Graphical abstract
Keywords: COVID-19, Chloroquine, Dosage regimens, Dose related toxicity, Modeling and simulation
Highlights
-
•
Available information on chloroquine dosage schemes for COVID19 patients is lacking.
-
•
Safety concerns have been raised from chloroquine administration due to dosing.
-
•
Simulations were performed to analyze the safety of dosing regimens.
-
•
New dosage regimens are proposed according to patients’ characteristics.
-
•
A more frequent dosing is favorable in terms of safety.
Abstract
Currently no specific medicinal treatment exists against the new SARS-CoV2 and chloroquine is widely used, since it can decrease the length of hospital stay and improve the evolution of the associated COVID-19 pneumonia. However, several safety concerns have been raised from chloroquine use due to the lack of essential information regarding its dosing. The aim of this study is to provide a critical appraisal of the safety information regarding chloroquine treatment and to apply simulation techniques to unveil relationships between the observed serious adverse events and overdosing, as well as to propose optimized dosage regimens. The dose related adverse events of chloroquine are unveiled and maximum tolerated doses and concentration levels are quoted. Among others, treatment with chloroquine can lead to severe adverse effects like prolongation of the QT interval and cardiomyopathy. In case of chloroquine overdosing, conditions similar to those produced by SARS-CoV2, such as pulmonary oedema with respiratory insufficiency and circulatory collapse, can be observed. Co-administration of chloroquine with other drugs for the treatment of COVID-19 patients, like azithromycin, can further increase the risk of QT prolongation and cardiomyopathy. For elder patients there is a high risk for toxicity and dose reduction should be made. This study unveils the risks of some widely used dosing regimens and binds the observed serious adverse events with dosing. Based on simulations, safer alternative dosage regimens are proposed and recommendations regarding chloroquine dosing are made.
1. Introduction
Since the pandemic outbreak of the new Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS-CoV-2) and the exponentially rising number of infected humans and deaths worldwide, there is an urgent need for the development of the appropriate pharmacotherapy and vaccines. Patients with Coronavirus Disease-2019 (COVID-19) are currently millions, while a portion around 15% of them contracts the severe form of the disease (Cortegiani et al., 2020). Since currently there is no specific medicinal treatment, existing medicines used to treat other diseases are administered and tested in COVID-19 patients in the light of drug repositioning. In this context, chloroquine (CQ) is widely used in treating SARS-CoV-2 infection since it can decrease the length of hospital stay and improve the evolution of the associated pneumonia (Colson et al., 2020, Yao et al., 2020).
Chloroquine has been used worldwide since 1930s for the treatment of malaria (and other parasitic infections) and it is a cheap drug belonging to the World Health Organization (WHO) list of essential drugs (Cortegiani et al., 2020). It is worth mentioning that CQ apart from its antimalarial efficacy, exerts also anti-inflammatory and direct antiviral effects against retroviruses, flaviviruses, HIV, Zika virus, and coronaviruses (Savarino et al., 2003, Delvecchio et al., 2016). CQ interferes with the glycosylation of the SARS-CoV2 cellular receptors and also alters the endosomal pH needed for virus-cell fusion (Avdic, 2020). CQ inhibits the attachment of viruses to the human respiratory cells, which is facilitated by the spike SARS-CoV2 protein (Fantini et al., 2020). The immunomodulant effects are mediated by the reduction in T cell activation and differentiation, and suppression of cytokines produced by B- and T- lymphocytes (such as IL-1, IL-6) which are elevated in the body inflammatory response against the viruses (Avdic, 2020). The first intimations of the possible CQ effect on SARS viruses were proposed as early as in 2003 (Savarino et al., 2003). A common characteristic of the COVID-19 patients is the probability of a clinical worsening in the second week of infection which cannot be directly attributed to SARS-CoV2, but it might be linked to an immunological response. Thus, both the anti-inflammatory and the direct antiviral properties of CQ make it capable of treating COVID-19 (Cortegiani et al., 2020). Currently, CQ is fully considered for controlling the late phase cytokine storm occurring in COVID-19 patients and the US FDA has released on March 28, 2020 an emergency use authorization for CQ to treat COVID-19 patients (Bright, 2020).
Due to the disease outbreak, the large numbers of fatalities worldwide, the possible collapse of national health systems, and the impact on the global economy, many clinical trials are now on-going aiming at testing new medicines, identifying the role of existing drugs, and among others to elucidate the appropriate dosage regimens of CQ (Cortegiani et al., 2020). Modeling and simulation methodologies have also joined the global efforts to find the most appropriate dosing scheme for COVI-19 patients (Perinel et al., 2020). However, for the moment, there are no explicitly proven effective and safe CQ dosage treatments for SARS-CoV2 pneumonia.
The aim of this study is to provide a critical assessment of safety information of CQ in regard to its dosing and use these data in a subsequent step to apply simulation techniques in order to optimize the dosage regimens. Several aspects of CQ dosing are explored including the impact of existing dosing schemes on CQ plasma levels, accumulation and long residence time in the body, the association of overdosing with frequently observed adverse events, the impact of renal/liver impairment of elder patients leading to toxic levels, the benefits of early initiation of CQ treatment, and the advantages of the twice daily over the once daily dosing in terms of safety. At the end, specific dosing recommendations are made to the health care specialists.
2. Critical appraisal of the relationship between dosing and safety
2.1. Adverse effects
Chloroquine has been in use for more than 80 years and its clinical/safety profile is known for the malaria and other parasitic infections. Several adverse events can occur after treatment with CQ, with some of them being more frequent and/or more severe requiring special attention. The most important/frequent are cardiovascular problems (QT interval prolongation, arrhythmias, cardiomyopathy), hypoglycemia, eye or vision problems (e.g. retinopathy macular degeneration), gastrointestinal disorders, muscle weakness, hypokalemia, increased risk for seizures, hearing problems, extrapyramidal disorders, and hemolytic anemia in patients with G6PD deficiency (Chatre et al., 2018, Singhi et al., 1979, Avloclor® Tablets, 2016, Mayo Clinic, 2020).
