Abstract
Gestational diabetes mellitus (GDM) is a condition in which a hormone made by the placenta prevents the body from using insulin effectively. It is important to find an effective treatment. A mouse model of GDM was used to testify the effects of astaxanthin on glucose tolerance and insulin sensitivity. Production of inflammatory cytokines, reactive oxygen species (ROS), and glucose transporter type 4 (GLUT4) translocation and insulin-related signaling were measured in the presence of astaxanthin both in vivo and in vitro. It was found that astaxanthin improved insulin sensitivity, glucose tolerance, and litter size of offspring and reduced birth weight of offspring and inflammation in GDM mouse. Moreover, astaxanthin increased GLUT4 translocating to membrane without altering its secretion/expression and glucose uptake and consumption in C2C12 skeletal muscle cells. Furthermore, ROS generation and insulin-related signaling inhibited by tumor necrosis factor α was restored by astaxanthin. It is concluded that astaxanthin has the potential to attenuate GDM symptoms by regulating inflammation and insulin resistance in skeletal muscle of pregnant mice. Our findings suggest that astaxanthin could be a promising and effective molecule to treat GDM.
Keywords: GDM, astaxanthin, insulin resistance, inflammation, skeletal muscle
Introduction
Diabetes mellitus (DM) is a metabolic disorder disease that is characterized by high blood glucose levels in the body over a prolonged period. Diabetes is caused due to the following 2 reasons: one is the pancreas does not produce enough insulin, which is a hormone that regulates blood glucose metabolized for energy, and another is insulin-resistant cells do not respond properly to the insulin. There are 3 major types of DM: type 1 diabetes mellitus (T1DM), an autoimmune disease due to failure of pancreas to produce enough insulin; type 2 diabetes mellitus (T2DM) due to insulin-resistant cells, and gestational diabetes mellitus (GDM). Gestational diabetes mellitus is a conditional diabetes in which a woman with potentially impaired glucose tolerance or normal glucose metabolism develops high blood glucose levels during pregnancy. According to World Health Organization report, hyperglycemia detected at any time during pregnancy is classified as GDM. It is a severe pregnant symptom, and its prevalence is growing in recent years.1 The babies born from mothers with poorly treated GDM result in high risk of being too large/overweight, having low blood glucose level at birth, and developing T2DM in the long term. Therefore, GDM needs effective treatment.2,3 Unlike T1DM, GDM is not caused by a lack of insulin but by other hormones produced during pregnancy that can make insulin resistance.
Healthy pregnant women could produce more insulin to accommodate the body changes of a 60% decrease in insulin sensitivity and a 30% increase in glycogenesis to provide energy for baby, contributing to maintaining normal blood glucose level. However, women with GDM have metabolic dysfunction due to the imbalance of glucose metabolism, including impaired insulin response, decreased hepatic inhibition of glycogenesis, and downregulated glucose uptake in skeletal muscle (SM).4 Among all above-mentioned dysfunction, SM is the most important organ for whole-body glucose homeostasis and is responsible for almost 80% of insulin-stimulated whole-body glucose uptake and distribution under normal conditions.5-7 Therefore, insulin resistance in SM is the key factor in T2DM, and this situation also applies in GDM. Moreover, multiple factors contribute to insulin resistance in SM, such as intramyocellular lipids, mitochondria defects, endocrine effects of adipokines, and inflammation.
Astaxanthin (AX), a ketocarotenoid red pigment, exists in red yeast, microalgae, and marine animals. Astaxanthin possesses pharmacological functions including anti-inflammation, antiapoptosis, antioxidation, anticancer, neuroprotective, and immunomodulative effects. Because it can reduce blood glucose levels, it has been applied to alleviate diabetic complications.8-10 However, it has not been reported yet whether AX could attenuate GDM. Previous study shows that AX improves insulin sensitivity via activating insulin signaling and reducing inflammation in rat L6 cells.11 Taken together, we suppose that AX might improve insulin resistance by regulating inflammation and its downstream pathway in SM to alleviate GDM complications.
Methods and Materials
Animal Model
Six- to 8-week-old C57BL/KsJ+/+ (wild-type) and C57 BL/KsJdb/+(db/+) mice used for GDM model as previously described3 were purchased from CAVENS Lab Animal Ltd (Changzhou, China). Mice were kept in an environment with 25 °C ± 1 °C, 40% to 70% humidity under a 12-hour light–dark shift and were allowed food and water ad libitum throughout the experiment. All the operations were carried out in the guidelines on the Care and Use of Animals in Northwest Women’s and Children’s Hospital and approved by the Ethical Committee of Northwest Women’s and Children’s Hospital. Briefly, at 10 to 12 weeks of age, female mice were mated, which was confirmed by the presence of a copulatory plug in the next morning, which was counted as gestation day (GD) 0. Pregnant female mice were randomly divided into 3 groups: wild-type (normal pregnancy, C57BL/KsJ+/+), GDM (C57BL/KsJdb+, db/+ mice), and GDM + AX group; n = 20 for each group. Astaxanthin (Sigma; purity >97%, dissolved in dimethyl sulfoxide in stock) was orally administered daily from GD 0 to GD 21 in the GDM + AX group (10, 25, or 40 mg/kg body weight). At the same time, mice in wild-type and GDM groups were gavaged with phosphate-buffered saline (PBS) daily as vehicle. On 22nd day of AX treatment, half of the experimental mice, after fasting overnight, were killed at 1 hour following last dose for further measurement. Skeletal muscle was carefully collected without fat.
Measurement of Body Weight and Blood Glucose Level
Body weight, blood glucose levels, and insulin levels in the serum were measured at GD 0, 9, and 18. Nonfasting blood samples were obtained from tail venipuncture, and blood glucose levels were analyzed by glucometer (Roche Diagnostics). All samples were detected directly except those for adiponectin measurement in which a dilution factor of 1:400 was applied.
Enzyme-Linked Immunosorbent Assay
Insulin levels and adiponectin levels were measured by Ultra-Sensitive Mouse Insulin Enzyme-Linked Immunosorbent Assay (ELISA) kit (Mercodia) and adiponectin kits (Abcam) according to the manufacturer’s instruction. The plates were read with a microplate reader (SpectraMax MS; Molecular Devices). The coefficient of variance between replicates was 4.5% and 4.3%, and the corresponding detection range was 1.56 to 100 ng/mL and 62.5 to 4000 pg/mL. Blood samples were collected from tail. For measurement of insulin and adiponectin levels in SM, tissues were first lysed with RIPA lysis buffer (Biovision) and centrifuged at 12 000g at 4 °C for 20 minutes. The supernatants were collected for analysis.
