Summary
Diffuse large B-cell lymphoma, not otherwise specified (DLBCL NOS) is subdivided according to the cell-of-origin (COO) classification into germinal centre B-cell (GCB) and activated B-cell (ABC) subtypes, each with different molecular profiles and clinical behaviour. This study aims to describe the pattern of the COO subtypes, the proportion of Epstein–Barr virus (EBV) co-infection, and their influence on survival outcomes in a setting of high HIV prevalence.
This retrospective cohort study included patients diagnosed with de novo DLBCL NOS at our tertiary academic centre in Cape Town, South Africa over a 14-year period. Immunohistochemical stains were performed for COO classification, according to the Hans algorithm. Tumour EBV co-infection was established by EBV-encoded ribonucleic acid in situ hybridisation (EBER-ISH) staining. The effect of the COO subtypes and EBV co-infection on overall survival were described by means of univariate, bivariate and multivariate analyses.
A total of 181 patients with DLBCL NOS were included, which comprised 131 HIV-uninfected and 50 HIV-infected patients. There was an equal distribution of GCB and ABC subtypes in the HIV-infected and HIV-uninfected groups. EBV co-infection was detected in 16% of the HIV-infected cases and in 7% of the HIV-uninfected cases (p=0.09). There was no significant difference in the incidence of EBV co-infection between the GCB and ABC subtypes (p=0.67). HIV-infected patients with CD4 ≥150 cells/mm3 had similar survival to HIV-uninfected patients (p=0.005). Multivariate regression analysis showed that in the HIV-infected group with marked immunosuppression (CD4 <150 cells/mm3), there was significantly poorer overall survival compared to the HIV-uninfected group (HR 2.4, 95% CI 1.3–4.1). There were no statistically significant differences in overall survival by DLBCL COO subtype.
There was no difference in the proportion of DLBCL COO subtypes, regardless of HIV status. EBV co-infection was more common in the HIV-infected group, but less than described in the literature. Unexpectedly, there were no significant differences in survival outcomes between the GCB and ABC subtypes. Higher CD4 counts in the HIV-infected group had good survival outcomes, while lower CD4 counts predicted adverse survival outcomes. Further research is needed to explore the genetic mutational landscape of HIV-associated DLBCL.
Keywords: Diffuse large B-cell lymphoma, cell-of-origin, HIV, Epstein–Barr virus, survival, South Africa
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common type of lymphoid malignancy in adults worldwide,1 accounting for approximately one-third of all non-Hodgkin lymphomas in developed countries and a greater proportion in developing countries.2 The cell-of-origin (COO) classification, based on gene expression profiling studies, delineates two molecular subtypes of DLBCL, not otherwise specified (NOS) namely germinal centre B-cell (GCB) and activated B-cell (ABC).3 The ABC subtype is reported to be associated with the poorest prognosis and highest relapse rates, in the well-resourced HIV-uninfected setting.4
Human immunodeficiency virus (HIV) has been shown to play an important role in lymphomagenesis.5 South Africa is home to the largest HIV epidemic in the world, with 20% of the global population of HIV-infected people.6 In 2019, the total number of people living with HIV among the South African population was estimated at 7.97 million, with a prevalence of approximately 19.1% amongst adults aged 15–49 years and an overall prevalence of approximately 13.5%.7 Universal antiretroviral therapy (ART) coverage for HIV has been implemented in South Africa since 2016, but 20% of people living with HIV still remain untreated.8 With the advent of ART, the risk of developing acquired immunodeficiency syndrome (AIDS)-defining cancers has reduced.5 However, in South Africa, late institution of ART and late diagnosis of AIDS-defining cancers remain common. In the setting of HIV, lymphomas constitute more than half of all AIDS-defining malignancies,9 and are characterised by high-grade features including extranodal involvement and advanced stage presentation.5
Tumour Epstein–Barr virus (EBV) co-infection has been well described in immunocompromised hosts and is more frequently linked with HIV-associated DLBCL than with HIV-unassociated DLBCL.10 Tumour EBV infection enables the tumour cells to evade the immune response and become immortalised.11 It is not known whether EBV infection is more likely to drive the development of the GCB or ABC subtype of DLBCL.
DLBCL NOS has been extensively studied for prognostic factors that may inform treatment decisions. These methods include tumour subtyping by immunohistochemistry and genomic sequencing techniques. However, regardless of HIV status, reports of DLBCL patient cohorts are mostly from higher income countries, and very limited information is available from patients in low and middle income countries, particularly those infected with HIV. The association of molecular subtypes with outcome in HIV-associated DLBCL is by no means settled.12–15 Therefore, it is of interest to determine DLBCL COO subtypes and their outcome in an HIV-endemic setting.
We performed this study among DLBCL NOS patients to describe the pattern of the COO subtypes, hereafter referred to as ‘DLBCL subtypes’, as well as the proportion of cases with concurrent EBV infection. We also evaluated the effect of DLBCL subtype, HIV status and EBV co-infection on survival outcomes.
MATERIALS AND METHODS
Study design and setting
This was a retrospective cohort study set in Groote Schuur Hospital (GSH), Cape Town, South Africa. GSH is one of two tertiary academic centres for public patients in the Western Cape Province. Study patients were identified from the pathology and clinical databases at GSH. Consecutive adults (age ≥18 years) diagnosed with de novo DLBCL NOS between 1 January 2005 and 31 December 2018 were selected. This study was approved, including a waiver of consent, by the University of Cape Town Human Research Ethics Committee (reference number: 441/2018).
Patient selection
All cases were diagnosed by qualified histopathologists, at the Division of Anatomical Pathology, GSH. The diagnosis of DLBCL was made based on histological and immunophenotypic findings, according to the World Health Organization (WHO) classification in use at the time.2,16,17 The DLBCL cases were reported as demonstrating large lymphoid cells with a diffuse growth pattern. The morphology displayed was centroblastic, immunoblastic or mixed centroblastic and immunoblastic (Fig. 1). All cases demonstrated diffuse CD20 immunohistochemical (IHC) expression. In the years prior to 2016, many of our cohort cases lacked the specific IHC stains used for COO subtyping according to the Hans algorithm; therefore, only cases with sufficient stored tissue blocks for histological review and additional testing were included for this study.
Fig. 1.
