Abstract
Study objective
We seek to describe the medical history and clinical findings of patients attending the emergency department (ED) with suspected coronavirus disease 2019 (COVID-19) and estimate the diagnostic accuracy of patients’ characteristics for predicting COVID-19.
Methods
We prospectively enrolled all patients tested for severe acute respiratory syndrome coronavirus 2 by reverse-transcriptase polymerase chain reaction in our ED from March 9, 2020, to April 4, 2020. We abstracted medical history, physical examination findings, and the clinical probability of COVID-19 (low, moderate, and high) rated by emergency physicians, depending on their clinical judgment. We assessed diagnostic accuracy of these characteristics for COVID-19 by calculating positive and negative likelihood ratios.
Results
We included 391 patients, of whom 225 had positive test results for severe acute respiratory syndrome coronavirus 2. Reverse-transcriptase polymerase chain reaction result was more likely to be negative when the emergency physician thought that clinical probability was low, and more likely to be positive when he or she thought that it was high. Patient-reported anosmia and the presence of bilateral B lines on lung ultrasonography had the highest positive likelihood ratio for the diagnosis of COVID-19 (7.58, 95% confidence interval [CI] 2.36 to 24.36; and 7.09, 95% CI 2.77 to 18.12, respectively). The absence of a high clinical probability determined by the emergency physician and the absence of bilateral B lines on lung ultrasonography had the lowest negative likelihood ratio for the diagnosis of COVID-19 (0.33, 95% CI 0.25 to 0.43; and 0.26, 95% CI 0.15 to 0.45, respectively).
Conclusion
Anosmia, emergency physician estimate of high clinical probability, and bilateral B lines on lung ultrasonography increased the likelihood of identifying COVID-19 in patients presenting to the ED.
Introduction
Background
The novel coronavirus disease 2019 (COVID-19) outbreak has led to major reorganizations of emergency departments (EDs) to face the significant increase of patients with suspected severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 The clinical description of hospitalized patients has been reported in the literature,2, 3, 4, 5, 6 but to our knowledge no study has focused on clinical and diagnostic findings in the ED setting.
Editor’s Capsule Summary.
What is already known on this topic
The number of coronavirus disease 2019 (COVID-19) cases has been increasing significantly, but it can be challenging to diagnose this clinically.
What question this study addressed
What features are associated with a greater likelihood of COVID-19 in the emergency department?
What this study adds to our knowledge
In this prospective observational study of 391 patients for whom COVID-19 testing was performed, anosmia, emergency physician estimation of high clinical probability, and bilateral B lines on ultrasonography were associated with COVID-19 positivity.
How this is relevant to clinical practice
Emergency physicians should have a higher suspicion for COVID-19 in patients with these features.
Importance
Among patients attending EDs, rapid triage of those with suspected COVID-19 is mandatory to appropriately isolate them and avoid secondary transmissions. Clinical diagnosis can be challenging because the disease may present with nonspecific symptoms such as myalgia, cough, or fever.3 , 6 Medical history and clinical presentations of COVID-19 patients attending EDs need to be precisely described to facilitate early recognition by emergency physicians and promptly trigger diagnostic procedures such as real-time reverse-transcriptase polymerase chain reaction (RT-PCR).
Goals of This Investigation
The objectives of this study were to collect and describe the medical history and clinical findings of patients attending the ED who had suspected COVID-19, and to assess utility of clinical parameters, physician gestalt (clinical judgment), and lung ultrasonography to accurately identify COVID-19 patients at ED presentation.
