Abstract
Schizophrenia (Sz) is a chronic mental disorder characterized by disturbances in thought (such as delusions and confused thinking), perception (hearing voices), and behavior (lack of motivation) (American_Psychiatric_Association, 2013). The lifetime prevalence of Sz is between 0.3 to 0.7% (Perala et al., 2007), with late adolescence and early adulthood the peak period for the onset of psychotic symptoms (American_Psychiatric_Association, 2013). Causal factors in Sz include environmental and genetic factors and especially their interaction (Belbasis et al., 2018; Duncan & Keller, 2011; Guloksuz et al., 2019; Radua et al., 2018). About 50% of individuals with a diagnosis of Sz have lifelong impairment (American_Psychiatric_Association, 2013).
Keywords: Schizophrenia, neuroimaging, magnetoencephalography, neuroradiologists
Although radiologists have a large arsenal of imaging tools at their disposal (including x-ray, CT, MRI, ultrasound), these tools are not routinely used for clinical assessment of Sz. Indeed, although increased ventricular volume (Berger et al., 2017; Pantelis et al., 2005; van Erp et al., 2016) and reduced superior temporal gyrus gray matter (J. C. Edgar et al., 2014; Shenton, Dickey, Frumin, & McCarley, 2001; Smiley et al., 2009) in Sz are robust findings at the group level, brain imaging does not provide clinically usable information (diagnostic or prognostic) regarding Sz at the individual level due to insufficient sensitivity and specificity. As such, the relatively uncommon radiology clinical referral of someone having or suspected of having Sz is to rule out conditions such as a brain tumor or encephalitis.
MEG has been used for over 25 years in research studies examining neural activity in Sz. Although abnormalities in resting-state activity as well as basic sensory processing and cognitive processes are reliably reported in MEG studies (J. C. Edgar et al., 2018; Grent-’t-Jong et al., 2016; Huang et al., 2003; Popov et al., 2011; Rojas, Arciniegas, Teale, & Reite, 1999; Silverstein et al., 2006; Teale, Reite, Rojas, Sheeder, & Arciniegas, 2000; Thune, Recasens, & Uhlhaas, 2016; Wilson et al., 2008; Zeev-Wolf et al., 2018), analogous to other brain imaging modalities, no MEG marker for Sz exists for use at the individual level. MEG provides the ability to investigate brain activity at specific brain regions as well as to create whole-brain 3D images from the MEG sensor data. As radiologists excel at interpreting 3D brain images, neuroradiologists are well placed to make good use of MEG brain images. However, as detailed throughout this chapter, the MEG brain images of interest for Sz will be different from the structural brain images neuroradiologists typically encounter. This is because MEG records brain neural activity, and thus MEG brain images are images of brain function rather than brain structure. Although functional MRI (fMRI) clinical studies are sometimes obtained by radiologists, MEG images differ from fMRI images, as MEG records neural activity directly, in ‘real time’, rather than time-delayed and time-blurred hemodynamic activity related to neural activity, and thus millisecond-by-millisecond images (or even movies) are potentially available with MEG.
This chapter on Sz considers the possible future use of MEG by radiologists via focusing on areas of active research: research studies examining whole-brain resting-state activity, studies examining auditory encoding processes, and studies examining functional connectivity. Rather than provide a comprehensive review, this chapter considers the types of MEG images that neuroradiologists might one day read as part of a clinical radiology Sz exam. Given significant heterogeneity in Sz due to individual differences in genetic risk (Consortium, 2014; Henriksen, Nordgaard, & Jansson, 2017), environmental risk (Dean & Murray, 2005; van Os, Kenis, & Rutten, 2010), and symptoms (Andreasen, 1997), the brain neural abnormalities observed across individuals with Sz are likely to differ considerably. Indeed, growing clinical research evidence indicates that traditionally diagnosed Sz is diverse in etiology and heterogeneous in clinical presentation. Attention is turning to specific symptoms and risk factors as potential clinical targets for detection, diagnosis, and preventive and ameliorative intervention (J. C. Edgar, Miller, G.A., In Press; G. A. Miller, Rockstroh, B., 2013, 2016; Yee, Javitt, & Miller, 2015). The clinical payoff in examining activity throughout the brain is identifying abnormalities specific to each patient (e.g., observing abnormalities in superior temporal gyrus or frontal lobes), with individual findings hopefully one day guiding diagnosis and treatment. In addition, given that it is unlikely that brain pathology in Sz will be easily observed, the use of normative whole-brain MEG databases to identify abnormality at the individual level is highlighted, a procedure that allows for a more quantitative assessment of neural activity.
Resting-state Activity in Schizophrenia
Information related to collecting MEG data and obtaining source images is provided in Chapter 1, and Chapters 2 and 3 discuss resting-state recordings. Briefly, research studies examining resting-state (RS) brain activity in SZ typically collect spontaneous MEG in continuous 5-minute segments in an awake state. Given greater artifact in patients with Sz than in controls, multiple RS recordings are typically obtained in order to have a sufficient amount of artifact-free RS data (Lund, Sponheim, Iacono, & Clementz, 1995).
Although MEG Sz research has focused on RS brain activity across different frequency ranges such as alpha (9 to 12 Hz) and gamma (30 to 50 Hz) (Canive et al., 1998; Canive et al., 1996; Grent-’t-Jong et al., 2018; Rutter et al., 2009; Zeev-Wolf et al., 2018), RS low-frequency (delta and theta) oscillatory activity has received the most attention. Studies examining delta (1 to 4 Hz) and theta (4 to 8Hz) activity in Sz have built upon MEG and EEG studies observing more delta and theta RS activity in Sz than controls (Canive et al., 1998; Fehr et al., 2001; Pascual-Marqui et al., 1999; B. Rockstroh et al., 1997; Sponheim, Clementz, Iacono, & Beiser, 1994; Weinberger, 1988; Wienbruch et al., 2003; Winterer et al., 2000), with a meta-analysis concluding that enhanced low-frequency activity in SZ is a robust finding (Galderisi, 2009). Given increased low-frequency activity during the waking state in Sz and given that individuals with Sz typically do not show frank pathology on structural MRIs, it has been suggested that portions of the brain in Sz tend to be in an inactive, ‘sleep-like state’ (Lisman, 2012; Llinas, Urbano, Leznik, Ramirez, & van Marle, 2005) or that slowing in Sz reflects subtle brain pathology manifested in low-frequency MEG.
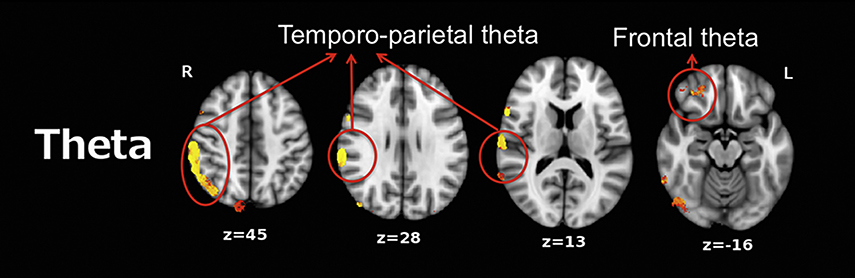
Rather than merely examining low-frequency activity at the sensor level (EEG or MEG), the brain areas associated with such slowing can be identified via source localization (typically co-registering MEG data with anatomical MRI data). Several MEG studies have examined RS low-frequency activity throughout the brain in Sz, using one of many source-localization methods that can be used to obtain a map of RS theta activity. Using L2-minimum norm estimate localization, Fehr et al. (Fehr et al., 2001) observed higher frontotemporal and posterior delta/theta activity in Sz than in adult controls. Using frequency-domain VEctor-based Spatio-Temporal analysis using Ll-minimum norm (VESTAL, (Huang et al., 2012; Huang et al., 2009)), as shown in Figure 1, Chen et al. (Chen et al., 2016) observed abnormal low-frequency theta activity in frontal and temporo-parietal regions in adults with Sz.
