Abstract
Background:
Long QT syndrome (LQTS) is a leading cause of sudden cardiac death in early life and has been implicated in ~10% of sudden infant deaths and unexplained stillbirths. The purpose of our study was to use fetal magnetocardiogaphy (fMCG) to characterize the electrophysiology and rhythm phenotypes of fetuses with de novo and inherited LQTS variants and identify risk factors for sudden death before birth.
Methods:
We reviewed the fMCG database from the University of Wisconsin Biomagnetism Laboratory for fetuses with confirmed LQTS. We assessed waveform intervals, heart rate, and rhythm, including the signature LQTS rhythms: functional 2° atrioventricular block, T-wave alternans, and torsade de pointes (TdP)
Results.
Thirty-nine fetuses had pathogenic variants in LQTS genes: 27 carried the family variant, 11 had de novo variants, and 1 was indeterminate. De novo variants, especially de novo SCN5A variants, were strongly associated with a severe rhythm phenotype and perinatal death: 9 (82%) showed signature LQTS rhythms, 6 (55%) showed TdP, 5 (45%) were stillborn, and 1 (9%) died in infancy. Those that died exhibited novel fetal rhythms, including AV block with 3:1 conduction ratio, QRS alternans in 2:1 AV block, long-cycle length TdP, and slow monomorphic ventricular tachycardia. Premature ventricular contractions were also strongly associated with TdP and perinatal death. Fetuses with familial variants showed a lower incidence of signature LQTS rhythm (6/27=22%), including TdP (3/27= 11%). All were live born.
Conclusions:
The malignancy of de novo LQTS variants was remarkably high and demonstrate that these mutations are a significant cause of stillbirth. Their ability to manifest rhythms not known to be associated with LQTS increases the difficulty of echocardiographic diagnosis and decreases the likelihood that a resultant fetal loss is attributed to LQTS.
Keywords: long QT syndrome, fetal, magnetocardiography, arrhythmia, alternans
Journal Subject Terms: arrhythmias, sudden cardiac death, genetic, association studies
Introduction
Over the last few decades our understanding of long QT syndrome (LQTS) has advanced remarkably; however, investigation of LQTS in the fetus has been impeded by the difficulty of recording the fetal ECG. Although echocardiography can detect some LQTS rhythms in utero, electrophysiological monitoring is imperative. Fetal magnetocardiography (fMCG), the magnetic analog of fetal ECG, is currently the most accurate and comprehensive method of assessing fetal rhythm.1, 2 In recent years, the efficacy of fMCG for diagnosis and management of serious fetal arrhythmia has become widely recognized.3 Its ability to diagnose LQTS based on QTc prolongation and detect LQTS rhythms indicative of severe disease has been demonstrated in numerous reports.4–8
This paper presents the results of an fMCG investigation of 39 cases of fetal LQTS, comprising by far the largest fMCG study to date. We sought to characterize the electrophysiology and rhythm phenotypes of fetuses with de novo and inherited LQTS variants and identify risk factors for sudden death before birth.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. The research was approved by the University of Wisconsin-Madison Health Sciences IRB. Written informed consent was obtained from all subjects. One author (RTW) had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Subjects
We searched the fMCG database from the University of Wisconsin Biomagnetism Lab for fetuses with confirmed LQTS based on genetic testing or a clinical diagnosis of LQTS. This yielded a cohort of 39 subjects that were studied in 1–4 sessions at gestational ages ranging from 20 to 37 weeks. Fifteen of the 39 subjects were reported in a prior publication.4 The subjects were referred by pediatric cardiologists from across the US due to fetal arrhythmia or a family history of LQTS. In addition, online advertising was used to recruit subjects with a family history of LQTS. We reviewed the pre- and post-natal medical records, including results of fetal echocardiograms, postnatal ECG, and postnatal genetic testing.
Data acquisition
The fMCG was recorded using a 37-channel (Magnes, 4D Neuroimaging, Inc., San Diego, CA) or 21-channel (Model 624, Tristan Technologies, San Diego, CA) superconducting quantum interference device magnetometer, housed in a magnetically-shielded room. Both devices have FDA 510(k) clearance. At least four 10-minute recordings were taken from each fetus.
In-lab echocardiography was performed using a SonoSite M Turbo scanner (Fujifilm SonoSite, Inc., Bothwell, Washington).
Signal processing and averaging
A digital filter with a 1–80 Hz passband was applied to band-limit the data. Signal processing was used to remove the maternal MCG and other interferences.9 Signal averaging was performed to increase the signal-to-noise ratio of the waveforms. The averaged waveforms were computed during periods of fetal quiescence, using the QRS complexes as triggers. Typically, 50–100 consecutive complexes were averaged, depending on the signal-to-noise ratio of the raw recording.
QTc measurement
The QT interval was measured from averaged waveforms, except for one fetus (#33) with a rhythm so irregular that averaging could not be performed. The heart rate corrected QT interval, QTc, was computed using Bazett’s formula: QTc= QT/(RR)1/2. QTc was measured in sinus rhythm during 1:1 conduction, except in four fetuses (#30, #31, #35, # 37) in which it was measured during 2:1 functional AV block because little or no 1:1 conduction was seen. A fetal QTc>500 ms was considered prolonged, based on two previously published reports.4, 10 The neonatal QTc was measured from an ECG taken within 48 hours of delivery. Neonatal QTc> 450 ms was considered prolonged.11
Rhythm assessment
Fetal rhythm was assessed from continuous rhythm recordings. In the three fetuses with little or no 1:1 conduction, sinus rate was assessed from measurement of the PP interval. We documented the presence of the three characteristic LQTS rhythms that throughout the paper will be called “signature” —second-degree atrioventricular block (2° AVB), T-wave alternans (TWA), and torsade de pointes (TdP). We defined 2° AVB as a rhythm with regular P-P interval and conduction of every other atrial beat, and TdP as paroxysms of a wide complex rhythm with a variable QRS morphology. We described the QRS morphology as narrow (< 95th percentile for gestational age) or wide (>95th percentile), based on comparison with normal fetuses.10 We also inspected the recordings for beat-to-beat changes in QRS and T-wave morphology; i.e., alternans. Premature ventricular contractions (PVCs) were considered present if they occurred with a frequency of at least one per minute.