Special attention should be paid to some adverse events occurring after CQ treatment, since COVID-19 patients treated with CQ commonly complaint about them. A serious condition is prolongation of the QT interval, with a potential to induce cardiac arrhythmias especially after long-term treatment (Avloclor® SmPC, 2016). The extent of QT prolongation can increase with high doses. Cardiomyopathy is another serious incident that might occur with CQ treatment leading to heart failure, with sometimes fatal outcome (Chatre et al., 2018). Again, high CQ plasma levels and long-term therapy are risk factors for cardiomyopathy. It should be underlined that the risk of QT prolongation and cardiomyopathy are highly increased after co-administration with azithromycin and warnings have already imposed by the FDA (Svanström et al., 2013, Ray et al., 2012, Mosholder et al., 2013, FDA Drug Safety Communication, 2013). In addition, the CQ-azithromycin combination with fluoroquinolones (moxifloxacin, levofloxacin) is definitely cardiotoxic and should be avoided (Svanström et al., 2013, Ray et al., 2012, Mosholder et al., 2013, FDA Drug Safety Communication, 2013). Diffuse parenchymal lung disease can appear to a small portion of patients, while respiratory failure and subsequent death can occur at high doses. Severe hypoglycemia, with the accompanying symptoms like loss of consciousness, can be life threatening for the patients treated with CQ and therefore they should have their blood glucose levels monitored closely. Eye/vision disorders can appear usually after long term CQ treatment, whereas when total CQ intake exceeds 1.6 g/kg, corneal changes and retinal damage can be irreversible. Some COVID-19 patients complaint also for symptoms like dizziness, drowsiness, headache, blurred vision, diplopia, increased excitability, and convulsions which typically can occur at higher CQ doses. In addition, there are complaints from patients for symptoms like involuntary movements of the tongue, tonic muscular spasms, dystonia (namely, extrapyramidal symptoms), which can be a result from CQ treatment. Myasthenia type symptoms and liver disorders can also happen. It has to be highlighted that patients with G6PD deficiency should take CQ with special caution due to the risk of hemolysis.
2.2. Toxicity due to overdosing
Chloroquine overdosing can result in life threatening situations requiring immediate intensive supportive treatment (Mayo Clinic, 2020, Chatre et al., 2018, Stiff et al., 1991, Albertson, 2012). Mild to moderate CQ overdose leads to nausea and vomiting, hypokalemia, metabolic acidosis, neuropsychiatric side effects, headache, and visual disturbances (e.g. blindness). Severe overdose can result in patient convulsions, depressed myocardial contractility, cardiac arrhythmias (however, correction with anti-arrhythmic drugs like those with quinidine-like effect should be avoided), severe hypokalemia, shock, and finally death through respiratory and circulatory collapse. Therapeutic treatments of CQ overdosing include early administration of adrenaline (to restore systolic blood pressure) and diazepam administration (to reduce CQ induced cardiotoxicity). Since CQ is eliminated from the body after several weeks/months, due to extended distribution to peripheral tissues, caution is needed to avoid overdosage. Hemodialysis or other types of dialysis (e.g. peritoneal dialysis) do not appear to exert benefit.
Lethal doses result in pulmonary oedema, subsequent respiratory insufficiency and finally death regardless the existence of mechanical ventilation and other treatment (Ursing et al., 2009, Ndiaye et al., 1999). In adults, the lethal dose is estimated between 30 and 50 mg/kg, while doses higher than 20 mg/kg can also be toxic (Olson, 2004). Doses of more than 40 mg/kg are lethal if no early intensive treatment is made. Other studies indicate fatalities after acute CQ intake of more than 2 g or 2.3 mg/kg (daily) (WHO, 1994). Parenteral doses higher than 5 g are also lethal (Hardman et al., 2001, McEvoy, 2006). Special caution with serum CQ levels above 1,000 ng/ml since cardiotoxicity can be observed (Olson, 2004). For children, a single dose of 10 mg CQ base/kg, followed by 5 mg base/kg six hours later can be considered safe (Avloclor® SmPC, 2016). However, intake greater than 10 mg/kg of CQ base may require immediate health care assistance (Smith and Klein-Schwartz, 2005).
2.3. Harmful interactions
Interactions of CQ with other drugs can arise, among others, due to the fact that CQ is substrate and inhibitor of CYP2D6 (Avdic, 2020, Chatre et al., 2018). It is better to avoid co-administration with a series of frequently prescribed drugs, but if it is completely needed upon doctor’s judgment, special attention should be paid for the following CQ combinations: amiodarone, azithromycin, carbamazepine, chlorpromazine, cimetidine, citalopram, clarithromycin, clomipramine, clozapine, desipramine, digoxin, domperidone, erythromycin, escitalopram, fluconazole, fluoxetine, hydroxychloroquine, imipramine, isoflurane, levofloxacin, lopinavir, metronidazole, ofloxacin, procainamide, quinidine, risperidone, sotalol, sunitinib, tacrolimus, tamoxifen, and vasopressin. It should also be kept in mind that CQ can reduce the convulsive threshold and antagonize the antiepileptic actions. It is important to highlight these interactions, since the abovementioned drugs can be administered in COVID-19 patients.
2.4. Overview of pharmacokinetic properties
2.4.1. General
In terms of pharmacokinetics, chloroquine is a highly water soluble and highly permeable drug belonging to class I of the biopharmaceutical classification system (Verbeeck et al., 2005). This implies that it is rapidly absorbed and in high percentages. Indeed, the bioavailable fraction of CQ absorption is 89% and peak plasma concentration are reached within 1–3 h after intake (Avdic, 2020, Avloclor® Tablets, 2016, Verbeeck et al., 2005). Antacids and acidic beverages can reduce absorption, while food increases bioavailability (and also reduces nausea/vomiting). A portion of 50–65% CQ is bound to plasma proteins, while the volume of distribution can be very high (up to 800 l/kg) indicating the extensive CQ distribution in peripheral tissues like lungs, eyes, heart, liver, kidneys, and brain. Due to its extensive distribution, the elimination half-life of CQ ranges from 20 to 60 days. Elimination occurs due to renal clearance and metabolism (in an almost equal proportion). The CQ kinetics have been found to be linear over the dose range 2–15 mg/kg (of CQ base).
2.4.2. Risks in elder, pregnant, and pediatric populations
In patients with impaired renal or liver function (e.g. elder people), officially, no dossing adjustment is required (Avloclor® SmPC, 2016). However, for elder patients, special concerns are raised, because it more likely to have kidney and/or liver problems and dose adjustment might be needed to avoid CQ accumulation (Mayo Clinic, 2020). In the subsequent analysis performed in this study, the impact of renal/liver impairment on the rise of concentration levels is revealed. In case of children, despite the fact that they exhibit higher clearance values than adults, children appear more sensitive to the effects of CQ (Zhao et al., 2014, Avloclor® Tablets, 2016, Mayo Clinic, 2020). Pregnant women are advised to avoid CQ, since high CQ doses can lead to fetal abnormalities like ototoxicity, cochlear dysfunction, and visual loss (Avloclor® SmPC, 2016). However, depending on the judgment of the physician the potential benefit can outweigh risk. Reported results on the safety of CQ during pregnancy reveal that CQ can be safe even after a dosage of 500 mg/day (Klinger et al., 2001). CQ is excreted in breast milk at around 2.8%, a portion which is inadequate for infant chemoprophylaxis, but it may cause disorders (even though it is considered low to be harmful). The physician should weigh the therapeutic benefit against potential risk for the infant. However, adequate studies are missing (Avdic, 2020, Avloclor® Tablets, 2016, Mayo Clinic, 2020). Finally, is should be kept in mind that CQ is metabolized by CYP2D6, an enzyme system with variable expression among individuals. For example, 7% of North Americans are poor CYP2D6 metabolizers, while a portion 1–2% of them are ultra-rapid metabolizers (Juurlink, 2020). Since, genetic variability, can significantly alter CQ levels (and therefore efficacy/safety profile), it has to be taken into account where possible.