Materials and Methods
Study Design and Setting
This prospective observational study was conducted in the ED of Saint-Louis University Hospital, Paris, France. Starting March 9, 2020, we prospectively enrolled a cohort of all adult patients with suspected COVID-19 who were tested for SARS-CoV-2. This study reports the results of patients enrolled until April 4, 2020. The study was approved by the institutional review board of the French Speaking Society for Respiratory Medicine–Société de Pneumologie de Langue Française. Our study followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.7
Selection of Participants and Methods of Measurement
All adult patients (≥18 years) who were tested for SARS-CoV-2 in our ED were included after giving oral consent. Cases were identified and enrolled 24 hours per day, 7 days per week during the study period by the attending emergency physician or resident who was in charge of the patient. Testing for SARS-CoV-2 in patients with suspected COVID-19 was left to the clinician’s discretion, but most of the time, patients were tested when they were dyspneic or reported shortness of breath; when they had comorbidities that put them at risk of severe infection such as immunosuppression, chronic respiratory insufficiency, cardiovascular diseases, and obesity; if they were older than 70 years; or if they were too weak to be discharged home. Some patients without clinical suspicion of COVID-19 but needing hospitalization in non–COVID-19 areas were also tested. Patients younger than 70 years, with no comorbidities, and with no respiratory symptoms were not tested. Before the outbreak, our ED received approximately 110 to 120 patients per day, and this number decreased to approximately 50 to 60 from the beginning of the pandemic in March. During the study period, we tested approximately 10 to 20 patients per day. If patients attended the ED more than once, only the last visit was included. There were no other exclusion criteria.
When testing for SARS-CoV-2, the attending emergency physician or resident physician was asked to report in a dedicated form the patient’s medical history; Eastern Cooperative Oncology Group Performance Status, which is a scale that describes patients’ ability to care for themselves and perform daily activities, ranging from 0 (fully active) to 4 (completely disabled); physical examination; and chest radiograph and lung ultrasonographic findings when those were performed. Lung ultrasonography was performed with a pocket-size device (VSCAN; GE Healthcare, Chicago, IL). After medical history, physical examination, ultrasonography, and chest radiographs, attending physicians were asked to rate the clinical probability of COVID-19 based on both their clinical judgment and a predefined 3-level scale (low, moderate, and high). Because anosmia was reported in Europe at approximately the end of March, this clinical sign was added to the form on March 24, 2020.
All study data and variables with their categories were defined before the beginning of the study. Four residents who were previously trained in data abstraction completed the forms with a dedicated spreadsheet. Age, sex, vital signs at ED arrival, and any data that were missing in the forms were abstracted retrospectively from the patients’ ED medical files, with the exception of clinical probability, which could be determined only prospectively. An emergency physician with expertise in research periodically monitored data abstraction. When there was disagreement between abstractors or if data were ambiguous, this emergency physician made the final decision. No assessment of interrater reliability was performed.
The criterion standard for diagnosis was the result of SARS-CoV-2 RT-PCR via nasal swab (Cobas SARS-CoV-2 Test; Roche, Meylan, France). The patients who initially had a negative RT-PCR result in the ED but a positive test result in the next 48 hours were considered as having positive results (initial false negative).
Primary Data Analysis
Continuous variables were reported as medians with their interquartile ranges. To assess the performance of each characteristic to accurately identify COVID-19, we calculated the sensitivity, specificity, positive predictive value, negative predictive value, positive and negative likelihood ratios, and their 95% confidence intervals (CIs). We calculated posttest probabilities depending on both pretest probabilities (5%, 10%, 25%, 50%, and 75%) and the presence or absence of bilateral B lines on lung ultrasonography. Accuracy of the physician clinical probability in identifying patients with COVID-19 was assessed with a receiver operating characteristic (ROC) curve and by calculating the area under the curve with its 95% CI. Data were analyzed with R 3.5.0 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of Study Subjects
During the study period, 400 patients were tested for SARS-CoV-2. After excluding 9 patients who were tested 2 times during 2 ED visits, we included 391 patients. Among those patients, 225 (57.6%) had positive test results for SARS-CoV-2 (including 5 initial false-negative results). Median age was 62 years (IQR, 48 to 71 years) and 150 (38.4%) were women. General characteristics of these patients with suspected COVID-19 are presented in Table 1 . Among patients with confirmed COVID-19, 67 (29.8%) were discharged home from the ED, 134 (59.5%) were hospitalized in wards, 22 (9.8%) were admitted to the ICU, and 2 (0.9%) died in the ED.
Table 1.
