Abstract
This study investigated the removal efficiency of micro- and nanoplastics (180 nm – 125 μm) during drinking water treatment, particularly coagulation/flocculation combined with sedimentation (CFS) and granular filtration under ordinary working conditions at water treatment plants (WTPs). It also studied the interactions between biofilms and microplastics and the consequential impact on treatment efficiency. Generally, CFS was not sufficient to remove micro- and nanoplastics. The sedimentation rate of clean plastics was lower than 2.0% for all different sizes of plastic particles with coagulant Al2(SO4)3. Even with the addition of coagulant aid (PolyDADMAC), the highest removal was only 13.6% for 45 – 53 μm of particles. In contrast, granular filtration was much more effective at filtering out micro- and nanoplastics, from 86.9% to nearly complete removal (99.9% for particles larger than 100 μm). However, there existed a critical size (10 – 20 μm) where a significant lower removal (86.9%) was observed. Biofilms were easily formed on microplastics. In addition, biofilm formation significantly increased the removal efficiency of CFS treatment from less than 2.0% to 16.5%. This work provides new knowledge to better understand the fate and transport of emerging micro- and nanoplastic pollutants during drinking water treatment, which is of increasing concern due to the potential human exposure to micro- and nanoplastics in drinking water.
Keywords: Micro- and nanoplastics, Water Treatment, Coagulation/Flocculation/Sedimentation, Filtration, Biofilm
Graphical Abstract
1. Introduction
Microplastics are defined as plastics smaller than 5 mm in size, including microspheres, fragments, and fibers (Arthur et al., 2009; Eerkes-Medrano et al., 2015). Water pollution due to microplastics is a top environmental concern and has been reported everywhere around the world, from wastewater, marine environment, freshwater, to drinking water (Amélineau et al., 2016; Andrady, 2011; Baldwin et al., 2016; Carr et al., 2016; Duis and Coors, 2016; Eerkes-Medrano et al., 2015; Hartmann et al., 2019; Kosuth et al., 2018a; Murphy et al., 2016; Tyree, 2017; Yonkos et al., 2014). For instance, surveys of 159 tap water samples taken from several countries and 259 bottled water samples across 11 brands showed that 81% of tap water and 93% of bottled water contained microplastics (Kosuth et al., 2018a; Mason et al., 2018; Oßmann et al., 2018). Research indicates that the average person ingests over 5,800 particles of synthetic debris each year, with the largest contribution coming from drinking water (88%) (Kosuth et al., 2018a). The critical questions are (1) how do these microplastics pass through water treatment and get into drinking water and (2) is drinking water treatment, the critical public service protecting human beings from exposure to pollutants, able to remove microplastics and to what extent? Currently, the fate of microplastics in water treatment has not been adequately investigated, and there is no definitive understanding of the significance and persistence of these pollutants in municipal water systems. Many questions remain unanswered, and data is missing, particularly for treatment processes where little information is available regarding how microplastics behave during drinking water treatment. These critical knowledge gaps need extensive research. For instance, the World Health Organization (WHO) recently released a report “Microplastics in Drinking-Water” and stated that “there is a need to better characterize the effectiveness of water treatment” (WHO, 2019).
Currently, limited studies have been conducted to evaluate microplastics removal in drinking water treatment. Mintenig et al. (Mintenig et al., 2019), Pivokonsky et al. (Pivokonsky et al., 2018a), and Wang et al. (Wang et al., 2020) surveyed microplastic content in raw and treated water samples from different water treatment plants (WTPs). The results of these studies demonstrate the ubiquitous presence of microplastics in the municipal water supply. Three studies investigated the removal efficiency of microplastics in different treatment processes. Wang et al. (Wang et al., 2020) examined the occurrence and removal of microplastics in an advanced WTP for each treatment step, including coagulation combined with sedimentation, sand filtration, ozonation, and GAC filtration. Ma et al. (Ma et al., 2019a; Ma et al., 2019b) studied microplastic removal in coagulation/sedimentation and ultrafiltration under controlled experiments. However, results from these studies were significantly different. For instance, the removal efficiency of coagulation/sedimentation was 40.5% – 54.5% in Wang et al.’s survey, but was only ~5% under an ordinary coagulant dosage (20 ppm) and below 40% even with an extreme high coagulant dosage (430 ppm) in Ma et al.’s experiments. More research is needed to investigate microplastics removal under WTPs’ ordinary working conditions (Novotna et al., 2019; WHO, 2019). In addition, given the results of the current literature, more attention should be focused on smaller microplastics (in the micrometer to nanometer range) that are most commonly detected in drinking water (Novotna et al., 2019). Particularly for nanoplastics, little information is currently available regarding their presence in the environment due to the limitation of analytical methods. Yet, a number of studies have indicated that the number of plastic particles increases steeply for smaller sizes (Andrady, 2017; Cózar et al., 2014) and follows a power law increasing with a decreasing particle size when smaller than 100 μm (Enders et al., 2015; Pivokonsky et al., 2018b). Therefore, many water sources may contain a considerable amount of nanoplastics, suggesting a critical need to investigate the fate and transport of nanoplastics during drinking water treatment. Furthermore, previous studies have suggested that biofilms are easily formed on microplastics and can significantly change the characteristics of microplastics (size, shape, and density) (Harrison et al., 2011; Harrison et al., 2014; McCormick et al., 2014; Oberbeckmann et al., 2015), which could subsequently alter the removal efficiency of microplastics. To date, there is no study to investigate the impact of biofilms on microplastic removal in water treatment. To address these critical knowledge gaps, the objectives of this work are to (1) compare the effectiveness of different water treatment processes under WTPs’ ordinary working conditions, (2) characterize the removal efficiency of small microplastics at micro- and nanosize (180 nm – 125 μm), and (3) to explore the interactions of biofilms and microplastics and the consequential impact on treatment efficiency of microplastics.
