. Compared with a large consumption of catalyst for synthesizing HPGAC in traditional chemical activation, the hydrothermal pretreatment-assisted method meets the environmental protection and low-cost preparation requirements.
1. Introduction
Supercapacitors are the most promising energy storage devices owing to their high power density, long cycle, fast charging, and discharging characteristics.1,2 The electrode materials as crucial components determine the electrochemical performance of supercapacitors. The ideal electrode materials should have an abundant pore structure with a high specific surface area (SBET) to strengthen ion transport capability and an ordered graphitic crystallite to promote good conductivity.3 Highly porous graphitic activated carbon (HPGAC) has attracted intense interest owing to its easy processability, adjustable porosity/crystallite, and excellent stability.4 At present, the preparation of HPGAC usually adopts the chemical activation method.5−7 Chen et al.8 found that when the ratio of chemical agents and raw materials increased from 2:1 to 5:1, the SBET value of the as-obtained sample rose from 878 to 2000 m2 g–1, forming an ordered three-dimensional structure. However, the addition of excessive activation agents inevitably causes the high cost and severe environmental pollution, resulting in the limited development of HPGAC using the chemical activation method.9 Therefore, it is very valuable to reduce the dosage of activation agents during the traditional chemical activation process to meet the environmental protection and low-cost preparation requirements for synthesizing HPGAC.
Many researchers had done great works to reduce the dosage of chemical agents.10−12 They found that the existence of oxygen-containing structures (such as cross-linking bonds, oxygen heterocycles, functional groups, etc.) in biomass/low-rank coals promoted the formation of disordered microcrystals and macropores. Our previous studies13,14 found that the thermal stability of the oxygen-containing structure in the coal framework increased with the increase in pyrolysis temperature. Some functional groups fixed on the edge of the aromatic ring were more stable, and the corresponding decomposition temperature was usually between 550 and 3000 °C.15 Therefore, excessive agents are added to cut these oxygen-containing structures for smoothly synthesizing HPGAC during chemical activation. If these oxygen-containing structures in biomass/low-rank coals can be removed basically during the pretreatment (<300 °C), then it is easier to obtain HPGAC using a small amount of agent during chemical activation. At present, hydrothermal carbonization (HTC) is a new technology for processing coal or waste materials over traditional pyrolysis (TP). Oliveira et al. and Petrovic et al.16,17 found that strong hydrolysis could cause the dehydration and decarboxylation reaction, reducing the oxygen content of the sample during HTC. He et al.18 also found an increase in FC (fixed carbon)/VM (volatile matter) and a decrease in O/C and H/C in hydrochar produced by HTC. Therefore, HTC is an excellent pretreatment method to remove the oxygen-containing structures in coal/biomass. However, the effects of hydrothermal pretreatment-assisted chemical activation on the preparation of HPGAC were not discussed systematically in previous studies.
The primary objective of this study was to study the feasibility of hydrothermal pretreatment assisted by a small amount of chemical reagent in preparing HPGAC from lignite.
The changes of the physical–chemical structure of hydrochar and pyrochar in the pretreatment and activation stages were discussed systematically. Finally, the electrochemical performance of the as-obtained samples was evaluated.
2. Results and Discussion
2.1. Yield, Proximate, and Ultimate Analyses of Hydrochar and Pyrochar
Yield, proximate, and ultimate analyses of hydrochar and pyrochar at different temperatures are shown in Table 1. The solid product yield and volatile content of hydrochar and pyrochar decrease gradually with an increase in pretreatment temperature; in contrast, the fixed carbon content increases gradually, indicating that the two pretreatment methods can promote the depolymerization and recombination of the molecular structure. However, a large number of soluble units in lignite are resolved by subcritical water during the hydrothermal process.13 Therefore, hydrochar exhibits the lower solid product yield, volatile content, and higher fixed carbon content at the same reaction temperature. Besides, the ash content of hydrochar decreases but that of pyrochar increases with the increase in pretreatment temperature. The inorganic matter in lignite can dissolve in subcritical water,19 but it is more likely to aggregate and precipitate on the particle surface in the pyrolysis environment.20
Table 1. Yield, Proximate, and Ultimate Analyses of Hydrochar and Pyrochar.
| proximate analysis (wt %) |
ultimate analysis (wt %) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| char | yield (%) | Vd | FCd | Ad | Cdaf | Hdaf | Oa | Ndaf | Sdaf |
| HTC-180 | 72.7 | 30.17 | 63.12 | 6.71 | 72.46 | 13.61 | 12.76 | 0.97 | 0.80 |
| HTC-240 | 65.3 | 23.21 | 71.94 | 3.51 | 80.25 | 11.20 | 8.12 | 0.32 | 0.11 |
| HTC-300 | 59.6 | 16.34 | 80.85 | 2.81 | 84.78 | 9.34 | 5.81 | 0.04 | 0.03 |
| TP-180 | 81.8 | 32.33 | 59.25 | 8.42 | 69.84 | 12.06 | 15.66 | 1.45 | 0.99 |
| TP-240 | 77.7 | 26.45 | 63.21 | 10.34 | 73.18 | 9.67 | 14.74 | 1.44 | 0.97 |
| TP-300 | 73.1 | 18.89 | 70.05 | 11.06 | 77.31 | 6.44 | 13.88 | 1.42 | 0.95 |
By difference; d (dry basis): the coal in the anhydrous state was used as a benchmark; daf (dry ash-free basis): the remaining component after the removal of water and ash in coal was used as a benchmark.