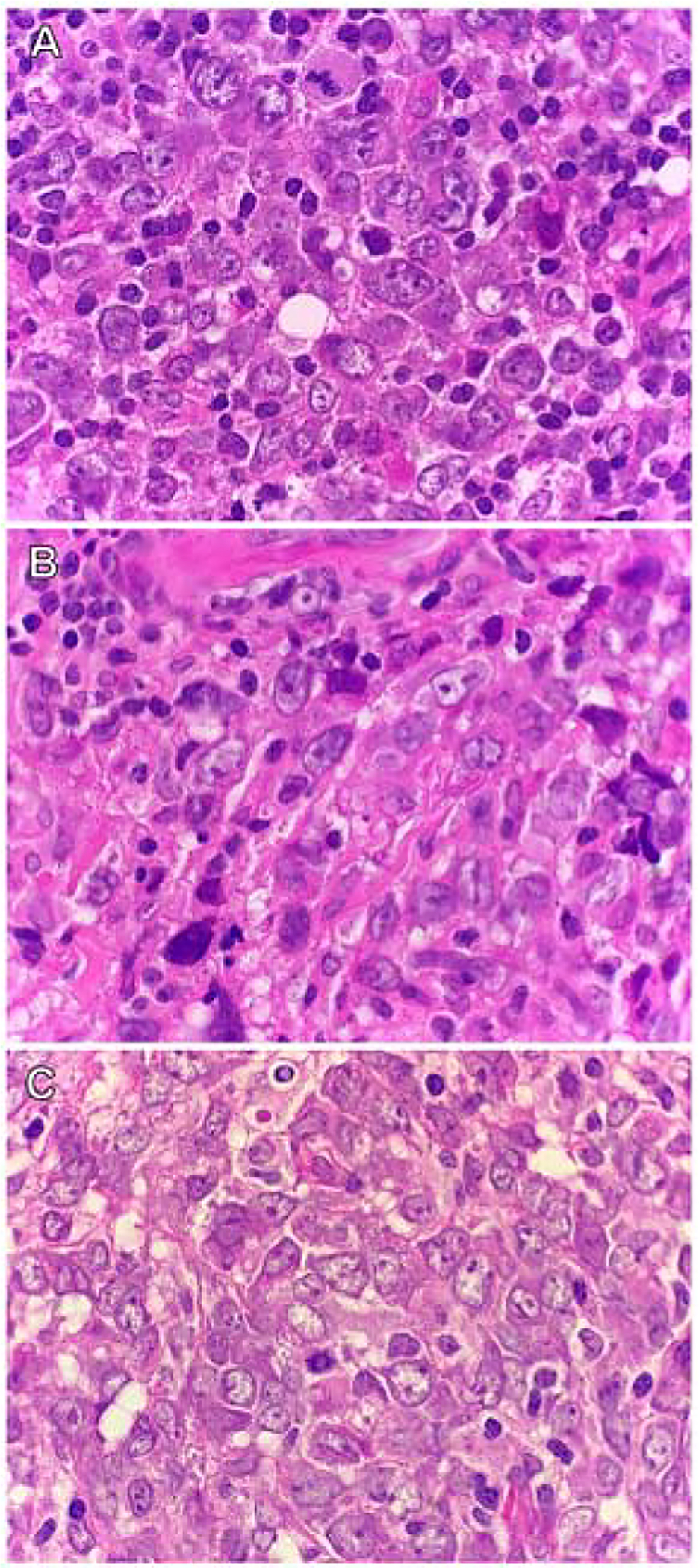
Morphological variants of DLBCL (H&E). Cases of DLBCL demonstrating the (A) centroblastic variant; (B) immunoblastic variant; and (C) mixed centroblastic and immunoblastic variant.
Research procedures
Patient demographic characteristics and disease variables were recorded including gender, age at diagnosis, HIV status, CD4 count if HIV-infected, primary biopsy site, DLBCL subtype, double expressor profile, Ki-67 proliferation index and tumour EBV status. Survival data were obtained from medical records and confirmed by the South African Government Department of Home Affairs. We did not assess individual treatment response; therefore, death by any cause was included.
Archived tissue specimens in the form of formalin-fixed, paraffin wax-embedded tissue blocks were obtained for cases requiring further stains. Stains that were not performed at the initial diagnostic work-up were determined for each case, and the corresponding number of slides prepared. Sections 3μm thick were cut from the tissue blocks, placed onto silanised slides and heat fixed on a hotplate at 75°C for 30 min. Tissue sections were then dewaxed through xylene, cleared in ethanol and rehydrated in water. All IHC stains were performed with the Envision Detection System on a Dako Autostainer (Universal Staining System; Dako, Denmark) using routine staining protocols and the antibodies listed in Supplementary Table 1 (Appendix A). The EBV-encoded ribonucleic acid in situ hybridisation (EBER-ISH) made use of the ISH iView Blue Plus Detection Kit on the BenchMark Ultra IHC/ISH System automated slide stainer (Ventana, USA).
IHC stains performed included CD10, BCL6 and MUM1 in order to classify the cases according to the WHO 2017 classification2 of DLBCL molecular subtypes, using the Hans algorithm.18 The ‘non-GCB’ group is referred to as the ABC subtype in this study. Cases were assigned as the GCB subtype if CD10 was positive; or as the ABC subtype if both CD10 and BCL6 were negative. If CD10 was negative and BCL6 was positive, the expression of MUM1 determined the subtype; with MUM1 negativity assigning the GCB subtype, and MUM1 positivity assigning the ABC subtype (Fig. 2). The stains were interpreted as either positive or negative with a threshold of ≥30% tumour cell staining to determine positivity.
Fig. 2.