Figure 1.
Between-group VEctor-based Spatio-Temporal analysis using L1-minimum norm (VESTAL) analyses for theta (4 Hz to 8 Hz). Clusters in yellow/red show more right-hemisphere slow-wave activity in the schizophrenia group than in the control group (P<0.05, family-wise corrected). Adapted from YH Chen, B Stone-Howell, Edgar, J. C., et al. Frontal slow-wave activity as a predictor of negative symptoms, cognition and functional capacity in schizophrenia. Br J Psychiatry. 2016 Feb; 208(2), 160–167. doi:10.1192/bjp.bp.114.156075
A few general features of the whole-brain RS theta images in Figure 1 are of note. To obtain a RS theta 3D map for each subject, each subject’s RS eyes-closed data were filtered to examine theta activity (4–8 Hz). To this end, the MEG continuous raw data were divided into 2.5-s epochs, which provides 0.40 Hz resolution. Epochs were overlapped 50% to adjust for windowing of each epoch. For each epoch, a Fast Fourier Transform (FFT) provided a measure of theta activity (summed across 4 Hz to 8 Hz). The frequency-domain VESTAL source grid (5 mm isotropic resolution) was obtained by sampling gray-matter areas from the T1-weighted MRI of each subject. The sensor-space frequency-domain data were used by frequency-domain VESTAL to obtain the theta amplitude (root mean squared) at each source location. These values were then used to generate MEG theta source images for each subject. Additional details of frequency-domain VESTAL source imaging are provided in Huang et al. (Huang et al., 2012) and in Chapter 3. Figure 1 is thresholded to reveal areas with significant control versus patient group differences in RS theta activity, displayed as a color overlay on the anatomical MRI. Figure 1 illustrates the value of MEG in identifying region-specific functional abnormalities, with higher spatial fidelity than scalp EEG provides.
Of note, Fehr et al. (2001) and Chen et al. (2016) showed control and patient low-frequency differences only at the group level. Fehr et al. and Chen et al. did not show that increased low-frequency activity was observed in each of the individuals with Sz included in their samples. Some low-frequency activity is normal and observed in most non-patient adults (see Chapter 3). As such, except in extreme cases (e.g., very significant slowing from cortex adjacent to a tumor (Lewine, 1995)), identification of significantly elevated low-frequency activity at the individual level is difficult and in our judgment generally not ready for routine clinical use. Thus, similar to identifying abnormal low-frequency activity in individuals with mTBI (Chapter 3), an objective method is needed to identify abnormal low-frequency activity at the individual level. Using whole-brain MEG source-space measures, Wienbruch et al. (Wienbruch et al., 2003) described a method to apply a Z-score-based analysis for single subjects (a similar procedure was described in Chapter 3). Once a normative database is established, comparison of individual data against scores from a demographically matched control group will allow fine distinctions and comparisons unattainable by clinical observation or traditional neuroimaging alone. Via this normative approach, Rockstroh et al. (B. S. Rockstroh, Wienbruch, Ray, & Elbert, 2007) reported that the spatial topography of low-frequency activity distinguishes individuals with Sz from individuals with neurotic/affective diagnoses.
In our judgment the field is ready to begin producing data bases of this sort that will have routine clinical utility. However, before assessment of low-frequency activity in Sz can be of use in the clinic, research is needed to determine how often low-frequency abnormalities are observed at the individual level in Sz. Given changes in brain activity across the lifespan, research is also needed to determine whether different RS normative databases are needed for different age groups (J. C. Edgar et al., 2019; Miskovic et al., 2015; Somsen, van’t Klooster, van der Molen, van Leeuwen, & Licht, 1997; Szava et al., 1994; Valdes et al., 1992). Research is also needed to determine whether analogous findings are observed across source localization methods (e.g., L1 and L2 source localization methods, see Chapter 1) and different MEG recording systems vs. whether particular methods are superior for this purpose. Finally, research is needed to determine whether abnormal low-frequency activity identified at the individual level provide sufficient sensitivity and specificity to be used to guide treatment (e.g., low-frequency abnormalities at a particular brain region indicating that patient would respond best to a specific therapy) as well as predict outcome (i.e., a prognostic marker).
Auditory Studies
In addition to examining RS activity in Sz, MEG is collected in paradigms where sensory stimuli are presented (e.g., auditory tones, visual images). In some instances, the subject is instructed to rest while stimuli are presented. In other instances, the subject is given a task and makes a response each time a stimulus is presented (e.g., a button press). MEG data are time-locked to the presentation of each stimulus and/or button press. Because the response to a single stimulus is small relative to ongoing oscillatory activity, an averaged response to repeated stimuli is often computed. Typically, over one hundred stimuli in each condition (e.g., 130 tones, 200 pictures of faces) are presented to obtain an average evoked response providing a signal-to-noise ratio sufficient for source localization.
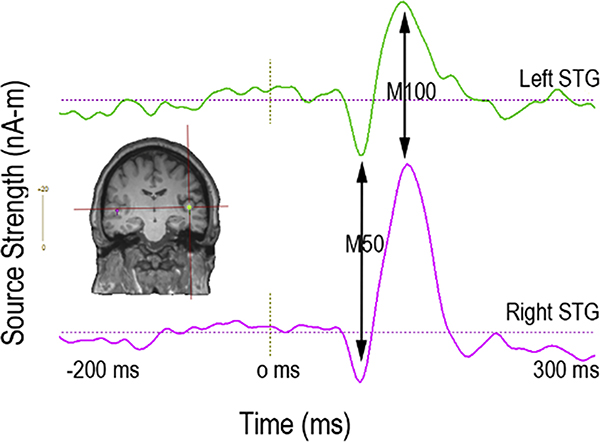
A very large EEG and MEG literature reports on auditory encoding abnormalities in individuals with Sz, typically using either pure tone stimuli (Chen et al., 2018; Greenwood et al., 2007; Kustermann, Rockstroh, Kienle, Miller, & Popov, 2016; Rosburg, Boutros, & Ford, 2008; Schubring, Popov, Miller, & Rockstroh, 2018; Smith et al., 2010; Turetsky et al., 2008) or steady-state auditory stimuli (J. C. Edgar et al., 2018; Thune et al., 2016). As the primary generators of early auditory encoding processes are in left and right superior temporal gyrus (STG) (J. C. Edgar et al., 2003; Huang et al., 2003), MEG studies have used source localization to examine auditory encoding processes in left and right STG. Figure 2 shows an auditory evoked response to tone stimuli in the left and right auditory cortex (activity measures obtained using single-dipole source localization). The x axis shows that auditory activity is revealed at the millisecond level, and the y axis shows that the strength of the response is measured in units of nano-amps per meter (nA-m). Figure 2 arrows show the typical adult auditory evoked responses at 50 ms and 100 ms.
Figure 2.
Left (green) and right (purple) superior temporal gyrus (primary/secondary auditory cortex) evoked source waveforms to tone stimuli. Arrows show typical adult auditory evoked responses at 50 ms and 100 ms.