3. Methods
A literature search was initially made to find as much as possible information regarding the safety profile of chloroquine. Dosage regimens that are currently proposed either for the treatment of COVID-19, or for parasitic infections were investigated through simulations in order to explore their impact on safety. The simulations were performed using population pharmacokinetic models already proposed in the literature for CQ (Zhao et al., 2014, Höglund et al., 2016, Karunajeewa et al., 2010, Salman et al., 2017). For adults, all proposed models were quite similar and their differences refer to the use either of lag-time during absorption, or a transition compartment. For the simulations performed in this study, the selected model refers to a two-compartment model, with absorption lag time and first order kinetics for all transfers (absorption, elimination, inter-compartmental) (Zhao et al., 2014). In the literature models, body weight was found to be a significant covariate for volumes of distribution and clearances. For the volume of distribution of the central and peripheral compartment an allometric exponent of 1 was used, while for clearance and inter-compartmental clearance an allometric exponent of 0.75 was utilized (Zhao et al., 2014). The reference patient of the study was considered to be: 70 kg, 40 years old, with body mass index 15 kg/m2, and body surface area of 1.73 m2. Other situations like patients with different weights and altered renal/liver function were also simulated. All population pharmacokinetic parameter estimates, as well as the between-subject and residual error models, were those quoted in the literature (Zhao et al., 2014).
Initially, simulated profiles of the dosing schemes found in the literature were generated in order to unveil the impact of these profiles on the achieved plasma levels and the subsequent risk for toxicity. Based on the critical assessment of the safety issues of CQ and taking into consideration the currently proposed dosing schemes, additional dosage regimens were simulated and explored aiming to serve as potential substitutes in clinical practice. Special focus was placed on performing simulations towards answering several questions imposed by the physicians like the time needed to achieve steady state levels of CQ, the residence time in the body after stopping administration, the conditions of early initiation of therapy, the effect of patients’ body weight, the frequency of daily administrations, and finally the impact of impaired renal and/or liver impairment. In order to avoid complexity in the simulated concentration – time profiles, only the average performance is depicted in the results. The entire modeling work was performed in Mlxplore® 2019R2 (Lixoft, Orsay, France).
4. Results and discussion
4.1. Safety assessment of existing dosage regimens
Table 1 summarizes the characteristics of dosage regimens already proposed and used, as well as those that are now evaluated in on-going clinical trials. Even though, some of these regimens are not specifically proposed for SARS-CoV2, however, they are utilized worldwide in patients with COVID-19, given that no other information is currently available.
Table 1.
Chloroquine dosage regimens currently in use. Dosing scenarios indicated by an asterisk (*) were explored in simulations.
| No | Dosing scheme | Indication | Reference |
|---|---|---|---|
| 1* | Adults: 500 mg × 2 for no more than 10 days | COVID-19 | Wong et al., 2020 |
| 2 | Adults: 500 mg × 2 for no more than 7 days A lower dose for patients weighing less than 50 kg |
COVID-19 | Wong et al., 2020 |
| 3* | Adults: At first 1000 mg once a day, then 500 mg 6 to 8 h after the first dose and then 500 mg on the second and third day of treatment Children and adults with low body weight: At first 10 mg/kg, then 5 mg/kg taken 6 h, 24 h, and 36 h after the first dose |
Malaria | Mayo Clinic, 2020 |
| 4* | Adults: 1000 mg once a day taken for 2 days. This is followed by 500 mg once a day for at least 2 to 3 weeks | Treatment of liver infection caused by protozoa | Mayo Clinic, 2020 |
| 5* | Adults, including pregnant women, and children: Total dose 25 mg/kg given over 3 days as follows: Day 1: 10 mg/kg, followed by 5 mg/kg 6–8 h later [this is equivalent for a 70 kg adult to: (700 + 350)*1.613 = 1,693 mg for the first day] Days 2 and 3: 5 mg/kg in a single dose [i.e. 350 mg for a 70Kg adult] (Since, dosages above are described in terms of chloroquine base, a conversion factor of 1.613 is used for converting to chloroquine phosphate) |
Parasitic diseases | WHO, 2020 |
| 6* | 500 mg daily for 2–10 days. It is also suggested the potential of a loading dose of 1 g | COVID-19 | Avdic, 2020 |
| 7 |
Treatment: 1000 mg once, then 500 mg at 6 h, 24 h and 48 h Prophylaxis (in chloroquine-sensitive regions): 500 mg (300 mg base) starting one week prior to entry, continue once weekly and then four weeks after leaving the endemic region |
Malaria | Avdic, 2020 |
| 8 | Days 1 and 2: 1000 mg/daily, From Day 3: 500 mg for 12 days | COVID-19 | Cortegiani et al., 2020 |
| 9 | 150 mg chloroquine phosphate every 12 h, inhaled by atomization for one week | COVID-19 | Cortegiani et al., 2020 |
| 10 | Two tablets chloroquine twice daily | Mild and common COVID-19 pneumonia | Cortegiani et al., 2020 |
| 11 | Two tablets chloroquine phosphate twice daily | Critically ill COVID-19 pneumonia | Cortegiani et al., 2020 |
| 12 | 12.600 mg twice daily for 10 days versus 450 mg twice daily (Day 1) followed by 450 mg once daily, for 4 days | Critically ill COVID-19 pneumonia | Borba et al., 2020 |
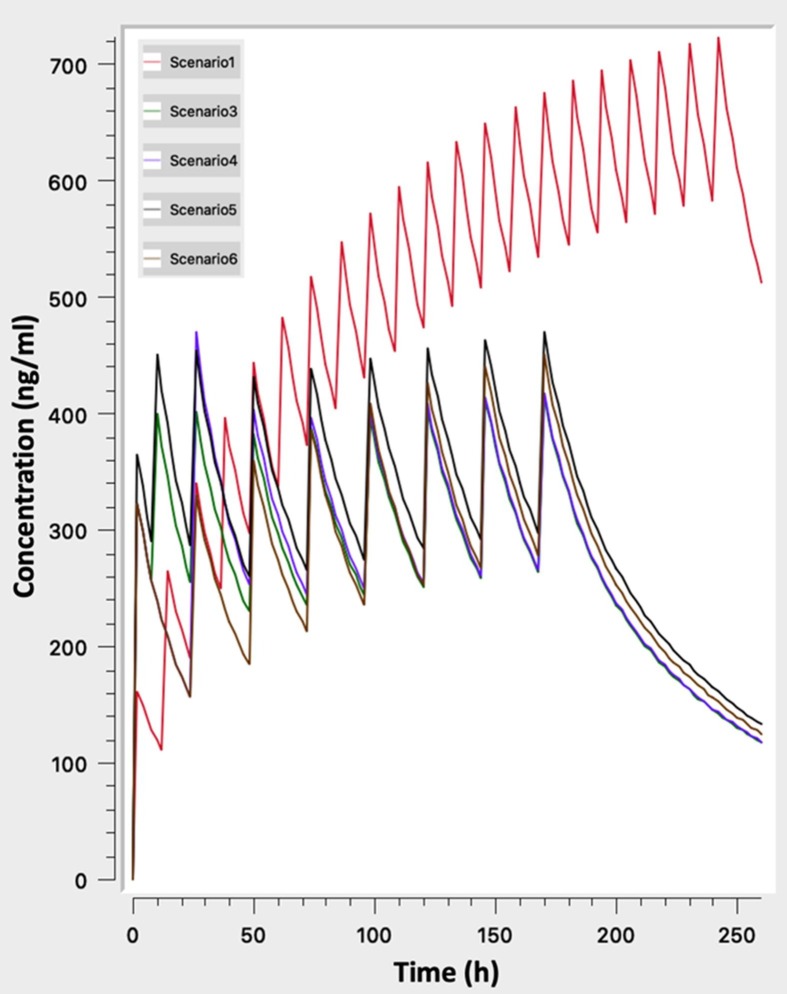
Simulated profiles of the dosing schemes listed in Table 1 were generated in order to unveil the impact of these profiles on the achieved plasma levels and the subsequent potential safety risk (Fig. 1 ).