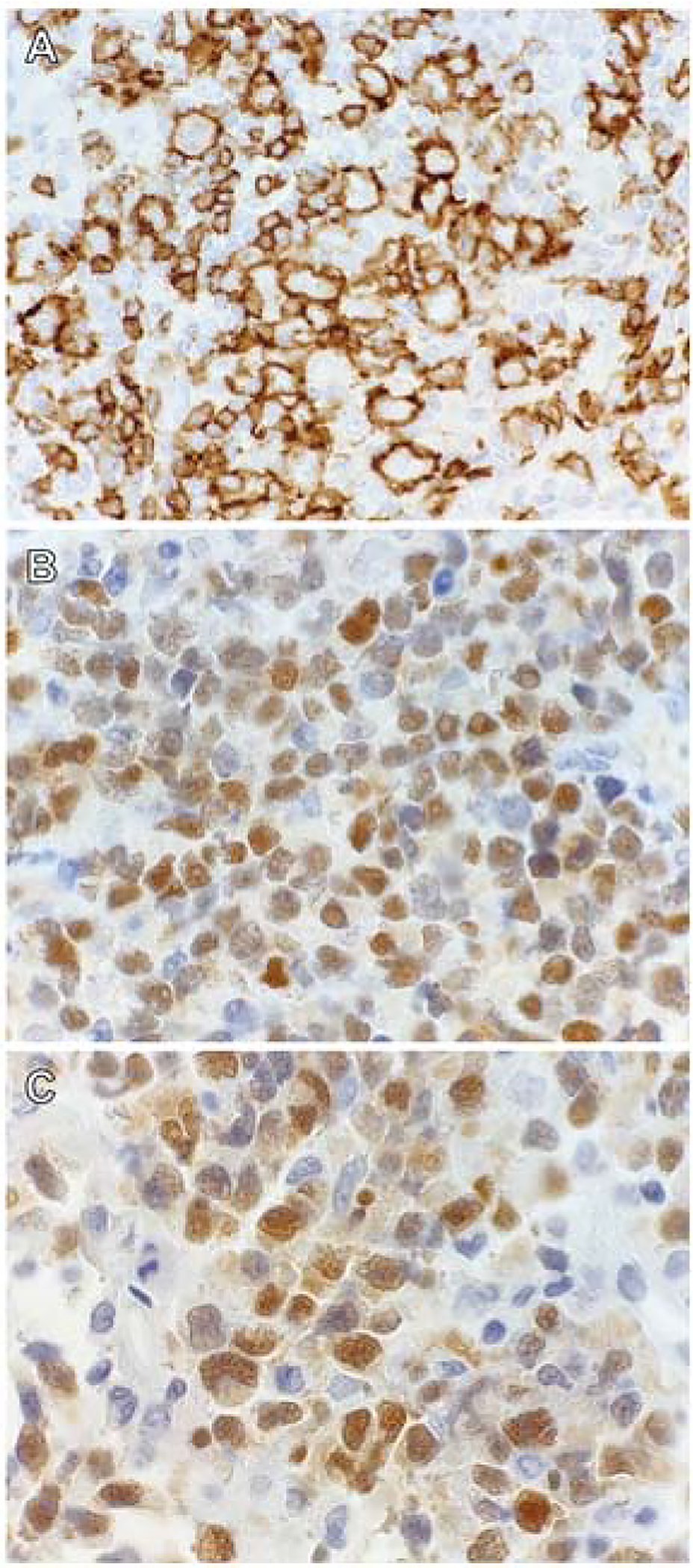
Immunohistochemical staining patterns used to classify DLBCL NOS according to the Hans algorithm. (A) CD10 membrane staining positivity in a case of the germinal centre B-cell (GCB) subtype. (B) BCL6 nuclear staining and (C) MUM1 nuclear staining in a case of the activated B-cell (ABC) subtype.
The c-MYC and BCL2 IHC stains were performed to determine double-expressor profiles of the DLBCL cases (Fig. 3). The stain thresholds to determine positivity were ≥50% tumour cytoplasmic staining for BCL2, and ≥40% tumour nuclear staining for MYC.
Fig. 3.
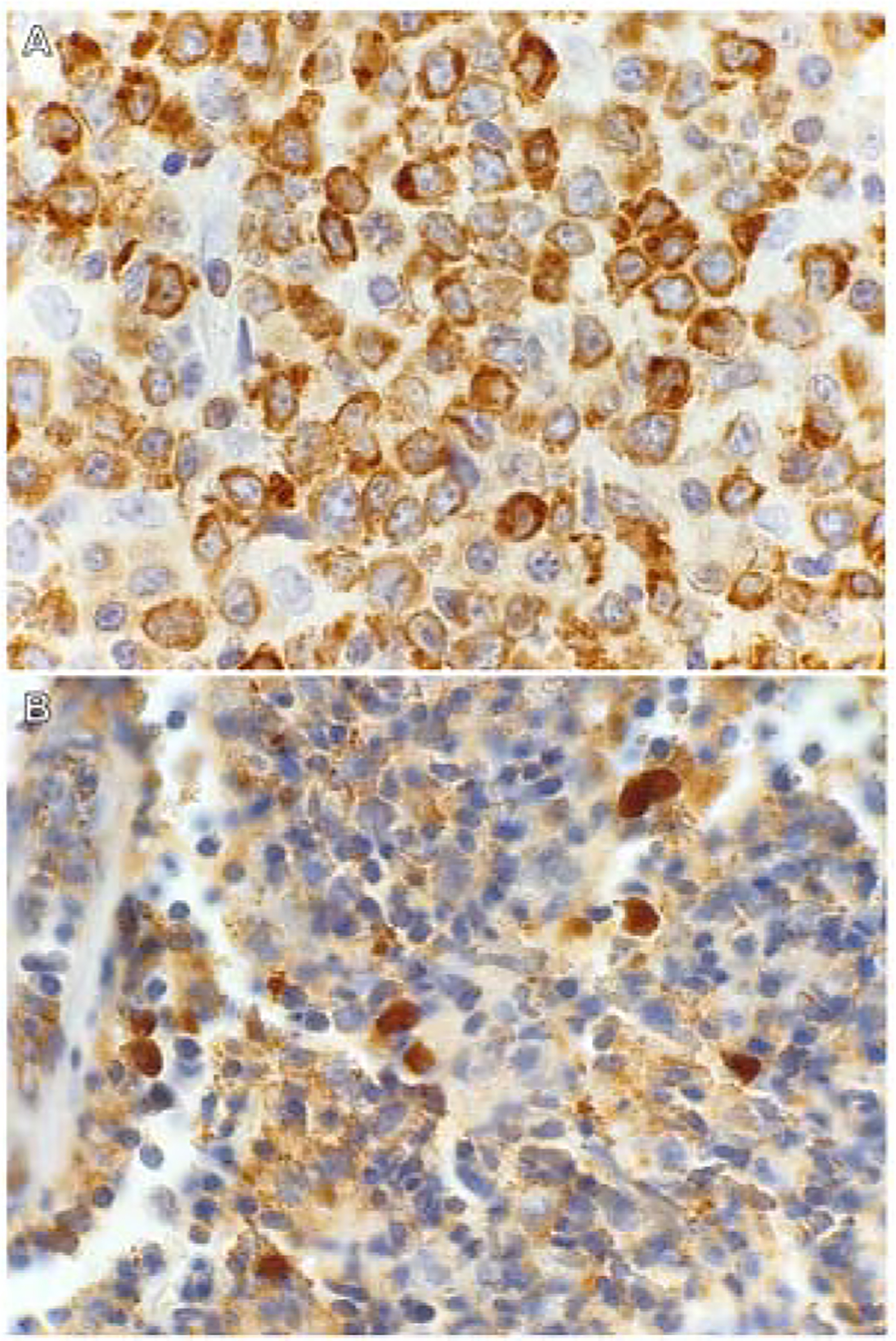
Immunohistochemical staining patterns used to determine double-expressor profiles of DLBCL. A case of double-expressor DLBCL with (A) BCL2 cytoplasmic staining and (B) c-MYC nuclear staining.
