Abstract
In Ontario on March 16, 2020, a directive was issued to all acute care hospitals to halt nonessential procedures in anticipation of a potential surge in COVID-19 patients. This included scheduled outpatient cardiac surgical and interventional procedures that required the use of intensive care units, ventilators, and skilled critical care personnel, given that these procedures would draw from the same pool of resources required for critically ill COVID-19 patients. We adapted the COVID-19 Resource Estimator (CORE) decision analytic model by adding a cardiac component to determine the impact of various policy decisions on the incremental waitlist growth and estimated waitlist mortality for 3 key groups of cardiovascular disease patients: coronary artery disease, valvular heart disease, and arrhythmias. We provided predictions based on COVID-19 epidemiology available in real-time, in 3 phases. First, in the initial crisis phase, in a worst case scenario, we showed that the potential number of waitlist related cardiac deaths would be orders of magnitude less than those who would die of COVID-19 if critical cardiac care resources were diverted to the care of COVID-19 patients. Second, with better local epidemiology data, we predicted that across 5 regions of Ontario, there may be insufficient resources to resume all elective outpatient cardiac procedures. Finally in the recovery phase, we showed that the estimated incremental growth in waitlist for all cardiac procedures is likely substantial. These outputs informed timely data-driven decisions during the COVID-19 pandemic regarding the provision of cardiovascular care.
Résumé
Le 16 mars 2020, le gouvernement de l’Ontario a émis une directive demandant à tous les hôpitaux de soins de courte durée de cesser d’effectuer des interventions non essentielles en prévision d’une envolée possible du nombre de cas de COVID-19. Les interventions à reporter comprenaient les opérations et les autres interventions en cardiologie ambulatoire pouvant exiger un séjour à l’unité de soins intensifs, l’utilisation d’un respirateur et l’intervention du personnel spécialisé en soins intensifs, puisque de telles interventions font appel aux mêmes ressources que celles qui sont nécessaires pour traiter les patients atteints de COVID-19 dont l’état est critique. Nous avons adapté le modèle d’analyse décisionnelle CORE (COVID-19 Resource Estimator) en y intégrant une composante cardiologique, afin de déterminer l’incidence de diverses décisions stratégiques sur l’allongement des listes d’attente et sur le taux de mortalité estimatif des patients inscrits sur les listes d’attente pour trois grandes catégories de maladies cardiovasculaires : les coronaropathies, les cardiopathies valvulaires et les arythmies. Nous avons formulé des prédictions à partir des données épidémiologiques sur la COVID-19 disponibles en temps réel à trois phases de la crise. Durant la première phase de la crise, nous avons d’abord montré, à l’aide d’une simulation fondée sur la situation la plus défavorable, que le nombre potentiel de décès d’origine cardiovasculaire chez les patients inscrits sur les listes d’attente serait de plusieurs fois inférieur au nombre de décès attribuables à la COVID-19 si les ressources essentielles en cardiologie étaient réorientées vers les soins aux patients atteints de COVID-19. Ensuite, grâce à de meilleures données épidémiologiques locales, nous avons prédit que dans cinq régions de l’Ontario, les ressources pourraient être insuffisantes pour permettre la reprise de toutes les interventions non urgentes en cardiologie ambulatoire. Enfin, durant la phase de stabilisation, nous avons montré que l’allongement estimatif des listes d’attente pour toutes les interventions en cardiologie est vraisemblablement important. Ces résultats ont éclairé la prise rapide de décisions fondées sur des données en matière de prestation de soins cardiovasculaires durant la pandémie de COVID-19.
The rapidly evolving COVID-19 pandemic has necessitated anticipatory policy decision making in the presence of uncertainty. In the initial crisis phase of the pandemic, there were substantial concerns that available health care resources would be overwhelmed. In Ontario, the Ministry of Health and Long-Term Care (MOHLTC) issued a directive on March 16, 2020, to all acute care hospitals to halt nonessential procedures in anticipation of a potential surge in COVID-19 patients. This included scheduled outpatient cardiac surgical and interventional procedures that required the use of intensive care units (ICUs), ventilators, and skilled critical care personnel and was broadly consistent with early guidance from the Canadian Cardiovascular Society and later recommendations from the Canadian Association of Interventional Cardiology and the Canadian Society of Cardiac Surgeons.1 , 2 However socially responsible, the magnitude of the immediate and long-term consequences of delaying cardiac care is not known. The use of simulation modelling allows one to balance the potential needs of COVID-19 patients against that of delaying non–COVID-19 care by explicitly estimating resource utilization and constraints in conjunction with predictions in COVID-19 infection rates and hospitalizations. Herein, we provide an overview of an approach to modelling coronary artery disease, electrophysiology procedures, and valvular heart disease with the use of historic referral and procedural volume data from a provincial clinical registry along with ICU and ward length of stay data from provincial administrative data. Our overall goals were to better understand the consequences of initially curtailing and subsequently resuming cardiac procedures during the pandemic, and to be able to provide timely data-informed predictions to facilitate policy decisions. We provide an example of how such modelling was used through the arc of the COVID-19 pandemic in Ontario, through the initial crisis phase of the pandemic with substantial unknowns, a second phase with local epidemiology data available, and finally the recovery phase.
