Abstract
Background:
Residual immune activation after successful antiretroviral therapy (ART) in HIV-1 infected patients is associated with the increased risk of complications. Cytokines, both soluble and extracellular vesicle (EV)-associated, may play an important role in this immune activation.
Setting:
Ex vivo tissues were infected with X4LAI04 or R5SF162 HIV-1. Virus replicated for 16 days, or tissues were treated with the anti-retroviral drug ritonavir.
Methods:
Viral replication and production of 33 cytokines in soluble and EV-associated forms were measured with multiplexed bead-based assays.
Results:
Both variants of HIV-1 efficiently replicated in tissues and triggered upregulation of soluble cytokines, including IL-1β, IL-7, IL-18, IFN-γ, MIP-1α, MIP-1β, and RANTES. A similar pattern was observed in EV-associated cytokine release by HIV-infected tissues. Additionally, TNF-α and RANTES demonstrated a significant shift to more soluble form compared to EV-associated. Ritonavir treatment efficiently suppressed viral replication; however, both soluble and EV-associated cytokines remained largely upregulated after 13 days of treatment. EV-associated cytokines were more likely to remain elevated after ART. Treatment of uninfected tissues with ritonavir itself did not affect cytokine release.
Conclusions:
We demonstrated that HIV-1 infection of ex vivo lymphoid tissues resulted in their immune activation as evaluated by upregulation of various cytokines, both soluble and EV-associated. This upregulation persisted despite inhibition of viral replication by ART. Thus, similar to in vivo, HIV-1 infected human tissues ex vivo continue to be immune activated after viral suppression, providing a new laboratory model to study this phenomenon.
Keywords: cellular factors/cytokines, HIV, ART, extracellular vesicles, ex vivo tissues
Introduction
Cytokines, cell signaling proteins that mediate cell-to-cell communication in multicellular organisms 1, are an essential part of immune responses. Cytokines were considered to be classical soluble factors, but recently, it was found that they can be associated with extracellular vesicles (EVs) 2,3. Soluble cytokines form complex networks that are altered in many diseases including cancer, autoimmune disorders, pregnancy complications, and viral infections 3,4, in particular, in HIV-1 disease.
Currently, a combination of antiretroviral therapy (ART) has proven to be efficient in suppressing viral replication 5–7. However, lengthy suppression of HIV-1 replication by ART is associated with the increased risk of complications, including neurological and cardiovascular diseases 7–9. These diseases seem to be related to immune activation in patients undergoing ART. Cytokines may play an important role in this residual immune activation 10, however the mechanisms of this phenomenon are largely unknown. To decipher them, it is necessary to develop an ex vivo laboratory-controlled system reflecting what happens in vivo. Here, we report on such a system.
As an experimental model we used ex vivo human lymphoid tissues where critical events in HIV-1 infection occur in vivo. We found that HIV-1 infection in these tissues led to upregulation of several key cytokines, both in soluble and EV-associated forms. ART, while suppressing HIV, did not restore cytokine production back to control levels.
Materials and Methods
Sample preparation and storage
Tonsillar tissues (n = 8) were obtained from routine tonsillectomies at the Children’s National Medical Center in Washington DC as anonymous pathological samples according to an Institutional Review Board approved protocol. Healthy tonsil tissues were dissected and cultured as previously described 11. Medium was changed at day 3, 6, 9, 12 and 16, samples were collected and centrifuged at 2000 × g for 10 minutes to remove any residual cells and then frozen at −80 °C.
HIV infection and antiretroviral treatment
Tonsils were infected with HIV-1 strains, X4LAI04 or R5SF162, at the beginning of culture as previously described 11. HIV was allowed to replicate for 16 days, or tissues were treated with a protease inhibitor, 5μM of ritonavir, beginning at day 3 following HIV inoculation, and subsequently at every medium change.
Evaluation of HIV-1 replication
We evaluated HIV-1 replication in tissue by measurement of HIV-1 p24gag antigen in the culture medium of tonsil cultures, using a cytometric bead assay, as described previously 12.
Preparation of EV fractions
Culture supernatant samples were treated with ExoQuick TC (System Biosciences, Palo Alto, CA) according to manufacturer’s protocols. The EV pellet was resuspended in 1X PBS in the original volume. Cytokines were measured on EV-free supernatant and intact and lysed (1% Triton X-100) EVs.
NanoSight measurement of EVs
Aliquots of supernatant from all conditions at day 9 from three representative tissues were characterized with Nanoparticle Tracking Analysis software using a NanoSight NS300 (Malvern, UK). Two video captures of 60 seconds each were used to generate average concentration (EV/ml) and particle size (nm)(mean± SEM).