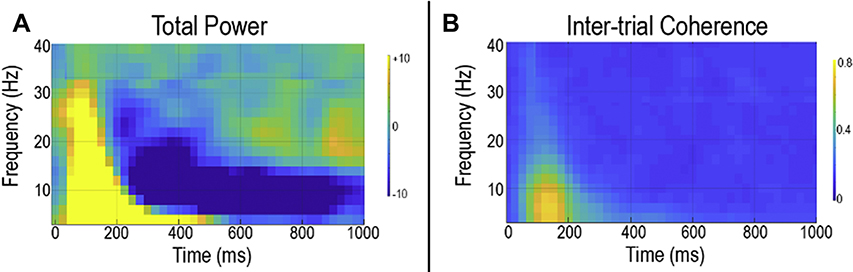
When there are brain areas of a priori interest, such as left and right STG auditory areas, other measures of neural activity are often obtained via computing what are often called time-frequency measures. Figure 3 shows auditory time-frequency plots from the right STG. The time-frequency measures for this figure were obtained via computing the continuous time course for a location within the right STG, and then computing from the single trials (i.e., each click or tone stimulus) the average strength of the response in terms of modulation of frequency (depicted in what is called a total power or an event-related spectral perturbation plot - Figure 3a) and the average phase of the response (depicted in what is called an inter-trial coherence or phase-locking plot - Figure 3b), with “phase” defined in trigonometric functions.
Figure 3.
Total power (a) and inter-trial coherence (b) activity in response to pure tone stimuli. The x axis shows time and the y axis the percent change in activity from baseline. The tone is presented at 0 ms.
Total power plots show the pre- to post-stimulus change in neural activity in terms of frequency modulation. Inter-trial coherence plots show the trial-to-trial similarity in frequency-domain neural activity. Figure 3 illustrates some general features of time-frequency plots. First, the x axis shows activity across time in milliseconds, and the y axis shows neural activity as a function of frequency. Color codes intensity of activity at a given time and frequency. As shown in Figure 3, around 50 to 100 ms after tone onset, increased pre- to post-stimulus neural activity (total power plot) and increased similarity in the trial-to-trial response (inter-trial coherence plot) are observed across a wide range of frequencies. At later times, such increases are observed only in lower frequencies (4 to 20 Hz). The total power plot also shows at later times a decrease in pre- to post-stimulus neural activity in frequencies lower than 20 Hz, with such later deceases (event-related desynchronization) observed in most sensory systems. Depending on the research question of interest, in some cases the full time-frequency plot might be examined, and in some cases a particular time and frequency region of interest is examined (e.g., theta and alpha activity from 50 to 200 ms).
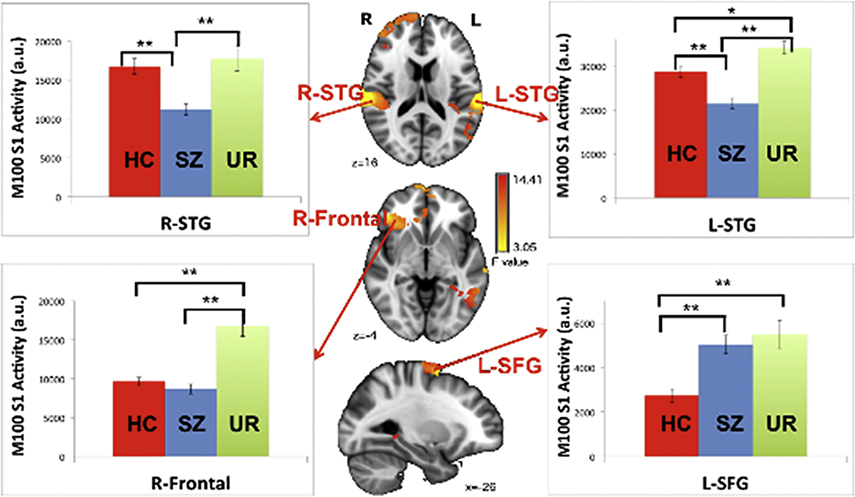
Another example of MEG auditory measures are whole-brain images. Chen et al. (Chen et al., 2018) examined auditory encoding processes in controls and patients with Sz throughout the brain. As shown in Figure 4, Chen et al. observed weaker post-stimulus auditory encoding activity in controls than in patients with Sz in right frontal areas as well as STG regions. Chen et al. also recruited unaffected relatives of the patients with Sz. As shown in Figure 4, auditory encoding abnormalities in the patients and the unaffected relatives of the patients with SZ were shared only in left STG, thus indicating that the identification of markers in Sz must consider specific brain regions. As noted above, MEG shows particular promise in providing region-specific activity with high temporal resolution.
Figure 4.
Analysis of variance (ANOVA) group differences and associated bar charts showing results of 100 ms (M100) simple-effects analyses at the 4 identified regions of interest (ROIs) (*p < .05, **p < .001). From YH Chen, B Howell, JC Edgar. Associations and Heritability of Auditory Encoding, Gray Matter, and Attention in Schizophrenia. Schizophr Bull. 2018 doi:10.1093/schbul/sby111
A few aspects of the whole-brain auditory encoding image are of note. First, whereas whole-brain images of RS activity generally provide a measure of the amount of activity within a specific frequency range across the RS recordings, whole-brain evoked response images focus on a particular time period. For example, Figure 4 shows activity averaged across 80 to 130 ms. Other time periods could be examined to address specific research questions. With respect to translating research findings to the clinic, a major hurdle will be to identify, from a large amount of data, measures that are most clinically informative. Similar to the RS measures, this work will also involve determining whether analogous clinical findings are observed across MEG recording systems and across analysis methods.
Finally, radiologists interested in this area are likely to come across a very active line of research examining the hypothesis that sensory overload and attention dysfunction in patients with schizophrenia is due to inadequate inhibition of redundant information. In many research studies, this is experimentally demonstrated as a failure of individuals with Sz to habituate to repeated auditory stimuli(Adler et al., 1982; Braff, 1993; Yee, Nuechterlein, Morris, & White, 1998). As an example, using the ‘paired-click paradigm’ where subjects are presented two click separated by 500 milliseconds, MEG studies compare primary/secondary auditory cortex activity to the first and second clicks in controls and individuals with Sz. MEG studies have provided mixed findings for the ‘gating’ hypothesis, with some MEG studies showing the individuals with Sz fail to show gating/habituation to the second click (i.e., a similar or larger response to the second than first click)(Thoma et al., 2003), while other studies find no gating/habituation abnormality in Sz(Bachmann et al., 2010; Smith et al., 2010). Although most studies have examined the time-domain M50 and M100 paired-click responses, other studies employ time-frequency analyses to examine the frequencies associated with gating(J. C. Edgar et al., 2008; Popov et al., 2011) (refs). And although the auditory system has been the primary focus of this line of research, researchers have examined gating in the somatosensory system(J. C. Edgar et al., 2005). Finally, within this line of research, an interesting direction that will surely develop over the next decade is the use of therapy (e.g., cognitive training) to normalize auditory processes in Sz(Popov, Rockstroh, Weisz, Elbert, & Miller, 2012; Popova et al., 2018).
