Abstract
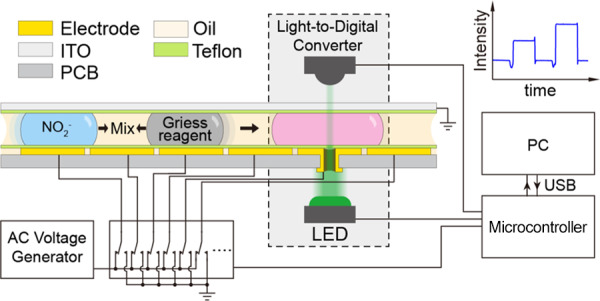
In this paper, a palm-size digital microfluidic (DMF) platform integrated with colorimetric analysis was developed for quantifying the concentration of nitrite. To realize the on-chip repeatable colorimetric analysis, a novel printed circuit board (PCB)-based DMF chip was designed with an embedded aperture on the actuator electrode, forming a vertical light path for online measurement of the droplets. The capabilities of the DMF platform enable automatic manipulation of microliter-level droplets to implement Griess assay without the use of external systems such as syringe, pump, or valve, which provides the benefits including high flexibility, portability, miniature size, and low cost. Results indicated the characteristics of good linearity (R2 = 0.9974), the ignorable crosstalk for reusability, and the limit of detection (LOD) of nitrite as low as 5 μg/L. Furthermore, the presented platform was successfully applied to determine nitrite levels in food products with reliable results and satisfactory recoveries. This integrated DMF platform can be a promising new tool for a wide range of applications involving step-by-step solution mixing and optical detection in environmental monitoring, food safety analysis, and point-of-care testing.
Introduction
Nitrite has been known as an essential and ubiquitous inorganic ion in the natural environment such as soil, water, and food, playing a significant role in various food, environmental, industrial, and physiological systems.1 Excessive intake and long-term accumulation of nitrite ions cause serious hazards to human health due to interference with the human oxygen transport system and the formation of potent carcinogenic N-nitrosamines.2,3 Due to the wide distributions of nitrite, it is desirable to develop portable, simple, quantitative, low-cost, and low-reagent methods for the detection of nitrite. Although a variety of analytical methods based on spectrophotometric,4 ion chromatography,5 capillary electrophoresis,6 and electrochemical techniques7,8 have been developed for the quantification of nitrite, common limitations of the methods include the need of trained operators, large devices (such as spectrophotometer, potentiostat, etc.), and large reagent and sample volumes.
In the last decade, microfluidics has witnessed explosive growth since its introduction.9,10 Nitrite analysis can benefit from the advantages of microfluidics, such as reduced reagent consumption, portability, fast operation, and high compatibility with multiplexing detection means, which facilitate the development of portable devices for an onsite analysis.11 A number of studies have focused on channel-based microfluidics with colorimetric assay of nitrite by implementing the Griess reaction.12−16 However, cross-contamination in the enclosed microchannels induced by the gaseous intermediate species from nitrite raises the difficulty for accurate measurement.13 In addition, the usage of pumps and valves in the channel-based microfluidics increases the volume of the device and power consumption. Microfluidic paper-based analytical devices (μPADs) have also been developed to conduct inexpensive and rapid onsite analysis of nitrite by manipulating liquid samples and reagents on the patterned paper for colorimetric sensing.17−20 Since μPADs are low-cost and nonreusable, they are mostly used in disposable applications, and a scanner or a smartphone with camera is needed to transmit the testing results to a digital format for quantification of the nitrite. This method with paper-based microfluidics may suffer from drawbacks including uncontrollable fluid velocity, low mixing efficiency, and adverse color development.20
An alternative microfluidic technique called digital microfluidic (DMF) has emerged as a promising platform for portable analytical systems with a unique capacity to operate fluids as individual droplets (picoliters to microliters).21−23 It enables manipulation of multiple droplets in parallel on an open surface to move, mix, split, and dispense from reservoirs by applying a series of potentials to a grid of actuator electrodes coated with a hydrophobic dielectric. Therefore, multistep protocols can be programmed on a DMF platform for automatic sample preparation and content analysis.24,25 Compared to channel-based microfluidics, the DMF platform has a smaller size as well as lower cost and power consumption, as the droplets are electrostatically manipulated without the usage of pumps, syringes, or valves. The DMF also offers high flexibility to combine with a number of analytical techniques such as Raman spectroscopy,26 colorimetry,27 electrochemistry,28,29 mass spectrometer,30 and electrochemiluminescence,31 highly extending its applications in the field of analytical chemistry, chemical biology, environmental monitoring,32 and food safety. It is apparent that the DMF technique can benefit the nitrite detection in both onsite and deployable applications. Up to now, a DMF system for sensing nitrite has not yet been developed and validated. The concern may come from two aspects: (1) the corrosion of the Griess reagent to the hydrophobic surface of the DMF chip and (2) how to set up the light path above the opaque actuation electrodes for the colorimetric assay of portable applications.