Glucose and Insulin Tolerance Tests
Mice on GD 18 were fasted overnight and intraperitoneally injected with glucose at a dose of 2 g/kg for glucose tolerance test (GTT). Blood glucose levels were immediately measured by glucometer (Roche Diagnostics) from tail venipuncture at 0, 30, 60, 90, and 120 minutes after injection.2,12 After 3 days of recovery from GTT, the animals received insulin tolerance test. Briefly, after fasting for 6 hours, mice were injected with insulin at a dose of 1 U/kg. Blood samples were collected and measured as the same way as in GTT following insulin injection.
C2C12 Cell Culture
C2C12 cells (ATCC) were cultured in high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco) supplemented with 10% vol/vol fetal bovine serum (Invitrogen), 2% vol/vol penicillin streptomycin at 37 °C, 5% CO2, and 5% to 7% O2.12 Cells were treated with insulin (10 nM; Sigma) for 10 minutes and tumor necrosis factor α (TNF-α, 2 ng/mL; R&D Biosystem) for 36 hours with or without AX (5 µM).
The Isolation and Culture of Primary SM Cells
Primary SM cells were used to further investigate the effects of AX on glucose uptake and its downstream insulin signaling pathway. Fresh SM tissue was obtained from consented women who were diagnosed with GDM and delivered healthy, singleton infants at term (>37 weeks’ gestation). After washing twice in PBS, the tissue was carefully dissected and digested with 0.25% (wt/vol) trypsin (Hyclone) and 1 mM EDTA in DMEM for about 1 hour. Then, the cells were centrifuged at 500g for 10 minutes, transferred into a Petri dish, and cultured at 21% O2, 5% CO2, at 37°C for 30 minutes. Next, the nonattached cells were transferred into a 0.2% gelatin precoated 25-cm2 flask and cultured in DMEM containing 10% heat-inactivated fetal calf serum (Gibco), 100 U/mL penicillin, and 100 mg/mL streptomycin (Hyclone) under a condition of 37°C with 5% CO2 and 21% O2. This procedure would eliminate the adhesive fibroblasts. Skeletal muscle cells of the first 1 to 5 passages were differentiated into myotubes with DMEM containing 100 U/mL penicillin and 100 mg/mL streptomycin and 2% horse serum (Gibco). After differentiation for 4 to 6 days, cells were used for further experiment.
Quantitative Real-Time Polymerase Chain Reaction
RNA was extracted from SM by homogenizing in TRIzol (Thermo Fisher Scientific). Complementary DNA (cDNA) was performed by High-Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific). SYBR green (Molecular Probes) was used to detect polymerase chain reaction (PCR) products. All reactions were performed in a 20 µL reaction volume in triplicate. The PCR amplification consisted of an initial denaturation step at 95°C for 5 minutes, followed by 40 cycles of PCR at 95°C for 20 seconds, and 60°C for 30 seconds. Standard curves were generated, and the relative amount of target gene messenger RNA (mRNA) was normalized to GADPH mRNA.
The sequences of primers are as follows:
Interleukin-1β: 5′-GCAACTGTTCCTGAACTCAACT-3′ (forward), 5′-ATCTTTTGGGGTCCGTCAACT-3′ (reverse)
Interleukin-6: 5′-TAGTCCTTCCTACCCCAATTTCC-3′ (forward), 5′-TTGGTCCTTAGCCACTCCTTC-3′ (reverse)
Tumor necrosis factor α: 5′-CCCTCACACTCAGATCATCTTCT-3′ (forward), 5′-GCTACGAGTGGGCTACAG-3′ (reverse)
Glucose transporter type 4 (GLUT4): 5′-ACACTGGTCCTAGCTGTATTCT-3′ (forward), 5′-CCAGCCACGTTGCATTGTA-3′ (reverse)
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 5′-AGGTCGGTGTGAACGGATTTG-3′ (forward), 5′-TGTAGACCATGTAGTTGAGGTCA-3′ (reverse)
Western Blot
At GD18, mice were killed and SM was collected. C2C12 cells were also collected after treatment. After lysed with RIPA lysis buffer (Biovision) and centrifuged at 12 000g at 4°C for 20 minutes, protein extracts were loaded onto sodium dodecyl sulfate polyacrylamide gel and then transferred to polyvinyl difluoride membranes. Membranes were blocked with 2% skim milk dissolved in Tris-buffered saline containing 0.1% Tween 20 overnight at 4°C. After that, GLUT4 (1:1000 dilution), phosphorylated insulin receptor substrate 1 (IRS-1) Tyr (1:2000 dilution), phosphorylated-IRS-1 Ser (1:2000 dilution), IRS-1, phosphorylated JNK (1:1000 dilution), JNK (1:1000 dilution), phosphorylated Akt (1:2000), Akt (1:2000), and GAPDH (1:10,000) were probed with corresponding mouse monoclonal primary antibody at the indicated concentration (Cell Signaling Technology). Then, the membrane was incubated with peroxidase-conjugated goat antimouse immunoglobulin G (IgG) secondary antibodies (1:5000 dilution) or antirabbit IgG secondary antibodies (1:5000 dilution; Santa Cruz). Blots were visualized by chemiluminescence (Pierce).
Transportation of GLUT4
Plasma membrane lawns from C2C12 cells were performed as previous study. Briefly, cells were treated with or without AX (5 µM) for 12 hours followed by serum starvation for 4 hours. Cells were sonicated 3 times for 1 to 2 seconds on ice in sonication buffer. Lawns were fixed and blocked and labeled for 1 hour at room temperature with GLUT4 antibody and with secondary antibody (A488-conjugated donkey antigoat IgG) for 45 minutes. Lawns were measured and quantified by ImageJ software.12
Glucose Uptake and Consumption
Glucose uptake assays were conducted using 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-d-glucose (2-NBDG) Glucose Uptake Assay Kit (Cell-Based; BioVision) according to the manufacturer’s introduction. After pretreated with 2 ng/mL TNF-α for 36 hours with or without 5 μM AX for 12 hours, the differentiated cells were plated in 6-well plates at a density of 1 × 105 cells/well; 2-NBDG was used to measure glucose uptake. Briefly, the pretreated cells were trypsinized and detached from the plates and resuspended in PBS which contained 50 μM 2-NBDG and 1 μM insulin for another 30 minutes at 37°C in darkness. Then the remaining 2-NBDG was washed away with cold PBS. After that, cells were resuspended in PBS and analyzed with flow cytometer (Beckman) with at least 10 000 events per sample. The mean fluorescence intensity was used as measurement of 2-NBDG uptake on a per cell basis. Cells untreated with TNF-α or AX were defined as basal. Results were reported as fold change relative to the basal.
