Expanding from China around the world, coronavirus 2019 (COVID-19) is the disease caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). COVID-19 primarily manifests by hypoxic normo-hypocapnia with preserved lung compliance [1]. In the absence of targeted treatment, sub-intensive clinicians support patients with noninvasive ventilation and anti-inflammatory/anti-viral agents waiting for status improvement. Angiotensin-converting enzyme (ACE)2, highly expressed on the external membrane of lungs, heart, kidney and gastrointestinal tract cells, displays the binding site for the spike protein of SARS-CoV-2 [2]. ACE2, identified as a counterpart of the Renin-Angiotensin-Aldosterone System (RAAS), converts angiotensin (Ang) II to Ang-(1-7) and Ang I to Ang-(1-9). ACE2 activity induces vasodilatation and reduces cell growth and inflammatory response.
Short abstract
In COVID-19 patients respiratory failure is associated with increased systemic blood pressure, conceivably due to the modulation of the renin-angiotensin-aldosterone system by SARS-CoV-2 infection https://bit.ly/3cINsHB
To the Editor:
Expanding from China around the world, coronavirus 2019 (COVID-19) is the disease caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). COVID-19 primarily manifests by hypoxic normo-hypocapnia with preserved lung compliance [1]. In the absence of targeted treatment, sub-intensive clinicians support patients with noninvasive ventilation and anti-inflammatory/anti-viral agents waiting for status improvement. Angiotensin-converting enzyme (ACE)2, highly expressed on the external membrane of lungs, heart, kidney and gastrointestinal tract cells, displays the binding site for the spike protein of SARS-CoV-2 [2]. ACE2, identified as a counterpart of the Renin-Angiotensin-Aldosterone System (RAAS), converts angiotensin (Ang) II to Ang-(1-7) and Ang I to Ang-(1-9). ACE2 activity induces vasodilatation and reduces cell growth and inflammatory response. In experimental models that mimic viral acute respiratory distress syndrome, the absence of Ace2 led to inflammation, vascular permeability and lung injury via activation of the Ang II pathway [3, 4]. The decrease in ACE2 activity by SARS-CoV-2 can unleash a cascade of injurious effects through a heightened imbalance in the actions of the products of ACE versus ACE2. Moving to a clinical setting, the ACE2 downregulation may be one of the pathways sustaining arterial hypertension [5] and pulmonary arterial hypertension [6]. Therefore, it is conceivable that in COVID-19 a cleavage of membrane ACE2 along with its circulatory levels could impact on the disease progression and clinical worsening [7]. Thus, to support a pathophysiological role of ACE2, the present report shares clinical data from an observational study conducted on 40 patients with a diagnosis of COVID-19, hospitalised in the Cardiorespiratory Sub-Intensive COVID-19 Unit at the Fondazione IRCCS Ca' Granda Policlinico Hospital (Milan, Italy).
40 consecutive patients with COVID-19 were recruited. At that time, standardised treatment was hydroxychloroquine and lopinavir/ritonavir. Blood pressure (BP), arterial oxygen tension/ inspiratory oxygen fraction (PaO2/FIO2) and alveolar–arterial oxygen tension difference (PA–aO2) were measured two to four times per day according to standard clinical protocol. Median value of plasma potassium concentration ([K+]plasma) was also evaluated. In the case of any supplementation of potassium, or administration of mineral corticoid receptor antagonists or diuretic stimulators, the [K+]plasma we considered was referred prior to the pharmacological intervention. The relationship between respiratory and haemodynamic variables, i.e. PA–aO2 versus mean BP was evaluated by Poon's analysis which allows us to normalise the inter-individual variability [8]. The composite of death and invasive ventilation were evaluated after 28 days of hospitalisation.