In this study, we present a portable microfluidic platform for nitrite sensing by developing a novel DMF chip constructed based on the printed circuit board (PCB) technique for droplet manipulation and online colorimetric assay. Taking the advantages of the PCB technique, an aperture is fabricated in the actuator electrode to form a vertical light path for optical sensing (Figure 1). To form a robust, flat, and ultrathin hydrophobic dielectric on the PCB, we introduce an electrostatic film-posting method instead of the conventional spin-coating method. Therefore, the reaction steps of the Griess assay was realized on the presented DMF platform. An optical sensor module is integrated to the platform for the online colorimetric assay. The results indicated a good linearity for nitrite determination (R2 = 0.9974). The detection limit of nitrite was 5 μg/L, which was comparable to those previously obtained for traditional continuous-flow microfluidics.15,16 Moreover, our platform also gave satisfactory and reliable results in automated, simple, fast, and quantitative analysis of nitrite in food samples.
Figure 1.
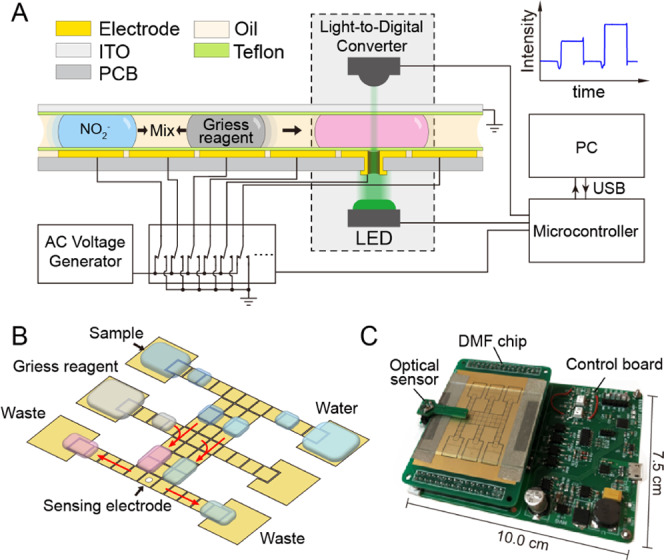
(A) Cross-sectional setup of the two-plate DMF platform integrated with a colorimetric analysis system. (B) Design of the PCB pattern on the bottom plate of DMF chip and the programmable process for nitrite detection; the actuator electrode embedded with a 1 mm aperture also works as the sensing electrode. (C) Photograph of the whole platform with a size of 10.0 cm × 7.5 cm × 3.0 cm.
Results and Discussion
Griess Reaction on Digital Microfluidics
The layers of hydrophobic dielectric are essential for droplet manipulation, which is commonly fabricated using a spin-coating method.29,33 However, the spin-coating method may not be suitable for the PCB-based DMF chip, as the aperture fabricated inside the actuator electrodes can hardly be evaluated through the method. Here, an electrostatic film-posting method is introduced to form a robust and ultrathin hydrophobic dielectric layer on the electrodes with a poly(tetrafluoroethylene) (PTFE) film. It is notable that this method is easy to implement without the requirement of any device. Preliminary results show that the PTFE film with a thickness of 10 μm is thin enough to be used as a hydrophobic dielectric for manipulating the droplet of deionized water. To test the possibility of DMF for conducting the Griess reaction, nitrite sample (3 μL, 500 μg/L) and the Griess reagent (3 μL) were added to the corresponding reservoirs. As shown in Figure 2A, the droplets of nitrite sample and the Griess reagent were successfully disposed from the reservoirs using a bottleneck cutoff method and merged together to be one droplet for the reaction. The merged droplet was manipulated to move back and forth at a speed of 4.5 mm/s for 60 s to promote the mixing of the sample and reagent. It is obvious that the DMF enables flexible control of the time for mixture and reaction according to the diffusion rate and the reaction rate. During the process, the color of the droplet gradually changed from transparent to red, suggesting that the reaction occurred on the DMF chip, and corrosion of the hydrophobic layer or dielectric breakdown is not observed.
Figure 2.
