Abstract
Three novel coronaviruses have emerged as new lethal zoonotic pathogens of humans during the past 17 years: The Severe Acute Respiratory Syndrome (SARS) coronavirus (SARS-CoV), the Middle East Respiratory Syndrome (MERS) coronavirus (MERS-CoV), and most recently SARS-CoV-2.
SARS-CoV first surfaced as a human pathogen in Guangdong, China in November 2002 and rapidly spread worldwide with 8098 cases and 774 deaths before the end of the epidemic. SARS-like CoVs have been detected in horseshoe bats with high sequence homology with human or civet isolates, suggesting that bats could be a natural reservoir of a close ancestor of SARS-CoV. No cases of SARS have been reported since January 2004.
MERS-CoV was first reported in September 2012, after it was isolated from respiratory samples from a patient in Jeddah, Saudi Arabia who died in June 2012. How humans acquire MERS-CoV infection is not yet known although bats and dromedary camels are intermediary reservoirs.
MERS-CoV continues to circulate in the Middle East. As of May 22, 2019, 2428 cases of laboratory-confirmed MERS-CoV cases reported to the World Health Organization, including 838 deaths (34.5% mortality) have been reported from 27 countries. While the majority of MERS cases occur in the Middle East, travel related MERS cases have been reported from all continents. Large health care associated outbreaks of MERS-CoV have occurred in Saudi Arabia, United Arab Emirates, and the Republic of Korea.
SARS-CoV-2 emerged from Wuhan, China in December 2019, and by March 2020 had established as a pandemic which has caused massive disruption in multiple countries. The eventual mortality caused by this virus remains to be seen.
All three viruses cause a similar wide range of nonspecific clinical manifestations from mild upper respiratory tract illness to severe respiratory, gastrointestinal and other extra-pulmonary disease. Early recognition of cases, improved compliance with internationally recommended infection control protocols, and rapid implementation of infection control measures are required to prevent health care facility-associated outbreaks, and in the case of SARS-CoV-2 for control of community spread as well. Treatment is supportive and there are no specific antivirals or vaccines available for both SARS and MERS.
Keywords: Coronavirus, Diagnosis, Epidemiology, MERS-CoV, Middle East Respiratory Syndrome (MERS), SARS-CoV, SARS-CoV-2, Severe acute Respiratory Syndrome (SARS), Treatment
Introduction
Coronaviruses are enveloped RNA viruses belonging to the Coronaviridae family and are named after the “crown-like spikes” present on their surface. Before 2002, known human coronaviruses HCoV-229E, NL63, OC43, and HKU1 were known to cause only mild symptoms similar to the common cold in immunocompetent individuals (Corman et al., 2018). More severe disease such as pneumonia and bronchitis occur in infants, the elderly and the immunosuppressed (Zumla et al., 2016a, Zumla et al., 2016b, Zumla et al., 2016c). Over the past 17 years, three previously unknown zoonotic coronaviruses, the Severe Acute Respiratory Syndrome (SARS) coronavirus (SARS-CoV), the Middle East Respiratory Syndrome (MERS) coronavirus (MERS-CoV), and SARS coronavirus 2 (SARS-CoV-2) have focused global attention due to their epidemic potential and high mortality rates (WHO, 2019a, WHO, 2019b). SARS-CoV and MERS-CoV are listed in the 2019 WHO list of blueprint priority diseases since they are considered potential threats to global public health security (WHO, 2019c). In early 2020 the WHO declared SARS-CoV-2 had established a pandemic infection and was added to the WHO list of blueprint priority diseases. The rest of this article provide detailed information on SARS-CoV and MERS-CoV, with SARS-CoV-2 discussed briefly (due to its novelty) at the end of the article.
Historical
SARS first emerged as a human zoonoses in November 2002 in Guangdong, China where a 64-year old renal physician who had travelled from southern China to Hong Kong (HK) on February 21, 2003 was the index case for the global outbreak (Lee et al., 2003; Peiris et al., 2003a, Peiris et al., 2003b). At least 16 people at his hotel were infected by the index case (Tsang et al., 2003). SARS-CoV subsequently spread rapidly worldwide through international travel to 29 countries and regions with a total of 8098 cases and a mortality rate of 774 (9.6%) before the epidemic ended in July 2003 (Peiris et al., 2003a, Peiris et al., 2003b). No cases of SARS have been reported after detection of four sporadic community-acquired cases over a six-week period from December 2003 to January 2004 with history of exposure to animals or environmental sources.
MERS-CoV was first reported in September 2012, after MERS-CoV was identified in a lung sample of a 60-year-old patient who had died in June 2012 of severe pneumonia and multi-organ failure in at a hospital in Jeddah, Saudi Arabia (Zaki et al., 2012). Retrospective analysis of a cluster of hospital cases dated back to Apr 2012 in Jordan also confirmed MERS-CoV as the etiology of the outbreak (Hijawi et al., 2013). MERS-CoV continues to circulate and remains endemic to the Middle East causing sporadic cases and occasional nosocomial and community outbreaks—which are a hallmark of MERS-CoV infection (Hui et al., 2018). It has remained on the radar of global public health authorities (WHO, 2019b) because of recurrent nosocomial and community outbreaks, its association with severe disease and high mortality rates, and its epidemic potential (Hui et al., 2015). Intermittent sporadic cases, community clusters and nosocomial outbreaks of MERS-CoV continue to occur in Saudi Arabia (WHO, 2019b).
Epidemiology
Geographical Distribution
Fig. 1 shows the geographical map of distribution of SARS cases (Fig. 1A) and MERS cases (Fig. 1B). Cases of MERS from outside the Middle East have been reported from all continents, and these were linked with travel to the Middle East (WHO, 2019b). Large health care associated outbreaks of MERS-CoV have occurred in Saudi Arabia, United Arab Emirates, Jordan, and the Republic of Korea (Hui et al., 2015; Seong et al., 2016; Oh et al., 2015). The largest outbreak of MERS outside the Arabian Peninsula occurred in the Republic of Korea resulting in 186 confirmed MERS cases with 38 deaths during the period between June 1 and July 31, 2015. The outbreak was caused by a Korean traveler returning from a trip to Qatar, UAE, Saudi Arabia and Bahrain became ill (Seong et al., 2016; Oh et al., 2015).
Figure 1.
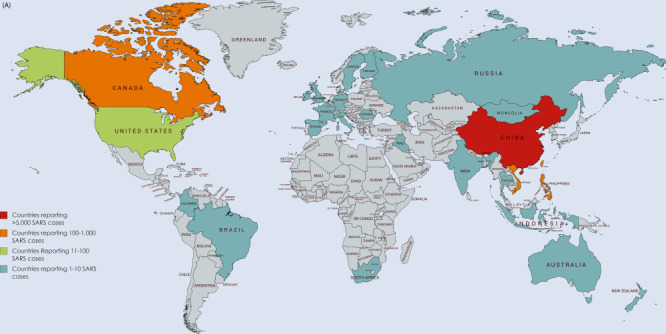


(A) Countries reporting SARS cases (2002–14). (B) Countries reporting Human MERS cases (2012–19). (C) Global cases of MERS-CoV infection reported to WHO (2012–18).
Courtesy of WHO-EMRO-FAO.
The number of MERS-CoV cases reported to the WHO have steadily increased, with seasonal variations since September 2012 (Fig. 1c). MERS-CoV cases have been reported from the community and hospitals across the Arabian Peninsula, with occasional cases in other continents resulting from travelers returning from the Middle East leading to non-sustained outbreaks in health care settings (Hui et al., 2018). Up to 50% of human MERS-CoV infection have been classified as primary infections, 11% as household and 38% as nosocomial and 3% of cases were not classifiable (WHO, 2019b; Choi et al., 2017).
As of the end of April 2019, worldwide there have been 2428 cases of laboratory-confirmed MERS cases have been reported to the World Health Organization, including 838 deaths (35% mortality) (WHO, 2019b). Only three MERS cases have been reported in children under 5 years of age. Of 27 countries who have reported cases of MERS, approximately 80% of human cases have occurred in Saudi Arabia. Cases identified outside the Middle East are usually in travelers who were infected in the Middle East and then travelled to areas outside the Middle East. Countries in or near the Arabian Peninsula with MERS cases are Bahrain, Iran, Jordan, Kuwait, Lebanon, Oman, Qatar, Saudi Arabia, United Arab Emirates (UAE), and Yemen. Countries outside of the Arabian Peninsula with travel-associated MERS cases: Algeria, Austria, China, Egypt, France, Germany, Greece, Italy, Malaysia, Netherlands, Philippines, Republic of Korea, Thailand, Tunisia, Turkey, the United Kingdom, and the United States (WHO, 2019b).
