Abstract
Homoisoflavonoids are in the subclass of the larger family of flavonoids but have one more alkyl carbon than flavonoids. Among them, 5,7,8-trioxygenated homoisoflavonoids have not been extensively studied for synthesis and biological evaluation. Our current objective is to synthesize 2 5,7,8-trioxygenated chroman-4-ones and 12 5,7,8-trioxygenated homoisoflavonoids that have been isolated from the plants Bellevalia eigii, Drimiopsis maculata, Ledebouria graminifolia, Eucomis autumnalis, Eucomis punctata, Eucomis pallidiflora, Chionodoxa luciliae, Muscari comosum, and Dracaena cochinchinensis. For this purpose, 1,3,4,5-tetramethoxybenzene and 4′-benzyloxy-2′,3′-dimethoxy-6′-hydroxyacetophenone were used as starting materials. Asymmetric transfer hydrogenation using Noyori’s Ru catalyst provided 5,7,8-trioxygenated-3-benzylchroman-4-ones with R-configuration in high yield and enantiomeric excess. By selective deprotection of homoisoflavonoids using BCl3, the total synthesis of natural products including 10 first syntheses and three asymmetric syntheses has been completed, and three isomers of the reported dracaeconolide B could be provided. Our research on 5,7,8-trioxygenated homoisoflavonoids would be useful for the synthesis of related natural products and pharmacological applications.
Introduction
Homoisoflavonoids are a subclass of the larger family of flavonoids, with a 16-carbon skeleton that has 1 more carbon (C9 position) than flavonoids and isoflavonoids (Figure 1). They have a structure including two aromatic rings and a heterocycle containing oxygen. As some review articles dealt with the natural origins and structures of homoisoflavonoids, they are mainly found in Asparagaceae and Fabaceae families and are rarely seen in Polygonaceae, Portulacaceae, Orchidaceae, and Gentianaceae families.1,2 Homoisoflavonoids have been reported to have various biological activities, including antiangiogenic, antibacterial, antimutagenic, antioxidant, and anti-inflammatory effects.3 Initially, the structural types of homoisoflavonoids were categorized into four scaffolds—3-benzylchroman-4-one, 3-benzylidenechroman-4-one, 3-benzyl-3-hydroxychroman-4-one, and scillascillins—by du Toit’s group.4 As some isolated natural products were not included in such a category of homoisoflavonoids, the newly classified five types of homoisoflavonoids are as follows: sappanin-type, scillascillin-type, brazilin-type, caesalpin-type, and protosappanin-type (Figure 1).1
Figure 1.
Categories of homoisoflavonoids.
Among them, sappanin-type natural products are the most isolated and studied. The methoxy and hydroxy groups are mainly substituted in the A ring and the B ring, respectively. In the A ring, naturally occurring homoisoflavonoids having mono- to tetra-substituents have been reported so far. In particular, we have been interested in the total synthesis and biological activities of 5,6,7-trisubstituted homoisoflavonoids. Naturally occurring homoisoflavonoids that contain either 5,7-dihydroxy-6-methoxy or 7-hydroxy-5,6-dimethoxy groups in the A ring have been synthesized by us.5,6 Cremastranone and its synthetic analogs having the 5,6,7-trimethoxy group in the A ring were biologically evaluated against retinal and choroidal neovascularization as a promising drug candidate for wet age-related macular degeneration and other neovascular eye diseases.7−12 Moreover, we developed an enantioselective synthesis of 5,6,7-trisubstituted homoisoflavonoids such as cremastranone having the C3-stereogenic center, which was confirmed to have an R-configuration, and the antiangiogenic activity of the unnatural S-isomer was found to be more potent than the R-isomer.13 While synthetic and medicinal chemistry on 5,6,7-trisubstituted homoisoflavonoids were established by us, 5,7,8-trisubstituted homoisoflavonoids have not been well studied yet, although isolated natural products have been reported.
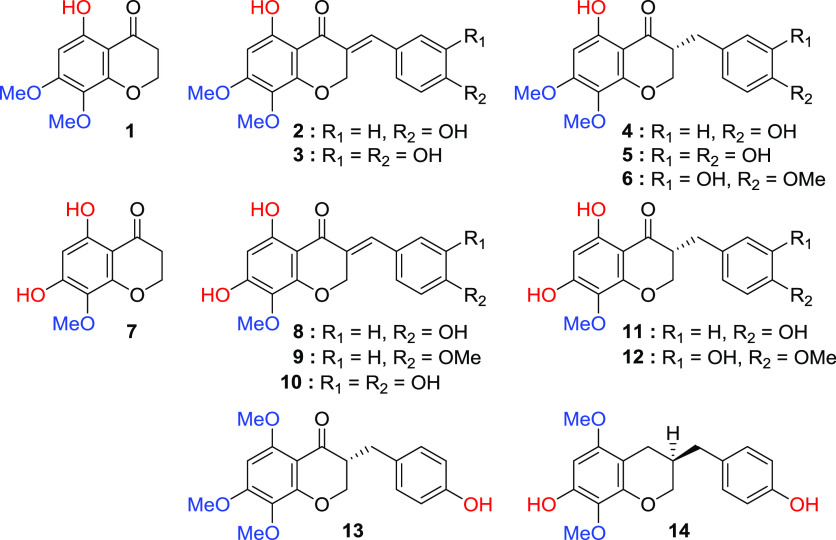
Naturally occurring 5,7,8-trioxygenated homoisoflavonoids have been isolated from the plants Bellevalia eigii, Drimiopsis maculata, Ledebouria graminifolia, Eucomis autumnalis, Eucomis punctata, Eucomis pallidiflora, Chionodoxa luciliae, Muscari comosum, and Dracaena cochinchinensis (Figure 2).14−23 Punctatin is known as the representative natural product for 5,7,8-trioxygenated homoisoflavonoids, and its congeners can be given a chemical name based on punctatin. B. eigii is a perennial plant belonging to the family Asparagaceae, subfamily Scilloideae. The Alali group reported the cytotoxic activity of the natural products isolated from the bulb of this plant.14 Among them, 5-hydroxy-7,8-dimethoxychroman-4-one (1), 7-O-methylpunctatin (2), 7-O-methyl-3′-hydroxypunctatin (3), 7-methyl-3,9-dihydropunctatin (4), 7-O-methyl-3′-hydroxy-3,9-dihydropunctatin (5), and 7,4′-di-O-methyl-3′-hydroxy-3,9-dihydropunctatin (6) were isolated. D. maculata is distributed mainly in South Africa and belongs to the family Asparagaceae, subfamily Scilloideae. The Mulholland group isolated 7-methyl-3,9-dihydropunctatin (4) from D. maculata, which has traditionally been used as a medicine for stomach ailments in children.15,1615,16Eucomis genus is a perennial bulb of the plant belonging to the family Asparagaceae, subfamily Scilloideae. The plants are widely distributed mainly in South Africa. The bulbs are toxic but have been used in South Africa as traditional medicines for urologic diseases, abdominal pain, and as an antipyretic. The Tamm group isolated 4′-O-methylpunctatin (9) from the bulb of E. autumnalis.17 Punctatin (8) and 3,9-dihydropunctatin (11) were isolated from the bulbs of E. punctata, Eucomis comosa, and E. pallidiflora.18,19C. luciliae and M. comosum are perennial plants distributed in Southeast Europe belonging to the family Asparagaceae and subfamily Scilloideae. The Parrilli group isolated punctatin (8), 3′-hydroxypunctatin (10), 3,9-dihydropunctatin (11), and 4′-O-methyl-3,9-dihydropunctatin (12) from bulbs of plants.20,21D. cochinchinensis belongs to the family Asparagaceae and subfamily Nolinoideae in the APG IV system. L. graminifolia is one of the 16 species comprising the Ledebouria genus in Botswana. Ledebouria belongs to the family Asparagaceae, subfamily Scilloideae. The Abegza group isolated 5,7-O-dimethyl-3,9-dihydropunctatin (13) by separating the organic extract from the bulb of this plant, which has traditionally been widely used mainly for skin irritations, wound dressing, sores, coughs, backaches, gastroenteritis, and as a sedative during pregnancy.22 The Jiang group isolated dracaeconolide B (14) from the resin of this plant, which is called dragon’s blood, and it has traditionally been used to treat traumatic injury, fractures, diarrhea, and dysmenorrhea.23
Figure 2.
Naturally occurring 5,7,8-trioxygenated chromanones and homoisoflavonoids.
Although various synthetic studies of the homoisoflavonoids have been introduced, there have been few reports toward 5,7,8-trioxygenated homoisoflavonoids except Strelisky’s synthesis in 1971.24 Facile synthesis of 5,7,8-trioxygenated homoisoflavonoids has the potential to provide a general and expedient entry into a plethora of analogues for interesting biological activities. Herein, we report the first synthesis of naturally occurring and synthetic 5,7,8-trioxygenated homoisoflavonoids from the proper acetophenones, moreover, in an enantioselective fashion by asymmetric transfer hydrogenation and subsequent alcohol oxidation.13,25
Results and Discussion
The syntheses of 5,7-dihydroxy-8-methoxychroman-4-one (7) and three homoisoflavonoids (8, 9, and 11) were studied by Strelisky et al.24 (Scheme 1). Using the Hoesch reaction, 1,3-bis(benzyloxy)-2,5-dimethoxybenzene was converted into the corresponding acetophenone, and a four-step synthesis was carried out to synthesize 7. The acetate of 3-benzylidenechroman-4-one was obtained by a condensation reaction using Ac2O, and finally natural products 8, 9, and 11 were synthesized via saponification and hydrogenation. To obtain 5,7,8-trioxygenated homoisoflavonoids with improved chemical yield and reproducibility, we contemplated aldol condensation with methoxy- and benzyl-protected chroman-4-ones. Instead, mild and facile deprotection by BCl3 was carried out to synthesize various 5,7-dihydroxy-8-methoxy or 5-hydroxy-7,8-dimethoxy homoisoflavonoids. Referring to enantioselective total synthesis of cremastranone by asymmetric transfer hydrogenation, we planned to synthesize 5,7,8-trioxygenated homoisoflavonoids with an R-configuration (Scheme 1).
Scheme 1. Strelisky’s and Our Synthetic Approaches of 5,7,8-Trioxygenated Homoisoflavonoids.
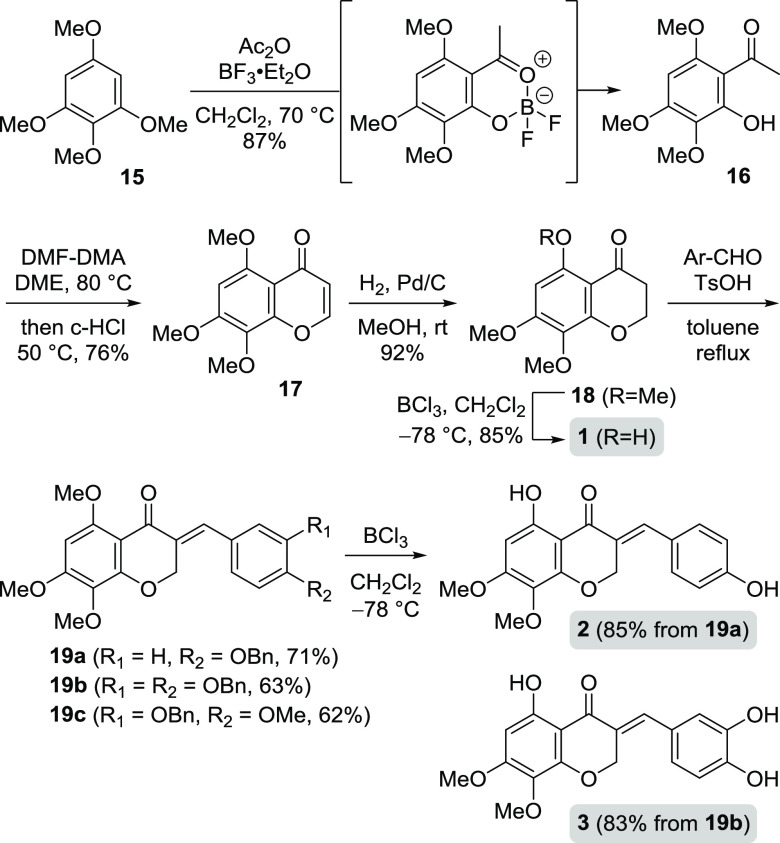
Our synthesis of 5,7,8-trioxygenated homoisoflavonoids commenced with the synthesis of 3-benzylidenechroman-4-ones having 5-hydroxy-7,8-dimethoxy groups, which were prepared from 1,2,3,5-tetramethoxybenzene 15 via 5,7,8-trimethoxychroman-4-one 18 as shown in Scheme 2. The acetylation of 15 provided by methylation of 3,4,5-trimethoxyphenol was conducted using Ac2O and BF3·OEt2, and 3 equiv of BF3·OEt2 affected simultaneous demethylation in 87% yield. On the other hand, when 1 equiv of BF3·OEt2 was used, only the acetyl group was introduced without demethylation. During the removal of the methyl group, the sterically hindered methoxy group was more easily removed among the four methoxy groups to obtain only the desired acetophenone 16. With the acetophenone 16 in hand, the 4H-chromen-4-one 17 was provided by treatment with dimethylformamide-dimethyl acetal (DMF-DMA) and subsequent c-HCl solution in good yield. Catalytic hydrogenation of 17 converted it to the chroman-4-one 18. BCl3-mediated demethylation afforded the naturally occurring chroman-4-one 1 in 85% yield, and the NMR spectral data of 1 were identical to those reported.14 Three benzaldehydes, 4-benzyloxybenzaldehyde, 3-benzyloxy-4-methoxybenzaldehyde, and 3,4-bis(benzyloxy)benzaldehyde, were used for the condensation with chroman-4-one 18 to provide the three benzylidene compounds (19a–c) in 62–71% yield, respectively. Unfortunately, general aldol condensation did not proceed with chroman-4-one 1 having a free phenol group instead of 18. Finally, the benzyl and methyl groups of the benzylidenechroman-4-ones 19a and 19b under BCl3-mediated conditions were removed to provide the desired natural products 2 and 3, respectively.
Scheme 2. Synthesis of 3-Benzylidenechroman-4-ones Having the 5-Hydroxy-7,8-dimethoxy Group.
The gray box is the reported natural product and in all other schemes.