Western Blot characterization of EVs
Total proteins were extracted from EV pellets with RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific, Waltham, MA). 10 μg of proteins were loaded on a 4–20% precast polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA) and separated by SDS-PAGE, then transferred to PVDF membranes. Proteins were detected with anti-CD63, anti-Rab27A, anti-TSG101 and anti-Calnexin (Thermo Fisher Scientific), followed by species specific horse-radish peroxidase labeled antibodies (Bio-Rad) and signal detection by V3 Western Workflow™ (Bio-Rad).
Cytokine measurement
In-house multiplexed bead-based assay were used to measure 33 cytokines as described previously: Interleukin-1α (IL-1a), IL-1β, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IL-15, IL-16, IL-17, IL-18, IL-21, IL-22, IL-33, Calgranulin A (S100A8), Eotaxin (CCL11), granulocyte-macrophage colony-stimulating factor (GM-CSF), growth regulated-α (GRO-α or CXCL1), interferon gamma (IFN-γ), interferon-inducible protein 10 (IP-10 or CXCL10), interferon-inducible T-cell alpha chemoattractant (ITAC or CXCL11), macrophage colony-stimulating factor (M-CSF), monocyte chemoattractant protein-1 (MCP-1 or CCL2), monokine induced by IFN-γ (MIG or CXCL9), macrophage inflammatory protein-1α (MIP-1α or CCL3) MIP-1β (CCL4), MIP-3α (CCL20), regulated on activation normally T-cell expressed and secreted (RANTES or CCL5), transforming growth factor-β (TGF-β) and tumor necrosis factor-α (TNF-α) 3. Lower limits of detection for analytes are listed in Table 1, Supplemental Digital Content. EV-free supernatants, intact EV samples and lysed (1% Triton X-100 final) EV samples were run in separate wells, and standard curves were also generated with and without detergent. Intact EV measurements reflect EV surface associated cytokine, EV encapsulated cytokines are calculated as EV lysed minus EV surface. Total EV-associated cytokine equals EV surface plus EV encapsulated. Data was acquired and analyzed on a BioPlex 200 with Bioplex Manager software (BioRad).
Cytokine bioassay
Cell line-based cytokine driven proliferation assays were used to test bioactivity of cytokines as previously described 3. Ultracentrifugation (WX ultra 80, TH660 rotor, Thermo Fisher Scientific) of culture supernatants at 100,000 x g for 70 min at 15°C was performed to obtain supernatants free of EVs and EV fractions (washed pellet resuspended in PBS at 4X concentration). TF-1 (ATCC), a human erythroleukemic cell line, was used to measure responsiveness to IL-6, and MC/9 (ATCC), a mouse mast cell line, was used to measure activity of IL-10. Briefly, 75 μl of cells at a concentration of 2 × 105 cells/ml per well were added to 96 well flat bottom plates and then treated with 25 μl of supernatant free of EVs or EV fractions from X4LAI.04 infected or X4LAI.04 ART infected tissues at day 9. Positive control wells consisted of cells with complete growth medium (plus 10ng/ml of IL-6 for TF-1 and plus 10ng/ml IL-10 for MC/9), and negative controls consisted of cells with complete medium without additional cytokines. Cells were incubated for 48 hours at 37°C and 5% CO2. Metabolic activity was measured by an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) based cell growth determination kit assay (Sigma). Results were expressed as % of negative control.
Statistical Analysis
Results from all timepoints were analysed by pairwise comparison with Wilcoxon signed-rank test with Benjamini-Hochberg correction for multiple comparison between different pairs of treatments in a day-to-day manner. Values of p < 0.05 were considered statistically significant; graphs are presented with p-values in log10-scale. Results analyzed for single timepoints are represented as means ± SEM, and statistical significances were determined by two-tailed paired Student’s t-test using Microsoft Excel version 16.15.
Results
HIV-1 infection and anti-retroviral treatment of ex vivo human lymphoid tissues
Tonsillar explants were inoculated with HIV-1 strains, X4LAI04 or R5SF162, at the beginning of culture and became productively infected in agreement with earlier reports 13,14. Between days 6 to 16 of post-inoculation, X4LAI04-infected tissues released on average 416.92 ± 168.55 ng/ml p24 into culture medium, while R5SF162 -infected tissues released on average 340.57 ± 92.64 ng/ml p24 into culture medium. Treatment with ritonavir inhibited viral replication by 99.66 ± 0.22% and 99.74 ± 0.09%, respectively in X4LAI04 and R5SF162 infected tissues (Figure 1).
Fig. 1. Cumulative p24 production by HIV-1 infected human lymphoid tissues ex vivo.
