To the Editor:
Chronic liver disease (CLD) and cirrhosis are common conditions1 associated with immune dysregulation,2 leading to concerns that these patients are at increased risk of complications from COVID-19 resulting from infection with SARS-CoV-2.3 However, the effects of COVID-19 among patients with pre-existing liver disease are currently undefined.
We report the outcomes of the first 152 consecutive submissions of clinician-reported cases of laboratory-confirmed COVID-19 in patients with CLD to two international reporting registries (COVID-Hep.net and COVIDCirrhosis.org) between 25 March 2020 and 20 April 2020. Our combined database includes 103 patients with cirrhosis and 49 with non-cirrhotic CLD from 21 countries across 4 continents (59.9% male, median age 61 years, aetiology: 22.4% non-alcoholic fatty liver disease, 19.7% alcohol, 11.8% hepatitis B, 10.5% hepatitis C, 35.6% other/combination).
Contributors were encouraged to enter data at the end of the patient's disease course. For patients admitted to hospital, cases were only included in the analysis if a definitive outcome of death or discharge was reported. 95.2% of patients with cirrhosis were hospitalised with a median length of hospital stay until discharge or death of 10 days (IQR 5–14 days). Outcomes for patients with cirrhosis included admission to intensive care unit (ICU) in 23.3%, invasive ventilation in 17.5%, non-invasive ventilatory support in 18.6%, renal replacement therapy in 4.9% and death in 39.8%. Mortality far exceeded that reported in unselected populations,4 hospitalised patients with cirrhosis in the era preceding COVID-19,5 and in patients with cirrhosis admitted with influenza.6 In patients not admitted to ICU, 59.5% had non-severe disease, 27.8% had severe disease but escalation of care was deemed inappropriate, and 3.7% were considered to require ICU but were not admitted due to lack of availability. Targeted antiviral therapy was used in 38.1% of total cases. The most frequently used treatments were chloroquine/hydroxychloroquine (23.0%), lopinovir/ritonavir (6.6%), tocilizumab (3.3%), and interferon-alpha (3.3%).
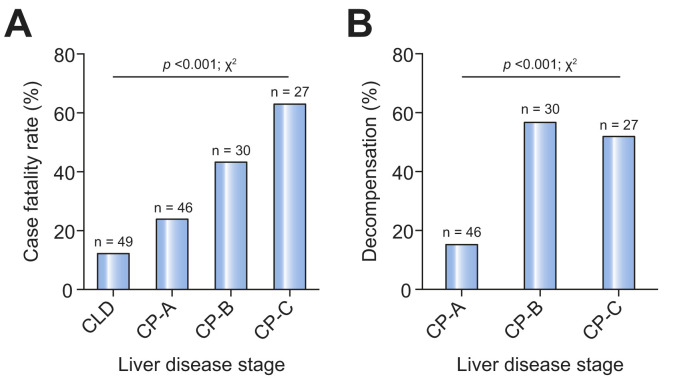
Cause of death in patients with cirrhosis was reported as COVID-19 lung disease in 78.7%, cardiac-related in 4.3%, and liver-related in 12.2%. Risk factors for poor COVID-19 outcomes in the general population, including advanced age, obesity, renal impairment, heart disease, and diabetes mellitus, were over-represented among those who died, although male sex and non-white ethnicity were not.7 Mortality correlated strongly with baseline Child-Pugh class and model for end-stage liver disease (MELD) score (Table 1 ). Deaths occurred in 12.2% of patients with CLD without cirrhosis, 23.9% with Child-Pugh class A cirrhosis, 43.3% with Child-Pugh class B cirrhosis, and 63.0% with Child-Pugh class C cirrhosis (Fig. 1 A). Child-Pugh class B and C cirrhosis remained associated with death after adjusting for baseline characteristics including comorbidities (Table 1). Child-Pugh class B and C cirrhosis remained significant predictors of mortality when analysis was restricted to those with cirrhosis.
Table 1.