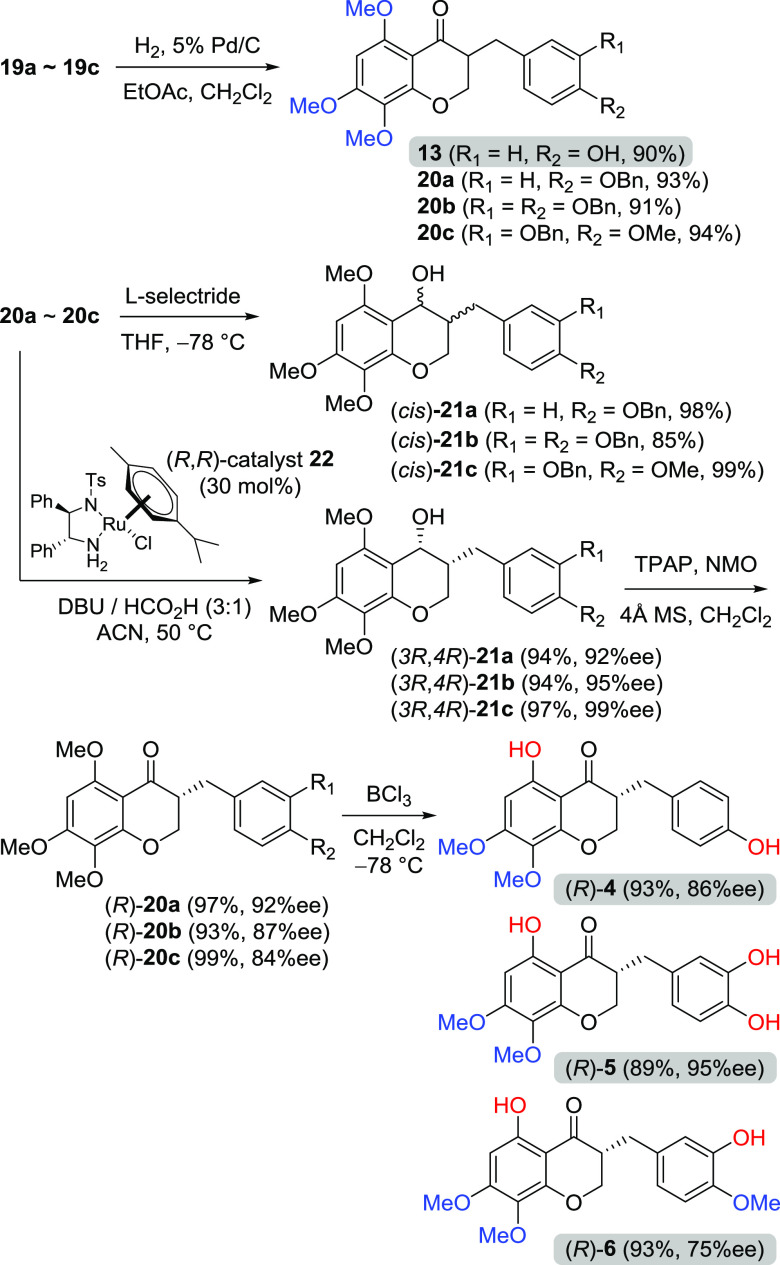
With 3-benzylidenechroman-4-ones (19a–c) in hand, four benzylchroman-4-ones, including naturally occurring homoisoflavonoid 13, were prepared by catalytic hydrogenation as shown in Scheme 3. The double bond of 3-benzylidenechroman-4-ones (19a–c) could be hydrogenated to afford 3-benzylchroman-4-ones (20a–c) without the removal of benzyl groups by controlling the catalyst loading and reaction time. As in our recent report on asymmetric transfer hydrogenation by Noyori’s Ru catalyst,26 the 3-benzylchroman-4-one (20a–c) was treated with 30 mol % of the (R,R)-catalyst, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), and formic acid to afford (3R,4R)-3-benzylchroman-4-ols (21a–c) in excellent yields (94–97%) and enantioselectivity (92–99% enantiomeric excess (ee)) along with the preparation of racemic cis-3-benzylchroman-4-ols by l-selectride reduction. Ley’s oxidation of a secondary alcohol with tetrapropylammonium perruthenate (TPAP) and N-methylmorpholine N-oxide (NMO) was chosen as a suitable alcohol oxidation of (3R,4R)-3-benzylchroman-4-ols (21a–c) to afford three kinds of the corresponding (R)-3-benzylchroman-4-ones ((R)-20a–c).27 Finally, BCl3-mediated deprotection was carried out to provide naturally occurring 4–6 through the cleavage of the C5-methyl in the A ring and benzyl group(s) in the B ring.28
Scheme 3. Synthesis of 3-Benzylchroman-4-ones Having 5-Hydroxy-7,8-dimethoxy Groups.
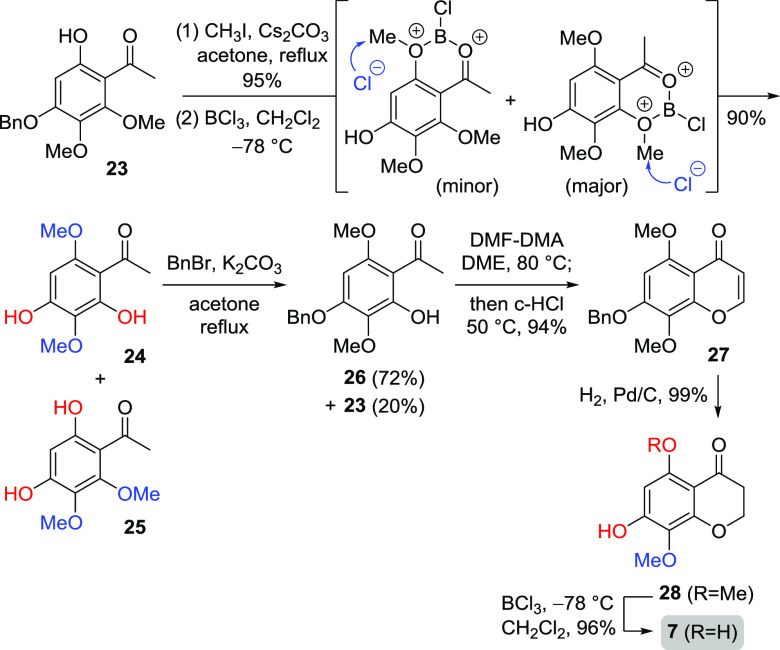
Based on the successful synthesis of 5-hydroxy-7,8-dimethoxychroman-4-one 1, we turned to consider the desired acetophenone for the synthesis of 5,7-dihydroxy-8-methoxychroman-4-one 7. Thus, the known acetophenone 23 was used as a starting material as shown in Scheme 4, and the hydroxy group of 23 was converted into methoxy by CH3I and Cs2CO3, followed by selective demethylation under BCl3-mediated conditions in which it was reported to occur selectively at a hindered methyl ether in polymethoxyarenes.29 Two isomers 24 and 25 were generated by BCl3-mediated demethylation, followed by the introduction of a benzyl group on C4 to afford the desired acetophenones 26 and 23 with 72 and 20% yields, respectively. With 26 in hand, the chromen-4-one 27 was afforded by the treatment of DMF–DMA and subsequent c-HCl. Finally, the conversion of 27 to the debenzylated chroman-4-one 28 by general catalytic hydrogenation, followed by BCl3-mediated demethylation on the C5 position, provided the naturally occurring chroman-4-one 7 in 96% yield.30
Scheme 4. Synthesis of 5,7-Dihydroxy-8-methoxychroman-4-one.
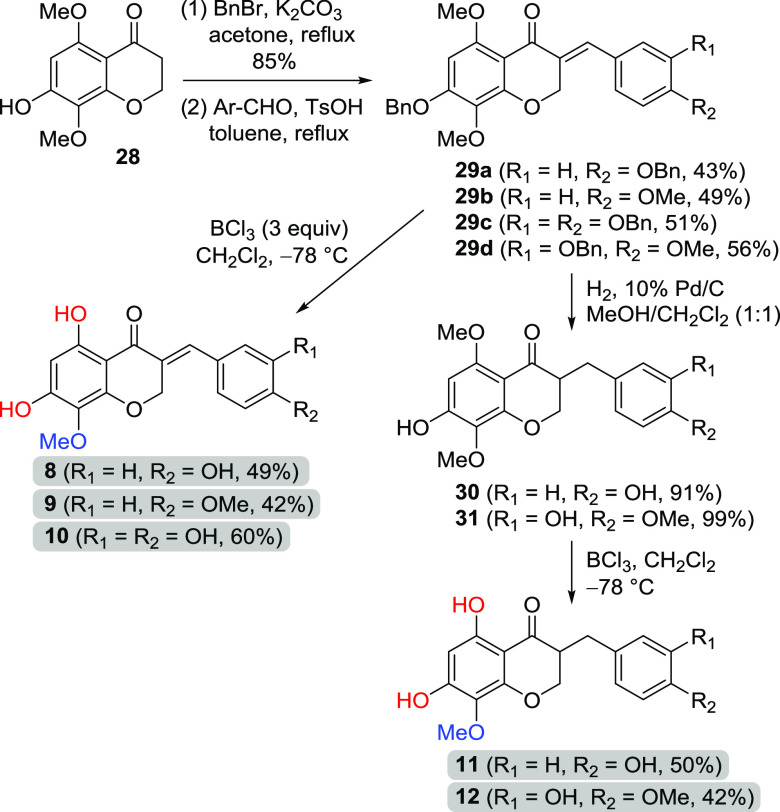
For the synthesis of 5,7-dihydroxy-8-methoxy homoisoflavonoids, the protection of the phenol group was necessary; otherwise, the yield of condensation was quite low. Thus, 28 was benzylated on the C7 position and condensed with four benzaldehydes to afford the corresponding 5,7,8-trioxygenated-3-benzylidenechroman-4-ones (29a–d) as shown in Scheme 5. BCl3-mediated deprotection was carried out to provide naturally occurring 5,7-dihydroxy-8-methoxy homoisoflavonoids (8–10) in moderate yield (42–60%). Also, 3-benzylchroman-4-ones (30 and 31) were obtained by catalytic hydrogenation of 29a and 29d, and both the methyl on C5 and the benzyl on the B ring were smoothly removed by BCl3-mediated reactions to afford two naturally occurring 3-benzylchroman-4-ones (11 and 12).
Scheme 5. Synthesis of Homoisoflavonoids Having 5,7-Dihydroxy-8-methoxy Groups.
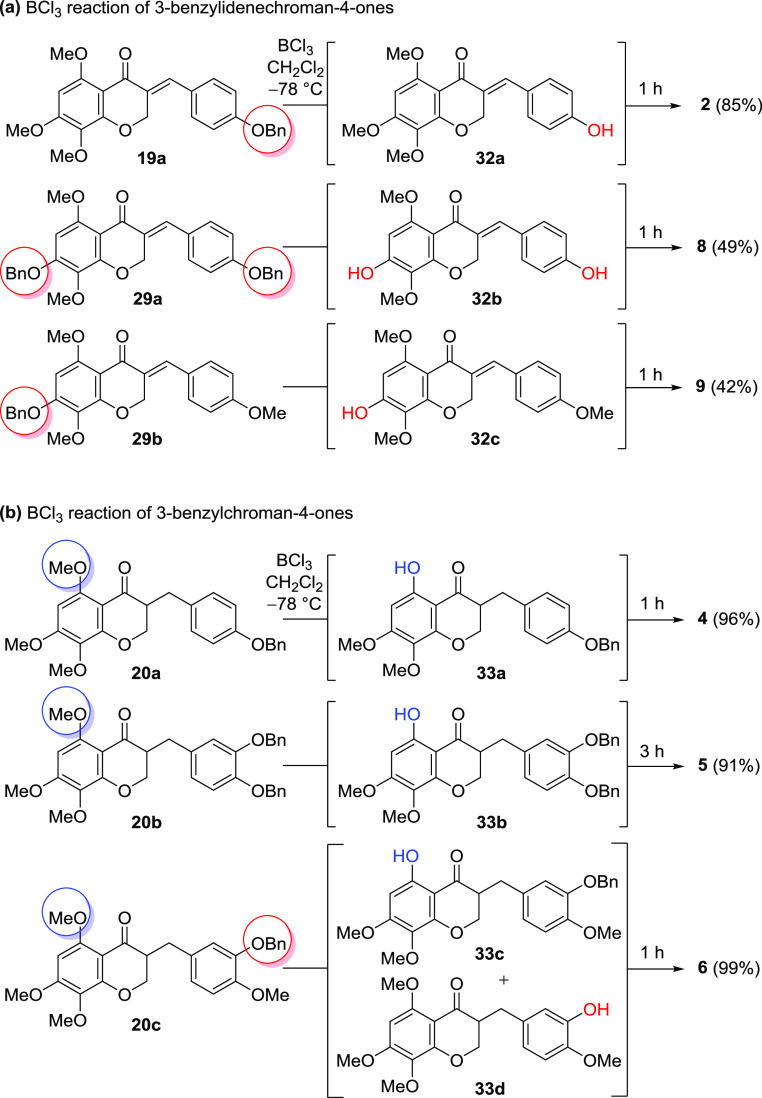
To obtain homoisoflavonoids containing either 5-hydroxy-7,8-dimethoxy or 5,7-dihydroxy-8-methoxy groups, it was important to study deprotection conditions to properly remove methyl and benzyl groups from the advanced intermediates of 5,7,8-trioxygenated homoisoflavonoids such as (R)-20a–c, 29a, and 29b, as shown in Scheme 6. BCl3 was considered an excellent reagent for deprotection owing to its mild and site-selective conditions. Interestingly, the order and process by which benzyl and methyl are removed could be understood through the structural confirmation of isolated intermediates (32a–c). In the case of phenol-deprotection of 3-benzylidenechroman-4-one (19a, 29a, and 29b), which was a highly conjugated system, the benzyl group(s) was removed first followed by methyl on the C5 position. However, slightly lower yields resulted in the overreaction of demethylation and instability of benzylidene derivatives. In 3-benzylchroman-4-ones ((R)-20a and (R)-20b), on the other hand, a methyl group neighboring the carbonyl group was first removed using BCl3 to yield the resulting intermediates (33a and 33b) having a hydroxy group on the C5 position, followed by the removal of benzyl group(s) on the B ring finally to afford 4 and 5, respectively. During BCl3-mediated deprotection of (R)-20c, the product 6 was provided via a mixture of 33c and 33d, and so it was concluded that the reactivity to removal of methyl and benzyl groups was similar.
Scheme 6. Sequential BCl3-Mediated Deprotection of (a) 3-Benzylidenechroman-4-one and (b) 3-Benzylchroman-4-one.
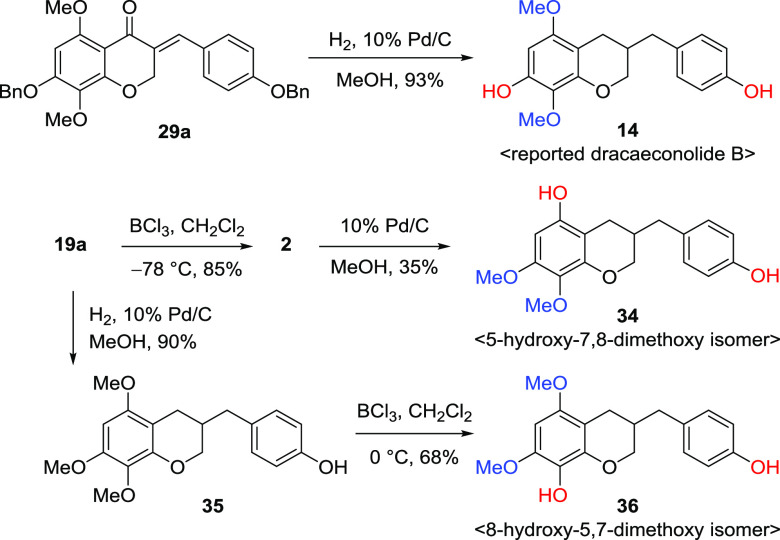
Dracaeconolide B (14), 3-(4-hydroxybenzyl)-7-hydroxy-5,8-dimethoxychromane, is unique in having the combination of a hydroxy at the C7 position and two methoxys on C5 and C8 positions of the homoisoflavonoid and a natural chromane skeleton, deoxygenated chroman-4-one, compared to other 5,7,8-trioxygenated homoisoflavonoids. We undertook catalytic hydrogenation of the previous intermediate 29a to reduce the double bond and cleave two benzyl groups as shown in Scheme 7. Unfortunately, the spectral data of our synthetic compound 14 were not identical to the reported ones of 1H and 13C NMR spectroscopies.23 We attempted to synthesize other regioisomers of dracaeconolide B to determine whether its structure would be corrected. 3-Benzylidenechroman-4-one 19a was converted to 5-hydroxy-7,8-dimethoxy isomer 34 by BCl3 reaction and catalytic hydrogenation of 2. In contrast, 19a was reduced to 3-(4-hydroxybenzyl)-5,7,8-trimethoxychromane 35 in which the sterically hindered C8-methoxy group could be cleaved predominantly under the BCl3-mediated condition to provide the 8-hydroxy-5,7-dimethoxy isomer 36. The structural elucidation of three different regioisomers (14, 34, and 36) was confirmed by comparison of the 1H and 13C NMR data (Table S14) and two-dimensional (2D) NMR spectroscopy (Figures S1–S3). The position and combination of one hydroxy and two methoxy groups on the C5, C7, and C8 positions of the A ring were assigned by heteronuclear multiple bond correlation (HMBC) correlations from H-6 to C-4a, C-5, C-7, and C-8; H2-4 to C-4a, C-5, C-6, C-8a, and C-8; and H2-2 to C-2 and C-8a.