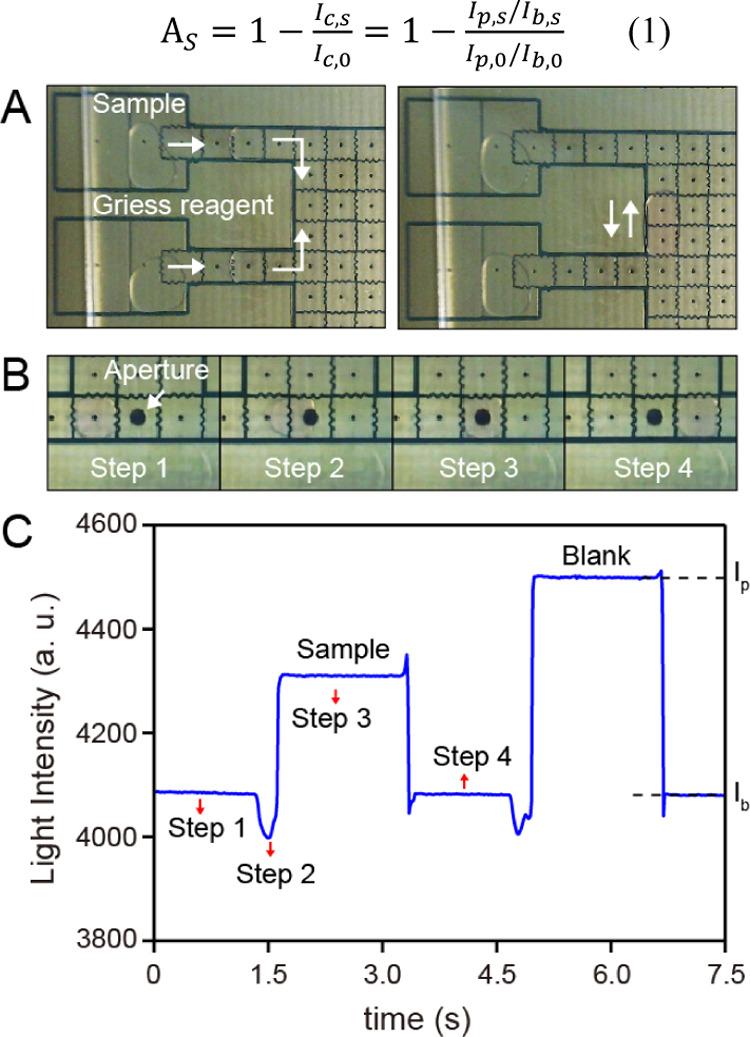
(A) Photographs of the droplets disposing for nitrite sample and Griess reagent (left) and the droplet-based Griess reaction (right) on the DMF. (B) Photographs of the droplet movements (steps 1–4) on the sensing electrode. For steps 1 and 4, the droplet is outside the sensing electrode. For step 2, part of the droplet enters the sensing electrode. For step 3, the whole droplet moves to and is stopped at the center of sensing electrode; except the position labeled as “Aperture”, the other aperture-like structure is just the linkage of each electrode for applying voltage. (C) Light response corresponding to the four steps; definitions of the Ip, Ib is labeled on the light pulse of the blank droplet as an example.
Measurement of Nitrite Concentration
To determine the concentration of nitrite based on the Griess assay, a pair of LED and photodiode has been commonly used in the studies based on channel-based microfluidics. Since the electrodes on the DMF chip are opaque, the vertical distance in the gap between the top and bottom electrodes is used as the light path for the detection. However, the measuring accuracy and linearity are unsatisfied using the gap as the light path, since it may cause unfavorable scattering of the light.27 To solve this problem, we fabricated an aperture in the center of sensing electrode based on the PCB technique, which works as the vertical light path for optical measurement. The droplet movements on the sensing electrode are shown in Figure 2B (the LED and light-to-digital converter are not shown in the photographs to clearly demonstrate the process of droplet movements). To ensure the stability and accuracy of the measurement, a sufficiently high voltage (150 Vrms) was used in this study, and hence the force driving the droplet toward the sensing electrode was high enough to cause the droplet to move to the center of the sensing electrode. During the colorimetric assay, the transmitted light intensity measured by the light-to-digital converter (integrated a photodiode and the analog-to-digital converter) was recorded as shown in Figure 2C. Without the droplets on the sensing electrode (steps 1 and 4), the light intensity is regarded as the baseline, which appears to be slowly drifting during the experiment. When the droplet moved to the sensing electrode, a transient decrease in light intensity was induced by the coverage of the edge of the droplet at the aperture (step 2 in Figure 2). During this process, the moving speed of the droplet was reduced due to the obstruction of the aperture, leading to the slow response of light intensity. Subsequently, the light intensity abruptly increased and then reached a plateau as the droplet covered the whole aperture (step 3). The enhancement of light intensity is caused by the collimation of light as the droplet plays the role of a liquid lens.34
We used the corrected light intensity (Ic) to describe the light response of the moving of a droplet on the sensing electrode using the ratio of the high plateau (Ip) to its local baseline (Ib) to eliminate the influence of the baseline drift. Ip was evaluated by averaging the last 30 data points before the signal returned to the baseline, and Ib was evaluated by averaging the following 30 data points after the signal returned to the baseline. Ultimately, the absorption of the sample droplet (As) was calculated as the ratio of the corrected light intensity of the sample droplet (Ic,s) to the corrected light intensity of the blank droplet (Ic,0) as follows1.