General characteristics of patients with suspected COVID-19.
| Variable | Total (N=391) | Missing Data, No. (%) |
|---|---|---|
| Age, median (IQR), y | 62 (48–71) | 0 |
| Female sex | 150 (38.4) | 0 |
| ECOG PS | 104 (26.6) | |
| 0–2 | 257 (89.5) | |
| 3–4 | 30 (10.5) | |
| Immunosuppression | 195 (50.5) | 5 (1.3) |
| Diabetes mellitus | 68 (17.6) | |
| Solid cancer | 58 (15.0) | |
| Hematologic malignancy | 47 (12.2) | |
| Solid organ transplant | 14 (3.6) | |
| HIV | 23 (6.0) | |
| Extended corticosteroid course | 22 (5.7) | |
| Other | 16 (4.1) | |
| Chronic lung disease | 85 (22.1) | 6 (1.5) |
| COPD | 24 (6.2) | |
| Asthma | 22 (5.7) | |
| Lung cancer | 8 (2.0) | |
| Bronchiectasis/emphysema | 8 (2.0) | |
| Sarcoidosis/fibrosis | 7 (1.8) | |
| Other | 23 (6.0) | |
| Cardiovascular disease | 156 (40.4) | 5 (1.3) |
| Hypertension | 122 (31.6) | |
| Atrial fibrillation | 29 (7.4) | |
| Coronary disease | 22 (5.7) | |
| Chronic heart failure | 16 (4.1) | |
| Other | 5 (1.3) | |
| Obesity | 58 (15.2) | 9 (2.3) |
| No comorbidity | 70 (18.1) | 5 (1.3) |
IQR, Interquartile range; ECOG PS, Eastern Cooperative Oncology Group Performance Status; COPD chronic obstructive pulmonary disease.
Data are provided as No. (%) unless otherwise indicated.
Main Results
Patient-reported symptoms, physical examination, lung ultrasonography, and chest radiographic findings in patients with or without COVID-19 are summarized in Table 2 . Among patients with confirmed COVID-19, 53 (23.6%) reported gastrointestinal symptoms such as vomiting, diarrhea, or abdominal pain, 147 (65.6%) had a temperature below 38°C (100.4°F), and 97 (43.3%) had a temperature below 37.5°C (98.6°F) on ED arrival (temperature was missing for 1 patient). When lung ultrasonography was performed on 48 patients (21.4%) with COVID-19, bilateral B lines were present in 36 (76.6%) of them.
Table 2.
Patient-reported symptoms and clinical and chest radiographic findings in patients with or without COVID-19.
| Variable | Total (N=391) | COVID-19 Positive (n=225) | COVID-19 Negative (n=166) | Missing Data, No. (%) |
|---|---|---|---|---|
| Patient-reported symptoms (%) | 1 (0.2) | |||
| Classic symptoms | ||||
| Fever | 259 (66.4) | 176 (78.2) | 83 (50.3) | |
| Cough | 239 (61.3) | 158 (70.2) | 81 (49.1) | |
| Dyspnea | 197 (50.5) | 131 (58.2) | 66 (40.0) | |
| Myalgia | 93 (23.8) | 71 (31.6) | 22 (13.3) | |
| Rhinitis/pharyngitis | 45 (11.5) | 19 (8.4) | 26 (15.8) | |
| Anosmia | 34 (8.7) | 31 (13.8) | 3 (1.8) | |
| None | 41 (10.5) | 10 (4.4) | 31 (18.8) | |
| Less classic symptoms | ||||
| Headache | 27 (6.9) | 15 (6.7) | 12 (7.3) | |