Scheme 7. Synthesis of Suggested Dracaeconolide B and Its Two Isomers.
Conclusions
We have developed total syntheses of 2 naturally occurring 5,7,8-trioxygenated chroman-4-ones and 12 5,7,8-trioxygenated homoisoflavonoids, including 10 synthesized for the first time. 1,3,4,5-Tetramethoxybenzene and 4′-benzyloxy-2′,3′-dimethoxy-6′-hydroxyacetophenone were used as starting materials. Asymmetric transfer hydrogenation using Noyori’s Ru catalyst provided three 5,7,8-trioxygenated homoisoflavanones having an R-configuration in >94% yield and >92% ee. During BCl3-mediated deprotection, we observed that the order in which the protecting groups are removed differs depending on the substrate. To synthesize the 5,7,8-trioxygenated benzylchromane dracaeconolide B, the synthesis and structural analysis of three regioisomers were executed. Our research on 5,7,8-trioxygenated homoisoflavonoids will be useful for the synthesis of related natural products and drug discovery.
Experimental Section
All starting materials and reagents were obtained from commercial sources and used without further purification. Air- and moisture-sensitive reactions were performed under nitrogen. Flash column chromatography was performed using silica gel 60 (230–400 mesh, Merck) with the indicated solvents. Thin-layer chromatography (TLC) was performed using 0.25 mm silica gel plates (Merck). 1H and 13C{1H} NMR spectra were recorded on a Bruker 600 MHz spectrometer as solutions in deuterochloroform (CDCl3) or methanol-d4 (CD3OD). 1H NMR data were reported on the order of chemical shift, multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet and/or multiplet resonances), number of protons, and coupling constant (J value) in hertz (Hz). Enantiomeric excesses were determined by high-performance liquid chromatography (HPLC) on an Agilent 1100 using one chiral column (CHIRALPAK IA, IB, IC, ID, IG). High-resolution mass spectra (HRMS) were recorded on an Agilent 6530 quadrupole time-of-flight (Q-TOF) liquid chromatography (LC)/tandem mass spectrometry (MS/MS) system (electrospray ionization (ESI)).
1,2,3,5-Tetramethoxybenzene (15)
To an N,N-dimethylformamide (20 mL) solution of 3,4,5-trimethoxy-phenol (3.0 g, 16 mmol), dimethyl sulfate (4.6 mL, 49 mmol) and Cs2CO3 (10 g, 33 mmol) were added. The reaction mixture was refluxed for 4 h. After cooling to ambient temperature, the reaction mixture was diluted with ethyl acetate and the organic phase was washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:5) to afford 1,2,3,5-tetramethoxybenzene (15) (3.2 g, 98%). 1H NMR (600 MHz, CDCl3) δ 6.15 (s, 2H), 3.84 (s, 6H), 3.78 (s, 3H), 3.78 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 156.4, 153.8, 132.4, 91.7, 61.1, 56.2, 55.6. HRMS (ESI) m/z: [M + H]+ calcd for C10H14O4 199.0970; found, 199.0966.
1-(2-Hydroxy-3,4,6-trimethoxyphenyl)ethan-1-one (16)
To a chloroform (15 mL) solution of 1,2,3,5-tetramethoxybenzene (15) (3.2 g, 16 mmol), acetic anhydride (1.7 mL, 18 mmol) and BF3·OEt2 (6.9 mL, 56 mmol) were added at 0 °C. After stirring at 70 °C for 4 h, the reaction mixture was cooled, and ice-cold water and 5% NaOH (5 mL) were poured into the mixture. The reaction mixture was diluted with ethyl acetate, and the organic phase was washed with water and brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography (ethyl acetate/n-hexane = 1:3) to afford 1-(2-hydroxy-3,4,6-trimethoxyphenyl)ethan-1-one (16) (3.1 g, 87%). 1H NMR (600 MHz, CDCl3) δ 13.80 (s, 1H), 5.95 (s, 1H), 3.93 (s, 3H), 3.88 (s, 3H), 3.80 (s, 3H), 2.61 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 203.9, 159.2, 158.9, 158.5, 130.6, 106.4, 86.5, 60.8, 56.1, 55.7, 33.3. HRMS (ESI) m/z: [M + H]+ calcd for C11H14O5 227.0919; found, 227.0914.
5,7,8-Trimethoxy-4H-chromen-4-one (17)
To a solution of 1-(2-hydroxy-3,4,6-trimethoxyphenyl)ethan-1-one (16) (2.7 g, 12 mmol) in dimethoxyethane (DME) (30 mL) was added N,N-dimethylformamide dimethyl acetal (4.8 mL, 36 mmol). After stirring for 24 h at 80 °C, the reaction mixture was cooled to 0 °C and c-HCl (6 mL) was added. After stirring for 1 h at 50 °C, the reaction mixture was diluted with ethyl acetate and the organic phase was washed with water and brine and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and purified by flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:1:0.1) to afford 5,7,8-trimethoxy-4H-chromen-4-one (17) (2.2 g, 76%). 1H NMR (600 MHz, CDCl3) δ 7.70 (d, 1H, J = 5.9 Hz), 6.43 (s, 1H), 6.18 (d, 1H, J = 5.9 Hz), 3.99 (s, 3H), 3.96 (s, 3H), 3.88 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 176.9, 156.7, 156.6, 152.9, 152.4, 130.7, 114.2, 110.2, 92.7, 61.8, 56.7, 56.4. HRMS (ESI) m/z: [M + H]+ calcd for C12H12O5 237.0763; found, 237.0760.
5,7,8-Trimethoxychroman-4-one (18)
5,7,8-Trimethoxy-4H-chromen-4-one (17) (2.8 g, 12 mmol) and 10% Pd/C (0.38 g, 3.5 mmol) in methanol (40 mL) were placed under an atmosphere of hydrogen. After stirring for 1 h, the mixture was filtered through a Celite pad. After the filtrate was concentrated under reduced pressure, purification of the residue via flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:1:0.1) afforded 5,7,8-trimethoxychroman-4-one (18) (2.6 g, 92.%). 1H NMR (600 MHz, CDCl3) δ 6.09 (s, 1H), 4.50 (t, 2H, J = 6.4 Hz), 3.93 (s, 3H), 3.90 (s, 3H), 3.79 (s, 3H), 2.72 (t, 2H, J = 6.4 Hz). 13C{1H} NMR (150 MHz, CDCl3) δ 189.5, 158.6, 158.1, 156.7, 130.8, 106.7, 89.3, 67.3, 61.3, 56.3, 56.1, 39.0. HRMS (ESI) m/z: [M + H]+ calcd for C12H14O5 239.0919; found, 239.0912.
5-Hydroxy-7,8-dimethoxychroman-4-one (1)
To a solution of 5,7,8-trimethoxychroman-4-one (18) (50 mg, 0.21 mmol) in CH2Cl2 (3 mL) was added boron trichloride (0.62 mL, 1.0 M solution in CH2Cl2) at −78 °C. After stirring for 1 h, the reaction mixture was diluted with CH2Cl2 and washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to afford 5-hydroxy-7,8-dimethoxychroman-4-one (1) (40 mg, 85%). 1H NMR (600 MHz, CDCl3) δ 11.98 (s, 1H), 6.08 (s, 1H), 4.52 (t, 2H, J = 6.3 Hz), 3.88 (s, 2H), 3.77 (s, 2H), 2.79 (t, 2H, J = 6.3 Hz). 13C{1H} NMR (150 MHz, CDCl3) δ 196.2, 161.5, 160.3, 154.0, 129.6, 103.3, 93.1, 67.1, 61.5, 56.4, 36.8. HRMS (ESI) m/z: [M + H]+ calcd for C11H12O5 225.0763; found, 225.0760.
(E)-3-(4′-(Benzyloxy)benzylidene)-5,7,8-trimethoxychroman-4-one (19a)
To a solution of 5,7,8-trimethoxychroman-4-one (18) (0.50 g, 2.1 mmol) in toluene (20 mL) were added 4-benzyloxybenzaldehyde (0.67 g, 3.1 mmol) and p-toluenesulfonic acid (36 mg, 0.21 mmol) at 0 °C. The reaction mixture was refluxed for 12 h. After cooling to ambient temperature, the mixture was concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:2:0.1) to afford (E)-3-(4′-(benzyloxy)benzylidene)-5,7,8-trimethoxychroman-4-one (19a) (0.64 g, 71%). 1H NMR (600 MHz, CDCl3) δ 7.69 (s, 1H), 7.43 (d, J = 7.4 Hz, 2H), 7.37 (t, 2H, J = 7.6 Hz), 7.30 (t, 1H, J = 7.3 Hz), 6.92 (d, 1H, J = 8.4 Hz), 6.88 (dd, 1H, J = 8.4, 1.7 Hz), 6.78 (d, 1H, J = 1.7 Hz), 6.15 (s, 1H), 5.17 (s, 2H), 5.13 (d, 2H, J = 1.5 Hz), 3.93 (s, 3H), 3.92 (s, 3H), 3.92 (s, 3H), 3.78 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 179.8, 159.6, 158.4, 158.3, 155.9, 136.5, 135.9, 131.8, 130.9, 130.1, 128.7, 128.1, 127.6, 127.5, 115.0, 107.6, 90.1, 70.1, 67.9, 61.3, 56.3, 56.1. HRMS (ESI) m/z: [M + H]+ calcd for C26H24O6 433.1651; found, 433.1650.
(E)-3-(3′,4′-Bis(benzyloxy)benzylidene)-5,7,8-trimethoxychroman-4-one (19b)
To a solution of 5,7,8-trimethoxychroman-4-one (18) (0.50 g, 2.1 mmol) in toluene (20 mL) were added 3,4-bis(benzyloxy)benzaldehyde (1.0 g, 3.2 mmol) and p-toluenesulfonic acid (36 mg, 0.21 mmol) at 0 °C. The reaction mixture was refluxed for 12 h. After cooling to ambient temperature, the mixture was concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/CH2Cl2 = 1:5) to afford (E)-3-(3′,4′-bis(benzyloxy)benzylidene)-5,7,8-trimethoxychroman-4-one (19b) (0.71 g, 63%). 1H NMR (600 MHz, CDCl3) δ 7.70 (s, 1H), 7.47–7.43 (m, 4H), 7.38 (td, 4H, J = 7.5, 2.1 Hz), 7.34–7.30 (m, 2H), 6.97–6.94 (m, 1H), 6.84 (dd, 2H, J = 5.4, 1.9 Hz), 6.17 (s, 1H), 5.22 (s, 2H), 5.19 (s, 2H), 5.15 (d, 2H, J = 1.7 Hz), 3.94 (s, 3H), 3.94 (s, 3H), 3.79 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 179.9, 158.5, 158.5, 156.0, 150.1, 148.6, 137.0, 136.9, 136.1, 131.1, 130.4, 128.8, 128.7, 128.2, 128.1, 127.4, 127.4, 124.3, 116.9, 114.4, 107.8, 90.2, 71.6, 71.2, 67.9, 61.5, 56.5, 56.2. HRMS (ESI) m/z: [M + H]+ calcd for C33H30O7 539.2070; found, 539.2067.
(E)-3-(3′-(Benzyloxy)-4′-methoxybenzylidene)-5,7,8-trimethoxychroman-4-one (19c)
To a solution of 5,7,8-trimethoxychroman-4-one (18) (0.50 g, 2.1 mmol) in toluene (20 mL) were added 3-(benzyloxy)-4-methoxybenzaldehyde (0.77 g, 3.2 mmol) and p-toluenesulfonic acid (36 mg, 0.21 mmol) at 0 °C. The reaction mixture was refluxed for 13 h. After cooling to ambient temperature, the mixture was concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:2:0.1) to afford (E)-3-(3′-(benzyloxy)-4′-methoxybenzylidene)-5,7,8-trimethoxychroman-4-one (19c) (0.60 g, 62%). 1H NMR (600 MHz, CDCl3) δ 7.77 (s, 1H), 7.41 (d, 2H, J = 7.3 Hz), 7.37 (t, 2H, J = 7.3 Hz), 7.32 (d, 1H, J = 7.3 Hz), 7.23 (d, 2H, J = 8.7 Hz), 7.00 (d, 2H, J = 8.7 Hz), 6.16 (s, 1H), 5.28 (d, 2H, J = 1.6 Hz), 5.08 (s, 2H), 3.92 (s, 3H), 3.91 (s, 3H), 3.77 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 179.8, 158.4, 158.4, 155.9, 150.8, 147.9, 136.8, 136.1, 131.0, 130.2, 128.8, 128.1, 127.5, 127.3, 124.2, 115.9, 111.6, 107.7, 90.2, 71.2, 67.9, 61.4, 56.4, 56.1, 56.1. HRMS (ESI) m/z: [M + H]+ calcd for C27H26O7 463.1757; found, 463.1754.
7-O-Methoxypunctatin (2)
To a solution of (E)-3-(4′-(benzyloxy)benzylidene)-5,7,8-trimethoxychroman-4-one (19a) (31 mg, 0.07 mmol) in CH2Cl2 (2 mL) was added boron trichloride (0.21 mL, 1.0 M solution in CH2Cl2) at −78 °C. After stirring for 1 h, the reaction mixture was diluted with CH2Cl2, washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:1:0.1) to afford 7-O-methoxypunctatin (2) (20 mg, 85%). 1H NMR (600 MHz, CDCl3) δ 12.66 (s, 1H), 7.79 (s, 1H), 7.20 (d, 2H, J = 8.6 Hz), 6.91 (d, 2H, J = 8.6 Hz), 6.11 (s, 1H), 5.55 (s, 1H), 5.34 (d, 2H, J = 1.7 Hz), 3.88 (s, 3H), 3.76 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 185.8, 161.5, 161.1, 157.5, 153.0, 137.7, 132.5, 129.5, 127.6, 127.0, 116.1, 103.2, 93.4, 67.8, 61.6, 56.4. HRMS (ESI) m/z: [M + H]+ calcd for C18H16O6 329.1025; found, 329.1023.
7-O-Methyl-3′-hydroxypunctatin (3)
To a solution of (E)-3-(3′,4′-bis(benzyloxy)benzylidene)-5,7,8-trimethoxy-chroman-4-one (19b) (31 mg, 0.06 mmol) in CH2Cl2 (2 mL) was added boron trichloride (0.17 mL, 1.0 M solution in CH2Cl2) at 0 °C. After stirring for 30 min, the reaction mixture was diluted with CH2Cl2, washed with water, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:1:0.1) to afford (E)-3-(3,4-dihydroxybenzylidene)-5-hydroxy-7,8-dimethoxychroman-4-one (3) (16 mg, 83%). 1H NMR (600 MHz, CD3OD) δ 7.72 (s, 1H), 6.89 (d, 1H, J = 8.2 Hz), 6.85 (s, 1H), 6.81 (d, 1H, J = 8.2 Hz), 6.19 (s, 1H), 5.40 (s, 1H), 3.89 (s, 1H), 3.72 (s, 1H). 13C{1H} NMR (150 MHz, CD3OD) δ 187.1, 162.9, 162.2, 154.5, 149.4, 146.8, 139.3, 130.7, 128.0, 127.5, 125.1, 118.6, 116.8, 104.1, 94.2, 69.1, 61.7, 56.9. HRMS (ESI) m/z: [M + H]+ calcd for C18H16O7 345.0974; found, 345.0978.