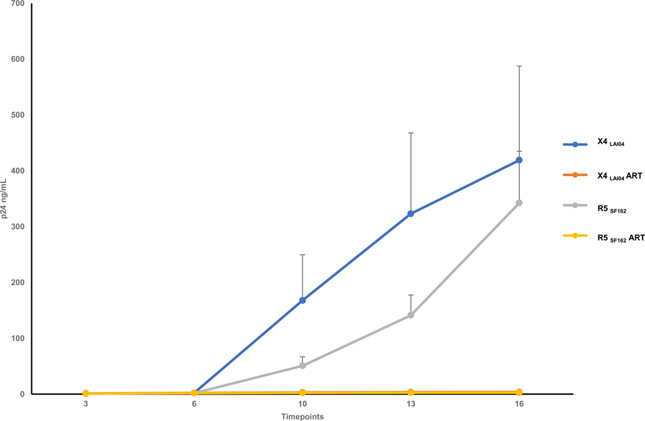
Tissue blocks were inoculated with HIV-1 X4LAI04 or R5SF162 variants and incubated for 16 days. Matched tissue blocks were treated with ritonavir (5μM) at day 3 and ritonavir was added with every medium change through day 16. Cumulative HIV-1 replication as evaluated by p24 measurement is shown (Mean ± SEM, n=8).
Characterization of EVs from ex vivo lymphoid tissue cultures
We quantified EV released by ex vivo tissues in infected, ritonavir-treated and control tissues. Tonsils released on average 3.14 ± 0.35 × 1010 EVs/ml with an average size of 160±5.75 nm and no significant differences were observed between conditions (see Table 2, Supplemental Digital Content, representative histogram Fig. 1 Supplemental Digital Content). EVs from all conditions were also characterized by Western blot, and the presence of CD63, Rab27A, and TSG101 was confirmed (Fig. 2 Supplemental Digital Content). The presence of calnexin was also noted, which is a negative marker for exosomes, but may be present on other vesicles in our preparations.
Cytokine release by HIV-1 infected ex vivo human lymphoid tissue
We measured the concentrations of 33 soluble and EV-associated cytokines released by HIV-1 infected and uninfected tissues over 16 days (Table 3 and 4, Supplemental Digital Content). HIV infection led to increases in a number of cytokines beginning at day 3 post-inoculation and continuing throughout the culture length. Over 16 days of culture, X4LAI04 infection significantly increased the production of soluble IL-1β, IL-2, IL-7, IL-12, IL-18, IL-21, GM-CSF, IFN-γ, M-CSF, MIP-1α, MIP-1β, RANTES and TNF-α compared to uninfected tissues (p<0.05, n=8, cumulative values in Table 3 Supplemental Digital Content, statistical comparisons in Fig. 2). R5SF162 infection resulted in significant increases in soluble IL-1β, IL-7, IL-18, IFN-γ, MIP-1α, MIP-1β, RANTES (p<0.05, n=8, Fig. 2). HIV-1 infection also led to significant increases in EV-associated cytokines: IL-2, IL-7, GRO-α, IFN-γ, M-CSF, MIP-1α, MIP-1β, RANTES and TNF-α with X4LAI04 infection, and MIP-1α, MIP-1β, and RANTES with R5SF162 (p<0.05, n=8, cumulative values in Table 4, Supplemental Digital Content, statistical comparisons in Fig. 2).
Fig. 2. Comparison of cytokine levels between uninfected, HIV-1 infected, and antiretroviral-treated human lymphoid tissues ex vivo.
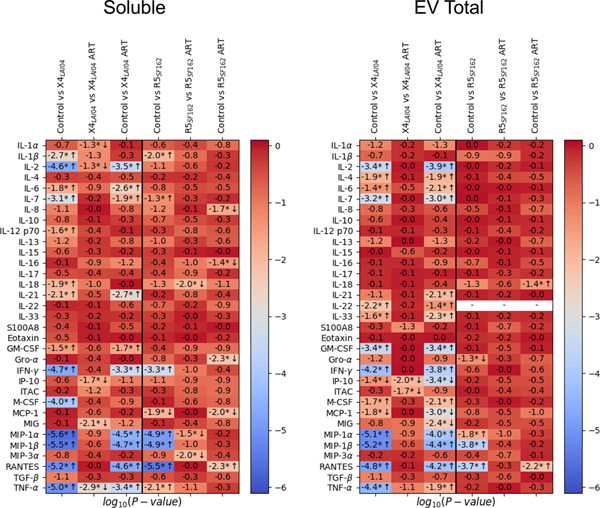
Presented are p-values for the Wilcoxon signed-rank test for comparison of the levels of soluble and EV-associated cytokines between the uninfected (control) group, HIV-1 infected (16 days, X4LAI04 and R5SF162), and HIV-1 infected and treated for 13 days with 5μM ritonavir (X4LAI04 ART and R5SF162 ART); p-values are presented in log10-scale with Benjamini-Hochberg correction (* indicates significance, n=8, arrows indication direction of change up or down).