The Ki-67 stain was performed to assess the proliferation index with a percentage of tumour cell positivity assigned (Fig. 4).
Fig. 4.
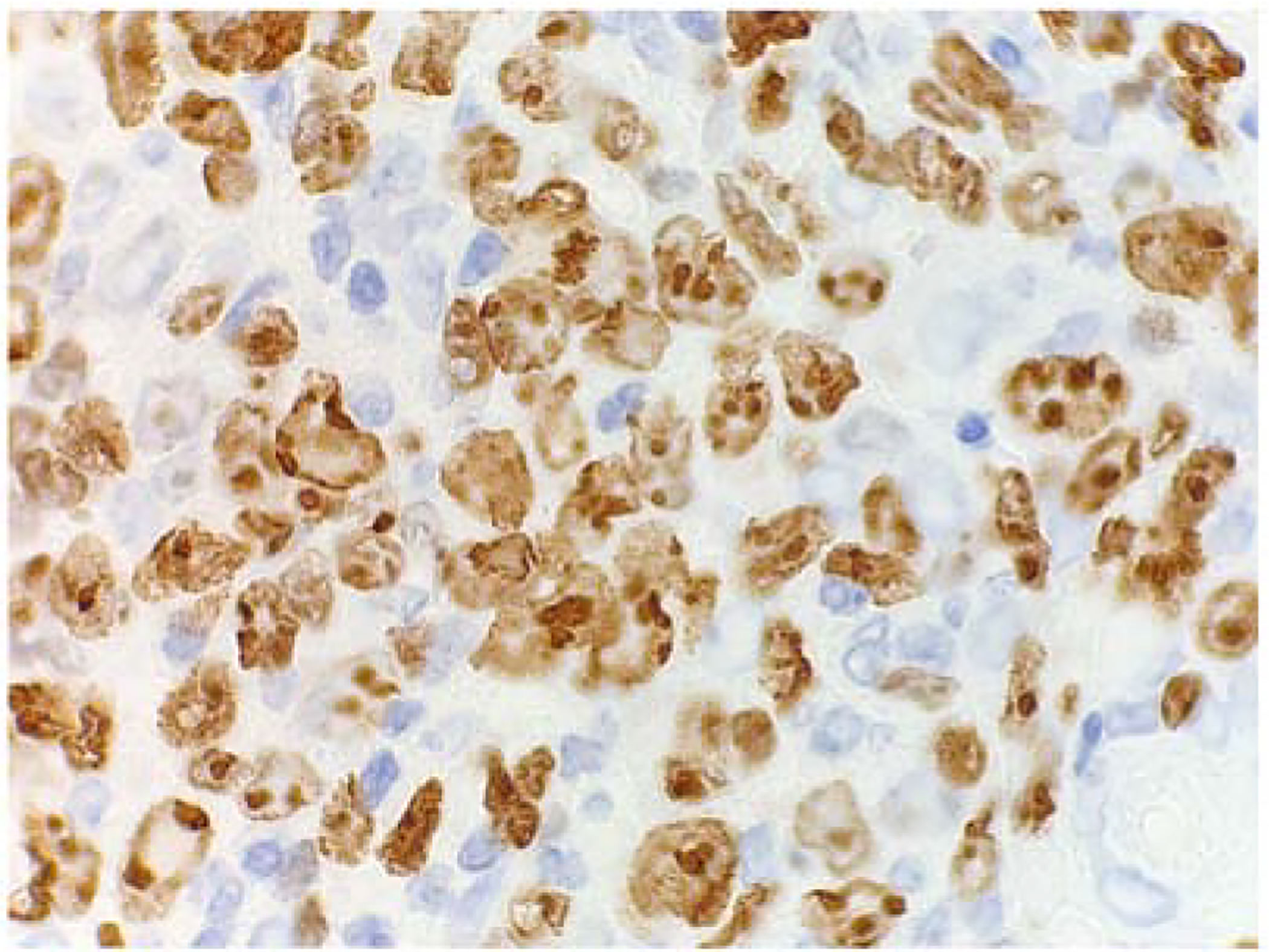
Ki-67 immunohistochemical staining to determine the proliferation index of DLBCL. A case of Ki-67 nuclear staining of >75% tumour cell positivity, in an HIV-infected patient.
EBER-ISH was performed to enable identification of the proportion of EBV-positive DLBCL cases and was interpreted as positive with any proportion of tumour cell reactivity up to 80% (Fig. 5).
Fig. 5.
