Abstract
Introduction.
The opioid epidemic in the United States is a public health crisis. Breast surgeons are obligated to provide good pain control for their patients after surgery but also must minimize administration of narcotics to prevent a surgical episode of care from becoming a patient’s gateway into opioid dependence.
Methods.
A survey to ascertain pain management practice patterns after breast surgery was performed. A review of currently available literature that was specific to breast surgery was performed to create recommendations regarding pain management strategies.
Results.
A total of 609 surgeons completed the survey and demonstrated significant variations in pain management practices, specifically within regards to utilization of regional anesthesia (e.g., nerve blocks), and quantity of prescribed narcotics. There is excellent data to guide the use of local and regional anesthesia. There are, however, fewer studies to guide narcotic recommendations; thus, these recommendations were guided by prevailing practice patterns.
Conclusions.
Pain management practices after breast surgery have significant variation and represent an opportunity to improve patient safety and quality of care. Multimodality approaches in conjunction with standardized quantities of narcotics are recommended.
INTRODUCTION
Deaths related to opioid overdose continue to be a public health crisis in the United States. Nearly a quarter of the 63,600 deaths from drug overdoses in 2016 were attributed to prescription opioids.1 Large numbers of the American populations indicate that their initial encounter with opioids was in the surgical setting.1 This is potentially a gateway to addiction and abuse.2 Nearly 300,000 patients are diagnosed with breast cancer in the United States every year. Subsequently, interventions targeted toward optimizing pain management in this population could have significant impact on decreasing the use and abuse of opioids.
METHODS
The American Society of Breast Surgeons (ASBrS) is the primary leadership organization for surgeons who treat patients with breast disease in the United States. The society currently has 3000 members and was founded in 1995. The Patient Safety and Quality Committee (PSQC) along with the Research Committee (RC) created a combined workgroup to evaluate opioids and pain management in breast surgery patients. Representatives from both committees were engaged and involved in this project. The workgroup initially performed a review of the data with brief summaries of the modalities utilized for breast surgery pain control. Subsequently, a survey of the ASBrS membership was performed to evaluate current practices and allow for comparison. Analysis of the survey data along with the review of the literature on pain management in breast surgery are the focus of this summary.
The Survey
The membership of ASBrS was surveyed to identify the most common practices currently in place with regards to pain management (supplemental Fig. 1). The survey collected data on the demographics of surgeons completing the survey (Table 1), as well as current approaches to the utilization of local anesthesia, nonsteroidal anti-inflammatories (NSAIDs), regional anesthesia (e.g., paravertebral/regional blocks), and narcotics in the perioperative setting. Wherever appropriate, delineation by breast procedure type was included. The survey was administered utilizing SurveyMonkey and had a 20% response rate from the ASBrS membership, with 609 complete responses.
TABLE 1.
Information regarding survey respondents
| Factor | N (%) |
|---|---|
| Practice type | |
| Hospital-employed | 228 (38) |
| Academic | 127 (21) |
| Multi-specialty group | 107 (18) |
| Single specialty group | 59 (10) |
| Solo private practice | 58 (10) |
| Other | 30 (3) |
| Years in practice | |
| ≤ 10 | 177 (30) |
| 11–20 | 172 (28) |
| 21–30 | 171 (28) |
| > 30 | 81 (13) |
| NA or skipped | 8 (1) |
| Practice location | |
| Large urban city (> 1,000,000) | 195 (32) |
| Urban city (< 1,000,000) | 196 (32) |
| Small city (50,000–100,000) | 156 (26) |
| Other and skipped | 62 (10) |
| Number of breast cancers treated per year | |
| < 100 | 220 (36) |
| 101–150 | 118 (19) |
| 151–300 | 223 (37) |
| > 300 | 36 (6) |
| Other | 12 (2) |
RESULTS
Local Anesthetic Infiltration
Survey Results Local anesthesia was the most common pain control approach utilized in the perioperative setting, with 98% (n = 534) of surgeons giving local anesthesia for excisional biopsies and lumpectomies. Additionally, more than half the surgeons gave local anesthetic for mastectomies either with (49%, n = 264) or without reconstruction (55%, n = 298), and 76% (n = 410) of surgeons gave local anesthesia when axillary lymph node dissection was performed.