Fig. 1.
Simulated concentration – time profiles for dosing regimens currently used in clinical practice (scenarios “1”, “3”, “4”, “5”, “6” in Table 1).
Visual inspection if Fig. 1 reveals that apart from the 500 mg x2 scheme, all other regimens lead to almost the same steady state levels. The differences among them can only be attributed to the time needed to reach steady state levels, where the dosing scenarios with loading doses (scenarios “3”, “4”, “5” of Table 1) achieve earlier plateau levels. The 500 mg x2 scheme lead to plasma concentrations very close to the reported toxic levels of 800 ng/ml and therefore a high toxicity risk exists. Obviously, a patient with impaired clearance would face an even higher risk due to the higher plasma levels. Another worth notable finding is the fact that large fluctuations can be observed at steady state. The impact of these fluctuations can be two-fold: a) sometimes sub-therapeutic levels may exist and b) the steep increases in the concentration might trigger adverse events by themselves (Ursing et al., 2009).
4.2. Accumulation, residence in the body, and frequency of dosing
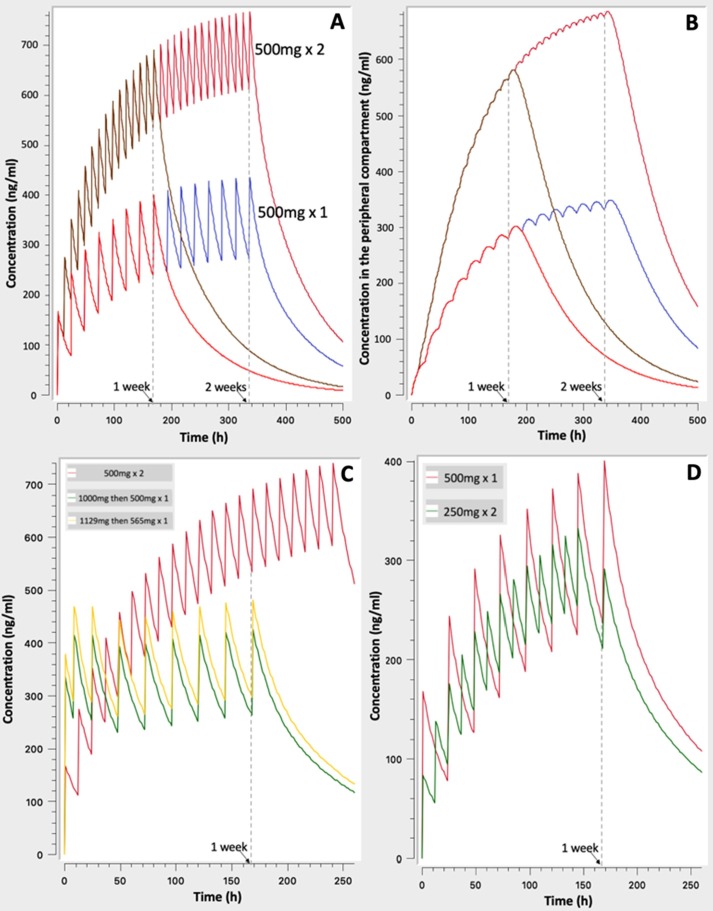
In order to highlight on some specific properties of the CQ pharmacokinetics, simulations continued with the investigation of the time required to achieve steady state levels, the residence in the body and the impact of dosing frequency on safety risks (Fig. 2 ).
Fig. 2.
Simulated concentration – time profiles to unveil the time required to reach steady state levels and the long residence of drug in body (A, B, C), as well as the benefits of more frequent dosing (D): A. 500 mg × 2 vs. 500 mg × 1 for one- and two- week treatment, B. As in ‘A’ but for the concentration in peripheral compartment, C. Comparison of three schemes of Table 1 (scenario “1” vs. scenario “3” vs. scenario “5”), D. 500 mg once daily vs. 250 mg twice daily.
Fig. 2A reveals that the time needed to reach steady state levels is almost two weeks, irrespectively of the dosing regimen (for example, 500 mg once or twice daily). A finding which can be attributed to the long half-life of CQ. Secondly, as it is expected from the linear CQ kinetics, the levels achieved after the 500 mg x2 dose is twice as those occurring from the once daily 500 mg dose. In addition, Fig. 2A reveals the long time required for CQ to be removed from the body after its withdrawal.
Fig. 2B presents the hypothetical concentration of CQ achieved in the peripheral tissues. It is called “hypothetical” since it is assumed that outside plasma the CQ concentration is everywhere the same. Even though, this is not completely true, however, Fig. 2B offers us the opportunity to realize that much more time is needed to reach saturation with drug in the peripheral tissues than that observed in plasma. Indeed, even after two weeks of continuous treatment, and although steady state levels are achieved in plasma, the concentration of CQ in tissues still rises and no saturation has occurred. This finding has also a significant impact on the observed CQ disappearance from plasma, since it explains why drug decay is so slow and why adverse events can be observed after withdrawal from CQ treatment.
