Abstract
Background:
Disabling persistent perceived fatigue occurs in 50% of people with multiple sclerosis (MS), but mechanisms are poorly understood. Low histidine could contribute to fatigue since it is the neurotransmitter histamine precursor and low serum levels are reported in other diseases where fatigue is common (e.g., breast cancer, kidney disease, diabetes). Serum histidine is also inversely correlated with proinflammatory cytokines (e.g., TNF, IFN-y), which have been linked to MS fatigue.
Purpose:
To determine if serum histidine is low in fatigued women with MS, and if histidine is related to differences in proinflammatory cytokines.
Methods:
Participants were classified as having elevated (n = 19) or normal (n = 18) perceived fatigue based on a median sample split using Profile of Mood States fatigue scale scores, with the elevated fatigue group having scores >7. Histidine and gene-expression of TNF, IFN-y, and leptin were assayed from a serum sample.
Results:
After adjustment for depression, serum histidine was significantly lower in women with MS with elevated fatigue, compared to normal fatigue (64.57 vs. 70.48 nmol/ml, p = .048, g = 0.75). There were no differences between groups in cytokine expression (all p > .24). Gene expression of TNF correlated with histidine only in people with normal fatigue (r = .51, p = .034), while no other cytokines related to histidine levels.
Conclusions:
These results provide evidence that serum histidine is lower in fatigued women with MS, but the study did not find a relationship between histidine and TNF, IFN-y, or leptin gene expression.
Keywords: amino acid, cytokine, histamine, leptin, proinflammatory, TNF
1. Introduction
Fatigue is a common multiple sclerosis (MS) symptom. About half of the people with MS report persistent fatigue, with many ranking fatigue as their most debilitating symptom (1–3). Fatigue is also a primary reason that persons with MS reduce work hours or become unemployed (4, 5). Still unknown is why only some with MS report elevated, persistent fatigue. Despite the prevalence and burden, the neurobiological mechanisms of fatigue are poorly understood, and there are no FDA approved treatments for MS fatigue (6).
Low serum histidine could play a role in MS fatigue. Histidine is the precursor for the neurotransmitter histamine, which has a demonstrated role in fatigue (7–9). Histidine is readily shuttled across the blood-brain barrier by amino acid transporters where it is converted by the enzyme histidine decarboxylase to histamine (7, 10). The synthesis rate of histamine is determined by histidine availability, as demonstrated by both depletion (11, 12) and loading studies (13). Giving histidine as a dietary supplement reduces fatigue in otherwise healthy people who report high fatigue (14). Conversely, drugs that block the histamine H1 receptor, such as low-dose doxepin, increase perceived fatigue (9, 15). These studies demonstrate that histidine is the rate-limiting step to histamine neurotransmitter synthesis, and that histidine and histamine have important roles in fatigue.
Both histidine and histamine are also related to proinflammatory cytokine activity in a number of disease states. Specifically, serum histidine levels were found to be inversely associated with C-reactive protein and interleukin 6 (IL-6) in a large sample of obese women and people with kidney disease (16, 17). Histidine given to women with insulin resistance as part of a randomized, double-blind, placebo-controlled trial also resulted in decreased tumor necrosis factor alpha (TNF) (18). Animal models suggest that histidine may also influence inflammation in MS. Histamine receptor knockout mice and histidine decarboxylase knockout mice given experimental autoimmune encephalomyelitis have elevated production of the proinflammatory cytokines TNF, interferon gamma (IFN-y) and leptin, in addition to early disease progression (19, 20). Many proinflammatory cytokines are themselves linked to fatigue. TNF mRNA is higher in persons with MS with elevated fatigue, when compared to persons with MS who had normal fatigue (21).
Several studies have found relationships between low histidine levels and elevated proinflammatory cytokine production in diseases where fatigue is common, including rheumatioid arthritis (22, 23), kidney disease (17), breast cancer (24), obesity (16), and diabetes (25). These relationships have not been fully explored in MS. The purpose of this study was to determine if serum histidine levels were different in women with MS reporting normal and elevated fatigue. The second purpose was to test if serum histidine levels were related to differences in proinflammatory cytokine gene-expression, and if these relationships differed between normal and elevated fatigued women with MS.
2. Methods
A cross-sectional design was used for this study. The Oregon Health & Science University (OHSU) institutional review board approved all methods and written informed consent was obtained using a paper form in a private exam room before data collection began.
2.1. Participants
Pre-existing MS patient databases, flyers, and websites were used to recruit study participants. Women with MS were included who were free of neurological impairment (other than MS), did not regularly use (> 2 times/week) fatigue-reducing drugs or supplements (including histidine or carnosine), and did not currently take antihistamine drugs. The sample (n = 37) was restricted to females because females have greater histamine H1 receptor density (26) and increased sensitivity to dietary histidine, possibly due to histamine-estrogen interactions (27, 28).