Whole-brain Functional Connectivity Maps
Recruitment and management of neural networks, consisting of spatially separate yet functionally related brain regions, is critical to diverse perceptual and higher-order processes. Accordingly, interest is increasing in elucidating the abnormal functional connectivity of such networks in Sz. MEG is particularly well equipped to address coordinated activity of neural networks due to its ability to measure neural oscillatory processes, its insensitivity to distortion by inhomogeneous conductivity in the head, and its excellent spatial resolution given advanced source-reconstruction algorithms and the large number of sensors now commonly used (Brookes et al., 2011; Cohen, 1972; Schoffelen & Gross, 2009; Zumer, Attias, Sekihara, & Nagarajan, 2007). Additionally, co-registering MEG data with high-resolution structural MRI improves the ability to estimate the neural generators of MEG signals by incorporating anatomical information. Findings of both hypo- and hyper-connectivity in Sz have been found across various studies using task-based and RS data, but particular patterns of connectivity may reflect specific symptoms that vary across samples.
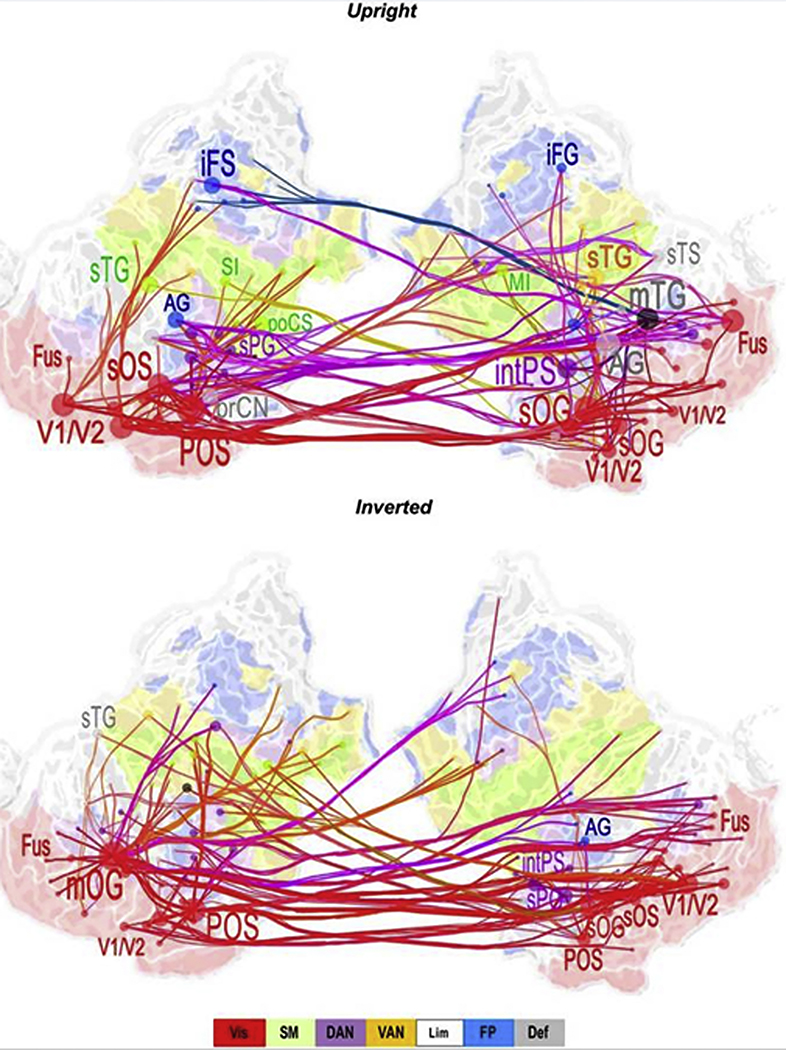
Because disrupted information processing is a hallmark of Sz and related to a variety of symptoms, whole-brain connectivity assessed during various cognitive tasks may be clinically relevant (Ioannides, Poghosyan, Dammers, & Streit, 2004). As an example, Hirvonen et al. (Hirvonen et al., 2017) found that both local and cross-region gamma-band (30–120 Hz) synchronization (a mechanism of connectivity) was lower in Sz patients than in controls during a task requiring perceptual integration. The functional connectivity measures were of clinical interest, with reduced gamma-band synchronization associated with more severe disorganization symptoms. The Figure 5 functional connectivity map is from Hirvonen et al. Although this image might look to some degree like a diffusion image (e.g., a fractional anisotropy brain diffusion map familiar to neuroradiologists), several features of this gamma-band functional connectivity image are of note. First, the functional connectivity information (colored lines) is superimposed on an inflated and flattened cortical surface. This facilitates visualization of the connections between brain areas. Second, measures of functional connectivity were computed for regions of a priori interest. This was done to reduce the number of calculations (from many, many thousands to tens or hundreds). Third, the functional connectivity data were scaled to show only connections with the greatest group difference (in this figure, showing the 200 most significant connections).
Figure 5.
Low gamma (30–40 Hz) band cortical networks stronger for controls than Sz for upright faces (top) and inverted faces (bottom). Graphs display the 200 strongest connections on a flattened, inflated cortical surface. Red = visual network (Vis); Green = sensorimotor network (SM); Purple = dorsal attention network (DAN); Yellow = ventral attention network (VAN); Blue = Frontoparietal network. AG = angular gyrus; iFS/G = inferior frontal sulcus/gyrus; MI = primary motor cortex; mOG = middle occipital gyrus, mTG = middle temporal gyrus; Fus = fusiform gyrus; POS = parieto-occipital sulcus; prCN = precuneus; SI = primary somatosensory cortex; sTG = superior temporal gyrus. From J Hirvonen, M Wibral, JM Palva. Whole-Brain Source-Reconstructed MEG-Data Reveal Reduced Long-Range Synchronization in Chronic Schizophrenia. eNeuro, 2017 4(5).
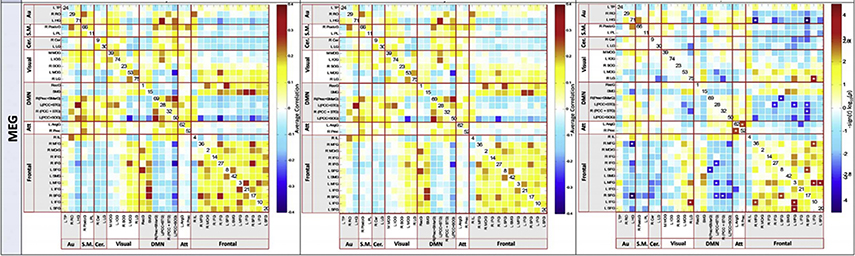
Research investigating RS MEG connectivity also shows abnormal network connectivity in adults with Sz (Cetin et al., 2016; Hinkley et al., 2011; Robinson & Mandell, 2015; Rutter et al., 2013). As an example, using pairwise correlations in ICA-derived RS networks across all frequency bands, Houck et al. (Houck et al., 2017) observed enhanced functional connectivity in frontal networks during RS in Sz patients. Figure 6 from Houck et al. shows a common way of plotting functional connectivity maps. Figure 6 show connectivity results for controls (left), patients (center), and the group difference (right). The x and y axes list brain regions, with the color scale showing the significance of the connection for each pair of brain regions.
Figure 6.