For glucose consumption assays, after the same pretreatment as glucose uptake assays, cells were then incubated with low-glucose DMEM which contained 100 nM insulin for another 12 hours. Glucose concentration in the culture medium after 12-hour incubation was determined by the glucose assay kit. Glucose consumption was determined by subtracting the remaining glucose in the medium from the total glucose in blank wells. Cells untreated with TNF-α or AX was defined as basal. Results were reported as fold change relative to the basal.
Reactive Oxygen Species Detection
Reactive oxygen species (ROS) generation was measured by 2′,7′-Dichlorofluorescin diacetate (DCFH-DA) staining. After pretreated with 2 ng/mL TNF-α for 36 hours with or without 5 μM AX for 12 hours, the differentiated cells were plated on coverslips in 24-well plates at a density of about 2.5 × 105 cells/well and cultured overnight at 37°C, 5% CO2. The medium was then moved away. Cells were washed twice with PBS and stained by adding DCFH-DA (10 µM) for 30 minutes at 37°C and then washed 3 times with PBS. The fluorescence images were taken by a single rapid scan (Leica) and quantified by ImageJ software. Cells untreated with TNF-α or AX were defined as basal. Results were reported as fold change relative to the basal. About 30 cells were analyzed for each experiment.
Statistical Analysis
Data were analyzed by statistical product and service solutions (SPSS 16.0; SPSS Inc) and expressed as means ± standard error of the mean. Student t test was used to compare the difference between the 2 groups, and 1-way analysis of variance followed by least significant difference as its post hoc test or 2-way analysis were used to compare multiple groups. Insulin resistance score was determined using Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), which was calculated as below:
HOMA-IR = (fasting glucose [mmol/L] × fasting insulin [mU/L])/22.5.
Results
Astaxanthin Ameliorates DM Symptoms in GDM Mice
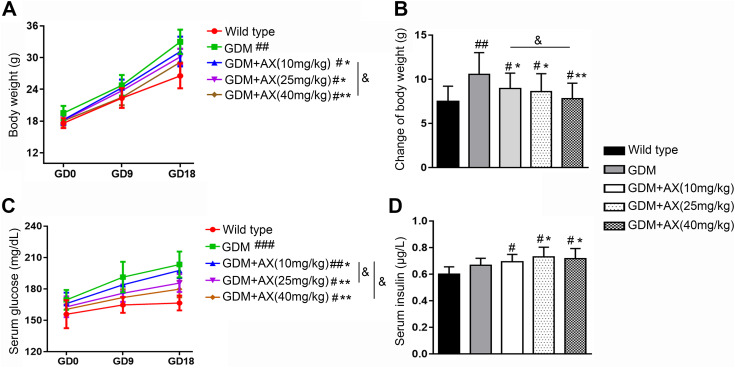
Compared to the normal pregnancy wild-type mice, the body weight of GDM mice was significantly increased (Figure 1A), while AX (10, 25, or 40 mg/kg) treatment could significantly decrease the body weight back to normal (Figure 1B). However, no animal death or abnormal behavior was found in the AX-treated group. Moreover, blood glucose levels of wild-type mice remained stable throughout the pregnancy, but GDM mice showed a dramatic increase in blood glucose levels as a marker of hyperglycemia symptom of GDM, which can be partly reversed by AX administration at all 3 doses (10, 25, or 40 mg/kg; Figure 1C). Comparatively, insulin levels in GDM mice that should have a higher insulin production to regulate the remarkable increase of glucose did not change compared with the normal pregnancy mice; thus, this result suggested that GDM affected insulin signal. After AX treatment, mice had higher insulin levels than both wild-type and GDM mice, which might explain the glucose decrease in GDM + AX mice (Figure 1D). Interestingly, there was no obvious difference between 25 mg/kg AX group and 40 mg/kg AX group in all above-mentioned experimental results, indicating that 25 mg/kg is the minimum dose that could exhibit the maximum effects. Thus, 25 mg/kg was chosen for the following studies.
Figure 1.
Gestational diabetes mellitus (GDM) symptoms were relieved by astaxanthin (AX) administration. A, Maternal body weight was recorded on gestation day (GD) 0, 9, and 18 in wild-type group, GDM group, and GDM + AX group (AX doses: 10, 25, or 40 mg/kg). B, Body weight changes from GD 0 to GD 18 were calculated. C, Serum glucose of each group was measured on GD 0, 9, and 18. D, The efficacy of AX in the maintenance of insulin levels. n = 12 for each group. Data were presented as means ± standard error of mean (SEM). # P < .05, ## P < .01, ### P < .001 compared to the wild-type group. *P < .05, **P < .01 between the comparison of GDM group and GDM + AX group. &P < .05 between the indicated groups.