2.2. Measures
Perception of fatigue was assessed using the fatigue subscale from the Profile of Mood States-Brief Form (POMS-BF). Five different adjectives were rated on a 0 (not at all) to 4 (extremely) scale over “the past two weeks, including today” (29). The Profile of Mood States has been widely used to assess fatigue in both people with MS (30) and healthy control participants (31, 32).
Depression, sleep, and physical activity can also be strongly related to MS fatigue (33–35), and were measured to control for potential confounding and for descriptive purposes. Depression was measured using the Beck Depression Inventory (36), a 21 item questionnaire with good validity evidence in MS (37). Sleep was measured using the Pittsburgh Sleep Quality Index (38–40), a self-rated inventory that measures sleep duration and quality over the prior month. Finally, physical activity behavior was measured using the Godin Leisure Time Exercise Questionnaire (GLTEQ), which asks participants to indicate the number of times in a typical week they participate in mild, moderate, and vigorous intensity exercise for more than 15 minutes at a time (41). The GLTEQ is strongly correlated with objective accelerometry measures of physical activity in persons with MS (42). The self-administered Expanded Disability Status Scale (EDSS), which assesses neurologic disability, was used to characterize the study participants (43, 44).
2.3. Procedure
Compliance with the pre-test instructions (i.e., minimum 2 hour fast) was checked before 6 ml of blood was drawn from the arm and stored in two different tubes. Blood in the tube for histidine analysis was allowed to clot before serum was separated by centrifugation. Tubes were stored at −80°C until histidine and gene-expression batch analysis. Lastly, participants completed fatigue, depression, sleep, physical activity, and additional demographic questionnaires using REDCap web-based software and a tablet.
2.4. Histidine Assay
The procedure to quantify serum histidine consisted of a solid phase extraction step, followed by a derivatization procedure using the EZ:faast amino acid analyses kit from Phenomenex (45, 46). Briefly, internal standard (d3-methionine) was added to diluted serum (10 μL serum plus 90 μL of phosphate buffered saline, PBS) and amino acids were extracted using sorbent tips. Extracted amino acids were converted to chloroformates using reagents and instructions as described in the EZ:faast kit from the manufacturer (46). Standards containing from 0.1 to 20 nmol/mL were prepared in 100 μL PBS at the same time.
Derivatized amino acids were analyzed using a 4000 QTRAP hybrid/triple quadrupole linear ion trap mass spectrometer (SCIEX, Foster City, CA) with electrospray ionization (ESI) in positive mode. The mass spectrometer was interfaced to a Shimadzu (Columbia, MD) SIL-20AC XR auto-sampler followed by 2 LC-20AD XR LC pumps. The instrument was operated with the following settings: source voltage 4500 kV, GS1 50, GS2 50, CUR 20, TEM 350 and CAD gas medium. The multiple reaction monitoring (MRM) transitions were as follows: Histidine, m/z 370→196 (quantifier ion) m/z 370→110 (qualifier ion); d3-methionine, m/z 281→193 and m/z 281→142. All transitions were obtained with a DP 56, CE 25, CXP 10 and each was monitored with a 50 ms dwell time. The gradient mobile phase was delivered at a flow rate of 0.25 ml/min and consisted of two solvents, 10 mM ammonium formate (solvent A) and 10 mM ammonium formate in methanol (solvent B). Initial concentration of B was 70%, which was held for 0.1 min followed by an increase to 98% B by 6 minutes, held at 98% to 8 min decreasing to 70% again over 0.1 minutes, followed by re-equilibration for 5 min. The column was a EZ:faast AAA-MS column, 250×2 mm, maintained at 40°C using a Shimadzu CTO-20AC column oven. Data were acquired using Analyst 1.6.2 software and analyzed using Multiquant 3.0.1 software.
2.5. Gene-Expression Analyses for pro-inflammatory cytokine expression
RNA isolation, quality assessments and qPCR assays were performed in the OHSU Gene Profiling Shared Resource in order to quantify expression of TNF, IFN-y, and leptin from blood. The sample RNA was isolated using the PAXgene QIAsymphony Kit (Qiagen). RNA assessments were performed on the samples using the Bioanalyzer 2100 and a NanoDrop 8000 for UV analysis. Samples displayed excellent RNA quality consistent with intact RNA. All samples have RIN values ranging between 7.2–9.0. Due to inconsistent quantitation measurements between the bioanalyzer and UV spectrophotometry, Qubit fluorescent quantitation was performed and these values were used for qPCR. Reverse transcription was performed using 600ng of total RNA per 50μl reaction using the Vilo Superscript kit (Life Technologies) for mRNA analysis. Following cDNA synthesis, 2μl of cDNA was used in PCR reactions with 10μl TaqMan universal mastermix and 1μl of 20x gene specific TaqMan assay in a total volume of 20μl and loaded onto the QuantStudio instrument. All genes were run together on a single 384 well plate. All samples generated Ct values within an acceptable linear range (between 5 and 35 cycles). The standard deviation between replicates was very low (all samples < 0.2).
