Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can cause severe pneumonia requiring invasive mechanical ventilation [1], in the context of atypical acute respiratory distress syndrome (ARDS) [2]. The magnitude of the epidemic places an unprecedented pressure on intensive care units (ICUs), making avoidance of intubation a critical issue.
Short abstract
COVID-19 pneumonia can be life-threatening. Given the unprecedented burden placed on ICU resources by the epidemic, avoiding intubation is a major issue. This study suggests that CPAP can achieve this objective. https://bit.ly/2X4Q8Zj
To the Editor:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can cause severe pneumonia requiring invasive mechanical ventilation [1], in the context of atypical acute respiratory distress syndrome (ARDS) [2]. The magnitude of the epidemic places an unprecedented pressure on intensive care units (ICUs), making avoidance of intubation a critical issue.
Supplemental oxygen is the first-line treatment of ARDS. When escalation is needed, pre-intubation approaches carry the risk of delaying intubation and increasing mortality [3]. Noninvasive ventilation (NIV) is not recommended [4] but high-flow nasal oxygen (HFNO) may decrease the need for intubation without impacting mortality [4, 5]. Mostly because of an early negative report [6], continuous positive airway pressure (CPAP) remains largely undocumented in ARDS. In SARS-CoV-2 pneumonia, evidence-based guidelines are lacking [7] but CPAP could prove useful [8].
In this context, on 20 March, 2020, the French learned society for respiratory medicine circulated a clinical management algorithm derived from the Italian experience and suggesting the use of CPAP in SARS-CoV-2 patients requiring oxygen escalation [8]. This algorithm was implemented in our department on 24 March, 2020, in a context of limited HFNO availability and environmental contamination concerns.
We designed this retrospective study to evaluate the impact of the CPAP strategy on intubation rate. We compared the period immediately before the algorithm implementation (11–23 March, 2020) with the period immediately after (24 March to 8 April), testing the hypothesis that CPAP can avoid intubation in patients with severe forms of SARS-CoV-2 pneumonia over the first week of their management.
This observational study with short-term historical controls was conducted in the 25-bed pulmonology unit of a 1600-bed university hospital (Pitié-Salpêtrière, Paris, France). It was approved by the institutional review board of the French learned society for respiratory medicine (CEPRO2020-024). Patients were informed of the use of their anonymised data and given the opportunity to refuse it.
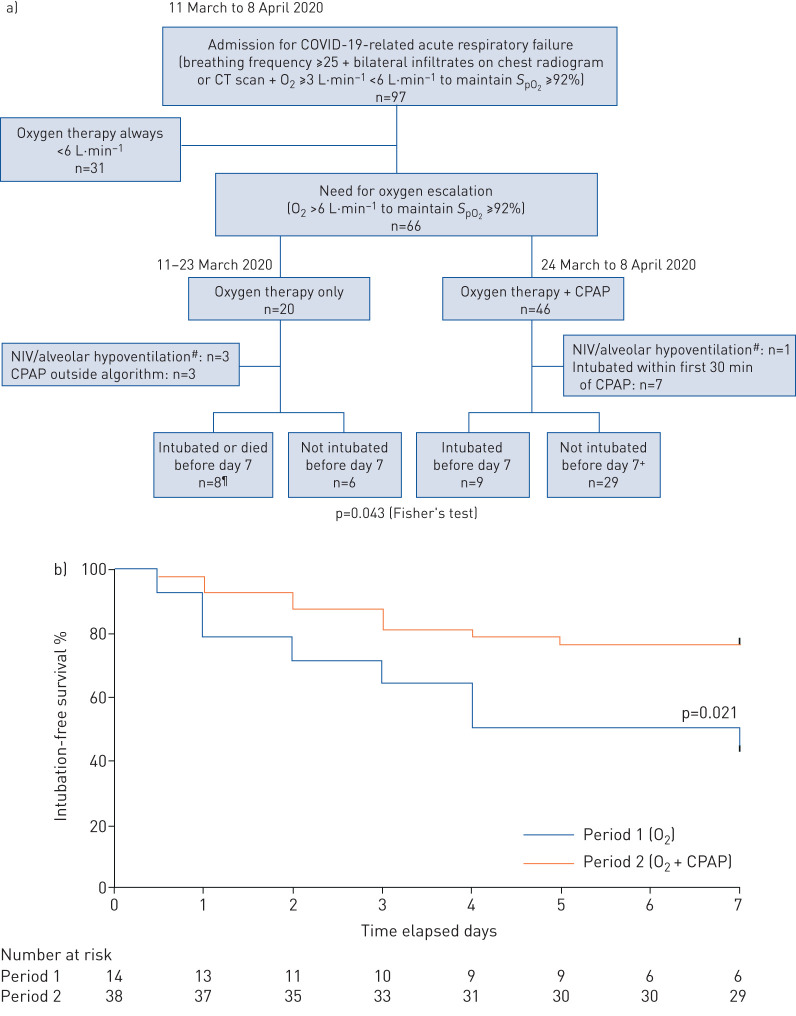
