Abstract
To thoroughly evaluate the quality of Citri Sarcodactylis Fructus (CSF) and acquire knowledge of the lipophilic components of CSF from different origins, a simple and efficient approach based on supercritical fluid extraction (SFE) combined with ultraperformance liquid chromatography plus Q-Exactive Orbitrap tandem mass spectrometry (UPLC-Q-Exactive Orbitrap/MS) detection for the discrimination of components from CSF was set up for the first time in this work. Eight batches of CSF samples from five main producing areas were extracted by SFE under optimized conditions, and then SFE extracts were dissected via UPLC-Q-Exactive Orbitrap/MS. The results indicated that 39 lipophilic compounds were successfully separated and unambiguously or tentatively identified, where 4 coumarins, 6 polymethoxyflavones, 3 phthalides, 6 terpenes, and 4 phenolics were not reported formerly. It was illustrated that CSF may be abundant in polymethoxyflavones, as in coumarins. Moreover, there were significant differences in the components of CSF from different origins. Especially, coumarin, dehydrocostus lactone, atractylenolide II, and atractylenolide I were exclusively found in CSF from the Guangdong province; isopsoralen was almost exclusively found in CSF from the Guangxi province; and ferulic acid was exclusively found in CSF from the Zhejiang province. These observations indicated that SFE joint with UPLC-Q-Exactive Orbitrap/MS owing to the potential of characterizing the lipophilic components could be used to promote quality assessment and chemotaxonomic investigation in phytology sciences of CSF.
1. Introduction
Citri Sarcodactylis Fructus (CSF), the dried fruit of Citrus medica L. var. sarcodactylis Swingle, which belongs to Citrus botany in Rutaceae,1 is widely cultivated in Guangdong, Guangxi, Zhejiang, Sichuan, and Yunnan provinces of China.2 It can be utilized as a traditional medicine for the cure of stomach ache, headache, edema, rheumatism, arthritis, and infectious hepatitis3 and also as a tonic material to make crispy cookies.4 As it is considered to be one of the homologies in medicine and food, CSF is full of potential for vast development and application prospects.5 However, the chemical composition of CSF from different origins has not been fully clarified. Therefore, a rapid and efficient method should be built for the systematic analysis and identification of phytochemicals in CSF.
As reported previously, many compounds such as coumarins and limonoids were separated and distinguished in the CSF extracts by use of methanol,6−8 although it is worth considering that some lipophilic components like nonpolar and low-polarity substances may not be easily obtained by the use of common organic solvents. Up to now, there have been a few studies wherein supercritical fluid CO2 extraction (SFE) was used to extract the chemical compounds of CSF prior to ingredient analysis. SFE is a low-temperature extraction technology and widely implemented to extract chemical components in plants, due to the potential to extract nonpolar and low-polarity substances.9,10 In addition, although high-performance liquid chromatography mass spectrometry (HPLC-MS) and gas chromatography mass spectrometry (GC-MS) are usually used for the analysis of plant chemicals, recently, ultraperformance liquid chromatography plus Q-Exactive Orbitrap tandem mass spectrometry (UPLC-Q-Exactive Orbitrap/MS) has proved to be a more rapid, efficient, and sensitive implement than HPLC-MS and GC-MS in the field of the analysis of extracts and bioactive constituents from medicine and food plants.11,12
Herein, the chemical components of CSF were extracted by SFE and discriminated via UPLC-Q-Exactive Orbitrap/MS technology in eight batches of CSF samples from several main producing areas in China. Notably, 39 lipophilic components were isolated and identified, some of which were first reported, for instance, 4 coumarins, 6 polymethoxyflavones (PMFs), and 3 phthalides. This study could lay the foundation for the quality evaluation and chemotaxonomic exploration of CSF in phytology sciences.
2. Results
2.1. Optimization of SFE and UPLC-Q-Exactive Orbitrap/MS Conditions
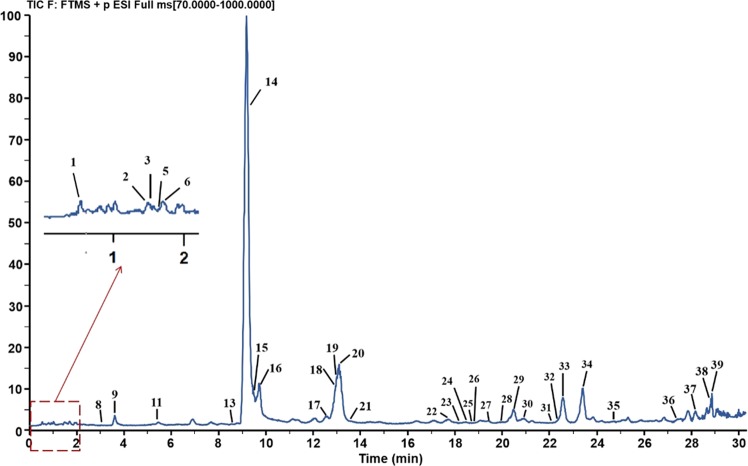
The SFE extracts of 8 batches of CSF (seen from Table 1) were analyzed via UPLC-Q-Exactive Orbitrap/MS. To acquire more precise information about compounds of CSF, analytical conditions were determined by optimizing a sequence of parameters like the elution gradient and flow rate of the mobile phase, whereby the components of SFE extracts in CSF were able to be isolated in the positive mode where the detection signal was better than that in the negative-ion mode within 30 min. The application method was efficient, and the mean SFE extraction rate of CSF was 1.42% (1.19–1.73%). Herein, the total ion chromatogram (TIC) of samples and mixed standard substances are displayed in Figure 1. Totally, 39 compounds were separated and identified and labeled 1–39 in accordance with the sequence of the peak time (seen in Table 2). In general, these extraction constituents were divided into several groups consisting of 14 coumarins, 7 PMFs, 3 phthalides, 3 limonoids, 7 terpenes, and 5 phenolics. Among these, 7 compounds were discerned via retention times and fragments by comparison with standard substances and 32 compounds were distinguished according to MS2 positive-ion fragments and the related information from these studies was reported formerly. All of these chemical structures are displayed in Figure 2.
Table 1. Representative Samples of CSF Investigated in This Studya.
| no. | collecting area (name) | sample source | collection time (yy/mm/dd) |
|---|---|---|---|
| S1 | Guangdong province | Lecheng town, Zhaoqing city, Guangdong province | 2019/09/25 |
| S2 | Guangxi province | Yongfu county, Guilin city, Guangxi province | 2019/10/29 |
| S3 | Zhejiang province | Luodian town, Jinhua city, Zhejiang province | 2019/11/07 |
| S4 | Zhejiang province | Chisong town, Jinhua city, Zhejiang province | 2019/11/15 |
| S5 | Sichuan province | Huidong county, Liangshan prefecture, Sichuan province | 2019/10/21 |
| S6 | Sichuan province | Peng’an county, Nanchong city, Sichuan province | 2019/11/17 |
| S7 | Sichuan provinceb | Baiyang town, Wanzhou county, Chongqing city | 2019/10/28 |
| S8 | Yunnan province | Huaning county, Yuxi city, Yunnan province | 2019/10/28 |
Guangdong province, Guangxi province, Zhejiang province, Sichuan province, and Yunnan province are the main producing areas.
Collecting area of Chongqing city was classified into main producing area of the Sichuan province by considering the geography.
Figure 1.
Representative total ion chromatogram of CSF in positive ionization mode. Compound 6: scopoletin; compound 9: scoparone; compound 14: 5,7-dimethoxycoumarin; compound 16: limonin; compound 20: nomilin; compound 29: nobiletin; compound 33: tangeretin.
