Abstract
Myofibroblastoma of the breast is a rare benign stromal tumor that occurs in both sexes with a higher prevalence in male breast of older populations. Furthermore, myofibroblastoma can arise in extra mammary sites, along the milk-line. A variety of morphological variants in addition to the classic type have been identified. The differential diagnosis includes both benign and malignant entities that, through the use of clinical and radiological imaging, is difficult to characterize. Histopathological examination and immunohistochemistry are fundamental in the establishment of appropriate management of the disease and avoidance of overtreatment. The present study focuses on two cases of male mammary myofibroblastoma, with a short literature review.
Keywords: breast, immunohistochemistry, male mammary myofibroblastoma, tumor of mammary stroma
Introduction
Mammary myofibroblastoma (MFB) is a rare benign mesenchymal tumor originating from mammary stromal that was described for the first time in 1981(1) and first named by Wargotz et al in 1987(2). Some years later, in 2001, McMenamin and Fletcher described the first case of extra mammary myofibroblastoma (MTM) (3). Both of these entities are histologically and immune-phenotypically identical. Traditionally, mammary myofibroblastoma mainly affects older men (between 60 and 70 years), although some cases were also described in postmenopausal women (4). In women, thanks to mammary screening, this entity can be detected at a smaller size and, in the last few years, the incidence has been on the increase. Conversely, in men it is not uncommon to identify at diagnosis a painless and palpable mass.
MFB was described in multiple races with no predilection for ethnicity. Owing to its rarity, this tumor can be confused, both clinically and radiologically, with other types of benign or malignant breast cancers. Differential diagnosis is fundamental to avoid excessive treatment in a condition where the correct approach is the excision of the lesion.
In the present study, we report two cases of male mammary myofibroblastoma treated in the Senology Unit of the University Hospital of Modena (Modena, Italy) between September 2010 and December 2018, with a short literature review.
Case reports
Case 1
In 2010, a 65-year-old man presented at the Division of Breast Surgery of the University Hospital of Modena (Modena, Italy) with a palpable mass in his left breast. He had no family history of breast cancer, ovarian cancer or other types of malignancies. At the clinical examination there were no skin changes, no nipple changes or retraction and there were not lymphadenopathies at the supraclavicular or axillary sites. No gynecomastia was identified.
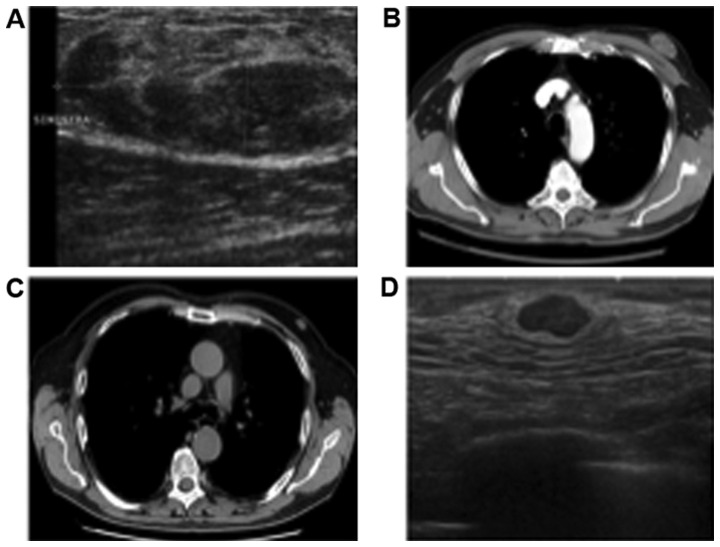
A diagnostic bilateral mammography and ultrasonography (Fig. 1A) were performed demonstrating a nodular, well-circumscribed lesion with no microcalcifications and an iso-hypoechoic, oval, solid mass, respectively. The lesion occupied the upper outer quadrant of the left breast and measured 41x18 mm. The right mammography and ultrasonography were normal. Fine needle aspiration cytology (FNAC) resulted in a suspicious specimen of malignant neoplasm (Category 4-C4). Pre-operatory abdomen and chest contrast-enhanced computed tomography (CT) scan (Fig. 1B) were negative and the patient underwent a radical left mastectomy plus axillary dissection.