The Origin and Source of Primary SARS-CoV Infections
Sero-prevalence surveys of asymptomatic animal handlers indicate that cross-species transmission of SARS-CoV occurs from various animal species to humans in the wet market. Palm civets were initially thought to be the source when a SARS-CoV variant was detected in palm civets in Dongmen market, Shenzhen, in 2003 (Guan et al., 2003; Peiris et al., 2003a, Peiris et al., 2003b; Wang et al., 2005; Song et al., 2005), and three of the four SARS patients had direct or indirect contact with palm civets in a restaurant in Guangzhou in the Winter of 2003 − 04 (Wang et al., 2005). Subsequent sequence analysis indicated that the SARS-CoV-like virus had not been circulating among masked civets for long. CoVs highly similar to SARS-CoV were isolated in horseshoe bats in 2005 with 88 − 92% sequence homology with human or civet isolates, suggesting that bats could be a natural reservoir of a close ancestor of SARS-CoV (Lau et al., 2010; Li et al., 2005).
Human to Human Transmission of SARS-CoV
SARS-CoV spreads by person-to-person contact via respiratory droplets transmission or contact with fomites (Zhao et al., 2003; WHO, 2019a). A super-spreading event at the Prince of Wales Hospital (PWH) in Hong Kong resulted in 138 people, including previously healthy healthcare workers (HCWs) being infected within 2 weeks after exposure to a patient with community acquired pneumonia (CAP) admitted to a general medical ward (Lee et al., 2003). The spread of SARS from the index case to other inpatients in the same medical ward of the PWH appeared consistent with airborne transmission (Yu et al., 2005). SARS-CoV has been detected in upper and lower respiratory tract secretions, feces, urine, and tears. SARS can also spread by airborne transmission as highlighted by a major community outbreak at Amoy Gardens, a private residential complex in Hong Kong. The outbreak also extended to nearby residential areas, thought to be by airborne transmission over a distance of 200 meters (Yu et al., 2014). In Toronto, Canada air samples from a SARS patient’s room, and swab samples taken from room surfaces and a nurses’ station were positive by SARS-CoV PCR (Booth et al., 2005).
The Origin and Source of Primary MERS-CoV Infections
The origin and primary source of primary and sporadic human MERS-CoV infections also remains unknown (WHO, 2019b). While bats were initially implicated, there are no definitive epidemiological links made between human MERS-CoV infections and bats to date (Memish et al., 2013a, Memish et al., 2013b, Memish et al., 2013c). However it appears contact with an intermediary animal or contaminated source other than bats is involved. Several estimates of the time frame of the emergence of MERS-CoV in humans suggest the emergence of MERS-CoV in mid-2011. In a study of 1100 bat samples, only one fragment of MERS CoV related to human MERS-CoV was found in one Taphozous bat (Memish et al., 2013a, Memish et al., 2013b, Memish et al., 2013c). MERS-related CoV was identified from a Neoromicia capensis bat sampled in South Africa and a phylogenetic study supports the hypothesis that bats are the evolutionary source of MERS-CoV (Corman et al., 2014, Corman et al., 2018).
Camel to Human Transmission of MERS-CoV
Dromedary camels are a host reservoir species for the MERS-CoV (Conzade et al., 2018). Overall one third of all MERS cases report contact with camels. Approximately half of the primary cases have had history of exposure to dromedary camels. Camel to human transmission of MERS-CoV has been confirmed by viral RNA sequencing of samples from infected dromedary camels (Azhar et al., 2014; Drosten et al., 2014a, Drosten et al., 2014b, Drosten et al., 2014c). MERS-CoV matched to those from symptomatic and asymptomatic patients with exposure to the infected camels (Corman et al., 2016). Full genome sequences of MERS-CoV camel isolates were identical to their corresponding patients although 71% of camels related to human cases had antibodies to MERS-CoV by ELISA assays (Kasem et al., 2017). MERS-CoV infection is widespread in dromedary camels although transmission of MERS-CoV from camels to humans appears relatively uncommon, and human disease is not directly proportional to exposure to camels (Conzade et al., 2018; Ommeh et al., 2018).
Person to Person Transmission of MERS-CoV
Human-to-human transmission occurs within communities, households and hospital settings. (Alanazi et al., 2019; Alenazi et al., 2017; Alfaraj et al., 2017; Hui et al., 2018). MERS-CoV does not seem to transmit easily from person to person unless there is close contact with MERS patients. MERS-CoV has been identified in a variety of clinical specimens such as sputum, endotracheal aspirate, bronchoalveolar lavage; nasal or nasopharyngeal swabs, urine, feces, blood and lung tissue, indicating widespread dissemination after infection. The precise modes of MERS-CoV transmission through direct or indirect contact, airborne, droplet or ingestion have yet to be defined (Hui et al., 2018).
Household Transmission of MERS-CoV
Transmission between persons in the community or those living in large households and family compounds is well described. A study of 280 household contacts of 26 index MERS-CoV-infected Saudi Arabian patients, with follow-up serologic analysis in 44 contacts determined the rate of “silent or subclinical” secondary infection after exposure to primary cases of MERS-CoV infection (Drosten et al., 2014a, Drosten et al., 2014b, Drosten et al., 2014c). Twelve probable cases of secondary transmission, and seven apparently healthy household contacts were MERS-CoV positive in their upper respiratory tract (Drosten et al., 2014a, Drosten et al., 2014b, Drosten et al., 2014c). Low levels of MERS-CoV RNA have been reported from asymptomatic subjects from MERS-CoV outbreaks in a Jeddah hospital indicating MERS-CoV carriage after exposure to infected patients (Oboho et al., 2015). A study of 79 extended family members after an MERS-CoV outbreak in Saudi Arabia in 2014, found that 19 (24%) were MERS-CoV positive; 11 were seriously ill and hospitalized, and 2 died (Arwady et al., 2016). Risk factors identified by the study for household transmission were: handling patient’s excreta and respiratory secretions and sleeping in the index patient’s room. Risk factors for MERS-CoV transmission are given in Table 1 .
Table 1.
Risk factors for MERS-CoV infection and outbreaks.
|
Adopted from Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al (2013a) Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East Respiratory Syndrome Coronavirus disease from Saudi Arabia: A descriptive study. The Lancet Infectious Diseases 13: 752–761; Assiri A, McGeer A, Perl TM, et al (2013b) Hospital outbreak of Middle East Respiratory Syndrome Coronavirus. The New England Journal of Medicine 2013b 369(5): 407–416; Al-Abdallat MM, Payne DC, Alqasrawi S, et al (2014) Hospital-associated outbreak of Middle East Respiratory Syndrome Coronavirus: A serologic, epidemiologic, and clinical description. Clinical Infectious Diseases 59(9): 1225–1233; Oboho IK, Tomczyk SM, Al-Asmari AM, et al (2015) 2014 MERS-CoV outbreak in Jeddah—A link to health care facilities. The New England Journal of Medicine 372(9): 846–854; Hui DS, Perlman S, Zumla A. Spread of MERS to South Korea and China. The Lancet Respiratory Medicine 3(7): 509–510; Zumla A, Hui DS, Perlman S (2015a) Middle East Respiratory Syndrome. Lancet 386 (9997): 995–1007; Zumla A, Rustomjee R, Ntoumi F, Mwaba P, Bates M, Maeurer M, Hui DS, Petersen E.(2015b) Middle East Respiratory Syndrome—Need for increased vigilance and watchful surveillance for MERS-CoV in sub-Saharan Africa. International Journal of Infectious Diseases 37: 77–79; Alenazi TH, Al Arbash H, El-Saed A, et al (2017) Identified transmission dynamics of Middle East Respiratory Syndrome Coronavirus infection during an outbreak: Implications of an Overcrowded Emergency Department. Clinical Infectious Diseases 65(4): 675–679; Nam HS, Park JW, Ki M, Yeon MY, Kim J, Kim SW (2017) High fatality rates and associated factors in two hospital outbreaks of MERS in Daejeon, the Republic of Korea. International Journal of Infectious Diseases 58: 37–42.