According to the element analysis data from Table 1, with the increase in reaction temperature, the content of O and H in hydrochar and pyrochar decreases slowly; in contrast, the content of C increases gradually. Hydrolysis is conducive to the dehydration and decarboxylation from the hydrochar in the form of H2O, CO2, and other small molecular matter;21 thus, the deoxidization effect of HTC on hydrochar is more significant at the same temperature. However, the dehydrogenation effect of TP on pyrochar is more remarkable during which a lot of volatile matter (such as alkenes, olefin, other kinds of tar, etc.) is released.22 Alternatively, S and N contents of pyrochar have little change with the increase in reaction temperature, but those of hydrochar decrease significantly, which might be related to the dissolution of nitrogen and sulfur oxides into the processing liquid.18 Besides, the high C content of hydrochar indicates that hydrothermal pretreatment is helpful in upgrading lignite coal.
2.2. FTIR Analysis of HLH, Hydrochar, and Pyrochar
To explore further the chemical structures of HLH, hydrochar, and pyrochar, the corresponding FTIR spectra are shown in Figure 1. According to the relevant literature,23−26 the absorption peak at 3420 cm–1 is related to the hydroxyl group (−OH). The absorption peaks at 2920 and 2830 cm–1 are related to the aliphatic C–H structure (such as −CH3 and −CH2 in the alkyl group). The absorption peak at 1730 cm–1 is related to the C=O structure (such as carbonyl, quinone, ester, and carboxyl groups). The absorption peak at 1600 cm–1 is attributed to the C=C bond in the aromatic framework. Some peaks at 1500–1000 cm–1 are related to the C–O structure (such as hydroxyl, carboxyl, ester, phenol, or ether and methoxy group). The absorption peaks at 810–750 cm–1 are related to the aromatic C–H bond.
Figure 1.
FTIR spectra of (a) HLH, (b) HTC-180, (c) HTC-240, (d) HTC-300, (e) TP-180, (f) TP-240, and (g) TP-300.
First, a wide absorption peak of HLH at 3420 cm–1 implies the existence of a lot of hydrogen bonds (−OH) owing to its high moisture content. Some absorption bands at 1500–1000 and 1730 cm–1 in HLH mean the existence of C=O and C–O bonds. Some absorption peaks at 810–750, 1600, 2920, and 2830 cm–1 mean that HLH is a three-dimensional macromolecular structure composed of a lot of aromatic rings, aliphatic side chains, and bridge bonds. Then, the gradually weakened peaks at 810–750, 2920, and 2830 cm–1 can be found in hydrochar with an increase in hydrothermal temperature. Hydrothermal pretreatment is conducive to the depolymerization of lignite into soluble or insoluble small molecular products, and some of them dissolve in water as acetic acid, alcohols, furan, and so on; the other part remains in hydrochar as an aliphatic carbon structure. Besides, the peak intensity at 3420 cm–1 (−OH) and 1500–1000 cm–1 (C–O) of hydrochar weakens gradually with an increase in hydrothermal pretreatment temperature. The characteristic peak at 1730 cm–1 (C–O) that disappeared means the removal of a lot of oxygen-containing structures with an increase in hydrothermal pretreatment temperature. However, the absorption peak at 1600 cm–1 of hydrochar hardly changes during the whole hydrothermal pretreatment, indicating that the stable C=C bond is not easily damaged even at the hydrothermal reaction. Finally, the absorption peaks at 1500–1000 cm–1 of pyrochar gradually weaken, whereas the C=O bond at 1730 cm–1 obviously enhances with an increase in pyrolysis pretreatment temperature. This indicates that some oxygen-containing groups with low thermal stability are broken and have been converted into the stable oxygen-containing structure by the cross-linking reaction during pyrolysis pretreatment; thus, more stable cross-linking bonds are still retained in pyrochar even at a high temperature. The absorption peaks of the aliphatic C–H bond at 2920 and 2830 cm–1 gradually weaken or even disappear, which are related to the depolymerization of the aliphatic structure. Still, the absorption peaks of aromatic C–H at 1600 and 810–750 cm–1 progressively increase; this results from the fact that the aromatization reaction of hydrocarbon substances (such as alkanes or cycloalkanes) is accompanied by dehydrogenation during pyrolysis pretreatment.
2.3. Crystal Structure Characterization of HLH, Hydrochar, and Pyrochar
The X-ray diffraction method (XRD) was used to explore the crystal structure of HLH, hydrochar, and pyrochar, and the corresponding XRD profiles are shown in Figure 2. There are two characteristic peaks including the (002) peak and (100) peak at 15°–32° and 35°–55°, respectively. The asymmetrical (002) peak of HLH originates from a weak diffraction peak (γ band) on its left side, presenting a lot of aliphatic structures (such as side chains or bridge bonds).27 As can be seen in Figure 2a, the (002) and (100) peaks of hydrochar gradually weaken, and the shape of (002) peak is always asymmetric; these changes are related to the strong depolymerization of the microcrystalline structure into the aliphatic structure caused by HTC. However, the (002) and (100) peaks of pyrochar gradually strengthen, and the shape of (002) peak gradually becomes symmetrical, as shown in Figure 2b, which is related to the disappearance of aliphatic side chains and bridge bonds and the cross-linking polymerization of aromatic compounds. The XRD profiles of these samples are treated by the fitting method to obtain the crystal structure parameters (such as layer distance (d002), stacking height (Lc), width (La), and layer number (N = Lc/d002)),23 as shown in Table 2.
Figure 2.
XRD profiles of (a) hydrochar (HTC-180/240/300) and (b) HLH and pyrochar (TP-180/240/300).