Table 2. Compounds Identified in CSF by UPLC-Q-Exactive Orbitrap/MSaFour.
| no. | tR (min) | theoretical [M + H]+ (m/z) | experimental [M + H]+ (m/z) | major secondary fragment ions (MS/MS) | compound formula | proposed compound | ref |
|---|---|---|---|---|---|---|---|
| Coumarins | |||||||
| 3 | 1.51 | 193.0495 | 193.0498 | 178.0263, 165.0548, 161.0539, 150.0313, 147.1172, 137.0599, 133.0286, 122.0365, 117.0337, 109.0651, 105.0339, 94.0420, 77.0393, 66.0472, 56.9658 | C10H8O4 | isoscopoletin | (14) |
| 6 | 1.70 | 193.0495 | 193.0498 | 178.0263, 165.0549, 161.0235, 150.0313, 137.0598, 133.0286, 122.0365, 117.0336, 105.0341, 89.0391, 77.0393, 66.0471, 53.0395 | C10H8O4 | scopoletin | c |
| 8 | 3.17 | 147.0440 | 147.0443 | 132.9590, 119.0858, 114.9491, 105.0703, 103.0546, 95.0494, 91.0548, 84.9603, 79.0553, 73.9930, 65.0393, 60.9770, 53.0395 | C9H6O2 | coumarin | (15) |
| 9 | 3.60 | 207.0651 | 207.0654 | 192.0417, 191.0340, 181.4489, 179.0704, 163.0390, 151.0755, 148.0520, 136.0520, 121.0651, 107.0496, 91.0549, 79.0549 | C11H10O4 | scoparoneb | c |
| 10 | 3.96 | 223.0601 | 223.0604 | 208.0370, 190.0263, 179.0343, 162.0314, 153.0105, 149.0964, 134.0364, 125.0154, 106.0418, 91.0547, 78.0472,58.0658 | C11H10O5 | fraxinolb | (16) |
| 11 | 5.50 | 177.0546 | 177.0547 | 162.0311, 149.0598, 133.0648, 121.0650, 118.0416, 103.0546, 91.0548, 79.0549, 66.0472, 53.0394 | C10H8O3 | 7-methoxycoumarin | (17) |
| 12 | 6.86 | 187.0389 | 187.0392 | 173.1181, 159.0441, 143.0493, 131.0494, 115.0546, 109.1017, 103.0548, 95.0495, 88.0224, 67.0550, 55.0550 | C11H6O3 | isopsoralenb | (18) |
| 13 | 8.71 | 305.1019 | 305.1020 | 287.1166, 203.0340, 192.0297, 175.0392, 159.0441, 147.0441, 131.0493, 119.0494, 91.0548, 59.0500 | C16H16O6 | oxypeucedan hydrate | (19) |
| 14 | 9.18 | 207.0651 | 207.0654 | 192.0418, 179.0703, 164.0469, 151.0755, 148.0520, 133.0650, 121.0651, 118.0471, 103.0547, 91.0548, 79.0549, 65.0393 | C11H10O4 | 5,7-dimethoxycoumarinb | c |
| 15 | 9.57 | 217.0495 | 217.0495 | 202.0261, 174.0312, 173.0598, 167.9620, 161.0596, 146.0361, 131.0491, 115.0545, 105.0704, 91.0548, 79.0549, 53.4664 | C12H8O4 | bergapten | (7) |
| 17 | 12.98 | 317.1019 | 317.1020 | 299.0896, 273.075, 245.0441, 233.0446, 218.0211, 203.0339, 188.0106, 175.0392, 162.0310, 147.0441, 119.0496, 91.0548, 85.0655, 67.0550, 57.0706 | C17H16O6 | byakangelicol | (20) |
| 19 | 12.99 | 287.0914 | 287.0913 | 203.0338, 175.0390, 174, 159.0441, 147.0440, 131.0492, 119.0491, 91.0546, 85.0653, 67.0550, 59.0500 | C16H14O2 | oxypeucedanin | (20) |
| 34 | 23.40 | 193.0495 | 193.0497 | 178.0261, 165.0547, 149.0598, 137.0598, 134.0364, 121.0650, 109.0652, 106.0418, 91.0549, 79.0549, 67.0186, 53.0394 | C10H8O4 | 5,7-dihydroxy-4-methylcoumarinb | (21) |
| 38 | 28.77 | 163.0389 | 163.0391 | 149.0450, 139.9821, 135.0441, 119.0493, 107.0494, 95.0495, 91.0547, 84.9604, 79.0548, 68.9979, 61.6360, 53.0395 | C9H6O3 | 7-hydroxycoumarin | (22) |
| Methoxyflavonoids | |||||||
| 23 | 18.02 | 361.0917 | 361.0920 | 346.0685, 331.0449, 315.0510, 303.0501, 285.0396, 257.0445, 229.0495, 201.0546, 181.0133, 169.0133, 121.0288, 105.0336, 68.9977 | C18H16O8 | 5,7,3′-trihydroxy-6,4′,5′-trimethoxyflavone | (8) |
| 25 | 18.72 | 343.1176 | 343.1178 | 328.0944, 313.0707, 285.0758, 270.0524, 257.0806, 242.0570, 211.0751, 199.0237, 181.0132, 153.0183, 135.0442, 125.0235, 107.0126, 85.0287, 69.0339 | C19H18O6 | 6-demethoxytangeretinb | (24) |
| 28 | 19.93 | 331.0812 | 331.0812 | 316.0578, 301.0347, 285.0393, 273.0393, 257.0445, 242.0571, 214.0622, 186.063, 169.0133, 135.0443, 121.0650, 108.0572, 91.0549, 68.9977 | C17H14O7 | iristectorigeninab | (25, 26) |
| 29 | 20.43 | 403.1387 | 403.1388 | 388.1152, 373.0919, 358.0682, 355.0811, 327.0860, 301.0706, 284.0680, 258.0524, 229.0340, 211.0237, 193.0129, 183.0290, 165.0546, 127.0392, 99.0441, 69.0344 | C21H22O8 | nobiletinb | c |
| 30 | 20.92 | 331.0812 | 331.0813 | 316.0579, 301.0344, 288.0629, 273.0395, 257.0448, 245.0444, 229.0493, 199.0389, 169.0133, 148.0520, 135.0442, 121.0651, 108.0574, 86.9453 | C17H14O7 | jaceosidinb | (27) |
| 32 | 22.31 | 389.1230 | 389.1234 | 374.0998, 359.0763, 341.0659, 331.0810, 316.0582, 285.0755, 260.0676, 227.0552, 215.0189, 197.0083, 163.0755, 148.0521, 113.0238, 85.0285 | C20H20O8 | 5-demethylnobiletinb | (28) |
| 33 | 22.51 | 373.1281 | 373.1286 | 358.1050, 343.0816, 328.0579, 297.0762, 285.0401, 271.0603, 254.0570, 229.0322, 211.0240, 183.0292, 135.0443, 127.0392, 99.0441, 69.0339 | C20H20O7 | tangeretinb | c |
| Phthalides | |||||||
| 18 | 12.66 | 191.1066 | 191.1068 | 173.0963, 163.1328, 158.0728, 149.0598, 145.1012, 130.0778, 117.0702, 111.0443, 105.0702, 99.0444, 91.0547, 79.0549, 71.0489, 55.0551 | C12H14O2 | 3-n-butylphthalideb | (30, 31) |
| 21 | 13.61 | 193.1223 | 193.0498 | 175.1120, 157.1016, 147.1170, 137.0599, 133.0286, 123.0444, 119.0859, 109.0652, 105.0703, 97.0652, 93.0705, 91.0548, 81.0705, 69.0706, 67.0550, 53.0395 | C12H16O2 | senkyunolide Ab | (31) |
| 24 | 18.42 | 191.1066 | 191.1068 | 173.0963, 163.1118, 149.0599, 145.1013, 135.0442, 130.0778, 121.0650, 117.0702, 105.0703, 91.0548, 79.0549, 67.0550, 55.0549 | C12H14O2 | ligustilideb | (30, 31) |
| Limonoids | |||||||
| 16 | 9.69 | 471.2013 | 471.2013 | 453.1893, 435.1798, 425.1957, 409.2002, 391.1882, 367.1909, 339.1952, 213.0910, 205.0498, 187.0754, 169.1006, 161.0598, 133.0649, 119.0858, 105.0702, 95.0132, 79.0549, 69.0706 | C26H30O8 | limoninb | c |