Astaxanthin Improves Glucose Tolerance and Insulin Sensitivity in GDM Mice
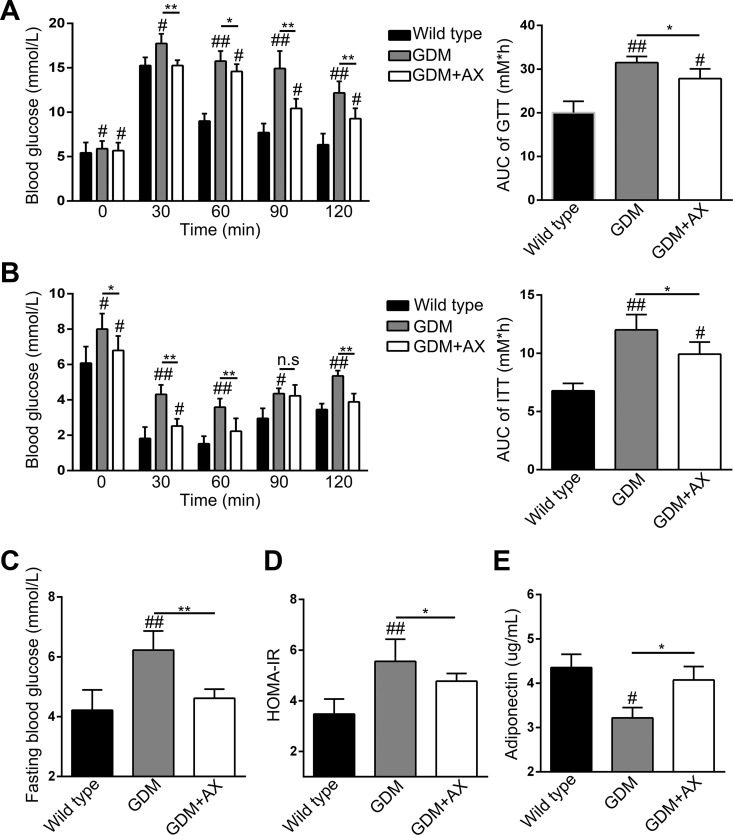
Compared to wild-type mice, blood glucose levels were elevated in the GDM mice, which could be ameliorated by AX administration after glucose injection (Figure 2A). On the contrary, compared to the dramatic downregulation in wild-type mice, blood glucose levels remained high in the GDM mice after insulin injection, which could also be partly reversed by AX treatment (Figure 2B). These results suggested that GDM mice had typical diabetic symptom of glucose intolerance and insulin resistance, which can be improved by AX supplement due to its benefit to glucose tolerance and insulin sensitivity. Similarly, AX administration could reduce the fasting blood glucose levels, which was induced in GDM mice (Figure 2C). Furthermore, we analyzed the HOMA-IR index that is a crucial parameter for DM, and low HOMA-IR means more sensitive to insulin. Not surprisingly, GDM mice had a higher HOMA-IR index compared to wild type, and AX downregulated HOMA-IR index (Figure 2D).
Figure 2.
Astaxanthin (AX) supplementation (25 mg/kg) improves glucose and insulin tolerance in gestational diabetes mellitus (GDM) mice. A, Effect of astaxanthin on glucose tolerance at gestational day (GD) 15 in GDM mice. B, Effect of astaxanthin on insulin tolerance at GD 15 in GDM mice. C, Effect of astaxanthin on fasting glucose level at GD 18 in GDM mice. D, Homeostatic Model Assessment of Insulin Resistance index of different groups at GD 18. E, Serum adiponectin levels of different groups were measured at GD 18. n = 7 to 12 for each group. Data were presented as means ± standard error of mean (SEM). # P < .05, ## P < .01 compared to the wild-type group. *P < .05, **P < .01 between the comparison of the GDM group and GDM + AX group.
Previous study reported that AX activates adiponectin pathway leading to decreasing blood glucose level; hence, we also testified adiponectin levels in the serum in different groups on GD 18. As expected, AX treatment reversely elevated adiponectin levels in the serum which was inhibited in GDM mice (Figure 2E).
Astaxanthin Inhibits Inflammation in GDM Mice
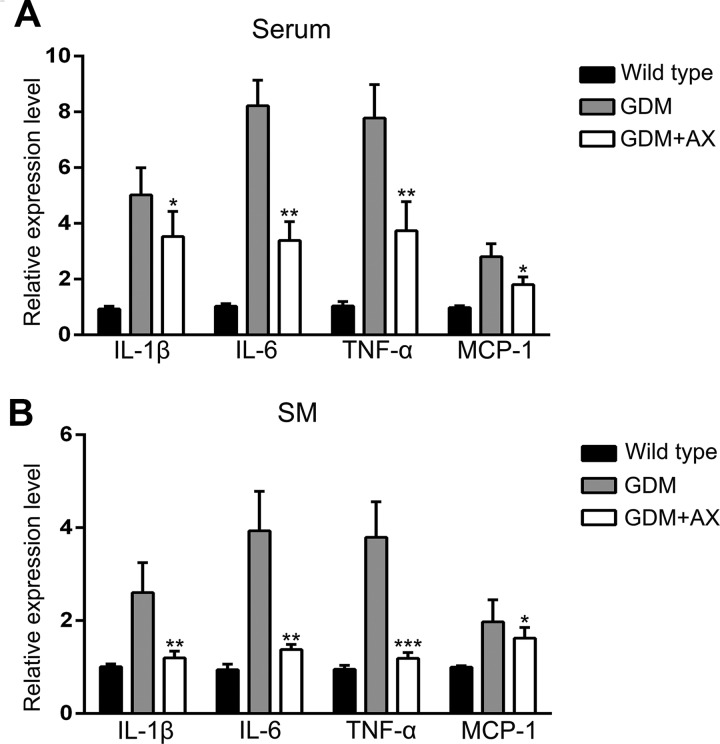
Chronic inflammation often accompanies with DM and takes a crucial role in the progression of GDM. Gestational diabetes mellitus is associated with the increased levels of inflammation cytokines. Therefore, we measured some important inflammatory cytokines by ELISA and quantitative reverse transcription polymerase chain reaction (RT-qPCR), respectively. Both the secretion in the serum and expression levels in the SM of these 4 inflammatory cytokines, includingIL-1α, IL-6, TNF-α, and monocyte chemoattractant protein 1 (MCP-1), were dramatically increased in GDM mice and markedly decreased after AX treatment (Figure 3A and B). These indicated that AX inhibited the inflammation caused by GDM in mice.
Figure 3.
Astaxanthin (AX; 25 mg/kg) inhibits inflammation in gestational diabetes mellitus (GDM) mice. Relative expression levels of interleukin (IL) 1β, IL-6, tumor necrosis factor α (TNF-α), and monocyte chemoattractant protein 1 (MCP-1) in mice serum (A) and skeletal muscle (SM) (B) of different groups. n = 7 to 12 for each group. Data were presented as means ± standard error of mean (SEM). *P < .05, **P < .01, ***P < .01 between the comparison of the GDM group and GDM + AX group.