HIV-1 infection altered the distribution of cytokines between soluble and EV-associated forms. On day 3 post infection, cytokines tended to increase in soluble form, reaching statistical significance for RANTES and TNF-α for both viruses (p<0.05, n=8, Figure 3a). At day 16, cytokines tended to increase in EV-associated form with X4LAI04 infection, reaching significance for IL-2, IFN-γ, M-CSF, MIP-1α, and TNF-α; however, R5SF162 led to a shift of more soluble TNF-α (p<0.05, n=8, Fig. 3b).
Fig. 3. Cytokine distribution between soluble and EV compartments.
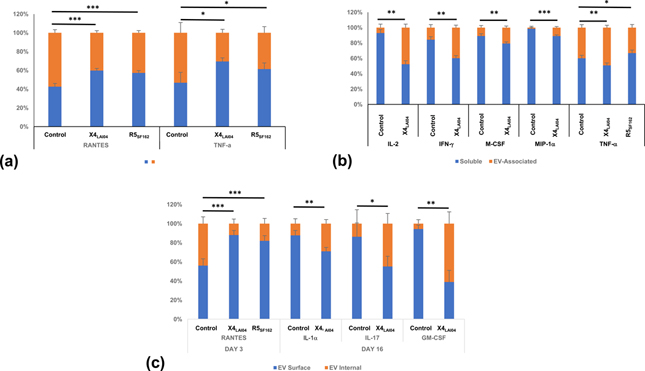
Cytokines were measured in soluble and EV-associated forms at various timepoints and ratios were determined between the cytokine concentrations in these two forms. (a) day 3; (b) day 16. (c) Cytokines encapsulated in EVs and associated with EV surface were measured at various timepoints and ratios between these amounts were determined. Mean ± SEM, n=8, *p<0.05, **p<0.01, ***p<0.001.
HIV-1 infection also significantly changed the distribution of some of EV-associated cytokines between the surface and internal space of EVs: RANTES distribution was shifted towards EV surface in both X4LAI04 and R5SF162 infections compared to matched uninfected tissues early in infection, while IL-1α, IL-17 and GM-CSF distribution shifted in the opposite direction late in X4LAI04 infection (p<0.05, n=8, Fig. 3c).
Cytokine release by HIV-1 infected lymphoid tissue ex vivo after antiretroviral treatment
Suppression of HIV-1 replication by ART does not reverse cytokine upregulation by HIV-1 infection. Most of the cytokines elevated in HIV-1 infected tissues remained upregulated after 13 days of ART. Comparison of HIV-1 infection and HIV-1 ART over the total length of culture demonstrated that few cytokines are significantly reduced with ART; and comparison of HIV-1 ART to control showed that many cytokines are likewise still upregulated (Fig. 2).
We also evaluated cytokines at the last day of culture, after maximal exposure to ART. The mean concentrations of many soluble cytokines were still elevated at least 1.5 times over control tissues: IL-2, IL-18, IL-21, S100A8, MIP-1α, MIP-1β, and RANTES for X4LAI04, and all of these remained elevated with ART treatment except for IL-18 (Fig. 4a, mean concentrations in Table 5, Supplemental Digital Content). For R5SF162 soluble IL-2, IL-7, IL-13, IL-18, IL-21, MIP-1α, MIP-1β, RANTES and TNF-α were elevated at least 1.5 times over control tissues at the final day of culture, and IL-2, IL-7, IL-13, and IL-21 remained elevated with R5SF162 ART (Fig. 4b).
Fig. 4. Soluble and EV-associated cytokines remained elevated after HIV-1 replication was suppressed by ART.
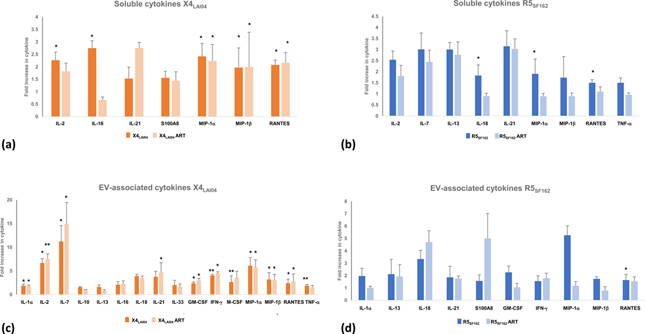
At day 16 of culture, cytokines were measured and their concentrations in uninfected, HIV-1 infected (X4LAI and R5SF162), and HIV-1 infected ritonavir-treated (X4LAI04 ART and R5SF162 ART) were compared. (a, b) soluble cytokines. (c, d) EV-associated cytokines. Presented are cytokines that remained at least 1.5-fold higher in HIV-1 infected compared to uninfected tissues. Mean ± SEM, n=8, *p<0.05, **p<0.01.