The qPCR cycle threshold (Ct) values were normalized relative to the stable reference gene UBE2D2 and transformed onto an expression scale. First, we evaluated data quality using standard metrics such as the amplification score. Based on these measures we found that essentially no meaningful amplification occurred after 35 cycles, although the amplification was allowed to run for a full 40 cycles before terminating. Thus, Ct values were censored at a value of 35 and may be viewed as gene expression below a lower limit of detection. To normalize the Ct values, we collapsed all triplicate wells to the median and subtracted the median Ct of each target gene from the median Ct of UBE2D2 for each subject-gene combination. Normalized Ct values were then transformed onto an expression scale by subtracting each normalized Ct from the nearest integer larger than the maximum normalized Ct across all plates, so that higher values on this expression scale represent relatively greater gene expression. Censored values are given a value of 0 on this scale, ultimately representing no measured expression.
2.6. Statistical Analysis
Statistical tests were conducted using SPSS 24.0 (IBM Corp., Armonk, NY). Complete and usable data were obtained from 37 of 44 participants in the study. Participants were grouped according to whether they had elevated (>7) or low (≤ 7) fatigue ratings, based on a median split of the POMS-BF fatigue subscale scores. This was done to improve clinical interpretation of the results (47), and because POMS-BF fatigue scores > 7 have previously been used as an indicator of high fatigue (9). Data were checked for normality using histograms, scatterplots, and Shapiro-Wilk tests. Differences in sample characteristics between the groups were detected using independent-samples t-tests. One-way analysis of covariance (ANCOVA) was used to test if serum histidine was different between fatigue groups, while controlling for differences in depression ratings between groups. Hedges’ g was calculated as a measure of effect size (48), with values ~ 0.20 considered small and those ~ 0.80 classified as large (49). Partial correlations, also controlling for depression, were used to determine if there were relationships between serum histidine and expression of proinflammatory cytokines (TNF, IFN-y, leptin). In order to test if these correlations differed by fatigue group, the correlation coefficients were converted using Fisher’s r-to-z transformation and the corresponding values were used in a z-test (50).
3. Results
Descriptive characteristics of the normal and elevated fatigue groups are reported in Table 1. Neither age nor EDSS were significantly different between the groups. Although the normal fatigue group was more physically active than the elevated fatigue group as measured by the GLTEQ, this difference was not statistically significant (p = .064). Depression was significantly higher in the elevated fatigue group (t = −4.74, p < .0001), as was poor sleep quality (t = −2.93, p = .006). Since depression and poor sleep quality were highly correlated here (r = .64, p < .0001), only depression was included as a covariate in further analyses.
Table 1.
Descriptive Characteristics of Fatigue Groups
| Variable | Entire Sample (n = 37) |
Normal Fatigue (≤ 7) (n = 18) |
Elevated Fatigue (> 7) (n = 19) |
Group Difference |
|---|---|---|---|---|
| Age (years) | 49.66 (9.32) | 51.02 (9.00) | 48.36 (9.68) | t = .86, p = .394 |
| Age at Diagnosis | 37.51 (9.85) | 38.11 (9.19) | 36.95 (10.66) | t = .35, p = .725 |
| EDSS | 4.09 (.79) | 3.97 (.58) | 4.21 (.96) | t = −.90, p = .371 |
| Sleep (PSQI) | 7.22 (3.31) | 5.72 (2.29) | 8.63 (3.56) | t = −2.93, p = .006* |
| Exercise (GLTEQ) | 37.35 (29.21) | 46.44 (27.24) | 28.74 (29.06) | t = 1.90, p = .064 |
| Depression (BDI) | 12.92 (8.89) | 7.28 (4.94) | 18.26 (8.54) | t = −4.74, p < .0001* |
| Histidine (nmol/ml) | ||||
| Unadjusted | 67.45 (14.13) | 70.48 (3.64) | 64.57 (2.86) | t = 1.28, p = .208 |
| Adjusted# | - | 73.54 (3.72) | 61.68 (3.59) | f = 4.22, p = .048 |
| Expression (normalized Ct) | ||||
| TNF | 4.96 (.31) | 4.94 (.31) | 4.99 (.32) | t = −.47, p = .640 |
| IFN-y | 3.53 (.67) | 3.49 (.63) | 3.56 (.73) | t = −.29, p = .769 |
| Leptin | 1.23 (.61) | 1.23 (.56) | .84 (.82) | t = 1.65, p = .107 |
Note. Values shown are unadjusted means (standard deviation), unless otherwise noted.
= values adjusted for depression.
= statistically significant.
PSQI = Pittsburgh Sleep Quality Index.
GLTEQ = Godin Leisure Time Exercise Questionnaire.
BDI = Beck Depression Inventory.
Nmol/ml = nanomoles of histidine per milliliter of serum.
= adjustment for Beck Depression Inventory score.
TNF = Tumor Necrosis Factor.
IFN-y = Interferon Gamma.