5,7,8-Trimethoxy-3-(4′-hydroxybenzyl)-4-chromanone (13)
(E)-3-(4′-(Benzyloxy)benzylidene)-5,7,8-trimethoxychroman-4-one (19a) (30 mg, 0.07 mmol) and 10% Pd/C (3.7 mg, 0.03 mmol) in methanol (3 mL) were placed under an atmosphere of hydrogen. After stirring for 2 h, the mixture was filtered through a Celite pad. After the filtrate was concentrated under reduced pressure, purification of the residue via flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:2:0.1) afforded 5,7,8-trimethoxy-3-(4′-hydroxybenzyl)-4-chromanone (13) (22 mg, 90%). 1H NMR (600 MHz, CD3OD) δ 7.08 (d, 2H, J = 8.1 Hz), 6.76 (d, 2H, J = 8.1 Hz), 6.32 (s, 1H), 4.34 (dd, 1H, J = 11.3, 3.8 Hz), 4.18 (dd, 1H, J = 11.3, 7.1 Hz), 3.96 (s, 3H), 3.90 (s, 3H), 3.73 (s, 3H), 3.06 (dd, J = 13.8, 4.6 Hz, 1H), 2.76–2.70 (m, 1H), 2.66 (dd, 1H, J = 13.6, 10.4 Hz). 13C{1H} NMR (150 MHz, CD3OD) δ 193.9, 160.4, 159.6, 157.4, 156.9, 131.4, 131.0, 130.2, 116.2, 106.1, 90.5, 70.1, 61.2, 56.5, 56.2, 49.7, 33.1. HRMS (ESI) m/z: [M + H]+ calcd for C19H20O6 345.1338; found, 345.1332.
3-(4′-Benzyloxy)-5,7,8-trimethoxychroman-4-one (20a)
An ethyl acetate (3 mL) and CH2Cl2 (1 mL) solution of (E)-3-(4-(benzyloxy)-benzylidene)-5,7,8-trimethoxychroman-4-one (19a) (27 mg, 0.06 mmol) as well as 5% wet Pd/C (3.5 mg, 0.03 mmol) was placed under an atmosphere of hydrogen. After stirring for 1 h, the mixture was filtered through a Celite pad. After the filtrate was concentrated under reduced pressure, purification of the residue via flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:3:0.1) afforded 3-(4′-(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-one (20a) (25 mg, 93%). 1H NMR (600 MHz, CDCl3) δ 7.43 (d, 2H, J = 7.3 Hz), 7.38 (t, 2H, J = 7.5 Hz), 7.32 (t, 1H, J = 7.3 Hz), 7.15 (d, 2H, J = 8.6 Hz), 6.92 (d, 2H, J = 8.6 Hz), 6.12 (s, 1H), 5.04 (s, 2H), 4.35 (dd, 1H, J = 11.3, 4.1 Hz), 4.18 (dd, 1H, J = 11.3, 7.5 Hz), 3.94 (s, 3H), 3.92 (s, 3H), 3.79 (s, 3H), 3.19 (dd, 1H, J = 14.0, 4.4 Hz), 2.77 (m, 1H), 2.64 (dd, 1H, J = 14.0, 10.6 Hz). 13C{1H} NMR (150 MHz, CDCl3) δ 191.7, 158.6, 158.3, 157.6, 156.4, 137.2, 130.9, 130.7, 130.3, 128.7, 128.0, 127.6, 115.1, 105.8, 89.3, 70.1, 69.3, 61.3, 56.3, 56.1, 48.7, 32.0. HRMS (ESI) m/z: [M + H]+ calcd for C26H26O6 435.1810; found, 435.1803.
3-(3′,4′-Bis(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-one (20b)
An ethyl acetate (9 mL) and CH2Cl2 (3 mL) solution of (E)-3-(3′,4′-bis(benzyloxy)-benzylidene)-5,7,8-trimethoxychroman-4-one (19b) (82 mg, 0.15 mmol) as well as 5% wet Pd/C (8.0 mg, 0.07 mmol) was placed under an atmosphere of hydrogen. After stirring for 1 h, the mixture was filtered through a Celite pad. After the filtrate was concentrated under reduced pressure, purification of the residue via flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:3:0.1) afforded 3-(3′,4′-bis(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-one (20b) (75 mg, 91%). 1H NMR (600 MHz, CDCl3) δ 7.47–7.43 (m, 4H), 7.38–7.34 (m, 4H), 7.33–7.28 (m, 2H), 6.87 (d, 1H, J = 8.1 Hz), 6.82 (d, 1H, J = 2.0 Hz), 6.74 (dd, 1H, J = 8.1, 2.0 Hz), 6.12 (s, 1H), 5.15 (d, 4H, J = 6.2 Hz), 4.28 (dd, 1H, J = 11.3, 4.2 Hz), 4.09 (dd, 1H, J = 11.3, 7.5 Hz), 3.94 (s, 3H), 3.92 (s, 3H), 3.80 (s, 3H), 3.15 (dd, 1H, J = 14.0, 4.3 Hz), 2.72 (m, 1H), 2.59 (dd, 1H, J = 14.0, 10.6 Hz). 13C{1H} NMR (150 MHz, CDCl3) δ 191.7, 158.6, 158.3, 156.5, 149.0, 147.8, 137.5, 137.34, 132.0, 130.7, 128.6, 127.9, 127.9, 127.6, 127.4, 122.3, 116.3, 115.5, 105.9, 89.4, 71.6, 71.5, 69.3, 61.3, 56.3, 56.2, 48.6, 32.4. HRMS (ESI) m/z: [M + H]+ calcd for C33H32O7 541.2226; found, 541.2224.
3-(3′-(Benzyloxy)-4′-methoxybenzyl)-5,7,8-trimethoxychroman-4-one (20c)
An ethyl acetate (9 mL) and CH2Cl2 (3 mL) solution of (E)-3-(3-(benzyloxy)-4-methoxybenzylidene)-5,7,8-trimethoxychroman-4-one (19c) (0.16 g, 0.35 mmol) as well as 5% wet Pd/C (18 mg, 0.17 mmol) was placed under an atmosphere of hydrogen. After stirring for 1 h, the mixture was filtered through a Celite pad. After the filtrate was concentrated under reduced pressure, purification of the residue via flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:3:0.1) afforded 3-(3′-(benzyloxy)-4′-methoxybenzyl)-5,7,8-trimethoxychroman-4-one (20c) (0.15 g, 94%). 1H NMR (600 MHz, CDCl3) δ 7.44 (d, 2H, J = 7.6 Hz), 7.35 (t, 2H, J = 7.6 Hz), 7.28 (t, 1H, J = 7.4 Hz), 6.83 (d, 1H, J = 8.1 Hz), 6.79–6.76 (m, 2H), 6.12 (s, 1H), 5.14 (s, 2H), 4.26 (dd, 1H, J = 11.3, 4.1 Hz), 4.07 (dd, 1H, J = 11.3, 7.5 Hz), 3.94 (s, 3H), 3.92 (s, 3H), 3.87 (s, 3H), 3.79 (s, 3H), 3.14 (dd, 1H, J = 13.9, 4.2 Hz), 2.70 (ddd, 1H, J = 11.6, 8.3, 4.2 Hz), 2.59 (dd, 1H, J = 13.9, 10.6 Hz). 13C{1H} NMR (150 MHz, CDCl3) δ 191.7, 158.6, 158.3, 156.5, 148.6, 148.2, 137.2, 131.1, 130.6, 128.7, 128.0, 127.6, 122.1, 115.4, 112.1, 105.9, 89.4, 71.2, 69.3, 61.3, 56.3, 56.2, 56.2, 48.7, 32.4. HRMS (ESI) m/z: [M + H]+ calcd for C27H28O7 465.1913; found, 465.1909.
3-(4′-(Benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-ol ((cis)-21a)
To a solution of 3-(4′-(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-one (20a) (30 mg, 0.069 mmol) in anhydrous tetrahydrofuran (THF) (2 mL), l-selectride (0.10 mL, 1.0 M in THF) was added dropwise at −78 °C. After 1 h, the reaction was completed and quenched with NH4Cl, extracted with ethyl ether, and dried over anhydrous Na2SO4. The residue was purified by flash chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to afford 3-(4′-(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-ol (cis-21a) (30 mg, 98%).
(3R,4R)-3-(4′-(Benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-ol ((3R,4R)-21a)
DBU and formic acid (3:1 (v/v)) were dissolved in acetonitrile. The solution was sparged with nitrogen for 15 min. Separately, 3-(4′-(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-one (20a) (53 mg, 0.12 mmol) and RuCl(p-cymene)[(R,R)-Ts-DPEN] (22) (23 mg, 0.036 mmol) were dissolved in acetonitrile and then added to the mixture of DBU and formic acid. The mixture was stirred for 24 h at 50 °C and then quenched by adding saturated NH4Cl solution at ambient temperature. After extraction with diethyl ether, the organic layer was washed with an additional portion of saturated NaHCO3 solution, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:1:0.1) to afford (3R,4R)-3-(4′-(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-ol ((3R,4R)-21a) (50 mg, 94%) [α]D24 = +100 (c 0.10, CH3OH). ee % = 92% ee. 1H NMR (600 MHz, CDCl3) δ 7.44 (d, 2H, J = 7.3 Hz), 7.39 (t, 2H, J = 7.5 Hz), 7.33 (t, 1H, J = 7.3 Hz), 7.20 (d, 2H, J = 8.6 Hz), 6.94 (d, 2H, J = 8.6 Hz), 6.09 (s, 1H), 5.06 (s, 2H), 4.73 (d, 1H, J = 2.5 Hz), 4.19–4.13 (m, 1H), 4.07–4.01 (m, 1H), 3.87 (s, 3H), 3.81 (s, 3H), 3.77 (s, 3H), 2.90 (dd, 1H, J = 13.8, 8.4 Hz), 2.63 (dd, 1H, J = 13.8, 7.3 Hz), 2.17 (m, 1H). 13C{1H} NMR (150 MHz, CDCl3) δ 157.4, 154.2, 153.3, 149.0, 137.3, 131.9, 131.5, 130.2, 128.7, 128.1, 127.6, 115.0, 107.9, 88.6, 70.2, 65.5, 61.2, 59.9, 56.4, 55.67, 40.1, 32.0. HRMS (ESI) m/z: [M + H]+ calcd for C26H28O6 541.2226; found, 541.2224. TR = 10 min (chiral IC, n-hexane/EtOH = 60:40).
3-(3′,4′-Bis(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-ol ((cis)-21b)
To a solution of 3-(3′,4′-bis(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-one (20b) (30 mg, 0.056 mmol) in anhydrous THF (2 mL), l-selectride (0.08 mL, 1.0 M in THF) was added dropwise at −78 °C. After 1 h, the reaction was completed, quenched with saturated NH4Cl solution, extracted with ethyl ether, and dried over anhydrous Na2SO4. The residue was purified by flash chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to afford 3-(3′,4′-bis(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-ol (cis-21b) (26 mg, 85%).
(3R,4R)-3-(3′,4′-Bis(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-ol ((3R,4R)-21b)
DBU and formic acid (3:1 (v/v)) were dissolved in acetonitrile. The solution was sparged with nitrogen for 15 min. Separately, 3-(3′,4′-bis(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-one (20b) (30 mg, 0.06 mmol) and RuCl(p-cymene)[(R,R)-Ts-DPEN] (22) (11 mg, 0.02 mmol) were dissolved in acetonitrile and then added to the mixture of DBU and formic acid. The mixture was stirred for 24 h at 50 °C and then quenched by adding saturated NH4Cl solution at ambient temperature. After extraction with diethyl ether, the organic layer was washed with an additional portion of saturated NaHCO3 solution, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:1:0.1) to afford (3R,4R)-3-(3′,4′-bis(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-ol ((3R,4R)-21b) (28 mg, 94%) [α]D24 = +103 (c 0.096, CH3OH). ee % = 95% ee. 1H NMR (600 MHz, CDCl3) δ 7.45 (dd, 4H, J = 7.2, 1.9 Hz), 7.36 (dt, 4H, J = 15.2, 7.6 Hz), 7.30 (dd, 2H, J = 14.6, 7.3 Hz), 6.89 (d, 1H, J = 8.2 Hz), 6.87 (d, 1H, J = 2.0 Hz), 6.78 (dd, 1H, J = 8.2, 2.0 Hz), 6.09 (s, 1H), 5.14 (dd, 4H, J = 10.7, 2.7 Hz), 4.65 (s, 1H), 4.10 (ddd, 1H, J = 10.4, 3.5, 1.0 Hz), 4.03–3.98 (m, 1H), 3.87 (s, 3H), 3.81 (s, 3H), 3.78 (s, 3H), 2.83 (dd, 1H, J = 13.8, 8.5 Hz), 2.59 (dd, 1H, J = 13.8, 7.2 Hz), 2.16–2.09 (m, 1H). 13C{1H} NMR (150 MHz, CDCl3) δ 154.2, 153.3, 149.0, 147.6, 137.6, 137.5, 133.0, 131.5, 128.6, 128.6, 127.9, 127.6, 127.5, 122.2, 116.6, 115.5, 107.9, 88.6, 71.6, 71.6, 65.5, 61.2, 59.8, 56.4, 55.7, 40.0, 32.4. HRMS (ESI) m/z: [M + Na]+ calcd for C33H34O7 565.2202; found, 565.2204. TR = 30 min (chiral ID, n-hexane/IPA/EtOH = 60:20:20).
3-(3′-(Benzyloxy)-4′-methoxybenzyl)-5,7,8-trimethoxychroman-4-ol ((cis)-21c)
To a solution of 3-(3′-(benzyloxy)-4′-methoxybenzyl)-5,7,8-trimethoxychroman-4-one (20c) (30 mg, 0.065 mmol) in anhydrous THF (2 mL), l-selectride (0.10 mL, 1.0 M in THF) was added dropwise at −78 °C. After 1 h, the reaction was completed and quenched with NH4Cl, extracted with ethyl ether, and dried over anhydrous Na2SO4. The residue was purified by flash chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to afford 3-(3′-(benzyloxy)-4′-methoxybenzyl)-5,7,8-trimethoxychroman-4-ol (cis-21c) (30 mg, 99%).
(3R,4R)-3-(3′-(Benzyloxy)-4′-methoxybenzyl)-5,7,8-trimethoxychroman-4-ol ((3R,4R)-21c)
DBU and formic acid (3:1 (v/v)) were dissolved in acetonitrile. The solution was sparged with nitrogen for 15 min. Separately, 3-(3′-(benzyloxy)-4′-methoxybenzyl)-5,7,8-trimethoxychroman-4-one (20c) (86 mg, 0.19 mmol) and RuCl(p-cymene)[(R,R)-Ts-DPEN] (22) (35 mg, 0.06 mmol) were dissolved in acetonitrile and then added to the mixture of DBU and formic acid. The mixture was stirred for 24 h at 50 °C and then quenched by adding saturated NH4Cl solution at ambient temperature. After extraction with diethyl ether, the organic layer was washed with an additional portion of saturated NaHCO3 solution, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:1:0.1) to afford (3R,4R)-3-(3′-(benzyloxy)-4′-methoxybenzyl)-5,7,8-trimethoxy-chroman-4-ol ((3R,4R)-21c) (84 mg, 97%) [α]D24 = +92 (c 0.098, CH3OH). ee % = 99% ee. 1H NMR (600 MHz, CDCl3) δ 7.44 (d, 2H, J = 7.3 Hz), 7.35 (t, 2H, J = 7.6 Hz), 7.29–7.25 (m, 1H), 6.86–6.81 (m, 3H), 6.08 (s, 1H), 5.14 (s, 2H), 4.63 (d, 1H, J = 2.8 Hz), 4.11–4.07 (m, 1H), 4.02–3.97 (m, 1H), 3.88 (s, 3H), 3.86 (s, 3H), 3.81 (s, 3H), 3.78 (s, 3H), 2.81 (dd, 1H, J = 13.8, 8.6 Hz), 2.58 (dd, 1H, J = 13.8, 7.2 Hz), 2.15–2.08 (m, 1H). 13C{1H} NMR (150 MHz, CDCl3) δ 154.2, 153.3, 148.91, 148.3, 148.1, 137.4, 132.0, 131.5, 128.6, 127.9, 127.5, 121.9, 115.6, 112.1, 107.8, 88.5, 71.2, 65.5, 61.1, 59.8, 56.4, 56.2, 55.6, 40.0, 32.3. HRMS (ESI) m/z: [M + H]+ calcd for C27H30O7 497.2070; found, 467.2063. TR = 26 min (chiral ID, n-hexane/IPA/EtOH = 60:20:20).