2. Materials and Methods
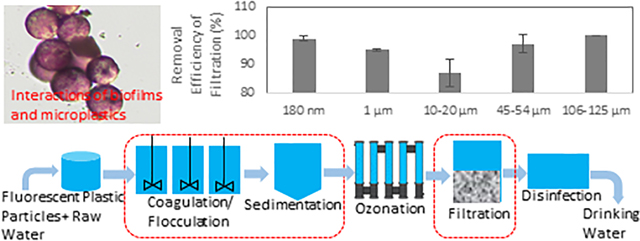
Coagulation/flocculation combined with sedimentation (CFS) and filtration were chosen for investigation in this work. These two processes are commonly employed at WTPs to remove different polluting agents (algal or cyanobacterial cells, dissolved natural organic matter, and clay particles) (Novotna et al., 2019). Bench-scale treatment of CFS and filtration was conducted to mimic the working conditions at the Detroit WTP operated by the Great Lakes Water Authority (GLWA). Figure 1 illustrates the treatment processes operated in the WTP, including CFS, ozonation, filtration, and chlorination. GLWA is one of the largest water utilities and operates five water treatment facilities that draw water from Lake Huron and the Great Lakes tributary, the Detroit River, serving nearly 4 million of the population. The Great Lakes are the largest group of freshwater lakes on Earth. Water resources in the Great Lakes basin are drinking water sources for eight U.S. states and one Canadian state, serving more than 30 million people - roughly 10% of the U.S. population and more than 30% of the Canadian population. In the Great Lakes, studies found an average concentration of 43,000 plastic particles per km2 in surface water with a maximum of nearly two million particles per km2 in the Detroit River (Baldwin et al., 2016; Cable et al., 2017; Eriksen et al., 2013). All tested tap water samples in the Great Lakes region were found to contain microplastics (Kosuth et al., 2018b). Therefore, the results from this study are informative for other WTPs in the Great Lakes basin with similar treatment processes.
Figure 1:
Drinking water treatment processes adopted in the Detroit WTP. Coagulation/flocculation combined with sedimentation (CFS) and filtration were chosen to study the removal efficiency of micro- and nanoplastics.
2.1. Materials
In this work, the removal efficiency of plastic particles at different sizes was systematically investigated. Fluorescent plastic particles (beads) at five different sizes (180 nm, 1.2 μm, 10–20 μm, 45–53 μm, and 106 – 125 μm) were purchased from Cospheric (Santa Barbara, CA 93160, USA) and Degradex (Hopkinton, MA 01748, USA) to be spiked in raw water for the experiments. The size range of microplastics was chosen because particles larger than 100 μm can be effectively removed by today’s WTPs and most microplastics found in treated drinking water are smaller than 100 μm (Pivokonsky et al., 2018a; Wang et al., 2020). Two types of plastics were included. One was polyethylene (PE) (10–20 μm, 45–53 μm, and 106–125 μm), and the other was polystyrene (PS) (180 nm and 1.2 μm). These plastics are some of the most common plastics and prevail in freshwaters and drinking water (Koelmans et al., 2019). In addition, both plastics are hydrophobic and have densities (~ 1.0 g/cm3) close to that of natural water and, as such, easily float in water (Hidalgo-Ruz et al., 2012), resulting in a high risk of staying in drinking water.
The number of plastic particles for each experiment was controlled at environmentally relevant levels reported in raw water (thousands of particles per litter) (Pivokonsky et al., 2018a; Wang et al., 2020). The quantity of each size of plastic particles was measured according to the fact sheet provided by the manufacturer. Specifically, for particles with the size of 106 – 125 μm, 45 – 53 μm, and 10 – 20 μm, about 0.00001 – 0.001 g of each size of particles was used for experiments. Particles with the size of 1.24 μm and 180 nm were diluted in 0.1% Tween 20 solution, and 1 μL of the diluted solution for each size of particles was used for experiments. The average concentration of spiked particles (particles/L) was approximately 1,800 (106 – 125 μm), 2,000 (45 – 53 μm), 7,000 (10 – 20 μm), and 9,500 (1.24 μm and 180 nm). The raw water, coagulant, coagulant aid, and filtration media used in this study were the same as those used in the GLWA’s WTP located in Detroit, MI.
2.2. Coagulation/Flocculation Combined with Sedimentation (CFS)
2.2.1. CFS
Raw water samples collected from the Detroit WTP were spiked with different sizes of fluorescent particles for the CFS test. Using a Phipps & Bird programmable jar test, a bench-scale model replicating the CFS processes at the Detroit WTP was implemented. Speeds of the paddle stirrers and the time durations/intervals of these speeds were determined based on the operation condition of the Detroit WTP (SI Figure S1). The coagulant and dosage was aluminum sulfate Al2(SO4)3 at 20 ppm, the same as used at the Detroit WTP during the time of the experiment corresponding to the turbidity of the raw water. 250 – 500 mL of raw water was spiked with fluorescent plastic particles at five different sizes (180 nm, 1.2 μm, 10–20 μm, 45–53 μm, and 106 – 125 μm), respectively. For each size of particles, there were: (1) positive control (nano-pure water with fluorescent particles), (2) negative control (raw water without fluorescent particles through the treatment), and (3) CFS treatment (raw water with fluorescent particles through the treatment). Triple replicates were included for each set of experiments. The sediment for each experiment was captured and filtered through filter membranes (0.025 μm of pore size for 180 nm particles and 0.2 μm of pore size for other particles). All the filter membranes were used for image analysis to quantify the removal efficiency of spiked particles (captured in the sediment). The image processing and analysis were described in section 2.5.
2.2.2. CFS with Coagulant Aid Diallyldimethylammonium Chloride (PolyDADMAC)
This experimental design was similar to the design of the CFS experiment. The only modification of this experiment included the addition of a coagulant aid (PolyDADMAC) to evaluate whether a coagulant aid would help increase particle collision for improved removal. 20 ppm of alum and 0.5 ppm of PolyDADMAC were used. This ratio between alum and PolyDADMAC was the same ratio used by the Detroit WTP. All other designs of this experiment and the analysis were performed in the same manner as the CFS experiment.
2.3. Filtration
Bench-scale filtration units were built with 45.7 cm long columns with diameters of 3.8 cm by supporting the filter media with two layers of 60-grade cheesecloth (648 μm pore size) on bottom and anthracite filled up to a height of 38.1 cm within the column (SI Figure S2). This created a porosity of 0.54 to replicate a standard pilot-scale drinking water filtration system at the Detroit WTP. The flow of water through the bench-scale filtration was controlled by gravity. To evaluate what percentage of plastic particles could flow through this filtration system and the effect of particle size, three sets of experiments were conducted. i.e., positive controls (nano-pure water with fluorescent particles), negative controls (raw water without fluorescent particles through filtration), and filtration tests (raw water with fluorescent particles through filtration). Triple replicates were included for each set of experiments. Membrane filtration with 0.025 μm (for 180 nm particles) and 0.2 μm (for other particles) pore sizes coupled with image analysis was used to quantify the removal efficiency of plastic particles during the filtration (plastic particles flowing through filtration). All the filter membranes were then examined and analyzed (Section 2.5).