According to an ad hoc hospital policy, all the patients admitted to the pulmonology unit between 11 March and 8 April, 2020) had to have laboratory-confirmed SARS-CoV-2 infection and acute respiratory distress (respiratory rate ≥25, bilateral pulmonary infiltrates on chest radiogram or computed tomography scan, and need for standard oxygen between 3 and 6 L·min−1 to maintain peripheral oxygen saturation (SpO2) ≥92%). Among them, those requiring escalating oxygen therapy ≥6 L·min−1 to maintain SpO2 ≥92% were included (figure 1). Two consecutive periods were compared (1: 11–23 March, controls; 2: 24 March to 8 April, cases). During period 1, escalation consisted only in increasing supplemental oxygen, up to 15 L·min−1. During period 2, CPAP was delivered on an as-needed basis, with a minimum of 2 h twice a day and continuous nocturnal administration. CPAP was administered using a face mask connected to a home mechanical ventilator in most cases, or to a Boussignac positive pressure valve. CPAP was initially set at 10 cmH2O and then adjusted between 8 and 12 cmH2O according to clinical tolerance, leaks and SpO2. The expiratory limb of the circuit was equipped with an antimicrobial filter. Patients were not included in the presence of alveolar hypoventilation (arterial carbon dioxide tension (PaCO2) ≥45 mmHg), if they received CPAP during period 1, or when they had to be intubated during the first 30 min of CPAP during period 2.
FIGURE 1.
a) Study flow chart. b) 7-day intubation-free survival. #: noninvasive ventilation (NIV) and alveolar hypoventilation defined as arterial carbon dioxide tension ≥45 mmHg; ¶: five intubations and three deaths; two of the deaths occurred in the two “do not intubate” patients; +: there were six patients with “do not intubate” decisions, none of whom died (p=0.036 versus controls; Fisher's exact test). COVID-19: coronavirus disease 2019; CT: computed tomography; SpO2: peripheral oxygen saturation; CPAP: continuous positive airway pressure.
The outcome was intubation at day 7, or death in the patients with a “do not intubate” (DNI) decision (collegial discussion between pulmonology and ICU staff, on admission or at any time thereafter, mainly taking into account the patient's own opinion when reliable, age, frailty score and comorbidities). Criteria for intubation were haemodynamic instability, neurological deterioration, worsening respiratory failure with respiratory rate above 40 breaths per min and high respiratory muscle recruitment, acidosis with a pH below 7.35, SpO2 below 90% for more than 5 min without response to pre-intubation approaches.
Clinical and laboratory data were obtained from electronic medical records. Missing data were not imputed. The statistical analysis was performed using Prism v6® (Graphpad, USA). Continuous variables are summarised as medians and interquartile ranges and compared using the Mann–Whitney U-test. Categorical variables are summarised as numbers and compared using Fisher's exact test. Intubation-free survival curves were compared by log-rank test. Differences were considered significant at p<0.05.