Overview of Model Structure and Inputs
The cardiac modelling group consisted of a multidisciplinary team of graduate students, clinician-scientists, and academics with expertise in cardiology/cardiac surgery, infectious disease, and advanced decision analytic modelling. We engaged our primary sponsor, CorHealth Ontario, to understand the needs of the type of predictions required to make timely policy decisions. CorHealth Ontario is responsible for providing recommendations to the MOHLTC on the provision of cardiac, vascular, and stroke care. The modelling was performed iteratively to contend with the rapid evolution of the pandemic, and the model output was assessed at biweekly meetings with participating entities to inform policy decisions.
A parallel, open-cohort, dynamic, individual patient–level microsimulation (COVID-19 Resource Estimator [CORE]) model to predict hospital resource utilization of COVID-19 patients presenting daily to hospital from the perspective of the Ontario health care system was created by researchers and clinicians at the University of Toronto’s COVID-19 Modelling Collaborative team.3 An interactive version of the model is publicly available (https://www.covid-19-mc.ca). In this model, a daily influx of symptomatic COVID-19 patients (based on patterns of predicted epidemiology at that time) entered the hospital system to compete for a pool of fixed health care resources. By running the model over the specified time horizon of the pandemic, this model allowed for the estimation of daily ward, ICU, and ventilator use at the provincial level over the course of the pandemic. Findings from the CORE model were used to set policy and inform technical press briefings by Ontario Health, the MOHLTC, and the Ontario Premier’s office.
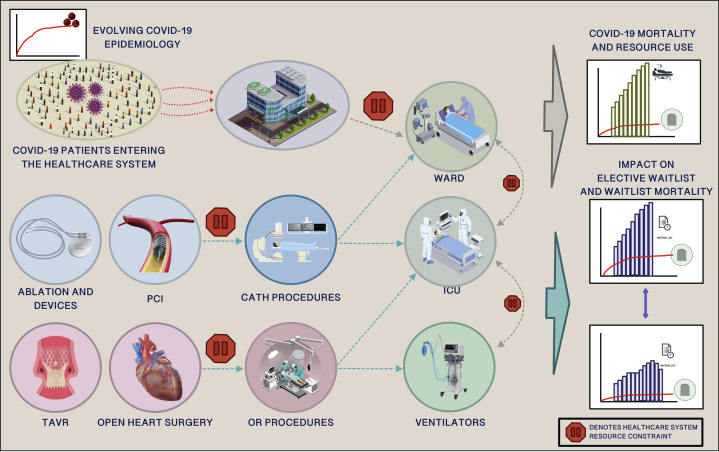
The CORE model was then adapted to include a cardiac submodule (Fig. 1 ). We chose to examine 3 areas of cardiovascular medicine with a poor natural history when left untreated: severe symptomatic valvular heart disease requiring open valve surgery or transcatheter aortic valve replacement, advanced coronary artery disease requiring revascularization with coronary artery bypass grafting or percutaneous coronary intervention (PCI), and arrhythmia disorders requiring ablation therapy or implantable cardiac defibrillator device implantation. We did not include heart transplantation, mechanical circulatory support, or cardiac catheterization procedures other than PCI.
Figure 1.
A schematic illustration of the COVID-19 Resource Estimator decision analytic model and the cardiac disease submodule that includes catheter-based procedures (electrophysiology procedures, transcatheter aortic valve replacement [TAVR], percutaneous coronary interventions [PCIs]) and open surgical procedures (coronary artery bypass grafting, valve surgery). Increasing numbers of COVID-19 patients and a steady number of elective cardiac outpatients compete for critical care resources. The stop sign denotes potential capacity constraints for resources. The model was adapted to measure incremental change in cardiac waitlist, consumption of critical care resources throughout the pandemic, and death while awaiting procedures for the entire province of Ontario and 5 geographic health regions with the use of historic referral data, real-time procedural data, and real-time resource intensive care unit (ICU) and ward bed capacity data.