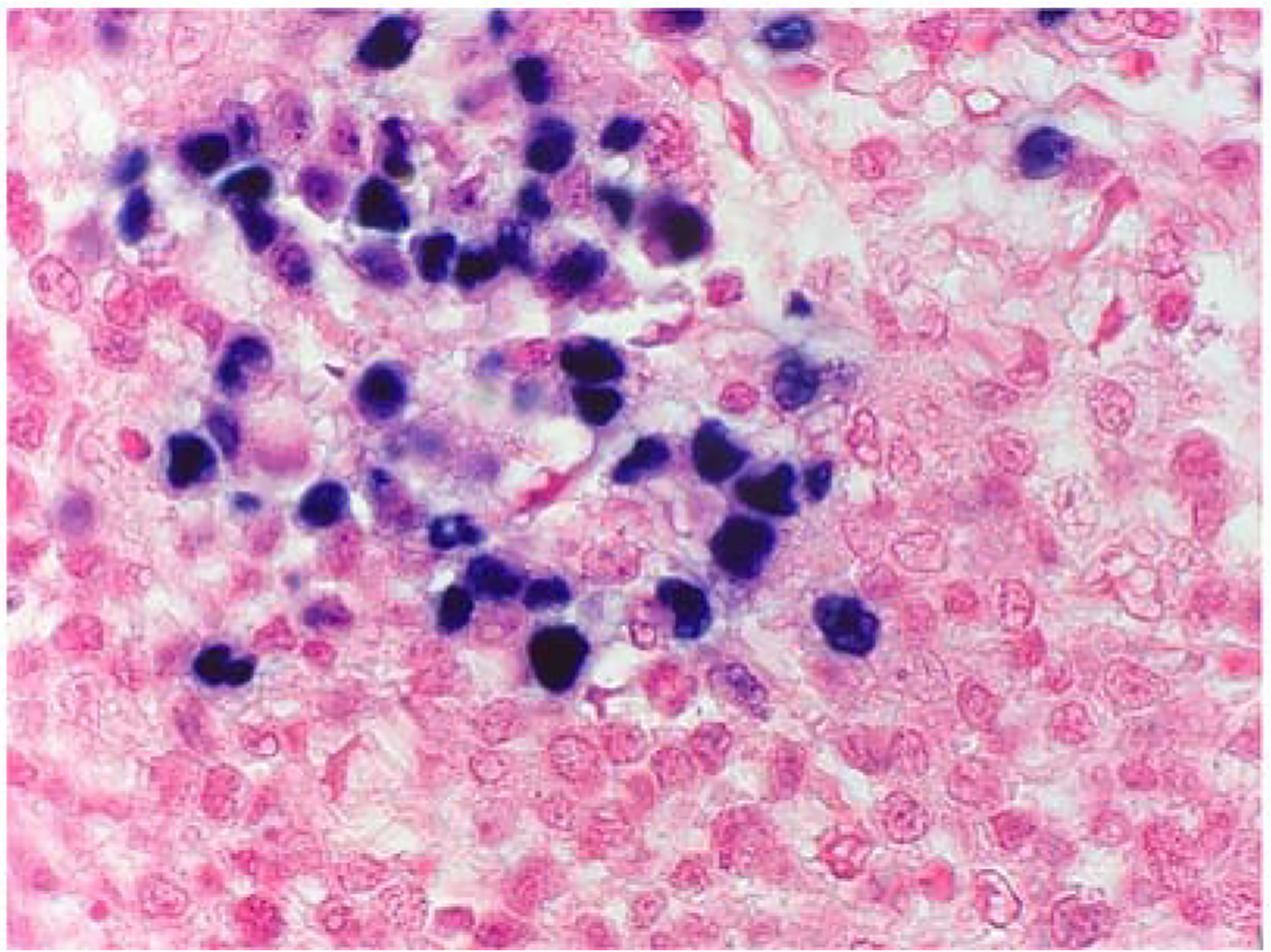
EBV-encoded ribonucleic acid in situ hybridisation (EBER-ISH) staining of DLBCL to determine the presence of Epstein–Barr virus (EBV). A case of EBER-ISH nuclear stain positivity, in an HIV-infected patient, confirming tumour EBV co-infection.
Statistical analysis
The data was collected and recorded into an online database (REDCap).19 Variables from all patients were obtained for analysis and included: gender, age at diagnosis, HIV status, CD4 count if HIV-infected, primary biopsy site, DLBCL subtype, double expressor profile, Ki-67 proliferation index and tumour EBV status. Statistical data analysis was performed using Stata v14.0 (StataCorp, USA).20
Dichotomous and categorical variables were characterised using frequencies and percentages and compared by HIV status and DLBCL subtype using the Pearson chi-squared test or Fisher’s exact test. Continuous non-parametric variables were summarised using medians and interquartile ranges (IQR) and compared using the Wilcoxon rank-sum test.
Survival analysis was estimated using the Kaplan–Meier method. Overall survival (OS) was measured as the time from date of diagnosis until date of death, regardless of cause, or date last seen alive. Patients who were lost to follow-up were censored at the last follow-up date. Kaplan–Meier survival curves were restricted to display 5-year OS. The comparison between survival distributions was determined by means of the log-rank test. A two-sided p value of <0.05 was considered statistically significant.
A Cox proportional hazards model was developed to calculate hazard ratios (HR) with 95% confidence intervals (CI) to assess the impact of prognostic factors on OS. Univariate analysis was performed to determine the potential association of prognostic factors with outcome. Factors with a p value of <0.2 or those that were thought to be clinically relevant were ultimately selected as covariates for multivariate analysis. These factors included: HIV status, DLBCL subtype, Ki-67 proliferation index and age at diagnosis.
RESULTS
A total of 362 de novo DLBCL cases were identified. Of this total, 181 cases had inadequate stored tissue specimens available for additional testing and therefore were excluded. As a result, 181 DLBCL NOS cases met eligibility criteria and included 131 HIV-uninfected patients and 50 HIV-infected patients.
Of the 181 patients included, 93 (51%) were men and the median age at diagnosis was 52 years (IQR 39–63). Fifty patients (28%) were HIV-positive with a median age of 39 years (IQR 34–49) and a median CD4 count of 148 cells/mm3 (IQR 72–337). There was a similar distribution of DLBCL subtypes, even when compared by age and HIV status. Nodal primary biopsy sites (71%) were more common than extranodal sites (29%). Further details of patient baseline characteristics are summarised in Table 1.
Table 1.
Baseline characteristics of DLBCL study patients
| Characteristic | Frequency (percentage) or Median (IQR) |
|---|---|
| Patients | 181 (100%) |
| Gender | |
| Male | 93 (51%) |
| Female | 88 (49%) |
| Age at diagnosis (years) | |
| <30 | 14 (8%) |
| 30–50 | 65 (36%) |
| 51–70 | 86 (47%) |
| >70 | 16 (9%) |
| Median age at diagnosis (years) | |
| Median (IQR) | 52 (39–63) |
| HIV -infected median (IQR) | 39 (34–49) |
| HIV-uninfected median (IQR) | 57 (48–65) |
| DLBCL ABC median (IQR) | 55 (39–61) |
| DLBCL GCB median (IQR) | 51(41–64) |
| HIV status | |
| Positive | 50 (28%) |
| Negative | 131 (72%) |
| CD4 count at diagnosis (cells/mm3) | |
| Median (IQR) | 148 (72–337) |
| DLBCL subtype | |
| ABC | 94 (52%) |
| GCB | 87 (48%) |
| HIV status & DLBCL subtype | |
| HIV-infected + ABC | 24 (48%) |
| HIV-infected + GCB | 26 (52%) |
| Primary biopsy site | |
| Nodal | 128 (71%) |
| Extranodal | 53 (29%) |
ABC, activated B-cell; DLBCL, diffuse large B-cell lymphoma; GCB, germinal centre B-cell; HIV, human immunodeficiency virus; IQR, interquartile range.
