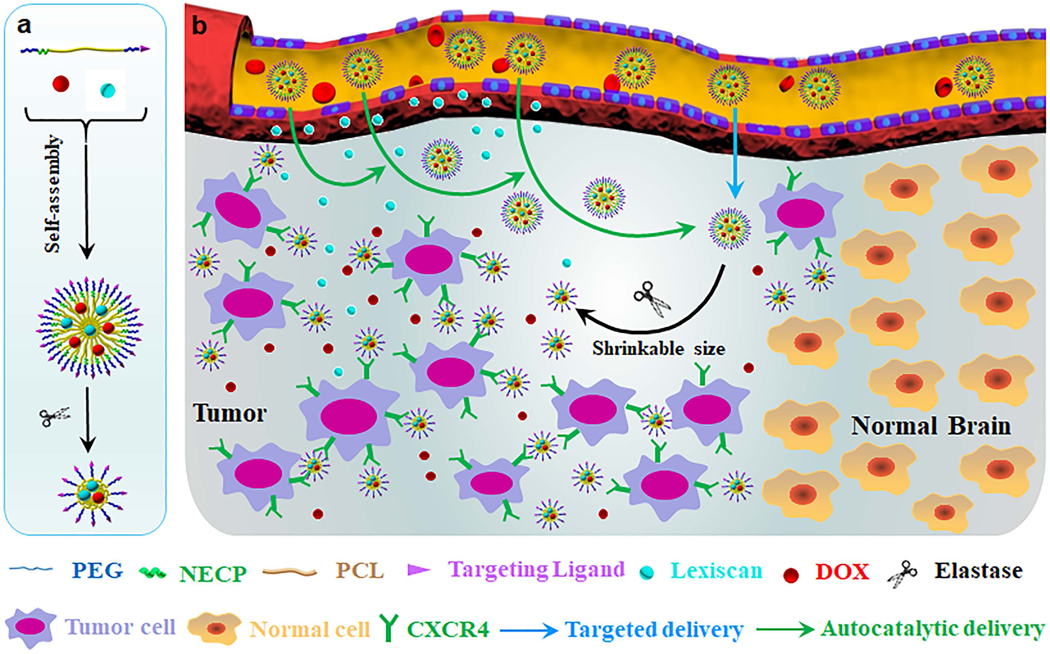
Abstract
Breast cancer brain metastases (BCBMs) represent a major cause of morbidity and mortality among patients with breast cancer. Chemotherapy, which is widely used to treat tumors outside of the brain, is often ineffective on BCBMs due to its inability to efficiently cross the blood-brain barrier (BBB). Although the BBB is partially disrupted in tumor lesions, it remains intact enough to prevent most therapeutics from entering the brain. Here, we report a nanotechnology approach that can overcome the BBB through synthesis of lexiscan-loaded, AMD3100-conjugated, shrinkable NPs, or LANPs. LANPs respond to neutrophil elastase-enriched tumor microenvironment by shrinking in size and disrupt the BBB in tumors through lexiscan-mediated modulation. LANPs recognize tumor cells through the interaction between AMD3100 and CXCR4, which are expressed in metastatic tumor cells. We demonstrate that the integration of tumor responsiveness, tumor targeting, and BBB penetration enables LANPs to penetrate metastatic lesions in the brain with high efficiency, and, when doxorubicin was encapsulated, LANPs effectively inhibited tumor growth and prolonged the survival of tumor-bearing mice. Due to their high efficiency in penetrating the BBB for BCBMs treatment, LANPs have the potential to be translated into clinical applications for improved treatment of patients with BCBMs.
Keywords: brain metastasis, nanoparticles, blood brain barrier, neutrophil elastase, CXCR4, autocatalytic delivery, BCBMs, neutrophil elastase-responsive
Graphical Abstract

1. Introduction
Over 30% of patients with breast cancer develop breast cancer brain metastases (BCBMs) [1]. Compared to other sites of metastatic spread, BCBMs are associated with the poorest prognosis [2]. With current state-of-the-art regimen, which mainly includes surgical resection and radiation therapy, the median survival for patients with BCBMs is limited to 4–6 months [3]. Chemotherapy, a major component of cancer therapy which contributes significantly in management of many cancers, is usually ineffective for BCBMs and thus excluded in the standard of care [4]. This lack of effective chemotherapy is largely due to its inability to deliver a pharmacologically significant amount of therapeutics to tumor lesions in the brain [5]. Therefore, improving drug delivery to BCBMs is in great demand.