2.4. Interactions of Biofilms and Microplastics
To understand the interactions of biofilms and microplastics and the consequential impact on treatment efficiency, one size of plastic particles was spiked in 500 mL raw water and then incubated on a shaker (100 RPM) up to seven days for biofilm formation. The incubation was set as light-dark (12 hrs – 12 hrs) cycles to mimic the condition of source water where microplastics were usually exposed to light-dark alternations. These light-dark cycles would also allow the growth of both phytoplankton and bacteria that are commonly found in natural biofilms on microplastics (Rummel et al., 2017). The particle size for this experiment was 45–53 μm. This size of particles showed medium removal in treatment experiments. In addition, this size was one size above the critical size, where the lowest removal was observed during filtration treatment. Therefore, the inclusion of 45 – 53 μm particles in this experiment would allow the observation of any significant impact of biofilm formation on removal efficiency. This experiment involved both CFS (without coagulant aid) and filtration. CFS tests (Phipps & Bird programmable jar test) and bench-scale filtration modules were the same conditions, processes, and analyses as described in Section 2.2.1, Section 2.3, and Section 2.5, respectively.
2.5. Image Processing and Analysis
A Nikon Eclipse Ts2 standing microscope was used in tandem with a Nikon Ds Fi3 camera and NISElements Microscope BR Ver 5.11 Imaging Software (Nikon) for membrane imaging under red, green, or blue light based on the fluorescence of the spiked particles. The magnification ranged from 1× – 5 × zoom for different sizes of particles. Each photo had a 1-s exposure and no analog gain. For each size of particles, membranes obtained from all the experiments were imaged under the required light for fluorescence measurement. For each membrane, 70–100 individual photos were taken and then stitched together using the “stitch image free shape” program that was included in the NIS-Elements software. The resulting stitched image was then saved as an Nd2 file and also saved as TIFF for image analysis. Background fluorescence was removed by adjusting the Look-Up Table (LUT) values within the NIS-Elements software and modifying the pixel values according to these adjustments with the “Modify Image through LUTs” option in the software. Specifically, the images of the negative controls were used to define LUT parameters in the positive controls and replicates. Further image analysis was performed using the developed algorithm in MATLAB. Within MATLAB, the total fluorescent intensity was computed by calculating the integrated density of the image. The quantity of spiked fluorescent particles in positive controls and treatment replicates was determined according to the fact sheet provided by the manufacturer. The number of particles remaining after each treatment experiment was calculated by the total fluorescent intensity of LUT adjusted image analyses of positive controls and treatment replicates (Eq. 1). Detailed information for image processing and analysis was described in SI Section S3.
| (Eq. 1) |
Where: NR was the number of fluorescent particles after each treatment experiment; FR was the total fluorescent intensity of LUT adjusted image analysis of each treatment replicate; FPC was the total fluorescent intensity of LUT adjusted image analysis of the positive control; NPC was the number of spiked microspheres in the positive control (determined according to the fact sheet provided by the manufacturer).
2.6. Data Analysis
The results of image analysis were used to evaluate the removal efficiency of plastic particles as described by Eq. 2 and Eq. 3.
| (Eq. 2) |
Where: RCFS was the removal efficiency of plastic particles during CFS (%); PS was the amount of plastic particles captured in the sediment; PT was the total amount of plastic particles spiked in water.
| (Eq. 3) |
Where: RF was the removal efficiency of plastic particles during filtration (%); Pf was the amount of plastic particles flowing through the filtration (remaining in the filtrate); PT was the total amount of plastic particles spiked in water.
Statistical analyses were performed using Microsoft Excel. ANOVA (analysis of variance) was used to analyze differences of removal efficiency of plastic particles at different sizes for different treatment, respectively. A two-sample t-test was used to compare the significance of different treatments, including (1) CFS versus CFS with PolyDADMAC, and (2) treatment with biofilm formation versus treatment without biofilm formation. For all statistical analyses, p-values <0.05 were considered to be significant.
3. Results and Discussion
3.1. Overall Experimental Design
This work investigated the removal efficiency of micro- and nanoplastics (180 nm – 125 μm) during different drinking water treatment processes, including CFS, CFS with PolyDADMAC, and filtration, as well as the interactions of biofilms and plastic particles and the consequential impact on the removal efficiency. The testing conditions for these treatment processes were set as WTPs’ ordinary working conditions, particularly those used in the Detroit WTP Pilot Plant operated by GLWA. The overall experimental design, testing conditions, and measured data were summarized in Table 1.
Table 1:
Experiments and testing conditions in this work
| Treatment | Testing Conditions | Data Measured | ||
|---|---|---|---|---|
| Agent | Working parameters | Biofilm formation | ||
| CFS | Al2(SO4)3 (20 ppm); plastic particles (180 nm– 125 μm) | Flash mix RPM and flash mix time interval, respectively: 200, 60 seconds;
|
Yes (45–53 μm) | Plastic particles in the sediment |
| Filtration | Anthracite; plastic particles (180 nm – 125 μm). | Porosity: 0.54 porosity; Filtration under gravity | Yes (45–53 μm) | Plastic particles in the filtrate |
| Positive Controls | Nona-pure water with fluorescent particles: positive controls were used for recognizing and quantifying spiked particles. | |||
| Negative Controls | Raw water went through the same treatment: negative controls were used to remove background fluorescence and to adjust LUTs. | |||
3.2. Effect of CFS
Before CFS experiments, a buoyancy test was conducted to observe the settleability of plastic particles in raw water. Specifically, different sizes of plastic particles were spiked into raw water. After 5 minutes of vigorous mixing and 10 minutes of sedimentation, the distribution of microbeads was examined. As shown in Figure S17 (SI Section S4), the settleability of plastic particles was extremely low. Most particles floated on the water surface. For the CFS treatment, Al- and Fe-based coagulants are widely used in drinking water treatment. Ma et al. (Ma et al., 2019b) compared the effect of these two coagulants on the removal efficiency of microplastics (< 500 μm - 5 mm), and found that Al-based coagulants performed significantly better than Fe-based coagulants. The concentration of flocculants used in WTPs is typically below 20 ppm for actual drinking water treatment (Sillanpää et al., 2018). Therefore, this work used Al-based coagulant, Al2(SO4)3, at the concentration at 20 ppm that was also the working condition in the Detroit WTP operated by GLWA. The pH value was 7.59±0.03, 7.55±0.05, and 7.43±0.11 for raw water, CFS treatment, and CFS with PolyDADMAC, respectively.
As summarized in Table 2, sedimented plastic particles of all sizes (180 nm – 125 μm) during the CFS treatment were neglectable (< 2%). No significant difference was observed for the removal efficiency among different sizes of particles (p = 0.5). When adding coagulant aid (PolyDADMAC), the removal efficiency was significantly different among different sizes (p = 0.001). This was mainly attributed to the significant improvement of removal efficiency for 45–54 μm particles that showed the highest removal (13.6 % ± 6.8 %). The removed fraction of this size of particles was significantly higher during the treatment of CFS with PolyDADMAC compared to the treatment of CFS without PolyDADMAC (p = 0.04).