(R)-3-(4′-(Benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-one ((R)-20a)
A solution of (3R,4R)-3-(4′-(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-ol ((3R,4R)-21a) (0.11 g, 0.24 mmol) in CH2Cl2 (5 mL) was treated with molecular sieves (4 Å, 0.10 g) and N-methylmorpholine N-oxide (0.1 g, 0.84 mmol), and the mixture was stirred for 15 min. Then, tetrapropylammonium perruthenate (85 mg, 0.24 mmol) was added, and stirring was continued at ambient temperature for 30 min. The reaction mixture was diluted with diethyl ether, washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to afford (R)-3-(4′-(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-one ((R)-20a) (0.10 g, 97%) [α]D24 = −83 (c 0.072, CH3OH). ee % = 92% ee. 1H NMR (600 MHz, CDCl3) δ 7.43 (d, 2H, J = 7.3 Hz), 7.38 (t, 2H, J = 7.5 Hz), 7.32 (t, 1H, J = 7.3 Hz), 7.15 (d, 2H, J = 8.6 Hz), 6.92 (d, 2H, J = 8.6 Hz), 6.12 (s, 1H), 5.05 (s, 2H), 4.35 (dd, 1H, J = 11.3, 4.1 Hz), 4.18 (dd, 1H, J = 11.3, 7.5 Hz), 3.94 (s, 3H), 3.92 (s, 3H), 3.79 (s, 3H), 3.19 (dd, 1H, J = 14.0, 4.3 Hz), 2.77 (m, 1H), 2.64 (dd, 1H, J = 14.0, 10.6 Hz). 13C{1H} NMR (150 MHz, CDCl3) δ 191.7, 158.6, 158.3, 157.6, 156.4, 137.2, 130.9, 130.7, 130.3, 128.7, 128.1, 127.6, 115.1, 105.8, 89.4, 70.2, 69.3, 61.3, 56.3, 56.2, 48.7, 32.0. HRMS (ESI) m/z: [M + H]+ calcd for C26H26O6 435.1808; found, 435.1803. TR = 21 min (chiral ID, n-hexane/EtOH = 70:30).
(R)-3-(3′,4′-Bis(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-one ((R)-20b)
A solution of (3R,4R)-3-(3′,4′-bis(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-ol ((3R,4R)-21b) (17 mg, 0.03 mmol) in CH2Cl2 (2 mL) was treated with molecular sieves (4 Å, 13 mg) and N-methylmorpholine N-oxide (13 mg, 0.11 mmol), and the mixture was stirred for 15 min. Then, tetrapropylammonium perruthenate (11 mg, 0.03 mmol) was added, and stirring was continued at ambient temperature for 30 min. The reaction mixture was diluted with diethyl ether, washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to afford (R)-3-(3′,4′-bis(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-one ((R)-20b) (69 mg, 93%) [α]D24 = −87 (c 0.058, CH3OH). ee % = 87% ee. 1H NMR (600 MHz, CDCl3) δ 7.44 (dd, 4H, J = 7.1, 4.1 Hz), 7.35 (td, 4H, J = 7.5, 2.5 Hz), 7.30 (td, 2H, J = 7.2, 5.5 Hz), 6.86 (d, 1H, J = 8.2 Hz), 6.82 (d, 1H, J = 1.9 Hz), 6.74 (dd, 1H, J = 8.2, 1.9 Hz), 6.12 (s, 1H), 5.15 (s, 2H), 5.13 (s, 2H), 4.27 (dd, 1H, J = 11.3, 4.1 Hz), 4.08 (dd, 1H, J = 11.3, 7.5 Hz), 3.94 (s, 3H), 3.92 (s, 3H), 3.79 (s, 3H), 3.14 (dd, 1H, J = 14.0, 4.3 Hz), 2.71 (m, 1H), 2.59 (dd, 1H, J = 14.0, 10.6 Hz). 13C{1H} NMR (150 MHz, CDCl3) δ 191.7, 158.6, 158.3, 156.4, 149.0, 147.8, 137.5, 137.3, 132.0, 130.7, 128.6, 127.9, 127.9, 127.6, 127.4, 122.3, 116.3, 115.5, 105.9, 89.3, 71.6, 71.5, 69.3, 61.3, 56.3, 56.2, 48.6, 32.4. HRMS (ESI) m/z: [M + H]+ calcd for C33H32O7 541.2226; found, 541.2222. TR = 27 min (chiral ID, n-hexane/EtOH = 70:40).
(R)-3-(3′-(Benzyloxy)-4′-methoxybenzyl)-5,7,8-trimethoxychroman-4-one ((R)-20c)
A solution of (3R,4R)-3-(3′-(benzyloxy)-4′-methoxybenzyl)-5,7,8-trimethoxy-chroman-4-ol ((3R,4R)-21c) (70 mg, 0.15 mmol) in CH2Cl2 (3 mL) was treated with molecular sieves (4 Å, 60 mg) and N-methylmorpholine N-oxide (60 mg, 0.51 mmol), and the mixture was stirred for 15 min. Then, tetrapropylammonium perruthenate (54 mg, 0.15 mmol) was added, and stirring was continued at ambient temperature for 20 min. The reaction mixture was diluted with diethyl ether, washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to afford (R)-3-(3′-(benzyloxy)-4′-methoxybenzyl)-5,7,8-trimethoxychroman-4-one ((R)-20c) (69 mg, 99%) [α]D24 = −57 (c 0.088, CH3OH). ee % = 84% ee. 1H NMR (600 MHz, CDCl3) δ 7.43 (d, 2H, J = 7.5 Hz), 7.34 (t, 2H, J = 7.5 Hz), 7.29–7.25 (m, 1H), 6.82 (d, 1H, J = 7.9 Hz), 6.77 (m, 2H), 6.11 (s, 1H), 5.12 (s, 2H), 4.25 (dd, 1H, J = 11.2, 3.7 Hz), 4.06 (dd, 1H, J = 11.2, 7.7 Hz), 3.93 (s, 3H), 3.90 (s, 3H), 3.85 (s, 3H), 3.78 (s, 3H), 3.76–3.76 (m, 1H), 3.12 (dd, 1H, J = 13.6, 3.8 Hz), 2.69 (m, 1H), 2.58 (dd, 1H, J = 13.6 10.9 Hz). 13C{1H} NMR (150 MHz, CDCl3) δ 191.6, 158.6, 158.3, 156.4, 148.5, 148.1, 137.1, 131.0, 130.6, 128.6, 127.9, 12 127.5, 122.0, 115.3, 112.0, 105.8, 89.3, 71.1, 69.2, 61.3, 56.3, 56.2, 56.1, 48.6, 32.4. HRMS (ESI) m/z: [M + H]+ calcd for C27H28O7 465.1913; found, 465.1908. TR = 24 min (chiral ID, n-hexane/EtOH = 70:40).
(R)-7-Methyl-3,9-dihydropunctatin ((R)-4)
To a solution of (R)-3-(4-(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-one ((R)-20a) (32 mg, 0.08 mmol) in CH2Cl2 (2 mL) was added boron trichloride (0.23 mL, 1.0 M solution in CH2Cl2) at −78 °C. After stirring for 40 min, the reaction mixture was diluted with CH2Cl2, washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:1:0.1) to afford (R)-7-methyl-3,9-dihydropunctation ((R)-4) (23 mg, 93%). [α]D24 = −74 (c 0.068, CH3OH). ee % = 86% ee. 1H NMR (600 MHz, CD3OD) δ 7.03 (d, 2H, J = 8.5 Hz), 6.70 (d, 2H, J = 8.5 Hz), 6.13 (s, 1H), 4.29 (dd, 1H, J = 11.4, 4.3 Hz), 4.14 (dd, 1H, J = 11.4, 7.3 Hz), 3.84 (s, 3H), 3.66 (s, 3H), 3.08 (dd, 1H, J = 14.0, 4.7 Hz), 2.83 (m, 1H), 2.64 (dd, 1H, J = 14.0, 10.1 Hz). 13C{1H} NMR (150 MHz, CD3OD) δ 199.8, 162.5, 161.3, 157.1, 154.9, 131.0, 130.2, 129.8, 116.2, 103.1, 93.6, 70.4, 61.3, 56.5, 48.0, 32.7. HRMS (ESI) m/z: [M + H]+ calcd for C18H18O6 331.1181; found, 331.1181. TR = 7.7 min (chiral IB, 0.1% diethanolamine (DEA) in n-hexane/EtOH = 70:30).
(R)-7-O-Methyl-3′-hydroxy-3,9-dihydropunctatin ((R)-5)
To a solution of (R)-3-(3,4-bis(benzyloxy)benzyl)-5,7,8-trimethoxychroman-4-one ((R)-20b) (30 mg, 0.06 mmol) in CH2Cl2 (3 mL) was added boron trichloride (0.16 mL, 1.0 M solution in CH2Cl2) at −78 °C. After stirring for 90 min, the reaction mixture was diluted with CH2Cl2, washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:1:0.1) to afford (R)-7-O-methyl-3′-hydroxy-3,9-dihydropunctatin ((R)-5) (17 mg, 89%). [α]D24 = −75 (c 0.066, CH3OH). ee % = 95% ee. 1H NMR (600 MHz, CDCl3) δ 12.02 (s, 1H), 6.78 (d, 1H, J = 8.0 Hz), 6.73 (d, 1H, J = 2.0 Hz), 6.63 (dd, 1H, J = 8.0, 2.0 Hz), 6.08 (s, 1H), 5.54 (br s, 1H), 5.43 (br s, 1H), 4.32 (dd, 1H, J = 11.4, 4.2 Hz), 4.17 (dd, 1H, J = 11.5, 6.9 Hz), 3.87 (s, 3H), 3.76 (s, 3H), 3.08 (dd, 1H, J = 14.0, 4.6 Hz), 2.79 (m, 1H), 2.65 (dd, 1H, J = 14.0, 10.3 Hz). 13C{1H} NMR (150 MHz, CDCl3) δ 198.4, 161.5, 160.5, 153.8, 144.0, 142.6, 130.8, 129.4, 121.8, 116.3, 115.7, 102.5, 93.2, 69.5, 61.6, 56.4, 47.0, 32.4. HRMS (ESI) m/z: [M + H]+ calcd for C18H18O7 347.1131; found, 347.1125. TR = 26 min (chiral IG, 0.1% trifluoroacetic acid (TFA) in n-hexane/EtOH = 70:30).
(R)-7,4′-Di-O-methyl-3′-hydroxy-3,9-dihydropunctatin ((R)-6)
To a solution of (R)-3-(3-(benzyloxy)-4-methoxybenzyl)-5,7,8-trimethoxy-chroman-4-one ((R)-20c) (25 mg, 0.05 mmol) in CH2Cl2 (2 mL) was added boron trichloride (0.16 mL, 1.0 M solution in CH2Cl2) at −78 °C. After stirring for 1 h, the reaction mixture was diluted with CH2Cl2, washed with water, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:1:0.1) to afford (R)-7,4′-di-O-methyl-3′-hydroxy-3,9-dihydropunctatin ((R)-6) (18 mg, 93%). [α]D24 = −48 (c 0.10, CH3OH). ee % = 75% ee. 1H NMR (600 MHz, CDCl3) δ 12.04 (s, 1H), 6.80 (d, 1H, J = 2.1 Hz), 6.78 (d, 1H, J = 8.2 Hz), 6.69 (dd, 1H, J = 8.2, 2.1 Hz), 6.09 (s, 1H), 5.62 (s, 1H), 4.34 (dd, 1H, J = 11.4, 4.3 Hz), 4.19 (dd, 1H, J = 11.4, 7.3 Hz), 3.88 (s, 3H), 3.87 (s, 3H), 3.76 (s, 3H), 3.15 (dd, 1H, J = 14.0, 4.6 Hz), 2.87–2.82 (m, 1H), 2.67 (dd, 1H, J = 14.0, 10.4 Hz). 13C{1H} NMR (150 MHz, CDCl3) δ 198.3, 161.5, 160.4, 153.9, 145.9, 145.6, 131.1, 129.5, 120.8, 115.3, 110.9, 102.5, 93.2, 69.5, 61.5, 56.4, 56.2, 47.0, 32.3. HRMS (ESI) m/z: [M + H]+ calcd for C19H20O7 361.1287; found, 361.1283. TR = 12 min (chiral IB, 0.1% diethanolamine (DEA) in n-hexane/EtOH = 70:30).
1-(4-(Benzyloxy)-2-hydroxy-3,6-dimethoxyphenyl)ethan-1-one (26)
First step: To an acetone (20 mL) solution of 1-(4-(benzyloxy)-6-hydroxy-2,3-dimethoxyphenyl)ethan-1-one (23) (2.0 g, 6.6 mmol), 2.0 M in t-butyl methyl ether of solution iodomethane (3.6 mL, 7.3 mmol) and Cs2CO3 (4.3 g, 13 mmol) were added. The reaction mixture was refluxed for 12 h. After cooling to ambient temperature, the reaction mixture was diluted with ethyl acetate and the organic phase was washed with water and saturated NH4Cl solution, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to afford 1-(4-(benzyloxy)-2,3,6-trimethoxyphenyl)ethan-1-one (2.0 g, 95%). 1H NMR (600 MHz, CDCl3) δ 7.44 (d, J = 7.2 Hz, 2H), 7.39 (t, J = 7.3 Hz, 2H), 7.34 (d, J = 7.1 Hz, 1H), 6.29 (s, 1H), 5.14 (s, 2H), 3.90 (s, 3H), 3.83 (s, 3H), 3.71 (s, 3H), 2.47 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 201.4, 154.0, 152.5, 151.4, 136.9, 136.7, 128.8, 128.3, 127.4, 119.1, 94.7, 71.4, 62.2, 61.3, 56.2, 32.7. HRMS (ESI) m/z: [M + H]+ calcd for C18H20O5 317.1389; found, 317.1384. Second step: To a solution of 1-(4-(benzyloxy)-2,3,6-trimethoxyphenyl)ethan-1-one (1.1 g, 5.4 mmol) in CH2Cl2 (20 mL) was added boron trichloride (16 mL, 1.0 M solution in CH2Cl2) at −78 °C. After stirring for 1 h, the reaction mixture was diluted with CH2Cl2, washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane/methanol = 1:5) to afford intermediates (24 and 25) (1.0 g, 90%). Third step: To an acetone (20 mL) solution of intermediates (24 and 25) (1.0 g, 4.8 mmol), benzyl bromide (0.6 mL, 5.3 mmol) and K2CO3 (1.3 g, 9.6 mmol) were added. The reaction mixture was refluxed for 2 h. After cooling to ambient temperature, the reaction mixture was diluted with ethyl acetate and the organic phase was washed with water and saturated NH4Cl solution, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:2) to afford 1-(4-(benzyloxy)-2-hydroxy-3,6-dimethoxyphenyl)ethan-1-one (26) (1.0 g, 72%). 1H NMR (600 MHz, CDCl3) δ 13.82 (s, 1H), 7.43 (d, J = 7.4 Hz, 2H), 7.39 (t, J = 7.5 Hz, 2H), 7.33 (t, J = 7.3 Hz, 1H), 5.97 (s, 1H), 5.22 (s, 2H), 3.84 (s, 3H), 3.78 (s, 3H), 2.59 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 203.9, 159.2, 158.8, 157.6, 136.4, 131.2, 128.8, 128.3, 127.3, 106.6, 88.6, 70.9, 60.9, 55.6, 33.3. HRMS (ESI) m/z: [M + H]+ calcd for C17H18O5 303.1232; found, 303.1227.