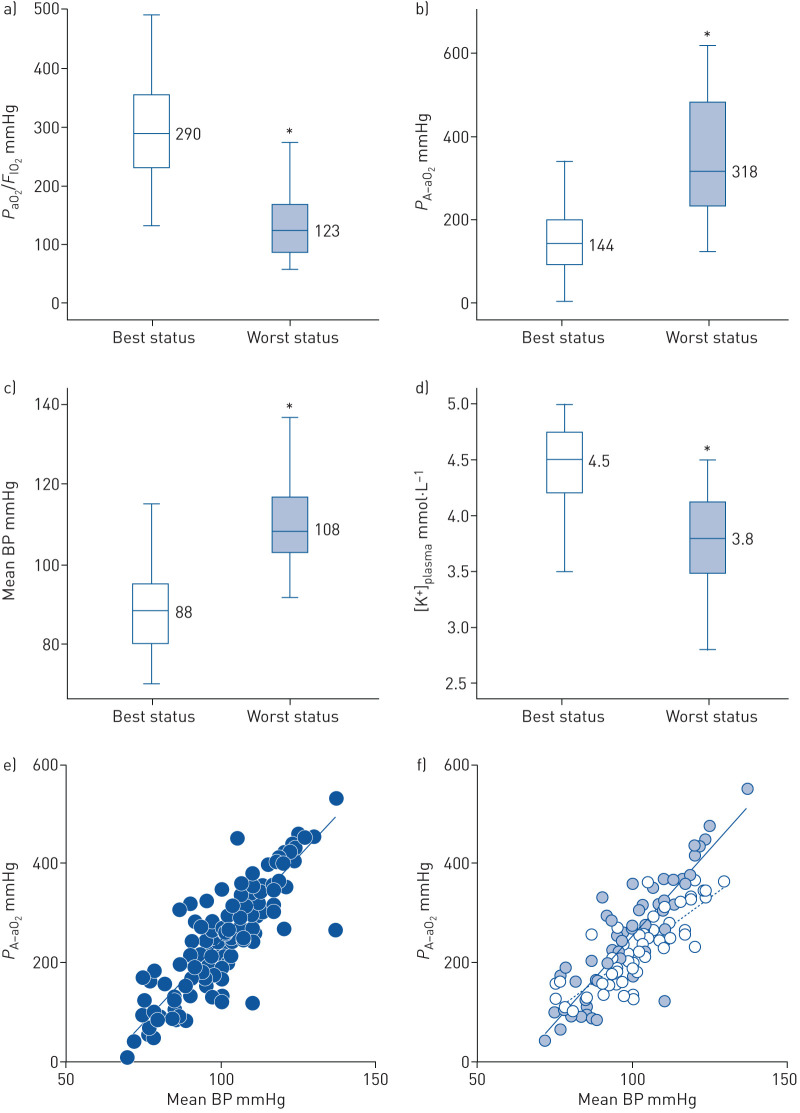
Mean age was 64±11 years and 29 out of 40 patients were male. All patients had normal heart function, but one had stable heart failure with reduced ejection fraction. Despite only 23 patients presenting with a pre-existing history of hypertension (preHT), all patients, although under optimised noninvasive ventilatory treatment (optimal FIO2 and positive end-respiratory pressure), showed a degradation of PaO2/FIO2 and PA–aO2 concomitant with a raised BP and average drop in [K+]plasma (figure 1a–d). Median [K+]plasma was 3.8 mmol·L−1. The median time-period leading to a negative clinical picture was 4.25 days. According to haemodynamic and respiratory changes, patients were grouped as follows: group 1 (8/40 patients, 4/8 with preHT) and group 2 (32/40 patients, 15/32 with preHT). Group 1 showed temporary oscillations towards high BP with contrary changes in lung function (best versus worst status: PaO2/FIO2=311 versus 130 mmHg; PA–aO2=107 versus 312 mmHg; mean BP=83 versus 89 mmHg). After 28 days, these patients showed better outcomes, i.e. no deaths and clinical improvement, even after the need of invasive ventilatory support (two cases). Patients in group 2 were in critical disease (best status: PaO2/FIO2=286 mmHg; PA–aO2=158 mmHg; mean BP=88 mmHg). They experienced a rapid deterioration of clinical conditions with linear increasing of BP and progressive worsening in gas exchange (worst status: PaO2/FIO2=122 mmHg; PA–aO2=364 mmHg; mean BP=111 mmHg). Figure 1e shows a positive correlation between PA–aO2 and mean BP as assessed by Poon's analysis (slope=6.666, R2=0.757; p<0.0001). According to our hypothesis, [K+]plasma was considered a marker of RAAS activation and in group 2 the median value of 3.8 mmol·L−1 was used to stratify the patients. The slope of PA–aO2/mean BP relationship was significantly (U-test p<0.001) steeper in those with [K+]plasma <3.8 mmol·L−1 (figure 1f). After 28 days, compared to group 1, those in group 2 had a greater prevalence of intensive care need (six out of 32) and a higher mortality (16 out of 32) in a very short period time (6.1 days). As of 12 April 2020, the remaining 10 patients were still alive or discharged at home.
FIGURE 1.
Box plots of a) oxygen and inspiratory fraction of oxygen ratio, b) alveolar–arterial oxygen gradient, c) mean blood pressure (BP) and d) plasma potassium levels ([K+]plasma). Statistical significance was obtained through Mann–Whitney U-test comparing best status with worst status. *: p<0.001. e) Poon's analysis of PA–aO2/mean BP: n=137, slope=6.666, R2=0.757; p<0.0001. f) Poon's analysis of PA–aO2/mean BP according to [K+]plasma stratum. In (f) pale blue dots represent the group with [K+]plasma≤3.8 mmol·L−1 (n=78; slope=6.686, R2=0.774; p<0.0001) and white dots represent the group with [K+]plasma >3.8 mmol·L−1 (n=59; slope=4.491, R2=0.670; p<0.0001). PaO2: arterial oxygen tension; FIO2: inspiratory oxygen fraction; PA–aO2: alveolar–arterial oxygen tension difference.