Bivariate analysis comparing the various IHC and EBER-ISH results by HIV status and DLBCL subtype are presented in Table 2. DLBCL subtypes were evenly distributed amongst the HIV-infected and HIV-uninfected groups. EBV co-infection was detected in eight (16%) of the HIV-infected cases and in nine (7%) of the HIV-uninfected cases, though this did not reach statistical significance (p=0.09), with no significant difference found between DLBCL subtypes (p=0.67). The proliferation index, Ki-67>75%, occurred significantly more frequently in HIV-infected patients (p=0.004) and approached significance in the ABC subtype (p=0.05). Sixteen patients were double expressors, but there were no statistically significant correlations with HIV status (p=0.77) nor DLBCL subtype (p=0.87).
Table 2.
Bivariate analysis comparing immunohistochemical and EBER-ISH results by HIV status & DLBCL subtype
| HIV-uninfected (n=131) |
HIV-infected (n=50) |
P value |
ABC subtype (n=94) |
GCB subtype (n=87) |
p value | |
|---|---|---|---|---|---|---|
| Hans algorithm | ||||||
| CD10 positive | 46 (35%) | 23 (46%) | 0.18 | 0 (0%) | 69 (79%) | |
| BCL6 positive | 86 (66%) | 31 (62%) | 0.65 | 42 (45%) | 75 (86%) | <0.001 |
| MUM1 positive | 70 (53%) | 30 (60%) | 0.67 | 79 (84%) | 21 (24%) | |
| EBV infection | ||||||
| EBER-ISH positive | 9 (7%) | 8 (16%) | 0.09 | 8 (9%) | 9 (10%) | 0.67 |
| EBER-ISH negative | 122 (93%) | 42 (84%) | 86 (91%) | 78 (90%) | ||
| Proliferation index | ||||||
| Ki-67 >75% | 78 (60%) | 41 (82%) | 68 (72%) | 51 (59%) | ||
| 0.004 | 0.05 | |||||
| Ki-67 ≥75% | 53 (40%) | 9 (18%) | 26 (28%) | 36 (41%) | ||
| Expressor profile | ||||||
| Double expressor | 11 (8%) | 5 (10%) | 0.77 | 8 (9%) | 8 (9%) | 0.87 |
| MYC positive | 18 (14%) | 7 (14%) | 0.96 | 12 (13%) | 13 (15%) | 0.67 |
| BCL2 positive | 78 (60%) | 24 (48%) | 0.16 | 58 (62%) | 44 (51%) | 0.13 |
ABC, activated B-cell; EBER-ISH, Epstein–Barr virus-encoded ribonucleic acid in situ hybridisation; EBV, Epstein–Barr virus; GCB, germinal centre B-cell; HIV, human immunodeficiency virus.
Survival analysis of the total DLBCL patient population showed that by the end of the study period, 95 patients (52%) had died and seven patients had been lost to follow-up. The median survival time was 30 months (95% CI 16–61 months), based on a total patient follow-up time of 4982 months and median follow-up time of 15 months (range 3 days–14 years). The 1-year, 2-year and 5-year OS were 65%, 52% and 40%, respectively.
HIV-infected patients with a CD4 <150 cells/mm3 had a median survival time of 6 months (95% CI 4–16 months). The Kaplan–Meier curves depicting 5-year OS estimates (Fig. 6) showed that HIV-infected patients with a CD4 ≥150 cells/mm3 had similar survival to HIV-uninfected patients; and HIV-infected patients with a CD4 <150 cells/mm3 had a significantly shorter survival than both HIV-infected patients with a CD4 ≥150 cells/mm3 and HIV-uninfected patients (p=0.005). No statistically significant differences in survival were found when the ABC subtype was compared with the GCB subtype (p=0.32), nor when both HIV status and DLBCL subtype were combined for analysis (p=0.14).
Fig. 6.
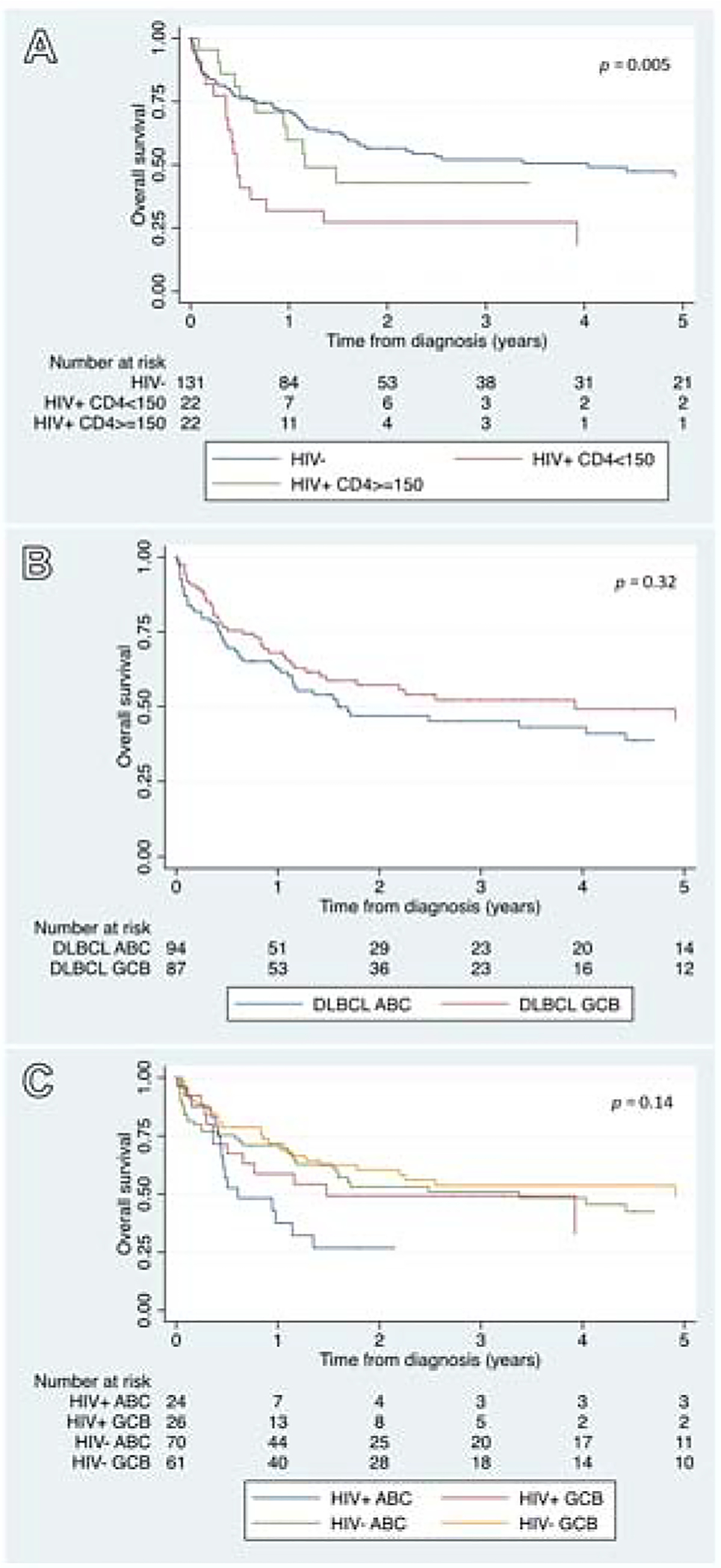
Kaplan–Meier survival estimates depicting 5-year overall survival of DLBCL study patients by: (A) HIV status, (B) DLBCL subtype, (C) HIV status and DLBCL subtype.