Nosocomial Transmission of MERS-CoV
Health care associated outbreaks of MERS-CoV are common and appear to be a hallmark of MERS-CoV infection. They account for upto 40% of MERS cases reported worldwide (Hui et al., 2018; Choi et al., 2017). The largest nosocomial outbreaks have been reported from Saudi Arabia, United Arab Emirates, and the Republic of Korea. An outbreak of MERS-CoV infection in Al Hasa, Saudi Arabia in 2013 involved 23 patients in 3 different hospitals and arose due to poor infection control practices, aerosol-generating procedures, CPAP, and cardiopulmonary resuscitation (Assiri et al., 2013a, Assiri et al., 2013b). In early 2017, two unlinked clusters of MERS cases occurred at the same hospital in Wadi ad-Dawasir City, Riyadh, Saudi Arabia involving household contacts, HCWs and patients (Memish et al., 2013a, Memish et al., 2013b, Memish et al., 2013c, Memish et al., 2013d). In June 2017 an outbreak of 34 MERS-CoV cases occurred in a hospital in Riyadh City, Riyadh (Alenazi et al., 2017). The primary case was a 47-year-old male admitted to the ED for intubation who at that time was not identified as infected with MERS-CoV. Prior to diagnosis, 220 HCWs, patients and visitors had contact with this patient. Contact tracing, identified an additional 33 MERS cases during this outbreak. Fifty percent of the cases associated with this outbreak were HCWs. In 2017 a large outbreak in Riyadh involving 4 hospitals, 48 MERS cases were identified involving patients, HCWs, and family members. The outbreak was ignited by four separate patients in the four HCF who were severely ill and not recognized early enough which led to superspreading events (Amer et al., 2018).
HCWs who put on masks covering their nose and mouth with medical mask or N95 mask were less likely to be infected with MERS-CoV compared to those who sometimes/never covered their nose and mouth during aerosol generating procedures. Over the years, a high degree of clinical awareness of MERS, and improvements in implementation of infection control measures (Table 2 ), have resulted in a decrease in the number of nosocomial MERS outbreaks which continue to be reported sporadically.
Table 2.
Epidemiological, clinical and laboratory features of SARS and MERS.
| SARS | MERS | |
|---|---|---|
| Date of first case report (place) | November 2002 (China) | April 2012 (Jordan) June 2012 (First KSA case) |
| Incubation period | Mean: 4.6 days (95% CI: 3.8–5.8) Range: 2–14 days |
Mean: 5.2 days (95%CI: 1.9–14.7) Range: 2–13 days |
| Age (years): Range, Median |
Range: 1–91 Mean: 39.9 |
Range:1–94; Median: 50 |
| Mortality | ||
| Case fatality rate (CFR)-overall | 9.6% | 41.8% |
| CFR in patients with co-morbidities | 1–2% | 13.3% |
| Gender (M, F) | M: 43%, F: 57% | M: 64.5%, F: 35.5% |
| Presenting symptoms | ||
| Fever > 38 °C | 99–100% | 98–100% |
| Chills/rigors | 15–73% | 87% |
Cough
|
62–100% 29–75% 4–29% |
83–100% 56% 44% |
| Hemoptysis | 0–1% | 17% |
| Headache | 20–56% | 11% |
| Myalgia | 45–61% | 32% |
| Malaise | 31–45% | 38% |
| Shortness of breath | 40–42% | 72% |
| Nausea | 20–35% | 21% |
| Vomiting | 20–35% | 21% |
| Diarrhea | 20–25% | 26% |
| Sore throat | 13–25% | 14% |
| Rhinorrhea | 2–24% | 6% |
| Disease progression | ||
| Time from onset to ventilatory support | Mean: 11 days | Median: 7 days |
| Time from onset to death | 23.7 days | Median 11.5 days |
| Co-morbidities | 10–30% | 76% |
| Diabetes | 24% | 10% |
| Chronic renal disease | 2–6% | 13% |
| Chronic heart disease | 10% | 7.5% |
| Malignancy | 3% | 2% |
| Hypertension | 19% | 34% |
| Obesity | N/A | 17% |
| Smoking | 17% | 23% |
| Viral hepatitis | 27% | Not known |
| Imaging and laboratory results | ||
| CXR abnormalities | 94–100% | 100% |
| Leukopenia (< 4.0 × 109/L) | 25–35% | 14% |
| Lymphopenia (< 1.5 × 109/L) | 68–85% | 32% |
| Thrombocytopenia (< 140 × 109/L) | 40–45% | 36% |
| Elevated LDH | 50–71% | 48% |
| Elevated ALT | 20–30% | 11% |
| Elevated AST | 20–30% | 14% |
| Ventilatory support required | 14–20% | 80% |
| Risk factors associated with poor outcome (severe disease or death) | Advanced age, male gender, high initial or peak LDH, high neutrophil count on presentation, diabetes mellitus or other comorbid conditions, low CD4 and CD8 lymphocyte counts at presentation | Any immunocompromised state, comorbid illness, concomitant infections, low albumin, age ≥ 65 years |
Adopted from Chan KS, Lai ST, Chu CM, et al (2003) Treatment of Severe Acute Respiratory Syndrome with lopinavir/ritonavir: A multicenter retrospective matched cohort study. Hong Kong Medical Journal 9: 399–406; Hui DS, Azhar EI, Kim YJ, Memish ZA, Oh MD, Zumla A (2018) Middle East Respiratory Syndrome Coronavirus: Risk factors and determinants of primary, household, and nosocomial transmission. The Lancet Infectious Diseases 18(8): e217–e227; Hui DS, Memish ZA, Zumla A (2014) Severe Acute Respiratory Syndrome vs. the Middle East Respiratory Syndrome. Current Opinion in Pulmonary Medicine 20(3): 233–241; Zumla A, Hui DS, Perlman S (2015a) Middle East Respiratory Syndrome. Lancet 386 (9997): 995–1007; Zumla A, Rustomjee R, Ntoumi F, Mwaba P, Bates M, Maeurer M, Hui DS, Petersen E (2015b) Middle East Respiratory Syndrome—Need for increased vigilance and watchful surveillance for MERS-CoV in sub-Saharan Africa. International Journal of Infectious Diseases 37: 77–79.
Pathology
SARS: SARS-CoV enters the body through the respiratory tract via droplet transmission. Human intestinal cells are also susceptible to SARS-CoV infection and replication, although the role of intestinal tract as a portal of entry remains unknown (Leung et al., 2003). SARS-CoV has been detected in cerebrospinal fluid (Hung et al., 2003) suggesting possible CNS infection (Lau et al., 2004). The lungs and intestines of SARS patients who died were extensively studied (Gu and Korteweg, 2007). Diffuse alveolar damage with varying degrees of edema, hyaline membranes, organization, and fibrosis are seen. Lung pathology is characterized by mixed cellular infiltration, macrophages, multinuclear giant cells, atypical reactive pneumocytes, and vascular injury. The SARS-CoV encoded 3a and 7a proteins have appear to be strong inducers of apoptosis in cell lines from lungs, kidneys and liver (Hui and Chan, 2010).
MERS: The DPP4 (dipeptidyl peptidase 4, also known as CD26), is the functional cellular receptor for MERS-CoV (Raj et al., 2013; Lu et al., 2013) and DPP4 homologues are present in a variety of bat, pig, civet and rabbit cell lines. From cell-based studies, innate immune responses appear to be ineffective, explaining the larger number of cases with severe disease in MERS than SARS. MERS-CoV is thought to induce more severe and rapid dysregulation of the host cellular transcriptome after infection than SARS-CoV, resulting in profound apoptosis of surrounding cells, through greater suppression of the antigen presentation pathway than SARS-CoV. Histopathological analyses studies are rare. A study of post-mortem needle biopsies from brain, heart, lung, liver, kidney and skeletal muscle (Alsaad et al., 2018) showed necrotising pneumonia, pulmonary diffuse alveolar damage, acute kidney injury, portal and lobular hepatitis and myositis with muscle atrophic changes. MERS-CoV particles were localized in the pneumocytes, pulmonary macrophages, renal proximal tubular epithelial cells and macrophages infiltrating the skeletal muscles.
Clinical Features
The symptoms, signs, laboratory, and imaging abnormalities associated with SARS and MERS are similar and thus are not disease specific. They are similar to those seen in patients with other acute lower respiratory tract infections. Table 2 compares and contrasts the epidemiological, clinical and laboratory features of both SARS and MERS. Both SARS and MERS cause a wide range of clinical manifestations from the asymptomatic, mild, moderate to severe and rapidly progressive and fulminant disease.
Incubation Periods
SARS: The mean incubation period of SARS is estimated at 4.6 days (95% CI: 3.8–5.8 days) and 95% of illness onset occurs within 10 days. The time from symptom onset to hospitalization varies between 2 and 8 days. The mean time from symptom onset to need for invasive mechanical ventilation (IMV) and death is recorded as 11 days and 23.7 days respectively. The mean time from onset to discharge was 26.5 days (95% CI: 25.8–27.2 days) (Leung et al., 2003).