Statistical analysis
For subjects that returned for follow-up visits we averaged the continuous variables over all visits to avoid biasing the data. If a rhythm was seen in any visit, they were considered positive for the rhythm.
A two-tailed t-test was used to identify differences in continuous variables between fetuses with de novo and familial LQTS. ANOVA was used to detect differences in continuous variables between fetuses with LQT1, LQT2, and LQT3. If a significant difference was found with ANOVA, pairwise comparisons were performed using the Tukey-Kramer test. A two-tailed Fisher exact test was performed to identify differences in categorical variables between fetuses with de novo and familial LQTS and between fetuses with LQT1, LQT2, and LQT3. For all tests a p-value less than 0.05 was considered significant. The p-values for each of the pair-wise categorical outcomes were adjusted for multiple comparisons using the Benjamini-Hochberg procedure.
Results
Postnatal genetic confirmation of a LQTS variant was obtained for 36 of 39 fetuses (Table 1).Of the three untested fetuses (#37-#39), two showed QTc prolongation on fMCG and postnatal ECG and had a parent with a diagnosis of LQTS, and the third died in utero and showed marked QTc prolongation and TdP.
Table 1.
Fetal cohort of 37 subjects with LQTS.
| ID | GA (weeks) | Indication | De novo | fQTc (ms) | nQTc (ms) | fHR (bpm) | TWA | QRS alt | TdP | 2° AVB | PVCs | LQTS mutation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LQT1 | ||||||||||||
| 1 | 30–1/7 | FH | N | 522 | 521 | 124 | N | N | N | N | N | KCNQ1 K422fs |
| 2 | 22 1/7 | FH+arrhytmia | N | 495 | 502 | 122 | N | N | N | N | N | KCNQ1 W176R |
| 28 5/7 | 536 | 115 | N | N | N | N | N | |||||
| 3 | 28 3/7 | FH | N | 517 | 545 | 118 | N | N | N | N | N | KCNQ1 G325R |
| 4 | 22 5/7 | FH | N | 360 | 556 | 139 | N | N | N | N | N | KCNQ1 S566Y |
| 28 2/7 | 486 | 138 | N | N | N | N | N | |||||
| 34 | 516 | 123 | N | N | N | N | N | |||||
| 5 | 23–6/7 | arrhythmia∥ | N | 445 | 468 | 126 | N | N | N | N** | N | KCNQ1 R518X |
| 6 | 22 6/7 | FH+arrhytmia | N | 535 | 517 | 121 | N | N | N | Y | N | KCNQ1 G314D |
| 27 | 550 | 120 | N | N | N | N | N | |||||
| 34 6/7 | 555 | 108 | N | N | N | N | N | |||||
| 7 | 20 6/7 | FH+arrhythmia | N | 561 | 580 | 116 | N | N | N | N | N | KCNQ1 A590T |
| 29 5/7 | 582 | 116 | Y | N | N | N | N | |||||
| 35 | 622 | 123 | Y | N | N | N | N | |||||
| 37 1/7 | 582 | 122 | Y | N | N | N | N | |||||
| 8 | 29 | FH | N | 529 | 467 | 106 | N | N | N | N | N | KCNQ1 A341V |
| 9 | 31 4/7 | FH | N | 524 | 573 | 122 | N | N | N | N | N | KCNQ1 |
| 10 | 28 3/7 | FH | N | 528 | 610 | 104 | N | N | N | N | N | KCNQ1 F339S |
| 11 | 25 | FH | N | 545 | 568 | 116 | N | N | N | N | N | KCNQ1 R594Q |
| 12 | 28 | FH | N | 547 | 544 | 112 | N | N | N | N | N | KCNQ1 R594Q |
| 34 | 536 | 112 | N | N | N | N | N | |||||
| 13 | 32 | FH | N | 560 | 522 | 118 | N | N | N | N | N | KCNQ1 R190W |
| 14 | 36 | arrhythmia | N | 533 | 554 | 116 | N | N | N | N | N | KCNQ1 R539W |
| 15 | 26 | arrhythmia | N | 563 | 462 | 119 | N | N | N | N | N | KCNQ1 G314D |
| 16 | 23 | arrhythmia | N | 505 | 488 | 115 | N | N | N | N | N | KCNQ1 R258C |
| 17 | 25 4/7 | arrhythmia | Y | 552 | 582 | 113 | N | N | N | N | N | KCNQ1 V110fs132X |
| 34 2/7 | 540 | 112 | N | N | N | N | N | |||||
| 18 | 36 | FH | N | 547 | 500 | 122 | N | N | N | N | N | KCNQ1 R594Q |
| AVG | 28.8 | 6% | 531 | 531 | 118 | 6% | 0% | 0% | 6% | 0% | ||
| LQT2 | ||||||||||||