| 1 |
For an online analysis, the prepared Griess reagent solution and the deionized water were loaded on the corresponding reservoirs, and all experiments are conducted at room temperature of 25 °C. The nitrite-sensing experiments were performed in four steps: (1) one 3 μL aliquot of the sample solution was added on the electrode of the sample reservoir, and one sample droplet and blank droplet (deionized water) were then dispensed from this reservoir; (2) two droplets were dispensed from the Griess reagent reservoir to merge with the sample droplet and blank droplet, respectively; (3) as shown by an arrow in Figure 1B, the merged droplets were fully mixed by continuous stirring for 60 s, and meanwhile, the Griess reaction occurred in the sample droplet; (4) the reacted sample droplet and blank droplet were driven to the sensing electrode in sequence for measuring their light response and then driven to waste reservoirs. All of the detection steps of the Griess assay can be realized on the chip within 2 min through a fully automated continuous merging, mixing, and dispensing process driven by the programmatic DMF with a synchronous manipulation of all of the droplets, showing high efficiency and automation of this method (see Supporting Information Video 1). In addition, the consumption of the reagent is only ∼1.5 μL for a single detection.
The linearity and limit of detection (LOD) were determined to assess the performance and reliability of the method. As shown in Figure 3B, the linearity was evaluated by analyzing the nitrite standards at nine different concentrations. The calibration curve was established by plotting the measured absorption values against analyte concentrations and was found to be linear between 10 and 1000 μg/L, with a correlation coefficient of 0.9974 (y = 4 × 10–5x). The LOD of nitrite, based on a signal-to-noise ratio of 3:1, was 5 μg/L for the nitrite sensor. This value was comparable to the values previously reported by the continuous-flow microfluidics method implementing the Griess reaction and reached the minimum value of the LODs achieved by spectrometric standard approaches for drinking water according to the World Health Organization (WHO) (13 μg/L for nitrite).35 The satisfactory linearity, precision, and LOD values show that the method can be applied for the analysis of nitrite in food samples.
Figure 3.
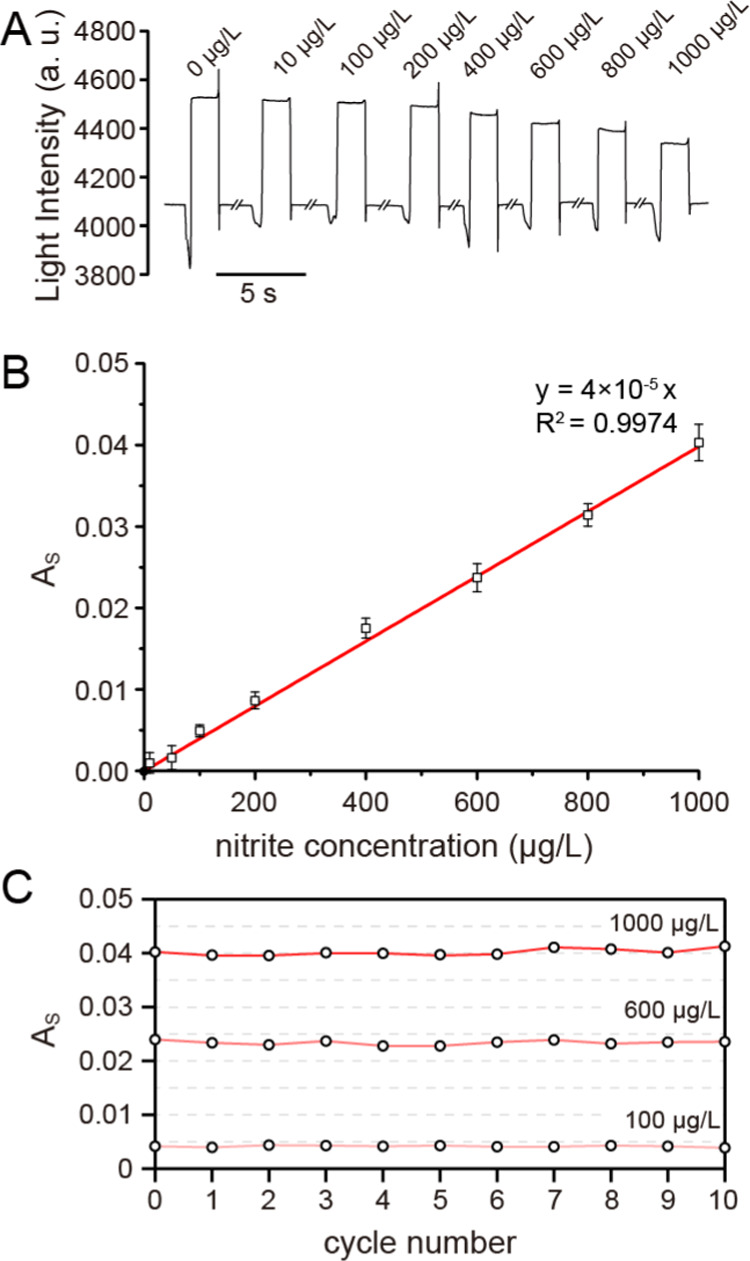
(A) Raw data of light response to the reacted droplet with different nitrite concentrations. (B) Standard linear curve of the light absorption versus the nitrite concentration. (C) Change in the measured As in the 10 cycles corresponding to the samples with concentrations of 1000, 600, and 100 μg/L.