In Fig. 2C the impact of loading doses on the CQ levels is underlined by simulating the typical case of 500 mg x2 (red line) and two other scenarios with loading dose (see Table 1). It becomes obvious that the use of loading doses, allows much earlier achievement of steady state levels. At first sight, this leads to the conclusion that the use of loading doses is favorable. However, this is only true in a pharmacokinetic point of view and not in terms of safety, since literature data reveal that steep increases of CQ levels can result in adverse/toxic effects (Ursing et al., 2009). Therefore, the practice of using loading doses of CQ should be considered very carefully due to the high risk of toxic effects (Borba et al., 2020).
Finally, Fig. 2D compares the frequency of two dosage regimens and in particular the 500 mg x1 versus 250 mg x2 daily. As it is expected, due to the linearity of CQ pharmacokinetics and the fact that in both cases the same total daily amount is used, both schemes lead to the same average concentration values at steady state. However, much less fluctuations are observed in case of the twice daily regimen. Bearing in mind the higher risk of toxic effects due to CQ concentrations changes, the use of more frequent regimens appears to be more favorable.
4.3. Proposed safer dosage regimens
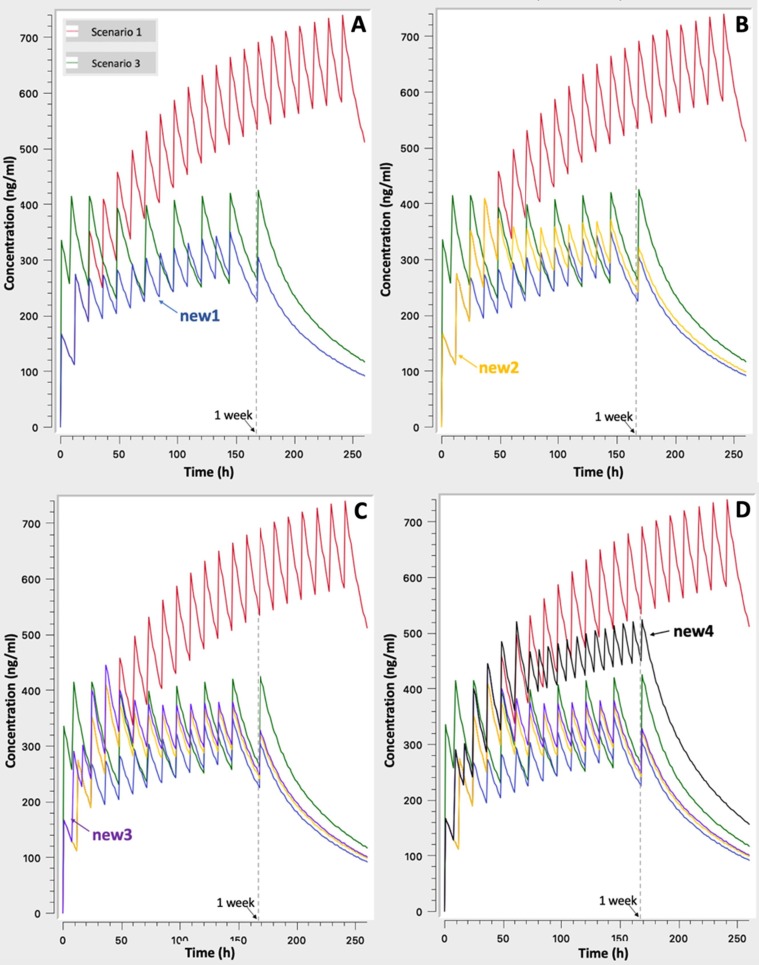
Taking into consideration the abovementioned findings, four additional dosage regimens (termed as “new” in comparison to the existing typical ones) are defined and further explored through simulations (Table 2 ).
Table 2.
Dosage regimens proposed in this study and further explored in the simulations.
| No | Dosing scheme |
|---|---|
| new1 | Day1: 500 mg x2 Day 2 and afterwards: 250 mg x2 |
| new2 | Day 1: 500 mg x2 Day 2: 500 mg x2 Day 3 and afterwards: 250 mg x2 |
| new3 | Day 1: 500 mg + 500 mg + 250 mg (with 8 h intervals) Day 2: 500 mg x2 Day 3: 250 mg x2 |
| new4 | Day 1: 500 mg + 500 mg + 250 mg (with 8 h intervals) Day 2: 500 mg x2 Day 3: 500 mg x2 Day 4 and afterwards: 250 mg x3 |
In Fig. 3 the evaluation of additional regimens starts with the simulation of the milder (i.e. new1) scenario and goes gradually to more severe regimens with loading doses and larger total doses in general (e.g. scenario “new4”). The addition of each new dosing scheme is made in subsequent plots in order to allow the reader to identify it easier. As it is expected, increasing the “severity” of dosing scenario higher concentrations are achieved, but in a different way. It is worth mentioning that the already proposed scenario “3” of Table 1 results in very steep increases during the first day of the treatment, while from the second day the achieved levels are almost equivalent to those from the scenarios “new2” and “new3”. Bearing in mind that steep increases can be harmful, it is proposed that in case of need, scenarios “new2” and “new3” should be chosen instead of the existing scenario “3”. If the physician decides for even higher CQ levels, then the “new4” scenario can be used, since it leads to high concentration levels in a mild way, namely, avoiding steep increases. It is interesting to note some additional characteristics of the “new4” scenario which is considered as the most aggressive of all the “new” scenarios: a) the use of a total dose of 1,250 mg (i.e. 500 + 500 + 250) the first day is in accordance with the maximum safe dose of 20 mg/kg outlined above, b) It achieves higher concentrations than the existing scenario “3” and lower than the typical case of scenario “1” (500 mg × 2), c) The initial concentration increases are milder than scenario 3, since the total daily dose is split into three doses (instead of two). These features make scenarios “new3” and “new4” safer dosing alternative for the treatment of severe COVID-19 patients, whereas scenarios “new1” and “new2” can be used in patients with mild-moderate symptoms of COVID-19 pneumonia.
Fig. 3.
Simulated concentration – time profiles for the “newly” proposed dosing schemes: A. Scenarios “1” and “3” of Table 1 and “new1” of Table 2, B. As in A plus “new2”, C. As in B plus “new3”, D. As in C plus “new4”.