Table 2:
Removal Efficiency of micro- and nanoplastics during the treatment of CFS and CFS with PolyDADMAC
| Treatment | Removal Efficiency | p-value (ANOVA) | ||||
|---|---|---|---|---|---|---|
| 180 nm | 1 μm | 10–20 μm | 45–53 μm | 106–125 μm | ||
| CFS | Not observeda | < 0.1 % | 1.8 % ± 1.2 % | 0.3 % ± 0.3 %b | 1.4 % ± 1.2 % | 0.5 |
| CFS + PolyDADMAC | Not observeda | < 0.1 % | 1.0 % ± 0.2 % | 13.6 % ± 6.8 %b | 2.1 % ± 0.6 % | 0.001 |
“Not observed” indicates that no spiked fluorescent particles were observed in the sediment. The filter membranes capturing spiked fluorescent particles in the sediment were completely black. The entire membrane was scanned for any sign of fluorescence and determined not to contain any spiked fluorescent particles.
A significant difference in removal efficiency was observed between the treatment of CFS and CFS with PolyDADMAC (p = 0.04) for 45–53 μm of particles.
To represent the ordinary working conditions in WTPs, this work only investigated the coagulant dosage at 20 ppm, which is the typical maximum concentration for actual drinking water treatment. The results of this work are consistent with other experimental studies where the removal efficiency of microplastics during CFS treatment was insufficient (Ma et al., 2019a; Ma et al., 2019b; Wang et al., 2020). For instance, Ma et al. (2019b) found that < 5% of microplastics could be removed by CFS at regular coagulant dosage. One possible approach to improve the sedimentation efficiency is increasing the dosage of coagulants and coagulants aid. Ma et al. (2019b) reported that when increasing the coagulant dosage to 430 pm (more than 20 times higher than the ordinary working condition), the removal efficiency of microplastics could be significantly improved. However, the tradeoff of this approach is the increased sedimentation sludge, which would add extra cost for WTPs. In addition, increasing of coagulant dosage may increase the turbidity of treated water, which could pose challenges for the subsequent filtration process.
Our results indicate that CFS coupled with PolyDADMAC may enhance the sedimentation of some small microplastics at a certain size (45 – 53 μm). However, other sizes of the tested particles did not show significant improvement. PolyDADMAC is one type of high-molecular polymer coagulant aids to help bridge and bind particles, as well as to strength the floc and reach to its optimum size to increase sedimentation rate. A number of parameters (such as particle size/concentration, polymer size/concentration, polymer molecular weight, and the velocity gradient or shear rate) can affect the efficiency of CFS coupled with coagulant polymers (Hogg, 2013; Moudgil, 1993). In term of particle size, coarse particles (> 100 μm) are usually hard to flocculate due to the small rate of collisions, polymer molecules incapable of attaching to the surface during collisions, and the easy breakage of coarse flocs (Moudgil, 1993). In our results, particles with the size of 106 – 125 μm did not show any improved flocculation/sedimentation with the addition of PolyDADMAC. Interestingly, the sedimentation efficiency of particles smaller than 45 – 53 μm did not improve neither. In polymer-induced flocculation, flocs form under three common mechanisms, i.e., charge neutralization, charge-patch interaction, and particle bridging (Hogg, 2013; Zhou and Franks, 2006). The formation of flocs could be dominated by one mechanism or be collectively affected by all the mechanisms dependent on particular conditions of the aforementioned parameters (i.e., particle size/concentration, polymer size/concentration, polymer molecular weight, and the velocity gradient or shear rate), resulting in different sedimentation rates. It is possible that, under the experimental condition of this study, only particles at the size of < 45 – 53 μm were able to form flocs at the optimal size for sedimentation. More research is warranted to investigate the sedimentation rate of plastic particles under different conditions by changing the operational parameters.
These observations suggest that CFS (with or without coagulant aid) may not be an effective process to remove micro- and nanoplastics. This is not surprising given the low density of plastic particles that makes them easy to float in water. Further study should focus on alternative approaches appropriate for removing light particles. For example, the Dissolved Air Flotation (DAF) is another clarification process. During DAF, the air is diffused as fine bubbles, and suspended particles are floated to the surface and removed by skimming (EPA). This method could be more useful for small and low-density particles like micro- and nanoplastics. In a survey of the occurrence of microplastics in raw and treated drinking water in three WTPs, the WTP with the addition of the flotation process achieved the highest removal rate of microplastics (83%) compared to the other two WTPs without flotation (70% – 81%) (Pivokonsky et al., 2018a). However, further investigations are warranted to evaluate the effectiveness of these alternative approaches.
3.3. Effect of Filtration
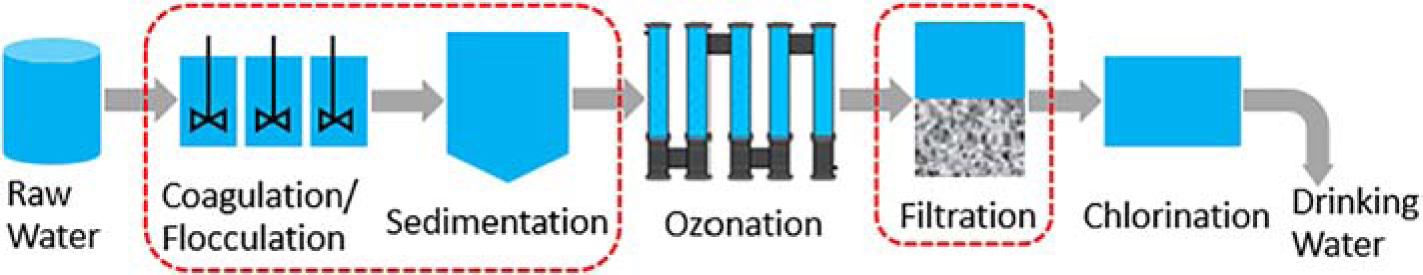
Overall, the filtration process is much more effective in removing small plastic particles than the CFS process (p < 0.001). The fraction removed during the filtration ranged from 86.9% ± 4.88% to nearly complete removal (99.9% ± 0.03% removal for 106 – 125 μm particles). The removal efficiency was significantly affected by the size of particles (p = 0.02) (Figure 2). However, the removal efficiency was not proportional to the change of particle sizes. A smaller particle size did not necessarily mean a lower removal efficiency. Among all the particles tested, the lowest removal efficiency was observed for the middle size of particles (10–20 μm) at a removal fraction of 86.9% ± 4.9%, followed by 1 μm particles (94.9% ± 0.4%), 45–54 μm particles (97.0% ± 3.0%), 180 nm particles (98.9% ± 0.7%), and 106 – 125 μm particles (99.9% ± 0.1%). When the size of the particle was larger than 10 – 20 μm, the larger the particle size was, the higher the fraction removed through filtration was observed. In contrast, when the size of the particle was smaller than 10–20 μm, the smaller size of particles showed a more significant removal.