Figure 1.
Radiological examination of mammary myofibroblastoma. The images represent the MFB mass observed in ultrasonography in (A) the first and (D) second patient. Both images show a well-circumscribed, oval, lesion. The axial imaging represents a CT scan in (B) the first and (C) second patient, with both patients showing a breast mass, well circumscribed. MFB, mammary myofibroblastoma; CT, computed tomography.
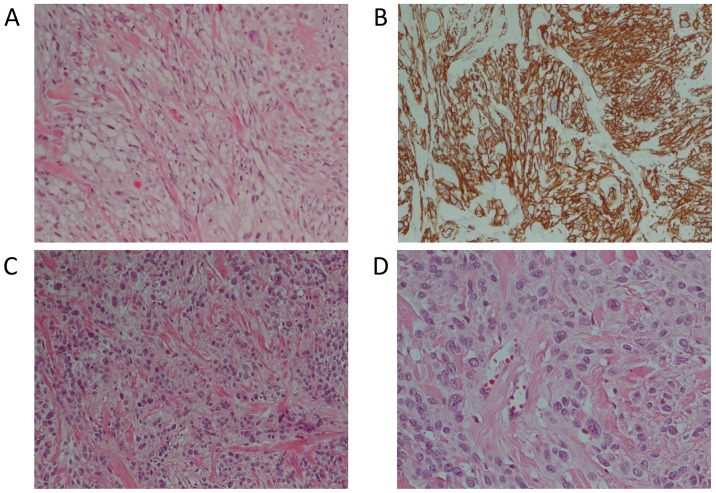
The excised mass measured 31 mm in the major axis and was mainly composed of epithelioid cells with some spindle cells, adipocytes and hyalinized collagen fibers in hematoxylin and eosin (H&E) stain (Fig. 2A). Some nuclear atypia were observed in epithelioid cells. Histopathologic examination also showed immunoreactivity for CD34 (Fig. 2B), desmin, alpha smooth muscle actin (α-SMA) and myosin. Tumor cells were negative for S100, p63 and cytokeratins (Table I). The Ki67 proliferative index was <5%. No pathological lymph node was found. The immunohistochemical pattern supported a mammary stromal origin and the diagnosis of epithelioid mammary myofibroblastoma was performed with the support of Professor C.D.M. Fletcher at Harvard Medical School, Brigham and Women's Hospital, Boston, USA.
Figure 2.
Histological examination of mammary myofibroblastoma. Fig. 2A, C and D represents myofibroblastoma cell population in H&E stain in the first and second patients. The lesion cells (from the first patient) are diffusely immunoreactive with CD34 (B). (A-C) magnification, x200; (D) magnification, x400. H&E, hematoxylin and eosin.
Table I.
Summary of the immunohistochemical findings in our cases.
| Variables | Case 1 | Case 2 |
|---|---|---|
| Vimentin | / | / |
| CD34 | + | + |
| Desmin | + | + |
| Bcl-2 | / | + |
| SMA | + | +/- |
| Myosin | + | / |
| ER | / | + (80%) |
| PgR | / | + (80%) |
| AR | / | + (98%) |
| CD99 | / | - |
| S100 | - | - |
| Cytokeratins | - | - |
| Melan A | / | - |
| p63 | - | - |
α-SMA, α-smooth muscle actin; ER, estrogen receptor; PgR, progesteron receptor; AR, androgen receptor; +, positive; -, negative; /, information not available.
Case 2
In 2017, a 76-year-old man came to our attention with a 13 mm oval mass in his left breast found during a chest CT scan (Fig. 1C). He was a strong smoker and he had a severe cough for a long period. This patient also had no family history of breast cancer, ovarian cancer or other type of malignancies. At clinical inspection there were no skin changes, no nipple changes or retraction and there were no lymphadenopathies at the supraclavicular or axillary sites. There were no breast masses or gynecomastia.