Table 2. XRD Data of HLH, Hydrochar, and Pyrochar.
| parameter | HLH | HTC-180 | HTC-240 | HTC-300 | TP-180 | TP-240 | TP-300 |
|---|---|---|---|---|---|---|---|
| La (Å) | 23.66 | 22.15 | 21.88 | 20.67 | 22.74 | 23.68 | 24.23 |
| Lc (Å) | 12.33 | 11.81 | 11.46 | 11.13 | 12.01 | 12.45 | 13.31 |
| d002 (Å) | 3.75 | 3.68 | 3.76 | 3.81 | 3.71 | 3.67 | 3.60 |
| N | 3.29 | 3.21 | 3.05 | 2.92 | 3.24 | 3.39 | 3.69 |
First, compared to the crystal structure parameters of HLH, La, Lc, and N values of hydrochar steadily decrease, and the d002 value first decreases and then increases with the increase in hydrothermal temperature. At the beginning stage of hydrothermal pretreatment, the decomposition of some aliphatic and oxygen-containing structures at low temperature destroys the stability of the macromolecular structure and makes it split into several “small aromatic units”; thus, La, Lc, and N values of HTC-180 decrease. In this process, the removal of intercalation materials under subcritical water is conducive to the longitudinal aggregation of aromatic layers, leading to the reduction in the d002 value of HTC-180. With the increase in hydrothermal temperature from 240 to 300 °C, the strong hydrothermal reaction gradually promotes the depolymerization of the aromatic structure into the amorphous structure, resulting in the significant reduction of the size of aromatic units (La, Lc, and N values); meanwhile, the formation of more aliphatic structures also facilitates the increase in layer distance (d002). Nomura and Thomas28 found that the thin layers were easier to transform into a graphite-like structure at a high activation stage. Then, the La, Lc, and N values of pyrochar first decrease and then increase, but the d002 value constantly decreases with an increase in pyrolysis pretreatment temperature. It is easier to remove the aliphatic structure (such as bridge bonds, side chains, and the intercalation material) at low pyrolysis temperature, leading to the modest depolymerization of the macromolecular structure; thus, all parameters of TP-180 are reduced. When the pyrolysis pretreatment temperature increases from 240 to 300 °C, the cross-linking reaction of the oxygen-containing structure and aromatization reaction of hydrocarbon substances strengthen the formation of the aromatic units with the interior opening space. Quite evidently, the oxygen-containing structure has an essential impact on the reorganization of the spatial structure of aromatic units with an increase in pyrolysis pretreatment temperature.
2.4. Surface Morphology Analysis of HLH, Hydrochar, and Pyrochar
SEM images of HLH, hydrochar, and pyrochar are shown in Figure 3. Some metaplast-like layer structure can be found in HLH, as shown in Figure 3a, and its surface is soft, loose, and irregular with a few cracks. With the increase in hydrothermal temperature from 180 to 300 °C, the distinct particle characteristic of HTC-180 with the rough and porous surface can be found in Figure 3b, which is related to the shrinkage of particles caused by the initial dehydration and degassing process,29 and then a gradually smooth and compact surface of HTC-240 and HTC-300 can be found in Figure 3c,d; the strong hydrolysis reaction at high hydrothermal temperature caused the melting, softening and pore shrinkage of particles, producing soluble and insoluble products (such as different kinds of tar).30 The aggregation of insoluble products on the surface of particles can reshape the morphology of hydrochar. With an increase in pyrolysis pretreatment temperature from 180 to 300 °C; the gradually rough and porous surface of pyrochar can be observed in Figure 3e–g. In the process, a large number of small molecular compounds are released from the inside of the particles in the form of gas (such as CO, CO2, CxHy, etc.) instead of metaplasts that block the pores in a relatively open pyrolysis environment.
Figure 3.
SEM images of (a) HLH, (b) HTC-180, (c) HTC-240, (d) HTC-300, (e) TP-180, (f) TP-240, and (g) TP-300.
2.5. Hybrid Carbon Structure Analysis of C-HTC/TP-180/240/300
The Raman spectra of C-HTC/TP-180/240/300 are shown in Figure 4. The D band at 1362 cm–1 and G band at 1569 cm–1 can be observed in Figure 4. Specifically, the D band and G band are related to the defect sites and disordered sp2-hybridized carbon atoms and the phonon mode in-plane vibration of sp2-bonded carbon atoms, respectively; thus, the ID/IG value means the disordered degree of the microstructure of carbon materials.13 Additionally, the second-order D band (2D band) at 2692 cm–1 can be observed in C-HTC-180/240/300, and the I2D/IG value means the highly ordered degree of the microstructure of carbon materials. The result of the relative intensity ratio (ID/IG) and (I2D/IG) of all samples is summarized in Table 3.
Figure 4.
Raman spectra of (a) C-TP-180, (b) C-TP-240, (c) C-TP-300, (d) C-HTC-180, (e) C-HTC-240, and (f) C-HTC-300.
Table 3. Relative Intensity Ratios (ID/IG and I2D/IG) of C-HTC/TP-180/240/300.
| ratio | C-HTC-180 | C-HTC-240 | C-HTC-300 | C-TP-180 | C-TP-240 | C-TP-300 |
|---|---|---|---|---|---|---|
| ID/IG | 0.43 | 0.27 | 0.14 | 1.05 | 1.12 | 1.23 |
| I2D/IG | 0.36 | 0.45 | 0.78 |
First, the widening peaks of the D band and G band and the increased ID/IG value from 1.05 to 1.23 can be observed for C-TP-180/240/300 in Figure 4a–c and Table 3, implying an increasing amorphization degree and a small number of graphene-like sheets. As an analysis, a lot of stable chemical bonds and oxygen-containing structures in pyrochar can be formed with the increase in pyrolysis pretreatment temperature. In the subsequent chemical activation stage, these stable chemical bonds (including functional groups and heterocycles within/connected to microcrystals) not only limit the free movement of the Na-based catalyst between the microcrystalline layers but also hinder the growth and condensation of microcrystals, leading to the disorder development of the microstructure of C-TP-180/240/300. Then, the gradually narrow characteristic peaks of D and G bands and a gradually strong characteristic peak of the 2D band can be observed for C-HTC-180/240/300 in Figure 4d–f. A decreasing ID/IG value from 0.43 to 0.14 and an increased I2D/IG value from 0.36 to 0.78 for C-HTC-180/240/300 in Table 3 imply an increasing graphitization degree, which means that the removal of a lot of oxygen-containing structure during hydrothermal pretreatment is favorable to the formation of the majority of graphene-like sheets with a small fraction of amorphous carbon or defects in the subsequent chemical activation stage. More concretely, the Na-based catalyst can easily move freely between layers to promote its catalytic graphitization effect, resulting in the condensation of microcrystals and the transformation of the aliphatic structure into graphene-like sheets.31 Therefore, the hydrothermal pretreatment-assisted chemical activation method is effective in synthesizing the ordered graphene-like structure even under a small amount of Na-based catalysts (20 wt %) during chemical activation.