Characteristics of patients with laboratory-confirmed chronic liver disease and COVID-19 submitted to COVIDCirrhosis.org and COVID-Hep.net reporting registries between 25th March 2020 and 20th April 2020.
| Variable | Univariable analysis |
Multivariable analysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total; n = 152 |
Survived; n = 105 |
Died; n = 47 |
p value† | OR (95%CI) for death | p value§ | ||||
| Median or n | IQR or % | Median or n | IQR or % | Median or n | IQR or % | ||||
| Age (years) | 61 | 48–71 | 60 | 46–70 | 64 | 57–73 | 0.025 | 1.04 (1.00–1.09) | 0.048 |
| Sex (male) | 91 | 59.9% | 61 | 58.1% | 30 | 63.8% | 0.666 | – | – |
| White ethnicity | 86 | 56.6% | 56 | 53.3% | 30 | 63.8% | 0.228 | – | – |
| Smoker | 9 | 5.9% | 7 | 6.7% | 2 | 4.3% | 0.560 | – | – |
| Obese (BMI >30 kg/m2) | 33 | 21.7% | 18 | 17.1% | 15 | 31.9% | 0.017 | 3.59 (1.10–10.47) | 0.033 |
| Cardiovascular disease | 33 | 21.7% | 18 | 17.1% | 15 | 31.9% | 0.041 | 1.87 (0.57–6.15) | 0.303 |
| Diabetes mellitus | 54 | 35.5% | 30 | 28.6% | 24 | 51.1% | 0.007 | 2.86 (1.00–8.20) | 0.051 |
| Hypertension | 60 | 39.5% | 35 | 33.3% | 25 | 53.2% | 0.021 | 0.71 (0.22–2.24) | 0.555 |
| Liver disease severity | |||||||||
| CLD without cirrhosis | 49 | 32.2% | 43 | 41.0% | 6 | 12.8% | <0.001 | 1.00 | – |
| Child-Pugh A | 46 | 30.3% | 35 | 33.3% | 11 | 23.4% | <0.001 | 1.21 (0.30–4.90) | 0.789 |
| Child-Pugh B | 30 | 19.7% | 17 | 16.2% | 13 | 27.7% | <0.001 | 4.90 (1.16–20.61) | 0.030 |
| Child-Pugh C | 27 | 17.8% | 10 | 9.5% | 17 | 36.2% | <0.001 | 28.07 (4.42–178.46) | <0.001 |
| MELD score∗ | 10 | 7–17 | 9 | 7–17 | 13 | 9–17 | 0.014 | – | – |
| Laboratory (baseline) | |||||||||
| Sodium (mmol/L) | 138 | 135–141 | 139 | 136–141 | 137 | 134–140 | 0.058 | 1.06 (0.93–1.22) | 0.377 |
| Prothrombin time (s) | 13 | 12–17 | 13 | 12–15 | 15 | 13–18 | 0.011 | – | – |
| Bilirubin (mg/dl) | 1.1 | 0.6–1.9 | 0.9 | 0.5–1.5 | 1.4 | 0.8–2.0 | 0.013 | – | – |
| Albumin (g/dl) | 3.4 | 2.8–4 | 3.8 | 3.0–4.0 | 2.9 | 2.4–3.3 | <0.001 | – | – |
| Creatinine (mg/dl) | 0.9 | 0.6–1.1 | 0.8 | 0.6–1.0 | 0.9 | 0.7–1.1 | 0.010 | 0.88 (0.53–1.47) | 0.634 |
| Events after diagnosis | |||||||||
| Any decompensation | 39 | 25.7% | 15 | 14.3% | 24 | 51.1% | <0.001 | – | – |
| New jaundice | 27 | 17.8% | 14 | 13.3% | 13 | 27.7% | 0.067 | – | – |
BMI, body mass index; CLD, chronic liver disease; MELD, model for end-stage liver disease (2016 revision); OR, odds ratio.