7-(Benzyloxy)-5,8-dimethoxy-4H-chromen-4-one (27)
To a solution of 1-(4-(benzyloxy)-2-hydroxy-3,6-dimethoxyphenyl)ethan-1-one (26) (1.0 g, 3.3 mmol) in DME (20 mL) was added N,N-dimethylformamide dimethyl acetal (1.3 mL, 9.9 mmol). After stirring for 24 h at 80 °C, the mixture was cooled to 0 °C and c-HCl (5 mL) was added. After stirring for 1 h at 50 °C, the reaction mixture was diluted with ethyl acetate and the organic phase was washed with water and brine and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and purified by flash column chromatography on silica gel (ethyl acetate/CH2Cl2 = 1:3) to afford 7-(benzyloxy)-5,8-dimethoxy-4H-chromen-4-one (27) (0.97 g, 94%). 1H NMR (600 MHz, CDCl3) δ 7.69 (d, 1H, J = 5.9 Hz), 7.45 (d, 2H, J = 7.4 Hz), 7.40 (t, 2H, J = 7.4 Hz), 7.35 (t, 1H, J = 7.3 Hz), 6.45 (s, 1H), 6.17 (d, 1H, J = 5.9 Hz), 5.26 (s, 2H), 3.90 (s, 3H), 3.86 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 176.9, 156.3, 155.6, 152.9, 152.5, 136.1, 131.2, 128.9, 128.6, 127.4, 114.2, 110.5, 94.7, 71.4, 61.8, 56.6. HRMS (ESI) m/z: [M + H]+ calcd for C18H16O5 313.1076; found, 313.1071.
7-Hydroxy-5,8-dimethoxychroman-4-one (28)
7-(Benzyloxy)-5,8-dimethoxy-4H-chromen-4-one (27) (0.30 g, 0.96 mmol) and 10% Pd/C (0.01 g, 0.10 mmol) in anhydrous methanol (15 mL) were placed under an atmosphere of hydrogen. After stirring for 4 h, the mixture was filtered through a Celite pad. After the filtrate was concentrated under reduced pressure, purification of the residue via flash column chromatography on silica gel (ethyl acetate/CH2Cl2 = 1:3) afforded 7-hydroxy-5,8-dimethoxychroman-4-one (28) (0.22 g, 99%). 1H NMR (600 MHz, CD3OD) δ 6.13 (s, 1H), 4.48 (t, 2H, J = 6.4 Hz), 3.78 (s, 3H), 3.74 (s, 3H), 2.69 (t, 2H, J = 6.4 Hz). 13C{1H} NMR (150 MHz, CD3OD) δ 191.9, 159.6, 159.3, 158.6, 130.6, 106.4, 94.0, 68.1, 61.3, 56.1, 39.5. HRMS (ESI) m/z: [M + H]+ calcd for C11H12O5 225.0763; found, 225.0759.
5,7-Dihydroxy-8-methoxychroman-4-one (7)
To a solution of 7-hydroxy-5,8-dimethoxy-chroman-4-one (28) (30 mg, 0.13 mmol) in CH2Cl2 (3 mL) was added boron trichloride (0.4 mL, 1.0 M solution in CH2Cl2) at −78 °C. After stirring for 1 h, the reaction mixture was diluted with CH2Cl2, washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to afford 5,7-dihydroxy-8-methoxychroman-4-one (7) (27 mg, 96%). 1H NMR (600 MHz, dimethyl sulfoxide (DMSO)-d6) δ 11.95 (s, 1H), 10.64 (s, 1H), 5.93 (s, 1H), 4.49 (t, 2H, J = 6.4 Hz), 3.62 (s, 3H), 2.77 (t, 2H, J = 6.4 Hz). 13C{1H} NMR (150 MHz, DMSO-d6) δ 196.4, 159.8, 158.7, 154.6, 128.3, 102.1, 95.7, 66.7, 60.4, 35.9. HRMS (ESI) m/z: [M + H]+ calcd for C10H10O5 211.0606; found, 211.0601.
7-(Benzyloxy)-5,8-dimethoxychroman-4-one
To an acetone (10 mL) solution of 7-hydroxy-5,8-dimethoxychroman-4-one (28) (0.21 g, 0.94 mmol), benzyl bromide (0.1 mL, 1.0 mmol) and K2CO3 (0.39 g, 2.8 mmol) were added. The reaction mixture was refluxed for 48 h. After cooling to ambient temperature, the reaction mixture was diluted with ethyl acetate and the organic phase was washed with water and saturated NH4Cl solution, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to afford 7-(benzyloxy)-5,8-dimethoxychroman-4-one (0.25 g, 85%). 1H NMR (600 MHz, CDCl3) δ 7.42 (d, 2H, J = 7.3 Hz), 7.38 (m, 2H), 7.32 (m, 1H), 6.12 (s, 1H), 5.20 (s, 2H), 4.50 (t, 2H, J = 6.4 Hz), 3.81 (s, 3H), 3.79 (s, 3H), 2.71 (t, 2H, J = 6.4 Hz). 13C{1H} NMR (150 MHz, CDCl3) δ 189.4, 157.8, 157.7, 156.8, 136.2, 131.3, 128.8, 128.4, 127.3, 106.9, 91.2, 70.9, 67.2, 61.3, 56.2, 38.9. HRMS (ESI) m/z: [M + H]+ calcd for C18H18O5 315.1232; found, 315.1230.
(E)-7-(Benzyloxy)-3-(4′-(benzyloxy)benzylidene)-5,8-dimethoxychroman-4-one (29a)
To a solution of 7-(benzyloxy)-5,8-dimethoxychroman-4-one (0.10 g, 0.32 mmol) in toluene (8 mL) were added 4-benzyloxybenzaldehyde (0.11 g, 0.49 mmol) and p-toluenesulfonic acid (5.5 mg, 0.03 mmol) at 0 °C. The reaction mixture was refluxed for 12 h. After cooling to ambient temperature, the mixture was concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/CH2Cl2 = 1:3) to afford (E)-7-(benzyloxy)-3-(4′-(benzyloxy)benzylidene)-5,8-dimethoxychroman-4-one (29a) (69 mg, 43%). 1H NMR (600 MHz, CDCl3) δ 7.78 (s, 1H), 7.44 (d, 4H, J = 7.8 Hz), 7.40–7.39 (m, 4H), 7.34–7.33 (m, 2H), 7.25 (d, 2H, J = 8.7 Hz), 7.02 (d, 2H, J = 8.7 Hz), 6.20 (s, 1H), 5.31 (d, 2H, J = 1.6 Hz), 5.23 (s, 2H), 5.11 (s, 2H), 3.84 (s, 3H), 3.81 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 180.0, 159.7, 158.2, 157.6, 156.2, 136.6, 136.3, 136.1, 131.9, 131.6, 130.2, 128.9, 128.8, 128.4, 128.3, 127.7, 127.6, 127.4, 115.2, 108.1, 92.2, 71.1, 70.2, 68.1, 61.5, 56.4. HRMS (ESI) m/z: [M + H]+ calcd for C32H28O6 509.1964; found, 509.1961.
(E)-7-(Benzyloxy)-5,8-dimethoxy-3-(4′-methoxybenzylidene)chroman-4-one (29b)
To a solution of 7-(benzyloxy)-5,8-dimethoxychroman-4-one (50 mg, 0.16 mmol) in toluene (5 mL) were added 4-methoxybenzaldehyde (0.03 mL, 0.24 mmol) and p-toluenesulfonic acid (2.8 mg, 0.02 mmol) at 0 °C. The reaction mixture was refluxed for 12 h. After cooling to ambient temperature, the mixture was concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/CH2Cl2 = 1:5) to afford (E)-7-(benzyloxy)-5,8-dimethoxy-3-(4′-methoxybenzylidene)chroman-4-one (29b) (34 mg, 49%). 1H NMR (600 MHz, CDCl3) δ 7.78 (s, 1H), 7.44 (d, 2H, J = 7.3 Hz), 7.41–7.39 (m, 2H), 7.35–7.33 (m, 1H), 7.26–7.25 (m, 2H), 6.95 (d, 2H, J = 8.7 Hz), 6.20 (s, 1H), 5.31 (d, 1H, J = 1.7 Hz), 5.23 (s, 1H), 3.85 (s, 1H), 3.85 (s, 1H), 3.81 (s, 1H). 13C{1H} NMR (150 MHz, CDCl3) δ 180.1, 160.6, 158.2, 157.5, 156.2, 136.3, 136.2, 131.9, 131.6, 130.1, 128.9, 128.4, 127.5, 127.4, 114.3, 108.1, 92.3, 71.1, 68.1, 61.5, 56.4, 55.5. HRMS (ESI) m/z: [M + H]+ calcd for C26H24O6 433.1651; found, 433.1649.
(E)-7-(Benzyloxy)-3-(3′,4′-bis(benzyloxy)benzylidene)-5,8-dimethoxychroman-4-one (29c)
To a solution of 7-(benzyloxy)-5,8-dimethoxychroman-4-one (0.10 g, 0.32 mmol) in toluene (8 mL) were added 3,4-bis(benzyloxy)benzaldehyde (0.15 g, 0.48 mmol) and p-toluenesulfonic acid (6.2 mg, 0.04 mmol) at 0 °C. The reaction mixture was refluxed for 13 h. After cooling to ambient temperature, the mixture was concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane/CH2Cl2 = 1:3:0.1) to afford (E)-7-(benzyloxy)-3-(3′,4′-bis(benzyloxy)benzylidene)-5,8-dimethoxychroman-4-one (29c) (0.10 g, 51%). 1H NMR (600 MHz, CDCl3) δ 7.69 (s, 1H), 7.46–7.43 (m, 6H), 7.42–7.37 (m, 6H), 7.34–7.30 (m, 3H), 6.95 (d, 1H, J = 8.8 Hz), 6.84–6.82 (m, 2H), 6.19 (s, 1H), 5.22 (s, 2H), 5.21 (s, 2H) 5.18 (s, 2H), 5.15 (d, 2H, J = 1.6 Hz), 3.83 (s, 3H), 3.82 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 179.8, 158.1, 157.5, 156.1, 150.1, 148.6, 137.0, 136.8, 136.3, 136.1, 131.5, 130.4, 128.8, 128.7, 128.7, 128.4, 128.1, 128.1, 127.3, 127.3, 124.2, 116.9, 114.3, 107.9, 92.2, 71.5, 71.1, 71.0, 67.9, 61.4, 56.3. HRMS (ESI) m/z: [M + H]+ calcd for C39H34O7 615.2383; found, 615.2379.
(E)-7-(Benzyloxy)-3-(3′-(benzyloxy)-4′-methoxybenzylidene)-5,8-dimethoxychroman-4-one (29d)
To a solution of 7-(benzyloxy)-5,8-dimethoxychroman-4-one (0.27 g, 0.87 mmol) in toluene (15 mL) were added 3-(benzyloxy)-4-methoxybenzaldehyde (0.32 mg, 1.3 mmol) and p-toluenesulfonic acid (14 mg, 0.09 mmol) at 0 °C. The reaction mixture was refluxed for 13 h. After cooling to ambient temperature, the mixture was concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane/CH2Cl2 = 1:1:0.1) to afford (E)-7-(benzyloxy)-3-(3′-(benzyloxy)-4′-methoxybenzylidene)-5,8-dimethoxychroman-4-one (29d) (0.26 g, 56%). 1H NMR (600 MHz, CDCl3) δ 7.69 (s, 1H), 7.46–7.43 (m, 4H), 7.41–7.38 (m, 4H), 7.36–7.30 (m, 2H), 6.93 (d, 1H, J = 8.4 Hz), 6.89 (dd, 1H, J = 8.4, 1.8 Hz), 6.79 (d, 1H, J = 1.8 Hz), 6.19 (s, 1H), 5.23 (s, 2H), 5.18 (s, 2H), 5.14 (d, 2H, J = 1.7 Hz), 3.94 (s, 3H), 3.84 (s, 3H), 3.82 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 179.9, 158.2, 157.5, 156.2, 150.9, 148.0, 136.9, 136.3, 136.3, 131.6, 130.3, 128.9, 128.8, 128.4, 128.2, 127.6, 127.4, 127.3, 124.2, 115.9, 111.6, 108.0, 92.2, 71.3, 71.1, 67.9, 61.5, 56.4, 56.2. HRMS (ESI) m/z: [M + H]+ calcd for C33H30O7 539.2070; found, 539.2071.
Punctatin (8)
To a solution of (E)-7-(benzyloxy)-3-(4′-(benzyloxy)benzylidene)-5,8-dimethoxychroman-4-one (29a) (30 mg, 0.06 mmol) in CH2Cl2 (2 mL) was added boron trichloride (0.18 mL, 1.0 M solution in CH2Cl2) at −78 °C. After stirring for 1 h, the reaction mixture was diluted with CH2Cl2, washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/CH2Cl2 = 1:3) to afford punctatin (8) (9.1 mg, 49%). 1H NMR (600 MHz, DMSO-d6) δ 12.62 (s, 1H), 10.74 (br s, 1H), 10.17 (br s, 1H), 7.69 (s, 1H), 7.35 (d, 2H, J = 8.6 Hz), 6.89 (d, 2H, J = 8.6 Hz), 5.99 (s, 1H), 5.41 (d, 3H, J = 1.4 Hz), 3.63 (s, 3H). 13C{1H} NMR (150 MHz, DMSO-d6) δ 184.6, 160.1, 159.5, 159.5, 153.4, 136.9, 132.9, 128.3, 126.1, 124.7, 115.8, 101.8, 96.2, 67.4, 60.5. HRMS (ESI) m/z: [M + H]+ calcd for C17H14O6 315.0869; found, 315.0865.
4′-O-Methylpunctatin (9)
To a solution of (E)-7-(benzyloxy)-5,8-dimethoxy-3-(4-methoxybenzylidene)-chroman-4-one (29b) (34 mg, 0.08 mmol) in CH2Cl2 (2 mL) was added boron trichloride (0.25 mL, 1.0 M solution in CH2Cl2) at −78 °C. After stirring for 1 h, the reaction mixture was diluted with CH2Cl2, washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/CH2Cl2 = 1:3) to afford 4′-O-methylpunctatin (9) (11 mg, 42%). 1H NMR (600 MHz, DMSO-d6) δ 12.58 (s, 1H), 10.77 (br s, 1H), 7.73 (s, 1H), 7.45 (d, 2H, J = 8.7 Hz), 7.06 (d, 2H, J = 8.8 Hz), 5.99 (s, 1H), 5.41 (d, 2H, J = 1.8 Hz), 3.83 (s, 3H), 3.63 (s, 3H). 13C{1H} NMR (150 MHz, DMSO-d6) δ 184.6, 160.7, 160.2, 159.6, 153.4, 136.4, 132.6, 128.4, 127.1, 126.2, 114.4, 101.9, 96.2, 67.4, 60.5, 55.4. HRMS (ESI) m/z: [M + H]+ calcd for C18H16O6 329.1025; found, 329.1025.
3-(3′,4′-Dihydroxybenzylidene)-5,7-dihydroxy-8-methoxychroman-4-one (10)
To a solution of (E)-7-(benzyloxy)-3-(3,4-bis(benzyloxy)benzylidene)-5,8-dimethoxy-chroman-4-one (29c) (45 mg, 0.07 mmol) in CH2Cl2 (2 mL) was added boron trichloride (0.37 mL, 1.0 M solution in CH2Cl2) at −78 °C. After stirring for 3 h, the reaction mixture was diluted with CH2Cl2, washed with water, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/CH2Cl2 = 1:3) to afford 3-(3′,4′-dihydroxybenzylidene)-5,7-dihydroxy-8-methoxychroman-4-one (10) (14 mg, 60%). 1H NMR (600 MHz, CD3OD) δ 12.58 (s, 1H), 7.69 (s, 1H), 6.87 (d, 1H, J = 8.2 Hz), 6.84 (d, 1H, J = 2.0 Hz), 6.78 (dd, 1H, J = 8.2, 2.0 Hz), 5.96 (s, 1H), 5.39 (d, 2H, J = 1.7 Hz), 3.74 (s, 3H). 13C{1H} NMR (150 MHz, CD3OD) δ 186.6, 161.6, 161.2, 154.9, 149.1, 146.7, 138.8, 129.7, 128.0, 127.5, 124.8, 118.4, 116.6, 103.6, 97.3, 69.0, 61.6. HRMS (ESI) m/z: [M + H]+ calcd for C17H14O7 331.0818; found, 331.0813.