Our work took place over 3 phases. In the initial “crisis” phase of the pandemic, we examined the impact of increasing numbers of COVID-19 patients under various epidemiologic scenarios (expected scenario, worst case, “Italy scenario,” best case, “South Korea scenario”)3 entering hospitals, requiring the use of cardiac care resources, such as dedicated cardiac ICU and ward beds, and thereby displacing non–COVID-19 cardiac patients. We used epidemiology scenarios from other countries, because that in Ontario was evolving and not known. Nonetheless, pandemic planning was required and could not be delayed until the Ontario epidemiology became apparent. The model estimated unplanned cardiovascular admissions and waitlist mortality for the 3 modelled cardiovascular disease states based on the published literature.4 The second phase of our model examined the impact of a policy decision to further curtail elective outpatient procedures (April 5, 2020) on an increasing waitlist as the Ontario epidemiology became apparent. The third and on-going phase of the model is recovery, because the Ontario COVID-19 pandemic is beyond its peak and currently in a plateau. Our work now focuses on the time required to clear the backlog of outpatient cardiac procedures by modelling various scenarios of the health care system’s capacity to perform cardiac procedures relative to baseline volume before the COVID-19 pandemic. Please see the Supplemental Appendix for further details of methods and the results for each phase.
Findings Throughout the Different Phases of the COVID-19 Pandemic
First phase
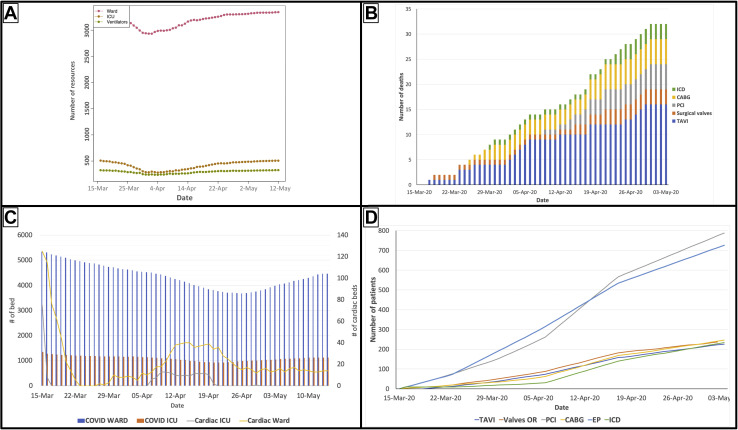
Initial work performed in late March using the CORE model showed that in the worst-case COVID-19 transmission scenario (Fig. 2 A; Supplemental Table S1), the increasing rate of COVID-19 cases in Ontario raised substantial concerns that the system was at an inflection point, whereby a surge of critically ill patients could overwhelm the acute care system.3 We compared the potential deaths from COVID-19 patients in a hypothetical ICU and ventilator shortage scenario vs the potential deaths of elective patients waiting for their cardiac procedures. We showed that the potential number of deaths for patients awaiting their elective cardiac procedures would be orders of magnitude less than those who would die of COVID-19 if there was a depletion of critical care resources (Fig. 2B). This led to the urgent release of CorHealth COVID-19 Cardiac Memo no. 7 on April 4, 2020, to all cardiac hospital chief executive officers, reinforcing the need for an immediate cessation in all elective outpatient cardiac procedures and surgeries across Ontario.
Figure 2.
Examples of different model outputs from the COVID-19 Resource Estimator model and cardiac submodule. (A) Base case scenario for resource consumption by COVID-19 patients in Ontario based on what was known about COVID-19 epidemiology in late March (modified from www.covid-19-mc.ca). (B) Estimated number of elective patient deaths over time with the cessation of elective cardiac procedures in early April followed by gradual resumption of procedures in mid-April to early May. (C) The potential depletion of cardiac critical care resources if 75% of cardiac beds are reserved for COVID-19 patients and cardiac procedures are performed at 100% capacity. (D) The predicted growth in waitlist as a consequence of holding all elective outpatient cardiac procedures for 1 week in April followed by gradual resumption of procedural activity. CABG, coronary artery bypass grafting; EP, electrophysiology study; ICD, implantable cardioverter-defibrillator; ICU, intensive care unit; PCI, percutaneous coronary intervention; TAVI, transcatheter valve replacement (implantation).
Second phase
Over the following week, as the epidemiology of COVID-19 was better known, it became clear that public health interventions, such as physical distancing, were effective and Ontario would not follow the worst-case transmission scenario (Supplemental Fig. S3).3 Under the new epidemiology pattern, our model predicted adequate global and cardiac resources across Ontario to resume selected outpatient procedures. However, our model suggested that across 5 regions of Ontario, there may not be sufficient resources to resume all elective outpatient cardiac procedures, if cardiac beds remained allocated to COVID-19 patients (Fig. 2C). As such, CorHealth COVID-19 Cardiac Memo no. 8 was released on April 13 and recommended that cardiac centres could resume scheduled outpatient cardiac procedures, restricted to those at highest risk for mortality based on local resource circumstances. We modelled the impact of resumption of cardiac procedures in mid-April at 50% of baseline volumes followed by normal volumes by early May, specifically on the estimated growth in the waitlist (Fig. 2D). The above memorandums are briefly summarized in Supplemental Table S1 along with the COVID-19 epidemiology at the time of the memo release.