Bilateral mammogram and ultrasonography (Fig. 1D) showed a 15 mm oval solid mass in the retroareolar region of the left breast (BIRADS R5 and US5). The right mammography and ultrasonography were normal. The subsequent ultrasound-guided needle biopsy resulted in C4.
The patient underwent a radical left mastectomy and the removal of the sentinel lymph node. The removed mass was 12 mm in its major axis. Histological examination in the H&E stain showed epithelioid and mesenchymal cells with hyalinized collagen fibers (Fig. 2C and D). There was no necrosis. Immunohistochemistry showed positive reaction for desmin, actin, Bcl-2 and CD34. Neoplastic cells were also positive for estrogen (ER), androgen (AR) and progesterone (PR) receptors while S100, p63, CD99 and cytokeratins were negative (Table I). The Ki67 proliferative index was 1%. No pathological lymph nodes were identified. All of these findings indicated the diagnosis of myofibroblastoma.
Discussion
Myofibroblastoma is a tumor with myofibroblastic differentiation, most frequently detected in men, and which represents the most common type of benign spindle cell lesion (1,5). At clinical examination, it generally presents as a unilateral, solitary, firm, mobile and painless breast mass with slow growth (6). Bilaterality and unilateral multicentricity are very rare. Otherwise, mammary-type myofibroblastoma occurs predominantly along the embryonic milk-line such as the axillary, perianal, vulvar and para-testicular regions (3,5,6).
Usually, the tumor mass is <40 mm but the literature also reports larger tumors and some cases of giant masses (around 150-160 mm) (7). Some patients were documented with gynecomastia (8) and that evidence suggests a role of the estrogen pathway. Interestingly, O'Bryan et al recently published the first case of MFB occurring in a transgender individual after 13 months of treatment with hormone replacement therapy (9). In 1998, Morgan and Pitha postulated that androgen receptor or its ligands could be pathologically related to the development of MFB, but the results did not resolutely prove a causal mechanism of hormonal tumorigenesis (10). MFB has also been described at the surgical scar after breast cancer excision (11), and after wide excision and radiation therapy for ductal carcinoma in situ (12). Several publications have also reported MFB in the setting of prior cancers such as prostatic, renal and pancreatic tumors (3,13).
Radiological features of MFB are non-specific and it is often mistaken for fibroadenomas or other benign and malignant lesions. Breast ultrasound usually reveals a well-circumscribed, oval and dense mass, with variable echogenicity and rare calcifications that are more common in cases of fibroadenoma (13-15). The mammography shows a well-circumscribed dense mass, typically round to oval without calcifications (14). For these reasons, immunohistochemistry and histological examination play major roles in making the correct diagnosis.
According to the macroscopic aspect, MFB is an unencapsulated mass, well circumscribed from the adjacent parenchyma (4,6). Necrosis, hemorrhage and cystic degeneration are not characteristics of MFB (6). At the microscopic level, the classical type is composed of uniform, slender, spindle cells arranged in clusters separated by broad bands of hyalinized collagen (13). A variable adipocyte component and mast cells are also described. Mammary ducts and lobules are absent. The tumor vascular component is variably represented by small to medium-sized vessels frequently showing hyalinization and foamy histiocytes in their walls. In most cases immunohistochemistry is positive for CD34 and vimentin (5,6). It is also frequently positive, with variable extension of immunoreactivity, for SMA, desmin, CD99, Bcl-2, CD10, ER, PR and AR, while it does not express cytokeratin, EMA, c-Kit (CD117), p63 and S-100 protein (5,6). Proliferative activity is low with two or fewer mitoses per 10 high-power fields (HPF) (5,6). In both our cases, immunohistochemical analysis was in line with this evidence, but for the first patient there was no information with regard to ER and PR status. In addition, there was no information regarding the expression of vimentin, which is typically positive in MFB.