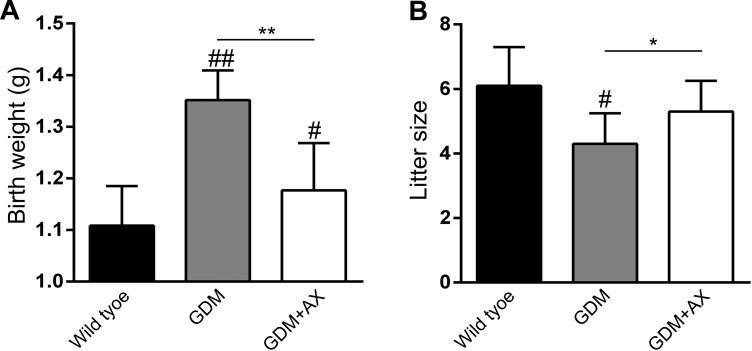
Astaxanthin Restores Body Weight of the Offspring and Litter Size in GDM Mice
The offspring of GDM increases body weight gain at birth and the risk of diabetes in the future and decreases fetal survival and birth rate. Consistent with previous results, body weight of offspring significantly elevated in the GDM group compared to the wild-type group, which can be restored by AX administration (Figure 4A). Diminished birth rate as a reflection of little size in GDM group was also reversed in the GDM + AX group (Figure 4B).
Figure 4.
Astaxanthin (AX; 25 mg/kg) improves the reproductive outcome of gestational diabetes mellitus (GDM) mice. Body weight at birth (A) and litter size (B) of offspring born from each female mouse in different groups were recorded. n = 7 to 12 for each group. Data were presented as means ± standard error of mean (SEM). # P < .05, ## P < .01 compared to the wild-type group. *P < .05, **P < .01 between the comparison of the GDM group and GDM + AX group.
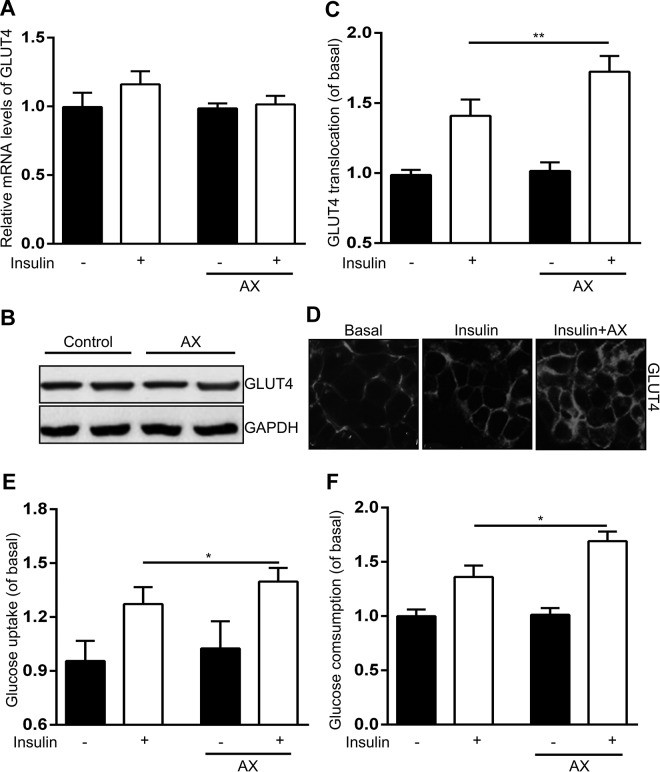
Astaxanthin enhances insulin-stimulated GLUT4 translocation, glucose uptake, and glucose consumption both in C2C12 cells and human primary SM cells. Glucose transporter type 4 is an insulin-induced GLUT that transports circulating glucose into cells, primarily adipocytes and muscle cells, which plays an important role in glucose homeostasis.
First, we monitored the effects of AX on GLUT4 mRNA and protein expression and its translocation in C2C12 cell line. The GLUT4 translocation toward plasma membrane could be induced by insulin and further increased by AX + insulin with no alteration in the presence of AX alone (Figure 5C and D). However, the mRNA and protein expression of GLUT4 did not change no matter treated with insulin, AX, or both (Figure 5A and B). Furthermore, glucose uptake was induced by insulin and further promoted with AX together (Figure 5E). Similarly, AX significantly enhanced muscle glucose consumption, which was also increased by insulin (Figure 5F).
Figure 5.
Astaxanthin (5 µM) enhances insulin-stimulated glucose transporter type 4 (GLUT4) translocation toward the plasma membrane and glucose uptake and consumption in C2C12 cells. A and B, Effects of astaxanthin on messenger RNA (mRNA) expressions (A) and protein levels (B) of GLUT4 in C2C12 cells. C and D, Astaxanthin promotes plasma membrane translocation of GLUT4. The GLUT4 translocation toward the plasma membrane was measured using plasma membrane lawn assay. Scale bar = 20 μm. E and F, Effects of astaxanthin on glucose uptake (E) and glucose consumption (F) in C2C12 cells. Data were presented as means ± standard error of mean (SEM) of 6 independent experiments. *P < .05, **P < .01.
To further investigate the effects of AX in SM, we isolated human primary SM cells from GDM pregnancy patients. Human primary SM cells treated with insulin with or without AX displayed the same pattern with C2C12 cells (Figure S1). Astaxanthin in the presence of insulin further increased GLUT4 translocation (Figure S1B), glucose uptake (Figure S1C), and glucose consumption (Figure S1D) with affecting GLUT4 expression (Figure S1A). All these data revealed that AX improved insulin sensitivity via stimulating GLUT4 translocation toward plasma membrane, leading to the consumption of circulating glucose in SM cells.