MERS: Since the exposure that leads to sporadic infection of MERS is unknown, it is impossible to estimate the incubation period in these primary cases. However, based on data related to human-to-human transmission in several clusters, the incubation period has been estimated to be over 5 days, but could be as long as two weeks [median 5.2 days (95% CI: 1.9–14.7)].
Symptoms and Signs
SARS: The major clinical features of SARS on presentation include persistent fever, chills/rigor, myalgia, dry cough, headache, malaise and dyspnoea. Sputum production, sore throat, coryza are less common. Gastrointestinal symptoms such as nausea and vomiting and watery diarrhea are also presenting features (Table 2).
Clinical course: In children < 12 years of age the disease is mild with no mortality (Hon et al., 2003). The clinical course of SARS in adults (Hui et al., 2003; Peiris et al., 2003a, Peiris et al., 2003b) generally follows a typical pattern grouped into two stages: Phase 1 in the first week is characterized by fever, myalgia, and other systemic symptoms and occurs during viral replication. Despite increasing viral load and lung consolidation, these start to improve after a few days. Phase 2 is characterized by an inverted v shaped peaking of viral load on day 10, recurrence of fever, hypoxemia, and progression of pneumonia in the second week of illness. Clinical worsening during phase 2 is attributed to excessive inflammatory response and immune-mediated lung injury. In elderly patients, particularly those with other comorbidities the disease is severe and often fatal. SARS in pregnancy carries a significant risk of mortality (Wong et al., 2004).
MERS: Like SARS, the clinical presentation of MERS ranges from asymptomatic to very severe pneumonia with Acute Respiratory Distress Syndrome (ARDS), septic shock and multi-organ failure resulting in death (Zumla et al., 2015a, Zumla et al., 2015b; Assiri et al., 2013a, Assiri et al., 2013b; Drosten et al., 2014a, Drosten et al., 2014b, Drosten et al., 2014c). MERS also manifests with fever, cough, chills, sore throat, myalgia and arthralgia, followed by dyspnea. In contrast to SARS, the clinical course of MERS is more severe in a larger proportion of cases with respiratory failure that requires mechanical ventilation and support in an intensive care unit and the mortality rates are higher. MERS-CoV causes more severe disease in older people, the immunocompromised, and those with chronic co-morbid diseases such as renal disease, cancer, chronic lung disease, and diabetes (Arabi et al., 2017; Zumla et al., 2015a, Zumla et al., 2015b; Hui et al., 2014).
Making an Early Diagnosis of MERS-CoV Infection
The diagnosis of MERS can be easily missed since the presentation is that of any other causes of viral upper respiratory tract infection; community acquired pneumonia or gastrointestinal illness. Thus, distinguishing MERS from other viral respiratory illness such as influenza A and B respiratory syncytial virus, parainfluenza viruses, rhinoviruses, adenoviruses, enteroviruses, human metapneumovirus, and endemic human coronaviruses is almost impossible. Most nosocomial outbreaks of MERS-CoV infection have been associated with a delay in diagnosis and late incorporation of infection control procedures. Early and accurate diagnosis of MERS-CoV infection is important for the clinical management, instituting infection control and epidemiological control measures of MERS-CoV infections. A high degree of clinical awareness of the possibility of MERS-CoV infection is required in all healthcare settings so that an accurate diagnosis can be made and infection control measures instituted as soon as the diagnosis is entertained clinically. A history of travel to the Middle East is important for patients presenting in non-Middle Eastern countries, while in endemic countries the use of point of care MERS-CoV testing is appropriate.
Clinical Samples for Laboratory Testing for MERS-CoV
For laboratory testing of MERS, WHO guidelines for testing should be followed (WHO, 2018 https://apps.who.int/iris/bitstream/handle/10665/259952/WHO-MERS-LAB-15.1-Rev1-2018-eng.pdf?sequence=1). Both upper respiratory tract specimens (oropharyngeal or nasopharyngeal) and lower respiratory tract specimens (sputum, endotracheal aspirate or lavage) should be analyzed whenever possible. Lower respiratory specimens have a higher diagnostic value than upper respiratory tract specimens for detecting MERS-CoV infection. If patients do not have signs or symptoms of lower respiratory tract disease and the collection of lower tract specimens is not possible or clinically indicated, upper respiratory tract specimens such as a nasopharyngeal aspirate or combined nasopharyngeal and oropharyngeal swabs should be collected. When taking nasopharyngeal and oropharyngeal specimens, dacron or rayon swabs specifically designed for collecting specimens for virology must be used. These swab kits should contain virus transport medium. A single negative test result does not exclude the diagnosis and repeat sampling and testing is strongly recommended (Alfaraj et al., 2017). A minimum of two samples are needed to exclude MERS-coV after initial assessment. To confirm clearance of the virus, respiratory samples should be collected sequentially (every 2–4 days) over ensuing days until there are two consecutive negative results in clinically recovered persons.
Laboratory Tests for MERS-CoV
Laboratory confirmation of MERS-CoV infection is obtained by detection of the virus by: (a) MERS-CoV specific nucleic acid amplification test with up to two separate targets and/or sequencing; or (b) virus isolation in tissue culture; or (c) serology on serum tested in a WHO collaborating center with established testing methods (WHO, 2018). MERS-CoV testing must be performed in appropriately equipped biosafety laboratories by staff trained in the relevant technical and safety procedures. National or WHO guidelines on the laboratory biosafety should be followed in all circumstances (WHO, 2013).
Molecular tests: Three rRT-PCR assays for routine detection of MERS-CoV have been developed targeting upstream of the E protein gene (upE) and the open reading frame 1b (ORF 1b), and ORF 1a. The assay for the upE target is considered highly sensitive and is recommended for screening, with the ORF 1a assay considered of equal sensitivity.
Serological assays: Serological tests such as ELISAs for MERS-CoV are being developed (Hashem et al., 2019). A case confirmed by serology requires demonstration of sero-conversion in two samples ideally taken at least 14 days apart, by a screening (ELISA, IFA) and a neutralization assay (WHO, 2018).
Imaging
SARS: Radiographic features of SARS show predominant involvement of lung periphery and the lower zone and absence of cavitation, hilar lymphadenopathy or pleural effusion. The intensity of clinical morbidity observed does not correlate with the scanty findings on imaging (Hui et al., 2004; Wong et al., 2003). Radiographic progression from unilateral focal air-space opacity to either multi-focal or bilateral involvement during the second phase of the disease, followed by radiographic improvement with treatment, is commonly observed. In a case series, 12% of patients developed spontaneous pneumo-mediastinum and 20% of patients developed evidence of ARDS over a period of 3 weeks. High resolution chest tomography (HRCT) may show ground-glass opacification, sometimes with consolidation, and interlobular septal and intralobular interstitial thickening, with predominantly a peripheral and lower lobe involvement (Hui et al., 2004).
MERS: As in SARS, a range of imaging (chest X-ray and chest CT) findings consistent with viral pneumonitis and ARDS are seen and none of them are MERS-specific (Das et al., 2017; Ajlan et al., 2014). Lower lobes tend to be affected more than upper lobes early in the course of illness with rapid radiographic progression.
Laboratory Features
In MERS, the laboratory findings are similar to SARS and other severe viral illness, with leucopoenia, particularly lymphopenia (Table 2). Several studies of MERS-CoV viral load measurements show upper respiratory tract specimens have general lower viral loads than in the lower respiratory specimens (Oh et al., 2016) MERS-CoV RNA has been detected in blood, urine and stool but at much lower viral loads than in the respiratory tract. Co-infection with other respiratory viruses such as parainfluenza, rhinovirus, influenza A and influenza B is possible. Nosocomial bacterial infections are common patients receiving in intensive care (Arabi et al., 2016).
Treatment
There are no specific treatments for eradicating MERS-CoV or SARS.
Table 3 summarizes the treatments that have been tried or are in development for SARS and MERS.
Table 3.
Potential therapies for the treatment of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection.
Antibodiesa
siRNA to key MERS-CoV genes |
Treatment benefits likely to exceed risks.
Risks likely to exceed benefits.