In the EV-associated form, even more cytokines remained at least 1.5 times higher than control cultures: IL-1α, IL-2, IL-7, IL-10, IL-13, IL-16, IL-18, IL-21, IL-33, GM-CSF, IFN-γ, M-CSF, MIP-1α, MIP-1β, and RANTES and TNF-α for X4LAI04; and only IL-10 and IL-13 were no longer elevated with X4LAI04 (Fig. 4c). IL-1α, IL-13, IL-18, IL-21, S100A8, GM-CSF, IFN-γ, MIP-1α, MIP-1β, and RANTES were elevated in R5SF162, and only IL-1α, GM-CSF, MIP-1α, and MIP-1β decreased with R5SF162 ART (Fig. 4d).
Cytokine release by uninfected lymphoid tissue ex vivo after antiretroviral treatment
We investigated whether ART itself may be responsible for elevating cytokines in the absence of HIV-1 infection by treating uninfected cultures with ritonavir in the same manner as HIV-1 ART cultures. After 13 days of treatment with ritonavir, tissues had small but significant decreases in soluble IL-1α and ITAC (10.1 and 30.8%, respectively; n=8, p<0.05) and in EV-associated IL-16, IP-10 and MCP-1 (21.5 – 39.2%; n=8, p<0.05) compared to matched control tissues (Fig. 5).
Fig. 5. Cytokines in uninfected tissues treated with ritonavir were not upregulated.
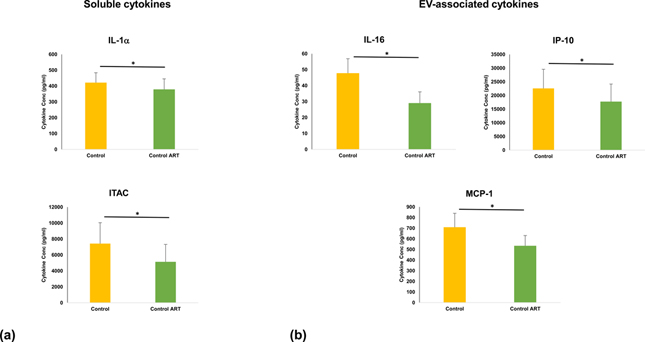
After 13 days of ART, cytokines were measured and their amounts in untreated (control) and matched ritonavir-treated (control ART) were compared. (a) soluble cytokines, (b) EV-associated cytokines. Mean ± SEM, n=8.
Soluble and EV-associated cytokines trigger responses in sensitive cells
We investigated whether these upregulated cytokines in both soluble and EV-associated forms are biologically active. To answer this question, indicator cell lines TF-1 and MC/9, that are dependent on particular cytokines for their proliferation 15, were treated with soluble and EV-associated cytokines from culture supernatants of X4LAI.04 infected or X4LAI.04 infected ART treated tissues at day 9. We found that both supernatants and EVs were active in eliciting responses from sensitive cells. TF-1 cells were 309.5 ± 28.1% and 307.2 ± 31.2% and MC/9 cells were 159.9 ± 8.9% and 294.0 ± 1.1% of negative control cells (no stimulation) for X4LAI.04 infected and X4LAI.04 infected ART treated tissue supernatants respectively (n=3, p<0.01). In response to EV fractions, TF-1 cells were 145.6 ± 10.3% and 141.8 ± 24.0% and MC/9 cells were 217.4 ± 5.3 % and 202.8 ± 2.8% of negative controls for X4LAI.04 infected and X4LAI.04 infected ART treated EV fractions respectively (n=3, p<0.05).
Discussion
Immune activation is now considered to be a driving force of HIV-1 disease 16. Moreover, residual immune activation was detected in patients in which HIV-1 replication was suppressed by ART for many years. This improper immune activation is associated with the development of various pathologies ~15 years earlier than in control population 17. Numerous studies demonstrated that HIV-1 triggered immune activation is associated with the upregulation of many cytokines and with the changes in the entire cytokine network 18,19. Some of these cytokines remain upregulated after patients’ HIV-1 infection was suppressed by ART and may contribute to the development of the above-mentioned pathologies 20. A laboratory-controlled experimental model is needed to study mechanisms of HIV-1 triggered immune activation. Our work describes such a model.
We evaluated 33 cytokines released by donor-matched human lymphoid tissues ex vivo productively infected with a prototypic X4 (LAI.04) or a prototypic R5 (SF162) HIV-1 over 16 days of infection and treated or not with ritonavir. We evaluated not only soluble cytokines but also recently discovered 3 “insoluble” cytokines, namely those associated with EVs.
EVs are released by virtually all cells in the human body and in viral infection EVs may incorporate viral-encoded molecules 21,22. These EVs could potentially cause inflammation, neurodegenerative disorders and immunological dysfunction 23–29. In ART treated HIV-1 patients EVs carry proteins related to immune activation 30.