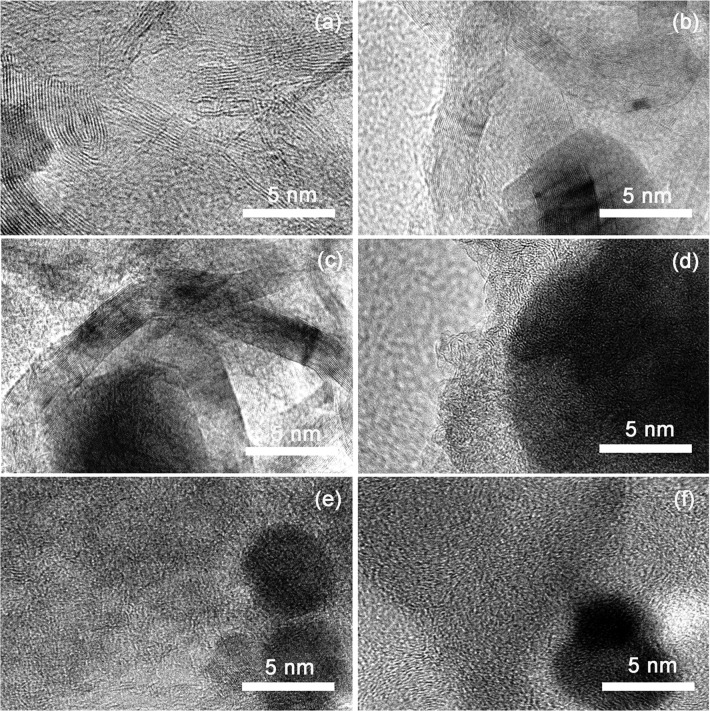
2.6. Microstructure Analysis from C-HTC/TP-180/240/300
TEM images of C-HTC/TP-180/240/300 are given in Figure 5. First, some multilayer graphitic microcrystals are shown in Figure 5a–c; more concretely, C-HTC-180 presents some interlaced arrangements of about three to eight thin crystalline layers in Figure 5a. Then, there are a lot of multilayer microcrystalline structures (more than 10 layers) with the same orientation for C-HTC-240/300 in Figure 5b,c, indicating the highly ordered microcrystalline layers. Mori et al.33 found that some metal-based compounds (such as FeCl3, NaOH, NaCl, etc.) had catalytic graphitization. These catalysts (M) could react with the sp3 hybrid (such as amorphous carbon) to form metal carbide (MxC) at high temperature. Then, the metal carbide (MxC) could decompose to form graphitized carbon under high-temperature conditions. The reaction formula was as follows
| 1 |
| 2 |
Figure 5.
TEM images of (a) C-HTC-180, (b) C-HTC-240, (c) C-HTC-300, (d) C-TP-180, (e) C-TP-240, and (f) C-TP-300.
According to the mechanism of metal-catalyzed graphitization,33 the free movement of a small number of Na-based catalysts (20 wt %) between crystalline layers within hydrochar with the lack of oxygen-containing structure can promote the transformation of its amorphous carbon into graphitized carbon during chemical activation. Then, a lot of amorphous carbon and crystalline layers are intricately arranged in C-TP-180/240/300, as shown in Figure 5d–f, indicating a clear increase of disordered crystalline carbon and a lot of amorphous carbon with an increase in pyrolysis pretreatment temperature. Our previous studies found that oxygen functional groups could fix the catalysts to perform catalytic cracking rather than catalytic graphitization, leading to the formation of more amorphous regions in oxidized samples.14
2.7. Pore Structure Analysis of C-HTC/TP-180/240/300
The N2 adsorption isotherm of C-HTC/TP-180/240/300 and their pore-size distribution are shown in Figure 6, and the corresponding pore structure parameters are given in Table 4. First, according to the IUPAC classification, the N2 adsorption isotherms of C-HTC-180/240/300 are all related to type I, revealing the typical microporous materials. More concretely, because of the high mobility of alkali metals in biomass/coal, when the pressure generated by the movement of Na exceeds the van der Waals force between the layers, the graphite layers were peeled off and then micropores are formed between the layers.32 In addition, the N2 adsorption capacity of C-HTC-180/240/300 at low pressures increases evidently, and C-HTC-180/240/300 have a rapid increase in Vt, Vmic, and SBET values and a little change in the non-Vmic value, as shown in Table 4, revealing the constant formation of a lot of micropores. Meanwhile, the knees of N2 adsorption isotherms of C-HTC-240 and C-HTC-300 gradually become broader, presenting an obvious increase in the size range from 0 to 4 nm in Figure 6b. The removal of a lot of oxygen-containing structures during hydrothermal pretreatment can greatly reduce the van der Waals force between the layers to enhance the free movement of Na between the layers, resulting in the formation of more micropores. Therefore, a small amount of Na-based catalysts (20 wt %) can significantly strengthen the stripping effect between layers of hydrochar to form a lot of micropores during chemical activation. Alternatively, adsorption isotherms of C-TP-180/240/300 all exhibit types I and IV, and they begin to branch and exhibit a hysteresis loop. These changes indicate simultaneous existence of mesopores and micropores (namely, the hierarchical porous materials). Then, the N2 adsorption capacities of C-TP-240 and C-TP-300 at low pressures decrease gradually, and the corresponding steeper isotherms and larger hysteresis loops also are observed as the relative pressure increases.