| 20 | 13.08 | 515.2272 | 515.2272 | 497.2172, 469.2216, 455.2065, 437.1960, 419.1853, 411.2165, 393.2059, 369.2074, 341.2097, 279.1383, 243.1000, 231.0645, 187.0754, 161.0598, 133.0649, 105.0703, 95.0132, 79.0548, 69.0705 | C28H34O9 | nomilinb | c |
| 22 | 17.73 | 455.2064 | 455.2068 | 437.1958, 419.1862, 409.2012, 391.1910, 377.1368, 359.1272, 349.1434, 331.1335, 303.1391, 273.1281, 235.1120, 209.0965, 187.0757, 161.0599, 133.0650, 119.0860, 105.0703, 95.0133, 81.0341, 67.0551 | C26H30O7 | obacunone | (33) |
| Terpenoids | |||||||
| 5 | 1.66 | 153.1273 | 153.1274 | 135.1169, 125.0599, 120.9808, 111.0444, 109.1015, 107.0859, 95.0860, 93.0704, 88.9530, 84.9603, 81.0705, 79.0549, 65.0394, 59.0500, 55.0551 | C10H16O | camphor | d |
| 27 | 19.59 | 231.1379 | 231.1381 | 213.1277, 203.1432, 195.1168, 189.0915, 185.1328, 175.0752, 163.0391, 157.1013, 145.1015, 143.0857, 131.0858, 119.0859, 105.0702, 91.0548, 79.0549, 69.0707, 55.0550 | C15H18O2 | dehydrocostus lactoneb | (42) |
| 31 | 22.13 | 233.1536 | 233.1539 | 215.1434, 197.1322, 187.1486, 177.0912, 173.0965, 159.0810, 151.0756, 145.1015, 131.0858, 105.0703, 95.0861, 91.0549, 81.0706, 67.0549, 56.9656 | C15H20O2 | atractylenolide IIb | (34) |
| 35 | 24.70 | 231.1379 | 231.1380 | 213.1274, 203.1068, 189.0910, 185.1325, 175.0756, 161.1327, 157.1013, 147.0800, 143.0856, 129.0700, 105.0702, 91.0548, 81.0705, 67.0549, 55.0549 | C15H18O2 | atractylenolide Ib | (35) |
| 36 | 27.43 | 219.1743 | 219.1745 | 203.1433, 187.0761, 173.0234, 163.0390, 145.0284, 131.0857, 119.0858, 107.0859, 95.0860, 91.0547, 81.0705, 79.0549, 69.0706, 67.0550, 55.0551 | C15H22O | (+)-nootkatoneb | (43) |
| 37 | 28.37 | 219.1743 | 219.1745 | 203.1430, 191.1794, 187.0763, 173.1328, 161.1326, 145.1013, 135.1169, 119.0858, 107.0859, 95.0860, 93.0704, 91.0548, 81.0705, 67.0550, 55.0551 | C15H22O | α-cyperoneb | (44) |
| 39 | 28.85 | 219.1743 | 219.1746 | 203.1433, 201.1436, 191.1792, 187.0761, 175.1479, 161.1328, 159.1017, 151.1120, 145.1013, 133.1017, 121.1016, 107.0860, 95.0861, 91.0548, 81.0705, 67.0550, 55.0551 | C15H22O | germacroneb | (36) |
| Phenolics | |||||||
| 1 | 0.48 | 123.0553 | 123.0555 | 106.0292, 96.0448, 95.0498, 90.9482, 81.0706, 80.0501, 79.0549, 72.9379, 67.0550, 60.9389, 56.9430, 53.0395 | C6H6N2O | nicotinamideb | d |
| 2 | 1.49 | 153.0546 | 153.0547 | 135.1170, 125.0599, 111.0444, 107.0859, 93.0340, 84.9602, 81.0706, 79.0548, 71.0500, 65.0394, 59.0500 | C8H8O3 | vanillinb | (45) |
| 4 | 1.59 | 183.0651 | 183.0653 | 165.0911, 155.0704, 140.0468, 137.0959, 125.0235, 123.0443, 111.0443, 105.0451, 97.0284, 95.0496, 90.9480, 81.0704, 71.0501, 67.0550, 53.0394 | C9H10O4 | syringaldehydeb | (45) |
| 7 | 1.71 | 195.0651 | 195.0544 | 187.4464, 177.0549, 163.0392, 153.0553, 149.0599, 145.0286, 135.0443, 125.0599, 117.0339, 107.095, 95.0494, 89.0392, 84.9606, 79.0549, 63.0237 | C10H10O4 | ferulic acid | (46) |
| 26 | 18.85 | 179.0702 | 179.0710 | 161.0599, 147.0442, 136.0521, 133.0651, 119.0495, 115.0548, 105.0703, 95.0499, 91.0549, 84.9603, 79.0550, 65.0394, 55.0187 | C10H10O3 | ferulaldehydeb | (47) |
coumarins (compounds 9, 10, 12, and 34), 6 polymethoxyflavones (compounds 25, 28, 29, 30, 32, and 33), 3 phthalides (compounds 18, 21, and 24), 6 terpenes (compounds 27, 31, 35, 36, 37, and 39), and 4 phenolics (compounds 1, 2, 4, and 26) were not reported previously.
Compounds were not reported in CSF extracts previously.
Further confirms the comparison with standard compounds.
Refers to the database.
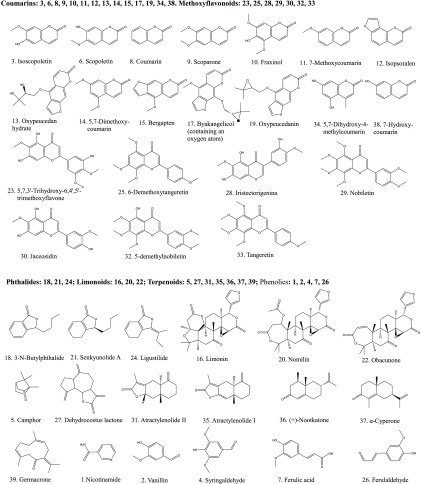
Figure 2.
Chemical structure of 39 compounds that were unequivocally and tentatively identified.
2.2. Identification of Coumarins
Coumarins are a kind of natural compound with benzo-α-pyranone parent nucleus, which are the primary constituents in the SFE extracts of CSF. Fourteen coumarins were identified in CSF extracts. In the positive mode, the fragmentation of coumarins from mass spectrometry was characterized by the loss of neutral molecules, such as CO, CO2, and CH3, because of high-energy collisions.13
Compound 3 produced a protonation ion at m/z 193.0498 and fragment ions at m/z 178.0263 [M + H – CH3]+, 165.0548 [M + H – CO]+, 150.0313 [M + H – CH3 – CO]+, and 147.1172 [M + H – CO2]+. Referring to mass spectrum cracking information from a previous study,14 compound 3 was identified as isoscopoletin. The precursor ion of compound 8 was m/z 147.0443, which gave daughter ions such as m/z 132.9590, m/z 119.0858, m/z 103.0546, and m/z 91.0548 successively. According to the structure cracking rule of coumarins, compound 8 was detailed to be coumarin as done previously.15 Meanwhile, compound 10 produced a strong molecular ion at m/z 223.0604 and formed fragments at m/z 208.0370 [M + H – CH3]+ and 179.0343 [M + H – CO2]+. Thus, compound 10 was tentatively identified as fraxinol from a previous study.16 Moreover, compound 11 produced a molecular ion at m/z 177.0547 and formed fragments at m/z 162.0311 [M + H – CH3]+, 133.0647 [M + H – CO2]+, and 121.0650 [M + H – 2CO]+. Therefore, compound 11 was identified as 7-methoxycoumarin as before.17
Compound 6, 9, and 14 were unambiguously identified as scopoletin, scoparone, and 5,7-dimethoxycoumarin, respectively, by referring to the document and the standard substance with the same fragmentation traits.