| Gastrointestinal symptoms | 94 (24.1) | 53 (23.6) | 41 (24.8) | |
| Fatigue | 55 (14.1) | 34 (15.1) | 21 (12.7) | |
| Chest pain | 24 (6.2) | 11 (4.9) | 13 (7.9) | |
| Dizziness/syncope | 21 (5.4) | 8 (3.6) | 13 (7.9) | |
| Hemoptysis | 4 (1.0) | 3 (1.3) | 1 (0.6) | |
| Symptom duration, median (IQR), days | 5 (3–8) | 7 (3–9) | 4 (2–7) | 37 (9.5) |
| Vital signs at ED arrival, median (IQR) | ||||
| SBP, mm Hg | 130 (113–143) | 129 (111–141) | 131 (116–145) | 6 (1.5) |
| DBP, mm Hg | 75 (65–85) | 75 (65–85) | 75 (66–85) | 7 (1.8) |
| PR, beats/min | 94 (84–109) | 93 (85–106) | 99 (82–110) | 8 (2.0) |
| Temperature, °C | 37.3 (36.6–38.0) | 37.6 (36.9–38.1) | 36.9 (36.4–37.6) | 6 (1.5) |
| RR, breaths/min | 22 (19–28) | 25 (20–30) | 20 (17–24) | 70 (17.9) |
| Oxygen saturation, % | 100 (96–100) | 99 (96–100) | 100 (97–100) | 1 (0.2) |
| Need for oxygen therapy, No. (%) | 131 (34.0) | 96 (43.0) | 35 (21.6) | 6 (1.5) |
| Oxygen delivery, median (IQR), L/min | 3 (2–5) | 3 (2–6) | 2 (2–3) | 5 (3.8) |
| Physical examination, No. (%) | ||||
| Altered mental status | 28 (7.2) | 15 (6.7) | 13 (7.8) | 1 (0.2) |
| Mottled skin | 34 (9.1) | 23 (10.7) | 11 (7.0) | 19 (4.9) |
| Lung auscultation | 7 (1.8) | |||
| Normal | 218 (56.8) | 106 (48.4) | 112 (67.9) | |
| Unilateral crackles | 33 (8.6) | 21 (9.6) | 12 (7.3) | |
| Bilateral crackles | 95 (24.7) | 80 (36.5) | 15 (9.1) | |
| Wheezing | 17 (4.4) | 4 (1.8) | 13 (7.9) | |
| Lung ultrasonography | 4 (1.0) | |||
| Not performed | 303 (78.3) | 176 (78.9) | 127 (77.4) | |
| No bilateral B lines | 44 (11.4) | 11 (4.9) | 33 (20.1) | |
| Bilateral B lines | 40 (10.3) | 36 (16.2) | 4 (2.5) | |
| Chest radiograph | 0 | |||
| Not performed | 262 (67.0) | 145 (64.4) | 117 (70.5) | |
| Normal | 48 (12.3) | 19 (8.4) | 29 (17.5) | |
| 1 lung involved | 11 (2.8) | 6 (2.7) | 5 (3.0) | |
| Both lungs involved | 43 (11.0) | 35 (15.6) | 8 (4.8) | |
| Other | 27 (6.9) | 20 (8.9) | 7 (4.2) |
SBP, Systolic blood pressure; DBP, diastolic blood pressure; PR, pulse rate; RR, respiratory rate.
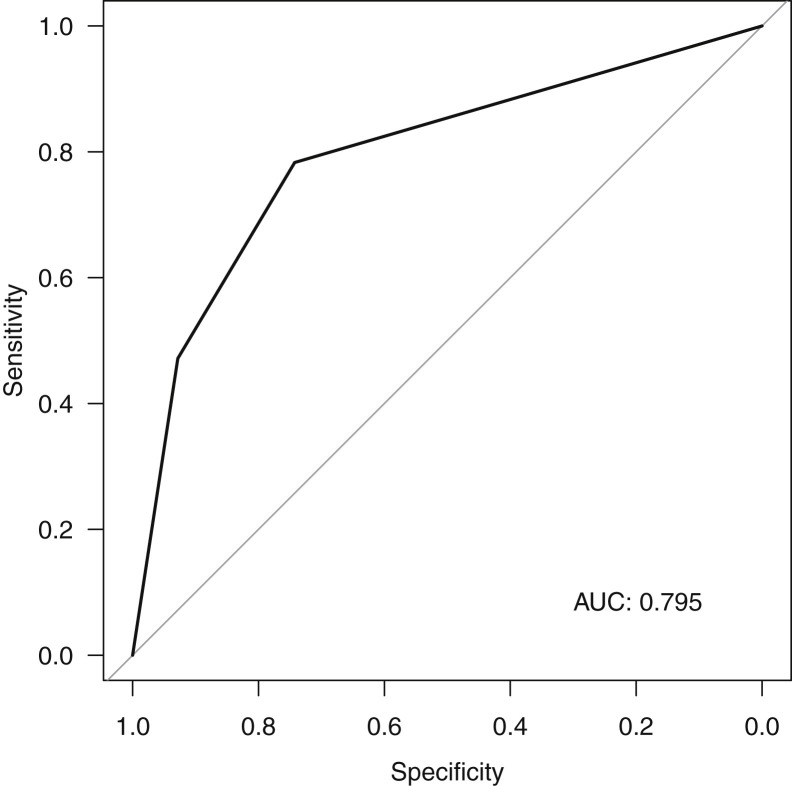
Emergency physicians rated the clinical probability for 273 patients. Table 3 shows the proportion of patients with or without COVID-19, depending on the emergency physician’s clinical probability. RT-PCR was more likely to be negative for SARS-CoV-2 when the emergency physician thought that clinical probability was low and more likely to be positive when he or she thought that it was high. The Figure shows the accuracy of physician clinical judgment in identifying COVID-19 patients (area under the curve=0.795; 95% CI 0.743 to 0.848).