Figure 6.
(a, c) N2 adsorption isotherm and (b, d) pore-size distribution of C-HTC/TP-180/240/300.
Table 4. Pore Structure Parameters of C-HTC/TP-180/240/300.
| parameter | C-HTC-180 | C-HTC-240 | C-HTC-300 | C-TP-180 | C-TP-240 | C-TP-300 |
|---|---|---|---|---|---|---|
| SBET (m2/g) | 1217.55 | 1588.46 | 1945.33 | 718.45 | 608.54 | 513.12 |
| Vt (m3/g) | 0.705 | 0.813 | 0.998 | 0.757 | 0.897 | 1.221 |
| Vmic (m3/g) | 0.656 | 0.756 | 0.919 | 0.401 | 0.348 | 0.311 |
| non-Vmic (%) | 6.95 | 7.01 | 7.92 | 47.03 | 61.20 | 74.53 |
There is a significant increase in Vt and non-Vmic values and a rapid decrease in SBET and Vmic values for C-TP-180/240/300 with wider size distribution from 20 to 100 nm; these changes indicate their mesopore development during chemical activation. Our previous studies13,14 found that the evolution of oxygen-containing structures in char during a high temperature was more conducive to the development of mesopores. Then, the micropore development was closely related to the free movement of the Na-based catalyst between the layers. Therefore, the results of N2 adsorption indicate that the hydrothermal pretreatment-assisted chemical activation method is useful in preparing the ideal porous materials, such as a high SBET of 1945.33 m2/g, Vt of 0.998 m3/g, and Vmic of 0.919 m3/g values for C-HTC-300.
2.8. Electrochemical Measurements
According to the results and analysis from Sections 2.5–2.7, C-HTC-300 is the most suitable electrode material in all samples owing to its high SBET and Vmic values and highly ordered microstructure. To determine the electrochemical advantages of C-HTC-300, we also compare the supercapacitive performances of C-TP-300, and the corresponding results of the electrochemical experiment are given in Figure 7.
Figure 7.
Electrochemical performances of C-HTC-300 and C-TP-300 in the 6 M KOH electrolyte. (a) CV curves of C-HTC-300 at the scan rates of 20 to 200 mV s–1; (b) CV curves of C-HTC-300 and C-TP-300 at the scan rates of 100 mV s–1; (c) galvanostatic charge–discharge curves of C-HTC-300 at different current densities; (d) gravimetric capacitances of C-HTC-300 and C-TP-300 at different current densities; and (e) Nyquist plots of C-HTC-300 and C-TP-300.
The cyclic voltammetry (CV) curves of the C-HTC-300 electrode always present an excellent rectangular characteristic, as shown in Figure 7a, indicating that the charge exchange rate between the electrolyte and active substance is constant. At different scanning rates from 20 to 200 mV s–1, the current of the C-HTC-300 electrode can turn quickly, showing that the electrode has small internal resistance and good reversibility in the charge–discharge process.18 Even when the scanning rate increases to 200 mV s–1, the C-HTC-300 electrode also has a quasi-rectangular shape, indicating that there are still more protons or ions in the C-HTC-300 electrode to enter the bulk phase for charge exchange. The CV curves of C-HTC-300 and C-TP-300 electrodes at 100 mV s–1 are shown in Figure 7b; the distorted CV curve of the C-TP-300 electrode indicates deteriorative electrolyte ion transfer and adsorption.34 Then, the rectangular area of C-HTC-300 in the CV curve is much larger than that of the C-TP-300 electrode, indicating that the C-HTC-300 electrode has a better capacitive performance. The galvanostatic charge–discharge curves of the C-HTC-300 electrode at different current densities are shown in Figure 7c, and its isosceles triangle shape presents a slight deformation due to polarization; this change indicates the excellent charge and discharge qualities for an electric double-layer capacitor (EDLC).35Figure 7d shows the specific capacitances of C-HTC-300 and C-TP-300 electrodes at different current densities, in which the C-HTC-300 electrode always presents higher capacitances relative to the C-TP-300 electrode at the same current densities. More concretely, when the current density is 1 A g–1, the gravimetric capacitances of C-HTC-300 and C-TP-300 electrodes are ∼230 and 101 F g–1, respectively. Then, the C-HTC-300 electrode exhibits slight capacitance decay with the increase in current density, but visible capacitance decay for the C-TP-300 electrode can be found. Finally, the C-HTC-300 electrode still presents a high specific capacitance (170 F g–1) even at a current density of 50 A g–1, indicating an excellent charge–discharge capability performance of the C-HTC-300 electrode. Nyquist plots of the C-HTC-300 and C-TP-300 electrodes of the frequency range from 10 mHz to 100 kHz are shown in Figure 7e. C-HTC-300 and C-TP-300 electrodes all display semicircles in the high-frequency region, suggesting charge transfer resistances in both samples. It is noteworthy that the C-HTC-300 electrode displays a high slope in the low-frequency region and a relatively small semicircle radius in the high-frequency region relative to those of the C-TP-300 electrode, indicating a low charge transfer resistance for the C-HTC-300 electrode. A lot of micropores and the highly ordered microstructure of the C-HTC-300 can greatly promote the ion/electron conductivity within the carbon material to enhance the rate performance.36 The results of electrochemical measurements directly prove that the C-HTC-300 electrode material prepared by the hydrothermal pretreatment-assisted chemical activation method exhibits an excellent electrochemical performance.