Functional network connectivity (FNC) for MEG, for healthy controls (left column), patients with schizophrenia (center column), and false discovery rate corrected group differences (right column). Color scale describes the p-value after FDR correction. From JM Houck, MS Cetin, AR Mayer. Magnetoencephalographic and functional MRI connectomics in schizophrenia via intra- and inter-network connectivity. Neuroimage. 2017 Jan 15;145(Pt A), 96–106. doi:10.1016/j.neuroimage.2016.10.011
Functional connectivity methods add another layer of complexity to be considered, as the functional connectivity measures that can be computed are nearly infinite, and the clinical research literature has not settled on a standard set of measures. Studies to date primarily examine functional connectivity (amplitude or phase) within a specific frequency band (e.g., connectivity between the strength of occipital gamma and frontal gamma activity). Other connectivity measures are of interest, such as phase-amplitude coupling (PAC) measures that assess how the phase of low-frequency activity in one brain region modulates the strength of high-frequency activity in another brain region (Berman et al., 2012; Canolty et al., 2006; Osipova, Hermes, & Jensen, 2008). The principle is that higher-frequency activity facilitates and reflects the operation of local brain networks and that lower-frequency activity facilitates and reflects control of one region by another (Canolty & Knight, 2010). For example, it has been hypothesized that a failure in theta and alpha activity modulation of gamma activity is a mechanism that fosters poor cognitive interference control in Sz. In support of this proposal, Popov et al. (Popov, Wienbruch, Meissner, Miller, & Rockstroh, 2015) reported weaker coupling of the phase of theta-band activity in anterior cingulate cortex to the amplitude of gamma-band activity in middle frontal gyrus in individuals with Sz than in healthy comparison participants.
Although relationships between disruptions in neural network dynamics and pathological symptoms in Sz are observed in many studies utilizing MEG recordings (as well as those employing EEG and fMRI), findings vary across studies. This variability may correspond to methodological differences in collection and processing of MEG data as well as nosographic variability (Alamian et al., 2017) in line with the growing consensus that traditional psychiatric diagnostic categories are etiologically and mechanistically diverse (e.g., (Yee et al., 2015)). It may be preferable to utilize MEG connectivity measures to assess specific functional and symptom domains and constructs relevant to Sz, as highlighted by the Research Domain Criteria Framework (Kozak & Cuthbert, 2016; Lake, 2017; G. A. Miller, in press).
Despite the diversity of findings in the literature, analysis of MEG connectivity can provide important information regarding neural network dysfunction that disrupts information processing in Sz, resulting in a variety of clinical symptoms and cognitive performance deficits. Although use of MEG connectivity measures in Sz is in its infancy, it is a promising tool for elucidating the unique functional architecture of Sz, including aberrant neural integration and/or segregation, related to clinical presentation. Finally, and analogous to the RS and auditory Sz studies, functional connectivity in Sz has not yet demonstrated the degree of sensitivity and specificity that would be needed for routine clinical use.
General Comments and Future Directions
MEG systems are much less common in and outside of radiology departments than are EEG and MRI systems and thus less available for both clinical and research use. This scarcity is a primary factor holding back clinical application of MEG, with the resulting corollary that the expert user community is small as well. As such, there is not the large established base of EEG users nor the growth of fMRI users pursuing the translational research needed to move MEG technology into the clinic.
Aside from the need to develop a larger user base, as detailed in this Chapter and also Chapter 3, the future of clinical MEG likely depends on increased standardization and a gradual move from a qualitative to a quantitative/actuarial framework. At present, clinical MEG assessment relies primarily on careful and intensive examination of each subject’s MEG data. This qualitative tradition likely reflects the development of clinical use of MEG to date principally as a tool to localize epileptiform activity (see Chapter 2). In such an application, individual aspects of each clinical case (including seizure source, trade-offs of extent of surgical recision vs. risk of functional loss) are critical and not yet sufficiently subject to quantitative standardization. Clinical-theoretical and actuarial approaches can be thought of as extremes on a continuum of quantification (Lezak, 1995). At the qualitative end are assessment approaches built on detailed observations of MEG activity, with particular weight given to human judgment about the manner in which MEG activity is abnormal, but which lack objective standardization. In contrast, actuarial systems rely on statistical evaluations of scores, derived from a standard set of protocols and analysis methods. Whereas at present much more emphasis on quantitative approaches is needed, Lezak (Lezak, 1995) notes that “... to do justice to a field of inquiry as complex as brain-behavior relationships in adult human beings requires an adaptable assessment methodology that incorporates the strengths of both quantitative and qualitative approaches” (pg. 4). Because clinical judgment can be used as an input to such quantitative methods, the field is well positioned for such an integration.
For routine clinical practice, more fundamental than standardization of data-integration methods is standardization of data-acquisition methods. At present, there are no MEG clinical protocols that are standardized beyond individual centers. MEG research exam protocols vary widely as well. In addition, MEG recording systems and associated analysis algorithms vary across labs. For example, for auditory tasks, some labs use insert headphones, whereas other labs use freefield speakers. Even for spontaneous resting data, labs differ in how resting data are obtained. Similar variability is observed in data analysis procedures. Whereas some labs use single dipole strategies to localize auditory cortex activity, the use of whole-brain distributed source localization techniques to obtain whole-brain images is of interest and is growing, with different laboratories using different source localization routines (e.g., beamforming, L1, or L2).
Development of new clinical indications for MEG requires a multi-pronged approach. This will include standardization of protocols, data collection, and data analysis procedures. Whereas such procedural standardization is possible and surely inevitable, some variability will remain as MEG hardware, software, and other site-specific factors (e.g., environmental electromagnetic noise levels) differ across sites. It is hoped that the gradual establishment and validation of standard presentation and analysis protocols will allow merging of data across sites and the development of normative databases. Development of normative databases in turn will foster a quantitative and generalizable approach to clinical interpretation.
Key Points.
This chapter on schizophrenia considers the possible future clinical use of MEG by radiologists via focusing on areas of active research: research studies examining whole-brain resting-state activity, studies examining auditory encoding processes, and studies examining functional connectivity.
Rather than provide a comprehensive review, this chapter considers the types of MEG images that neuroradiologists might one day read as part of a clinical radiology schizophrenia exam.
The use of normative whole-brain MEG databases to identify abnormality at the individual level is highlighted, a procedure that allows for a more quantitative assessment of neural activity.
The clinical payoff in examining activity throughout the brain is identifying abnormalities specific to each patient (e.g., observing abnormalities in superior temporal gyrus or frontal lobes), with individual findings hopefully one day guiding diagnosis and treatment.