Table 3.
Proportion of COVID-19 positive or negative results, depending on the emergency physician clinical probability.
| Clinical Probability (N=273) | RT-PCR Result for SARS-CoV-2, |
|
|---|---|---|
| No. (%) | ||
| Positive (n=167) | Negative (n=106) | |
| Low | 12 (19.4) | 50 (80.6) |
| Moderate | 31 (48.4) | 33 (51.6) |
| High | 124 (84.4) | 23 (15.6) |
Figure.
ROC curve. Accuracy of the emergency physician clinical probability in identifying patients with COVID-19. AUC, Area under the curve.
Table 4 shows the sensitivity, specificity, positive and negative predictive value, and positive and negative likelihood ratio for some of the patient-reported symptoms, clinical ultrasonographic findings, and chest radiographic findings. Patient-reported anosmia and the presence of bilateral B lines on lung ultrasonography had the highest positive likelihood ratio for the diagnosis of COVID-19 (7.58, 95% CI 2.36 to 24.36; and 7.09, 95% CI 2.77 to 18.12 respectively). The absence of a high clinical probability determined by the emergency physician and the absence of bilateral B lines on lung ultrasonography had the lowest negative likelihood ratio for the diagnosis of COVID-19 (0.33, 95% CI 0.25 to 0.43; and 0.26, 95% CI 0.15 to 0.45, respectively).
Table 4.
Clinical and chest radiographic findings accuracy for the diagnosis of COVID-19.
| Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR– (95% CI) | |
|---|---|---|---|---|---|---|
| Patient-reported symptoms | ||||||
| Fever | 0.78 (0.72–0.83) | 0.50 (0.42–0.58) | 0.68 (0.62–0.74) | 0.63 (0.54–0.71) | 1.56 (1.32–1.84) | 0.44 (0.33–0.59) |
| Dyspnea | 0.58 (0.51–0.65) | 0.60 (0.52–0.68) | 0.66 (0.59–0.73) | 0.51 (0.44–0.59) | 1.46 (1.17–1.81) | 0.70 (0.57–0.85) |
| Myalgia | 0.32 (0.26–0.38) | 0.87 (0.81–0.91) | 0.76 (0.66–0.85) | 0.48 (0.42–0.54) | 2.37 (1.53–3.65) | 0.79 (0.71–0.88) |
| Anosmia | 0.14 (0.10–0.19) | 0.98 (0.95–1.00) | 0.91 (0.76–0.98) | 0.46 (0.40–0.51) | 7.58 (2.36–24.36) | 0.88 (0.83–0.93) |
| Vital signs at ED arrival | ||||||
| Temperature ≥38 °C | 0.34 (0.28–0.41) | 0.83 (0.76–0.88) | 0.73 (0.64–0.81) | 0.47 (0.42–0.54) | 1.98 (1.35–2.90) | 0.79 (0.71–0.89) |
| Oxygen saturation <95% | 0.17 (0.12–0.22) | 0.91 (0.85–0.95) | 0.72 (0.58–0.83) | 0.45 (0.39–0.50) | 1.86 (1.06–3.26) | 0.91 (0.85–0.99) |
| RR >25 breaths/min | 0.47 (0.39–0.54) | 0.78 (0.70–0.85) | 0.76 (0.68–0.84) | 0.49 (0.42–0.56) | 2.13 (1.49–3.06) | 0.68 (0.58–0.80) |
| Oxygen flow ≥6 L/min | 0.28 (0.19–0.38) | 0.91 (0.75–0.98) | 0.90 (0.73–0.98) | 0.30 (0.21–0.40) | 2.95 (0.96–9.09) | 0.80 (0.68–0.94) |
| Physical examination | ||||||
| Bilateral lung crackles | 0.37 (0.30–0.43) | 0.91 (0.85–0.95) | 0.84 (0.75–0.91) | 0.52 (0.46–0.58) | 4.02 (2.41–6.71) | 0.70 (0.62–0.78) |
| Lung ultrasonography | ||||||
| Bilateral B lines | 0.77 (0.62–0.88) | 0.89 (075–0.97) | 0.90 (0.76–0.97) | 0.75 (0.60–0.87) | 7.09 (2.77–18.12) | 0.26 (0.15–0.45) |
| Chest radiograph | ||||||
| Both lungs involved | 0.16 (0.11–0.21) | 0.96 (0.91–0.98) | 0.83 (0.69–0.93) | 0.45 (0.40–0.51) | 3.67 (1.67–8.05) | 0.88 (0.83–0.94) |