Literature Review
Local anesthetic use in breast surgery is well known to reduce perioperative pain. The use of traditional local anesthetic is widely accepted and is performed with relative ease in the operating room. Multiple studies have evaluated the use of wound infiltration, and two systematic reviews demonstrated that local anesthetic consistently reduces pain in the immediate postoperative period (2 h after surgery) but generally does not provide longer term pain control.3,4 Injection of local anesthesia does not compromise the diagnostic accuracy of radiotracer utilized for sentinel node biopsy.5
Liposomal bupivacaine is increasingly used in breast surgery, and anecdotally is reported to be a very effective medication. However, there is a lack of randomized, prospective studies comparing this modality to traditional local anesthetics. Many of the published studies contain small numbers of patients and show that liposomal bupivacaine is equivalent, and maybe better, than traditional local anesthetics 4-8 Most of these studies report on lower patient reported pain scores and decreased opioid use.6 A Cochrane review of 1377 patients undergoing all surgery types concluded that “Liposomal bupivacaine at the surgical site does appear to reduce postoperative pain compared to placebo; however, at present the limited evidence does not demonstrate superiority to bupivacaine hydrochloride.” One study of plastic surgery patients (including implant-based and autologous breast reconstruction) showed decreased cost of the entire care episode in patients who were given liposomal bupivacaine compared with those who were not.7
The use of long-acting liposomal local anesthesia has not demonstrated any benefit over traditional local in any breast study to-date; in fact, of all the RCTs looking at liposomal bupivacaine, only one has demonstrated statistically significant benefit and was in patients undergoing hemorrhoidectomy.8
REGIONAL ANESTHESIA
Survey Results
For patients undergoing mastectomy without reconstruction, there was a marked practice variation in the administration of regional anesthesia (i.e., blocks); 43% (n = 263) of surgeons reported no blocks being given versus the next most common practice (26%, n = 158) of surgeons giving blocks for 75–100% of these patients. Surgeons in academic practices (65%, n = 82) were more likely to give blocks versus those in nonacademic practices (53%, n = 255; p = 0.02).
For patients undergoing mastectomy with reconstruction, a similar practice variation was seen with 33% (n = 203) of surgeons never offering regional blocks and 33% (n = 201) offering blocks to 75–100% of all these patients. The majority (75%, n = 461) of the surgeons indicated that blocks were performed by anesthesia. Liposomal bupivacaine is utilized for blocks by 27% (n = 166) of surgeons who responded to this survey; and it is most commonly utilized for patients undergoing mastectomy with and without reconstruction. Liposomal bupivacaine is currently only approved for infiltration as an interscalene brachial plexus nerve block. Safety and efficacy have not been established for its use in other nerve blocks.
Literature Review
There are many regional analgesic procedures available for breast surgery, depending on the extent of the surgery and the experience of the anesthesia provider. There exist a great variety of techniques that can be segregated by the goal of the block: control of breast pain versus management of myofascial pain associated with reconstruction.8 Despite theoretical concerns, the use of regional anesthesia does not impact the accuracy of sentinel node biopsy utilizing radiotracer.9
Blocks may be as simple as intercostal nerve blocks by direct injection at the mid-axillary line and just lateral to the sternum; interfacial plane blocks (pectoralis I and II blocks, serratus blocks; can be done using ultrasound guidance or direct visualization), or regional blocks, such as thoracic epidurals or paravertebral blocks.8,10
The greatest amount of research is available for paravertebral blocks. Randomized, controlled trials have demonstrated efficacy, however, use is limited by concern for complications, such as pneumothorax, symptomatic bradycardia, hypotension, and vasovagal episodes. These potential issues are especially pertinent in an outpatient environment, even though data reveal the overall rate of these complications to be low.8,10,11
The interfacial plane blocks may provide the best pain control at the lowest complication rate, but a strong recommendation is limited by the small number of studies.8,10
NONSTEROIDAL ANTI-INFLAMMATORIES
Survey Results
Nonsteroidal anti-inflammatories (NSAIDs) were frequently used across all operations. Fewer surgeons (75%) recommended NSAIDs to patients after mastectomy with reconstruction compared with lumpectomy or excisional biopsy (96%). Nearly half of surgeons prescribed a particular number of NSAIDs, although there was wide, scattered variation (1–15 pills) across all procedure types. Additionally, for the 56% (n = 344) of surgeons who gave preemptive anesthesia (medications for pain control given in the immediate preoperative setting), acetaminophen (76%, n = 269) and NSAIDs (57%, n = 196) were the most commonly given preemptive medications.