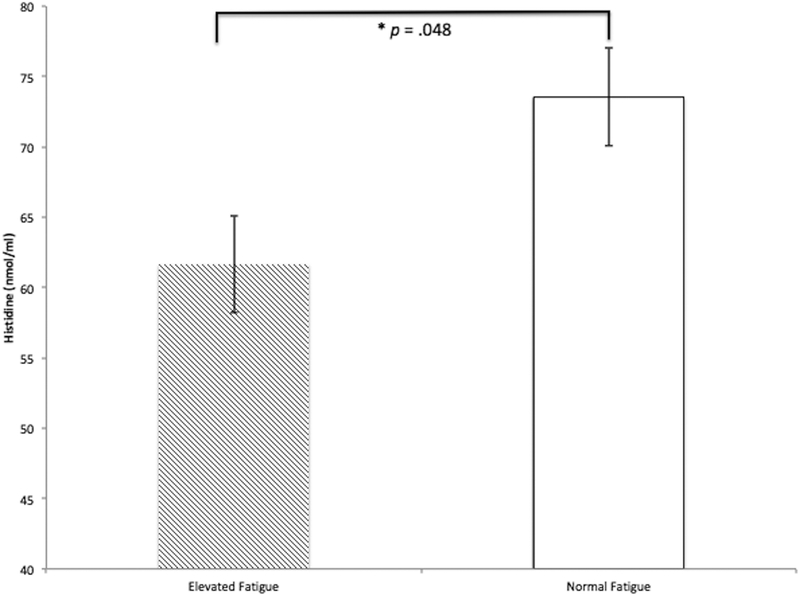
Histidine was significantly higher in the normal fatigue group than in the elevated fatigue group, as shown in Figure 1 and Table 1. The ANCOVA testing if histidine was different between fatigue groups was significant, F(1,34) = 4.22, p = .048, g = 0.75 (95% CI = 0.08, 1.42). ANCOVAs identified no difference between fatigue groups for TNF, F(1,34) = .10, p = .746, g = 0.09 (95% CI = −0.55, 0.73); IFN-y, F(1,34) = 1.40, p = .244, g = 0.36 (95% CI = −0.28, 1.01) or leptin, F(1,34) = .88, p = .355, g = −0.25 (95% CI = −0.90, 0.39). Descriptive statistics of expression are provided in Table 1.
Figure 1.
Serum Histidine in Women with MS with Elevated and Normal Fatigue. There was a significant difference between groups, F(1,34) = 4.22, p = .048. Values shown are adjusted for Beck Depression Inventory scores. Bars are standard errors. Nmol/ml = nanomoles of histidine per milliliter of serum.
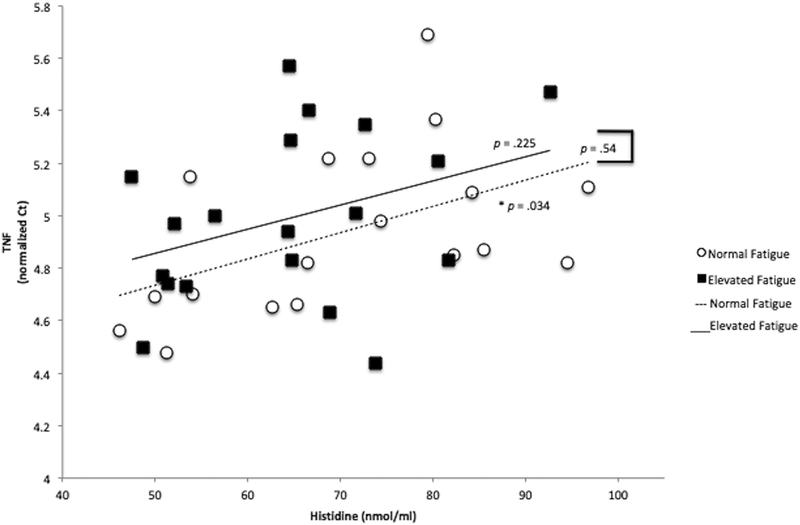
TNF expression correlated significantly with histidine in persons with MS with normal fatigue (r = .51, p = .034) but not among those with elevated fatigue (r = .30, p = .225), as shown in Figure 2. However, the relationship between TNF and histidine was not statistically different by fatigue group (z = −.598, p = .549). IFN-y was not significantly correlated with histidine among either MS with elevated fatigue (r = .34, p = .165) or normal fatigue (r = −.07, p = .785). The correlations between IFN and histidine in the elevated and normal fatigue group were not statistically different from each other (z = 1.151, p =.249). Leptin was not significantly correlated with histidine in those with elevated fatigue (r = −.093, p =.714) or normal fatigue (r = −.12, p = .627). Leptin correlations with histidine also did not differ by fatigue group (z = .094, p = .924).
Figure 2.
Relationship Between TNF Expression and Serum Histidine in People with MS with Normal and Elevated Fatigue. TNF expression was significantly correlated with histidine in people with normal fatigue (r = .51, p = .034) but not those with elevated fatigue (r = .30, p = .225). TNF cycle threshold (Ct) values are normalized to a reference gene (UBE2D2). Nmol/ml = nanomoles of histidine per milliliter of serum.