Over the whole period of interest, 97 coronavirus disease 2019 (COVID-19) patients with acute respiratory failure criteria were admitted 66; patients were eligible for inclusion, and 52 were included (14 controls and 38 cases) (figure 1). There was no significant difference between controls and cases regarding age (62 (54–72) versus 63 (55–70) years), gender (13 men versus 26), body mass index (26.7 (23.0–33.0) versus 27.6 (24.2–34.4) kg·m−2), tobacco smoking (3 versus 9), respiratory and cardiovascular comorbidities, clinical data upon admission (body temperature, heart rate and arterial pressure), biological data (white cell count, lymphocyte count, C-reactive protein, procalcitonin, D-dimers) or radiological data (bilateral infiltrates in 100% of cases). Physiological severity on admission was not different (simplified acute physiology score II 24 (21–28) versus 27 (22–30)) and respiratory severity was comparable (respiratory rate 30 (20–35) versus 27 (23–32); use of accessory muscles 100%; PaO2 under oxygen 69.0 (61.0–83.0) versus 71 (63.5–88.5) mmHg; PaCO2 32.0 (28.0–38.0) versus 30.5 (30.5–37) mmHg; pH 7.48 (7.46–7.51) versus 7.48 (7.46–7.51); oxygen needed to maintain SpO2 ≥92% 3 (2–6) versus 5 (3–6) L·min−1). There was also no difference during the two periods between treatment received by controls and cases (hydroxychloroguine 8 versus 20; antiviral drugs 0 versus 1; tocilizumab 0 versus 1; corticosteroids 0 versus 1) and between the number of inclusions in randomised trials (0 versus 6). Cases received CPAP for 5 (2–7.5) days with a daily use of 8 (4–11) h.
Six intubations and two deaths without intubation were recorded at day 7 during period 1 (57%), versus nine intubations and no deaths during period 2 (23%) (p=0.043). Among DNI patients there were two deaths in two patients during period 1, versus no deaths in six patients during period 2 (p=0.036). 7-day intubation-free survival rate was significantly better during period 2 (p=0.021) (figure 1). Median time to intubation or death was 5.5 days during period 1 and was not reached at day 7 during period 2. Identical results were observed at day 14.
Our findings indicate that CPAP is feasible in deteriorating COVID-19 patients managed in a pulmonology unit. They suggest that CPAP can avoid intubation at 7 days and at 14 days, particularly in patients with a previous DNI decision, which resembles similar observations with NIV in patients having declined intubation [9].
This result must be interpreted very cautiously due to the monocentric retrospective and non-randomised nature of the study, its small size and a smaller number of controls than cases. Yet several elements compensate for these weaknesses. The two periods were short and consecutive, limiting the risk of practice variations. Identical treatment protocols and personnel-to-patient ratio were used; intubation criteria were unchanged and there was no shortage of ICU beds. Patients treated during period 1 did not significantly differ from patients treated during period 2. Because we generally used high-end home mechanical ventilators to apply CPAP (due to immediate availability), we could ascertain the quality and duration of treatment. Of note, simpler devices have been used to apply CPAP [8], including in our patients, and even if potential “technical” differences may be found between methods, there is no reason to think they should provide different results.
Regarding safety, none of our patients receiving CPAP had to be intubated under high emergency or cardiac arrest conditions. We acknowledge that CPAP may unduly delay intubation in non-expert hands, and insist on the notion that intubation should not be delayed in the absence of a rapid and clear response to treatment. The proportion of caregivers contaminated by SARS-CoV-2 was similar during period 2 (6%) and during period 1 (10%).
Our observations need to be corroborated and can only justify prospective randomised trials. If confirmed, they would be of particular interest in the context of mass critical care or healthcare systems in low income countries.