Our findings showed that in COVID-19 a degradation of lung function may be associated with a rise in BP. Though COVID-19 is primarily known as a respiratory disease, it seems to move progressively to a vascular disease resulting in haemodynamic instability. In line with the evidence that SARS-CoV-2 knocks out the vasodilatory modulation driven by ACE2 [7, 9], we indirectly documented the RAAS activation by monitoring [K+]plasma changes. Indeed, there is particular concern about hypokalemia in COVID-19, due to interaction of SARS-CoV-2 with RAAS [10]. According to [K+]plasma and BP variability, an upregulation of aldosterone might be one of the fatal mechanisms leading to a negative prognosis. Aldosterone which is a potent arteriolar vasoconstrictor directly acts on salt and water retention, as well as on inflammation [6]. Considering that an increased BP could mirror the systemic vasoconstriction due to ACE2 depletion, the increased ventilatory dead space (PA–aO2) may be an expression of changes in pulmonary vessel tone leading to blood flow redistribution. Indeed, in stable condition, hypoxic pulmonary vasoconstriction is physiologically protective allowing a perfusion steering blood flow toward functionally preserved lung regions [11]. When COVID-19 reaches a certain stage there is disproportionate endothelial damage that disrupts pulmonary vasoregulation, promotes ventilation–perfusion mismatch (the primary cause of initial hypoxaemia), and fosters thrombogenesis [12]. Overall, our evidence, that has to be further verified, suggests that COVID-19 patients are less capable to counteract the progressive activation of the RAAS. The disequilibrium of AngII/ATR1 balance may be effective on the cardiovascular system once COVID-19 progresses and ACE2 reduces. In group 1 a sort of physiological or pharmacological counterbalance restored the best possible status. In group 2, the extreme severity of the disease has led to worst outcome. The quicker the haemodynamic changes, the greater the severity of COVID-19 syndrome. While the results of the trial on recombinant-human-ACE2 are still awaited (clinicaltrials.gov identifier NCT04335136), COVID-19 patients may benefit from known endothelial active agents, i.e. ACE-inhibitors, angiotensin II type-I receptor blockers or mineral corticoid-receptor antagonists.
Shareable PDF
Supplementary Material
Footnotes
Conflict of interest: M. Vicenzi reports personal fees from Janssen-Cilag SpA and MSD Italia, outside the submitted work.
Conflict of interest: R. Di Cosola has nothing to disclose.
Conflict of interest: M. Ruscica has nothing to disclose.
Conflict of interest: A. Ratti has nothing to disclose.
Conflict of interest: I. Rota has nothing to disclose.
Conflict of interest: F. Rota has nothing to disclose.
Conflict of interest: V. Bollati has nothing to disclose.
Conflict of interest: S. Aliberti reports grants and personal fees from Bayer Healthcare, Aradigm Corporation, Grifols, INSMED and Chiesi, personal fees from AstraZeneca, Basilea, Zambon, Novartis, Raptor, Actavis UK Ltd and Horizon, outside the submitted work.
Conflict of interest: F. Blasi reports grants and personal fees from AstraZeneca, Chiesi, GSK, Insmed and Pfizer, grants from Bayer, personal fees from Guidotti, Grifols, Menarini, Mundipharma, Novartis and Zambon, outside the submitted work.
References
- 1.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020; 367: 1444–1448. doi: 10.1126/science.abb2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005; 436: 112–116. doi: 10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu H, Xie Z, Li T, et al. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci Rep 2016; 6: 19840. doi: 10.1038/srep19840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ Res 2020; 126: 1456–1474. doi: 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guignabert C, de Man F, Lombes M. ACE2 as therapy for pulmonary arterial hypertension: the good outweighs the bad. Eur Respir J 2018; 51: 1800848. doi: 10.1183/13993003.00848-2018 [DOI] [PubMed] [Google Scholar]
- 7.Wang K, Gheblawi M, Oudit GY. Angiotensin converting enzyme 2: a double-edged sword. Circulation 2020; in press [ 10.1161/CIRCULATIONAHA.120.047049]. [DOI] [PubMed] [Google Scholar]
- 8.Poon CS. Analysis of linear and mildly nonlinear relationships using pooled subject data. J Appl Physiol 1988; 64: 854–859. doi: 10.1152/jappl.1988.64.2.854 [DOI] [PubMed] [Google Scholar]
- 9.Vaduganathan M, Vardeny O, Michel T, et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020; 382: 1653–1659. doi: 10.1056/NEJMsr2005760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr 2020; 14: 247–250. doi: 10.1016/j.dsx.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunham-Snary KJ, Wu D, Sykes EA, et al. Hypoxic pulmonary vasoconstriction: from molecular mechanisms to medicine. Chest 2017; 151: 181–192. in press [doi: 10.1016/j.chest.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA 2020; in press [ 10.1001/jama.2020.6825]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01157-2020.Shareable (426KB, pdf)