4. Discussion
This study found that mean serum histidine was lower in women with MS who reported elevated fatigue, compared to those with normal fatigue. This finding was consistent with our hypotheses that low serum histidine could potentially play a role in reducing brain histamine synthesis and result in fatigue. Another possible implication is that low histidine availability could reduce the effectiveness of some treatments used to reduce MS fatigue, such as medications (i.e., modafinil, methylphenidate) or exercise, which may work in part by increasing histamine release (9, 51, 52). Although it is unclear why histidine may be lower in fatigued persons with MS, our findings are consistent with others who found alterations in histidine metabolism in MS (53), and in fatigued groups with other diseases (24).
The current study did not find that cytokine expression (TNF, IFN-y, leptin) was different between fatigue groups. We expected to see higher cytokine expression in the elevated fatigue group given that previous reports found higher TNF expression in persons with MS with fatigue (21, 54). Although other researchers have not always adjusted for depression, we did not find that TNF was higher in the elevated fatigue group even when models were unadjusted (data not shown). Other researchers have also included males in their MS samples, and it is unknown if the relationships between cytokine expression and fatigue vary by sex. Other investigators also sometimes report large variability in cytokine expression between fatigue groups (21). If the true relationship between cytokine expression and fatigue is highly variable or not particularly strong, authors may not always find a statistically significant difference in cytokine expression between fatigue groups. If null findings are not reported, this could create a publication bias and lead researchers to believe that proinflammatory cytokines are much higher in fatigued persons with MS when this is not true.
We also did not find that histidine was related to cytokine gene expression, except to TNF in the low fatigue group. This was unexpected since supplemental histidine in humans reduces TNF (18) and histidine decarboxylase knockout mice given experimental autoimmune encephalomyelitis have higher TNF, IFN-y, and leptin production (20). There are several reasons this could have occurred. One possibility is that only extreme levels of histidine and histamine (i.e., supplementation or complete absence) influence measured cytokines to a degree that can be detected, whereas individual variability of histidine is not related to cytokine expression. Numerous confounders can also impact proinflammatory cytokines, including time of day, body fat, physical activity, and medication use (55). Although these confounders were not related to expression in our sample (analyses not reported), not all investigators report considering these confounders. Since this study also found that cytokine expression was not different between fatigue groups but that histidine was, it may not be important in future studies to reduce proinflammatory cytokine expression in order to also improve fatigue in MS.
A strength of this study is that it was hypothesis-driven, in that only serum histidine and expression of select genes (TNF, IFN-y, leptin) were measured. No other amino acids or expression of additional cytokines were measured. Another strength is that all analyses adjusted for depression differences between fatigue groups. Although depression and fatigue are highly comorbid in MS (33), further efforts to separate fatigue and depression research in MS are needed to develop effective treatments. Limitations of the study are that men with MS and a healthy control group were not included, small sample size, and a cross-sectional design was used. This limits generalizability and the ability to infer a causal relationship between low histidine and fatigue. The study also did not examine metabolic differences in MS that could explain fatigue. Some researchers have noted that dysregulated mitochondrial function could increase fatigue in persons with MS (56). Differences between MS and control participants have been noted for sorbitol and fructose metabolites from cerebrospinal fluid (57), serum lactate (58), and, among others, serum creatinine and xanthine (59). However, these studies do not specifically examine differences in perceived fatigue among those MS, so the relationship between mitochondrial dysfunction and perceived fatigue within groups of persons with MS remains unclear and needs further research.
In summary, this study found that serum histidine is lower in women with MS who report elevated fatigue, and that serum histidine and fatigue levels are not related to the proinflammatory cytokine gene expression of TNF, IFN-y and leptin. Future studies are needed to confirm these results and test if serum histidine is also related to fatigue in males with MS. Researchers could also determine if serum histidine is lower in other conditions marked by fatigue including cancer, heart failure, and myalgic encephalomyelitis/chronic fatigue syndrome. Finally, since dietary histidine reduces fatigue in apparently healthy people (14), future research could also test histidine supplementation as a treatment for MS fatigue.
Acknowledgement/funding
We thank Nick Coddington, Rachel Murdock, and Kayla Warner for assistance with participant recruitment and data collection. We would like to thank Ms. Jenny Luo and Dr. Dennis Koop for the assistance with the histidine analysis that was conducted in the Bioanalytical Shared Resource/Pharmacokinetics Core. The facility is part of the Universiy Shared Resource Program at Oregon Health and Sciences University. We thank Anna Booman for assistance with data reduction and analysis. This work was supported by the National Institutes of Health (NIH-NCCIH T32 AT002688), the National Multiple Sclerosis Society (MB0011) and the Medical Research Foundation of Oregon. Data collection supported by Oregon Clinical and Translational Research Institute (1 UL1 RR024140 01) and REDCap electronic data capture tools at Oregon Health & Science University.