Acknowledgements
The authors report no conflicts of interest. This paper was supported in part by NIH grant R01MH107506 (JCE), R21MH098204 (JCE), and R21 NS090192 (JCE), NICHD grant R01HD093776 (JCE), and the Intellectual and Developmental Disabilities Research Center funded by NICHD grant 5U54HD086984 (principal investigator, M. Robinson, PhD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, & Freedman R (1982). Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry, 17(6), 639–654. [PubMed] [Google Scholar]
- Alamian G, Hincapie AS, Pascarella A, Thiery T, Combrisson E, Saive AL, … Jerbi K. (2017). Measuring alterations in oscillatory brain networks in schizophrenia with resting-state MEG: State-of-the-art and methodological challenges. Clin Neurophysiol, 128(9), 1719–1736. doi: 10.1016/j.clinph.2017.06.246 [DOI] [PubMed] [Google Scholar]
- American_Psychiatric_Association. (2013). Diagnostic and statistical manual of mental disorders 5th edition. Washington, DC: American Psychiatric Association. [Google Scholar]
- Andreasen NC (1997). The evolving concept of schizophrenia: from Kraepelin to the present and future. Schizophr Res, 28(2–3), 105–109. [DOI] [PubMed] [Google Scholar]
- Bachmann S, Weisbrod M, Rohrig M, Schroder J, Thomas C, Scherg M, & Rupp A (2010). MEG does not reveal impaired sensory gating in first-episode schizophrenia. Schizophr Res, 121(1–3), 131–138. doi: 10.1016/j.schres.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Belbasis L, Kohler CA, Stefanis N, Stubbs B, van Os J, Vieta E, … Evangelou E (2018). Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta-analyses. Acta PsychiatrScand, 137(2), 88–97. doi: 10.1111/acps.12847 [DOI] [PubMed] [Google Scholar]
- Berger GE, Bartholomeusz CF, Wood SJ, Ang A, Phillips LJ, Proffitt T, … Pantelis C (2017). Ventricular volumes across stages of schizophrenia and other psychoses. Aust N ZJ Psychiatry, 51(10), 1041–1051. doi: 10.1177/0004867417715914 [DOI] [PubMed] [Google Scholar]
- Berman JI, McDaniel J, Liu S, Cornew L, Gaetz W, Roberts TP, & Edgar JC (2012). Variable bandwidth filtering for improved sensitivity of cross-frequency coupling metrics. Brain Connect, 2(3), 155–163. doi: 10.1089/brain.2012.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL (1993). Information processing and attention dysfunctions in schizophrenia. Schizophr Bull, 19(2), 233–259. doi: 10.1093/schbul/19.2.233 [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Hale JR, Zumer JM, Stevenson CM, Francis ST, Barnes GR, … Nagarajan SS (2011). Measuring functional connectivity using MEG: methodology and comparison with fcMRI. Neuroimage, 56(3), 1082–1104. doi: 10.1016/j.neuroimage.2011.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canive JM, Lewine JD, Edgar JC, Davis JT, Miller GA, Torres F, & Tuason VB (1998). Spontaneous brain magnetic activity in schizophrenia patients treated with aripiprazole. Psychopharmacol Bull, 34(1), 101–105. [PubMed] [Google Scholar]
- Canive JM, Lewine JD, Edgar JC, Davis JT, Torres F, Roberts B, … Tuason VB (1996). Magnetoencephalographic assessment of spontaneous brain activity in schizophrenia. Psychopharmacol Bull, 32(4), 741–750. [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, … Knight RT (2006). High gamma power is phase-locked to theta oscillations in human neocortex. Science, 313(5793), 1626–1628. doi: 10.1126/science.1128115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, & Knight RT (2010). The functional role of cross-frequency coupling. Trends Cogn Sci, 14(11), 506–515. doi: 10.1016/j.tics.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin MS, Houck JM, Rashid B, Agacoglu O, Stephen JM, Sui J, … Calhoun VD (2016). Multimodal Classification of Schizophrenia Patients with MEG and fMRI Data Using Static and Dynamic Connectivity Measures. Front Neurosci, 10, 466. doi: 10.3389/fnins.2016.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Howell B, Edgar JC, Huang M, Kochunov P, Hunter MA, … Canive JM (2018). Associations and Heritability of Auditory Encoding, Gray Matter, and Attention in Schizophrenia. Schizophr Bull doi: 10.1093/schbul/sby111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Stone-Howell B, Edgar JC, Huang M, Wootton C, Hunter MA, … Canive JM (2016). Frontal slow-wave activity as a predictor of negative symptoms, cognition and functional capacity in schizophrenia. Br J Psychiatry, 208(2), 160–167. doi: 10.1192/bjp.bp.114.156075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D (1972). Magnetoencephalography: detection of the brain’s electrical activity with a superconducting magnetometer. Science, 175(4022), 664–666. doi: 10.1126/science.175.4022.664 [DOI] [PubMed] [Google Scholar]
- Consortium, S. W. G. o. t. P. G. (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature, 511(7510), 421–427. doi: 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean K, & Murray RM (2005). Environmental risk factors for psychosis. Dialogues Clin Neurosci, 7(1), 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, & Keller MC (2011). A critical review of the first 10 years of candidate gene-byenvironment interaction research in psychiatry. Am J Psychiatry, 168(10), 1041–1049. doi: 10.1176/appi.ajp.2011.11020191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Chen YH, Lanza M, Howell B, Chow VY, Heiken K, … Canive JM (2014). Cortical thickness as a contributor to abnormal oscillations in schizophrenia? Neuroimage Clin, 4, 122–129. doi: 10.1016/j.nicl.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Dipiero M, McBride E, Green HL, Berman J, Ku M, … Roberts TPL (2019). Abnormal maturation of the resting-state peak alpha frequency in children with autism spectrum disorder. Hum Brain Mapp. doi: 10.1002/hbm.24598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Fisk C. L. t., Chen YH, Stone-Howell B, Liu S, Hunter MA, … Miller GA (2018). Identifying auditory cortex encoding abnormalities in schizophrenia: The utility of low-frequency versus 40 Hz steady-state measures. Psychophysiology, 55(8), e13074. doi: 10.1111/psyp.13074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Hanlon FM, Huang MX, Weisend MP, Thoma RJ, Carpenter B, … Miller GA (2008). Superior temporal gyrus spectral abnormalities in schizophrenia. Psychophysiology, 45(5), 812–824. doi: 10.1111/j.1469-8986.2008.00682.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Huang MX, Weisend MP, Sherwood A, Miller GA, Adler LE, & Canive JM (2003). Interpreting abnormality: an EEG and MEG study of P50 and the auditory paired-stimulus paradigm. Biol Psychol, 65(1), 1–20. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Miller GA, Moses SN, Thoma RJ, Huang MX, Hanlon FM, … Canive JM (2005). Cross-modal generality of the gating deficit. Psychophysiology, 42(3), 318–327. doi: 10.1111/j.1469-8986.2005.00292.x [DOI] [PubMed] [Google Scholar]
- Edgar JC, Miller GA (In Press). Identifying neural abnormalities in schizophrenia In Papanicolaou AC, Roberts TPL., Wheless JW (Ed.), Fifty Years of MEG: The Oxford handbook of magnetoencephalography: Oxford University Press. [Google Scholar]
- Fehr T, Kissler J, Moratti S, Wienbruch C, Rockstroh B, & Elbert T (2001). Source distribution of neuromagnetic slow waves and MEG-delta activity in schizophrenic patients. Biol Psychiatry, 50(2), 108–116. [DOI] [PubMed] [Google Scholar]