Literature Review
Data on perioperative use of NSAIDs in the setting of breast surgery is relatively limited. The NSAIDs that have been most studied in association with breast surgery are ketorolac and ibuprofen. Several studies suggest that perioperative use of ketorolac improves short-term pain outcomes after breast surgery12-14 There also is some limited data regarding improved oncologic outcomes with the use of perioperative ketorolac.15 A meta-analysis of six studies of plastic surgery patients demonstrated no difference in hematoma formation between those treated with versus without ketorolac in the subgroup of patients that underwent aesthetic breast surgery (11/257 [4.3%] vs. 6/277 [2.2%]; p = 0.59).16 Likewise, several more recent retrospective studies of breast plastic surgeries (one study also included body contouring surgery) have not demonstrated an increase in hematoma incidence associated with the use of ketorolac.14,17,18 It is noteworthy that most of these studies were retrospective, and administration of ketorolac was based on clinical judgment. Clinicians are therefore advised to use clinical judgement and avoid use of ketorolac in a patient at high risk of hemorrhage or with other contraindications to its use. Additionally, because many of the studies are from the plastic surgery literature, little information is available on the safety of ketorolac after axillary procedures. Ibuprofen has been less well-studied in the setting of breast surgery, but limited evidence suggests that it is associated with reduced narcotic use postoperatively and that ibuprofen-containing regimens are less likely to be discontinued due to adverse effects than are codeine-containing regimens.19,20
Surgeons may consider perioperative NSAID at the time of breast surgery for patients at low risk for postoperative hemorrhage, and without other contraindications to NSAID use. One reasonable regimen for inpatient procedures is ketorolac 30 mg IV intraoperatively, followed by ketorolac 15 mg or 30 mg IV q 8 h postoperatively, with an ibuprofen-containing regimen at discharge. For outpatient procedures, a reasonable regimen is ketorolac 30 mg IV intraoperatively, with an ibuprofen-containing regimen at discharge.
NARCOTICS
Survey Results
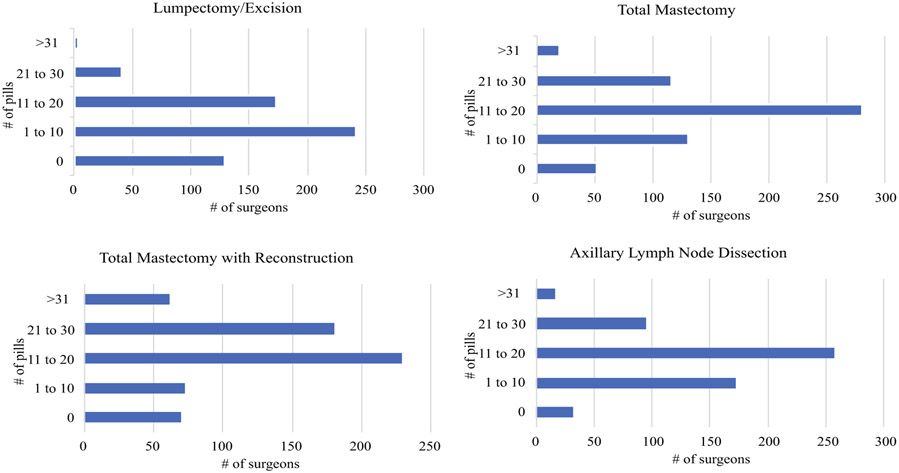
Surgeons do report recent changes in their pain management strategies. Most notably, 76% (n = 457) report decreasing opioid use. Only 14% (n = 86) utilize a standard enhanced recovery after surgery (ERAS) pathway. There was remarkably wide variation in the number of pills prescribed across the various procedure types (Fig. 1). A proportion of surgeons even report not giving any narcotics, irrespective of procedures, and some surgeons report prescribing > 31 pills even for lumpectomies or excisional biopsies (Table 2).
FIG. 1.
Number of surgeons prescribing specific quantities for different operations
TABLE 2.