Figure 2:
The removal efficiency of plastic particles during the filtration process.
These results show that filtration could be the most effective process to remove micro- and nanoplastics. This process could completely remove microplastics larger than 100 μm, which is consistent with other findings where very few microplastics larger than 100 μm were found in treated drinking water (Koelmans et al., 2019; Pivokonsky et al., 2018a; Wang et al., 2020). However, our results also suggest that filtration may not be able to filter out all microplastics, particularly for particle size ranging from 1 μm to 10–20 μm that were the top two least removed particles in our study. This implies that a considerable amount of microplastics may remain in treated water if the source water is contaminated. For example, Mintenig et al. (Mintenig et al., 2019) found very low numbers of microplastics (ranging from 0 to 7 particle/m3) in raw water and treated water from groundwater sources. In contrast, Pivokonsky et al. (Pivokonsky et al., 2018a) investigated the occurrence of microplastics in raw and treated drinking water at three WTPs using surface water sources, and reported much higher concentrations of microplastics. Their average abundance ranged from 1473 ± 34 to 3605 ± 497 particle/L in raw water and from 338 ± 76 to 628 ± 28 particle/L in treated drinking water (Pivokonsky et al., 2018a). Wang et al. (Wang et al., 2020) also found 6614 ± 1132 particle/L (raw water) and 930 ± 71 particle/L (effluent) in an advanced WTP.
Granular media filters are widely used in WTPs to remove small particles. The removal efficiency of particles during filtration treatment is affected by multiple mechanisms, including straining, interception, gravitational sedimentation, diffusion, and particle attachment/detachment (Bai and Tien, 1997; Logan et al., 1995). The overall removal rate is a result of the collective effects of all these mechanisms that are affected by many factors such as diameters of the particles, bed porosity, diameters of filter grains, and flow rate (Bai and Tien, 1997; Logan et al., 1995). Studies have demonstrated that the removal efficiency of particles at different sizes follows a parabolic change. There exists a critical size of particles that has the lowest filtration efficiency in the transition range among different dominated removal behaviors due to the absence of the collective filtration mechanisms (Schmidt et al., 1978). For example, Logan et al. (1995, Figure 2) assessed the filtration efficiency of different sizes of particles by using different filtration models. They observed the lowest removal efficiency for particles in size range of approximately 1 – 5 μm (Logan et al., 1995). Bai and Tien (1996) studied the filtration efficiency controlled by the mechanism of particle attachment/detachment with particle size ranging from less than 0.5 to 14 μm. They found that smaller particles (< 2μm) were less likely to detach and showed better removal efficiency (Bai and Tien, 1997). Our results show that the top two least removal particles under the specific filtration conditions in this study were those with the size from 1 μm to 10–20 μm. This observation could be the result of the collective effect of different filtration mechanisms. When particle size was smaller than 1 μm, particles could be more likely to attach on (or to be absorbed to) filtration grains and showed improved removal efficiency. When particles were larger than 10 – 20 μm, the opening between filtration grains was smaller than the diameter of the particle. Therefore, these particles could be easily captured by the filtration media. Our results are also consistent with other survey studies in actual WTPs. For instance, Pivokonsky et al. (Pivokonsky et al., 2018a) reported that microplastics in size range of 1 – 10 μm were the most plentiful (up to 95%) in the treated drinking water. Wang et al. (Wang et al., 2020) found that 1–5 μm and 5–10 μm of microplastics, compared to other sizes of plastic particles, had low removal efficiency during filtration. The overall removal efficiency during filtration in their studies ranged from 56.8% to 60.9%, which was significantly lower compared to our experimental results. This is due to the low removal efficiency of microfibers (< 50%) in their study. When considering the removal of spheres and fragments, our results are comparable with theirs (~ 70% of removal for fragments and over 80% of removal for microspheres).
Currently, little knowledge is available for the occurrence and removal of plastic particles at nanosize due to the limitation of analytical methods. This study provides new information by investigating fluorescent nanoplastics in simulated water treatment processes. The removal efficiency of 180 nm particles was actually higher than that of 1.24 μm and 10–20 μm particles. The possible reason could be that smaller particles would be more likely to retain in filtration grains through particle attachment or diffusion. However, our results suggest that granular filtration may not be able to eliminate all nanoparticles, implying a possible presence of nanoplastics in drinking water. Further research is needed to identify nanoplastics naturally present in raw and treated water with the advancement of analytical methods.
Collectively, these results indicate that microplastics smaller than 10–20 μm are most likely present in drinking water. This could be a significant health concern because these small particles, if ingested, present a higher chance of translocation across the gut barrier and could cause adverse health impacts (Chain, 2016). Future research should focus on the investigation of small microplastics (< 10–20 μm) including nanoplastics during water treatment to minimize their presence in drinking water. Besides, other filtration approaches such as dual media filter beds or ultra-filtration should be investigated to increase the removal efficiency of these small plastic particles.