| High clinical probability | 0.74 (0.67–0.81) | 0.78 (0.69–0.86) | 0.84 (0.77–0.90) | 0.66 (0.57–0.74) | 3.42 (2.36–4.97) | 0.33 (0.25–0.43) |
PPV, Positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR–, negative likelihood ratio.
Table E1 (available online at http://www.annemergmed.com) shows the posttest probability, depending on the pretest probability (ie, prevalence) and the results of lung ultrasonography.
Limitations
Our study has several limitations. First, not every patient was tested for SARS-CoV-2 and testing was left to the clinician’s discretion. However, despite the absence of clear predefined inclusion criteria, testing was performed in the majority of cases for patients who had severe symptoms such as dyspnea, reported shortness of breath, presented with comorbidities (eg, immunosuppression, chronic pulmonary or cardiovascular diseases), or were older than 70 years. Thus, our results may not be valid in other populations such as young people without comorbidities and those with few symptoms. Also, because our results are from a single center in France, they may not be generalizable to other centers. Second, it is possible that patients did not systematically report their symptoms, and this might have decreased the estimates of their prevalence. Third, we may have underestimated anosmia because we added this to the form only on March 24, 2020. Fourth, lung ultrasonography was not systematic and occurred for only 22.3% of the patients, which contributed to the large 95% CI observed. It is possible that emergency physicians performed lung ultrasonography in the most severe cases and that the accuracy of this examination in predicting COVID-19 is likely to be lower in patients with fewer symptoms. Fifth, our criterion standard to diagnose COVID-19 was based on the RT-PCR, which may have yielded false-negative results. To address this, we also evaluated patients who initially had negative test results for SARS-CoV-2 but were then hospitalized and secondarily had positive results, and considered them as having positive results.
Discussion
To the best of our knowledge, this is the first prospective study that described patient-reported symptoms and physical examination findings in a large cohort of patients with suspected COVID-19 who attended the ED, as well as the first prospective study that estimated the accuracy of clinical findings for the diagnosis of COVID-19.
At the beginning of the epidemic in France, knowledge of the COVID-19 clinical picture was mainly extrapolated from cases in Wuhan, China. Emergency physicians prepared to face an unknown disease and attend to patients with nonspecific influenza-like symptoms with clinical signs of lung infection, or acute respiratory failure for the most severe cases.3 , 6 Since then, studies have been published about the epidemiology,1 the risk factors for severe disease,4 and the description of critically ill patients8, 9, 10, 11 infected with SARS-CoV-2, but there are limited data regarding the accuracy of clinical findings in patients with suspected COVID-19. Moreover, most studies have focused on patients who were already hospitalized for the disease, which may differ from the ED population.