Recommended narcotic-prescribing guidelines
| Procedure | Number of narcotic tablets for initial prescription |
|---|---|
| Biopsy or lumpectomy, no surgery for axilla | 10 or fewer |
| Sentinel node biopsy only | 10 or fewer |
| Lumpectomy, sentinel node biopsy | 10 or fewer |
| Oncoplastic re-arrangement | 10 or fewer |
| Axillary Dissection | 20 or fewer |
| Mastectomy ± surgery for axilla | 20 or fewer |
| Mastectomy with reconstruction | 30 or fewer |
Literature Review
Although there has been a recent consideration toward removing all narcotics for patients undergoing breast surgery, including with the use of ERAS pathways, it is unclear whether this is the optimal approach given the current small sample sizes.21,22 In October 2014, the Drug Enforcement Agency (DEA), in an effort to decrease the rate of prescription drug abuse and addiction, reclassified hydrocodone combination products (HCPs) from Schedule III to Schedule II. This change was meant to limit easy access to HCPs and prevents routine refills of HCPs. Studies have revealed changes in prescribing patterns.23 As would be expected, higher dosages of opioids are associated with a higher risk of overdose and death. Narcotics have differing morphine milligram equivalents (MME); however, studies consistently demonstrate that taking more than 50 MME per day significantly increased the risk of overdose and death.24 Subsequently, surgeons can modulate the number and dose of pills to minimize daily MME. A recent consensus panel evaluated and made recommendations regarding opioid administration across several surgical procedures, including two breast operations: 0-10 pills after partial mastectomy, and 0-15 pills after partial mastectomy with sentinel node biopsy.1
CONCLUSIONS
Nearly all surgeons reported utilizing some type of multimodality pain management for breast patients. As expected, there has been an increased use of blocks and NSAIDs in an effort to decrease narcotic administration. There is tremendous variation in the amount of narcotics prescribed, which presents an opportunity for quality improvement.
Supplementary Material
RECOMMENDATIONS.
Strongly consider an ERAS protocol standard.
Given the significant amount of data supporting use of regional anesthesia, as well as the evident practice pattern differences with regards to these blocks, there is a need for more widespread use of blocks for pain management in breast patients. This education could be disseminated through ASBrS avenues as surgeons are in an optimal position to administer this regional anesthesia.
Specific recommendations for the acceptable number of pills given for individual breast operations are made in Table 1. This is for opioid naïve patients without chronic pain issues and incorporates efforts to minimize MME.
ACKNOWLEDGEMENT
The authors thank Sharon Grutman for her fantastic efforts on this project and the executive board of the American Society of Breast Surgeons for supporting this work group. Dr. Fayanju is supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Number 1KL2TR002554 (PI: Svetkey) and the Duke Cancer Institute through NIH Grant P30CA014236 (PI: Kastan).
Footnotes
DISCLOSURES Dr. Steven Chen is a part time employee for Avelas Biosciences—a company that develops imaging techniques to be used to identify margins during breast surgery. Dr. Lee Wilke is founder and minority stock owner for Elucent Medical-a tumor localization device company.
Electronic supplementary material The online version of this article (https://doi.org/10.1245/s10434-020-08197-z) contains supplementary material, which is available to authorized users.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Overton HN, Hanna MN, Bruhn WE, et al. Opioid-prescribing guidelines for common surgical procedures: an expert panel consensus. J Am Coll Surg. 2018;227:411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoicea N, Costa A, Periel L, Uribe A, Weaver T, Bergese SD. Current perspectives on the opioid crisis in the US healthcare system: a comprehensive literature review. Medicine (Baltimore). 2019;98:e15425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tam KW, Chen SY, Huang TW, et al. Effect of wound infiltration with ropivacaine or bupivacaine analgesia in breast cancer surgery: a meta-analysis of randomized controlled trials. Int J Surg. 2015;22:79–85. [DOI] [PubMed] [Google Scholar]
- 4.Byager N, Hansen MS, Mathiesen O, Dahl JB. The analgesic effect of wound infiltration with local anaesthetics after breast surgery: a qualitative systematic review. Acta Anaesthesiol Scand. 2014;58:402–410. [DOI] [PubMed] [Google Scholar]