7-Hydroxy-3-(4′-hydroxybenzyl)-5,8-dimethoxychroman-4-one (30)
(E)-7-(Benzyloxy)-3-(4-(benzyloxy)benzylidene)-5,8-dimethoxychroman-4-one (29a) (21 mg, 0.04 mmol) and 10% Pd/C (1.5 mg, 0.01 mmol) in methanol/dichloromethane (1 mL, 1 mL) were placed under an atmosphere of hydrogen. After stirring for 3 h, the mixture was filtered through a Celite pad. After the filtrate was concentrated under reduced pressure, purification of the residue via flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) afforded 7-hydroxy-3-(4′-hydroxybenzyl)-5,8-dimethoxychroman-4-one (30) (12 mg, 91%). 1H NMR (600 MHz, CD3OD) δ 7.05 (d, 2H, J = 8.4 Hz), 6.73 (d, 2H, J = 8.4 Hz), 6.14 (s, 1H), 4.31 (dd, 1H, J = 11.3, 3.9 Hz), 4.16 (dd, 1H, J = 11.3, 6.6 Hz), 3.79 (s, 3H), 3.74 (s, 3H), 3.03 (dd, 1H, J = 13.3, 4.2 Hz), 2.70–2.66 (m, 1H), 2.63 (dd, 1H, J = 13.3, 10.2 Hz). 13C{1H} NMR (150 MHz, CD3OD) δ 194.0, 159.7, 159.0, 158.1, 157.1, 131.2, 130.4, 116.4, 105.6, 94.1, 70.2, 61.4, 56.2, 49.8, 33.4. HRMS (ESI) m/z: [M + H]+ calcd for C18H18O6 331.1181; found, 331.1181.
7-Hydroxy-3-(3′-hydroxy-4′-methoxybenzyl)-5,8-dimethoxychroman-4-one (31)
(E)-7-(Benzyloxy)-3-(3-(benzyloxy)-4-methoxybenzylidene)-5,8-dimethoxychroman-4-one (29d) (0.10 mg, 0.19 mmol) and 10% Pd/C (6.0 mg, 0.06 mmol) in methanol/dichloromethane (3 mL, 3 mL) were placed under an atmosphere of hydrogen. After stirring for 1 h, the mixture was filtered through a Celite pad. After the filtrate was concentrated under reduced pressure, purification of the residue via flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) afforded 7-hydroxy-3-(3′-hydroxy-4′-methoxybenzyl)-5,8-dimethoxychroman-4-one (31) (67 mg, 98%). 1H NMR (600 MHz, CD3OD) δ 6.85 (d, 1H, J = 8.2 Hz), 6.71 (d, 1H, J = 2.0 Hz), 6.67 (dd, 1H, J = 8.2, 2.0 Hz), 6.14 (s, 1H), 4.31 (dd, 1H, J = 11.3, 4.0 Hz), 4.16 (dd, 1H, J = 11.3, 6.8 Hz), 3.82 (s, 3H), 3.79 (s, 3H), 3.74 (s, 3H), 3.01 (dd, 1H, J = 13.7, 4.6 Hz), 2.71–2.67 (m, 1H), 2.60 (dd, 1H, J = 13.7, 10.3 Hz). 13C{1H} NMR (150 MHz, CD3OD) δ 193.9, 159.7, 159.1, 158.1, 147.8, 147.7, 132.6, 130.4, 121.4, 117.1, 112.9, 105.6, 94.1, 70.2, 61.4, 56.4, 56.2, 49.7, 33.6. HRMS (ESI) m/z: [M + H]+ calcd for C19H20O7 361.1287; found, 361.1284.
(E)-3-(4′-Hydroxybenzylidene)-5,7,8-trimethoxychroman-4-one (32a)
1H NMR (600 MHz, CDCl3) δ 7.79 (s, 1H), 7.19 (d, 2H, J = 8.3 Hz), 6.94 (d, 2H, J = 8.3 Hz), 6.45 (s, 1H), 6.17 (s, 1H), 5.30 (s, 2H), 3.95 (s, 3H), 3.92 (s, 3H), 3.79 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 180.4, 158.7, 158.7, 157.5, 156.1, 136.8, 132.1, 131.0, 129.7, 127.1, 116.0, 107.6, 90.1, 68.0, 61.5, 56.4, 56.2.
(E)-7-Hydroxy-3-(4′-hydroxybenzylidene)-5,8-dimethoxychroman-4-one (32b)
1H NMR (600 MHz, CD3OD) δ 7.71 (s, 1H), 7.24 (d, 2H, J = 8.5 Hz), 6.88 (d, 2H, J = 8.5 Hz), 6.20 (s, 1H), 5.30 (d, 2H, J = 1.7 Hz), 3.82 (s, 3H), 3.75 (s, 3H). 13C{1H} NMR (150 MHz, CD3OD) δ 180.4, 159.0, 158.5, 157.6, 156.3, 136.3, 131.9, 129.4, 129.2, 125.8, 115.3, 106.0, 93.4, 67.5, 60.2, 54.8.
(E)-7-Hydroxy-5,8-dimethoxy-3-(4′-methoxybenzylidene)chroman-4-one (32c)
1H NMR (600 MHz, DMSO-d6) δ 10.39 (s, 1H), 7.55 (s, 1H), 7.38 (d, 2H, J = 8.8 Hz), 7.03 (d, 2H, J = 8.8 Hz), 6.20 (s, 1H), 5.27 (d, 2H, J = 1.6 Hz), 3.82 (s, 3H), 3.73 (s, 3H), 3.63 (s, 3H). 13C{1H} NMR (150 MHz, DMSO-d6) δ 177.9, 160.1, 157.5, 156.8, 155.9, 134.1, 132.0, 130.3, 129.3, 126.7, 114.3, 106.1, 94.2, 67.5, 60.6, 55.7, 55.3.
3-(4′-(Benzyloxy)benzyl)-5-hydroxy-7,8-dimethoxychroman-4-one (33a)
1H NMR (600 MHz, CDCl3) δ 12.06 (s, 1H), 7.44 (d, 2H, J = 7.5 Hz), 7.39 (t, 2H, J = 7.5 Hz), 7.33 (t, 1H, J = 7.3 Hz), 7.16 (d, 2H, J = 8.6 Hz), 6.94 (d, 2H, J = 8.6 Hz), 6.11 (s, 1H), 5.06 (s, 2H), 4.36 (dd, 1H, J = 11.5, 4.2 Hz), 4.21 (dd, 1H, J = 11.5, 7.1 Hz), 3.89 (s, 3H), 3.78 (s, 3H), 3.18 (dd, 1H, J = 14.0, 4.5 Hz), 2.85 (m, 1H), 2.74 (dd, 1H, J = 14.0, 10.3 Hz). 13C{1H} NMR (150 MHz, CDCl3) δ 198.3, 161.4, 160.4, 157.8, 153.8, 137.1, 130.3, 130.1, 129.5, 128.7, 128.1, 127.6, 115.2, 102.5, 93.1, 70.2, 69.5, 61.5, 56.4, 47.1, 32.1.
3-(3′,4′-Bis(benzyloxy)benzyl)-5-hydroxy-7,8-dimethoxychroman-4-one (33b)
1H NMR (600 MHz, CDCl3) δ 12.04 (s, 1H), 7.44 (d, 4H, J = 7.7 Hz), 7.36 (td, 4H, J = 7.4, 4.1 Hz), 7.30 (dd, 2H, J = 14.3, 7.1 Hz), 6.88 (d, 1H, J = 8.2 Hz), 6.80 (d, 1H, J = 2.0 Hz), 6.74 (dd, 1H, J = 8.2, 2.0 Hz), 6.10 (s, 1H), 5.15 (d, 4H, J = 4.9 Hz), 4.27 (dd, 1H, J = 11.5, 4.2 Hz), 4.09 (dd, 1H, J = 11.5, 7.3 Hz), 3.89 (s, 3H), 3.77 (s, 3H), 3.12 (dd, 1H, J = 14.0, 4.4 Hz), 2.79 (m, 1H), 2.68 (dd, 1H, J = 14.0, 10.2 Hz).
3-(3′-(Benzyloxy)-4′-methoxybenzyl)-5-hydroxy-7,8-dimethoxychroman-4-one (33c)
1H NMR (600 MHz, CDCl3) δ 12.04 (s, 1H), 7.43 (d, 2H, J = 7.5 Hz), 7.36 (t, 2H, J = 7.6 Hz), 7.29 (t, 1H, J = 7.4 Hz), 6.84 (d, 1H, J = 8.1 Hz), 6.78 (dd, 1H, J = 8.1, 1.9 Hz), 6.75 (d, 1H, J = 1.9 Hz), 6.10 (s, 1H), 5.14 (s, 2H), 4.27 (dd, 1H, J = 11.5, 4.2 Hz), 4.09 (dd, 1H, J = 11.5, 7.3 Hz), 3.89 (s, 3H), 3.88 (s, 3H), 3.78 (s, 3H), 3.11 (dd, 1H, J = 13.9, 4.4 Hz), 2.78 (m, 1H), 2.69 (dd, 1H, J = 13.9, 10.1 Hz). 13C{1H} NMR (150 MHz, CDCl3) δ 198.2, 161.5, 160.4, 153.8, 148.8, 148.2, 137.1, 130.2, 129.5, 128.7, 128.0, 127.5, 122.1, 115.4, 112.1, 102.5, 93.1, 71.2, 69.4, 61.5, 56.4, 56.2, 46.9, 32.4.
3-(3′-Hydroxy-4′-methoxybenzyl)-5,7,8-trimethoxychroman-4-one (33d)
1H NMR (600 MHz, CDCl3) δ 6.81 (s, 1H), 6.78 (d, 1H, J = 8.1 Hz), 6.71 (d, 1H, J = 8.1 Hz), 6.12 (s, 1H), 5.59 (s, 1H), 4.35 (dd, 1H, J = 11.2, 3.6 Hz), 4.18 (dd, 1H, J = 10.8, 8.0 Hz), 3.94 (s, 3H), 3.92 (s, 3H), 3.87 (s, 3H), 3.79 (s, 3H), 3.18 (dd, J = 14.0, 4.0 Hz, 1H), 2.78 (m, 1H), 2.58 (dd, J = 14.0, 10.5 Hz, 1H). 13C{1H} NMR (150 MHz, CDCl3) δ 191.6, 158.5, 158.2, 156.3, 145.6, 145.3, 131.8, 130.6, 120.6, 115.2, 110.8, 105.7, 89.2, 69.3, 61.2, 56.2, 56.1, 56.0, 48.5, 32.1.
3,9-Dihydropunctatin (11)
To a solution of 7-hydroxy-3-(4′-hydroxybenzyl)-5,8-dimethoxy-chroman-4-one (30) (17 mg, 0.05 mmol) in CH2Cl2 (2 mL) was added boron trichloride (0.15 mL, 1.0 M solution in CH2Cl2) at −78 °C. After stirring for 2 h, the reaction mixture was diluted with CH2Cl2, washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to afford 3,9-dihydropunctatin (11) (8.0 mg, 50%). 1H NMR (600 MHz, DMSO-d6) δ 11.96 (s, 1H), 10.68 (s, 1H), 9.26 (s, 1H), 7.03 (d, 2H, J = 8.4 Hz), 6.69 (d, 2H, J = 8.4 Hz), 5.94 (s, 1H), 4.31 (dd, 1H, J = 11.3, 4.4 Hz), 4.13 (dd, 1H, J = 11.4, 7.9 Hz), 3.60 (s, 3H), 3.00 (dd, 1H, J = 13.7, 5.0 Hz), 2.98–2.93 (m, 1H), 2.61 (dd, 1H, J = 13.7, 9.3 Hz). 13C{1H} NMR (150 MHz, DMSO-d6) δ 198.0, 159.8, 158.8, 155.9, 154.3, 130.0, 128.2, 128.0, 115.2, 101.3, 95.9, 69.1, 60.4, 45.6, 31.2. HRMS (ESI) m/z: [M + H]+ calcd for C17H16O6 317.1025; found, 317.1019.
5,6-Dihydroxy-3-(3′-hydroxy-4′-methoxybenzyl)-8-methoxychroman-4-one (12)
To a solution of 7-hydroxy-3-(3′-hydroxy-4′-methoxybenzyl)-5,8-dimethoxy-chroman-4-one (31) (20 mg, 0.05 mmol) in CH2Cl2 (2 mL) was added boron trichloride (0.16 mL, 1.0 M solution in CH2Cl2) at −78 °C. After stirring for 3 h, the reaction mixture was diluted with CH2Cl2, washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to afford 5,6-dihydroxy-3-(3′-hydroxy-4′-methoxybenzyl)-8-methoxychroman-4-one (12) (8.1 mg, 42%). 1H NMR (600 MHz, CD3OD) δ 6.86 (d, 1H, J = 8.2 Hz), 6.72 (d, 1H, J = 2.1 Hz), 6.68 (dd, 1H, J = 8.2, 2.1 Hz), 5.94 (s, 1H), 4.33 (dd, 1H, J = 11.4, 4.3 Hz), 4.17 (dd, 1H, J = 11.4, 7.2 Hz), 3.83 (s, 3H), 3.73 (s, 3H), 3.08 (dd, 1H, J = 13.9, 4.7 Hz), 2.86–2.82 (m, 1H), 2.65 (dd, 1H, J = 13.9, 10.2 Hz). 13C{1H} NMR (150 MHz, CD3OD) δ 199.5, 161.1, 161.1, 155.7, 147.9, 147.7, 132.2, 129.6, 121.4, 117.1, 112.9, 102.9, 97.0, 70.6, 61.5, 56.4, 47.9, 33.2. HRMS (ESI) m/z: [M + H]+ calcd for C18H18O7 347.1131; found, 347.1125.
3-(4-Hydroxybenzyl)-5,8-dimethoxychroman-7-ol (14)
(E)-7-(Benzyloxy)-3-(4-(benzyloxy)benzylidene)-5,8-dimethoxychroman-4-one (29a) (30 mg, 0.06 mmol), and 10% Pd/C (1.8 mg, 0.006 mmol) in methanol (2 mL) were placed under an atmosphere of hydrogen. After stirring for 3 h, the mixture was filtered through a Celite pad. After the filtrate was concentrated under reduced pressure, purification of the residue via flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) afforded 3-(4-hydroxybenzyl)-5,8-dimethoxychroman-7-ol (14) (17 mg, 93%). 1H NMR (600 MHz, CD3OD) δ 7.01 (d, 2H, J = 8.5 Hz), 6.72 (d, 2H, J = 8.5 Hz), 6.03 (s, 1H), 4.11 (ddd, 1H, J = 10.5, 3.0, 1.5 Hz), 3.71–3.69 (m, 4H), 3.68 (s, 3H), 2.61 (ddd, 1H, J = 16.3, 5.4, 1.5 Hz), 2.58–2.49 (m, 2H), 2.18 (dd, 1H, J = 16.3, 8.5 Hz), 2.14–2.07 (m, 1H). 13C{1H} NMR (150 MHz, CD3OD) δ 156.8, 155.1, 149.6, 149.4, 131.7, 131.0, 130.8, 116.2, 103.8, 92.4, 70.78, 61.2, 55.8, 38.2, 35.2, 26.2. HRMS (ESI) m/z: [M + H]+ calcd for C18H20O5 317.1389; found, 317.1386.