Compounds 12, 13, 15, 17, and 19 belong to the linear furanocoumarins that are rich in Citrus genus. Compound 12 produced a molecular ion at m/z 187.0392 and formed characteristic fragmentations at m/z 159.0441 [M + H – CO]+, 143.0493 [M + H – CO2]+, 131.0494 [M + H – 2CO]+, and 115.0546 [M + H – CO – CO2]+. Thus, compound 12 was identified as isopsoralen from a previous study.18 Because of an [M + H]+ ion at m/z 305.1020, the fragment ion at m/z 203.0340 indicating loss of the neutral substituent [M + H – C5H10O2]+, and sequential cleavage losing the CO radical and generating the typical [203-2CO]+ fragment at m/z 147.0441, compound 13 was tentatively identified as oxypeucedanin hydrate, which was in accordance with a previous report.19 Similarly, compound 15 generated a precursor ion m/z 217.0495, while m/z 202.0261, m/z 174.0312, m/z 173.0598, and m/z 146.0361 were characteristic fragment ions that matched with the MS2 information as reported earlier.7 Therefore, compound 15 was identified as bergapten (fragmentation pattern is shown in Figure 3A). Compound 17 exhibited a precursor ion at m/z 317.1020 and fragment ions at m/z 299.0896, m/z 273.0750, and m/z 233.0446, while compound 19 exhibited a precursor ion at m/z 287.0913 and fragment ions at m/z 203.0338 and m/z 175.0390. According to the MS2 information supported by the previous investigation,20 compounds 17 and 19 were confirmed to be byakangelicol and oxypeucedanin, respectively.
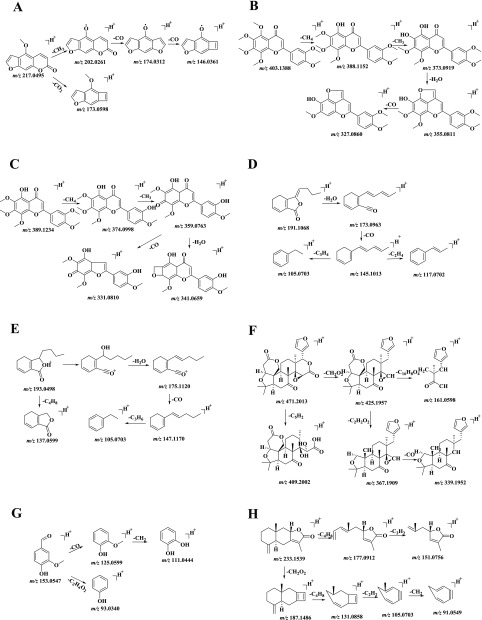
Figure 3.
Proposed fragmentation pathways of the main fragment ions in the positive-ion mode. (A) Bergapten; (B) nobiletin; (C) 5-demethylnobiletin; (D) ligustilide; (E) senkyunolide A; (F) limonin; (G) vanillin; and (H) atractylenolide II.
In addition, compound 34 exerted a protonated ion at m/z 193.0497 and fragment ions at m/z 178.0261, m/z 165.0547, m/z 149.0598, and m/z 137.0598. Thus, compound 34 was confirmed as 5,7-dihydroxy-4-methylcoumarin, as reported previously.21 Also, compound 38 produced a precursor ion at m/z 163.0391 and fragment ions at m/z 149.0450, m/z 135.0441, m/z 119.0493, and m/z 107.0494. Following the characteristic fragmentation profiles and a previous study,22 compound 38 was assigned as 7-hydroxycoumarin.
It was manifested that compounds 9, 10, 12, and 34 were not reported previously. These four coumarins have various functions; for example, compound 12 as isopsoralen has antibacterial, anti-inflammatory, antitumor, and antiosteoporotic properties.23
2.3. Identification of PMFs
PMFs are discovered nearly exclusively in Citrus species and possess the basic aglycone structure and are different from each other in the location and amount of substituted −OCH3 and/or −OH on the flavonoid mother nucleus. It was believed that characteristic fragment ions were formed by removal of −CH3, CO, and H2O. After scanning, seven PMFs were identified or tentatively discriminated in CSF.
Because of standard reference, compounds 29 and 33 were determined as nobiletin and tangeretin, respectively. The comprehensive fragmentation pattern of compound 29 is shown in Figure 3B, which included daughter ions at m/z 388.1152, m/z 373.0919, m/z 355.0811, and m/z 327.0860.
Compound 23 was assigned to be 5,7,3′-trihydroxy-6,4′,5′-trimethoxyflavone with reference to the literature,8 which gave a protonated molecular ion at m/z 361.0920 and fragment ions at m/z 346.0685, m/z 331.0449, and m/z 303.0501. Besides, compound 25 generated a protonated ion at m/z 343.1178 and fragment ions at m/z 328.0944, m/z 313.0707, and m/z 285.0758. Thus, compound 25 was indicated as 6-demethoxytangeretin from reference literature.24 Compound 28 was assigned as isoflavone for the protonated ion at m/z 331.0812 and fragment ions like m/z 316.0578, m/z 301.0347, m/z 285.0393, and m/z 273.0393. As a result, compound 28 was assigned as iristectorigenina from the literature reported.25,26 Compound 30 was plausibly regarded as jaceosidin from reference literature,27 which exhibited the [M + H]+ at m/z 331.0813 and MS2 ions at m/z 316.0597 [M + H – CH3]+, 301.0344 [M + H – 2CH3]+, and 288.0629 [M + H – CH3 – CO]+. The precursor ion of compound 32 was at m/z 389.1234, as well as fragment ions, at m/z 374.0998, m/z 359.0763, m/z 341.0659, and m/z 331.0818. By comparison with the previous report,28 compound 32 could be unambiguously identified as 5-demethylnobiletin (fragmentation pattern is shown in Figure 3C).
It was indicated that six PMFs including compounds 25, 28, 29, 30, 32, and 33 were not reported previously.
2.4. Identification of Phthalides
Phthalides are also known as γ-hydroxymethyl benzoate lactones, which are characterized by the double ring fusion of the inner ester and benzene. The fragment ions of H2O, CO, and alkyl chain are easily formed under high-energy collision.29 Herein, three phthalides were identified.
The protonated ions of compounds 18 and 24 were both at m/z 191.1068, with similar second-order fragment ions at m/z 173 [M + H – H2O]+, 149 [M + H – C3H6]+, 145 [M + H – H2O – CO]+, and 130 [M + H – H2O – C3H6]+; they were found to be isomers. Further, the retention times of compound 18 and compound 24 was 12.66 and 18.42 min, respectively; according to the polarity of the compound combined with the above information, compound 18 was finally determined as 3-n-butylphthalide and compound 24 (fragmentation pattern shown in Figure 3D), as ligustilide.30,31 Compound 21 had a precursor ion at m/z 193.0498 and secondary fragment ions at m/z 175.1120, m/z 147.1170, m/z 137.0599, and m/z 105.0703. Based on previous research,31 compound 21 was tentatively characterized as senkyunolide A (fragmentation pattern is shown in Figure 3E).