Compiled from Zumla A, Hui DS, Perlman S (2015a) Middle East Respiratory Syndrome. Lancet 386 (9997): 995–1007; Zumla A, Rustomjee R, Ntoumi F, Mwaba P, Bates M, Maeurer M, Hui DS, Petersen E (2015b) Middle East Respiratory Syndrome—Need for increased vigilance and watchful surveillance for MERS-CoV in sub-Saharan Africa. International Journal of Infectious Diseases 37: 77–79; Arabi YM, Balkhy HH, Hayden FG, Bouchama A, Luke T, Baillie JK, Al-Omari A, Hajeer AH, Senga M, Denison MR, Nguyen-Van-Tam JS, Shindo N, Bermingham A, Chappell JD, Van Kerkhove MD, Fowler RA (2017) Middle East Respiratory Syndrome. The New England Journal of Medicine 376(6): 584–594; Arabi YM, Alothman A, Balkhy HH, Al-Dawood A, Aljohani S, Al Harbi S, Kojan S, Al Jeraisy M, Deeb AM, Assiri AM, Al-Hameed F, Alsaedi A, Mandourah Y, Almekhlafi GA, Sherbeeni NM, Elzein FE, Memon J, Taha Y, Almotairi A, Maghrabi KA, Qushmaq I, Al Bshabshe A, Kharaba A, Shalhoub S, Jose J, Fowler RA, Hayden FG, Hussein MA; and The MIRACLE trial group (2018a) Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): Study protocol for a randomized controlled trial. Trials 19(1): 81. https://doi.org/10.1186/s13063-017-2427-0; Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al-Omari A, Kharaba A, Almotairi A, Al Khatib K, Alraddadi B, Shalhoub S, Abdulmomen A, Qushmaq I, Mady A, Solaiman O, Al-Aithan AM, Al-Raddadi R, Ragab A, Balkhy HH, Al Harthy A, Deeb AM, Al Mutairi H, Al-Dawood A, Merson L, Hayden FG, Fowler RA; Saudi Critical Care Trial Group (2018b) Corticosteroid therapy for critically Ill patients with Middle East Respiratory Syndrome. American Journal of Respiratory and Critical Care Medicine 197(6): 757–767.
SARS Treatment
Treatment is mostly symptomatic relief of pain and fever with rest. Due to its high infectivity with a basic reproduction number between 2 and 3, hospital admission is required for isolation and supportive therapy.
Systemic Corticosteroids: The use of rescue pulsed methyl prednisolone (MP) during clinical progression was associated with favorable clinical improvement in some patients with resolution of fever and radiographic lung opacities within 2 weeks in observational studies (Sung et al., 2004). However, a retrospective analysis showed that the use of pulsed MP was associated with an increased risk of 30-day mortality (adjusted OR 26.0, 95% CI: 4.4–154.8) (Tsang et al., 2003). Complications such as disseminated fungal disease and avascular osteonecrosis occurred following prolonged systemic corticosteroid therapy. Data based on a randomized controlled trial (RCT) suggest that pulsed MP given in the earlier phase prolonged viraemia in comparisons to the controlled group that received normal saline (Lee et al., 2004).
Antiviral agents: Ribavirin alone had no significant in vitro activity against SARS-CoV and it caused significant hemolysis in many patients (Sung et al., 2004). Lopinavir and ritonavir used as initial therapy was associated with lower overall death rate (2.3% vs. 15.6%) (Chan et al., 2003). Additional beneficial effects included a reduction in use of steroids, less nosocomial infections, a decreasing viral load and rising peripheral lymphocyte count. A subgroup analyses showed that in those who received LPV/r as late rescue therapy after receiving pulsed MP treatment for worsening respiratory symptoms, the outcome was not better than the matched cohort (Chan et al., 2003; Chu et al., 2004).
Convalescent plasma: Convalescent plasma obtained from patients and HCWs who recovered from SARS, contains high levels of neutralizing antibody. A study of 80 SARS patients, receiving early administration of convalescent plasma showed that discharge rate at day 22 was 58.3% for patients (n = 48) treated within 14 days of illness onset, compared to 15.6% in 32 patients treated beyond 14 days (Cheng et al., 2005).
Interferons: Use of IFN-α 1 plus systemic corticosteroids in SARS patients found improved oxygen saturation, and more rapid resolution of radiographic lung opacities than systemic corticosteroids alone. However, this study was uncontrolled with a small sample size (Loutfy et al., 2003).
MERS Treatment
Supportive treatment: As SARS, the clinical management of mild cases of MERS is largely symptomatic and supportive with pain and fever medications, rest staying at home. For serious cases, hospital inpatient care is required for supportive therapy, aiming to reduce the risk of complications such as organ failure and secondary infections. Non-invasive ventilation is associated with a high failure rate (92%) in patients with acute hypoxemic respiratory failure due to MERS-CoV infection (Alraddadi et al., 2016). Lung-protective ventilatory strategies for ARDS, inotropic support, antimicrobial therapy for co-infections, and renal replacement therapy for acute renal failure have been used.
Specific treatments tried: For MERS, a range of treatments have been considered or are under evaluation (Table 3). Currently there are no specific treatments for MERS-CoV infections.
Systemic corticosteroids: Studies show no benefit from the use of systemic corticosteroids. Systemic corticosteroids were shown to delay viral clearance in critically ill patients with MERS-CoV infection (Arabi et al., 2018a, Arabi et al., 2018b).
Other treatments: Treatment is mostly symptomatic relief of pain and fever with rest staying at home for the milder cases. For serious cases, hospital admission is required for supportive therapy. Several agents have shown inhibitory effects against MERS-CoV in cell cultures, including IFNs, ribavirin, cyclosporin A, and mycophenolic acid (Table 3). Empiric Lopinavir/ritonavir, pegulated IFN-α2a and ribavirin have been used empirically for serious cases but no efficacy data are available. Antibiotic (Macrolide) therapy is commonly started before the patient arrived in intensive care unit in Saudi Arabia. A retrospective study of 136 MERS patients found that macrolide therapy was not useful and there was no reduction in mortality or faster MERS-CoV RNA clearance (Arabi et al., 2019). Currently there is an ongoing RCT in progress in KSA comparing lopinavir/ritonavir, recombinant IFN-β1b and standard supportive care against placebo and standard supportive care in patients with lab-confirmed MERS requiring hospital admission. Properly designed studies are needed to answer several knowledge gaps for us understand the disease pathogenesis, viral kinetics, mode of disease transmission, and the intermediary source of MERS in order to guide infection control prevention measures and treatment responses in MERS-CoV infection (Table 4 ).
Table 4.
Global public health bodies - information on MERS.
All websites accessed June 6, 2019.
Host-directed therapies: A range of anti-MERS-CoV drugs and host-directed therapies are under discussion (Zumla et al., 2016a).
Mortality and Risk Factors
Several studies have reported a range of mortality and risk factor data (Ahmed, 2017) collected daily information on MERS-CoV cases posted by the Saudi Arabian Ministry of Health (MOH) between December 2, 2014, and November 12, 2016, reviewing 660 laboratory confirmed cases of MERS. They found that the 3-day, 30-day, and overall mortality as 13.8%, 28.3%, and 29.8% respectively. Patients over the age of 60 were more likely to die (45.2% mortality) from their infections than were younger patients (20%). Patients with pre-existing medical co-morbidities tend to have more severe disease and higher mortality rates. Other studies have also described other factors associated with severe disease or death in MERS patients. These include male gender, comorbid pre-existing illnesses such as obesity, diabetes mellitus, heart and lung disease, and immunocompromised states, low serum albumin, concomitant infections, positive plasma MERS-CoV RNA. DPP4 receptors are upregulated in the lungs of smokers and patients with COPD and this may explain why patients with comorbid lung diseases are prone to severe illness (Hui et al., 2018; Alfaraj et al., 2017).
Prevention
For both SARS and MERS, prevention of transmission in the community and in healthcare settings is critical to preventing outbreaks and further spread. Several substantive reviews and WHO guidelines are available on the subject.
General Preventive Measures
It is important to maintain good personal and environmental hygiene, and to implement stringent contact and droplet precautions among the healthcare workers. To prevent community transmission, contact tracing, quarantine/isolation of close contacts and public education are important measures
Hospital Infection Control Measures
Early case detection followed by isolation is essential, ideally in negative pressure isolation rooms if available. The main infection prevention and control measures for managing patients with MERS are well documented from the SARS epidemic and from experiences from managing MERS outbreaks. Early identification and isolation of suspected or confirmed cases and ongoing surveillance are key to preventing nosocomial spread. Droplet precaution (wearing a surgical mask within 1 m of the patient) and contact and droplet precautions (wearing gown, gloves, mask, eye protection on entering the room and removing them on leaving) when caring for patients with suspected MERS. Healthcare workers (HCWs) should implement airborne precautions and wear a fit-tested particulate respirator such as a US NIOSH-approved N95 filtering facepiece respirator [FFR] or a European EN-approved FFP2 or FFP3 filtering facepiece respirator) when performing aerosol-generating procedures for infected and potentially-infected patients. It is also prudent to use higher levels of protection for HCWs who extended close contact with MERS patients and those who are exposed to aerosols from high-risk procedures. Higher levels of ventilation (more air changes, higher air flow and velocity), greater effort to prevent air dispersion beyond the point of generation (enclosure, using capture ventilation) and higher levels of personal protective equipment (more coverage, more protective types of respiratory protection) are all necessary. To reduce room contamination in the hospital setting, the application of a minimum room ventilation rate of 12 air changes per hour in a single room or at least 160 liters/second/patient in facilities with natural ventilation is recommended when caring for patients receiving mechanical ventilation and during aerosol-generating procedures. Avoiding aerosolizing procedures in crowded hospital emergency or inpatient medical wards without adequate control measures may decrease MERS-CoV human-to-human spread and environmental contamination (Zumla and Hui, 2014).