| 19 | 25 5/7 | FH+arrhythmia | N | 562 | 459 | 126 | N | N | N | N | N | KCNH2 V94G |
| 20 | 34 6/7 | arrhythmia | Y | 694 | 675 | 109 | Y | Y | Y | N | Y | KCNH2 T613M |
| 21Ꞩ | 27 3/7 | FH | N | 548 | 521 | 141 | N | N | N | N | N | KCNH2 1157Pfs+111X |
| 22Ꞩ | 29 3/7 | FH | N | 555 | – | 140 | N | N | N | N | N | KCNH2 1157Pfs+111X |
| 23Ꞩ | 36 4/7 | FH | N | 649 | 524 | 127 | Y | Y | Y | N | Y | KCNH2 T613K |
| 24Ꞩ | 33 6/7 | FH | N | 646 | 568 | 123 | Y | Y | Y | N | Y | KCNH2 T613K |
| 25 | 25 6/7 | FH+arrhythmia | N | 606 | 492 | 126 | Y | Y | N | N | N | KCNH2 A614V |
| 33 | 595 | 115 | N | N | N | N | Y | |||||
| 26 | 29 | FH | N | 579 | 506 | 130 | N | N | N | N | N | KCNH2 W1001X |
| AVG | 30.8 | 13% | 604 | 535 | 127 | 50% | 50% | 38% | 0% | 50% | ||
| LQT3 | ||||||||||||
| 27 | 28 5/7 | FH | N | 483 | 430 | 138 | N | N | N | N | N | SCN5A E1784K |
| 28† | 21 | arrhythmia | Y | 652 | -- | 148 | Y | Y | Y | N | Y | SCN5A L409P |
| 29‡ | 31 | arrhythmia | Y | 693 | 540 | 124 | Y | Y | Y | Y | Y | SCN5A R1623Q |
| 30† | 21 3/7 | arrhythmia | Y | 610* | -- | 157# | Y | N | N | Y | Y | SCN5A R1623Q |
| 31† | 26 | arrhythmia | Y | 841* | -- | 138# | N | Y | Y | Y | Y | SCN5A V1763L |
| AVG | 25.5 | 75% | 656 | 485 | 141 | 60% | 60% | 60% | 60% | 80% | ||
| Rare mutations | ||||||||||||
| 32 | 32 | arrhythmia | Y | 592 | 645 | 133 | Y | Y | N | Y | N | CACNA1C (LQT8††) |
| 33 | 625 | 130 | Y | Y | N | N | N | |||||
| 35 | 663 | 123 | Y | Y | N | N | N | |||||
| 33 | 27 | arrhythmia | Y | 714 | 580 | 98 | Y | Y | N | Y | N | CALM2 |
| 34 | 33 2/7 | arrhythmia | Y | 524 | 588 | 120 | N | N | N | N | N | CALM2 |
| AVG | 31.2 | 100% | 622 | 604 | 116 | 67% | 67% | 0% | 67% | 0% | ||
| Uncharacterized mutations | ||||||||||||
| 35† | 33 4/7 | arrhythmia | Y | 769* | -- | 128# | Y | Y | Y | Y | Y | Uncharacterized |
| 36 | 35 | arrhythmia | -- | 574 | 540 | 104 | Y | N | N | N | N | Uncharacterized |
| AVG | 34.3 | 100% | 672 | 540 | 116 | 100% | 50% | 50% | 50% | 50% | ||
| Untested | ||||||||||||
| 37† | 26 1/7 | arrhythmia | Y | 850* | -- | 136# | N | Y | Y | Y | Y | untested |
| 38 | 33 3/7 | FH+arrhythmia | N | 606 | 554 | 99 | N | N | N | N | N | maternal LQTS |
| 39 | 25 | FH+arrhythmia | N | 652 | 664 | 116 | N | N | N | N | N | paternal LQTS |
| 33 | 682 | 115 | Y | Y | Y | N | Y | |||||
| AVG | 28.7 | 33% | 708 | 609 | 117 | 33% | 67% | 67% | 33% | 67% | ||
Subjects reported in a prior publication are denoted by an underlined subject number. De novo mutations (last column) are shown in bold typeface (ID= subject number, GA= gestational age, fQTc= fetal QTc, nQTc= neonatal QTc, fHR= fetal heart rate, FH=family history of LQTS, TWA= T-wave alternans, QRS alt= QRS alternans, TdP= torsade de pointes, 2° AVB= second-degree atrioventricular block, PVCs= premature ventricular contractions).
QTc in second-degree AV block
fetal loss
died postnatally at age 6 months
siblings: #21 and #22 are siblings; #23 and #24 are siblings
atrial rate in second-degree AV block
family history of Brugada’s Syndrome
referral diagnosis included second-degree AV block
type 1 Timothy Syndrome
Sixteen (41%) fetuses had no family history of LQTS at the time of study. Cascade testing of family members revealed that four of the fetuses were probands. One case (fetus #36) was not classified as familial or de novo; i.e. was indeterminate, because the parents refused testing. The remaining 11 (28%) fetuses were considered to have de novo variants (Fig. 1).
Fig. 1.