It was clarified that three phthalides including compounds 18, 21, and 24 were not reported previously in CSF. Phthalides, especially ligustilide, were effective in improving microcirculation, protecting against cerebral ischemia-reperfusion injury, and treating tumor.32
2.5. Identification of Limonoids
Three limonoids, including limonin, nomilin, and obacunone, were identified from the SFE extracts of CSF. Via standard reference, compounds 16 and 20 were identified as limonin and nomilin, respectively. The detailed cracking process of compound 16 is shown in Figure 3F, which included son ions at m/z 425.1957, m/z 409.2002, m/z 367.1909, m/z 339.1952, and m/z 161.0598. In addition, compound 22 had an [M + H]+ ion at m/z 455.2068 and a series of MS2 ions at m/z 437.1958, m/z 419.1862, and m/z 409.2012; thus, compound 22 was determined to be obacunone, which is also a known ingredient in Citrus fruits as reported previously.33
2.6. Identification of Terpenoids
In this study, seven terpenoids were identified from CSF where compounds 5, 27, 31, 35, 36, 37, and 39 were unequivocally identified as camphor, dehydrocostus lactone, atractylenolide II, atractylenolide I, (+)-nootkatone, α-cyperone, and germacrone, respectively.
Compound 31 (fragmentation pathway is shown in Figure 3H) exhibited the [M + H]+ ion at m/z 233.1539 in the MS spectrum, and the main fragment ion peaks were at m/z 187.1486, m/z 177.0712, m/z 151.0756, m/z131.0858, m/z 105.0703, and m/z 91.0549. Therefore, compound 31 was confirmed as atractylenolide II from a previous report.34 Compound 35 produced a molecular ion peak at m/z 231.1380 for [M + H]+ and daughter ions like m/z 213.1274, m/z 185.1325, and m/z 147.0800. On the basis of characteristic fragment profiles, compound 35 was determined to be atractylenolide I, as reported earlier.35
In addition, the deprotonated ion of compound 39 appeared at m/z 219.1746, further producing MS2 fragmentation at m/z 201.1436 and m/z 159.1017. According to the literature,36 compound 39 was determined to be germacrone.
Note that compounds 27, 31, 35, 36, 37, and 39 were not found in previous studies for identification of components in CSF extracts.
2.7. Identification of Phenolics
In addition, five constituents including compounds 1, 2, 4, 7, and 26 were unequivocally assigned as nicotinamide, vanillin, syringaldehyde, ferulic acid, and ferulaldehyde, respectively, integrated with the literature and the corresponding formula supplied by MS.
Taking compound 2 as an example, a precursor ion sign was observed at m/z 153.0547, while in the MS2 spectrum, the primary fragment ions were at m/z 125.0599 [M + H – CO]+, m/z 111.0444 [M + H – CO – CH2]+, and m/z 93.0340 [M + H – C2H4O2]+. Compound 2 was identified as vanillin by comparing with a previous study,37,38 which included a protonated ion at m/z 153.0547 and son ions at m/z 125.0599, m/z 111.0444, and m/z 93.0340. The fragmentation pathways of vanillin are shown in Figure 3G.
Of note, compounds 1, 2, 4, and 26 were not reported previously in CSF extracts.
2.8. Comparison of Citrus Samples from Different Origins
The established UPLC-Q-Exactive Orbitrap/MS method was applied to the coinstantaneous determination of the chemical markers in eight batches of CSF samples from several main producing areas, such as the Guangdong province, Guangxi province, Zhejiang province, Sichuan province, and Yunnan province. The results are shown in Table 3 as the mean values of two repeated injections. Twenty-five compounds were found in all of these CSF samples, which showed that they were common in places. Of course, there were differences among these samples. Coumarin, dehydrocostus lactone, atractylenolide II, and atractylenolide I were exclusively found in CSF samples from the Guangdong province by comparison with others. Moreover, compared with others, isopsoralen was exclusively found in CSF samples from the Guangxi province and ferulic acid was exclusively found in CSF samples from the Zhejiang province. In parallel, it should be noted that nicotinamide was not found in CSF samples from the Yunnan province, syringaldehyde was not found in CSF samples from the Guangdong province, and both 3-n-butylphthalide and ligustilide were deficient in CSF samples from a region of the Sichuan province (Nanchong city).
Table 3. Differences in Chemical Components Among Different Originsa.
| peak | compound | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | nicotinamidec | * | * | * | * | * | * | * | |
| 2 | vanillin | * | * | * | * | * | * | * | * |
| 3 | isoscopoletin | * | * | * | * | * | * | * | * |
| 4 | syringaldehydec | * | * | * | * | * | * | * | |
| 5 | camphor | * | * | * | * | * | * | * | * |
| 6 | scopoletin | * | * | * | * | * | * | * | * |
| 7 | ferulic acidb | * | * | ||||||
| 8 | coumarinb | * | |||||||
| 9 | scoparone | * | * | * | * | * | * | * | * |
| 10 | fraxinol | * | * | * | * | * | |||
| 11 | 7-methoxycoumarin | * | * | * | * | * | * | * | * |
| 12 | isopsoralenb | * | |||||||
| 13 | oxypeucedan hydrate | * | * | * | * | * | * | * | * |
| 14 | 5,7-dimethoxycoumarin | * | * | * | * | * | * | * | * |
| 15 | bergapten | * | * | * | * | * | * | * | * |
| 16 | limonina | * | * | * | * | * | * | * | * |
| 17 | byakangelicol | * | * | * | * | * | * | * | * |
| 18 | 3-n-butylphthalidec | * | * | * | * | * | * | * | |
| 19 | oxypeucedanin | * | * | * | * | * | * | * | * |
| 20 | nomilin | * | * | * | * | * | * | * | * |
| 21 | senkyunolide A | * | * | * | * | ||||
| 22 | obacunone | * | * | * | * | * | * | * | * |
| 23 | 5,7,3′-trihydroxy-6,4′,5′-trimethoxyflavone | * | * | * | * | * | * | * | * |
| 24 | ligustilidec | * | * | * | * | * | * | * | |
| 25 | 6-demethoxytangeretin | * | * | * | * | * | * | ||
| 26 | ferulaldehyde | * | * | * | * | * | |||
| 27 | dehydrocostus lactoneb | * | |||||||
| 28 | iristectorigenina | * | * | * | * | * | * | * | * |
| 29 | nobiletinb | * | * | * | * | * | * | * | * |
| 30 | jaceosidin | * | * | * | * | * | * | * | * |
| 31 | atractylenolide IIb | * | |||||||
| 32 | 5-demethylnobiletin | * | * | * | * | * | * | * | * |
| 33 | tangeretin | * | * | * | * | * | * | * | * |
| 34 | 5,7-dihydroxy-4-methylcoumarin | * | * | * | * | * | * | * | * |
| 35 | atractylenolide Ib | * | |||||||
| 36 | (+)-nootkatone | * | * | * | * | * | * | * | * |
| 37 | α-cyperone | * | * | * | * | * | * | * | * |
| 38 | 7-hydroxycoumarin | * | * | * | * | * | * | * | * |
| 39 | germacrone | * | * | * | * | * | * | * | * |
S1, CSF samples from the Guangdong province; S2, CSF samples from the Guangxi province; S3 and S4, CSF samples from the Zhejiang province; S5, S6, and S7, CSF samples from the Sichuan province; and S8, CSF samples from the Yunnan province.
The compound is exclusively found in the corresponding origins or main producing area.
The compound is exclusively deficient in the corresponding origins or main producing area.
*The compound is found in the corresponding origins or main producing area.
3. Discussion
The most widespread technique for isolation of bioactive components from plant materials is the traditional organic solvent extraction. The major drawbacks of this extraction method are high time consumption and relatively poor selectivity. Noticeably, the new generation of extraction techniques are evolving to overcome these shortcomings.39 SFC has proved to be a powerful separation technique. Because of the low viscosity and high diffusivity of supercritical fluids’ mobile phases, high flow rates and thus short analysis time as well as high efficiency could be easily achieved.40 The SFE approach showed an excellent functioning of extraction of nonpolar and some low-polarity substances. Herein, SFE combined with UPLC-Q-Exactive Orbitrap/MS method was established for identification of components from CSF, whereby 39 lipophilic compounds in SFE extracts were isolated and identified via UPLC-Q-Exactive Orbitrap/MS, among which 23 compounds including 4 coumarins, 6 PMFs, and 3 phthalides were first identified in CSF extracts.