3.4. Interactions of Biofilms and Microplastics and the Consequential Impact on Removal Efficiency
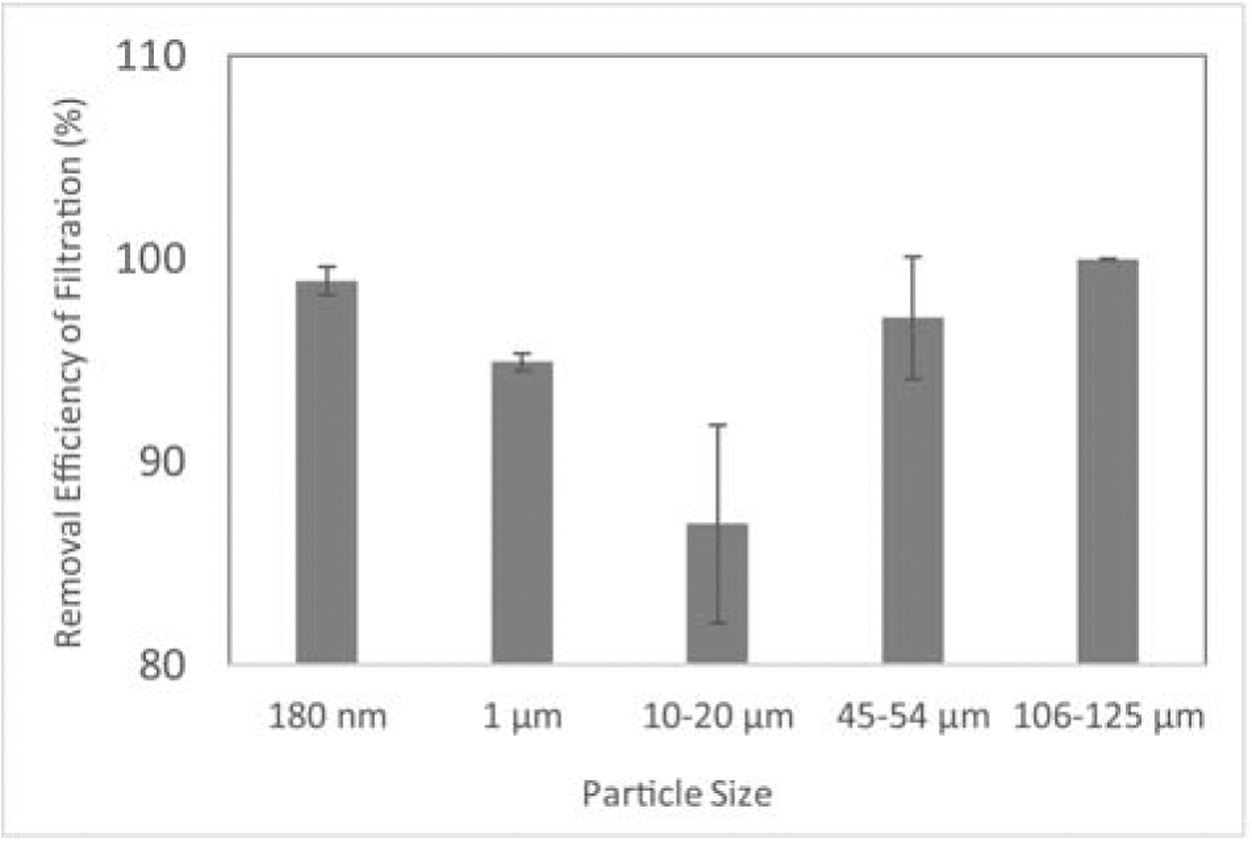
Plastic particles with the size of 45 – 53 μm was used to investigate the interactions of biofilms and microplastics, as well as the consequential impact on removal efficiency during CFS and filtration treatment. As shown in Figure 3, biofilms were able to form on microplastics. The formation of biofilms significantly increased the treatment efficiency of CFS from 0.3% ± 0.3% to 16.5% ± 7.3% (Figure 4). However, no significant difference was observed during filtration (99.7% ± 0.2% and 97.1% ± 3.0% of removal with and without biofilms, respectively).
Figure 3:
Interactions of microplastics and biofilms. Left, within 4 days, biofilms were observed on microplastics; Right, more biofilms were formed at day 7. Blue arrows, biofilms on microplastics.
Figure 4:
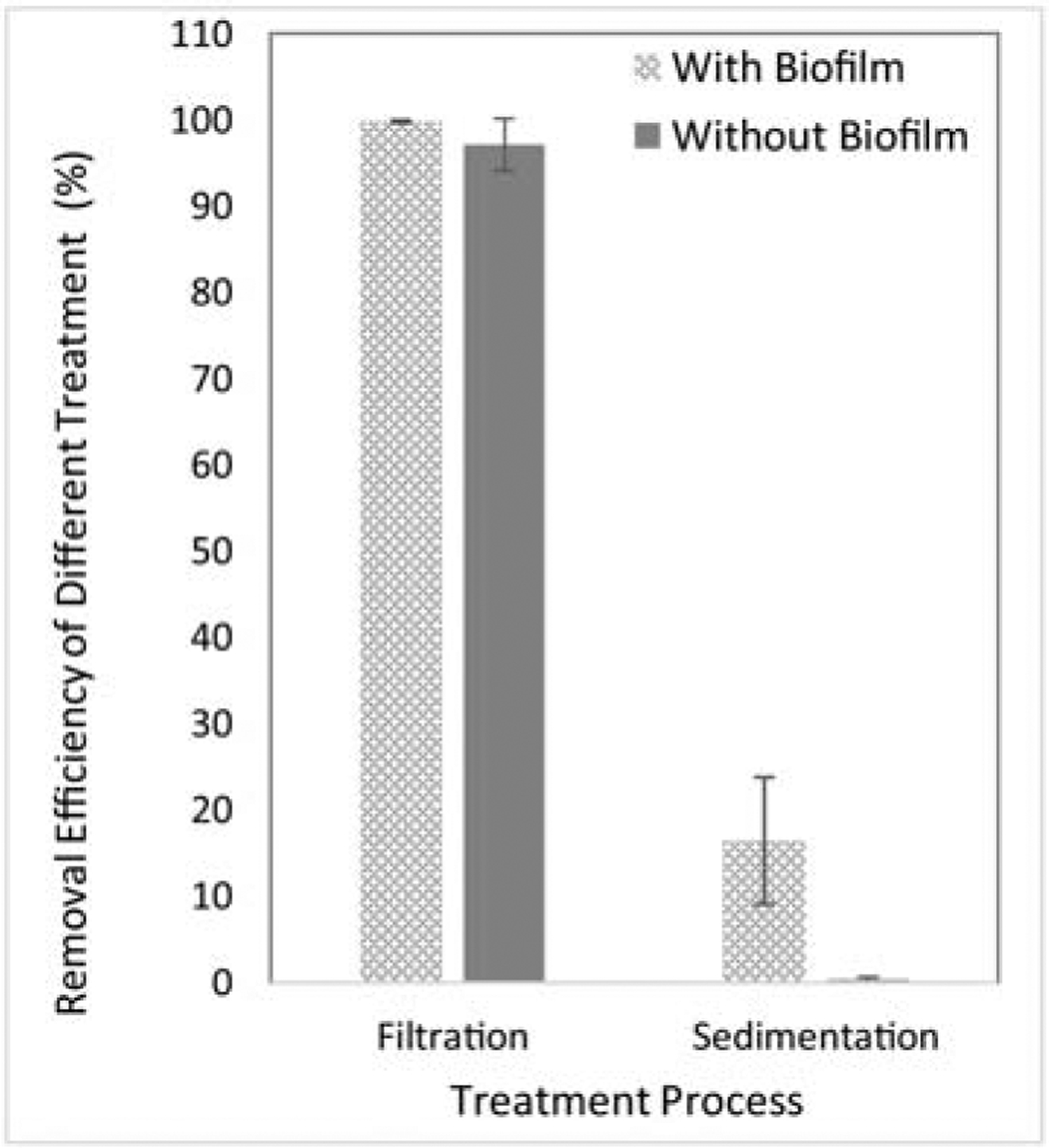
The removal efficiency of plastic particles (45 – 53 μm) with and without biofilm formation during CFS and filtration.