4.4. Early therapy initiation
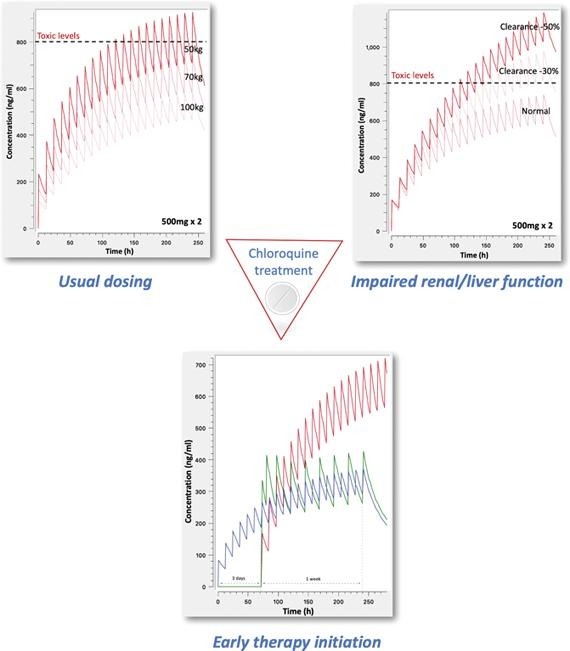
The importance of early initiation of therapy is further investigated in Fig. 4 . In this case, the situation of a patient who starts CQ treatment early after the development of first symptoms is simulated. This patient is considered to start CQ at low dose (250 mg × 2) immediately and is compared to other patients who start treatment three days later. Visual observation of Fig. 4 clearly reveals that the patient who initiated early CQ treatment exhibits the same plateau levels after three days with patients started later, but using much more aggressive regimens. In particular, in the early initiation patient a low dose of 250 mg × 2 is given, compared to the much higher doses of 500 mg x2 (scenario 1: red line) and the scheme with the loading dose (scenario 3: green line). In other words, the early initiation of treatment with low doses, offers several advantages such as the achievement of the desired levels in a non-aggressive way, avoidance of steep concentration increases and subsequent toxicities, cover the patient with low CQ doses which are rather safe, whereas depending on patient’s condition the dosing scheme can be increased. Even though, chemoprophylaxis against SARS-CoV2 using chloroquine is another issue, these results indicate the benefits of low dose CQ administration in terms of safety and its subsequent clinical implications. However, other studies are required to unveil the potential role of CQ in chemoprophylaxis against SARS-CoV2.
Fig. 4.
Simulated concentration – time profiles underlying the need of early initiation of therapy after the first appearance of symptoms. The early administration of 250 mg × 2 is compared with the existing dosing scenarios “1” and “3” (Table 1) started three days later.
4.5. Body weight, impaired clearance, and toxic chloroquine levels in patients’ plasma
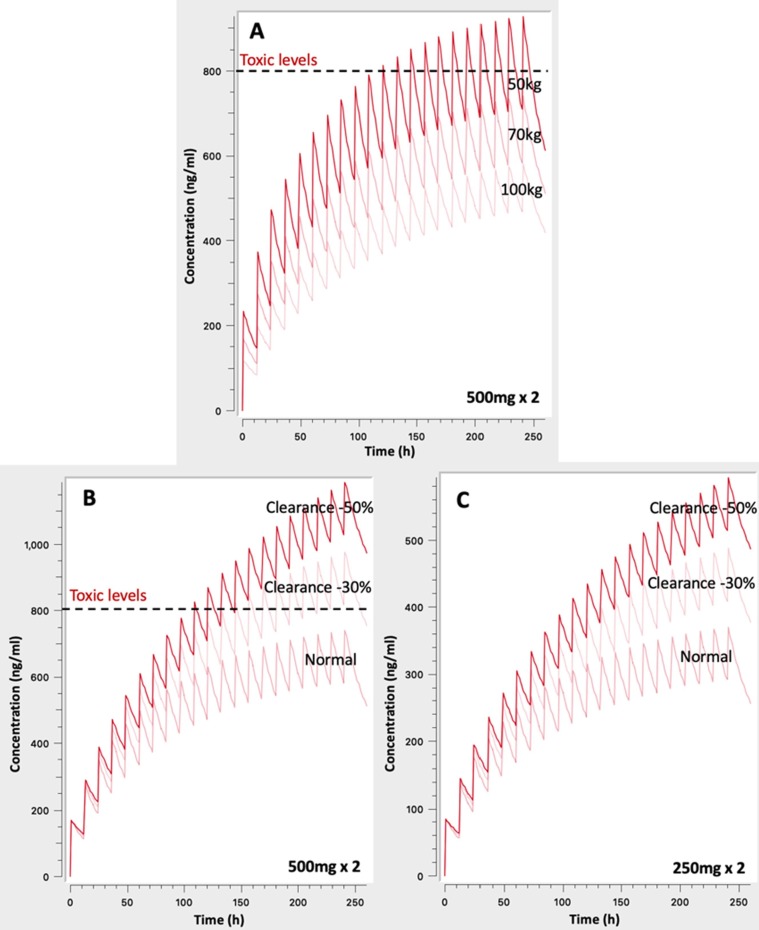
The next aim of this study was to identify the role of body weight towards dosage adjustment and the impact of renal/liver impairment on the CQ concentration levels and toxicity risks (Fig. 5 ).
Fig. 5.
Simulated concentration – time profiles to: A. Unveil the impact of body weight on chloroquine levels, B. Impaired renal/liver clearance by 30% and 50% for the 500 mg × 2 regimen, and C. Impaired renal/liver clearance by 30% and 50% for the 250 mg × 2 regimen.
Fig. 5A shows the achieved levels after 500 mg × 2 administration for ten days in three different subjects: the typical case of 70 kg, a heavier adult weighting 100 kg, and a lighter of 50 kg. Since weight was found to affect clearances and volumes of distribution (see ‘Methods’), the increase of body weight results in lower plasma levels. On the contrary, for low body weight individuals, administration of the typically used now 500 mg × 2 regimen can lead to concentrations above the toxic levels of 800 ng/ml (Furst, 1996). Besides, even for the case of typical patient of 70 kg, the observed levels are close to this toxic value increasing the risk for adverse events. These findings clearly indicate the high toxicity risk associated with the currently applied dosing scheme.
The impact of reduced clearance (e.g. in the elderly) is also depicted in Fig. 5B. It becomes evident that even a 30% decrease can lead to levels exceeding the toxic limit of 800 ng/ml for the typical 500 mg x2 dosing scheme. Much more pronounced is the impact of higher impairment (i.e. 50%) leading to very high concentrations with a definite toxic impact.
However, when a lower total dose is administered, as in case of a 250 mg × 2 scheme, approximation of toxic levels is avoided even after 10 days treatment (Fig. 5C).