It is noteworthy that it is well known that coumarins are the main components of CSF and that PMFs may not be abundant; however, this study raised the novel interpretation that SFE extracts of CSF may contain a series of PMFs as well as coumarins. Interestingly, seven PMFs composed of 6-demethoxytangeretin, iristectorigenina, nobiletin, jaceosidin, 5-demethylnobiletin, and tangeretin were identified in SFE extracts, illustrating that CSF may contain a series of PMFs. The result was different from that of the study performed by Li et al.,6 whose investigation demonstrated that PMFs were hardly detected in certain Citrus herbs including CSF based on LC-QTOF-MS/MS. The present study was of great significance for a strong supplement to the previous conclusion. In addition, these PMFs have beneficial pharmacodynamics, such as tangeretin exhibiting anti-inflammation, anti-oxidation, and neuroprotection properties.41
Moreover, a correlation between their composition and the collection areas was demonstrated. To a great extent, coumarin, dehydrocostus lactone, atractylenolide II, or atractylenolide I could be the marker compounds used to distinguish CSF of the Guangdong province from that of the other origins, while isopsoralen and ferulic acid may be the marker compounds that are exclusively found in CSF from the Guangxi province and Zhejiang province, respectively.
4. Conclusions
SFE coupled with UPLC-Q-Exactive Orbitrap MS analysis helped to explore more novel information on lipophilic components in CSF extracts: 23 compounds were not reported in CSF extracts earlier. Meanwhile, it was indicative that PMFs should be abundant in CSF. Moreover, some compounds may be recognized as a marker to distinguish CSF from different origins in China. In brief, the present study can boost the quality of assessment as well as future pharmacodynamics investigation of CSF and provide chemotaxonomic botanical knowledge for this functional food. Further, developing quality control methods is indispensable to ensure the identification of the product from different origins.
5. Materials and Methods
5.1. Reagents and Chemicals
Formic acid and methanol of MS grade were purchased from Merck KGaA Company of Germany, Ltd., and ThermoFisher Scientific (China) Co., respectively. Standard compounds 5,7-dimethoxycoumarin (compound 14), nomilin (compound 20), scopoletin (compound 6), and scoparone (compound 9) were provided by Sichuan Vicky Biotechnology Co. Ltd., and limonin (compound 16), nobiletin (compound 29), and tangeretin (compound 33) were provided by Chengdu Mansite Biotechnology Co. Ltd. The purity of these standard substances was over 98%. There were eight batches of CSF samples (shown in Table 1), which were identified from the dried fruits of Citrus medica L. var. sarcodactoxylis Swingle in Rutaceae by Professor Wu Bo of the School of Pharmacy, Guangzhou Medical University. The samples were kept in the Pharmacognosy Laboratory, School of Pharmacy, Guangzhou Medical University.
5.2. Instrumentation
SFE was operated with an ASI Supercritical Fluid Extraction system (SPE-ed SFE-2) (Applied Separations, Inc., Allentown, PA) equipped with a high-pressure CO2 ASF-100 pump. Hk-04b swing crusher was produced from Guangzhou Xuyang Machinery Equipment Co. Ltd., and the ME 104 electronic analytical balance was provided by Mettler Toledo Instrument Co. Ltd. The UPLC system mainly consisted of an Ultimate 3000 series ultra-high-performance liquid chromatograph (ThermoFisher Scientific) equipped with an online degasser, a quaternary pump, an autosampler, and a column temperature compartment; it was connected to a Q-Exactive Orbitrap tandem mass spectrometer (ThermoFisher Scientific) via an electrospray ionization interface. Besides, the ZORBAX C18 column (2.1 mm × 50 mm, 1.8 μm) was offered by Agilent Technologies.
5.3. SFE Conditions
All SFE extractions were carried out at 50 °C in a dynamic mode with a total CO2 flow rate of 3 L/min and extractive pressure of 33 MPa. The total process was within 120 min for each extraction.
5.4. Sample Preparation
These reference substances were weighed appropriately and then dissolved in dichloromethane. The CSF samples collected from different origins were cut into smaller pieces and smashed into powder by a swing crusher and sifted through a 40-mesh sieve. The accurately weighed powder (50 g) was directly put into the SFE ax, without any type of support materials or entrainers. SFE extracts were harvested, put into vials, and then stored at 4 °C. Prior to the detection test; the stock solution was diluted with dichloromethane to a suitable concentration. The diluent solution was filtered by a 0.22 μm microporous membrane and then shifted into an autosampler vial for MS analysis.
5.5. Analytical System
The separation was performed on a ZORBAX Rclipse Plus C18 column at 35 °C. The mobile phase consisted of 0.1% formic acid aqueous solution (phase A) and methanol (phase B), using a gradient elution of 30% B at 0–3 min; 30–45% B at 3–8 min; 45% B at 8–12 min; 45–50% B at 12–16 min; 50–70% B at 16–25 min; and 70–90% B at 25–30 min. The flow rate was maintained at 0.3 mL/min, and the injected sample volume was 1 μL.
For Q-exactive Orbit-MS analysis, the source settings were as follows: The spray voltage was 3.5 kV. The capillary temperature was 320 °C, and the auxiliary gas heating temperature was 300 °C. Sheath gas, auxiliary gas, and purge gas were set at 30 units, 10 units, and 5 units, respectively. Full scan data collection and dependent scan event data collection were executed from m/z 100 to m/z 1000 in the dd-MS2 mode with a resolution of 70 000. The system was regulated by Xcalibur software and collected data was processed by Metworks software (ThermoFisher Scientific).
Acknowledgments
This research was funded by National Major New Drug Creation Project of China (No. 2020ZX09201-010), the Cultivation Plan for High-level University Academic Backbone of Guangzhou Medical University in 2017 (No. gydf [2017] 210), and Scientific Research Project of Health Commission of Hunan province (No. 20201980).
Author Contributions
Conceptualization: G.Y., C.F., and G.Z.; formal analysis: C.F. and M.L.; investigation: B.Y., G.Z., and G.Y.; methodology: C.F., M.L., K.W., J.T., and L.D.; project administration: M.L.; resources: K.W. and J.T.; writing—original draft: C.F., M.L., and K.W.; writing—review and editing: C.F.
The authors declare no competing financial interest.