One knowledge gap in current studies of microplastics during drinking water treatment is the lack of understanding of the effects of biofilms on particle behavior. In most studies, the fate of microplastics mainly has been investigated using virgin and clean particles (Rummel et al., 2017). However, in a natural aquatic environment, biofilms are easily formed on microplastics due to the unique properties of microplastics, i.e., relatively high surface roughness, large surface area, and leaching of additives as a potential nutrient source. For instance, studies have found that microplastics in aquatic environments could rapidly form biofilms consisting of bacteria, phytoplankton, and other organic matters (Kaiser et al., 2017; Lagarde et al., 2016; Long et al., 2015; Rummel et al., 2017). The formation of biofilms can significantly alter the characteristics of microplastics (size, shape, and density) and consequently change the treatment efficiency during water treatment.
On the one hand, biofilm formation could enhance the sedimentation rate of microplastics. First, biofilm formation makes microplastics sticky because of the EPS matrix, promoting aggregation and sinking rate (Long et al., 2015). Secondly, the fouling organisms may also increase the density of the particle and, thus, reduce its buoyancy and facilitate sedimentation (Kaiser et al., 2017; Lagarde et al., 2016; Long et al., 2015). In real treatment conditions, microplastics in raw water are usually coated with biofilms and other organic matters, which could help the sedimentation of microplastics during water treatment. This work demonstrated that biofilm formation would significantly enhance the sedimentation rate of microplastics from 0.3% ± 0.3% to 16.5% ± 7.3% (Figure 4). This observation may explain why different results were reported in previous studies. For instance, the removal efficiency of clean microplastics was very low (~5% of removal) in the study conducted by Ma et al. (Ma et al., 2019b). In contrast, Wang et al. (2020) found a significantly higher sedimentation rate of microplastics (40.5 – 54.5%) in a real WTP. Our results suggest that the actual treatment efficiency of the CFS process for microplastics in WTPs would be much higher than those observed for clean plastic particles.
On the other hand, biofilm formation may lower filtration efficiency. Biofilms can break down microplastics into smaller particles, making them easier to penetrate filtration beds if the size of the broken microplastics reaches the critical size. To prevent the potential decrease of filtration efficiency, alternative filtration methods such as multi-layer filtration with different types of filtration media could be used to optimize the removal efficiency of different sizes of plastics. However, in this work, no significant change of filtration efficiency was observed with biofilm formation in one week. More research is needed to better understand the impact of biofilms on the treatment efficiency of microplastics by investigating different types of microplastics with more extended periods. In addition, further studies should systematically investigate the properties of microplastics (such as surface properties, chemical features, physical characteristics, and others) with biofilm formation.
4. Conclusions
This work characterized the removal efficiency of micro- and nanoplastics (180 nm – 125 μm) during drinking water treatment. Generally, the removal efficiency of clean microplastics during the CFS treatment was very low. Without coagulant aid, the removal rate was less than 2.0 %. Even with coagulant aid, the highest removal was only observed for particles with the size of 45–53 μm at a removal rate of 16.5%. Compared to CFS, filtration was a much more effective method in removing micro- and nanoplastics. The filtration efficiency ranged from 86.5% to 99.9%. Particles larger than 100 μm could be entirely filtered out (99.9%). However, there existed a critical particle size (10–20 μm) where a lower removal was observed, indicating a potential source for a considerable presence of microplastics (< 10–20 μm) in treated drinking water. Biofilms were easily formed on microplastics, and biofilm formation could significantly enhance the CFS treatment efficiency on microplastics removal. Future research needs to focus on the removal of small-sized plastics (< 10–20 μm) in water treatment processes, because these particles are more difficult to be removed yet could cause more significant health concerns if ingested. Furthermore, more work is needed to investigate the fate of other types of micro- and nanoplastics (different shape and polymer type) during water treatment.
Supplementary Material
Highlights.
CFS process was insufficient in removing microplastics.
Granular filtration entirely filtered out microplastics larger than 100 μm.
Microplastics smaller than 10 – 20 μm would be likely present in treated water.
Biofilms quickly formed on microplastics.
Biofilm formation significantly enhanced the treatment efficiency of CFS.
Acknowledgments
Funding for this work was provided by WSU Center for Urban Responses to Environmental Stressors (NIEHS P30 ES020957), Smart Management of Microplastic Pollution in the Great Lakes (Great Lakes Protection Fund, GLPF #1151), and the Richard Barber Interdisciplinary Research Program. We thank GLWA, Detroit Water Works Park Water Treatment Plant, and Mr. Michael Dunne for providing us water samples, water treatment materials, and water treatment operational parameters.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information: The supporting information for this work is available.
References
- Amélineau F, Bonnet D, Heitz O, Mortreux V, Harding AM, Karnovsky N, et al. Microplastic pollution in the Greenland Sea: Background levels and selective contamination of planktivorous diving seabirds. Environmental Pollution 2016; 219: 1131–1139. [DOI] [PubMed] [Google Scholar]
- Andrady AL. Microplastics in the marine environment. Marine pollution bulletin 2011; 62: 1596–1605. [DOI] [PubMed] [Google Scholar]
- Andrady AL. The plastic in microplastics: a review. Marine Pollution Bulletin 2017; 119: 12–22. [DOI] [PubMed] [Google Scholar]
- Arthur C, Baker J, Bamford H. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, September 9–11, 2008, 2009. [Google Scholar]
- Bai R, Tien C. Particle detachment in deep bed filtration. Journal of colloid and interface science 1997; 186: 307–317. [DOI] [PubMed] [Google Scholar]
- Baldwin AK, Corsi SR, Mason SA. Plastic debris in 29 Great Lakes tributaries: relations to watershed attributes and hydrology. Environmental science & technology 2016; 50: 10377–10385. [DOI] [PubMed] [Google Scholar]
- Cable RN, Beletsky D, Beletsky R, Wigginton K, Locke BW, Duhaime MB. Distribution and Modeled Transport of Plastic Pollution in the Great Lakes, the World’s Largest Freshwater Resource. Frontiers in Environmental Science 2017; 5: 45. [Google Scholar]
- Carr SA, Liu J, Tesoro AG. Transport and fate of microplastic particles in wastewater treatment plants. Water research 2016; 91: 174–182. [DOI] [PubMed] [Google Scholar]
- Chain EPoCitF. Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA Journal 2016; 14: e04501. [Google Scholar]
- Cózar A, Echevarría F, González-Gordillo JI, Irigoien X, Úbeda B, Hernández-León S, et al. Plastic debris in the open ocean. Proceedings of the National Academy of Sciences 2014; 111: 10239–10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duis K, Coors A. Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environmental Sciences Europe 2016; 28: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eerkes-Medrano D, Thompson RC, Aldridge DC. Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water research 2015; 75: 63–82. [DOI] [PubMed] [Google Scholar]
- Enders K, Lenz R, Stedmon CA, Nielsen TG. Abundance, size and polymer composition of marine microplastics≥ 10 μm in the Atlantic Ocean and their modelled vertical distribution. Marine pollution bulletin 2015; 100: 70–81. [DOI] [PubMed] [Google Scholar]
- EPA. Drinking Water Treatability Database. 2019.