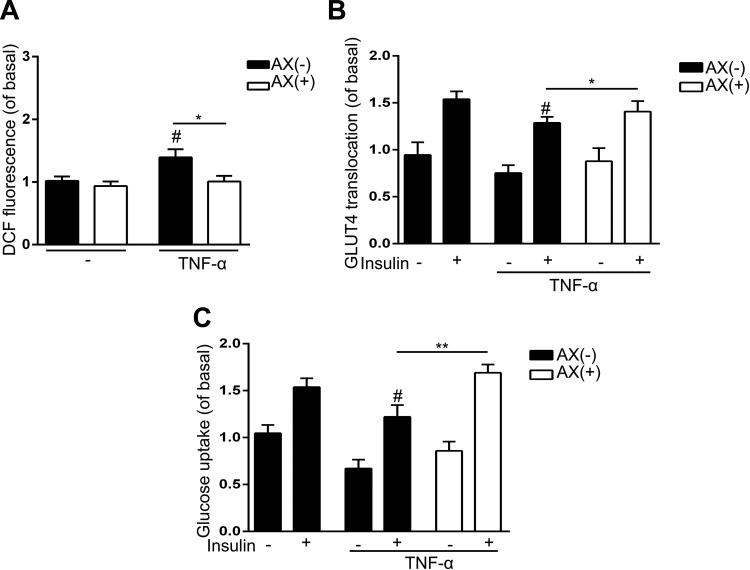
Astaxanthin Restores TNF-α and Induced Insulin Resistance in C2C12 Cells
In SM, inflammatory cytokines released from adipocytes, as well as myocytes and immune cells, play important roles in insulin resistance. Among all these cytokines, TNF-α is the most potential one. Therefore, we established an insulin resistance muscle cell model and induced TNF-α in C2C12 cell line. We testified the ability of AX to reduce ROS derived from TNF-α in C2C12 cells and found that AX markedly reduced TNF-α-generated ROS (Figure 6A). Figure 6B demonstrates that AX also elevated the amount of GLUT4 in plasma membrane, which was reduced by TNF-α. Both the decreased ROS generation and increased GLUT4 translocation to membrane induced by AX, which was impaired by TNF-α, contributed to improve insulin sensitivity in C2C12 cells. Accordingly, glucose uptake was also promoted by AX due to AX-stimulated insulin sensitivity (Figure 6C).
Figure 6.
Astaxanthin (5 µM) decreases reactive oxygen species (ROS) generated by tumor necrosis factor α (TNF-α) and improved impaired glucose transporter type 4 (GLUT4) translocation and glucose transport. A, Generation of ROS was monitored using 5 μM 2′,7′-Dichlorofluorescin diacetate. Astaxanthin decreases ROS generated by TNF-α. B, Astaxanthin restored the impaired GLUT4 translocation to the plasma membrane. C, Astaxanthin restored glucose transport in C2C12 cells after treatment with TNF-α. Data were presented as means ± standard error of mean (SEM). # P < .05 compared to untreated cells stimulated by insulin at the same dose. *P < .05, **P < .01.
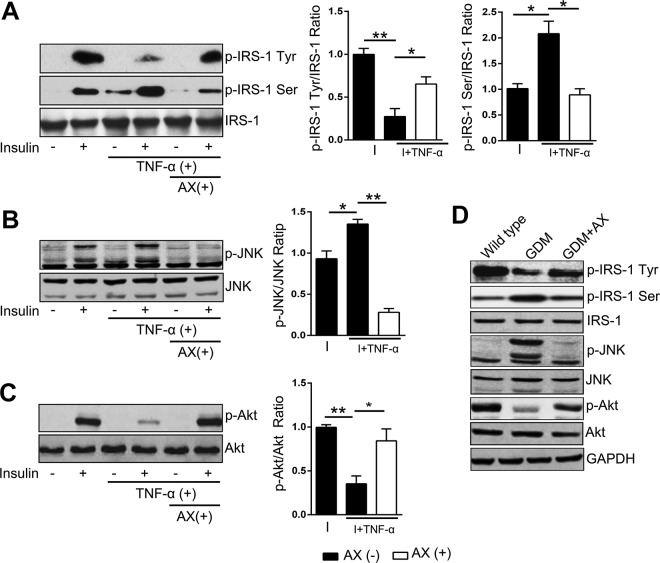
Astaxanthin Modulates Insulin Signaling Pathway Both in C2C12 Cells and Human Primary SM Cells
Insulin binds to the α-subunit of the insulin receptor, which autophosphorylated α-subunit, then followed by the phosphorylation of IRS-1. Insulin receptor substrate 1 transmits signals from insulin to intracellular pathways. However, different phosphorylation sites of IRS-1 have opposite roles in insulin signaling; tyrosine phosphorylation of IRS-1 activates Akt pathway and facilitates glucose uptake, but serine residues phosphorylation, which can be stimulated by JNK pathway, subsequently inhibits tyrosine phosphorylation leading to inhibiting glucose uptake. JNK, a seine–threonine kinase, is a well-known negative regulator of insulin signaling, which can be activated by TNF-α. Since the effects of JNK stimulation on IRS-1 serine phosphorylation was also observed in vivo, JNK might be a key target for regulating the inflammation-induced abnormal glucose tolerance. Therefore, we testified these insulin-related signaling and found that insulin could stimulate the phosphorylation of tyrosine IRS-1, serine IRS-1, JNK, and Akt (Figure 7A-C), and AX activated phosphorylation of tyrosine IRS-1 (Figure 7A) and Akt (Figure 7C), which signals were both inhibited by TNF-α. Besides that, AX also suppressed phosphorylation of serine IRS-1 and JNK pathway induced by TNF-α (Figure 7A and B). Moreover, in human primary SM cells, AX increased the phosphorylation of tyrosine IRS-1 (Figure S2A) and Akt (Figure S2C) in the presence of insulin and inhibited insulin-induced phosphorylation of serine IRS-1 (Figure S2A) and JNK (Figure S2B). Accordingly, GDM mice treated with AX showed the consistent results with in vitro study (Figure 7D).
Figure 7.
Astaxanthin (5 µM) can modulate the insulin signaling pathway in C2C12 cells. Cell lysates with different treatments were probed for insulin receptor substrate 1 (IRS-1) Tyr phosphorylation (A), IRS Ser phosphorylation-1 (A), JNK phosphorylation (B), Akt phosphorylation (C), and the corresponding total protein levels. Representative Western blots are shown from 6 independent experiments for each set. The summary of all experiments performed are presented in the respective graphs using densitometric quantification. Values are expressed as the ratio of phosphorylated protein relative to total protein. D, The phosphorylation of IRS-1 Tyr, IRS Ser, JNK, and Akt of SM in mice were assessed by Western blot. Data were presented as means ± standard error of mean (SEM) from 6 independent experiments. *P < .05, **P < .01. I indicates insulin.