To study the changes in the free and EV-associated cytokine network in HIV-1 infection and to investigate the effects of ART on these processes, in the present work we used a system of human lymphoid tissues ex vivo that maintain their cytoarchitecture and cell-cell interactions. It more faithfully reproduces important aspects of the in vivo situation than isolated cell cultures, thus constituting an adequate system to study tissue pathogenesis of various pathogens 11,13,14. In our current work, we used a single drug, rather than drug combinations that are used in vivo to prevent evolving of drug-resistant variants, since such variants do not seem to evolve ex vivo during the time-course of the experiment 31.
HIV-1 infection with both X4LAI04 and R5SF162 triggered significant upregulation of many soluble cytokines, in particular IL-1β, IL-7, IL-18, IFN-γ, MIP-1α, MIP-1β, and RANTES, suggesting the importance of these cytokines for general HIV-1 infection. Commonly, these cytokines are involved in the immune response to viral infection with proinflammatory and chemoattractive functions 32,33. IL-2, IL12, IL-21, GM-CSF, M-CSF, and TNF-α were also increased in X4LAI04 infection. Difference in cytokine upregulation between viral strains may be related to the difference in the CD4+ T cell populations infected with these viruses: while X4LAI04 infects a wide population of CD4+ T cells, R5SF162 infects only a subpopulation of these cells that express CCR5 14. A number of the above-mentioned cytokines that upon HIV-1 infection were upregulated in soluble form were upregulated in EV-associated form as well. GRO-α was uniquely upregulated only in EV form in X4LAI04 infection probably suggesting its special role in the anti-HIV-1 response.
Since it is HIV-1 infection that triggers immune activation in infected tissues, intuitively it was expected that suppression of HIV-1 infection would restore their immune systems to pre-infection levels. However, this is not the case and low-level immune activation persists in patients successfully treated with antivirals.
Several hypotheses regarding continuous immune activation have been suggested. In particular, bacteria translocated through the damaged gut epithelium in HIV-1 infected individuals were shown to trigger immune activation34. Apparently, this mechanism is not operating in our system. Activation may be not due to a single factor, but rather to a combination of different events such as residual viral replication, reactivation of latent viruses, opportunistic infections, etc. 35,36,37.
Here, the phenomenon of residual immune activation after successful ART is reproduced in an isolated human lymphoid tissue ex vivo system opening a way to study this phenomenon, under controlled laboratory conditions. As expected, ART suppressed productive HIV-1 infection in human tissue ex vivo, however cytokines remained upregulated after 13 days of ART. Of the 7 cytokines that remained elevated at late days of X4LAI04 infection, 6 of these remained elevated with ART; 4 of 9 cytokines remained elevated with R5SF162 ART.
These cytokines seem to be released predominantly by bystander cells since cytokine increase continues in the course of productive HIV-1 infection in spite of massive death of infected cells 38.
EV-associated cytokines were more likely to be elevated late in HIV-1 infection than soluble ones, and these also remained elevated in spite of ART. Several of these cytokines remained more upregulated in EV-associated form than in soluble form, suggesting they may have a different role in cellular communication, probably being delivered with EVs to specific cells using an address “barcode” on EV surface 3,39 and triggering a response in these cells. Besides cytokines, EVs released by HIV-1 infected cells may contain viral envelope proteins (Env) 41 and these EVs facilitate HIV-1 infection 42 or could influence non-infected and uninfectable cells through the release of negative regulatory factor (Nef) inside vesicles 43.
Here, we demonstrated that cytokines released both in soluble and EV-associated form are biologically active. These cytokines triggered biological response in indicator cells sensitive to IL-6 and IL-10. In vivo cytokine released in infected tissues in soluble or EV-associated forms may attract cells to the infected cell environment. Although such a redistribution of cells within a small tissue block was not observed in our earlier histological analysis 40, it cannot be excluded unless a thorough real-time confocal analysis is performed.
In our ex vivo system we were able to investigate whether there is an input from ART itself in upregulation of cytokines. In our experiments, treatment of uninfected tissues with ritonavir did not result in upregulation of any cytokine. Thus, it seems that other factors, not ART support immunoactivation in treated tissues.
Although reflecting in vivo in many aspects, our system has obvious limitations: Unlike in vivo where immune activation can be traced for years in individuals with suppressed HIV-1 infection, our ex vivo experiments are limited to two to three weeks for preservation of tissue cytoarchitecture. Also, unlike in vivo there is no recruitment of new cells from periphery. Regardless of these limitations, we were able to demonstrate that immune activation in isolated tissues remained despite viral control. Our work indicates that cytokine upregulation in HIV-1 infected patients constitutes a local response by the infected tissue rather than a systemic one. Various implicated factors can be studied in our model system including activation of other viral infections (i.e. herpesviruses, in particular CMV) common in the HIV-1 infected population 44–47, or undetectable viremia in tissues, etc.