3. Conclusions
This paper has demonstrated a novel hydrothermal pretreatment-assisted chemical activation method using Chinese large-reserve lignite coal and a small amount of Na-based catalysts (20 wt %) to prepare highly porous graphitic activated carbon (HPGAC) as electrode materials for supercapacitors. In the pretreatment stage, we compare the differences in the physical–chemical properties of hydrochar and pyrochar. The hydrothermal pretreatment can well control the depolymerization and reorganization of the coal framework, during which a large amount of oxygen-containing structures have been removed basically, and a lot of aliphatic structure also have been formed in HTC-180/240/300. At the same pretreatment temperature, hydrochar has lower solid yield, volatile and ash content, and higher fixed carbon content than pyrochar. With the increase in pretreatment temperature from 180 to 300 °C, the removal of a lot of oxygen functional groups, the formation of a disordered crystal structure, and a smooth surface appearance all indicate that the deoxidation effect of hydrothermal carbonization (HTC) on hydrochar is more significant; however, the dehydrogenation effect of traditional pyrolysis (TP) on pyrochar is more remarkable at the same temperature. In the subsequent chemical activation stage, such a microstructure of hydrochar not only strengthens the rapid condensation of microcrystals but also promotes free movement of the Na-based catalyst between the layers to enable its graphitization effect, which leads to the formation of a lot of ordered graphene-like structures and micropores even under a small amount of Na-based catalysts (20 wt %). The as-obtained C-HTC-300 with a highly ordered microstructure and high specific surface area (SBET of 1945.33 m2/g) exhibits an excellent electrochemical performance, including high capacitances, good rate capability, and excellent cycling stability. Therefore, the hydrothermal pretreatment method assisted by a small amount of metal catalysts for producing the coal-based HPGAC as electrode materials meets the environmental protection and low-cost preparation requirements.
4. Experimental Section
4.1. Sample Pretreatment
Huolinhe lignite collected from Inner Mongolia in China was shattered in a mechanical grinder and sieved to obtain a particle size of 180–250 μm (denoted as HLH). The proximate and ultimate analyses of HLH are given in Table 5.
Table 5. Proximate and Ultimate Analyses of HLH (wt %).
| Vd | FCd | Ad | Mad | Cdaf | Hdaf | Oa | Ndaf | Sdaf |
|---|---|---|---|---|---|---|---|---|
| 36.06 | 56.60 | 7.34 | 23.62 | 65.90 | 15.50 | 16.14 | 1.46 | 1.00 |
By difference; d (dry basis): the coal in anhydrous state was used as a benchmark; ad (air-dried basis): the coal in equilibrium with air humidity was used as a benchmark; daf (dry ash-free basis): the remaining component after the removal of water and ash in coal was used as a benchmark.
4.2. Experimental Arrangement
For the hydrothermal pretreatment experiment, 5 g of HLH and 50 mL of deionized water were mixed and placed in a microform high-pressure autoclave (100 mL). This reaction autoclave was closed tightly, heated to the setting temperature (180, 240, and 300 °C, respectively) at the speed of 5 °C/min, and held for 1 h. After naturally cooling them down to room temperature, the solid products in mixtures were obtained by filtration; the as-obtained hydrochar was marked as HTC-different temperature. For the traditional pyrolysis pretreatment experiment, 10 g of HLH was heated to the setting temperature (180, 240, and 300 °C, respectively) at the speed of 5 °C/min under a nitrogen (99.999%) flow of 400 mL/min in a horizontal tube reactor and held for 3 h. After naturally cooling it down to room temperature, the as-obtained pyrochar was marked as TP-different temperature. For the chemical activation experiment, 6 g of HTC/TP-different temperature and 1.5 g of NaOH (Kemiou, Tianjin, China) powder were mixed adequately into a homogeneous sample by the grinding method. The mixtures were heated to 900 °C at a constant rate of 10 °C/min in a nitrogen (99.999%) flow of 400 mL/min and held for 4 h before cooling down to ambient temperature. Na-based compounds in samples were removed by using 0.2 M HCl and thoroughly rinsing off acid solution with distilled water, and the as-obtained samples were marked as C-HTC/TP-different temperature.
4.3. Measurement Analysis
The microstructure of all samples was measured by TEM (Hitachi, H-9500, 300 kV). SEM (Hitachi, Regulus 8200, 200 keV) was used to obtain the surface topography of samples. The information of functional groups of samples was measured by FTIR (BRUKER, TENSOR II) at a resolution of 4 cm–1 and a scanning range from 500 to 4000 cm–1. The crystal information of samples was measured by XRD (Shimadzu, 6100) at 3°/min and a 2θ range from 15° to 60°. Raman analysis (Renishaw inVia, 532 nm wavelength) was used to obtain the carbon structures of samples. N2 adsorption/desorption analysis was measured using the N2 absorption apparatus (ASAP2020) under 77 K, and the parameters of the pore structure were calculated by corresponding formulas in the previous literature.37,38
4.4. Electrochemical Measurements
A three-electrode system with a basic aqueous solution (6 M KOH) was used to test the electrochemical performance of as-obtained samples. For preparing the electrodes, first, the mixtures including samples (80 wt %), carbon black (10 wt %), and polytetrafluoroethylene (PTFE) (10 wt %) were obtained by mechanical stirring and then further filled in a nickel foam and dried at 100 °C for 12 h in a vacuum oven. Finally, a thin sheet as the working electrode was made by pressing the above samples at 8 MPa for 2 min; each working electrode usually included about 3.0 mg of active materials. Moreover, the counter electrode and reference electrode were platinum foil and a saturated calomel electrode (SCE), respectively. The electrochemistry test, including galvanostatic charge/discharge (GC), cyclic voltammetry (CV), and electrical impedance spectroscopy (EIS), was performed by a CHI660E at room temperature. Specifically, Cs = IΔt/(mΔV) was used to calculate the specific capacitance (Cs, F g–1) of the sample. The I value means the constant discharge current, the Δt value means the discharge time, m means the weight of active substances, and ΔV means the voltage window in the above formula.39
Acknowledgments
This study was financially supported by National Natural Science Foundation of China (51806080), Jilin Province Education Department Science and Technology Program during the Thirteenth Five-year Plan Period (JJKH20200325KJ), and Scientific Research Fund Project of Jilin Agricultural University (201801).