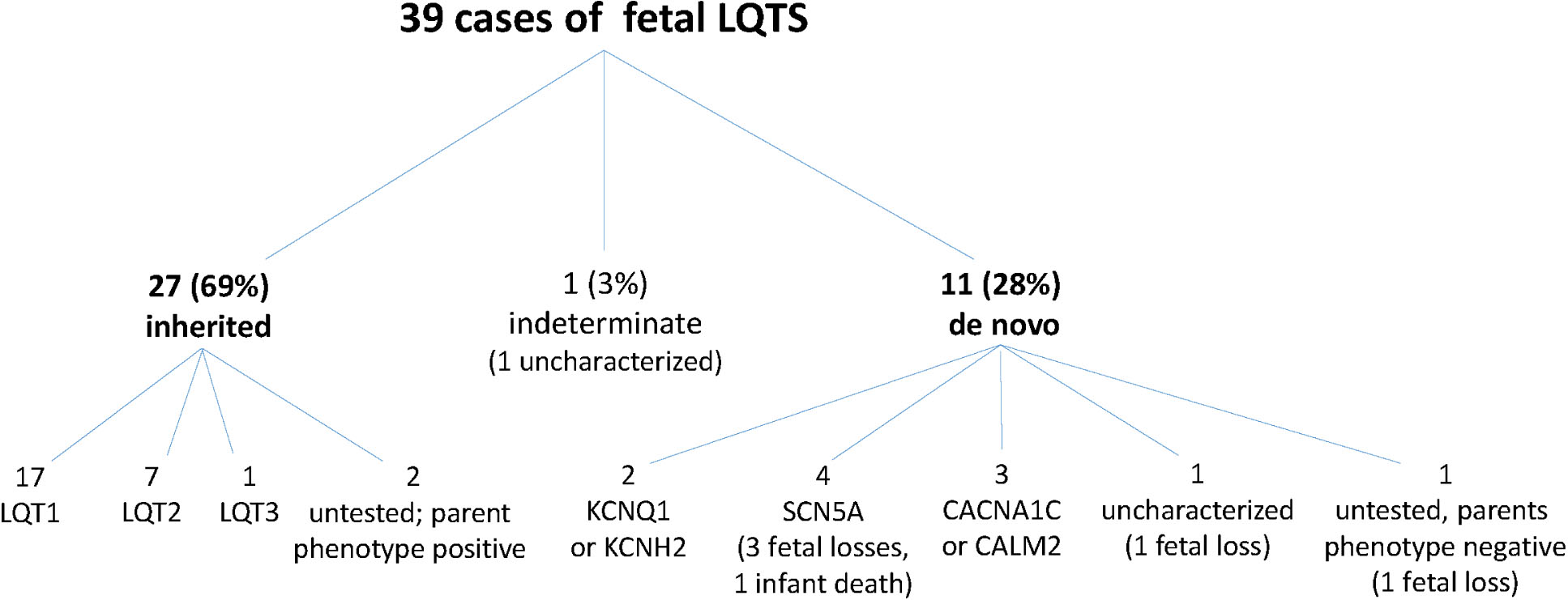
Genotype composition of fetal LQTS cohort. Within the cohort of 39 subjects there were 6 deaths. All involved de novo variants. Four were de novo SCN5A variants.
QTc prolongation
Thirty-seven of 39 (95%) fetuses showed QTc prolongation. One fetus (#27) did not show QTc prolongation or LQTS rhythms on fMCG or postnatal ECG, but carried the family SCN5A variant. Another fetus (#5) showed a normal QTc (445 ms) by fMCG at 23–6/7 weeks’ and a mildly prolonged QTc (468 ms) by postnatal ECG before testing positive for a KCNQ1 variant. Except for this case, the fMCG and postnatal ECG (Table 1) were concordant for QTc prolongation for the 33 subjects that had both in utero and postnatal measurements of QTc. Concordance could not be assessed in 6 subjects: five died before birth and postnatal records could not be obtained from one (#22). It is notable that in two KCNQ1 fetuses (#2 and #4) QTc was initially normal at 22 weeks’ but was prolonged at 28 and 34 weeks’, respectively, demonstrating the usefulness of serial monitoring.
Rhythms
A. Signature Rhythms
Sixteen (16/39= 41%) fetuses showed at least one of the three signature LQTS rhythms. TWA had the highest incidence (13/39= 33%), followed by TdP (9/39= 23%), and 2° AVB (8/39=21%).TWA potentially had an even higher incidence but could not be assessed in several fetuses that showed highly irregular rhythms. Of the 9 cases of TdP, 4 (44%) were unsuspected by echocardiography and first detected by fMCG, likely because the episodes were infrequent and brief and/or the cycle length was similar to that of sinus rhythm, as described below.
B. Novel Rhythms
Several novel and previously unreported rhythms were seen, all in fetuses with de novo variants that succumbed in utero or in infancy. The first was functional AV block with a 3:1 conduction ratio, seen in 2 fetuses. In one case, this manifested as periods of sustained 3:1 AV, which alternated with periods of sustained 2:1 AV block (fetus #35; Fig. 2A). In the other, 3:1 AV block alternated sporadically with 2:1 AV block and contributed to the exceedingly complex rhythm patterns, which also included frequent PVCs, QRS alternans and/or aberrantly conducted QRS complexes, and TdP (fetus #37; Fig. 2B). The second novel rhythm was atrial flutter (fetus #37; Fig. 2C). Atrial flutter was also part of the referral diagnosis for fetus #30 but resolved prior to the fMCG session. Third was an unusual form of QRS alternans, seen as an overt beat-to-beat variation in QRS amplitude with little or no broadening and occurring during 2:1 functional AV block (fetus #31; Fig. 2D). The fourth novel rhythm was TdP with a cycle length similar to that of sinus rhythm. Although the cycle length in TdP was variable, it was similar to or slower than that of sinus rhythm in three fetuses (#28, #31, #35; Fig. 2E–2G). The fifth novel rhythm, seen in two fetuses (#29 and #30), was slow monomorphic ventricular tachycardia (VT) with rates of 120–145 bpm (Fig. 2H).
Fig. 2.