References
- He Z.; Liang F.; Zhang Y.; Pan Y. Water-soluble polysaccharides from finger citron fruits (Citrus medica L. var. sarcodactylis). Carbohydr. Res. 2014, 388, 100–104. 10.1016/j.carres.2013.12.020. [DOI] [PubMed] [Google Scholar]
- Wang E.; Li Y.; Maguy B. L.; Lou Z.; Wang H.; Zhao W.; Chen X. Separation and enrichment of phenolics improved the antibiofilm and antibacterial activity of the fractions from Citrus medica L. var. sarcodactylis in vitro and in tofu. Food Chem. 2019, 294, 533–538. 10.1016/j.foodchem.2019.05.038. [DOI] [PubMed] [Google Scholar]
- Kim K. N.; Ko Y. J.; Yang H. M.; Ham Y. M.; Roh S. W.; Jeon Y. J.; Ahn G.; Kang M. C.; Yoon W. J.; Kim D.; Oda T. Anti-inflammatory effect of essential oil and its constituents from fingered citron (Citrus medica L. var. sarcodactylis) through blocking JNK, ERK and NF-kappaB signaling pathways in LPS-activated RAW 264.7 cells. Food Chem. Toxicol. 2013, 57, 126–131. 10.1016/j.fct.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Peng C. H.; Ker Y. B.; Weng C. F.; Peng C. C.; Huang C. N.; Lin L. Y.; Peng R. Y. Insulin secretagogue bioactivity of finger citron fruit (Citrus medica L. var. Sarcodactylis Hort, Rutaceae). J. Agric. Food Chem. 2009, 57, 8812–8819. 10.1021/jf902143x. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Zhu C.; Xu C.; Sun J.; Grierson D.; Zhang B.; Chen K. Integration of Metabolite Profiling and Transcriptome Analysis Reveals Genes Related to Volatile Terpenoid Metabolism in Finger Citron (C. medica var. sarcodactylis). Molecules 2019, 24, 2564 10.3390/molecules24142564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L.; Guo L.; Liu K.; Liu E. H.; Li P. Characterization and classification of seven Citrus herbs by liquid chromatography-quadrupole time-of-flight mass spectrometry and genetic algorithm optimized support vector machines. J. Chromatogr. A 2014, 1339, 118–127. 10.1016/j.chroma.2014.02.091. [DOI] [PubMed] [Google Scholar]
- Yanmei Z.; Yifan F.; Xia W.; Jiao G. Rapid Identification of Coumarins from Fructus Citri Sarcodactylis by UPLC/Q-TOF-MS. Nat. Prod. Res. 2015, 29, 53–58. 10.1080/14786419.2014.957700. [DOI] [PubMed] [Google Scholar]
- Chu J.; Li S. L.; Yin Z. Q.; Ye W. C.; Zhang Q. W. Simultaneous quantification of coumarins, flavonoids and limonoids in Fructus Citri Sarcodactylis by high performance liquid chromatography coupled with diode array detector. J. Pharm. Biomed. Anal. 2012, 66, 170–175. 10.1016/j.jpba.2012.03.041. [DOI] [PubMed] [Google Scholar]
- Zheng G.; Yang X.; Liu M.; Chao Y.; Chen B.; Yang D.; Wei M. Supercritical CO2 Fluid Extraction for the Identification of Compounds from Citrus reticulata Semen by Ultra-High-Performance Liquid Chromatography Combined with Q-Exactive Orbitrap Tandem Mass Spectrometry. ACS Omega 2020, 5, 2180–2186. 10.1021/acsomega.9b03123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaw K. Y.; Parat M. O.; Shaw P. N.; Falconer J. R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, e1186 10.3390/molecules22071186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Gao G.; Liu P.; Pan M.; Chai Y.; Liu X.; Lu C. Development and validation of an ultra performance liquid chromatography Q-Exactive Orbitrap mass spectrometry for the determination of fipronil and its metabolites in tea and chrysanthemum. Food Chem. 2018, 246, 328–334. 10.1016/j.foodchem.2017.11.017. [DOI] [PubMed] [Google Scholar]
- Zheng G.; Yang X.; Chen B.; Chao Y.; Hu P.; Cai Y.; Wu B.; Wei M. Identification and determination of chemical constituents of Citrus reticulata semen through ultra high performance liquid chromatography combined with Q Exactive Orbitrap tandem mass spectrometry. J. Sep. Sci. 2020, 43, 438–451. 10.1002/jssc.201900641. [DOI] [PubMed] [Google Scholar]
- Sun C.; Wang Y.; Sun S.; Chen X.; Shi X.; Fang H.; Zhang Y.; Fang Z. Fragmentation pathways of protonated coumarin by ESI-QE- Orbitrap-MS/MS coupled with DFT calculations. J. Mass Spectrom. 2020, e4496 10.1002/jms.4496. [DOI] [PubMed] [Google Scholar]
- Wang X.; Lv H.; Sun H.; Liu L.; Sun W.; Cao H. Development of a rapid and validated method for investigating the metabolism of scoparone in rat using ultra-performance liquid chromatography/electrospray ionization quadruple time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3883–3890. 10.1002/rcm.3296. [DOI] [PubMed] [Google Scholar]
- Stiefel C.; Schubert T.; Morlock G. E. Bioprofiling of Cosmetics with Focus on Streamlined Coumarin Analysis. ACS Omega 2017, 2, 5242–5250. 10.1021/acsomega.7b00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi S.; Lombardo D.; Abou-Zaid M. A Metabolomic and HPLC-MS/MS Analysis of the Foliar Phenolics, Flavonoids and Coumarins of the Fraxinus Species Resistant and Susceptible to Emerald Ash Borer. Molecules 2018, 23, 2734. 10.3390/molecules23112734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerković I.; Molnar M.; Vidović S.; Vladić J.; Jokić S. Supercritical CO2 Extraction ofLavandula angustifolia Mill. Flowers: Optimisation of Oxygenated Monoterpenes, Coumarin and Herniarin Content. Phytochem. Anal. 2017, 28, 558–566. 10.1002/pca.2705. [DOI] [PubMed] [Google Scholar]
- Gao J.; Yu J.; Xu P.; Chen J.; Gao G.; Li B.; Sun L. Established UPLC-MS/MS procedure for multicomponent quantitative analysis in rat plasma: A contrastive pharmacokinetics study of Qiangshen tablet in normal and kidney yang deficiency syndrome models. J. Chromatogr. B 2019, 1106–1107, 35–42. 10.1016/j.jchromb.2018.12.031. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Tan C.; Cai L.; Xia F.; Gao D.; Yang F.; Chen H.; Xia Z. Characterization of active antiplatelet chemical compositions of edible Citrus limon through ultra-performance liquid chromatography single quadrupole mass spectrometry-based chemometrics. Food Funct. 2018, 9, 2762–2773. 10.1039/C8FO00403J. [DOI] [PubMed] [Google Scholar]
- Park A. Y.; Park S.; Lee J.; Jung M.; Kim J.; Kang S. S.; Youm J.; Han S. B. Simultaneous determination of five coumarins inAngelicae dahuricae Radix by HPLC/UV and LC-ESI-MS/MS. Biomed. Chromatogr. 2009, 23, 1034–1043. 10.1002/bmc.1219. [DOI] [PubMed] [Google Scholar]
- Céspedes C. L.; Avila J. G.; Martínez A.; Serrato B.; Calderón-Mugica J. C.; Salgado-Garciglia R. Antifungal and Antibacterial Activities of Mexican Tarragon (Tagetes lucida). J. Agric. Food Chem. 2006, 54, 3521–3527. 10.1021/jf053071w. [DOI] [PubMed] [Google Scholar]
- Wei L.; Wang X.; Mu S.; Sun L.; Yu Z. Ultra high performance liquid chromatography with electrospray ionization tandem mass spectrometry coupled with hierarchical cluster analysis to evaluate Wikstroemia indica (L.) C. A. Mey. from different geographical regions. J. Sep. Sci. 2015, 38, 2093–2100. 10.1002/jssc.201401398. [DOI] [PubMed] [Google Scholar]
- Ge L.; Cui Y.; Cheng K.; Han J. Isopsoralen Enhanced Osteogenesis by Targeting AhR/ERα. Molecules 2018, 23, 2600 10.3390/molecules23102600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahata I.; Yoshida M.; Sun W.; Nakajima A.; Lai Y.; Osaka N.; Matsuzaki K.; Yokosuka A.; Mimaki Y.; Naganuma A.; Tomioka Y.; Yamakuni T. Potent activity of nobiletin-rich Citrus reticulata peel extract to facilitate cAMP/PKA/ERK/CREB signaling associated with learning and memory in cultured hippocampal neurons: identification of the substances responsible for the pharmacological action. J. Neural Transm. 2013, 120, 1397–1409. 10.1007/s00702-013-1025-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Wang Q.; Qi L.; Qin X.; Qin M. Characterization and determination of the major constituents in Belamcandae Rhizoma by HPLC–DAD–ESI-MSn. J. Pharm. Biomed. Anal. 2011, 56, 304–314. 10.1016/j.jpba.2011.05.040. [DOI] [PubMed] [Google Scholar]