References
- 1.Bakshi R Fatigue associated with multiple sclerosis: diagnosis, impact and management. Multiple sclerosis. 2003;9:219–27. [DOI] [PubMed] [Google Scholar]
- 2.Wood B, van der Mei IA, Ponsonby AL, Pittas F, Quinn S, Dwyer T, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19(2):217–24. Epub 2012/06/26. doi: 10.1177/1352458512450351. [DOI] [PubMed] [Google Scholar]
- 3.Vercoulen JHMM, Hommes OR, Swanink CMA, Jongen PJH, Fennis JFM, Galama JMD, et al. The measurement of fatigue in patients with multiple sclerosis: a multidimensional comparision with patients with chronic fatigue syndrome and healthy subjects. Archives of Neurology. 1996;53:642–9. [DOI] [PubMed] [Google Scholar]
- 4.Smith MM, Arnett PA. Factors related to employment status changes in individuals with multiple sclerosis. Multiple sclerosis. 2005;11:1–8. [DOI] [PubMed] [Google Scholar]
- 5.Julian LJ, Vella L, Vollmer T, Hadjimichael O, Mohr DC. Employment in multiple sclerosis. Exiting and re-entering the work force. J Neurol. 2008;255(9):1354–60. Epub 2008/08/05. doi: 10.1007/s00415-008-0910-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Management of MS-related fatigue. New York: National Clinical Advisory Board of the National Multiple Sclerosis Society, 2006. [Google Scholar]
- 7.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiological Reviews. 2008;88:1183–241. doi: 10.1152/physrev.00043.2007.-Histamine. [DOI] [PubMed] [Google Scholar]
- 8.Stahl SM. The pyschopharmacology of energy and fatigue. Journal of Clinical Psychiatry. 2002;63(1):7–8. [DOI] [PubMed] [Google Scholar]
- 9.Loy BD, O’Connor PJ. The effect of histamine on changes in mental energy and fatigue after a single bout of exercise. Physiol Behav. 2016;153:7–18. Epub 2015/10/21. doi: 10.1016/j.physbeh.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4(2):121–30. Epub 2003/02/04. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa T, Nakamura T, Shibakusa T, Sugita M, Naganuma F, Iida T, et al. Insufficient intake of L-histidine reduces brain histamine and causes anxiety-like behaviors in male mice. J Nutr. 2014;144(10):1637–41. Epub 2014/07/25. doi: 10.3945/jn.114.196105. [DOI] [PubMed] [Google Scholar]
- 12.van Ruitenbeek P, Sambeth A, Vermeeren A, Young SN, Riedel WJ. Effects of L-histidine depletion and L-tyrosine/L-phenylalanine depletion on sensory and motor processes in healthy volunteers. Br J Pharmacol. 2009;157(1):92–103. Epub 2009/05/06. doi: 10.1111/j.1476-5381.2009.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimatsu H, Chiba S, Tajima D, Akehi Y, Saketa T. Histidine suppresses food intake through its conversion into neuronal histamine. Exp Biol Med. 2002;227(1):63–8. [DOI] [PubMed] [Google Scholar]
- 14.Sasahara I, Fujimura N, Nozawa Y, Furuhata Y, Sato H. The effect of histidine on mental fatigue and cognitive performance in subjects with high fatigue and sleep disruption scores. Physiol Behav. 2015;147:238–44. Epub 2015/04/30. doi: 10.1016/j.physbeh.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 15.McDonald K, Trick L, Boyle J. Sedation and antihistamines: an update. Review of inter-drug differences using proportional impairment ratios. Hum Psychopharmacol. 2008;23(7):555–70. Epub 2008/07/12. doi: 10.1002/hup.962. [DOI] [PubMed] [Google Scholar]
- 16.Niu YC, Feng RN, Hou Y, Li K, Kang Z, Wang J, et al. Histidine and arginine are associated with inflammation and oxidative stress in obese women. Br J Nutr. 2012;108(1):57–61. Epub 2011/10/15. doi: 10.1017/S0007114511005289. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe M, Suliman ME, Qureshi AR, Garcia-Lopez E, Barany P, Heimburger O, et al. Consequences of low plasma histidine in chronic kidney disease patients: associations with inflammation, oxidative stress, and mortality. Am J Clin Nutr. 2008;87:1860–6. [DOI] [PubMed] [Google Scholar]
- 18.Feng RN, Niu YC, Sun XW, Li Q, Zhao C, Wang C, et al. Histidine supplementation improves insulin resistance through suppressed inflammation in obese women with the metabolic syndrome: a randomised controlled trial. Diabetologia. 2013;56(5):985–94. Epub 2013/01/31. doi: 10.1007/s00125-013-2839-7. [DOI] [PubMed] [Google Scholar]