As reported, the crosstalk in the droplet-based assay of nitrite is mainly caused by the gaseous intermediate species at low pH. Since low pH is necessary for the Griess reaction to render its constituent reactants soluble, it is hard to eliminate the crosstalk in the enclosed space of a channel-based microfluids. The conditions could be improved in the DMF for two reasons: (1) the distance between the droplets and the paths of each droplet can be optimized without magnifying the size of the chip and (2) the open space of the DMF chip highly decreases the possibility of a crosstalk through gaseous intermediate species. To see if the crosstalk occurred on the DMF-based assay, samples of three different nitrite concentrations (1000, 600, and 100 μg/L) were analyzed one after another for 10 cycles on the same chip. The results show that the absorbance of the droplets is barely changed during the experiment (Figure 3C), and the values of relative standard deviation (RSD) are 1.46, 1.75, and 3.51% for 1000, 600, and 100 μg/L, respectively. The results reveal that the crosstalk between the droplets is negligible for detection of nitrite through the DMF platform.
Detection of Nitrite in Food Products
To demonstrate how the DMF method can be applied for the analysis of food samples, the method was further investigated by the analysis of nitrite in ham, pickles (cabbage), vegetable (celery), orange, and bean curd. The samples were prepared and pretreated before use and then measured by the presented DMF platform (see the Supporting Information Sections S1–S4). Table 1 shows the nitrite content of blank food samples and the samples spiked with 500 μg/L nitrite before pretreatment, which is approximately the median concentration present in common nitrite residue foods.1 Nitrite is detected in the blank samples at different concentrations, which is consistent with the formal research studies.1,36 Satisfactory recovery results were obtained between 93.5 and 107.0% (see Table 1), which demonstrated that the matrices of real food samples have little interference in the quantification of nitrite. Therefore, it is self-evident that this approach could potentially be used for the nitrite analysis in food products.
Table 1. Content and Recovery of Nitrite in Food Products.
| food product | nitrite concentration in the blank sample (μg/L) | amount spiked (μg/L) | amount detected (μg/L) | recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|---|
| ham | 216.67 | 500 | 684.39 | 93.5 | 1.9 |
| pickles (cabbage) | 58.33 | 500 | 584.60 | 105.3 | 3.5 |
| bean curd | 53.17 | 500 | 588.38 | 107.0 | 2.7 |
| vegetable (Celery) | 42.75 | 500 | 575.48 | 106.6 | 2.4 |
Conclusions
In summary, we have proposed a portable, economical, and automated DMF platform with an integrated colorimetric analysis system for nitrite sensing. A novel PCB-based DMF chip was developed with an aperture embedded in the sensing electrode to form a vertical light path, and no other external system needed to be integrated. The characteristics of good linearity, high sensitivity, and robustness were approved using this platform. An LOD as low as 5 μg/L was achieved. Compared with conventional continuous-flow microfluidics, this DMF nitrite sensor provides many advantages, including low cost of apparatus, lower reagent consumption, high stability, and easy operation with high automation and flexibility. Besides, this method was also successfully applied to determine nitrite levels in several food samples with reliable results and satisfactory recoveries, representing a versatile system for microscale analysis of many analytes in practical use. By further combining with advanced techniques such as wireless communication and automatic sample injection, the applications of the platform could be greatly extended including household food analysis, point-of-care test, and field-deployable environment monitoring.
Experimental Section
Materials and Reagents
NaNO2 standard solution (sodium nitrite, 10 mg/L) was purchased from Shenzhen Bolinda Technology Co., Ltd. Other nitrite standard solutions of different concentrations were prepared by the dilution of these solutions with water. NaCl (AR), acetic acid (AR), sulfanilic acid (AR), and N-(1-naphthyl)ethylenediamine dihydrochloride (AR) were obtained from Sinopharm Chemical Reagent Co., Ltd. Water was supplied by the Milli-Q water purification system (Merck, Millipore). Acetonitrile (4 L, J.T. Baker, high-performance liquid chromatography (HPLC)) was bought from Fisher Scientifc Co., Ltd. Graphite carbon black (GCB, PestCarb 120–400 mesh) was purchased from Agela Technologies. Filters (0.22 μm, PTFE, hydrophobic) were supplied by Tianjin Branch billion Lung Experimental Equipment Co., Ltd. The PTFE film (0.01 mm) was purchased from Hongfu Insulating Material Co., Ltd., China. The Griess reagent was prepared by mixing sulfanilic acid (0.4% sulfanilic acid dissolved in 30% acetate acid) and N-(1-naphthyl)ethylenediamine dihydrochloride (0.2% N-(1-naphthyl)ethylenediamine dihydrochloride dissolved in water) before usage.