4.6. Dosing recommendations for enhanced safety
The above presented simulations showed that existing CQ dosing schemes can lead to high plasma levels which are associated with increased risk for adverse and toxic events including prolongation of the QT interval, cardiomyopathy, severe hypoglycemia, vision disorders, neurological problem. In case of chloroquine overdosing, conditions similar to those produced by SARS-CoV2 like pulmonary oedema with subsequent respiratory insufficiency and circulatory collapse can occur. Bearing in mind that toxic levels can be easily reached in elder patients (>65 years) under the treatment of even typical dosing schemes (e.g. 500 mg x2 even for few days, Fig. 5), it becomes evident the need for discriminating between the harmful impact of CQ and the COVID-19 pneumonia. In an attempt, to summarize the information provided by this analysis in a concise and easy to interpret way by the physicians, the following issues are highlighted.
General recommendations:
-
•
As chloroquine dose increases, toxicity risk also increases
-
•
Chloroquine toxicity is not only caused by high peak levels, but also due to steep increases of concentration
-
•
Doses up to 20 mg/kg can usually be considered safe
-
•
Typical regimens include administration of 500 mg/day. For a 500 mg/daily scheme, the typical duration is around 7 days
-
•
Accumulated CQ doses are responsible for increased toxicity risk. Therefore, duration of treatment is also important and the totally administered CQ should be taken into account
-
•
Much time is needed to reach steady state levels, irrespectively of dose
-
•
After chloroquine withdrawal, the drug remains in the body for weeks
-
•
Chloroquine plasma levels above 800 ng/ml result in increased risk for toxic effects, whereas patients with levels below 400 ng/ml usually have no side effects
-
•
Administration of chloroquine should be made after meals to increase bioavailability and avoid nausea and vomiting. Concomitant use of antacids and acidic beverages should be avoided, since they decrease absorption
Model based dosing recommendations:
-
•
Critical conditions similar to those produced by SARS-CoV2 (i.e. pulmonary oedema, respiratory insufficiency, circulatory collapse) can occur due to chloroquine overdosing
-
•
Safer dosage regimens (from “mild” to more “aggressive”) were evaluated through simulations and are proposed to physicians (Table 3 ). Physicians can select the appropriate model based on their patient characteristics and severity of symptoms.
-
•
For elder patients there is a high toxicity risk and dose reduction is proposed
-
•
For patients with low weight, there is an increased toxicity risk and therefore a milder dosing regimen should be used
-
•
A more frequent dosing is favorable in terms of safety. For example, 250 mg twice daily is better than 500 mg once daily, due to lower fluctuations and lower risk of adverse events from steep concentration increases
-
•
Treatment should be initiated as early as possible using the less aggressive dose regimens
Table 3.
Optimized dosage schemes ranked from the milder to the more aggressive depending on patients’ characteristics (age, weight) and needs (severity of disease).
| Dosing scheme | COVID severity | Patientsa, b |
|---|---|---|
| Start early: 250 mg x2 for all days (or increase after Day3 depending on the disease progress) |
Mild | Adults / Elder |
| Day1: 500 mg x2 Day 2 and afterwards: 250 mg x2 |
Mild / Moderate | Adults / Elder |
| Day 1: 500 mg x2 Day 2: 500 mg x2 Day 3 and afterwards: 250 mg x2 |
Moderate | Adults / Elder |
| Day 1: 500 mg + 500 mg + 250 mg (with 8 h intervals) Day 2: 500 mg x2 Day 3: 250 mg x2 |
Severe | Adults |
| Day 1: 500 mg + 500 mg + 250 mg (with 8 h intervals) Day 2: 500 mg x2 Day 3: 500 mg x2 Day 4 and afterwards: 250 mg x3 |
Severe | Adults |
Patients weighting more than 50% from the average (70 kg), require 25% higher doses.
Patients weighting less than 50% from the average (70 kg), require 25% lower doses.
In order to avoid complexity of the proposed dosing schemes, it was attempted to organize them (Table 3) in the simplest possible way, but at the meantime to cover special patients’ needs.
Thus, the physicians considering the severity of COVID-19 related pneumonia and taking into account the abovementioned factors can select the appropriate dosing regimen for their patient.
5. Conclusions
Concrete results from extensive clinical trials are currently missing and there is an urgent need for information regarding the safety of chloroquine dosage regimens. The fact that many COVID-19 patients face clinical conditions similar to those triggered by chloroquine overdosing (like pulmonary oedema, respiratory insufficiency, and circulatory collapse) underlines the necessity of appropriate dosing. Even though, clinical trials and everyday clinical practice should be performed to confirm the appropriate dosage regimens, modeling and simulation approaches can provide early recommendations for dosing. This study attempts to unveil the toxicity risks of some widely used dosage schemes and binds the observed serious adverse events with dosing. It also proposes safer dosage regimens tailored to patients’ characteristics (e.g. body weight, age) and the severity of COVID-19 pneumonia. Finally, it is suggested early chloroquine therapy initiation at low doses, upon the first symptoms, as a safe dosing alternative in high risk patients.
Declaration of Competing Interest
The authors have no competing interests to declare. This work has not received any funding.
References
- Albertson, T.E., 2012. Chapter 51. Chloroquine and Other Aminoquinolines in Poisoning & Drug Overdose 6th eds (editor: Olson KR) The McGraw-Hill Companies, Inc®2012. New York city (USA).
- Avdic, E., 2020. Chloroquine record. Johns Hopkins ABX Guide. Updated on April 2020. Available at: https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_ABX_Guide/540120/all/Chloroquine?q=chloroquine#0.
- Avloclor® Tablets, Summary of Product Characteristics (SmPC), 2016.
- Bright, R., 2019. Request for Emergency Use Authorization For Use of Chloroquine Phosphate or Hydroxychloroquine Sulfate Supplied From the Strategic National Stockpile for Treatment of 2019 Coronavirus Disease to the U.S. Food &Drugs Administration (US-FDA). March 2020. Available at: https://www.fda.gov/media/136534/download.
- Borba MS, Val FA, Sampaio VS. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Network Open. 2020 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatre C., Roubille F., Vernhet H., Jorgensen C., Pers Y.M. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41(10):919–931. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- Colson, P., Rolain, J.M., Lagier, J.C., Brouqui, P., Raoult, D., 2020. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020 Mar 4:105932. [Epub ahead of print] DOI: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed]
- Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020. pii: S0883-9441(20)30390-7. [Epub ahead of print] DOI: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed]
- Delvecchio R, Higa LM, Pezzuto P, Valadão AL, Garcez PP, Monteiro FL, Loiola EC, Dias AA, Silva FJ, Aliota MT, Caine EA, Osorio JE, Bellio M, O'Connor DH, Rehen S, de Aguiar RS, Savarino A, Campanati L, Tanuri A Chloroquine, an Endocytosis Blocking Agent, Inhibits Zika Virus Infection in Different Cell Models. Viruses. 2016;8(12). DOI: 10.1016/s1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed]
- Fantini, J., Di Scala, C., Chahinian, H., Yahi, N., 2020. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020 :105960. [Epub ahead of print] DOI: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed]
- FDA Drug Safety Communication: Azithromycin (Zithromax or Zmax) and the risk of potentially fatal heart rhythms. [3-12-2013] Available at.