- 19.Teuscher C, Subramanian M, Noubade R, Gao JF, Offner H, Zachary JF, et al. Central histamine H3 receptor signaling negatively regulates susceptibility to autoimmune inflammatory disease of the CNS. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(24):10146–51. doi: 10.1073/pnas.0702291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musio S, Gallo B, Scabeni S, Lapilla M, Poliani PL, Matarese G, et al. A key regulatory role for histamine in experimental autoimmune encephalomyelitis: Disease exacerbation in histidine decarboxylase-deficient mice. The Journal of Immunology. 2006;176:17–26. [DOI] [PubMed] [Google Scholar]
- 21.Flachenecker P, Bihler I, Weber F, Gottschalk M, Toyka KV, Rieckmann P. Cytokine mRNA expression in patients with multiple sclerosis and fatigue. Multiple sclerosis. 2004;10:165–9. [DOI] [PubMed] [Google Scholar]
- 22.Sitton NG, Dixon JS, Astbury C, Francis RJ, Bird HA, Wright V. Kinetic investigations into the possible cause of low serum histidine in rheumatoid arthritis. Annals of the Rheumatic Diseases. 1988;47:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerber DA. Low free serum histidine concentration in rheumatoid arthritis: a measure of disease activity. The Journal of Clinical Investigation. 1975;55:1164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hugh Dunstan R, Sparkes DL, Macdonald MM, Roberts TK, Wratten C, Kumar MB, et al. Altered amino acid homeostasis and the development of fatigue by breast cancer radiotherapy patients: A pilot study. Clin Biochem. 2011;44(2–3):208–15. Epub 2010/10/19. doi: 10.1016/j.clinbiochem.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Mihalik SJ, Michaliszyn SF, Heras J, Bacha F, Lee S, Chace DH, et al. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes Diabetes Care. 2012;35:605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshizawa M, Tashiro M, Fukudo S, Yanai K, Utsumi A, Kano M, et al. Increased brain histamine H1 receptor binding in patients with anorexia nervosa. Biol Psychiatry. 2009;65(4):329–35. Epub 2008/09/26. doi: 10.1016/j.biopsych.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Kasaoka S, Kawahara Y, Inoue S, Tsuji M, Kato H, Tsuchiya T, et al. Gender effects in dietary histidine-induced anorexia. Nutrition. 2005;21(7–8):855–8. Epub 2005/06/25. doi: 10.1016/j.nut.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Gotoh K, Masaki T, Chiba S, Higuchi K, Kakuma T, Shimizu H, et al. Hypothalamic neuronal histamine signaling in the estrogen deficiency-induced obesity. J Neurochem. 2009;110(6):1796–805. Epub 2009/07/22. doi: 10.1111/j.1471-4159.2009.06272.x. [DOI] [PubMed] [Google Scholar]
- 29.McNair DM, Lorr M, Heuchert JWP, Droppleman LF. Profile of Mood States: Brief Form. North Tonawanda, NY: Multi-Health Systems (MHS); 2003. [Google Scholar]
- 30.Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225–34. [DOI] [PubMed] [Google Scholar]
- 31.Yeun EJ, Shin-Park KK. Verification of the profile of mood states-brief: cross-cultural analysis. J Clin Psychol. 2006;62(9):1173–80. Epub 2006/05/12. doi: 10.1002/jclp.20269. [DOI] [PubMed] [Google Scholar]
- 32.Loy BD, O’Connor PJ, Dishman RK. The effect of a single bout of exercise on energy and fatigue states: a systematic review and meta-analysis. Fatigue: Biomedicine, Health & Behavior. 2013:1–20. doi: 10.1080/21641846.2013.843266. [DOI] [Google Scholar]
- 33.Bakshi R, Shaikh ZA, Miletich RS, Czarnecki D, Dmochowski J, Henschel K, et al. Fatigue in multiple sclerosis and its relationship to depression and neurologic disability. Multiple sclerosis. 2000;6:181–5. [DOI] [PubMed] [Google Scholar]
- 34.Attarian HP, Brown KM, Duntley SP, Carter JD, Cross AH. The relationship of sleep disturbances and fatigue in multiple sclerosis. Archives of Neurology. 2004;61:525–8. [DOI] [PubMed] [Google Scholar]
- 35.Herring MP, Fleming KM, Hayes SP, Motl RW, Coote SB. Moderators of Exercise Effects on Depressive Symptoms in Multiple Sclerosis: A Meta-regression. Am J Prev Med. 2017;53(4):508–18. Epub 2017/06/13. doi: 10.1016/j.amepre.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Steer RA, Garbin MG. Psychometric properties of the beck depression inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- 37.Moran PJ, Mohr DC. The Validity of Beck Depression Inventory and Hamilton Rating Scale for Depression Items in the Assessment of Depression Among Patients with Multiple Sclerosis. Journal of Behavioral Medicine. 2005;28(1):35–41. doi: 10.1007/s10865-005-2561-0. [DOI] [PubMed] [Google Scholar]
- 38.Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. Journal of Clinical Sleep Medicine.4(6):563–71. [PMC free article] [PubMed] [Google Scholar]