The Cox regression analysis, shown in Table 3, assessed the prognostic effect of associated variables on OS. HIV infection with a CD4 <150 cells/mm3 was a significantly poorer prognosis in comparison with no HIV infection (HR 2.4, 95% CI 1.3–4.1). DLBCL subtype (HR 1.2, 95% CI 0.8–1.9), Ki-67 (HR 1.2, 95% CI 0.8–1.9) and age (HR 1.0, 95% CI 0.6–1.6) were not associated with statistically significant differences in survival.
Table 3.
Multivariate model of prognostic factors associated with overall survival
| Selected variables | Hazard ratio | 95% confidence interval | p value |
|---|---|---|---|
| HIV status and CD4 count (cells/mm3) | |||
| (HIV-infected + CD4 <150 vs HIV-uninfected) | 2.4 | 1.3–4.1 | 0.003 |
| (HIV-infected + CD4 ≥150 vs HIV-uninfected) | 1.2 | 0.6–2.4 | 0.55 |
| DLBCL subtype (ABC vs GCB) | 1.2 | 0.8–1.9 | 0.35 |
| Ki-67 proliferation index (>75% vs ≤75%) | 1.2 | 0.8–1.9 | 0.44 |
| Age at diagnosis (years) (>45 vs ≤45) | 1.0 | 0.6–1.6 | 0.97 |
ABC, activated B-cell; DLBCL, diffuse large B-cell lymphoma; GCB, germinal centre B-cell; HIV, human immunodeficiency virus.
DISCUSSION
In this study, performed in a major South African academic referral centre, we found that the DLBCL subtypes (GCB and ABC) were equally distributed among HIV-infected and HIV-uninfected patients. Contrary to expectation, we found that EBV co-infection was detected in only 16% of HIV-associated DLBCLs, although it occurred more frequently in HIV-infected patients as opposed to 7% in HIV-uninfected patients. This is an interesting finding, as previous studies have shown that EBV is positive in 30–60% of HIV-associated DLBCL cases and has been thought to play a significant driving role in lymphomagenesis, compared with 10% EBV positivity in HIV-unassociated DLBCL cases.10,21
No significant difference in 5-year OS was demonstrated between the GCB and ABC subtypes. This is in keeping with results of a similar South African study by Pather et al.22 which, albeit a smaller cohort, also established no significant differences in survival between the subtypes. However, international studies from well-resourced countries with low HIV prevalence report the ABC subtype to have a poorer prognosis with a 5-year OS of approximately 40% compared to the GCB subtype, which has a 60% 5-year OS.4,23
HIV has been shown to play both an indirect and direct role in lymphomagenesis.5 Indirect mechanisms include chronic B-cell activation due to immune dysfunction, oncogenic viral co-infection (e.g., EBV) due to impaired immune surveillance, cytokine overproduction, and genetic alterations.5 Direct mechanisms involve HIV-encoded proteins and HIV virions that act as crucial microenvironmental factors in promoting lymphoma development.5 These aetiological mechanisms persist even in those on ART with undetectable viral loads.24 We hypothesise that these mechanisms are common to the development of either the ABC or GCB subtype, which may explain the equal incidence of the subtypes amongst our HIV-infected cases.
With regards to HIV-infected patients, there was no significant difference in OS by DLBCL subtype. Of four previous published studies, two reported poorer survival outcomes in patients with the ABC subtype,4,14 one found poorer survival in patients with the GCB subtype,15 and a fourth study found no significant difference in outcome between the subtypes.12
Our study showed that HIV-infected patients with CD4 counts of 150 cells/mm3 or more had similar survival outcomes to HIV-uninfected patients. It also showed that HIV-infected patients with CD4 counts of less than 150 cells/mm3 had a significantly shorter survival and a 2.4 times higher risk of mortality in comparison to HIV-uninfected patients.
A high Ki-67 proliferation index has been shown to indicate a poor prognosis with decreased survival outcomes in DLBCL patients treated with standard chemotherapy.25 We found that Ki-67 >75% occurred more commonly in HIV-infected patients and the ABC subtype, however its association with adverse survival was not significant.
Double-expressor lymphoma (DEL) refers to the co-expression of MYC and BCL2 proteins without underlying chromosomal rearrangements.26 DEL is an adverse prognostic indicator which is present in 20–30% of DLBCL cases, and has been reported to correlate highly with the ABC subtype.26 Our cohort of DLBCL patients yielded a small proportion (9%) of double expressors that showed no significant correlation with subtype.
Two recently published studies by Schmitz et al.27 and Chapuy et al.28 have identified genetic subtypes of DLBCL based on shared genomic aberrations involving, for example, MYD88, CD79B, BCL6, BCL2, EZH2, NOTCH, CARD11 and TP53. This has taken the COO classification of DLBCL to new depth and complexity. To our knowledge, these genetic subtypes have not been specifically investigated in HIV.
This study has several limitations. Firstly, due to lack of archived tissue, 50% of our patient cohort could not be subtyped and was excluded. Secondly, in our resource-constrained local setting, ancillary investigations such as fluorescent in situ hybridisation (FISH) and molecular testing are not routinely performed on the majority of cases, which may result in double/triple-hit lymphomas being erroneously classified as DLBCL NOS and thus being treated with standard chemotherapy instead of the recommended higher intensity regimens.29 Thirdly, it is difficult to compare the mortality rates of our study group with international data as rituximab was only implemented into our treatment protocols from 2013, and even then mostly in HIV-uninfected patients due to resource constraints and lack of supportive data. Lastly, we did not collect details on stage of disease, treatment regimens nor specific treatment outcomes other than survival which would have allowed for a more comprehensive analysis of the effect of DLBCL subtype on survival.