Prompt identification of possible cases is mandatory to avoid the spread of the virus by patients with mild or nonspecific symptoms.12 Therefore, emergency physicians need to be cautious when they evaluate such patients and be aware of some pitfalls. Whereas fever (temperature >37.3°C) was reported in more than 90% of the patients hospitalized with COVID-19,4 , 6 we found that even if 78.2% of the patients reported fever, only 34.4% of the patients had a temperature greater than or equal to 38°C and 56.7% had one greater than or equal to 37.5°C at ED triage. It is possible that the temperature was initially decreased by antipyretics and subsequently increased during the ED stay. Nevertheless, temperature should not be used in isolation to exclude COVID-19.13 Consistent with other studies, we found that the most frequent reported symptoms were fever, cough, dyspnea, and myalgia.2, 3, 4, 5, 6 Gastrointestinal symptoms were present in 23.6% of our patients, whereas 1 small study of 18 patients found a rate of 17%14 and larger cohorts have reported these symptoms in less than 10%.2, 3, 4, 5, 6 Anosmia was reported by 13.8% of the patients in our cohort and was the most specific sign of COVID-19. It is likely that we underestimated this sign, which was not initially described in the Chinese literature and was reported in Europe at approximately the end of March.15, 16, 17
Other findings such as bilateral crackles on lung auscultation or the rapid need for high levels of oxygen delivery at ED arrival were highly suggestive of COVID-19, especially among middle-aged or older patients with comorbidities such as diabetes or cardiovascular diseases.18 Because this disease induces endotheliitis, leading to vascular derangements,19 it is likely that new symptoms involving multiple organs such as neurologic20 or skin disorders21 will emerge.
Besides clinical signs, lung imaging and particularly computed tomographic scans have been shown to have a high sensitivity for the diagnosis of COVID-19, particularly in severe cases, and may be valuable in patients with high clinical probability but negative RT-PCR results.22, 23, 24 Another option might be to perform lung ultrasonography, allowing rapid diagnosis and severity assessment at patient bedside for suspected COVID-19.25 , 26 In our study, the presence or absence of bilateral B lines with a pocket-size ultrasonographic device had the higher positive likelihood ratio and a low negative likelihood ratio, respectively.
In a study with 20 patients with COVID-19, Xing et al27 found that all patients showed pleural-line abnormalities and bilateral B lines on lung ultrasonography, regardless of the severity or stage of the disease. In another retrospective study that included 30 patients with COVID-19, interstitial pulmonary edema was present on lung ultrasonography in 90.0% of the cases.28 Nonetheless, literature on this topic is scarce and more data are needed.29
In the present study, we found that emergency physician clinical judgment was accurate and that only 19.4% of the patients with low clinical probability had COVID-19, whereas 15.6% of the patients with high clinical probability did not have it. The area under the curve of 0.795 seems fair for a new disease with few specific clinical signs. Emergency physician gestalt has been studied in predicting other diseases and performed equally well, with an observed area under the curve of 0.83 for appendicitis in children,30 0.75 for acute coronary syndrome,31 0.86 for acute heart failure syndrome,32 and 0.81 for pulmonary embolism.33
In summary, in this large prospective cohort study of patients attending the ED for suspected COVID-19, anosmia, emergency physician estimate of high clinical probability, and bilateral B lines on lung ultrasonography increased the likelihood of identifying COVID-19. Future studies should assess this in other EDs and the role of combining findings to develop clinical decision tools.
Acknowledgments
The authors acknowledge Pierre Bourrier, MD, for his lessons about echostethoscopy and the medical emergency team of Saint-Louis Hospital for their participation in this study.
Footnotes
Please see page 406 for the Editor’s Capsule Summary of this article.
Supervising editors: Michael Gottlieb, MD; Steven M. Green, MD. Specific detailed information about possible conflict of interest for individual editors is available at https://www.annemergmed.com/editors.
Author contributions: OP, SE, and J-PF were responsible for study conception and design. OP, CM-G, VT, MG, CR, KK, LL, MS, AE, AP, PT, CO, and SE were responsible for provision of study materials for patients. OP, CM-G, VT, MG, CR, and SE were responsible for collection and assembly of data. OP was responsible for data analysis and interpretation. OP, MT, and JPF were responsible for writing the article. All authors approved the final article. OP takes responsibility for the paper as a whole.