Some case series described nuclear atypia without clinical consequences and, according to Howitt and Fletcher (4), atypical cells were present in approximately 10% of 141 MFB cases reviewed. According to their histological composition, several patterns of MFB have been identified in addition to the classical type: Collagenized/fibrous, cellular, lipomatous, infiltrative, myxoid, epitheliod and deciduoid-like variant (5,6). Two different morphological patterns may potentially albeit rarely coexist in the same MFB.
Cytogenetic studies have shown that MFB exhibits chromosome 13 rearrangements. In particular, in most cases it was associated with the 13q14 deletion that includes the loss of RB1 and/or FOXO1 loci (16). These deletions have been confirmed by FISH analyses and were also described in spindle cell lipoma and in cellular angiomiofibroma, suggesting a close relationship among these types of lesions (16).
The principal differential diagnosis includes tumors that can arise primarily in the breast parenchyma such as leiomyoma, spindle cell lipoma, solitary fibrous tumor, spindle cell sarcoma, nodular fasciitis, desmoid-type fibromatosis, angiomyolipomas, pseudoangiomatous stroma hyperplasia and spindle cell carcinoma (5). In particular, the epithelioid variant can be confused with invasive lobular carcinoma due to the pseudo-infiltrative growth pattern and the expression of ER and PR. As the name implies, the epitheliod variant is composed, exclusively or predominantly, by cells with epitheliod morphology (at least 50% of the entire tumor) (5) and for this reason, it is a rare subtype. The cases presented in this study emphasize that the correct diagnosis of MFB is fundamental to avoid its overtreatment. Generally, the absences of cytologic atypia and necrosis as well as the lack of high mitotic activity and atypical mitoses at the diagnostic biopsy are useful in the exclusion of malignancy. No therapies are necessary after surgical removal, since recurrence is unlikely following excision with clear resection margins (R0) and no distant metastasis has been described after a follow-up period of 15 years (17).
Non-specific imaging of this type of tumor necessitates the support of histopathological analysis for correct diagnosis. The careful analysis of cellular composition, growth pattern and immunoreaction should help to differentiate MFB from other benign or malignant tumors of the breast. In both our cases, patients underwent radical surgery after the only execution of fine needle aspiration cytology. No core biopsy was performed to help clinicians in differential diagnosis and to avoid overtreatment, in particular axillary dissection. For these reasons, a multidisciplinary approach is critical to establish the appropriate management. The long-term prognosis of MFB is excellent and the complete surgical excision is considered curative, no additional therapies, such as radiation or hormonal therapies are necessary.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
MV, GT, SC and LM conceived and designed this case report. MV and AT contributed to the writing of the manuscript. MV, LC and AT acquired the data in the diagnostic imaging and archives of pathology. AG, AA and GT surgically treated the two patients. LC, AT and LM made strict changes to the language of the manuscript and made suggestions. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The local 'Area Vasta Emilia Nord (AVEN)' Ethical Committee does not require official approval for the publication of single case reports. Nevertheless, written informed consent was obtained from both participants included in the pubblication.