The authors declare no competing financial interest.
References
- Winter M.; Brodd R. J. What are batteries, fuel cells, and supercapacitors. Chem. Rev. 2004, 104, 4245–4270. 10.1021/cr020730k. [DOI] [PubMed] [Google Scholar]
- Peng X.; Peng L.; Wu C.; Xie Y. Two dimensional nanomaterials for flexible supercapacitors. Chem. Soc. Rev. 2014, 43, 3303–3323. 10.1039/c3cs60407a. [DOI] [PubMed] [Google Scholar]
- Wang D.-W.; Li F.; Liu M.; Lu G. Q.; Cheng H.-M. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem., Int. Ed. 2008, 47, 373–376. 10.1002/anie.200702721. [DOI] [PubMed] [Google Scholar]
- Ma F.; Ma D.; Wu G.; Geng W.; Shao J.; Song S.; Wan J.; Qiu J. Construction of 3D nanostructure hierarchical porous graphitic carbons by charge-induced self-assembly and nanocrystal assisted catalytic graphitization for supercapacitors. Chem. Commun. 2016, 52, 6673–6676. 10.1039/C6CC02147F. [DOI] [PubMed] [Google Scholar]
- Li Y.-T.; Pi Y.-T.; Lu L.-M.; Xu S.-H.; Ren T.-Z. Hierarchical porous active carbon from fallen leaves by synergy of K2CO3, and their supercapacitor performance. J. Power Sources 2015, 299, 519–528. 10.1016/j.jpowsour.2015.09.039. [DOI] [Google Scholar]
- Liu D.; Gao J.; Wu S.; Qin Y. Effect of char structures caused by varying the amount of FeCl3 on the pore development during activation. RSC Adv. 2016, 6, 87478–87485. 10.1039/C6RA14712G. [DOI] [Google Scholar]
- Mahmoudian L.; Rashidi A.; Dehghani H.; Rahighi R. Single-step scalable synthesis of three-dimensional highly porous graphene with favorable methane adsorption. Chem. Eng. J. 2016, 304, 784–792. 10.1016/j.cej.2016.07.015. [DOI] [Google Scholar]
- Chen C.; Yu D.; Zhao G.; Du B.; Tang W.; Sun L.; Sun Y.; Besenbacher F.; Yu M. Three-dimensional scaffolding framework of porous carbon nanosheets derived from plant wastes for high-performance supercapacitors. Nano Energy 2016, 27, 377–389. 10.1016/j.nanoen.2016.07.020. [DOI] [Google Scholar]
- Wang L.; Sun F.; Gao J.; Pi X.; Pei T.; Qie Z.; Zhao G.; Qin Y. A novel melt infiltration method promoting porosity development of low-rank coal derived activated carbon as supercapacitor electrode materials. J. Taiwan. Inst. Chem. Eng. 2018, 91, 588–596. 10.1016/j.jtice.2018.06.014. [DOI] [Google Scholar]
- de la Puente G.; Marbán G.; Fuente E.; Pis J. J. Modelling of volatile product evolution in coal pyrolysis. The role of aerial oxidation. J. Anal. Appl Pyrolysis 1998, 44, 205–218. 10.1016/S0165-2370(97)00078-8. [DOI] [Google Scholar]
- Yürüm Y.; Altuntaş N. Air oxidation of beypazari lignite at 50°C, 100°C and 150°C. Fuel 1998, 77, 1809–1814. 10.1016/S0016-2361(98)00067-2. [DOI] [Google Scholar]
- Ndaji F. E.; Thomas K. M. The effects of oxidation on the macromolecular structure of coal. Fuel 1995, 74, 932–937. 10.1016/0016-2361(95)00019-2. [DOI] [Google Scholar]
- Liu D.; Gao J.; Cao Q.; Wu S.; Qin Y. Improvement of activated carbon from Jixi Bituminous coal by air preoxidation. Energy Fuels 2017, 31, 1406–1415. 10.1021/acs.energyfuels.6b02875. [DOI] [Google Scholar]
- Liu D.; Jia B.; Liu X. .; Zhao B.; Gao J.; Cao Q.; Wu S.; Qin Y. Effects of oxygen functional groups and FeCl3 on the evolution of physico-chemical structure in activated carbon obtained from Jixi bituminous coal. RSC Adv. 2018, 8, 8569–8579. 10.1039/C7RA12928A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasebi A.; Yu J.; Han Y.; Li X. A study of chemical structure changes of Chinese lignite during fluidized-bed drying in nitrogen and air. Fuel Process. Technol. 2012, 101, 85–93. 10.1016/j.fuproc.2012.04.005. [DOI] [Google Scholar]
- Oliveira I.; Blöhse D.; Ramke H.-G. Hydrothermal carbonization of agricultural residues. Bioresour. Technol. 2013, 142, 138–146. 10.1016/j.biortech.2013.04.125. [DOI] [PubMed] [Google Scholar]
- Petrović J.; Perišić N.; Maksimović J. D.; Maksimović V.; Kragović M.; Stojanović M.; Laušević M.; Mihajlović M. Hydrothermal conversion of grape pomace: Detailed characterization of obtained hydrochar and liquid phase. J. Anal. Appl. Pyrolysis 2016, 118, 267–277. 10.1016/j.jaap.2016.02.010. [DOI] [Google Scholar]
- He C.; Giannis A.; Wang J. Y. Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior. Appl. Energy 2013, 111, 257–266. 10.1016/j.apenergy.2013.04.084. [DOI] [Google Scholar]
- Wu R. M. Application of subcritical water extraction in analytical chemistry. Prog. Chem. 2002, 14, 32–36. [Google Scholar]
- Ma Z.; Bai J.; Li W.; Bai Z.; Kong L. Mineral transformation in char and its effect on coal char gasification reac-tivity at high temperatures, part 1: mineral transformation in char. Energy Fuels 2013, 27, 4545–4554. 10.1021/ef4010626. [DOI] [Google Scholar]