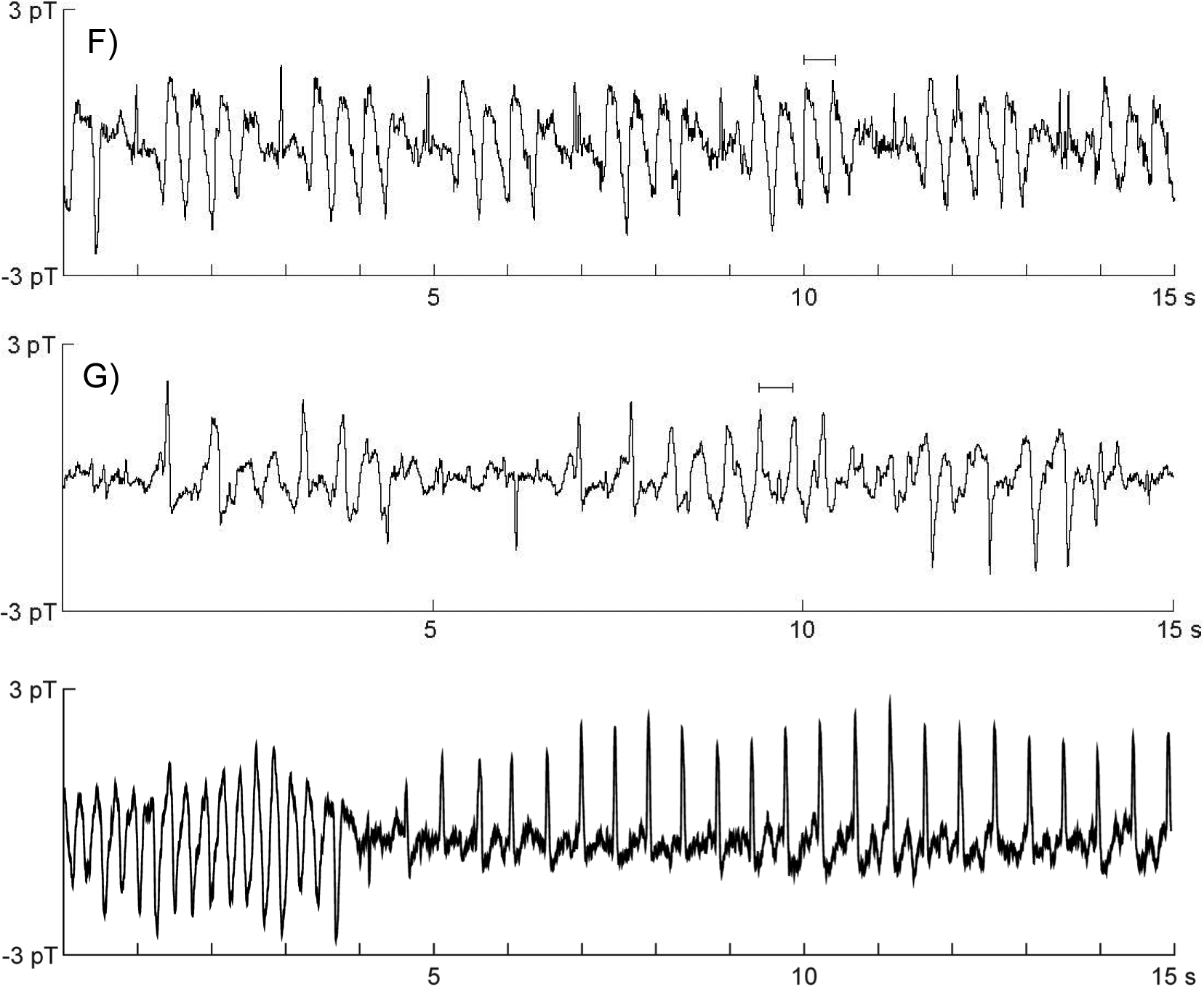
Novel LQTS rhythm patterns. A) Transition from 2:1 to 3:1 AV block in fetus #35. A premature ventricular contraction (PVC; asterisk) occurs at the transition, followed by an aberrantly conducted beat and a relatively short QT interval that progressively lengthens. In 2:1 AV block the T-waves are notched, which is commonly seen in LQT217. In 3:1 AV block the QRS complexes change morphology and become narrower. B-C) Complex rhythm with TdP, PVCs, (B) periods of AV block in a 3:1 or 2:1 conduction ratio and (C) atrial flutter in fetus #37. Sinus beats (upward arrows) were relatively rare due to AV block, PVCs (asterisks), and the prevalence of TdP. P-waves are indicated by downward arrows. The T-waves (T) show a negative-positive biphasic morphology. TdP was usually initiated by a PVC. Atrial flutter (330 bpm) was seen approximately 25% of the time and occurred with and without the presence of TdP. D) QRS alternans in 2:1 AV block in fetus #31. The QRS complexes (arrows) are narrow and on time, but show marked beat-to-beat variation in amplitude and polarity. Normally, QRS alternans occurs during 1:1 AV conduction in a regular ABAB pattern and is characterized by modest variation in QRS amplitude and prominent variation in QRS morphology. E-G) TdP with a cycle length similar to that of sinus rhythm in fetuses #35, #28, and #31, respectively. The horizontal bars indicate the duration of the PP intervals. H) Transition from typical TdP to slow VT with a rate of 127 bpm in fetus #29.
C. Ectopy
PVCs were seen in 11 (28%) fetuses, all with QTc> 600ms, including all 9 fetuses that showed TdP and all 5 that died in utero. TdP was commonly initiated by a PVC (Fig. 2B, 2C, 2E).
Mechanical Alternans
Of the 12 fetuses that showed TWA, 8 showed QRS alternans in an ABAB pattern due to “R-on-T” phenomenon. In-lab echocardiography was performed in 4 of the 8 with TWA and revealed mechanical alternans in 3 (Fig. 3). The fetus that did not show mechanical alternans showed only brief, intermittent periods of TWA
Fig. 3.