The strength of this study is that, for the first time in our HIV-endemic region, we describe a large proportion of our DLBCL population with detailed IHC and EBER-ISH findings as well as survival outcomes.
The information gained from our study will form a basis from which to further explore the pathobiology of DLBCL, particularly in HIV.
CONCLUSIONS
We found that DLBCL subtypes were present in equal proportions, regardless of HIV status. EBV co-infection was also associated equally with both the ABC and GCB subtypes. EBV was present in a much lower proportion of HIV-associated DLBCLs than described in the literature at only 16%. There were no significant differences in survival between the DLBCL subtypes. HIV infection with high CD4 counts had good survival outcomes, and HIV infection with low CD4 counts predict for markedly adverse survival outcomes.
Supplementary Material
Acknowledgements:
Edward L. Murphy, George Rutherford, Lawrence D. Kaplan and the rest of the UCSF team for assisting in the writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest and sources of funding: Research reported in this manuscript was supported by the Fogarty International Center of the National Institutes of Health grants (D43-TW010345, D43-TW010543) awarded to SC, EV and KA; the National Research Foundation Thuthuka grant (TTK14052267787) awarded to EV; and the Peter Jacobs Bursary Trust awarded to KA. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the supporters. The authors state that there are no conflicts of interest to disclose.
APPENDIX A: SUPPLEMENTARY DATA
Supplementary data related to this article can be found at http: http://dx.doi.org/10.17632/4m4dz4n2wn.1.
References
- 1.Martelli M, Ferreri AJM, Agostinelli C, et al. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol 2013; 87: 146–71. [DOI] [PubMed] [Google Scholar]
- 2.Gascoyne RD, Campo E, Jaffe ES, et al. Diffuse large B-cell lymphoma, NOS In: Swerdlow SH, Campo E, Harris NL, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed. Lyon: IARC, 2017; 291–7. [Google Scholar]
- 3.Chapuy B, Cheng H, Watahiki A, et al. Diffuse large B-cell lymphoma patient-derived xenograft models capture the molecular and biological heterogeneity of the disease. Blood 2016; 127: 2203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waibel M, Gregory G, Shortt J, et al. Rational combination therapies targeting survival signaling in aggressive B-cell leukemia/lymphoma. Curr Opin Hematol 2014; 21: 297–308. [DOI] [PubMed] [Google Scholar]
- 5.Dolcetti R, Gloghini A, Caruso A, et al. A lymphomagenic role for HIV beyond immune suppression? Blood 2016; 127: 1403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS. Geneva 2019. Cited 10 Oct 2019 http://www.unaids.org.
- 7.Statistics South Africa. Pretoria 2019. Accessed 10 Oct 2019 http://www.statssa.gov.za.
- 8.Larsen A, Cheyip M, Tesfay A, et al. Timing and predictors of initiation on antiretroviral therapy among newly-diagnosed HIV-infected persons in South Africa. AIDS Behav 2019; 23: 375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011; 103: 753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao C, Silverberg MJ, Martinez-Maza O, et al. Epstein-Barr virus infection and expression of B-cell oncogenic markers in HIV-related diffuse large B-cell lymphoma. Clin Cancer Res 2012; 18: 4702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roschewski M, Wilson WH. EBV-associated lymphomas in adults. Best Pract Res Clin Haematol 2012; 25: 75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chadburn A, Chiu A, Lee JY, et al. Immunophenotypic analysis of AIDS-related diffuse large B-cell lymphoma and clinical implications in patients from AIDS Malignancies Consortium clinical trials 010 and 034. J Clin Oncol 2009; 27: 5039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunleavy K, Little RF, Pittaluga S, et al. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood 2010; 115: 3017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morton LM, Kim CJ, Weiss LM, et al. Molecular characteristics of diffuse large B-cell lymphoma in human immunodeficiency virus-infected and -uninfected patients in the pre-highly active antiretroviral therapy and pre-rituximab era. Leuk Lymphoma 2014; 55: 551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao C, Silverberg MJ, Xu L, et al. A comparative study of molecular characteristics of diffuse large B-cell lymphoma from patients with and without human immunodeficiency virus infection. Clin Cancer Res 2015; 21: 1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein H, Warnke RA, Chan WC, et al. Diffuse large B-cell lymphoma, not otherwise specified In: Swerdlow SH, Campo E, Harris NL, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: IARC, 2008; 233–7. [Google Scholar]
- 17.Gatter KC, Warnke RA. Diffuse large B-cell lymphoma In: Jaffe ES, Harris NL, Stein H, editors. WHO Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC, 2001; 171–4. [Google Scholar]
- 18.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004; 103: 275–82. [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.StataCorp 2015. Stata statistical software: release 14. College Station, TX: StataCorp, 2015. [Google Scholar]
- 21.Cohen JI, Bollard CM, Khanna R, et al. Current understanding of the role of Epstein-Barr virus (EBV) in lymphomagenesis and therapeutic approaches to EBV-associated lymphomas. Leuk Lymphoma 2008; 49 (Suppl 1): 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pather S, Mohamed Z, McLeod H, et al. Large cell lymphoma: correlation of HIV status and prognosis with differentiation profiles assessed by immunophenotyping. Pathol Oncol Res 2013; 19: 695–705. [DOI] [PubMed] [Google Scholar]
- 23.Nowakowski GS, Czuczman MS. ABC, GCB, and double-hit diffuse large B-cell lymphoma: does subtype make a difference in therapy selection? In: Dizon DS, editor. American Society of Clinical Oncology Educational Book. 35th ed. Virginia: ASCO, 2015; 449–57. [DOI] [PubMed] [Google Scholar]
- 24.Popovic M, Tenner-Racz K, Pelser C, et al. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc Natl Acad Sci USA 2005; 102: 14807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li ZM, Huang JJ, Xia Y, et al. High Ki-67 expression in diffuse large B-cell lymphoma patients with non-germinal center subtype indicates limited survival benefit from R-CHOP therapy. Eur J Haematol 2012; 88: 510–7. [DOI] [PubMed] [Google Scholar]
- 26.Riedell PA, Smith SM. Double hit and double expressors in lymphoma: definition and treatment. Cancer 2018; 124: 4622–32. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med 2018; 378: 1396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 2018; 24: 679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barta SK, Xue X, Wang D, et al. Treatment factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: a pooled analysis of 1546 patients. Blood 2013; 122: 3251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