3-(4-Hydroxybenzyl)-7,8-dimethoxychroman-5-ol (34)
7-O-Methoxypunctatin (2) (10 mg, 0.03 mmol) and 10% Pd/C (1.4 mg, 0.01 mmol) in methanol (2 mL) were placed under an atmosphere of hydrogen. After stirring for 24 h, the mixture was filtered through a Celite pad. After the filtrate was concentrated under reduced pressure, purification of the residue via flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) afforded 3-(4-hydroxybenzyl)-7,8-dimethoxychroman-5-ol (34) (3.4 mg, 35%). 1H NMR (600 MHz, CD3OD) δ 7.03 (d, 2H, J = 8.4 Hz), 6.73 (d, 2H, J = 8.4 Hz), 6.06 (s, 1H), 4.13 (ddd, 1H, J = 10.4, 2.7, 1.2 Hz), 3.75 (s, 3H), 3.76–3.71 (m, 1H), 3.67 (s, 3H), 2.67 (ddd, 1H, J = 16.3, 5.4, 1.1 Hz), 2.56 (m, 2H), 2.24 (dd, 1H, J = 16.3, 8.5 Hz), 2.16–2.09 (m, 1H). 13C{1H} NMR (150 MHz, CD3OD) δ 156.6, 152.3, 152.2, 149.6, 131.5, 131.1, 130.8, 115.9, 103.8, 92.9, 70.6, 61.1, 56.1, 38.0, 35.0, 26.2.
3-(4-Hydroxybenzyl)-5,7-dimethoxychroman-8-ol (36)
First step: (E)-3-(4-(benzyloxy)benzylidene)-5,7,8-trimethoxychroman-4-one (19a) (0.15 g, 0.35 mmol) and 10% Pd/C (14 mg, 0.13 mmol) in methanol (6 mL) were placed under an atmosphere of hydrogen. After stirring for 24 h, the mixture was filtered through a Celite pad. After the filtrate was concentrated under reduced pressure, purification of the residue via flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) afforded 4-((5,7,8-trimethoxychroman-3-yl)methyl)phenol (35) (0.10 g, 90%). 1H NMR (600 MHz, CDCl3) δ 7.05 (d, 2H, J = 8.2 Hz), 6.77 (d, 2H, J = 8.5 Hz), 6.09 (s, 1H), 5.07 (br s, 1H), 4.21 (ddd, 1H, J = 10.5, 3.0, 1.6 Hz), 3.86 (s, 3H), 3.79 (s, 3H), 3.79–3.74 (m, 4H), 2.72 (ddd, 1H, J = 16.3, 5.3, 1.6 Hz), 2.59 (d, 2H, J = 7.2 Hz), 2.26 (dd 1H, J = 16.3, 8.6 Hz,), 2.23–2.16 (m, 1H). 13C{1H} NMR (150 MHz, CDCl3) δ 154.2, 153.6, 151.4, 148.9, 131.6, 130.2, 115.4, 104.4, 88.9, 70.0, 61.2, 56.5, 55.7, 37.5, 33.7, 25.5. HRMS (ESI) m/z: [M + H]+ calcd for C19H22O5 331.1545; found, 331.1540. Second step: To a solution of 4-((5,7,8-trimethoxychroman-3-yl)methyl)phenol (35) (0.10 g, 0.31 mmol) in CH2Cl2 (3 mL) was added boron trichloride (1.0 mL, 1.0 M solution in CH2Cl2) at 0 °C. After stirring for 3 h at ambient temperature, the reaction mixture was diluted with CH2Cl2, washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to afford 3-(4-hydroxybenzyl)-5,7-dimethoxychroman-8-ol (36) (67 mg, 68%). 1H NMR (600 MHz, CD3OD) δ 7.02 (d, 2H, J = 8.4 Hz), 6.72 (d, 2H, J = 8.4 Hz), 6.20 (s, 1H), 4.15 (ddd, 1H, J = 10.5, 2.9, 1.5 Hz), 3.82 (s, 3H), 3.73 (s, 3H), 3.75–3.70 (m, 1H), 2.66 (ddd, 1H, J = 16.5, 5.4, 1.3 Hz), 2.58–2.52 (m, 2H), 2.22 (dd, 1H, J = 16.5, 8.7 Hz), 2.12 (m, 1H). 13C{1H} NMR (150 MHz, CD3OD) δ 156.8, 151.7, 147.2, 144.9, 131.7, 131.0, 129.6, 116.2, 105.4, 90.6, 70.9, 57.0, 56.2, 38.3, 35.3, 26.3.
Acknowledgments
This work was supported by grants from the National Eye Institute (Grant number R01EY025641) and the BrightFocus Foundation (Grant number M2015301) to TWC and grants from the National Research Foundation funded by the Korean government, MSIP (NRF-2014M3C1A3054139 and NRF-2017M3A9C8027781), and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (Grant Number HI14C1135) to S.-Y.S.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00932.
1H and 13C{1H} NMR spectral data and HPLC analysis for synthesized chiral compounds; 2D NMR (HMBC) of 14, 34, and 36 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Lin L. G.; Liu Q. Y.; Ye Y. Naturally occurring homoisoflavonoids and their pharmacological activities. Planta Med. 2014, 80, 1053–1066. 10.1055/s-0034-1383026. [DOI] [PubMed] [Google Scholar]
- Mulholland D. A.; Schwikkard S. L.; Crouch N. R. The chemistry and biological activity of the Hyacinthaceae. Nat. Prod. Rep. 2013, 30, 1165–1210. 10.1039/c3np70008a. [DOI] [PubMed] [Google Scholar]
- Abegaz B. M.; Kinfe H. H. Naturally Occurring Homoisoflavonoids: Phytochemistry, Biological Activities, and Synthesis (Part II). Nat. Prod. Commun. 2019, 14, 475–498. 10.1177/1934578X19845813. [DOI] [Google Scholar]
- du Toit K.; Drewes S. E.; Bodenstein J. The chemical structures, plant origins, ethnobotany and biological activities of homoisoflavanones. Nat. Prod. Res. 2010, 24, 457–490. 10.1080/14786410903335174. [DOI] [PubMed] [Google Scholar]
- Lee B.; Basavarajappa H. D.; Sulaiman R. S.; Fei X.; Seo S. Y.; Corson T. W. The first synthesis of the antiangiogenic homoisoflavanone, cremastranone. Org. Biomol. Chem. 2014, 12, 7673–7677. 10.1039/C4OB01604A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J.; Yuan Y.; Rhee I.; Corson T. W.; Seo S.-Y. Synthesis of natural homoisoflavonoids having either 5,7-dihydroxy-6-methoxy or 7-hydroxy-5,6-dimethoxy groups. Molecules 2016, 21, 1058–1067. 10.3390/molecules21081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa H. D.; Lee B.; Fei X.; Lim D.; Callaghan B.; Mund J. A.; Case J.; Rajashekhar G.; Seo S. Y.; Corson T. W. Synthesis and mechanistic studies of a novel homoisoflavanone inhibitor of endothelial cell growth. PLoS One 2014, 9, e95694 10.1371/journal.pone.0095694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa H. D.; Lee B.; Lee H. J.; Sulaiman R. S.; An H. C.; Magaña C.; Shadmand M.; Vayl A.; Rajashekhar G.; Kim E.-Y.; Suh Y.-G.; Lee K.; Seo S.-Y.; Corson T. W. Synthesis and biological evaluation of novel homoisoflavonoids for retinal neovascularization. J. Med. Chem. 2015, 58, 5015–5027. 10.1021/acs.jmedchem.5b00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman R. S.; Merrigan S.; Quigley J.; Qi X.; Lee B.; Boulton M. E.; Kennedy B.; Seo S.-Y.; Corson T. W. A novel small molecule ameliorates ocular neovascularisation and synergises with anti-VEGF therapy. Sci. Rep. 2016, 6, 25509 10.1038/srep25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.; Sun W.; Lee H.; Basavarajappa H.; Sulaiman R. S.; Sishtla K.; Fei X.; Corson T. W.; Seo S.-Y. Design, synthesis and biological evaluation of photoaffinity probes of antiangiogenic homoisoflavonoids. Bioorg. Med. Chem. Lett. 2016, 26, 4277–4281. 10.1016/j.bmcl.2016.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa H. D.; Sulaiman R. S.; Qi X.; Shetty T.; Sheik Pran Babu S.; Sishtla K. L.; Lee B.; Quigley J.; Alkhairy S.; Briggs C. M.; Gupta K.; Tang B.; Shadmand M.; Grant M. B.; Boulton M. E.; Seo S.-Y.; Corson T. W. Ferrochelatase is a therapeutic target for ocular neovascularization. EMBO Mol. Med. 2017, 9, 786–801. 10.15252/emmm.201606561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman R. S.; Park B.; Sheik Pran Babu S. P.; Si Y.; Kharwadkar R.; Mitter S. K.; Lee B.; Sun W.; Qi X.; Boulton M. E.; Meroueh S. O.; Fei X.; Seo S.-Y.; Corson T. W. Chemical proteomics reveals soluble epoxide hydrolase as a therapeutic target for ocular neovascularization. ACS Chem. Biol. 2018, 13, 45–52. 10.1021/acschembio.7b00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo M.; Lee B.; Sishtla K.; Fei X.; Lee S.; Park S.; Yuan Y.; Lee S.; Kwon S.; Lee J.; Kim S.; Corson T. W.; Seo S.-Y. Enantioselective Synthesis of Homoisoflavanones by Asymmetric Transfer Hydrogenation and Their Biological Evaluation for Antiangiogenic Activity. J. Org. Chem. 2019, 84, 9995–10011. 10.1021/acs.joc.9b01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alali F.; El-Elimat T.; Albataineh H.; Al-Balas Q.; Al-Gharaibeh M.; Falkinham J. O. III; Chen W. L.; Swanson S. M.; Oberlies N. H. Cytotoxic Homoisoflavones from the Bulbs of Bellevalia eigii. J. Nat. Prod. 2015, 78, 1708–1715. 10.1021/acs.jnatprod.5b00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Toit K.; Elorashi E. E.; Malan S. F.; Drewes S. E.; van Staden J.; Crouch N. R.; Mulholland D. A. Anti-inflammatory activity and QSAR studies of compounds isolated from Hyacinthaceae species and Tachiadenus longiflorus Griseb. (Gentianaceae). Bioorg. Med. Chem. 2005, 13, 2561–2568. 10.1016/j.bmc.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Ngamga D.; Bipa J.; Lebatha P.; Hiza C.; Mutanyatta J.; Bezabih M.-T.; Tane P.; Abegaz B. M. Isoquinoline Alkaloids and Homoisoflavonoids from Drimiopsis barteri Bak and D. burkei Bak. Nat. Prod. Commun. 2008, 3, 769–777. 10.1177/1934578X0800300518. [DOI] [Google Scholar]
- Sidwell W. T. L.; Tamm C. Homo-isoflavones. II. Isolation and structure of 4′-O-methyl-punctatin, autumnalin and 3,9-dihydro-autumnalin. Tetrahedron Lett. 1970, 11, 475–478. 10.1016/0040-4039(70)89003-7. [DOI] [Google Scholar]
- Finckh R. E.; Tamm C. The homo-isoflavones III. isolation and structure of punctatin, 3,9-dihydro-punctatin, 4′-O-methyl-3,9-dihydro-punctatin, 4′-demethyl-eucomin and 4′-demethyl-5-O-methyl-3,9-dihydro-eucomin. Experientia 1970, 26, 472–473. 10.1007/BF01898447. [DOI] [PubMed] [Google Scholar]
- Koorbanally C.; Crouch N. R.; Langlois A.; Du Toit K.; Mulholland D. A.; Drewes S. E. Homoisoflavanones and spirocyclic nortriterpenoids from three Eucomis species: E. comosa, E. schijffii and E. pallidiflora subsp. pole-evansii (Hyacinthaceae). S. Afr. J. Bot. 2006, 72, 428–433. 10.1016/j.sajb.2005.12.006. [DOI] [Google Scholar]
- Corsaro M. M.; Lanzetta R.; Mancino A.; Parrilli M. Homoisoflavanones from Chionodoxa luciliae. Phytochemistry 1992, 31, 1395–1397. 10.1016/0031-9422(92)80299-T. [DOI] [Google Scholar]
- Adinolfi M.; Barone G.; Belardini M.; Lanzetta R.; Laonigro G.; Parrilli M. Homoisoflavanones from Muscari comosum bulbs. Phytochemistry 1985, 24, 2423–2426. 10.1016/S0031-9422(00)83055-1. [DOI] [Google Scholar]
- Mutanyatta J.; Matapa B. G.; Shushu D. D.; Abegaz B. M. Homoisoflavonoids and xanthones from the tubers of wild and in vitro regenerated Ledebouria graminifolia and cytotoxic activities of some of the homoisoflavonoids. Phytochemistry 2003, 62, 797–804. 10.1016/S0031-9422(02)00622-2. [DOI] [PubMed] [Google Scholar]
- Xu X.; Cheng K.; Cheng W.; Zhou T.; Jiang M.; Xu J. Isolation and characterization of homoisoflavonoids from Dracaena cochinchinensis and their osteogenic activities in mouse mesenchymal stem cells. J. Pharm. Biomed. Anal. 2016, 129, 466–472. 10.1016/j.jpba.2016.07.017. [DOI] [PubMed] [Google Scholar]
- Farkas L.; Gottsegen A.; Nogradi M.; Strelisky J. Synthesis of homoisoflavanones. II. Constituents of Eucomis autumnalis and E. punctata. Tetrahedron 1971, 27, 5049–5054. 10.1016/S0040-4020(01)98209-2. [DOI] [Google Scholar]
- a Qin T.; Metz P. Enantioselective synthesis of isoflavanones by catalytic dynamic kinetic resolution. Org. Lett. 2017, 19, 2981–2984. 10.1021/acs.orglett.7b01218. [DOI] [PubMed] [Google Scholar]; b Ashley E. R.; Sherer E. C.; Pio B.; Orr R. K.; Ruck R. T. Ruthenium-catalyzed dynamic kinetic resolution asymmetric transfer hydrogenation of β-chromanones by an elimination-induced racemization mechanism. ACS Catal. 2017, 7, 1446–1451. 10.1021/acscatal.6b03191. [DOI] [Google Scholar]
- a Fujii A.; Hashiguchi S.; Uematsu N.; Ikariya T.; Noyori R. Ruthenium(II)-Catalyzed Asymmetric Transfer Hydrogenation of Ketones Using a Formic Acid-Triethylamine Mixture. J. Am. Chem. Soc. 1996, 118, 2521–2522. 10.1021/ja954126l. [DOI] [Google Scholar]; b Ohkuma T.; Ishii D.; Takeno H.; Noyori R. Asymmetric hydrogenation of amino ketones using chiral RuCl2(diphophine)(1,2-diamine) complexes. J. Am. Chem. Soc. 2000, 122, 6510–6511. 10.1021/ja001098k. [DOI] [Google Scholar]
- Ley S. V.; Norman J.; Griffith W. P.; Marsden S. P. Tetrapropylammonium Perruthenate, Pr4N+RuO4–, TPAP: A Catalytic Oxidant for Organic Synthesis. Synthesis 1994, 639–666. 10.1055/s-1994-25538. [DOI] [Google Scholar]
- Adinolfi M.; Lanzetta R.; Laonigro G.; Parrilli M.; Breitmaier E. 1H and 13C Chemical Shift Assignments of Homoisoflavanones. Magn. Reson. Chem. 1986, 24, 663–666. 10.1002/mrc.1260240806. [DOI] [Google Scholar]
- Carvalho C. F.; Russo A. V.; Sargent M. V. Boron Trichloride as a Selective Demethylating Agent for Hindered Ethers: a Synthesis of the Phytoalexins and P-Pyrufuran, a Synthesis of Tri-O-methylleprolomin and its Demethylation. Aust. J. Chem. 1985, 38, 777–792. 10.1071/CH9850777. [DOI] [Google Scholar]
- Heller W.; Tamm C. 5,7-Dihydroxy-8-methoxy-chroman-4-on aus dem Zwiebelwachs von Eucomis comosa. Helv. Chim. Acta 1978, 61, 1257–1261. 10.1002/hlca.19780610408. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.