Discussion
To our best knowledge, the current study is the first report to explore the effect of AX in SM cells and mouse model with GDM. Our study demonstrates that AX improves insulin sensitivity and glucose tolerance by inhibiting inflammation in the SM, which leads to alleviate diabetic symptoms in GDM. Along with the life improvement worldwide, such as obesity, cardiovascular diseases, and T2DM, GDM is growing fast, leading to the high risk of developing potential diseases in patients with GDM and their children. Therefore, it is necessary to find a safe and effective drug for treating women with GDM.
As the most potent antioxidant found in nature,13 AX is proved to effectively alleviate obesity and T2DM both in diabetic animal models and in patients with T2DM, as it can attenuate oxidative stress and decrease insulin resistance.14-17 It functions in multiple mechanisms including improving glucose metabolism, reducing blood pressure, attenuating the apoptosis of retinal ganglion cells, ameliorating the redox imbalance in lymphocytes, protecting β-cells in pancreas, and so on. Moreover, clinical trials discovered that AX accelerates the consumption of triglyceride to increase the level of high-density lipoprotein whose accumulation can facilitate cardiovascular disease, and adiponectin takes an important role in regulating blood glucose.18-21 It may give us a hint that AX, an effective antioxidant, can be used as an antidiabetic drug for patients with GDM. Considering the particularity of pregnant women, it needs more studies to explore the efficacy and effectiveness of AX in GDM and the safety dose for both pregnant women and their fetus. However, even AX is effective to treat diabetes, it should still be applied cautiously in women who are pregnant or might become pregnant because AX may inhibit 5-α reductase whose deficiency is a condition that affects male sexual development before birth and during puberty. Thus, this might be the major limitation for using AX in pregnancy, and all the male offspring must be assessed when AX was applied.
Gestational diabetes mellitus often involves in the progression of chronic inflammation and might be associated with increased secretion of inflammatory cytokines and potential stimulation on producing inflammatory factors in placenta.22,23 Both maternal and fetal inflammatory factors result in insulin resistance by regulating myocyte metabolism.7 Oxidative stress is involved in the pathogenesis of insulin resistance by inflammation and lipotoxicity. Therefore, reducing oxidative stress could suppress insulin resistance by AX. Current result was consistent with previous evidence that cytokines such as TNF-α promote insulin resistance by generating ROS and activating JNK signaling in inflammatory pathway at target SM, which is responsible for abnormal glucose homeostasis.24-27 All these evidence are the reasons why we choose inflammation in SM to investigate the effect of AX in glucose metabolism and insulin resistance.
Inflammation plays important roles in the development of GDM. Of note, we only analyzed the inflammation in the SM in GDM, even SM is one of the major tissues that regulates inflammation, glucose homeostasis, and insulin signaling. However, inflammation occures in other tissues including placenta and adipose tissue as well during GDM. Therefore, this needs to be further explored to better elucidate the mechanism of AX in the development of GDM.
Although how TNF-α induces ROS generation remains mainly unknown, there are several publications that show ROS including hydrogen peroxide, hydroxyradical, and superoxide is generated largely from mitochondria. On the other hand, TNF-α suppresses JNK inactivating phosphatase leading to the sustained JNK activation both in vivo and in vitro, which is known to negatively regulate insulin signaling via IRS-1 serine phosphorylation that reversely inhibits IRS-1 tyrosine phosphorylation, although JNK could be regulated by mitogen-activated protein kinase pathway which is inhibited by TNF-α–induced ROS.
Previous study discovered that AX decreased nitric oxide (NO) production without clear mechanism. Nitric oxide could induce insulin resistance due to enhancement of insulin-stimulated IRS-1 serine phosphorylation and also reduces IRS-1 tyrosine phosphorylation in SM cells, although we did not measure the NO production or the level of nitric oxide synthase (NOS) which catalyzed the production of NO from arginine. It is possible that AX increases insulin sensitivity via the NO or NOS directly. An extended experiment is needed for clarifying this possibility.
However, excess antioxidant disturbs normal homeostasis in cells by modulating natural redox balance. Vitamin C, a famous antioxidant which exists mainly in cytosol, has been proved to inhibit in the physiological production of other antioxidants such as superoxide dismutase-1 and glutathione peroxidase-1, leading to the increased ROS production.28,29 Of note, after long-time exposure to AX in SM cells, the inhibitory effects mentioned above were not observed (data not shown) probably because of its specialty in either localization or action. Although this evidence is far from enough and more various signaling are required to explore, AX seems to have less side effect and less impact on positive physiological cellular function. However, AX still needs to be carefully evaluated for use in the women experiencing gestation diabetes. Because not only in the fetus but also in the pregnant women the physiological responses are all different from adults. Although AX is proved to have minimal adverse effect in patients, it is hard to say whether it is safe for the pregnant women or fetus. Our findings demonstrated that AX improves insulin sensitivity in GDM mouse model and C2C12 cells, and more extended studies need to be done in the future for the more detailed mechanism and also the safety, efficacy, and effectiveness in the women experiencing gestation diabetes. Hopefully, AX could be a promising drug for the patients with GDM. It is worth noting that there are other limitations for the current investigation. First, no positive control was introduced, making it hard to compare the effects of AX with any known drugs. Second, it would more convincing if more than 1 animal model was used to see if AX has similar protective effects in other models.
Conclusion
Astaxanthin improves glucose uptake and glucose consumption in GDM mice through multiple pathways, including inhibiting inflammatory cytokines secretion, ROS generation, stimulating GLUT4 translocation, and insulin-related signaling in C2C12 SM cells, which all contribute to promote glucose tolerance and insulin sensitivity. Our findings highlight the potential of AX as a promising therapy for treating GDM.
Supplemental Material
Supplemental_materials for Effects of Astaxanthin on Inflammation and Insulin Resistance in a Mouse Model of Gestational Diabetes Mellitus by Weihong Feng, Yanxia Wang, Na Guo, Pu Huang and Yang Mi in Dose-Response
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by Shaanxi Provincial Health Planning Commission Scientific Research Project (2014D87).