- Galderisi S, Mucci A, Volpe U, Boutros N (2009). Evidence-based medicine and electrophysiology in schizophrenia. Clin EEG Neurosci, 40, 62–77. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, …Schork NJ (2007). Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Arch Gen Psychiatry, 64(11), 1242–1250. doi: 10.1001/archpsyc.64.11.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grent-’t-Jong T, Gross J, Goense J, Wibral M, Gajwani R, Gumley AI, … Uhlhaas PJ. (2018). Resting-state gamma-band power alterations in schizophrenia reveal E/I-balance abnormalities across illness-stages. Elife, 7. doi: 10.7554/eLife.37799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grent-’t-Jong T, Rivolta D, Sauer A, Grube M, Singer W, Wibral M, & Uhlhaas PJ (2016). MEG-measured visually induced gamma-band oscillations in chronic schizophrenia: Evidence for impaired generation of rhythmic activity in ventral stream regions. Schizophr Res, 176(2–3), 177–185. doi: 10.1016/j.schres.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Guloksuz S, Pries LK, Delespaul P, Kenis G, Luykx JJ, Lin BD, … van Os J (2019). Examining the independent and joint effects of molecular genetic liability and environmental exposures in schizophrenia: results from the EUGEI study. World Psychiatry, 18(2), 173–182. doi: 10.1002/wps.20629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen MG, Nordgaard J, & Jansson LB (2017). Genetics of Schizophrenia: Overview of Methods, Findings and Limitations. Front Hum Neurosci, 11, 322. doi: 10.3389/fnhum.2017.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley LB, Vinogradov S, Guggisberg AG, Fisher M, Findlay AM, & Nagarajan SS (2011). Clinical symptoms and alpha band resting-state functional connectivity imaging in patients with schizophrenia: implications for novel approaches to treatment. Biol Psychiatry, 70(12), 1134–1142. doi: 10.1016/j.biopsych.2011.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Wibral M, Palva JM, Singer W, Uhlhaas P, & Palva S (2017). Whole-Brain Source-Reconstructed MEG-Data Reveal Reduced Long-Range Synchronization in Chronic Schizophrenia. eNeuro, 4(5). doi: 10.1523/ENEURO.0338-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck JM, Cetin MS, Mayer AR, Bustillo JR, Stephen J, Aine C, … Calhoun VD (2017). Magnetoencephalographic and functional MRI connectomics in schizophrenia via intra- and inter-network connectivity. Neuroimage, 145(Pt A), 96–106. doi: 10.1016/j.neuroimage.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MX, Edgar JC, Thoma RJ, Hanlon FM, Moses SN, Lee RR, … Canive JM. (2003). Predicting EEG responses using MEG sources in superior temporal gyrus reveals source asynchrony in patients with schizophrenia. Clin Neurophysiol, 114(5), 835–850. [DOI] [PubMed] [Google Scholar]
- Huang MX, Nichols S, Robb A, Angeles A, Drake A, Holland M, … Lee RR (2012). An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. Neuroimage, 61(4), 1067–1082. doi: 10.1016/j.neuroimage.2012.04.029 [DOI] [PubMed] [Google Scholar]
- Huang MX, Theilmann RJ, Robb A, Angeles A, Nichols S, Drake A, … Lee RR (2009). Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J Neurotrauma, 26(8), 1213–1226. doi: 10.1089/neu.2008.0672 [DOI] [PubMed] [Google Scholar]
- Ioannides AA, Poghosyan V, Dammers J, & Streit M (2004). Real-time neural activity and connectivity in healthy individuals and schizophrenia patients. Neuroimage, 23(2), 473–482. doi: 10.1016/j.neuroimage.2004.06.023 [DOI] [PubMed] [Google Scholar]
- Kozak MJ, & Cuthbert BN (2016). The NIMH Research Domain Criteria Initiative: Background, Issues, and Pragmatics. Psychophysiology, 53(3), 286–297. doi: 10.1111/psyp.12518 [DOI] [PubMed] [Google Scholar]
- Kustermann T, Rockstroh B, Kienle J, Miller GA, & Popov T (2016). Deficient attention modulation of lateralized alpha power in schizophrenia. Psychophysiology, 53(6), 776–785. doi: 10.1111/psyp.12626 [DOI] [PubMed] [Google Scholar]
- Lake JI, Yee CM, Miller GA (2017). Misunderstanding RDoC. Zietschriftfur Psychologie, 225(3), 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewine JD, Orrison WW (1995). Magnetoencephalography and magnetic source imaging. 369–417. [Google Scholar]
- Lezak MD (1995). Neuropsychological Assessment. New York: Oxford University Press. [Google Scholar]
- Lisman J (2012). Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol, 22(3), 537–544. doi: 10.1016/j.conb.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Urbano FJ, Leznik E, Ramirez RR, & van Marle HJ (2005). Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci, 28(6), 325–333. doi: 10.1016/j.tins.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Lund TR, Sponheim SR, Iacono WG, & Clementz BA (1995). Internal consistency reliability ofresting EEG power spectra in schizophrenic and normal subjects. Psychophysiology, 32(1), 66–71. [DOI] [PubMed] [Google Scholar]
- Miller GA (in press). Comments on Kendler’s “The Impact of Faculty Psychology and Theories of Psychological Causation on the Origins of Modern Psychiatric Nosology. New York: Cambridge University Press. [Google Scholar]
- Miller GA, Rockstroh B (2013). Endophenotypes in psychopathology research: Where do we stand. Annual Review of Clinical Psychology, 9, 177–213. [DOI] [PubMed] [Google Scholar]
- Miller GA, Rockstroh B (2016). Progress and prospects for endophenotypes for schizophrenia in the time of genemics, epigentics, oscillatroy dynamics, and RDOC
- Miskovic V, Ma X, Chou CA, Fan M, Owens M, Sayama H, & Gibb BE (2015). Developmental changes in spontaneous electrocortical activity and network organization from early to late childhood. Neuroimage, 118, 237–247. doi: 10.1016/j.neuroimage.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipova D, Hermes D, & Jensen O (2008). Gamma power is phase-locked to posterior alpha activity. PLoS One, 3(12), e3990. doi: 10.1371/journal.pone.0003990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C, Yucel M, Wood SJ, Velakoulis D, Sun D, Berger G, … McGorry PD (2005). Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull, 31(3), 672–696. doi: 10.1093/schbul/sbi034 [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Lehmann D, Koenig T, Kochi K, Merlo MC, Hell D, & Koukkou M (1999). Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Res, 90(3), 169–179. [DOI] [PubMed] [Google Scholar]
- Perala J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsa E, Pirkola S, … Lonnqvist J (2007). Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry, 64(1), 19–28. doi: 10.1001/archpsyc.64.1.19 [DOI] [PubMed] [Google Scholar]
- Popov T, Jordanov T, Weisz N, Elbert T, Rockstroh B, & Miller GA (2011). Evoked and induced oscillatory activity contributes to abnormal auditory sensory gating in schizophrenia. Neuroimage, 56(1), 307–314. doi: 10.1016/j.neuroimage.2011.02.016 [DOI] [PubMed] [Google Scholar]
- Popov T, Rockstroh B, Weisz N, Elbert T, & Miller GA (2012). Adjusting brain dynamics in schizophrenia by means of perceptual and cognitive training. PLoS One, 7(7), e39051. doi: 10.1371/journal.pone.0039051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov T, Wienbruch C, Meissner S, Miller GA, & Rockstroh B (2015). A mechanism of deficient interregional neural communication in schizophrenia. Psychophysiology, 52(5), 648–656. doi: 10.1111/psyp.12393 [DOI] [PubMed] [Google Scholar]
- Popova P, Rockstroh B, Miller GA, Wienbruch C, Carolus AM, & Popov T (2018). The impact of cognitive training on spontaneous gamma oscillations in schizophrenia. Psychophysiology, 55(8), e13083. doi: 10.1111/psyp.13083 [DOI] [PubMed] [Google Scholar]