In the event of a late detection of a nosocomial outbreak, hospital closure for any new admissions and stopping any transfers to other hospitals is required to contain the outbreak and any onward disease transmission.
Vaccines
There are no human or camel vaccines available yet although several efforts are ongoing to find an effective preventive or therapeutic vaccine.
SARS-CoV-2 (2019-nCoV)
In January 2020, another novel zoonotic coronavirus that causes lethal human disease, SARS-CoV-2, was included on the WHO priority Blueprint list (WHO, 2020a). This followed the appearance in December 2019 of a case cluster of patients with pneumonia of unknown cause in Wuhan, Hubei province in China. The Chinese Center for Disease Control and Prevention (China CDC) linked its source to Wuhan’s Seafood market (China CDC Weekly, 2020). A novel coronavirus was identified from patients’ samples using whole-genome sequencing and was provisionally named 2019-nCoV, now renamed as SARS-CoV-2. The disease caused by SARS-CoV-2 is abbreviated as COVID-19 (Zumla and Niederman, 2020). The clinical and virologic features of SARS-CoV-2 have been defined (Guan et al., 2020), and although there are similarities to SARS-CoV and MERS-CoV, some differences have also been observed. COVID-19 appears to replicate efficiently in the upper respiratory tract and seems to cause less abrupt onset of symptoms, which are similar to conventional human coronaviruses that are a major cause of common colds and URTIs in the winter seasons. The time from exposure to onset of symptoms is between 2 and 14 days, and infected subjects can probably transmit the infection before developing symptoms. This combined with a relatively high infectivity means that SARS-C0V-2 readily establishes community transmission and spread rapidly across the globe. As with SARS and MERS, data for COVID-19 available to date indicate a wide spectrum of clinical disease is being seen from asymptomatic, subclinical, mild, and self-limiting disease to severe disease and ARDS among the elderly or those with other comorbid diseases such as diabetes, chronic respiratory disease, and hypertension. As of 8 April, 2020, there have been 1,279,722 confirmed cases of COVID-19 (with 72,614 deaths) reported to the WHO (2020b). This pandemic has to date caused considerable social, economic, and political disruption globally with many countries adapting unprecedented social distancing policies that have paralyzed economic activity. The eventual total global mortality from COVID-19 will be significant.
Conclusions
The 2002–04 SARS-CoV epidemic and the emergence of MERS-CoV as a highly pathogenic zoonotic virus illustrates that novel zoonotic diseases remain a major threat to global health security. SARS posed a major challenge for global public health services due to its sudden appearance, rapid spread and disappearance. Whether SARS will reappear and cause another pandemic remains unknown. The appearance of MERS-CoV in 2012 and its continued circulation in the Middle East highlights the persistent threat of coronavirus outbreaks and the urgent need for a more concerted ONE-Human-Environmental-Animal-HEALTH collaborative approach to tackling new zoonotic threats to global health security (Zumla et al., 2016b). The SARS-CoV-2 pandemic of 2020 will have considerable more impact globally than either SARS-CoV or MERS-CoV, the consequences of which are unknown at time of publication.
Acknowledgments
Acknowledgments
AZ is in receipt of an National Institutes of Health Research (NIHR) senior investigator award and acknowledges support for the PANDORA-ID-NET EDCTP Reg/Grant RIA2016E-1609, which is funded by the European and Developing Countries Clinical Trials Partnership (EDCTP2) programme, Horizon 2020, the European Union’s Framework Programme for Research and Innovation.
Author Declarations
DH, EIA, ZAM and AZ have an interest in global public health, emerging and re-emerging infections, particularly respiratory tract infections. All authors have research interests in coronaviruses.
References
- Ahmed A.E. The predictors of 3- and 30-day mortality in 660 MERS-CoV patients. BMC Infectious Diseases. 2017;17(1):615. doi: 10.1186/s12879-017-2712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajlan A.M., Ahyad R.A., Jamjoom L.G., Alharthy A., Madani T.A. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: Chest CT findings. AJR. American Journal of Roentgenology. 2014;203(4):782–787. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- Alanazi K.H., Killerby M.E., Biggs H.M., Abedi G.R., Jokhdar H., Alsharef A.A., et al. Scope and extent of healthcare-associated Middle East Respiratory Syndrome Coronavirus transmission during two contemporaneous outbreaks in Riyadh, Saudi Arabia, 2017. Infection Control and Hospital Epidemiology. 2019;40(1):79–88. doi: 10.1017/ice.2018.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenazi T.H., Al Arbash H., El-Saed A., et al. Identified transmission dynamics of Middle East Respiratory Syndrome Coronavirus infection during an outbreak: Implications of an Overcrowded Emergency Department. Clinical Infectious Diseases. 2017;65(4):675–679. doi: 10.1093/cid/cix352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaraj S.H., Al-Tawfiq J.A., Altuwaijri T.A., Alanazi M., Alzahrani N., Memish Z.A. Middle East Respiratory Syndrome Coronavirus transmission among health care workers: Implication for infection control. American Journal of Infection Control. 2017 doi: 10.1016/j.ajic.2017.08.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alraddadi B.M., Al-Salmi H.S., Jacobs-Slifka K., et al. Risk factors for Middle East Respiratory Syndrome Coronavirus infection among healthcare personnel. Emerging Infectious Diseases. 2016;22(11):1915–1920. doi: 10.3201/eid2211.160920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaad K.O., Hajeer A.H., Al Balwi M., Al Moaiqel M., Al Oudah N., Al Ajlan A., AlJohani S., Alsolamy S., Gmati G.E., Balkhy H., Al-Jahdali H.H., Baharoon S.A., Arabi Y.M. Histopathology of Middle East Respiratory Syndrome Coronovirus (MERS-CoV) infection—Clinicopathological and ultrastructural study. Histopathology. 2018;72(3):516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer H., Alqahtani A.S., Alzoman H., Aljerian N., Memish Z.A. Unusual presentation of Middle East Respiratory Syndrome Coronavirus leading to a large outbreak in Riyadh during 2017. American Journal of Infection Control. 2018;46:1022–1025. doi: 10.1016/j.ajic.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Alomari A., MAndourah Y., et al. Critically ill healthcare workers with the Middle East Respiratory Syndrome (MERS) American Journal of Respiratory and Critical Care Medicine. 2016;193:A6892. [Google Scholar]
- Arabi Y.M., Balkhy H.H., Hayden F.G., Bouchama A., Luke T., Baillie J.K., Al-Omari A., Hajeer A.H., Senga M., Denison M.R., Nguyen-Van-Tam J.S., Shindo N., Bermingham A., Chappell J.D., Van Kerkhove M.D., Fowler R.A. Middle East respiratory syndrome. The New England Journal of Medicine. 2017;376(6):584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Alothman A., Balkhy H.H., Al-Dawood A., Aljohani S., Al Harbi S., Kojan S., Al Jeraisy M., Deeb A.M., Assiri A.M., Al-Hameed F., Alsaedi A., Mandourah Y., Almekhlafi G.A., Sherbeeni N.M., Elzein F.E., Memon J., Taha Y., Almotairi A., Maghrabi K.A., Qushmaq I., Al Bshabshe A., Kharaba A., Shalhoub S., Jose J., Fowler R.A., Hayden F.G., Hussein M.A., The MIRACLE trial group Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): Study protocol for a randomized controlled trial. Trials. 2018;19(1):81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Deeb A.M., Al-Hameed F., et al. Saudi Critical Care Trials group (2019) Macrolides in critically ill patients with Middle East Respiratory Syndrome. International Journal of Infectious Diseases. 2019;81:184–190. doi: 10.1016/j.ijid.2019.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., Jose J., Pinto R., Al-Omari A., Kharaba A., Almotairi A., Al Khatib K., Alraddadi B., Shalhoub S., Abdulmomen A., Qushmaq I., Mady A., Solaiman O., Al-Aithan A.M., Al-Raddadi R., Ragab A., Balkhy H.H., Al Harthy A., Deeb A.M., Al Mutairi H., Al-Dawood A., Merson L., Hayden F.G., Fowler R.A., Saudi Critical Care Trial Group Corticosteroid therapy for critically Ill patients with Middle East Respiratory Syndrome. American Journal of Respiratory and Critical Care Medicine. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- Arwady M.A., Alraddadi B., Basler C., et al. Middle East Respiratory Syndrome Coronavirus transmission in extended family, Saudi Arabia, 2014. Emerging Infectious Diseases. 2016;22(8):1395–1402. doi: 10.3201/eid2208.152015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East Respiratory Syndrome Coronavirus disease from Saudi Arabia: A descriptive study. The Lancet Infectious Diseases. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A., McGeer A., Perl T.M., et al. Hospital outbreak of Middle East Respiratory Syndrome Coronavirus. The New England Journal of Medicine. 2013;369(5):407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar E.I., El-Kafrawy S.A., Farraj S.A., Hassan A.M., Al-Saeed M.S., Hashem A.M., Madani T.A. Evidence for camel-to-human transmission of MERS coronavirus. The New England Journal of Medicine. 2014;370(26):2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- Booth T.F., Kournikakis B., Bastien N., et al. Detection of airborne Severe Acute Respiratory Syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. The Journal of Infectious Diseases. 2005;191:1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.S., Lai S.T., Chu C.M., et al. Treatment of Severe Acute Respiratory Syndrome with lopinavir/ritonavir: A multicenter retrospective matched cohort study. Hong Kong Medical Journal. 2003;9:399–406. [PubMed] [Google Scholar]