MELD score presented is as calculated for all patients; when restricted to patients with cirrhosis, MELD was 11 (IQR 7–19) in those who survived and 14 (9–17) in those who died, p = 0.136. To explore the relationship of MELD with death, multiple logistic regression was repeated with death as the dependent variable and age, baseline MELD, obesity, cardiovascular disease, diabetes mellitus, hypertension, and baseline albumin as independent variables; here the OR for death for MELD was 1.05 (0.98–1.11) p = 0.204; other variables with p <0.05 were age 1.05 (1.00–1.08) p = 0.038, obesity 3.61 (1.36–9.60) p = 0.010, and baseline albumin 0.97 (0.93–1.00) p = 0.029. Any decompensation defined as one or more of worsening ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, or variceal haemorrhage.
p values for univariable analyses calculated using chi-squared or Wilcoxon ranksum tests as appropriate. p values <0.05 in bold.
p values for multivariable analysis calculated by multiple logistic regression with the dependent variable as death and the following independent variables: age, obesity, cardiovascular disease, diabetes mellitus, hypertension, chronic liver disease status as Child-Pugh class, baseline serum sodium, and baseline serum creatinine. p values <0.05 in bold.
Fig. 1.
Outcome in patients with non-cirrhotic chronic liver disease or cirrhosis with COVID-19.
Graphs depict data from 152 submissions to COVID-Hep.net and COVIDCirrhosis.org registries between 25th March 2020 and 20th April 2020. (A) Case fatality rate by liver disease stage. (B) Rates of hepatic decompensation by stage of cirrhosis (defined as one or more of new or worsened ascites, spontaneous bacterial peritonitis, new or worsened hepatic encephalopathy, or variceal haemorrhage). p values derived using chi-squared test. CLD, chronic liver disease without cirrhosis; CP, Child-Pugh.
Hepatic decompensation occurred in 36.9% and was associated with baseline Child-Pugh class (Fig. 1B). Decompensation events included worsening ascites (27.2%), spontaneous bacterial peritonitis (2.9%), hepatic encephalopathy (16.5%), and variceal haemorrhage (1%). Hepatic decompensation during COVID-19 was strongly associated with a subsequent risk of death: 63.2% of those with new decompensation died compared to 26.2% of those without new decompensation. Notably, 24.3% of those with new hepatic decompensation had no respiratory symptoms of COVID-19 at the time of diagnosis.
This large, multicentre, international cohort of patients with chronic liver disease and cirrhosis allows for in-depth assessment of the clinical factors associated with poor outcomes from COVID-19. Accepting that data from registries are subject to selection bias, preliminary findings suggest that baseline liver disease severity is strongly associated with COVID-19-related morbidity and mortality. Furthermore, many SARS-CoV-2-infected patients with cirrhosis experienced hepatic decompensation even in the absence of respiratory symptoms. These findings have important implications for clinicians regarding risk stratification and prognostication for patients with cirrhosis and COVID-19 and suggest the need to maintain a low threshold for SARS-CoV-2 testing in the presence of new hepatic decompensation.
Financial support
This work was supported by the National Institutes of Health grant T32 DK007634 (AMM and TWJ). We acknowledge the assistance of the NC Translational and Clinical Sciences (NC TraCS) Institute, which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002489. COVID-Hep.net was supported by the European Association for Study of the Liver 2020RG03 (TM).
Authors' contributions
Concept and Design: TM, AMM, EB, ASB, GW, TWJ. Acquistion of data: CA, MJA, TC, RD, JG, USG, TWJ, PDJ, AM, GM, PVP, XQ, FS, NNU. Statistical analysis: TM, AMM, GW. Interpretation of data: TM, AMM, EB, ASB, GW. Drafting and critical revision of manuscript: TM, AMM, EB, ASB, GW.
Conflicts of interest
The authors have no conflicts of interest or competing interests to disclose.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2020.05.013.
Supplementary data
References
- 1.Moon A.M., Singal A.G., Tapper E.B. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2019;(S1542–3565(19)) doi: 10.1016/j.cgh.2019.07.060. 30849–30844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albillos A., Lario M., Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61(6):1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Boettler T., Newsome P.N., Mondelli M.U., Maticic M., Cordero E., Cornberg M. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2(3):100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;(NEJMoa2007621) doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Schmidt M.L., Barritt A.S., Orman E.S., Hayashi P.H. Decreasing mortality among patients hospitalized with cirrhosis in the United States from 2002 through 2010. Gastroenterology. 2015;148(5):967–977.e2. doi: 10.1053/j.gastro.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schütte A., Ciesek S., Wedemeyer H., Lange C.M. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatol. 2019;70(4):797–799. doi: 10.1016/j.jhep.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.