- Sannigrahi P.; Ragauskas A. J.; Tuskan G. A. Poplar as a feedstock for biofuels: A review of compositional characteristics. Biofuels, Bioprod. Biorefin. 2010, 4, 209–226. 10.1002/bbb.206. [DOI] [Google Scholar]
- Kang S.; Li X.; Fan J.; Chang J. Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, D-xylose, and wood meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. 10.1021/ie300565d. [DOI] [Google Scholar]
- Liu D. D.; Jia B. Y.; Li S.; Dong L. J.; Gao J. H.; Qin Y. K. Effect of pyrolysis conditions on the improvement of the physicochemical structure of activated carbon obtained from Jixi bituminous coal. Asia-Pac. J. Chem. Eng. 2019, e2289 10.1002/apj.2289. [DOI] [Google Scholar]
- Zhang J. H.; Lin Q. M.; Zhao X. R. The hydrochar characters of municipal sewage sludge under different hydrothermal temperatures and durations. J. Integr. Agric. 2014, 13, 471–482. 10.1016/S2095-3119(13)60702-9. [DOI] [Google Scholar]
- Meng F.; Yu J.; Tahmasebi A.; Han Y.; Zhao H.; Lucas J.; Wall T. Characteristics of chars from low-temperature pyrolysis of lignite. Energy Fuels 2014, 28, 275–284. 10.1021/ef401423s. [DOI] [Google Scholar]
- Ibarra J.; Muñoz E.; Moliner R. FTIR study of the evolution of coal structure during the coalification process. Org. Geochem. 1996, 24, 725–735. 10.1016/0146-6380(96)00063-0. [DOI] [Google Scholar]
- Sonibare O. O.; Haeger T.; Foley S. F. Structural characterization of Nigerian coals by X-ray diffraction, Raman and FTIR spectroscopy. Energy 2010, 35, 5347–5353. 10.1016/j.energy.2010.07.025. [DOI] [Google Scholar]
- Nomura S.; Thomas K. M. Fundamental aspects of coal structural changes in the thermoplastic phase. Carbon 1998, 77, 829–836. 10.1016/S0016-2361(97)00259-7. [DOI] [Google Scholar]
- Yu Y.; Lei Z.; Yang X.; Yang X.; Huang W.; Shimizu K.; Zhang Z. Hydrothermal carbonization of anaerobic granular sludge: effect of process temperature on nutrients availability and energy gain from produced hydrochar. Appl. Energy. 2018, 229, 88–95. 10.1016/j.apenergy.2018.07.088. [DOI] [Google Scholar]
- Zhai Y.; Liu X.; Zhu Y.; Peng C.; Wang T.; Zhu L.; Li C.; Zeng G. Hydrothermal carbonization of sewage sludge: the effect of feed-water pH on fate and risk of heavy metals in hydrochars. Bioresour. Technol. 2016, 218, 183–188. 10.1016/j.biortech.2016.06.085. [DOI] [PubMed] [Google Scholar]
- Liu D.; Su R.; Hao Z.; Zhao X.; Jia B.; Dong L. Catalytic effect of NaCl on the improvement of the physicochemical structure of coal-based activated carbons for SO2 adsorption. Processes 2019, 7, 338. 10.3390/pr7060338. [DOI] [Google Scholar]
- Liu D.; Zhao X.; Su R.; Hao Z.; Jia B.; Li S.; Dong L. Highly porous graphitic activated carbons from lignite via microwave pretreatment and iron-catalyzed graphitization at low-temperature for supercapacitor electrode materials. Processes 2019, 7, 300. 10.3390/pr7050300. [DOI] [Google Scholar]
- Mori H.; Asami K.; Ohtsuka Y. Role of iron catalyst in fate of fuel nitrogen during coal pyrolysis. Energy Fuels 1996, 10, 1022–1027. 10.1021/ef960035d. [DOI] [Google Scholar]
- Raymundo-Piñero E.; Cadek M.; Béguin F. Tuning carbon materials for supercapacitors by direct pyrolysis of seaweeds. Adv. Funct. Mater. 2009, 19, 1032–1039. 10.1002/adfm.200801057. [DOI] [Google Scholar]
- Liu H.-J.; Wang J.; Wang C.-X.; Xia Y.-Y. Ordered hierarchical mesoporous/microporous carbon derived from mesoporous titanium-carbide/carbon composites and its electrochemical performance in supercapacitor. Adv. Energy Mater. 2011, 1, 1101–1108. 10.1002/aenm.201100255. [DOI] [Google Scholar]
- He X.; Geng Y.; Qiu J.; Zheng M.; Long S.; Zhang X. Effect of activation time on the properties of activated carbons prepared by microwave-assisted activation for electric double layer capacitors. Carbon 2010, 48, 1662–1669. 10.1016/j.carbon.2010.01.016. [DOI] [Google Scholar]
- Yang K.; Peng J.; Xia H.; Zhang L.; Srinivasakannan C.; Guo S. Textural characteristics of activated carbon by single step CO2 activation from coconut shells. J. Taiwan Inst. Chem. Eng. 2010, 41, 367–372. 10.1016/j.jtice.2009.09.004. [DOI] [Google Scholar]
- Pietrzak R. XPS study and physico-chemical properties of nitrogen-enriched microporous activated carbon from high volatile bituminous coal. Fuel 2009, 88, 1871–1877. 10.1016/j.fuel.2009.04.017. [DOI] [Google Scholar]
- Wang L.; Mu G.; Tian C.; Sun L.; Zhou W.; Yu P.; Yin J.; Fu H. Porous graphitic carbon nanosheets derived from cornstalk biomass for advanced supercapacitors. ChemSusChem 2013, 6, 880–889. 10.1002/cssc.201200990. [DOI] [PubMed] [Google Scholar]