- Cheng Y., Wong R., Soo Y.O., et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. European Journal of Clinical Microbiology & Infectious Diseases. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China CDC Weekly The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases COVID-19-China, 2020. CCDC Weekly. 2020;2020(2):1–10. [PMC free article] [PubMed] [Google Scholar]
- Choi S., Jung E., Choi B.Y., Hur Y.J., Ki M. High reproduction number of Middle East Respiratory Syndrome Coronavirus in nosocomial outbreaks: Mathematical modelling in Saudi Arabia and South Korea. The Journal of Hospital Infection. 2017 doi: 10.1016/j.jhin.2017.09.017. pii: S0195-6701 (17)30526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C., Hung I.F., et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzade R., Grant R., Malik M.R., Elkholy A., Elhakim M., Samhouri D., Ben Embarek P.K., Van Kerkhove M.D. Reported direct and indirect contact with dromedary camels among laboratory-confirmed MERS-CoV cases. Viruses. 2018;10(8) doi: 10.3390/v10080425. pii: E425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Ithete N.L., Richards L.R., Schoeman M.C., Preiser W., Drosten C., et al. Rooting the phylogenetic tree of Middle East Respiratory Syndrome Coronavirus by characterization of a conspecific virus from an African bat. Journal of Virology. 2014;88:11297–11303. doi: 10.1128/JVI.01498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Eckerle I., Memish Z.A., Liljander A.M., Dijkman R., Jonsdottir H., Juma Ngeiywa K.J., Kamau E., Younan M., Al Masri M., Assiri A., Gluecks I., Musa B.E., Meyer B., Müller M.A., Hilali M., Bornstein S., Wernery U., Thiel V., Jores J., Drexler J.F., Drosten C. Link of a ubiquitous human coronavirus to dromedary camels. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(35):9864–9869. doi: 10.1073/pnas.1604472113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Muth D., Niemeyer D., Drosten C. Hosts and sources of endemic human coronaviruses. Advances in Virus Research. 2018;100:163–188. doi: 10.1016/bs.aivir.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K.M., Lee E.Y., Singh R., Enani M.A., Al Dossari K., Van Gorkom K., Larsson S.G., Langer R.D. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian Journal of Radiology and Imaging. 2017;27(3):342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Kellam P., Memish Z.A. Evidence for camel-to-human transmission of MERS coronavirus. The New England Journal of Medicine. 2014;371(14):1359–1360. doi: 10.1056/NEJMc1409847. [DOI] [PubMed] [Google Scholar]
- Drosten C., Meyer B., Müller M.A., Corman V.M., Al-Masri M., Hossain R., Madani H., Sieberg A., Bosch B.J., Lattwein E., Alhakeem R.F., Assiri A.M., Hajomar W., Albarrak A.M., Al-Tawfiq J.A., Zumla A.I., Memish Z.A. Transmission of MERS-coronavirus in household contacts. The New England Journal of Medicine. 2014;371(9):828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- Drosten C., Meyer B., Müller M.A., et al. Transmission of MERS-coronavirus in household contacts. The New England Journal of Medicine. 2014;371(9):828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- Gu J., Korteweg C. Pathology and pathogenesis of Severe Acute Respiratory Syndrome. The American Journal of Pathology. 2007;170(4):1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Zheng B.J., He Y.Q., et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicine. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem A.M., Al-Amri S.S., Al-Subhi T.L., Siddiq L.A., Hassan A.M., Alawi M.M., Alhabbab R.Y., Hindawi S.I., Mohammed O.B., Amor N.S., Alagaili A.N., Mirza A.A., Azhar E.I. Development and validation of different indirect ELISAs for MERS-CoV serological testing. Journal of Immunological Methods. 2019;466:41–46. doi: 10.1016/j.jim.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijawi B., Abdallat M., Sayaydeh A., et al. Novel coronavirus infections in Jordan, April 2012: Epidemiological findings from a retrospective investigation. Eastern Mediterranean Health Journal. 2013;19(Suppl 1):S12–S18. [PubMed] [Google Scholar]
- Hon K.L., Leung C.W., Cheng W.T., et al. Clinical presentations and outcome of Severe Acute Respiratory Syndrome in children. Lancet. 2003;561:1701–1703. doi: 10.1016/S0140-6736(03)13364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Chan P.K. Severe acute respiratory syndrome and coronavirus. Infectious Disease Clinics of North America. 2010;24(3):619–638. doi: 10.1016/j.idc.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Wong P.C., Wang C. Severe Acute Respiratory Syndrome: Clinical features and diagnosis. Respirology. 2003;8:S20–S24. doi: 10.1046/j.1440-1843.2003.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Wong K.T., Antonio G.E., et al. Severe Acute Respiratory Syndrome (SARS): Correlation of clinical outcome and radiological features. Radiology. 2004;233:579–585. doi: 10.1148/radiol.2332031649. [DOI] [PubMed] [Google Scholar]
- Hui D.S., Memish Z.A., Zumla A. Severe Acute Respiratory Syndrome vs. the Middle East Respiratory Syndrome. Current Opinion in Pulmonary Medicine. 2014;20(3):233–241. doi: 10.1097/MCP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- Hui D.S., Perlman S., Zumla A. Spread of MERS to South Korea and China. The Lancet Respiratory Medicine. 2015;3(7):509–510. doi: 10.1016/S2213-2600(15)00238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Azhar E.I., Kim Y.J., Memish Z.A., Oh M.D., Zumla A. Middle East Respiratory Syndrome Coronavirus: Risk factors and determinants of primary, household, and nosocomial transmission. The Lancet Infectious Diseases. 2018;18(8):e217–e227. doi: 10.1016/S1473-3099(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung E.C., Chim S.S., Chan P.K., et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with Severe Acute Respiratory Syndrome. Clinical Chemistry. 2003;49:2108–2109. doi: 10.1373/clinchem.2003.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasem S., Qasim I., Al-Hufofi A., et al. Cross-sectional study of MERS-CoV-specific RNA and antibodies in animals that have had contact with MERS patients in Saudi Arabia. Journal of Infection and Public Health. 2017 doi: 10.1016/j.jiph.2017.09.022. pii: S1876-0341(17)30257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K.K., Yu W.C., Chu C.M., et al. Possible central nervous system infection by SARS coronavirus. Emerging Infectious Diseases. 2004;10:342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K., Li K.S., Huang Y., et al. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. Journal of Virology. 2010;84(6):2808–2819. doi: 10.1128/JVI.02219-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Hui D.S., Wu A., et al. A major outbreak of Severe Acute Respiratory Syndrome in Hong Kong. The New England Journal of Medicine. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Lee N., Allen Chan K.C., et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. Journal of Clinical Virology. 2004;31(4):304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W.K., To K.F., Chan P.K., et al. Enteric involvement of Severe Acute Respiratory Syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Shi Z., Yu M., et al. Bats are natural reserviors of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Loutfy M.R., Blatt L.M., Siminovitch K.A., et al. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: A preliminary study. JAMA. 2003;290(24):3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- Lu G., Hu Y., Wang Q., et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish Z.A., Mishra N., Olival K.J., et al. Middle East Respiratory Syndrome Coronavirus in bats, Saudi Arabia. Emerging Infectious Diseases. 2013;19:1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish Z.A., Mishra N., Olival K.J., et al. Middle East Respiratory Syndrome Coronavirus in bats, Saudi Arabia. Emerging Infectious Diseases. 2013;19(11):1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish Z.A., Zumla A.I., Al-Hakeem R.F., Al-Rabeeah A.A., Stephens G.M. Family cluster of Middle East Respiratory Syndrome Coronavirus infections. The New England Journal of Medicine. 2013;368(26):2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- Memish Z.A., Zumla A.I., Assiri A. Middle East Respiratory Syndrome Coronavirus infections in health care workers. The New England Journal of Medicine. 2013;369(9):884–886. doi: 10.1056/NEJMc1308698. [DOI] [PubMed] [Google Scholar]