- Radua J, Ramella-Cravaro V, Ioannidis JPA, Reichenberg A, Phiphopthatsanee N, Amir T, … Fusar-Poli P. (2018). What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry, 17(1), 49–66. doi: 10.1002/wps.20490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SE, & Mandell AJ (2015). Mutual Information in a MEG complexity measure suggests regional hyper-connectivity in schizophrenic probands. Neuropsychopharmacology, 40(1), 251–252. doi: 10.1038/npp.2014.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockstroh B, Watzl H, Kowalik ZJ, Cohen R, Sterr A, Muller M, & Elbert T (1997). Dynamical aspects of the EEG in different psychopathological states in an interview situation: a pilot study. Schizophr Res, 28(1), 77–85. [DOI] [PubMed] [Google Scholar]
- Rockstroh BS, Wienbruch C, Ray WJ, & Elbert T (2007). Abnormal oscillatory brain dynamics in schizophrenia: a sign of deviant communication in neural network? BMC Psychiatry, 7, 44. doi: 10.1186/1471-244X-7-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Arciniegas DB, Teale PD, & Reite ML (1999). Magnetoencephalography and magnetic source imaging: technology overview and applications in psychiatric neuroimaging. CNS Spectr, 4(8), 37–43. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Boutros NN, & Ford JM (2008). Reduced auditory evoked potential component N100 in schizophrenia--a critical review. Psychiatry Res, 161(3), 259–274. doi: 10.1016/j.psychres.2008.03.017 [DOI] [PubMed] [Google Scholar]
- Rutter L, Carver FW, Holroyd T, Nadar SR, Mitchell-Francis J, Apud J, … Coppola R (2009). Magnetoencephalographic gamma power reduction in patients with schizophrenia during resting condition. Hum Brain Mapp, 30(10), 3254–3264. doi: 10.1002/hbm.20746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter L, Nadar SR, Holroyd T, Carver FW, Apud J, Weinberger DR, & Coppola R (2013). Graph theoretical analysis of resting magnetoencephalographic functional connectivity networks. Front Comput Neurosci, 7, 93. doi: 10.3389/fncom.2013.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelen JM, & Gross J (2009). Source connectivity analysis with MEG and EEG. Hum Brain Mapp, 30(6), 1857–1865. doi: 10.1002/hbm.20745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubring D, Popov T, Miller GA, & Rockstroh B (2018). Consistency of abnormal sensory gating in first-admission and chronic schizophrenia across quantification methods. Psychophysiology, 55(4). doi: 10.1111/psyp.13006 [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, & McCarley RW (2001). A review of MRI findings in schizophrenia. Schizophr Res, 49(1–2), 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S, Uhlhaas PJ, Essex B, Halpin S, Schall U, & Carr V (2006). Perceptual organization in first episode schizophrenia and ultra-high-risk states. Schizophr Res, 83(1), 41–52. doi: 10.1016/j.schres.2006.01.003 [DOI] [PubMed] [Google Scholar]
- Smiley JF, Rosoklija G, Mancevski B, Mann JJ, Dwork AJ, & Javitt DC (2009). Altered volume and hemispheric asymmetry of the superficial cortical layers in the schizophrenia planum temporale. Eur J Neurosci, 30(3), 449–463. doi: 10.1111/j.1460-9568.2009.06838.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Edgar JC, Huang M, Lu BY, Thoma RJ, Hanlon FM, … Canive JM. (2010). Cognitive abilities and 50- and 100-msec paired-click processes in schizophrenia. Am J Psychiatry, 167(10), 1264–1275. doi: 10.1176/appi.ajp.2010.09071059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsen RJ, van’t Klooster BJ, van der Molen MW, van Leeuwen HM, & Licht R (1997). Growth spurts in brain maturation during middle childhood as indexed by EEG power spectra. Biol Psychol, 44(3), 187–209. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Clementz BA, Iacono WG, & Beiser M (1994). Resting EEG in first-episode and chronic schizophrenia. Psychophysiology, 31(1), 37–43. [DOI] [PubMed] [Google Scholar]
- Szava S, Valdes P, Biscay R, Galan L, Bosch J, Clark I, & Jimenez JC (1994). High resolution quantitative EEG analysis. Brain Topogr, 6(3), 211–219. [DOI] [PubMed] [Google Scholar]
- Teale P, Reite M, Rojas DC, Sheeder J, & Arciniegas D (2000). Fine structure of the auditory M100 in schizophrenia and schizoaffective disorder. Biol Psychiatry, 48(11), 1109–1112. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Hanlon FM, Moses SN, Edgar JC, Huang M, Weisend MP, … Canive JM (2003). Lateralization of auditory sensory gating and neuropsychological dysfunction in schizophrenia. Am J Psychiatry, 160(9), 1595–1605. doi: 10.1176/appi.ajp.160.9.1595 [DOI] [PubMed] [Google Scholar]
- Thune H, Recasens M, & Uhlhaas PJ (2016). The 40-Hz Auditory Steady-State Response in Patients With Schizophrenia: A Meta-analysis. JAMA Psychiatry, 73(11), 1145–1153. doi: 10.1001/jamapsychiatry.2016.2619 [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Greenwood TA, Olincy A, Radant AD, Braff DL, Cadenhead KS, … Calkins ME (2008). Abnormal auditory N100 amplitude: a heritable endophenotype in first-degree relatives of schizophrenia probands. Biol Psychiatry, 64(12), 1051–1059. doi: 10.1016/j.biopsych.2008.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes P, Valdes M, Carballo JA, Alvarez A, Diaz GF, Biscay R, … Quesada ME (1992). QEEG in a public health system. Brain Topogr, 4(4), 259–266. [DOI] [PubMed] [Google Scholar]
- van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, … Turner JA (2016). Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry, 21(4), 547–553. doi: 10.1038/mp.2015.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Kenis G, & Rutten BP (2010). The environment and schizophrenia. Nature, 468(7321), 203–212. doi: 10.1038/nature09563 [DOI] [PubMed] [Google Scholar]
- Weinberger DR (1988). Schizophrenia and the frontal lobe. Trends Neurosci, 11(8), 367–370. [DOI] [PubMed] [Google Scholar]
- Wienbruch C, Moratti S, Elbert T, Vogel U, Fehr T, Kissler J, … Rockstroh B (2003). Source distribution of neuromagnetic slow wave activity in schizophrenic and depressive patients. Clin Neurophysiol, 114(11), 2052–2060. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Hernandez OO, Asherin RM, Teale PD, Reite ML, & Rojas DC (2008). Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb Cortex, 18(2), 371–378. doi: 10.1093/cercor/bhm062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, & Herrmann WM (2000). Frontal dysfunction in schizophrenia--a new electrophysiological classifier for research and clinical applications. Eur Arch Psychiatry Clin Neurosci, 250(4), 207–214. [DOI] [PubMed] [Google Scholar]
- Yee CM, Javitt DC, & Miller GA (2015). Replacing DSM Categorical Analyses With Dimensional Analyses in Psychiatry Research: The Research Domain Criteria Initiative. JAMA Psychiatry, 72(12), 1159–1160. doi: 10.1001/jamapsychiatry.2015.1900 [DOI] [PubMed] [Google Scholar]
- Yee CM, Nuechterlein KH, Morris SE, & White PM (1998). P50 suppression in recent-onset schizophrenia: clinical correlates and risperidone effects. J Abnorm Psychol, 107(4), 691–698. doi: 10.1037//0021-843x.107.4.691 [DOI] [PubMed] [Google Scholar]
- Zeev-Wolf M, Levy J, Jahshan C, Peled A, Levkovitz Y, Grinshpoon A, & Goldstein A (2018). MEG resting-state oscillations and their relationship to clinical symptoms in schizophrenia. Neuroimage Clin, 20, 753–761. doi: 10.1016/j.nicl.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumer JM, Attias HT, Sekihara K, & Nagarajan SS (2007). A probabilistic algorithm integrating source localization and noise suppression for MEG and EEG data. Neuroimage, 37(1), 102–115. doi: 10.1016/j.neuroimage.2007.04.054 [DOI] [PubMed] [Google Scholar]