DMF Chip Design and Fabrication
DMF chips were custom-designed and fabricated using standard printed circuit board techniques (FASTPCB TECH Co., Ltd., China). The structure of our DMF chip in a two-plate format is depicted with a detailed cross-sectional schematic diagram in Figure 1A. Electrodes on the PCB were fabricated using the gold electrodeposition method. The PCB pattern on the bottom plate comprised 51 actuator electrodes (2.25 mm × 2.25 mm) and 6 reservoir electrodes (9.72 mm × 6.95 mm) (Figure 1B). The reservoir electrodes can have different functions of loading and expelling different droplets of solution during the actual operation. As can be seen in Figure 1A,B, a 1 mm diameter aperture was fabricated at the center of an actuator electrode (labeled as the sensing electrode) to form the vertical light path. Besides, the hydrophobic dielectric of the bottom plate was formed using an electrostatic film-posting method in three steps: (1) the surface of the bottom plate was cleaned with acetone and dried in air for 5 min; (2) 10 μL of silicon oil (10 cst) was dropped to the bottom plate and applied evenly; and (3) a piece of PTFE film was carefully attached to the surface to form an insulating and hydrophobic layer. Indium tin oxide (ITO) glass was used as the top plate, and a 5 μm thick layer of Teflon AF 1600 (DuPont, Wilmington, DE) was applied to the conductive side of the ITO glass by spin coating at 1500 rpm for 5 min, followed by baking on a hot plate at 220 °C for 1 h. The ITO glass was mounted on the PCB pattern with the conductive tape as a support between two plates, and the top plates were kept 0.1 mm apart from the bottom plate as an optimized parameter for droplet manipulation. Silicone oil (0.65 cst, DuPont) was applied as the liquid filler between the two plates (Figure 1A), which was demonstrated to lubricate efficiently the movement of the droplets.
DMF Platform Setup and Operation
To realize the droplet manipulation with high automation, a custom-designed electronic circuit was used to control and manage the droplet movement. Voltage on each electrode was controlled by a high-voltage multiplexing chip (HV507, Microchip Technology Inc.) introduced by the OpenDrop project.37 Droplet actuation was achieved by applying ac voltages (150 Vrms, 1 kHz) between the top plate electrode and the bottom plate. The platform was directly powered by a USB port with low power consumption (∼0.42 W). The setup of the online colorimetric analysis system was effectively integrated into the DMF platform (Figure 1A) with a LED (520 nm, XLamp, Cree Inc.) and a light-to-digital converter (TSL2571, ams AG, Austria) with 16-bit resolution. The LED and light-to-digital converter are mounted on the two sides of the aperture to form a vertical light path at a distance of 2.8 mm. The microcontroller read out the light intensity data from the light-to-digital converter at a sampling rate of 100 Hz and sent it to a PC software for further data recording and analysis. The whole device was only 10.0 cm × 7.5 cm × 3.0 cm in size and weighed around 75 g. To avoid interference from ambient light, measurements were conducted in a case to create a dark environment.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (31701698) and the Shanghai Key Laboratory of Forensic Medicine (Academy of Forensic Science, KF1910).
Glossary
Abbreviations Used
- DMF
digital microfluidics
- PCB
printed circuit board
- LOD
limit of detection
- PTFE
poly(tetrafluoroethylene)
- LED
light-emitting diode
- ITO
indium tin oxide
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01274.