- Li S.; Li S.; Huang Y.; Liu C.; Chen L.; Zhang Y. Ionic-liquid-based ultrasound-assisted extraction of isoflavones fromBelamcanda chinensis and subsequent screening and isolation of potential α-glucosidase inhibitors by ultrafiltration and semipreparative high-performance liquid chromatography. J. Sep. Sci. 2017, 40, 2565–2574. 10.1002/jssc.201700258. [DOI] [PubMed] [Google Scholar]
- Xia J.-X.; Zhao B.; Zan J.; Wang P.; Chen L. Simultaneous determination of phenolic acids and flavonoids in Artemisiae Argyi Folium by HPLC-MS/MS and discovery of antioxidant ingredients based on relevance analysis. J. Pharm. Biomed. Anal. 2019, 175, 112734 10.1016/j.jpba.2019.06.031. [DOI] [PubMed] [Google Scholar]
- Ye X.; Cao D.; Zhao X.; Song F.; Huang Q.; Fan G.; Wu F. Chemical fingerprint and metabolic profile analysis of Citrus reticulata ‘Chachi’ decoction by HPLC-PDA-IT-MSn and HPLC-Quadrupole-Orbitrap-MS method. J. Chromatogr. B 2014, 970, 108–120. 10.1016/j.jchromb.2014.06.035. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Liu J.; Zhong X.; Li J.; Wang X.; Ji L.; Shang X. Rapid Characterization of Chemical Components in Edible Mushroom Sparassis crispa by UPLC-Orbitrap MS Analysis and Potential Inhibitory Effects on Allergic Rhinitis. Molecules 2019, 24, 3014 10.3390/molecules24163014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Y.; Pei K.; Cao G.; Cai H.; Liu X.; Cao L.; Zhang S.; Cai B. Comparative Study on Pharmacokinetics of Four Active Compounds in Rat Plasma after Oral Administration of Raw and Wine Processed Chuanxiong Rhizoma. Molecules 2020, 25, 93 10.3390/molecules25010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Yan T.; Jiang W.; Hu N.; Zhang S.; Yang P.; Zhang W.; Shi L.; Liu L. Simultaneous quantification of ligustilide, dl-3-n-butylphthalide and senkyunolide A in rat plasma by GC-MS and its application to comparative pharmacokinetic studies of Rhizoma Chuanxiong extract alone and Baizhi Chuanxiong Decoction. Biomed. Chromatogr. 2019, 33, e4625 10.1002/bmc.4625. [DOI] [PubMed] [Google Scholar]
- Ma J.; Mei J.; Lu J.; Wang Y.; Hu M.; Ma F.; Long H.; Qin Z.; Tao N. Ligustilide promotes apoptosis of cancer-associated fibroblasts via the TLR4 pathways. Food Chem. Toxicol. 2020, 135, 110991 10.1016/j.fct.2019.110991. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Zhang Z.; Zhang Y.; Zhang X.; Zhang Z.; Liao Y.; Zhang B. A New Method for Simultaneous Determination of Phenolic Acids, Alkaloids and Limonoids in Phellodendri Amurensis Cortex. Molecules 2019, 24, 709 10.3390/molecules24040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Bo C.; Fan Y.; An R.; Chen L.; Zhang Y.; Jia Y.; Wang X. Qualitative and quantitative determination of Atractylodes rhizome using ultra-performance liquid chromatography coupled with linear ion trap-Orbitrap mass spectrometry with data-dependent processing. Biomed. Chromatogr. 2019, 33, e4443 10.1002/bmc.4443. [DOI] [PubMed] [Google Scholar]
- Kim J. H.; Lee Y.; Lee G.; Doh E. J.; Hong S. Quantitative Interrelation between Atractylenolide I, II, and III in Atractylodes japonica Koidzumi Rhizomes, and Evaluation of Their Oxidative Transformation Using a Biomimetic Kinetic Model. ACS Omega 2018, 3, 14833–14840. 10.1021/acsomega.8b02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.; Yang S.; Sheng L.; Li Y. Simultaneous quantification of curdione, furanodiene and germacrone in rabbit plasma using liquid chromatography-tandem mass spectrometry and its application to a pharmacokinetic study. Biomed. Chromatogr. 2015, 29, 1393–1398. 10.1002/bmc.3436. [DOI] [PubMed] [Google Scholar]
- Wu X.; Jia L.; Wu J.; Liu Y.; Kang H.; Liu X.; Li P.; He P.; Tu Y.; Li B. Simultaneous Determination and Quantification of Triterpene Saponins from Camellia sinensis Seeds Using UPLC-PDA-QTOF-MS/MS. Molecules 2019, 24, 3794 10.3390/molecules24203794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P.; Gupta S.; Sharma M.; Kumar N.; Pruthi V.; Poluri K. M. Effectiveness of Phytoactive Molecules on Transcriptional Expression, Biofilm Matrix, and Cell Wall Components of Candida glabrata and Its Clinical Isolates. ACS Omega 2018, 3, 12201–12214. 10.1021/acsomega.8b01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastić N.; Borras-Linares I.; Lozano-Sanchez J.; Svarc-Gajic J.; Segura-Carretero A. Comparative Assessment of Phytochemical Profiles of Comfrey (Symphytum officinale L.) Root Extracts Obtained by Different Extraction Techniques. Molecules 2020, 25, 837 10.3390/molecules25040837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Feng Y.; Tang G.; Li M.; Zhang T.; Fillet M.; Crommen J.; Jiang Z. Development and validation of a fast SFC method for the analysis of flavonoids in plant extracts. J. Pharm. Biomed. Anal. 2017, 140, 384–391. 10.1016/j.jpba.2017.03.012. [DOI] [PubMed] [Google Scholar]
- Wunpathe C.; Maneesai P.; Rattanakanokchai S.; Bunbupha S.; Kukongviriyapan U.; Tong-Un T.; Pakdeechote P. Tangeretin mitigates l-NAME-induced ventricular dysfunction and remodeling through the AT1R/pERK1/2/pJNK signaling pathway in rats. Food Funct. 2020, 11, 1322–1333. 10.1039/C9FO02365H. [DOI] [PubMed] [Google Scholar]
- Peng Z.; Wang Y.; Gu X.; Wen Y.; Yan C. A platform for fast screening potential anti-breast cancer compounds in traditional Chinese medicines. Biomed. Chromatogr. 2013, 27, 1759–1766. 10.1002/bmc.2990. [DOI] [PubMed] [Google Scholar]
- Qi Y.; Cheng X.; Jing H.; Yan T.; Xiao F.; Wu B.; Bi K.; Jia Y. Comparative pharmacokinetic study of the components in Alpinia oxyphylla Miq.-Schisandra chinensis (Turcz.) Baill. herb pair and its single herb between normal and Alzheimer’s disease rats by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2020, 177, 112874 10.1016/j.jpba.2019.112874. [DOI] [PubMed] [Google Scholar]
- Liu P.; Li Z.; Qian D.; Li W.; Shang E. X.; Duan J. A. Simultaneous determination of bioactive components in essential oil of Xiang-Fu-Si-Wu Formula in Beagle dog plasma by UPLC-MS/MS and its application to pharmacokinetics. J. Chromatogr. B 2013, 929, 63–69. 10.1016/j.jchromb.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Flamini R.; Vedova A. D.; Cancian D.; Panighel A.; De Rosso M. GC/MS-positive ion chemical ionization and MS/MS study of volatile benzene compounds in five different woods used in barrel making. J. Mass Spectrom. 2007, 42, 641–646. 10.1002/jms.1193. [DOI] [PubMed] [Google Scholar]
- Xu M.; Xu Z.; Xu Q.; Zhang H.; Liu M.; Geng F.; Zhang N. UPLC-MS/MS Method for the Determination of 14 Compounds in Rat Plasma and Its Application in a Pharmacokinetic Study of Orally Administered Xiaoyao Powder. Molecules 2018, 23, 2514 10.3390/molecules23102514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hassan A.; El-Sayed M.; Hamed A. I.; Rhee I. K.; Ahmed A. A.; Zeller K. P.; Verpoorte R. Bioactive constituents of Leptadenia arborea. Fitoterapia. 2003, 74, 184–187. 10.1016/S0367-326X(02)00314-3. [DOI] [PubMed] [Google Scholar]