Third phase
Currently, we are working on estimating the necessary resource capacity and time required to bring the waitlist back to pre–COVID-19 levels. As real-world data is made available, we will be able to validate model outputs against observed outcomes and make iterative improvements to allow for more accurate predictions in the future. This is an important point because of the concern for multiple waves of the pandemic in the absence of a vaccine for COVID-19.5 In this first response to the pandemic, the public health mandate was prioritized over other concerns; we recognize that in hindsight, resources were left unused in Ontario. This reinforces the need for prediction models to be improved for the next phase of the pandemic, such that subsequent policy decisions are improved and spillover impacts on non–COVID-19 care is reduced.
Summary of Modeling Implications for Policy Making
Pandemic planning requires nimble decision making in the face of substantial unknowns. The consequences of delaying decisions until local epidemiology data is known with certainty can be catastrophic, as seen in jurisdictions such as Italy and New York, where the systems were overwhelmed. Modelling provides decision makers with a credible envelope of potential future scenarios, to aid with difficult decisions. The COVID-19 pandemic in Ontario followed this process. Initial worse-case scenarios required an immediate and almost complete shift of the health care system to care for those critically ill with the virus, at the exclusion of all but the most gravely ill non–COVID-19 patients. Although reasonable in the short term, once local data were known, it required a strategy shift. Understanding the impact of such a policy is crucial to understand the cardiac consequences of the decision and to estimate the size of the potential backlog of cardiovascular procedures so that decisions about resumption of activity can be informed. A unique aspect of this model is that COVID-19 patients and cardiovascular patients compete for shared resources. In essence, this explicitly models the fact that policy decisions regarding cardiac patients cannot be made in isolation from the reality of the pandemic and the needs of COVID-19 patients.
Limitations
There are important limitations in decision analytic modeling that must be addressed. First, accurate modelling depends on several factors: a model structure that closely resembles reality, reasonable model assumptions, and relevant inputs. We acknowledge that not every possible scenario can be modelled and there may be important nonfatal outcomes that are relevant to cardiovascular patients that are inadequately represented. Another limitation is that models may be complex and difficult to understand without specialized knowledge or understanding of modelling software. To address concerns about model transparency, we think such models should be public and to that end, we have provided a detailed Supplemental Appendix to describe the model structure and assumptions.
Conclusion
Decision modelling is not designed to predict the future, but rather to provide decision makers with a credible envelope of possible scenarios. As witnessed in other jurisdictions, in a pandemic, failure to make anticipatory decisions can have dire consequences. Balanced against the direct impacts of the pandemic, delayed presentation and treatment of non–COVID-19 conditions, such as cardiac disease, result in significant sequelae; these spillover impacts are also critical consequences of the pandemic. The use of decision analytic modelling that is iterated on rapidly evolving data is a tool to help inform health policy decisions to address these difficult trade-offs.
Funding Sources
D.Y.T. is supported by a Canadian Institutes of Health Research Fellowship Award. H.C.W. is supported by a Phase 2 Clinician Scientist Award from the Heart and Stroke Foundation of Canada, Ontario Office. This research was supported, in part, by a Canada Research Chair in Economics of Infectious Diseases held by B.S. (CRC-950-232429).
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 1312 for disclosure information.
To access the supplementary material accompanying this article, visit the online version of the Canadian Journal of Cardiology at www.onlinecjc.ca and at https://doi.org/10.1016/j.cjca.2020.05.024.
Supplementary Material
References
- 1.Hassan A, Arora RC, Adams C, et al. Cardiac surgery in Canada during the COVID-19 pandemic: a guidance statement from the Canadian Society of Cardiac Surgeons [e-pub ahead of print]. Can J Cardiol 10.1016/j.cjca.2020.04.001. [DOI] [PMC free article] [PubMed]
- 2.Wood D.A., Sathananthan J., Gin K. Precautions and procedures for coronary and structural cardiac interventions during the COVID-19 pandemic: guidance from Canadian Association of Interventional Cardiology. Can J Cardiol. 2020;36:780–783. doi: 10.1016/j.cjca.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett K, Khan YA, Mac S, Ximenes R, Naimark DMJ, Sander B. Estimation of COVID-19–induced depletion of hospital resources in Ontario, Canada [e-pub ahead of print]. CMAJ 10.1503/cmaj.200715. [DOI] [PMC free article] [PubMed]
- 4.Elbaz-Greener G., Masih S., Fang J. Temporal trends and clinical consequences of wait-times for trans-catheter aortic valve replacement: a population based study. Circulation. 2018;138:483–493. doi: 10.1161/CIRCULATIONAHA.117.033432. [DOI] [PubMed] [Google Scholar]
- 5.Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.