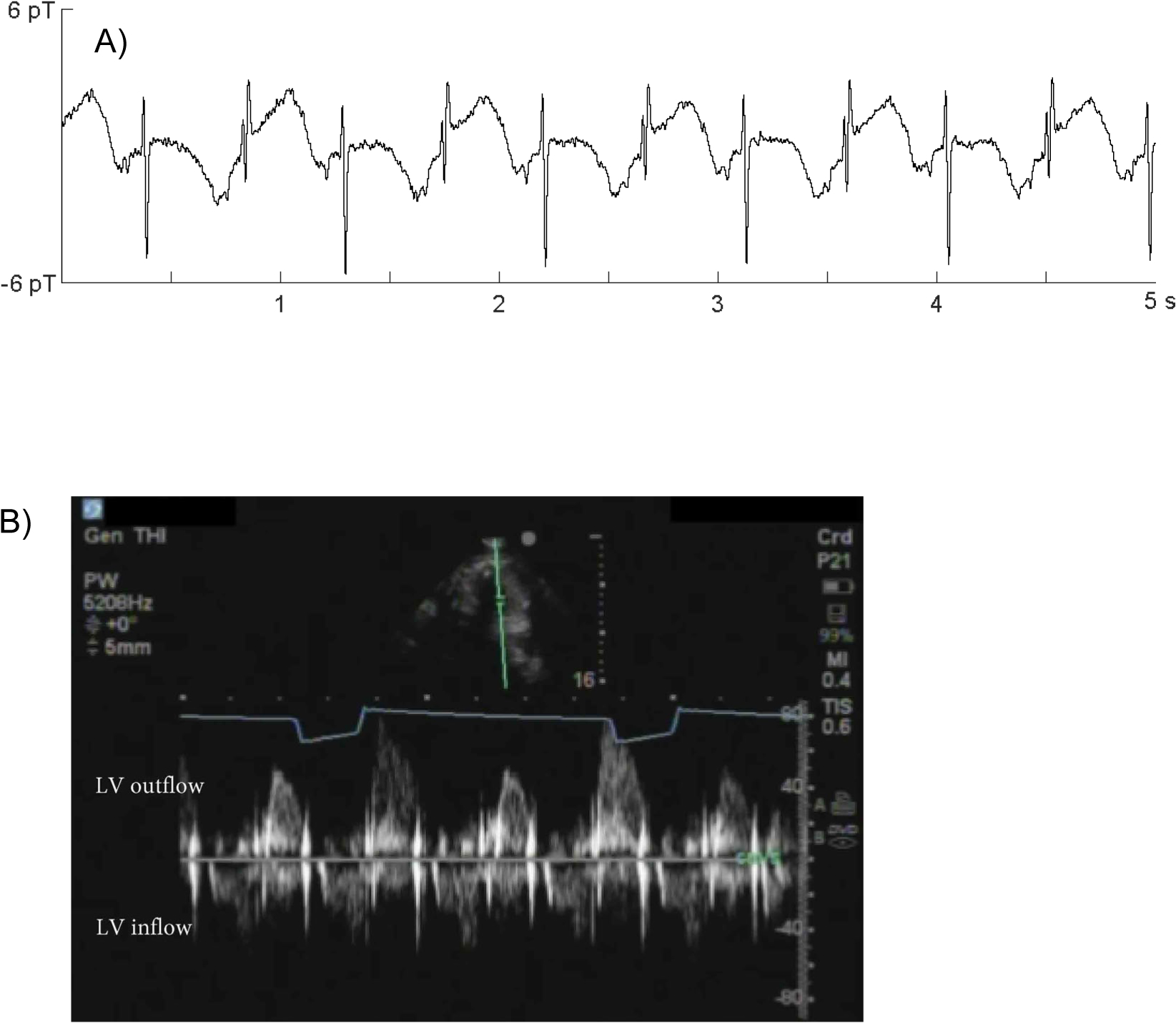
Magnetic and mechanical alternans. A) QRS and T-wave alternans in fetus #32. B) Pulsed Doppler tracing from the same fetus showing mechanical alternans. Notice the prolonged diastolic relaxation with every other beat. The flow pattern can mimic PVCs, but is distinguished by its uniform cycle length.
Disease severity and mortality: familial versus de novo variants
De novo variants were associated with extremely severe disease; all five fetuses that died in utero and another that died in infancy had de novo variants. SCN5A variants were involved in four cases (Fig. 1). The others that died had uncharacterized mutations or were not tested. To our knowledge, there were no other deaths in the cohort.
Four of the 5 fetuses that died in utero (#28, #31, #35, #37) showed TdP during the fMCG session and died within 10 days. Transplacental therapy was given in 3 cases and was declined in 1 due to the extremely poor clinical status. The fifth case of fetal demise (fetus #30) showed 2° AVB, intermittent TWA, and frequent PVCs with short runs of slow VT and died two months later despite transplacental treatment that restored sinus rhythm following fMCG diagnosis. The fetus that died in infancy (#29) showed TdP and slow VT in utero. Following fMCG diagnosis the fetus was transitioned to appropriate aggressive therapy that initially restored sinus rhythm, but death occurred at approximately age 6 months. These 6 cases presented with QTc> 600 ms and PVCs, and 5 of 6 presented with hydrops.
Phenotype-Genotype Correlations
QTc was much longer for de novo variants (684±107 ms) than for familial variants (554±50 ms; p<0.01) and the overall incidence of signature, as well as novel, LQTS rhythms was higher (Table 2). Although no fetuses with familial variants died in utero, the proportion that showed LQTS rhythms (LQT1: 2/16 (13%); LQT2: 3/7 (43%); LQT3: 0/1 (0%); untested: 1/2 (50%)) was nonetheless substantial, and included 3 (11%) cases of TdP The incidence of 2° AVB, however, was notably low (1/26= 4%) for familial variants, being present in only one case. This fetus (#6) showed 2° AVB in the first session at 22–6/7 weeks; however, it resolved spontaneously and was not seen in two follow-up fMCG sessions or with echocardiography.
Table 2.
Incidence of signature LQTS rhythms associated with familial and de novo genetic variants
| Variant | Gestational Age (wks) | QTc (ms) | Signature Rhythm(s) | 2° AVB | TWA | TdP | Fetal loss |
|---|---|---|---|---|---|---|---|
| Familial N=27 | 29.4±3.6 | 550±53 | 6 (22%) | 1 (4%) | 5 (19%) | 3 (11%) | 0 (0%) |
| De novo N=11 | 28.9±4.9 | 684±107 | 9 (82%) | 7 (64%) | 7 (64%) | 6 (55%) | 5 (45%) |
| p-value | -- | <0.01 | <0.05 | -- | -- | -- | <0.01 |
The bottom row shows the p-values for variables with statistically significant differences (2° AVB= second-degree AV block, TWA=T-wave alternans, TdP= torsade de pointes).