Shareable PDF
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the clinical help of the following persons, listed as collaborators of the study: Isabelle Arnulf, Valérie Attali, Zakaria Belhachimi, Alexandra Beurton, Yasmine Bouznad, Julie Delemazure, Robin Deleris, Pauline Dodet, Martin Dres, Alexandre Duguet, Fadwa El-Kouari, Christina Esteban-Amarilla, Maria Alejandra Galarza Jimenez, Claire Gazaniol, Luc Haudebourg, Noémie Haziot, Pierre Helly De Tauriers, Pierantonio Laveneziana, Marie Lecronier, Julien Lemarec, Cécile Londner, Jeanne Maisonobe, Roxane Malrin, Julien Mayaux, Alexis Mendoza, Pierre Mora, Elise Morawiec, Leila Mourtada, Safaa Nemlaghi, Raphaelle Ohayon, Brigitte Orcel, Stefania Redolfi, Claire Riquier, Bérénice Soyez, Christian Straus, Sara Virolle and Estelle Wozniak.
Footnotes
Data availability: The authors will provide raw data to other researchers upon reasonable request.
Conflict of interest: M. Oranger has nothing to disclose.
Conflict of interest: J. Gonzalez-Bermejo has nothing to disclose.
Conflict of interest: P. Dacosta-Noble has nothing to disclose.
Conflict of interest: C. Llontop has nothing to disclose.
Conflict of interest: A. Guerder has nothing to disclose.
Conflict of interest: V. Trosini-Desert has nothing to disclose.
Conflict of interest: M. Faure has nothing to disclose.
Conflict of interest: M. Raux has nothing to disclose.
Conflict of interest: M. Decavele has nothing to disclose.
Conflict of interest: A. Demoule has nothing to disclose.
Conflict of interest: C. Morélot-Panzini reports personal fees for lectures from AstraZeneca, Menarini, SOS Oxygene and Resmed, personal fees for advisory board work and lectures from Chiesi and ADEP, personal fees for advisory board work from Vivisol, outside the submitted work.
Conflict of interest: T. Similowski reports personal fees from AstraZeneca France, Boehringer Ingelheim France, GSK France, TEVA France, Chiesi France, Lungpacer Inc. and ADEP Assistance, personal fees and non-financial support from Novartis France, grants from Air Liquide Medical Systems, outside the submitted work.
References
- 1.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L, Coppola S, Cressoni M, et al. Covid-19 does not lead to a ‘typical’ acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 201: 1299–1300. doi: 10.1164/rccm.202003-0817LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demoule A, Girou E, Richard JC, et al. Benefits and risks of success or failure of noninvasive ventilation. Intensive Care Med 2006; 32: 1756–1765. doi: 10.1007/s00134-006-0324-1 [DOI] [PubMed] [Google Scholar]
- 4.Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017; 50: 1602426. doi: 10.1183/13993003.02426-2016 [DOI] [PubMed] [Google Scholar]
- 5.Wang K, Zhao W, Li J, et al. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care 2020; 10: 37. doi: 10.1186/s13613-020-00653-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delclaux C, L'Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA 2000; 284: 2352–2360. doi: 10.1001/jama.284.18.2352 [DOI] [PubMed] [Google Scholar]
- 7.Namendys-Silva SA. Respiratory support for patients with COVID-19 infection. Lancet Respir Med 2020; 8: e18. doi: 10.1016/S2213-2600(20)30110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harari SA, Vitacca M, Blasi F, et al. Italian Thoracic Society (AIPO – ITS), Italian Respirarory Society (SIP/IRS), 2020. http://www.aiponet.it/news/speciale-covid-19/2426-managing-the-respiratory-care-of-patients-with-covid-19-english-version.html Managing the respiratory care of patients with COVID-19 - English version.
- 9.Azoulay E, Kouatchet A, Jaber S, et al. Noninvasive mechanical ventilation in patients having declined tracheal intubation. Intensive Care Med 2013; 39: 292–301. doi: 10.1007/s00134-012-2746-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01692-2020.Shareable (323.8KB, pdf)