ORCID iD: Yang Mi  https://orcid.org/0000-0002-5127-8008
https://orcid.org/0000-0002-5127-8008
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. 2018;29(11):743–754. [DOI] [PubMed] [Google Scholar]
- 2. Plows JF, Budin F, Andersson RA. et al. The effects of myo-inositol and B and D vitamin supplementation in the DB/+ mouse model of gestational diabetes mellitus. Nutrients. 2017;9(2):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zou C, Zhang Q, Zhang S. Mogroside IIIE attenuates gestational diabetes mellitus through activating of AMPK signaling pathway in mice. J Pharmacol Sci. 2018;138(3):161–166. [DOI] [PubMed] [Google Scholar]
- 4. Catalano PM. Trying to understand gestational diabetes. Diabet Med. 2014;31(3):273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liong S, Lappas M. Endoplasmic reticulum stress regulates inflammation and insulin resistance in skeletal muscle from pregnant women. Mol Cell Endocrinol. 2016;425(1):11–25. [DOI] [PubMed] [Google Scholar]
- 6. Nair S, Jayabalan N, Guanzon D. et al. Human placental exosomes in gestational diabetes mellitus carry a specific set of miRNAs associated with skeletal muscle insulin sensitivity. Clin Sci (Lond). 2018;132(22):2451–2467. [DOI] [PubMed] [Google Scholar]
- 7. Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest. 2017;127(1):43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng Y, Chu A, Luo Q. et al. The protective effect of astaxanthin on cognitive function via inhibition of oxidative stress and inflammation in the brains of chronic T2DM rats. Front Pharmacol. 2018;9:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ying CJ, Zhang F, Zhou XY. et al. Anti-inflammatory effect of astaxanthin on the sickness behavior induced by diabetes mellitus. Cell Mol Neurobiol. 2015;35(7):1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou X, Zhang F, Hu X. et al. Inhibition of inflammation by astaxanthin alleviates cognition deficits in diabetic mice. Physiol Behav. 2015;151:412–420. [DOI] [PubMed] [Google Scholar]
- 11. Ishiki M, Nishida Y, Ishibashi H. et al. Impact of divergent effects of astaxanthin on insulin signaling in L6 cells. Endocrinology. 2013;154(8):2600–2612. [DOI] [PubMed] [Google Scholar]
- 12. Sun B, Zhong Z, Wang F. et al. Atorvastatin impaired glucose metabolism in C2C12 cells partly via inhibiting cholesterol-dependent glucose transporter 4 translocation. Biochem Pharmacol. 2018;150(2):108–119. [DOI] [PubMed] [Google Scholar]
- 13. Boursereau R, Abou-Samra M, Lecompte S, Noel L, Brichard SM. New targets to alleviate skeletal muscle inflammation: role of microRNAs regulated by adiponectin. Sci Rep. 2017;7:43437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baccouche B, Benlarbi M, Barber AJ, Ben Chaouacha-Chekir R. Short-term administration of astaxanthin attenuates retinal changes in diet-induced diabetic Psammomys obesus. Curr Eye Res. 2018;43(9):1177–1189. [DOI] [PubMed] [Google Scholar]
- 15. Dong LY, Jin J, Lu G, Kang XL. Astaxanthin attenuates the apoptosis of retinal ganglion cells in DB/DB mice by inhibition of oxidative stress. Mar Drugs. 2013;11(3):960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mashhadi NS, Zakerkish M, Mohammadiasl J. et al. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac J Clin Nutr. 2018;27(2):341–346. [DOI] [PubMed] [Google Scholar]
- 17. Zhao ZW, Cai W, Lin YL. et al. Ameliorative effect of astaxanthin on endothelial dysfunction in streptozotocin-induced diabetes in male rats. Arzneimittelforschung. 2011;61(4):239–246. [DOI] [PubMed] [Google Scholar]
- 18. Fassett RG, Coombes JS. Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiol. 2009;5(4):333–342. [DOI] [PubMed] [Google Scholar]
- 19. Nakano M, Onodera A, Saito E. et al. Effect of astaxanthin in combination with alpha-tocopherol or ascorbic acid against oxidative damage in diabetic ODS rats. J Nutr Sci Vitaminol (Tokyo). 2008; 54(4):329–334. [DOI] [PubMed] [Google Scholar]
- 20. Otton R, Marin DP, Bolin AP. et al. Astaxanthin ameliorates the redox imbalance in lymphocytes of experimental diabetic rats. Chem Biol Interact. 2010;186(3):306–315. [DOI] [PubMed] [Google Scholar]
- 21. Uchiyama K, Naito Y, Hasegawa G. et al. Astaxanthin protects beta-cells against glucose toxicity in diabetic db/db mice. Redox Rep. 2002;7(5):290–293. [DOI] [PubMed] [Google Scholar]
- 22. Sheu A, Chan Y, Ferguson A. et al. A proinflammatory CD4(+) T cell phenotype in gestational diabetes mellitus. Diabetologia. 2018;61(7):1633–1643. [DOI] [PubMed] [Google Scholar]
- 23. Yoshida H, Yanai H, Ito K. et al. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis. 2010;209(2):520–523. [DOI] [PubMed] [Google Scholar]
- 24. Evers-van Gogh IJ, Oteng AB, Alex S. et al. Muscle-specific inflammation induced by MCP-1 overexpression does not affect whole-body insulin sensitivity in mice. Diabetologia. 2016;59(3):624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jourdan T, Szanda G, Rosenberg AZ. et al. Overactive cannabinoid 1 receptor in podocytes drives type 2 diabetic nephropathy. Proc Natl Acad Sci U S A. 2014;111(50):E5420–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Z, Luo H, Zhang L. et al. Hyperhomocysteinemia exaggerates adventitial inflammation and angiotensin II-induced abdominal aortic aneurysm in mice. Circ Res. 2012;111(10):1261–1273. [DOI] [PubMed] [Google Scholar]
- 27. Patsouris D, Cao JJ, Vial G. et al. Insulin resistance is associated with MCP1-mediated macrophage accumulation in skeletal muscle in mice and humans. PLoS One. 2014;9(10):e110653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lang CH, Silvis C, Deshpande N, Nystrom G, Frost RA. Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin-1beta, -6, and high-mobility-group protein-1 in skeletal muscle. Shock. 2003;19(6):538–546. [DOI] [PubMed] [Google Scholar]
- 29. Nanobashvili K, Jack-Roberts C, Bretter R. et al. Maternal choline and betaine supplementation modifies the placental response to hyperglycemia in mice and human trophoblasts. Nutrients. 2018;10(10):1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_materials for Effects of Astaxanthin on Inflammation and Insulin Resistance in a Mouse Model of Gestational Diabetes Mellitus by Weihong Feng, Yanxia Wang, Na Guo, Pu Huang and Yang Mi in Dose-Response