- Eriksen M, Mason S, Wilson S, Box C, Zellers A, Edwards W, et al. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Marine pollution bulletin 2013; 77: 177–182. [DOI] [PubMed] [Google Scholar]
- Harrison JP, Sapp M, Schratzberger M, Osborn AM. Interactions between microorganisms and marine microplastics: a call for research. Marine Technology Society Journal 2011; 45: 12–20. [Google Scholar]
- Harrison JP, Schratzberger M, Sapp M, Osborn AM. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC microbiology 2014; 14: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann NB, Hüffer T, Thompson RC, Hassellöv M, Verschoor A, Daugaard AE, et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. ACS Publications, 2019. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environmental science & technology 2012; 46: 3060–3075. [DOI] [PubMed] [Google Scholar]
- Hogg R Bridging flocculation by polymers. 2013; 30: 3–14. [Google Scholar]
- Kaiser D, Kowalski N, Waniek JJ. Effects of biofouling on the sinking behavior of microplastics. Environmental Research Letters 2017; 12: 124003. [Google Scholar]
- Koelmans AA, Nor NHM, Hermsen E, Kooi M, Mintenig SM, De France J. Microplastics in freshwaters and drinking water: critical review and assessment of data quality. Water research 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuth M, Mason SA, Wattenberg EV. Anthropogenic contamination of tap water, beer, and sea salt. PloS one 2018a; 13: e0194970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuth M, Mason SA, Wattenberg EVJPo. Anthropogenic contamination of tap water, beer, and sea salt. 2018b; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde F, Olivier O, Zanella M, Daniel P, Hiard S, Caruso A. Microplastic interactions with freshwater microalgae: Hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environmental Pollution 2016; 215: 331–339. [DOI] [PubMed] [Google Scholar]
- Logan BE, Jewett D, Arnold RG, Bouwer E, O’Melia C. Clarification of clean-bed filtration models. Journal of Environmental Engineering 1995; 121: 869–873. [Google Scholar]
- Long M, Moriceau B, Gallinari M, Lambert C, Huvet A, Raffray J, et al. Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Marine Chemistry 2015; 175: 39–46. [Google Scholar]
- Ma B, Xue W, Ding Y, Hu C, Liu H, Qu J. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. Journal of Environmental Sciences 2019a; 78: 267–275. [DOI] [PubMed] [Google Scholar]
- Ma B, Xue W, Hu C, Liu H, Qu J, Li L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chemical Engineering Journal 2019b; 359: 159–167. [Google Scholar]
- Mason SA, Welch V, Neratko J. Synthetic Polymer Contamination in Bottled Water. Available from:. Department of Geology and Environmental Sciences, Fredonia University, New York: [Accessed 23 March 2018]. http://news.bbc.co.uk/2/shared/bsp/hi/pdfs/14_03_13_finalbottled.pdf 2018. [Google Scholar]
- McCormick A, Hoellein TJ, Mason SA, Schluep J, Kelly JJ. Microplastic is an abundant and distinct microbial habitat in an urban river. Environmental science & technology 2014; 48: 11863–11871. [DOI] [PubMed] [Google Scholar]
- Mintenig S, Löder M, Primpke S, Gerdts G. Low numbers of microplastics detected in drinking water from ground water sources. Science of the total environment 2019; 648: 631–635. [DOI] [PubMed] [Google Scholar]
- Moudgil BM, Sanjay B, Prakash TS Effect of particle size in flocculation. Journal of colloid and interface science 1993; 158: 511–512. [Google Scholar]
- Murphy F, Ewins C, Carbonnier F, Quinn B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environmental science & technology 2016; 50: 5800–5808. [DOI] [PubMed] [Google Scholar]
- Novotna K, Cermakova L, Pivokonska L, Cajthaml T, Pivokonsky M. Microplastics in drinking water treatment–Current knowledge and research needs. Science of The Total Environment 2019. [DOI] [PubMed] [Google Scholar]
- Oberbeckmann S, Löder MG, Labrenz M. Marine microplastic-associated biofilms–a review. Environmental Chemistry 2015; 12: 551–562. [Google Scholar]
- Oßmann BE, Sarau G, Holtmannspötter H, Pischetsrieder M, Christiansen SH, Dicke W. Small-sized microplastics and pigmented particles in bottled mineral water. Water research 2018; 141: 307–316. [DOI] [PubMed] [Google Scholar]
- Pivokonsky M, Cermakova L, Novotna K, Peer P, Cajthaml T, Janda V. Occurrence of microplastics in raw and treated drinking water. Science of The Total Environment 2018a; 643: 1644–1651. [DOI] [PubMed] [Google Scholar]
- Pivokonsky M, Cermakova L, Novotna K, Peer P, Cajthaml T, Janda VJSoTTE. Occurrence of microplastics in raw and treated drinking water. 2018b; 643: 1644–1651. [DOI] [PubMed] [Google Scholar]
- Rummel CD, Jahnke A, Gorokhova E, Kühnel D, Schmitt-Jansen M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environmental Science & Technology Letters 2017; 4: 258–267. [Google Scholar]
- Schmidt E, Gieseke J, Gelfand P, Lugar T, Furlong D. Filtration theory for granular beds. Journal of the Air Pollution Control Association 1978; 28: 143–146. [Google Scholar]
- Sillanpää M, Ncibi MC, Matilainen A, Vepsäläinen M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018; 190: 54–71. [DOI] [PubMed] [Google Scholar]
- Tyree CM, Dan. Invisibles: the plastic in side us, 2017.
- Wang Z, Lin T, Chen W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Science of The Total Environment 2020; 700: 134520. [DOI] [PubMed] [Google Scholar]
- WHO. Microplastics in Drinking-Water. Geneva: World Health Organization, 2019. [Google Scholar]
- Yonkos LT, Friedel EA, Perez-Reyes AC, Ghosal S, Arthur CD. Microplastics in four estuarine rivers in the Chesapeake Bay, USA. Environmental science & technology 2014; 48: 14195–14202. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Franks GVJL. Flocculation mechanism induced by cationic polymers investigated by light scattering. 2006; 22: 6775–6786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