- Furst D.E. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus. 1996;Suppl 1:S11–S15. [PubMed] [Google Scholar]
- Hardman J.G., Limbird L.E., Gilman P.B.A.G. 10th ed. McGraw-Hill; New York, NY: 2001. Goodman and Gilman's The Pharmacological Basis of Therapeutics; p. 1079. [Google Scholar]
- Höglund R., Moussavi Y., Ruengweerayut R., Cheomung A., Äbelö A., Na-Bangchang K. Population pharmacokinetics of a three-day chloroquine treatment in patients with Plasmodium vivax infection on the Thai-Myanmar border. Malar J. 2016;15:129. doi: 10.1186/s12936-016-1181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juurlink David N. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ. 2020;192(17):E450–E453. doi: 10.1503/cmaj.200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunajeewa H.A., Salman S., Mueller I., Baiwog F., Gomorrai S., Law I., Page-Sharp M., Rogerson S., Siba P., Ilett K.F., Davis T.M. Pharmacokinetics of chloroquine and monodesethylchloroquine in pregnancy. Antimicrob Agents Chemother. 2010;54(3):1186–1192. doi: 10.1128/AAC.01269-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger G., Morad Y., Westall C.A., Laskin C., Spitzer K.A., Koren G., Ito S., Buncic R.J. Ocular toxicity and antenatal exposure to chloroquine or hydroxychloroquine for rheumatic diseases. Lancet. 2001;358(9284):813–814. doi: 10.1016/S0140-6736(01)06004-4. [DOI] [PubMed] [Google Scholar]
- Mayo Clinic. Chloroquine (oral route) record. Available at: https://www.mayoclinic.org/drugs-supplements/chloroquine-oral-route/description/drg-20062834.
- McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006, p. 859.
- Mosholder A.D., Mathew J., Alexander J.J., Smith H., Nambiar S. Cardiovascular risks with azithromycin and other antibacterial drugs. N Engl. J. Med. 2013;368(18):1665–1668. doi: 10.1056/NEJMp1302726. [DOI] [PubMed] [Google Scholar]
- Ndiaye N., Petrognani R., Diatta B., Seck M., Theobald X., Adnet P. Chloroquine poisoning with respiratory distress and fatal outcome. Ann. Fr. Anesth Reanim. 1999;18(6):683–685. doi: 10.1016/s0750-7658(99)80157-9. [DOI] [PubMed] [Google Scholar]
- Olson K.R. 4th ed. Lange Medical Books/McGraw-Hill; New York, N.Y: 2004. Poisoning & Drug Overdose; p. 166. [Google Scholar]
- Perinel, S., Launay, M., Botelho-Nevers, É., Diconne, É., Louf-Durier, A., Lachand, R., Murgier, M., Page, D., Vermesch, R., Thierry, G., Delavenne, X., 2020. Towards Optimization of Hydroxychloroquine Dosing in Intensive Care Unit COVID-19 Patients. Clin Infect Dis. 2020 Apr 7. pii: ciaa394. [Epub ahead of print] DOI: 10.1093/cid/ciaa394. [DOI] [PMC free article] [PubMed]
- Ray W.A., Murray K.T., Hall K., Arbogast P.G., Stein C.M. Azithromycin and the risk of cardiovascular death. N. Engl. J. Med. 2012;366(20):1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman S., Baiwog F., Page-Sharp M., Kose K., Karunajeewa H.A., Mueller I., Rogerson S.J., Siba P.M., Ilett K.F., Davis T.M.E. Optimal antimalarial dose regimens for chloroquine in pregnancy based on population pharmacokinetic modelling. Int. J. Antimicrob. Agents. 2017;50(4):542–551. doi: 10.1016/j.ijantimicag.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3(11):722–727. doi: 10.1016/s1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhi S., Singhi P., Singh M. Extrapyramidal syndrome following chloroquine therapy. Indian J. Pediatr. 1979;46(373):58–60. doi: 10.1007/bf02811499. [DOI] [PubMed] [Google Scholar]
- Smith E.R., Klein-Schwartz W. Are 1–2 dangerous? Chloroquine and hydroxychloroquine exposure in toddlers. J. Emerg. Med. 2005;28(4):437–443. doi: 10.1016/j.jemermed.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Stiff G., Robinson D., Cugnoni H.L., Touquet R., Dalton A.M. Massive chloroquine overdose–a survivor. Postgrad. Med. J. 1991;67(789):678–679. doi: 10.1136/pgmj.67.789.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanström H., Pasternak B., Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl. J. Med. 2013;368(18):1704–1712. doi: 10.1056/NEJMoa1300799. [DOI] [PubMed] [Google Scholar]
- Ursing J., Kofoed P.E., Rodrigues A., Bergqvist Y., Rombo L. Chloroquine is grossly overdosed and overused but well tolerated in Guinea-bissau. Antimicrob. Agents Chemother. 2009;53(1):180–185. doi: 10.1128/AAC.01111-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeeck R.K., Junginger H.E., Midha K.K., Shah V.P., Barends D.M. Biowaiver monographs for immediate release solid oral dosage forms based on biopharmaceutics classification system (BCS) literature data: chloroquine phosphate, chloroquine sulfate, and chloroquine hydrochloride. J. Pharm. Sci. 2005;94(7):1389–1395. doi: 10.1002/jps.20343. [DOI] [PubMed] [Google Scholar]
- WHO model prescribing information: drugs used in parasitic diseases, 2nd ed. https://apps.who.int/medicinedocs/en/d/Jh2922e/2.5.1.html.
- Wong, Y.K., Yang, J., He, Y., 2020. Caution and clarity required in the use of chloroquine for COVID-19. The Lancet. 2020. [Epub ahead of print] DOI: 10.1016/S2665-9913(20)30093-X. [DOI] [PMC free article] [PubMed]
- World Health Organization (WHO). INCHEM Chloroquine (PIM 123) record. Reviewed on 1994. Available at: http://www.inchem.org/documents/pims/pharm/chloroqu.htm.
- Yao, .X, Ye, F., Zhang, M., Cui, C., Huang, B., Niu, P., Liu, X., Zhao, L., Dong, E., Song, C., Zhan, S., Lu, R., Li, H., Tan, W., Liu, D., In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020. pii: ciaa237. [Epub ahead of print] DOI: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed]
- Zhao Q., Tensfeldt T.G., Chandra R., Mould D.R. Population pharmacokinetics of azithromycin and chloroquine in healthy adults and paediatric malaria subjects following oral administration of fixed-dose azithromycin and chloroquine combination tablets. Malar J. 2014;13:36. doi: 10.1186/1475-2875-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]