Author Contributions
The manuscript was written with contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Kalaycıoğlu Z.; Erim F. B. Nitrate and Nitrites in Foods: Worldwide Regional Distribution in View of Their Risks and Benefits. J. Agric. Food Chem. 2019, 67, 7205–7222. 10.1021/acs.jafc.9b01194. [DOI] [PubMed] [Google Scholar]
- Balimandawa M.; de Meester C.; Léonard A. The mutagenicity of nitrite in the Salmonella/microsome test system. Mutat. Res., Genet. Toxicol. 1994, 321, 7–11. 10.1016/0165-1218(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Ma L.; Hu L.; Feng X.; Wang S. Nitrate and Nitrite in Health and Disease. Aging Dis. 2018, 9, 938–945. 10.14336/AD.2017.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poormoghadam P.; Larki A.; Rastegarzadeh S. Ion pair-based dispersive liquid–liquid microextraction combined with UV-Vis spectrophotometry as a circuitous assay for nitrite. Anal. Methods 2015, 7, 8655–8662. 10.1039/C5AY01812A. [DOI] [Google Scholar]
- Ito K.; Takayama Y.; Makabe N.; Mitsui R.; Hirokawa T. Ion chromatography for determination of nitrite and nitrate in seawater using monolithic ODS columns. J. Chromatogr. A 2005, 1083, 63–67. 10.1016/j.chroma.2005.05.073. [DOI] [PubMed] [Google Scholar]
- Öztekin N.; Nutku M. S.; Erim F. B. Simultaneous determination of nitrite and nitrate in meat products and vegetables by capillary electrophoresis. Food Chem. 2002, 76, 103–106. 10.1016/S0308-8146(01)00287-4. [DOI] [Google Scholar]
- Zhu W.; Zhang Y.; Gong J.; Ma Y.; Sun J.; Li T.; Wang J. Surface Engineering of Carbon Fiber Paper toward Exceptionally High-Performance and Stable Electrochemical Nitrite Sensing. ACS Sens. 2019, 4, 2980–2987. 10.1021/acssensors.9b01474. [DOI] [PubMed] [Google Scholar]
- Sun C.; Pan W.; Zheng D.; Zheng Y.; Zhu J. An electrochemical sensor for nitrite using a glassy carbon electrode modified with Cu/CBSA nanoflower networks. Anal. Methods 2019, 11, 4998–5006. 10.1039/C9AY01544B. [DOI] [Google Scholar]
- Ding Y.; Howes P. D.; deMello A. J. Recent Advances in Droplet Microfluidics. Anal. Chem. 2020, 92, 132–149. 10.1021/acs.analchem.9b05047. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B.; Hanson R. L.; Almughamsi H. M.; Pang C.; Fish T. R.; Woolley A. T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168. 10.1021/acs.analchem.9b04986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.; Chen W.; Wang G.; He P.; Wang Q. Analysis of acrylamide in food products by microchip electrophoresis with on-line multiple-preconcentration techniques. Food Chem. 2016, 209, 154–161. 10.1016/j.foodchem.2016.04.065. [DOI] [PubMed] [Google Scholar]
- Sieben V. J.; Floquet C. F. A.; Ogilvie I. R. G.; Mowlem M. C.; Morgan H. Microfluidic colourimetric chemical analysis system: Application to nitrite detection. Anal. Methods 2010, 2, 484–491. 10.1039/c002672g. [DOI] [Google Scholar]
- Nightingale A. M.; Hassan S.-u.; Evans G. W. H.; Coleman S. M.; Niu X. Nitrate measurement in droplet flow: gas-mediated crosstalk and correction. Lab Chip 2018, 18, 1903–1913. 10.1039/C8LC00092A. [DOI] [PubMed] [Google Scholar]
- Nightingale A. M.; Hassan S.-u.; Warren B. M.; Makris K.; Evans G. W. H.; Papadopoulou E.; Coleman S.; Niu X. A Droplet Microfluidic-Based Sensor for Simultaneous in Situ Monitoring of Nitrate and Nitrite in Natural Waters. Environ. Sci. Technol. 2019, 53, 9677–9685. 10.1021/acs.est.9b01032. [DOI] [PubMed] [Google Scholar]
- Lin B.; Xu J.; Lin K.; Li M.; Lu M. Low-Cost Automatic Sensor for in Situ Colorimetric Detection of Phosphate and Nitrite in Agricultural Water. ACS Sens. 2018, 3, 2541–2549. 10.1021/acssensors.8b00781. [DOI] [PubMed] [Google Scholar]
- Beaton A. D.; Sieben V. J.; Floquet C. F. A.; Waugh E. M.; Abi Kaed Bey S.; Ogilvie I. R. G.; Mowlem M. C.; Morgan H. An automated microfluidic colourimetric sensor applied in situ to determine nitrite concentration. Sens. Actuators, B 2011, 156, 1009–1014. 10.1016/j.snb.2011.02.042. [DOI] [Google Scholar]
- Smith G. T.; Dwork N.; Khan S. A.; Millet M.; Magar K.; Javanmard M.; Ellerbee Bowden A. K. Robust dipstick urinalysis using a low-cost, micro-volume slipping manifold and mobile phone platform. Lab Chip 2016, 16, 2069–2078. 10.1039/C6LC00340K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ruiz N.; Curto V. F.; Erenas M. M.; Benito-Lopez F.; Diamond D.; Palma A. J.; Capitan-Vallvey L. F. Smartphone-Based Simultaneous pH and Nitrite Colorimetric Determination for Paper Microfluidic Devices. Anal. Chem. 2014, 86, 9554–9562. 10.1021/ac5019205. [DOI] [PubMed] [Google Scholar]