- 39.Buysse DJ, Reynolds III CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 40.Carpenter JS, Andrykowski MA. Psychometric evaluation of the pittsburgh sleep quality index. Journal of psychosomatic research. 1998;45(1):5–13. [DOI] [PubMed] [Google Scholar]
- 41.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian Journal of Applied Sport Sciences. 1985;10(3):141–6. [PubMed] [Google Scholar]
- 42.Snook EM, Motl RW, Gliottoni RC. The effect of walking mobility on the measurement of physical activity using accelerometry in multiple sclerosis. Clinical rehabilitation. 2009;23:248–53. [DOI] [PubMed] [Google Scholar]
- 43.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–52. [DOI] [PubMed] [Google Scholar]
- 44.Bowen J, Gibbons L, Gianas A, Kraft GH. Self-administered expanded disability status scale with functional system scores correlates well with a physician-administered test. Multiple sclerosis. 2001;7:201–6. [DOI] [PubMed] [Google Scholar]
- 45.Fonteh AN, Harrington RJ, Harrington MG. Quantification of free amino acids and dipeptides using isotope dilution liquid chromatography and electrospray ionization tandem mass spectrometry. Amino Acids. 2007;32(2):203–12. Epub 2006/10/13. doi: 10.1007/s00726-006-0370-6. [DOI] [PubMed] [Google Scholar]
- 46.Phenomenex Phenomenex EZ:faast [easy fast] amino acid sample testing kit (2003) user guide 4111 Madrid Avenue, Torrance, CA: 90501–1430, USA. Available from: http://www.phenomenex.com. [Google Scholar]
- 47.Iacobucci D, Posavac SS, Kardes FR, Schneider MJ, Popovich DL. Toward a more nuanced understanding of the statistical properties of a median split. Journal of Consumer Psychology. 2015;25(4):652–65. doi: 10.1016/j.jcps.2014.12.002. [DOI] [Google Scholar]
- 48.Hedges LV, Olkin I. Statistical methods for meta-analysis. New York: Academic Press; 1985. [Google Scholar]
- 49.Cohen J A power primer. Psychological bulletin. 1992;112(1):155–9. [DOI] [PubMed] [Google Scholar]
- 50.Weaver B, Wuensch KL. SPSS and SAS programs for comparing Pearson correlations and OLS regression coefficients. Behav Res Methods. 2013;45(3):880–95. Epub 2013/01/25. doi: 10.3758/s13428-012-0289-7. [DOI] [PubMed] [Google Scholar]
- 51.Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. Journal of Clinical Psychiatry. 2006;67:554–66. [DOI] [PubMed] [Google Scholar]
- 52.Horner WE, Johnson DE, Schmidt AW, Rollema H. Methylphenidate and atomoxetine increase histamine release in rat prefrontal cortex. European Journal of Pharmacology. 2007;558:96–7. doi: 10.1016/j.ejphar.2006.11.04810.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 53.Bhargava P, Fitzgerald KC, Calabresi PA, Mowry EM. Metabolic alterations in multiple sclerosis and the impact of vitamin d supplementation. JCI Insight. 2017;2(19):e95302. doi: 10.1172/jci.insight.95302DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heesen C, Nawrath L, Reich C, Bauer N, Schulz KH, Gold SM. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry. 2006;77(1):34–9. Epub 2005/12/20. doi: 10.1136/jnnp.2005.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mokhtarzade M, Ranjbar R, Majdinasab N, Patel D, Molanouri Shamsi M. Effect of aerobic interval training on serum IL-10, TNFalpha, and adipokines levels in women with multiple sclerosis: possible relations with fatigue and quality of life. Endocrine. 2017;57(2):262–71. Epub 2017/06/16. doi: 10.1007/s12020-017-1337-y. [DOI] [PubMed] [Google Scholar]
- 56.Morris G, Berk M, Walder K, Maes M. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med. 2015;13:28 Epub 2015/04/10. doi: 10.1186/s12916-014-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Regenold WT, Phatak P, Makley MJ, Stone RD, Kling MA. Cerebrospinal fluid evidence of increased extra-mitochondrial glucose metabolism implicates mitochondrial dysfunction in multiple sclerosis disease progression. J Neurol Sci. 2008;275(1–2):106–12. Epub 2008/09/12. doi: 10.1016/j.jns.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amorini AM, Nociti V, Petzold A, Gasperini C, Quartuccio E, Lazzarino G, et al. Serum lactate as a novel potential biomarker in multiple sclerosis. Biochim Biophys Acta. 2014;1842(7):1137–43. Epub 2014/04/15. doi: 10.1016/j.bbadis.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 59.Lazzarino G, Amorini AM, Petzold A, Gasperini C, Ruggieri S, Quartuccio ME, et al. Serum Compounds of Energy Metabolism Impairment Are Related to Disability, Disease Course and Neuroimaging in Multiple Sclerosis. Mol Neurobiol. 2017;54(9):7520–33. Epub 2016/11/09. doi: 10.1007/s12035-016-0257-9. [DOI] [PubMed] [Google Scholar]