- 5.Stearns V, Blackford A, Kessler J, et al. Diagnostic accuracy of sentinel node identification is maintained with the addition of local lidocaine and subareolar radioactive colloid injection. Breast Cancer Res Treat. 2015;150:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vyas KS, Rajendran S, Morrison SD, et al. Systematic review of liposomal bupivacaine (exparel) for postoperative analgesia. Plast Reconstr Surg. 2016;138:748e–56e. [DOI] [PubMed] [Google Scholar]
- 7.Little A, Brower K, Keller D, Ramshaw B, Janis JE. A cost-minimization analysis evaluating the use of liposomal bupivacaine in reconstructive plastic surgery procedures. Plast Reconstr Surg. 2019;143:1269–74. [DOI] [PubMed] [Google Scholar]
- 8.Woodworth GE, Ivie RMJ, Nelson SM, Walker CM, Maniker RB. Perioperative breast analgesia: a qualitative review of anatomy and regional techniques. Reg Anesth Pain Med. 2017;42:609–31. [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, Kim S, Kim CS, et al. Efficacy of pectoral nerve block type II for breast-conserving surgery and sentinel lymph node biopsy: a prospective randomized controlled study. Pain Res Manag. 2018;2018:4315931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng GS, Ilfeld BM. An evidence-based review of the efficacy of perioperative analgesic techniques for breast cancer-related surgery. Pain Med. 2017;18:1344–65. [DOI] [PubMed] [Google Scholar]
- 11.Kelly ME, Mc Nicholas D, Killen J, Coyne J, Sweeney KJ, McDonnell J. Thoracic paravertebral blockade in breast surgery: Is pneumothorax an appreciable concern? A review of over 1000 cases. Breast J. 2018;24:23–7. [DOI] [PubMed] [Google Scholar]
- 12.Chen JY, Feng IJ, Loh EW, Wang LK, Lin CC, Tam KW. Analgesic effects of locally administered ketorolac-based analgesics after breast surgery: a meta-analysis of randomized controlled Trials. Clin J Pain. 2018;34:577–84. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S, Chang DW, Koutz C, et al. Incidence of hematoma associated with ketorolac after TRAM flap breast reconstruction. Plast Reconstr Surg. 2001;107:352–5. [DOI] [PubMed] [Google Scholar]
- 14.Kelley BP, Chung KC, Chung TT, et al. Postoperative ketorolac in breast and body contouring procedures: a nationwide claims analysis. Plast Reconstr Surg. 2018;142:472e–80e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desmedt C, Demicheli R, Fornili M, et al. Potential benefit of intra-operative administration of ketorolac on breast cancer recurrence according to the patient’s body mass index. J Natl Cancer Inst. 2018;110:1115–22. [DOI] [PubMed] [Google Scholar]
- 16.Stephens DM, Richards BG, Schleicher WF, Zins JE, Langstein HN. Is ketorolac safe to use in plastic surgery? A critical review. Aesthet Surg J. 2015;35:462–66. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen BN, Barta RJ, Stewart CE, Heinrich CA. Toradol following breast surgery: is there an increased risk of hematoma? Plast Reconstr Surg. 2018;141:814e–7e. [DOI] [PubMed] [Google Scholar]
- 18.Mikhaylov Y, Weinstein B, Schrank TP, et al. Ketorolac and hematoma incidence in postmastectomy implant-based breast reconstruction. Ann Plast Surg. 2018;80:472–4. [DOI] [PubMed] [Google Scholar]
- 19.Oh E, Ahn HJ, Sim WS, Lee JY. Synergistic effect of intravenous ibuprofen and hydromorphone for postoperative pain: prospective randomized controlled trial. Pain Physician. 2016;19:341–8. [PubMed] [Google Scholar]
- 20.Mitchell A, McCrea P, Inglis K, Porter G. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol. 2012;19:3792–800. [DOI] [PubMed] [Google Scholar]
- 21.Rojas KE, Manasseh DM, Flom PL, et al. A pilot study of a breast surgery Enhanced Recovery After Surgery (ERAS) protocol to eliminate narcotic prescription at discharge. Breast Cancer Res Treat. 2018;171:621–6. [DOI] [PubMed] [Google Scholar]
- 22.Kumar K, Kirksey MA, Duong S, Wu CL. A review of opioid-sparing modalities in perioperative pain management: methods to decrease opioid use postoperatively. Anesth Analg.2017;125:1749–60. [DOI] [PubMed] [Google Scholar]
- 23.Schultz S, Chamberlain C, Vulcan M, Rana H, Patel B, Alexander JC. Analgesic utilization before and after rescheduling of hydrocodone in a large academic level 1 trauma center. J Opioid Manag. 2016;12:119–22. [DOI] [PubMed] [Google Scholar]
- 24.Dowell D, Haegerich TM. Using the CDC guideline and tools for opioid prescribing in patients with chronic pain. Am Fam Physician. 2016;93:970–2. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.