Considering only the three main LQTS susceptibility genes, the proportion of fetuses with a signature LQTS rhythm showed a statistically significant association (p<0.01) with LQTS type—LQT1: 2/18 (11%), LQT2: 4/8 (50%), LQT3: 4/5 (80%). The pair-wise comparisons were statistically significant for LQT1 vs. LQT3 (p=0.042). The proportion of fetuses with TdP also showed a statistically significant association (p=0.010) with LQTS type—LQT1: 0/18 (0%), LQT2: 3/8 (50%), LQT3: 3/5 (60%). The pair-wise comparisons were statistically significant for LQT1 vs. LQT2 (p=0.047) and LQT1 vs. LQT3 (p=0.026).
FHR showed a statistically significant association (p<0.01) with LQTS type— LQT1: 118±7 bpm, LQT2: 127±10 bpm, LQT3: 141±12 bpm. All of the pair-wise comparisons were statistically significant: LQT1 vs. LQT2 (p<0.05), LQT1 vs. LQT3 (p<0.01), LQT2 vs. LQT3 (p<0.05). Lastly, QTc was significantly shorter in LQT1 fetuses (531±41) compared to LQT2 fetuses (604±53, p<0.05) and LQT3 fetuses (656±130, p<0.01).
Discussion
This study is the first to document the rhythms that precede stillbirth in fetuses with LQTS and demonstrate that de novo LQTS variants are associated with a high rate of stillbirth. A critically important discovery was that de novo variants can produce novel rhythms that are not known to be associated with LQTS. The fetuses and infant that died all had a de novo variant in combination with a pernicious phenotype characterized by signature and novel LQTS rhythms, QTc>600 ms, PVCs, and hydrops. For fetuses with inherited variants the prognosis was much more favorable. While variants other than KCNQ1 variants were associated with a substantial risk of signature LQTS rhythms, including TdP, no deaths occurred.
The true incidence of de novo variants in any population is difficult to estimate; however, our results imply that the incidence in utero may be significantly higher than estimates based on postnatal analyses. The incidence can be overestimated due to familial LQTS with low penetrance. On the other hand, it can be underestimated if deaths occur in utero prior to diagnosis. Indeed, the most notable finding of our study was the lethality and severity of symptoms associated with de novo variants. All 5 fetuses that died had de novo variants. It is very unlikely that these cases, and perhaps others, would have been recognized as de novo LQTS variants, if not for participation in the study. Previously, Crotti and co-workers used molecular autopsy to provide evidence that variants of the three main LQTS susceptibility genes contribute to unexplained fetal demise.12 Our work compliments their findings by highlighting the contribution of de novo variants, many of which do not involve the three main LQTS susceptibility genes. The ability of fMCG to document the presence of malignant rhythms leaves little doubt that LQTS arrhythmia was causative in these stillbirths.
We identified several unexpected, novel rhythms. All were seen in fetuses with de novo variants that died in utero or in infancy. The documentation of these rhythms is of considerable importance because it significantly expands the known phenotypes of fetal LQTS and demonstrates that the manifestations of LQTS can be more severe prenatally than postnatally. Ironically, the presence of such severe disease can confound diagnosis of LQTS and may explain why some cases involving de novo variants go unrecognized. Long-cycle length TdP can be difficult to diagnose with echocardiography because the range of heart rate is compatible with sinus rhythm with normal heart rate reactivity. Similarly, slow VT shows heart rate compatible with sinus rhythm, and atrial flutter does not result in tachycardia in the presence of marked QTc prolongation. To our knowledge, atrial flutter has not been noted previously in association with fetal LQTS. Atrial flutter and other unexpected rhythms may result from overlap syndromes, such as SCN5A overlap syndrome, which is associated with atrial arrhythmia. The novel rhythms were often seen in combination and contributed significantly to the extremely complex patterns observed in some fetuses. A final important observation was functional AV block witha 3:1 conduction ratio, which results when QTc is so prolonged that 2 consecutive sinus beats are blocked, resulting in severe bradycardia.
Consistent with postnatal findings, our results show that the least deleterious type of LQTS presenting in the fetus is LQT1 (KCNQ1). The LQT1 fetuses had the shortest QTc and the lowest incidence of signature LQTS rhythms. In contrast, de novo LQT3 variants were lethal. The four subjects with de novo SCN5A mutations all died, three in utero and one postnatally; however, a fifth SCN5A fetus with a familial variant was asymptomatic.
The findings of this study have implications for echocardiographic evaluation of fetal LQTS. Echocardiography cannot ascertain TWA, which was shown here to be more common than 2° AV block and is considered to be more specific for LQTS. We demonstrated, however, that echocardiography can detect mechanical alternans, which can be considered a surrogate of TWA and should be present in fetuses with overt QRS alternans resulting from TWA. The fetuses that showed 3:1 functional AV block were noted by echocardiography to have periods of 3° AV block. This made it more difficult to diagnose LQTS because the apparent 3° AV block was a confound. In the absence of structural disease and maternal anti-Ro/SSA antibodies, a finding of severe bradycardia due to 3° AV block should be investigated for the possibility of 3:1 functional AV block due to fetal LQTS. The observation of long-cycle length TdP and slow monomorphic VT provide a possible explanation for heart failure in the presence of a normal fetal heart rate.
Lastly, in the setting of fetal LQTS, PVCs and slow VT are ominous indicators that can be detected with echocardiography.