- Liu Y.-C.; Hsu C.-H.; Lu B.-J.; Lin P.-Y.; Ho M.-L. Determination of nitrite ions in environment analysis with a paper-based microfluidic device. Dalton Trans. 2018, 47, 14799–14807. 10.1039/C8DT02960A. [DOI] [PubMed] [Google Scholar]
- Bhakta S. A.; Borba R.; Taba M. Jr.; Garcia C. D.; Carrilho E. Determination of nitrite in saliva using microfluidic paper-based analytical devices. Anal. Chim. Acta 2014, 809, 117–122. 10.1016/j.aca.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.; Ng A. H. C.; Fobel R.; Wheeler A. R. Digital microfluidics. Annu. Rev. Anal. Chem. 2012, 5, 413–440. 10.1146/annurev-anchem-062011-143028. [DOI] [PubMed] [Google Scholar]
- Li J.; Ha N. S.; Liu T.; van Dam R. M.; Kim C. J. Ionic-surfactant-mediated electro-dewetting for digital microfluidics. Nature 2019, 572, 507–510. 10.1038/s41586-019-1491-x. [DOI] [PubMed] [Google Scholar]
- Zhai J.; Li H.; Wong A. H.-H.; Dong C.; Yi S.; Jia Y.; Mak P.-I.; Deng C.-X.; Martins R. P. A digital microfluidic system with 3D microstructures for single-cell culture. Microsyst. Nanoeng. 2020, 6, 6 10.1038/s41378-019-0109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.-S.; Hsu W.; Huang H.-Y.; Tseng H.-Y.; Lee C.-T.; Hsu C.-Y.; Shieh Y.-C.; Wang S.-H.; Yao D.-J.; Liu C.-H. Simultaneous detection of two growth factors from human single-embryo culture medium by a bead-based digital microfluidic chip. Biosens. Bioelectron. 2020, 150, 111851 10.1016/j.bios.2019.111851. [DOI] [PubMed] [Google Scholar]
- Rackus D. G.; de Campos R. P. S.; Chan C.; Karcz M. M.; Seale B.; Narahari T.; Dixon C.; Chamberlain M. D.; Wheeler A. R. Pre-concentration by liquid intake by paper (P-CLIP): a new technique for large volumes and digital microfluidics. Lab Chip 2017, 17, 2272–2280. 10.1039/C7LC00440K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Ruan Q.; Lei Z.-C.; Lin S.-C.; Zhu Z.; Zhou L.; Yang C. Highly Sensitive and Automated Surface Enhanced Raman Scattering-based Immunoassay for H5N1 Detection with Digital Microfluidics. Anal. Chem. 2018, 90, 5224–5231. 10.1021/acs.analchem.8b00002. [DOI] [PubMed] [Google Scholar]
- Huang S.; Fair R. B. Quantitative measurements of inorganic analytes on a digital microfluidics platform. SN Appl. Sci. 2019, 1, 1654 10.1007/s42452-019-1693-8. [DOI] [Google Scholar]
- Min X.; Bao C.; Kim W. S. Additively Manufactured Digital Microfluidic Platforms for Ion-Selective Sensing. ACS Sens. 2019, 4, 918–923. 10.1021/acssensors.8b01689. [DOI] [PubMed] [Google Scholar]
- Dryden M. D. M.; Rackus D. D. G.; Shamsi M. H.; Wheeler A. R. Integrated digital microfluidic platform for voltammetric analysis. Anal. Chem. 2013, 85, 8809–8816. 10.1021/ac402003v. [DOI] [PubMed] [Google Scholar]
- Mei N.; Seale B.; Ng A. H. C.; Wheeler A. R.; Oleschuk R. Digital Microfluidic Platform for Human Plasma Protein Depletion. Anal. Chem. 2014, 86, 8466–8472. 10.1021/ac5022198. [DOI] [PubMed] [Google Scholar]
- Shamsi M. H.; Choi K.; Ng A. H. C.; Chamberlain M. D.; Wheeler A. R. Electrochemiluminescence on digital microfluidics for microRNA analysis. Biosens. Bioelectron. 2016, 77, 845–852. 10.1016/j.bios.2015.10.036. [DOI] [PubMed] [Google Scholar]
- Han S.; Zhang Q.; Zhang X.; Liu X.; Lu L.; Wei J.; Li Y.; Wang Y.; Zheng G. A digital microfluidic diluter-based microalgal motion biosensor for marine pollution monitoring. Biosens. Bioelectron. 2019, 143, 111597 10.1016/j.bios.2019.111597. [DOI] [PubMed] [Google Scholar]
- Ng A. H. C.; Chamberlain M. D.; Situ H.; Lee V.; Wheeler A. R. Digital microfluidic immunocytochemistry in single cells. Nat. Commun. 2015, 6, 7513 10.1038/ncomms8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S.-K.; Wang F.-M. Multiphase optofluidics on an electro-microfluidic platform powered by electrowetting and dielectrophoresis. Lab Chip 2014, 14, 2728–2738. 10.1039/C4LC00317A. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Nitrate and Nitrite in Drinking-Water Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization, 2003.
- Zhou Z.-Y.; Wang M.-J.; Wang J.-S. Nitrate and nitrite contamination in vegetables in China. Food Rev. Int. 2000, 16, 61–76. 10.1081/FRI-100100282. [DOI] [Google Scholar]
- OpenDrop Project. http://www.gaudi.ch/OpenDrop/. 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