All authors attest to meeting the four ICMJE.org authorship criteria: (1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND (2) Drafting the work or revising it critically for important intellectual content; AND (3) Final approval of the version to be published; AND (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding and support: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Readers: click on the link to go directly to a survey in which you can provide feedback to Annals on this particular article.
A podcast for this article is available at www.annemergmed.com.
Supplementary Data
Post-test probability depending on the pre-test probability (i.e. disease prevalence) and the results of lung US (presence or absence of B-lines)
References
- 1.Park M., Cook A.R., Lim J.T. A systematic review of COVID-19 epidemiology based on current evidence. J Clin Med. 2020;9:967. doi: 10.3390/jcm9040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X.W., Wu X.X., Jiang X.G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandenbroucke J.P., von Elm E., Altman D.G. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G., Zangrillo A., Zanella A. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Y., Tu L., Zhu P. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region. Case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J.Y., Yun J.G., Noh J.Y. Covid-19 in South Korea. Challenges of subclinical manifestations. N Engl J Med. 2020;382:1858–1859. doi: 10.1056/NEJMc2001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y.Y., Jin Y.H., Ren X.Q., Zhongnan Hospital of Wuhan University Novel Coronavirus Management and Research Team Updating the diagnostic criteria of COVID-19 “suspected case” and “confirmed case” is necessary. Mil Med Res. 2020;7:17. doi: 10.1186/s40779-020-00245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young B.E., Ong S.W.X., Kalimuddin S. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gane S.B., Kelly C., Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020 doi: 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- 16.Vaira L.A., Salzano G., Deiana G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y., Yu X., Zhao H. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouaziz J.D., Duong T., Jachiet M. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long C., Xu H., Shen Q. Diagnosis of the coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126:108961. doi: 10.1016/j.ejrad.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He J.L., Luo L., Luo Z.D. Diagnostic performance between CT and initial real-time RT-PCR for clinically suspected 2019 coronavirus disease (COVID-19) patients outside Wuhan, China. Respir Med. 2020;168:105980. doi: 10.1016/j.rmed.2020.105980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vetrugno L., Bove T., Orso D. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography. 2020;37:625–627. doi: 10.1111/echo.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soldati G., Smargiassi A., Inchingolo R. Proposal for international standardization of the use of lung ultrasound for COVID-19 patients: a simple, quantitative, reproducible method. J Ultrasound Med. 2020 doi: 10.1002/jum.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing C., Li Q., Du H. Lung ultrasound findings in patients with COVID-19 pneumonia. Crit Care. 2020;24:174. doi: 10.1186/s13054-020-02876-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu W., Zhang S., Chen B. A clinical study of noninvasive assessment of lung lesions in patients with coronavirus disease-19 (COVID-19) by bedside ultrasound. Ultraschall Med. 2020 doi: 10.1055/a-1154-8795. [DOI] [PubMed] [Google Scholar]
- 29.Fiala M.J. A brief review of lung ultrasound in COVID-19: is it useful? Ann Emerg Med. 2020 doi: 10.1016/j.annemergmed.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon L.E., Kene M.V., Warton E.M. Diagnostic performance of emergency physician gestalt for predicting acute appendicitis in patients age 5 to 20 years. Acad Emerg Med. 2020 doi: 10.1111/acem.13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver G., Reynard C., Morris N. Can emergency physician gestalt “rule in” or “rule out” acute coronary syndrome: validation in a multicenter prospective diagnostic cohort study. Acad Emerg Med. 2020;27:24–30. doi: 10.1111/acem.13836. [DOI] [PubMed] [Google Scholar]
- 32.Roncalli J., Picard F., Delarche N. Predictive criteria for acute heart failure in emergency department patients with acute dyspnoea: the PREDICA study. Eur J Emerg Med. 2019;26:400–404. doi: 10.1097/MEJ.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 33.Penaloza A., Verschuren F., Meyer G. Comparison of the unstructured clinician gestalt, the Wells score, and the revised Geneva score to estimate pretest probability for suspected pulmonary embolism. Ann Emerg Med. 2013;62:117–124.e2. doi: 10.1016/j.annemergmed.2012.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Post-test probability depending on the pre-test probability (i.e. disease prevalence) and the results of lung US (presence or absence of B-lines)