Conclusions
The complex and novel rhythms documented in this study are causally linked to de novo LQTS stillbirth and help explain why these cases are often not recognized as resulting from LQTS. The unexpected findings of this study validate the need for systematic investigation of the fetus, versus extrapolation of postnatal findings to the prenatal period. In addition, the study further demonstrates the unrivaled capabilities of fMCG for investigation and clinical management of fetal LQTS. Although fMCG is currently confined to a small number of laboratories, we have recently demonstrated a practical, cost-effective fMCG system that can make the technique more widely available.16
Acknowledgements
We wish to thank the many fetal cardiologists and maternal-fetal medicine specialists who referred patients for this study. The research was funded by a grant from the NIH, which provides travel support for eligible subjects to participate in the study. For further information contact one of the study authors.
Sources of Funding: National Institutes of Health, Bethesda, MD, grant number R01-HL063174
Footnotes
Clinical Trial Registration: https://clinicaltrials.gov/ct2/show/NCT03047161
ClinicalTrials.gov Identifier: NCT03047161
Disclosures: The authors have no relationships with industry
REFERENCES
- 1.Hornberger LK and Collins K. New insights into fetal atrioventricular block using fetal magnetocardiography. J Am Coll Cardiol 2008;51:85–6. [DOI] [PubMed] [Google Scholar]
- 2.Van Hare GF. Magnetocardiography in the diagnosis of fetal arrhythmias. Heart Rhythm 2013;10:1199–200. [DOI] [PubMed] [Google Scholar]
- 3.Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, Cuneo BF, Huhta JC, Jonas RA, Krishnan A, Lacey S, Lee W, Michelfelder EC Sr., Rempel GR, Silverman NH, Spray TL, Strasburger JF, Tworetzky W, Rychik J, American Heart Association Adults With Congenital Heart Disease Joint Committee of the Council on Cardiovascular Disease in the Y, Council on Clinical Cardiology CoCS, Anesthesia, Council on C and Stroke N. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation 2014;129:2183–242 [DOI] [PubMed] [Google Scholar]
- 4.Cuneo BF, Strasburger JF, Yu SH, Horigome H, Hosono T, Kandori A and Wakai RT. In Utero Diagnosis of Long QT Syndrome by Magnetocardiography. Circulation 2013;128:2183–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horigome H, Iwashita H, Yoshinaga M and Shimizu W. Magnetocardiographic demonstration of torsade de pointes in a fetus with congenital long QT syndrome. J Cardiovasc Ectrophysiol. 2008;19:334–5. [DOI] [PubMed] [Google Scholar]
- 6.Schneider U, Haueisen J, Loeff M, Bondarenko N and Schleussner E. Prenatal diagnosis of a long QT syndrome by fetal magnetocardiography in an unshielded bedside environment. Prenat Diagn. 2005;25:704–8. [DOI] [PubMed] [Google Scholar]
- 7.Cuneo BF, Ovadia M, Strasburger JF, Zhao H, Petropulos T, Schneider J and Wakai RT. Prenatal diagnosis and in utero treatment of torsades de pointes associated with congenital long QT syndrome. Am J Cardiol. 2003;91:1395–8. [DOI] [PubMed] [Google Scholar]
- 8.Hosono T, Kawamata K, Chiba Y, Kandori A and Tsukada K. Prenatal diagnosis of long QT syndrome using magnetocardiography: a case report and review of the literature. Prenat Diagn. 2002;22:198–200. [DOI] [PubMed] [Google Scholar]
- 9.Yu S and Wakai RT. Maternal MCG interference cancellation using splined independent component subtraction. IEEE Trans Biomed Eng. 2011;58:2835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strand SA, Strasburger JF and Wakai RT. Fetal magnetocardiogram waveform characteristics. Physiol Meas. 2019;40:035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz PJ, Garson A Jr., Paul T, Stramba-Badiale M, Vetter VL, Wren C and European Society of C. Guidelines for the interpretation of the neonatal electrocardiogram. A task force of the European Society of Cardiology. 2002;23:1329–44. [DOI] [PubMed] [Google Scholar]
- 12.Crotti L, Tester DJ, White WM, Bartos DC, Insolia R, Besana A, Kunic JD, Will ML, Velasco EJ, Bair JJ, Ghidoni A, Cetin I, Van Dyke DL, Wick MJ, Brost B, Delisle BP, Facchinetti F, George AL, Schwartz PJ and Ackerman MJ. Long QT syndrome-associated mutations in intrauterine fetal death. JAMA. 2013;309:1473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinoshita K, Komatsu T, Nishide K, Hata Y, Hisajima N, Takahashi H, Kimoto K, Aonuma K, Tsushima E, Tabata T, Yoshida T, Mori H, Nishida K, Yamaguchi Y, Ichida F, Fukurotani K, Inoue H and Nishida N. A590T mutation in KCNQ1 C-terminal helix D decreases IKs channel trafficking and function but not Yotiao interaction. J Mol Cell Cardiol. 2014;72:273–80. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell JL, Cuneo BF, Etheridge SP, Horigome H, Weng HY and Benson DW. Fetal heart rate predictors of long QT syndrome. Circulation. 2012;126:2688–95. [DOI] [PubMed] [Google Scholar]
- 15.Choi G, Kopplin LJ, Tester DJ, Will ML, Haglund CM and Ackerman MJ. Spectrum and frequency of cardiac channel defects in swimming-triggered arrhythmia syndromes. Circulation. 2004;110:2119–24 [DOI] [PubMed] [Google Scholar]
- 16.Strand S, Lutter W, Strasburger JF, Shah V, Baffa O and Wakai RT. Low-Cost Fetal Magnetocardiography: A Comparison of Superconducting Quantum Interference Device and Optically Pumped Magnetometers. J Am Heart Assoc. 2019;8:e013436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lupoglazoff JM, Denjoy I, Berthet M, Neyroud N, Demay L, Richard P, Hainque B, Vaksmann G, Klug D, Leenhardt A, Maillard G, Coumel P and Guicheney P. Notched T waves on Holter recordings enhance detection of patients with LQt2 (HERG) mutations. Circulation. 2001;103:1095–101. [DOI] [PubMed] [Google Scholar]