Conclusion
Our analysis showed that HIV-1 infected lymphoid tissues ex vivo upregulated production of many cytokines, both free and EV-associated, and the majority of these cytokines remained upregulated despite suppression of viral replication by ART. These results are consistent with the findings in vivo 20 and demonstrate that HIV-1 infected human tissues ex vivo provide a valuable in vivo-relevant model to study the immune activation in HIV-1 infection after ART. Investigation of the mechanisms of the residual immune activation in the described ex vivo system under controlled laboratory conditions may help to decipher this phenomenon and lead to the development of new therapeutic strategies aimed at controlling or modulating cytokine network.
Supplementary Material
Acknowledgements
Funding Sources: The work of V.M, W.F. and L.M. was supported by the Intramural Program of the National Institute of Child Health and Human Development.
Conflicts of Interest and Source of Funding: The authors declare no conflicts of interest. The work of V.M, W.F. and L.M. was supported by the Intramural Program of the National Institute of Child Health and Human Development.
References
- 1.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts L, Passmore JA, Williamson C, et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS. 2010;24(6):819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald W, Freeman ML, Lederman MM, Vasilieva E, Romero R, Margolis L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci Rep. 2018;8(1):8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming A, Sampey G, Chung MC, et al. The carrying pigeons of the cell: exosomes and their role in infectious diseases caused by human pathogens. Pathog Dis. 2014;71(2):107–118. [DOI] [PubMed] [Google Scholar]
- 5.Passaes CP, Saez-Cirion A. HIV cure research: advances and prospects. Virology. 2014;454–455:340–352. [DOI] [PubMed] [Google Scholar]
- 6.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambrosioni J, Nicolas D, Sued O, Aguero F, Manzardo C, Miro JM. Update on antiretroviral treatment during primary HIV infection. Expert Rev Anti-Infe. 2014;12(7):793–807. [DOI] [PubMed] [Google Scholar]
- 8.Archin NM, Sung JM, Garrido C, Soriano-Sarabia N, Margolis DM. Eradicating HIV-1 infection: seeking to clear a persistent pathogen. Nat Rev Microbiol. 2014;12(11):750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garbelli A, Riva V, Crespan E, Maga G. How to win the HIV-1 drug resistance hurdle race: running faster or jumping higher? Biochem J. 2017;474(10):1559–1577. [DOI] [PubMed] [Google Scholar]
- 10.Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. Aids. 2015;29(4):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Introini A, Fitzgerald W, Vanpouille C, Margolis L. Histoculture and Infection with HIV of Functional Human Lymphoid Tissue on Gelfoam (R). Methods Mol Biol. 2018;1760:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biancotto A, Brichacek B, Chen SS, et al. A highly sensitive and dynamic immunofluorescent cytometric bead assay for the detection of HIV-1 p24. J Virol Methods. 2009;157(1):98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grivel JC, Elliott J, Lisco A, et al. HIV-1 pathogenesis differs in rectosigmoid and tonsillar tissues infected ex vivo with CCR5-and CXCR4-tropic HIV-1. Aids. 2007;21(10):1263–1272. [DOI] [PubMed] [Google Scholar]
- 14.Grivel JC, Margolis LB. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat Med. 1999;5(3):344–346. [DOI] [PubMed] [Google Scholar]
- 15.Mire-Sluis AR, Thorpe R. Laboratory protocols for the quantitation of cytokines by bioassay using cytokine responsive cell lines. J Immunol Methods. 1998;211(1–2):P199–P210. [DOI] [PubMed] [Google Scholar]
- 16.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual Immune Dysregulation Syndrome in Treated HIV infection. Adv Immunol. 2013;119:51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margolis L Immunoactivation at the Crossroads of Human Disease. Am J Med. 2015;128(6):562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paiardini M, Muller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev. 2013;254:78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katsikis PD, Mueller YM, Villinger F. The Cytokine Network of Acute HIV Infection: A Promising Target for Vaccines and Therapy to Reduce Viral Set-Point? Plos Pathog. 2011;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanpouille C, Introini A, Morris SR, et al. Distinct cytokine/chemokine network in semen and blood characterize different stages of HIV infection. AIDS. 2016;30(2):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nolte-’t Hoen E, Cremer T, Gallo RC, Margolis LB. Extracellular vesicles and viruses: Are they close relatives? P Natl Acad Sci USA. 2016;113(33):9155–9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeMarino C, Pleet ML, Cowen M, et al. Antiretroviral Drugs Alter the Content of Extracellular Vesicles from HIV-1-Infected Cells (vol 8, 7653, 2018). Sci Rep-Uk. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Meara T, Kong Y, Chiarella J, et al. Exosomal MicroRNAs Associate With Neuropsychological Performance in Individuals With HIV Infection on Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2019;82(5):514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Chi X, Li R, Ouyang J, Chen Y. HIV-1-infected cell-derived exosomes promote the growth and progression of cervical cancer. Int J Biol Sci. 2019;15(11):2438–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu W, Santini PA, Sullivan JS, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10(9):1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu G, Yang L, Cai Y, et al. Emerging roles of extracellular vesicles in neurodegenerative disorders: focus on HIV-associated neurological complications. Cell Death Dis. 2016;7(11):e2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahimian P, He JJ. Exosome-associated release, uptake, and neurotoxicity of HIV-1 Tat protein. J Neurovirol. 2016;22(6):774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crenshaw BJ, Gu L, Sims B, Matthews QL. Exosome Biogenesis and Biological Function in Response to Viral Infections. Open Virol J. 2018;12:134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukhamedova N, Hoang A, Dragoljevic D, et al. Exosomes containing HIV protein Nef reorganize lipid rafts potentiating inflammatory response in bystander cells. Plos Pathog. 2019;15(7):e1007907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chettimada S, Lorenz DR, Misra V, et al. Exosome markers associated with immune activation and oxidative stress in HIV patients on antiretroviral therapy. Sci Rep-Uk. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanpouille C, Lisco A, Introini A, et al. Exploiting the Anti-HIV-1 Activity of Acyclovir: Suppression of Primary and Drug-Resistant HIV Isolates and Potentiation of the Activity by Ribavirin. Antimicrob Agents Ch. 2012;56(5):2604–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mogensen TH, Paludan SR. Molecular pathways in virus-induced cytokine production. Microbiol Mol Biol Rev. 2001;65(1):131–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta D, Petes C, Gee K, Basta S. The Role of Virus Infection in Deregulating the Cytokine Response to Secondary Bacterial Infection. J Interferon Cytokine Res. 2015;35(12):925–934. [DOI] [PubMed] [Google Scholar]
- 34.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21(1):6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beltran LM, Rubio-Navarro A, Amaro-Villalobos JM, Egido J, Garcia-Puig J, Moreno JA. Influence of immune activation and inflammatory response on cardiovascular risk associated with the human immunodeficiency virus. Vasc Health Risk Man. 2015;11:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Voeght A, Martens H, Renard C, et al. Exploring the link between innate immune activation and thymic function by measuring sCD14 and TRECs in HIV patients living in Belgium. Plos One. 2017;12(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miedema F, Hazenberg MD, Tesselaar K, van Baarle D, de Deboer RJ, Borghans JAM. Immune activation and collateral damage in AIDS pathogenesis. Front Immunol. 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grivel JC, Malkevitch N, Margolis L. Human immunodeficiency virus type 1 induces apoptosis in CD4(+) but not in CD8(+) T cells in ex vivo-infected human lymphoid tissue. J Virol. 2000;74(17):8077–8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berenguer J, Lagerweij T, Zhao XW, et al. Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. J Extracell Vesicles. 2018;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glushakova S, Baibakov B, Zimmerberg J, Margolis LB. Experimental HIV infection of human lymphoid tissue: correlation of CD4+ T cell depletion and virus syncytium-inducing/non-syncytium-inducing phenotype in histocultures inoculated with laboratory strains and patient isolates of HIV type 1. AIDS Res Hum Retroviruses. 1997;13(6):461–471. [DOI] [PubMed] [Google Scholar]
- 41.Dias MVS, Costa CS, Dasilva LLP. The Ambiguous Roles of Extracellular Vesicles in HIV Replication and Pathogenesis. Front Microbiol. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arakelyan A, Fitzgerald W, Zicari S, Vanpouille C, Margolis L. Extracellular Vesicles Carry HIV Env and Facilitate Hiv Infection of Human Lymphoid Tissue. Sci Rep-Uk. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McNamara RP, Costantini LM, Myers TA, et al. Nef Secretion into Extracellular Vesicles or Exosomes Is Conserved across Human and Simian Immunodeficiency Viruses. Mbio. 2018;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang CC, Crane M, Zhou J, et al. HIV and co-infections. Immunol Rev. 2013;254(1):114–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christensen-Quick A, Vanpouille C, Lisco A, Gianella S. Cytomegalovirus and HIV Persistence: Pouring Gas on the Fire. Aids Res Hum Retrov. 2017;33:S23–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biancotto A, Grivel JC, Lisco A, et al. Evolution of SIV toward RANTES resistance in macaques rapidly progressing to AIDS upon coinfection with HHV-6A. Retrovirology. 2009;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shmagel KV, Korolevskaya LB, Saidakova EV, et al. HCV coinfection of the HIV-infected patients with discordant CD4(+) T-cell response to antiretroviral therapy leads to intense systemic inflammation. Dokl Biol Sci. 2017;477(1):244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