- Oboho I.K., Tomczyk S.M., Al-Asmari A.M., et al. 2014 MERS-CoV outbreak in Jeddah—A link to health care facilities. The New England Journal of Medicine. 2015;372(9):846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M.D., Choe P.G., Oh H.S., Park W.B., Lee S.M., Park J., Lee S.K., Song J.S., Kim N.J. Middle East Respiratory Syndrome Coronavirus superspreading event involving 81 persons, Korea 2015. Journal of Korean Medical Science. 2015;30(11):1701–1705. doi: 10.3346/jkms.2015.30.11.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M.D., Park W.B., Choe P.G., et al. Viral load kinetics of MERS coronavirus infection. The New England Journal of Medicine. 2016;375(13):1303–1305. doi: 10.1056/NEJMc1511695. [DOI] [PubMed] [Google Scholar]
- Ommeh S., Zhang W., Zohaib A., Chen J., Zhang H., Hu B., Ge X.Y., Yang X.L., Masika M., Obanda V., Luo Y., Li S., Waruhiu C., Li B., Zhu Y., Ouma D., Odendo V., Wang L.F., Anderson D.E., Lichoti J., Mungube E., Gakuya F., Zhou P., Ngeiywa K.J., Yan B., Agwanda B., Shi Z.L. Genetic evidence of Middle East Respiratory Syndrome Coronavirus (MERS-Cov) and widespread seroprevalence among Camels in Kenya. Virologica Sinica. 2018 doi: 10.1007/s12250-018-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Yuen K.Y., Osterhaus A.D., Stöhr K. The Severe Acute Respiratory Syndrome. The New England Journal of Medicine. 2003;349(25):2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- Raj V.S., Mou H., Smits S.L., et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong M.W., Kim S.Y., Corman V.M., Kim T.S., Cho S.I., Kim M.J., Lee S.J., Lee J.S., Seo S.H., Ahn J.S., Yu B.S., Park N., Oh M.D., Park W.B., Lee J.Y., Kim G., Joh J.S., Jeong I., Kim E.C., Drosten C., Park S.S. Microevolution of outbreak-associated Middle East Respiratory Syndrome Coronavirus, South Korea, 2015. Emerging Infectious Diseases. 2016;22(2):327–330. doi: 10.3201/eid2202.151700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H.D., Tu C.C., et al. Cross-host evolution of Severe Acute Respiratory Syndrome coronavirus in palm civet and human. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2430–2435. doi: 10.1073/pnas.0409608102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung J.J., Wu A., Joynt G.M., et al. Severe acute respiratory syndrome: Report of treatment and outcome after a major outbreak. Thorax. 2004;59(5):414–420. doi: 10.1136/thx.2003.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang K.W., Ho P.L., Ooi G.C., et al. A cluster of cases of Severe Acute Respiratory Syndrome in Hong Kong. The New England Journal of Medicine. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- Wang M., Yan M., Xu H., et al. SARS-CoV infection in a restaurant from palm civet. Emerging Infectious Diseases. 2005;11:1860–1865. doi: 10.3201/eid1112.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2013) Laboratory Biorisk Management for Laboratories Handling Human Specimens Suspected or Confirmed to Contain Novel Coronavirus: Interim Recommendations. WHO. https: //www.who.int/csr/disease/coronavirus_infections/Biosafety_InterimRecommendations_NovelCoronavirus_19Feb13.pdf?ua=1 (accessed April 4, 2019).
- WHO (2018) Laboratory Testing for Middle East Respiratory Syndrome Coronavirus. Geneva, Switzerland: World Health Organization. https: //apps.who.int/iris/bitstream/handle/10665/259952/WHO-MERS-LAB-15.1-Rev1-2018-eng.pdf?sequence = 1 (accessed March 28, 2019).
- WHO (2019a) SARS (Severe Acute Respiratory Syndrome). https: //www.who.int/ith/diseases/sars/en/ (accessed May 16, 2019).
- WHO (2019b) Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Update. http: //www.who.int/emergencies/mers-cov/en/ (accessed May 16, 2019).
- WHO (2019c) List of Blueprint Priority Diseases. https: //www.who.int/blueprint/priority-diseases/en/ (accessed May 16, 2019).
- WHO (2020a) A Research and Development Blueprint for Action to Prevent Epidemics. WHO. https://www.who.int/blueprint/en/ (accessed March 14, 2020).
- WHO (2020b) Cornavirus diseases (COVID-2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed April 8, 2020).
- Wong K.T., Antonio G.E., Hui D.S., et al. Severe Acute Respiratory Syndrome: Radiographic appearances and pattern of progression in 138 patients. Radiology. 2003;228:401–406. doi: 10.1148/radiol.2282030593. [DOI] [PubMed] [Google Scholar]
- Wong S.F., Chow K.M., Leung T.N., et al. Pregnancy and perinatal outcomes of women with Severe Acute Respiratory Syndrome. American Journal of Obstetrics and Gynecology. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.T., Wong T.W., Chiu Y.L., et al. Temporal-spatial analysis of Severe Acute Respiratory Syndrome among hospital inpatients. Clinical Infectious Diseases. 2005;40:1237–1243. doi: 10.1086/428735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.T., Qiu H., Tse L.A., Wong T.W. Severe Acute Respiratory Syndrome beyond Amoy gardens: Completing the incomplete legacy. Clinical Infectious Diseases. 2014;58(5):683–686. doi: 10.1093/cid/cit797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. The New England Journal of Medicine. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Zhang F., Xu M., et al. Description and clinical treatment of an early outbreak of Severe Acute Respiratory Syndrome (SARS) in Guangzhou, PR China. Journal of Medical Microbiology. 2003;52:715–720. doi: 10.1099/jmm.0.05320-0. [DOI] [PubMed] [Google Scholar]
- Zumla A., Hui D.S. Infection control and MERS-CoV in health-care workers. Lancet. 2014;383(9932):1869–1871. doi: 10.1016/S0140-6736(14)60852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Hui D.S., Perlman S. Middle East Respiratory Syndrome. Lancet. 2015;386(9997):995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Rustomjee R., Ntoumi F., Mwaba P., Bates M., Maeurer M., Hui D.S., Petersen E. Middle East Respiratory Syndrome—Need for increased vigilance and watchful surveillance for MERS-CoV in sub-Saharan Africa. International Journal of Infectious Diseases. 2015;37:77–79. doi: 10.1016/j.ijid.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses—Drug discovery and therapeutic options. Nature Reviews. Drug Discovery. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Dar O., Kock R., Muturi M., Ntoumi F., Kaleebu P., Eusebio M., Mfinanga S., Bates M., Mwaba P., Ansumana R., Khan M., Alagaili A.N., Cotten M., Azhar E.I., Maeurer M., Ippolito G., Petersen E. Taking forward a ‘One Health’ approach for turning the tide against the Middle East Respiratory Syndrome Coronavirus and other zoonotic pathogens with epidemic potential. International Journal of Infectious Diseases. 2016;47:5–9. doi: 10.1016/j.ijid.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Niederman M.S. The explosive epidemic outbreak of novel coronavirus disease 2019 (COVID-19) and the persistent threat of respiratory tract infectious diseases to global health security. Current Opinion in Pulmonary Medicine. 2020;26(3):193–196. doi: 10.1097/MCP.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Rao M., Wallis R.S., Kaufmann S.H., Rustomjee R., Mwaba P., Vilaplana C., Yeboah-Manu D., Chakaya J., Ippolito G., Azhar E., Hoelscher M., Maeurer M., Host-Directed Therapies Network consortium Host-directed therapies for infectious diseases: Current status, recent progress, and future prospects. The Lancet Infectious Diseases. 2016;16(4):e47–e63. doi: 10.1016/S1473-3099(16)00078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