Patient consent for publication
We obtained written informed consent for publication from the two patients.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Toker C, Tang CK, Whitely JF, Berkheiser SW, Rachman R. Benign spindle cell breast tumor. Cancer. 1981;48:1615–1622. doi: 10.1002/1097-0142(19811001)48:7<1615::aid-cncr2820480724>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Wargotz ES, Weiss SW, Norris HJ. Myofibroblastoma of the breast. Sixteen cases of a distinctive benign mesenchymal tumor. Am J Surg Pathol. 1987;11:493–502. doi: 10.1097/00000478-198707000-00001. [DOI] [PubMed] [Google Scholar]
- 3.McMenamin ME, Fletcher CD. Mammary-type myofibroblastoma of soft tissue: A tumor closely related to spindle cell lipoma. Am Surg Pathol. 2001;25:1022–1029. doi: 10.1097/00000478-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Howitt BE, Fletcher CD. Mammary-type myofibroblastoma: Clinicopathologic characterization in a series of 143 cases. Am J Surg Pathol. 2016;40:361–367. doi: 10.1097/PAS.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 5.Magro G. Mammary myofibroblastoma: An update with emphasis on the most diagnostically challenging variants. Histol Histopathol. 2016;31:1–23. [PubMed] [Google Scholar]
- 6.Magro G. Mammary myofibroblastoma: A tumor with a wide morphologic spectrum. Arch Pathol Lab Med. 2008;132:1813–1820. doi: 10.1043/1543-2165-132.11.1813. [DOI] [PubMed] [Google Scholar]
- 7.Kataria K, Srivastava A, Singh L, Suri V, Yadav R. Giant myofibroblastoma of the male breast: a case report and literature review. Malays J Med Sci. 2012;19:74–76. [PMC free article] [PubMed] [Google Scholar]
- 8.Reis-Filho JS, Faoro LN, Gasparetto EL, Totsugui JT, Schmitt FC. Mammary epithelioid myofibroblastoma arising in bilateral gynecomastia: Case report with immunohistochemical profile. Int J Surg Pathol. 2001;9:331–334. doi: 10.1177/106689690100900413. [DOI] [PubMed] [Google Scholar]
- 9.O'Bryan J, Wolf-Gould C, Matsuo Y. Mammary myofibroblastoma in a transgender patient on feminizing hormones: Literature review and case report. Transgend Health. 2018;3:1–9. doi: 10.1089/trgh.2017.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan MB, Pitha JV. Myofibroblastoma of the breast revisited: An etiologic association with androgens? Hum Pathol. 1998;29:347–351. doi: 10.1016/s0046-8177(98)90114-9. [DOI] [PubMed] [Google Scholar]
- 11.Gocht A, Bösmüller HC, Bässler R, Tavassoli FA, Moinfar F, Katenkamp D, Schirrmacher K, Lüders P, Saeger W. Breast tumors with myofibroblastic differentiation: Clinico-pathological observations in myofibroblastoma and myofibrosarcoma. Pathol Res Pract. 1999;195:1–10. doi: 10.1016/S0344-0338(99)80087-9. [DOI] [PubMed] [Google Scholar]
- 12.Yagmur Y, Prasad ML, Osborne MP. Myofibroblastoma in the irradiated breast. Breast J. 1999;5:136–140. doi: 10.1046/j.1524-4741.1999.00138.x. [DOI] [PubMed] [Google Scholar]
- 13.Comer JD, Cui X, Eisen CS, Abbey G, Arleo EK. Myofibroblastoma of the male breast: A rare entity with radiologic-pathologic correlation. Clin Imaging. 2017;42:109–112. doi: 10.1016/j.clinimag.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg JS, Kaplan SS, Grady C. Myofibroblastoma of the breast in women: Imaging appearances. AJR Am J Roentgenol. 1998;171:71–72. doi: 10.2214/ajr.171.1.9648767. [DOI] [PubMed] [Google Scholar]
- 15.Dockery WD, Singh HR, Wilentz RE. Myofibroblastoma of the male breast: Imaging appearance and ultrasound-guided core biopsy diagnosis. Breast J. 2001;7:192–194. doi: 10.1046/j.1524-4741.2001.007003192.x. [DOI] [PubMed] [Google Scholar]
- 16.Maggiani F, Debiec-Rychter M, Vanbockrijck M, Sciot R. Cellular angiofibroma: Another mesenchymal tumour with 13q14 involvement, suggesting a link with spindle cell lipoma and (extra)-mammary myofibroblastoma. Histopathology. 2007;51:410–412. doi: 10.1111/j.1365-2559.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 17.Magro G, Bisceglia M, Michal M, Eusebi V. Spindle cell lipoma-like tumor, solitary fibrous tumor and myofibroblastoma of the breast: A clinico-pathological analysis of 13 cases in favor of a unifying histogenetic concept. Virchows Arch. 2002;440:249–260. doi: 10.1007/s00428-001-0572-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.