To improve drug delivery to tumors, a common approach has been engineering nanocarriers through conjugation of ligands that recognize “receptor” molecules expressed on cancer cells, such as hyaluronic acid (HA) [6], TAT [7], iRGD [8], polysorbate 80 [1b], and chlorotoxin [9], which have been tested for BCBMs. Despite their potential, this approach has shown to be limited by various factors, including the lack of molecules that is specifically expressed on the surface of all cancer cells but not normal tissues [10]. As a result, the efficiency of ligand-mediated targeted delivery has been limited to 0.7% without significant improvement [11]. To overcome this limitation, a new approach has been proposed by designing nanomaterials to “intelligently” responsive to the tumor microenvironment and trigger a change in their physical properties [12]. This approach has shown potential in recent studies. For example, nanoparticles (NPs) have been designed to respond to matrix metalloproteinase-2 (MMP-2) or acidic pH by releasing small size NPs to significantly enhance drug delivery to tumors [13]. Drug delivery to BCBMs is further complicated by the blood brain barrier (BBB). The BBB in BCBMs is partially disrupted [14]. However, the degree of disruption does not allow for a clinically meaningful response, as the median drug levels in all lesions is one log lower than those achieved in systemic metastases [15]. As a result, most therapeutic agents after intravenous administration cannot penetrate the brain to elicit a therapeutic response [5]. Thus, effective chemotherapy for BCBMs that can overcome the BBB are urgently needed.
In this study, we developed novel NPs to improve drug delivery to BCBMs. First, by studying BCBM mouse xenografts derived from MDA-MB-231-Br-HER2 (231BR) cells, a model that has been well-characterized to recapitulate pathology of human BCBMs [14], we found the tumor microenvironment in BCBMs is highly enriched neutrophil elastase (NE). Accordingly, we designed and synthesized block copolymers consisting of polyethylene (PEG), poly(ε-caprolactone) (PCL), and RLQLKL, a NE-cleavable peptide (NECP). The resulting NPs can be activated by NE and can either shrink or expand in size. We found that both the size shrinkable and size expandable NPs delivered drugs to BCBMs in efficiency significantly greater than non-responsive NPs. Among the three NPs, the size shrinkable NPs demonstrated the greatest efficiency and thus were selected for further customization for tumor targeting through surface conjugation of AMD3100, a ligand binding with CXCR4 overexpressed in tumor cells [16]. We found that conjugation of AMD3100 further enhanced the delivery efficiency. The resulting AMD3100- conjugated, shrinkable NPs were then engineered for enhanced brain penetration through encapsulation of lexiscan, an adenosine receptor agonist that can pharmacologically modulate permeability of the BBB [17]. Consistent with our recent findings [9, 18], we found that this approach autocatalytically enhanced drug delivery to the brain. Lastly, we characterized the optimized NP formulation, lexiscan-loaded, AMD3100-conjugated, shrinkable NPs, or LANPs, for BCBMs treatment through encapsulation of doxorubicin (DOX). We found that DOX-loaded LANPs effectively enhanced the survival of mice bearing BCBMs. Due to their tremendous efficiency, LANPs represent a novel approach to delivering therapeutic agents to the brain for effective treatment of BCBMs.
2. Results
To address the challenges for drug delivery to BCBMs, we developed a nanotechnology approach that integrates intelligent responsiveness, tumor targeting, and BBB penetration by synthesizing LANPs (Figure 1a). Specifically, to achieve intelligent responsiveness, LANPs were designed to be activatable by NE, a protease aberrantly enriched in BCBMs tumor microenvironment, such that they can shrink in size. To enable tumor targeting, LANPs were synthesized with surface conjugation of AMD3100, which interacts with CXCR4 that is highly expressed in tumor cells. To overcome the BBB, LANPs were encapsulated with lexiscan, a BBB modulator. After reaching the tumor microenvironment, LANPs locally release lexiscan, which in turn transiently enhance BBB permeability to allow additional NPs to enter the same region. Consequently, the delivery process establishes a positive feedback loop, which autocatalytically increases the delivery efficiency with time and subsequent administrations (Figure 1b). We assessed LANPs for BCBMs treatment by synthesizing DOX-loaded LANPs and evaluated them in mouse BCBMs xenografts established through intracardiac injection of 231Br cells.
Figure 1.
Schematic diagrams of LANPs synthesis (a) and application (b) for drug delivery to BCBMs.
2.1. Design and synthesis of NE-responsive polymers
Tumor-responsive nanomaterials are often designed to be activated by proteases that are preferentially enriched in the tumor microenvironment, such as MMPs [12, 19]. A recent study showed that NE could be a promising alternative [20]. Compared to PLGLAG, a MMP-2-cleavable peptide that was commonly used to mediate tumor-responsiveness in previous studies [19a], a NE-cleavable peptide, RLQLKL, allows for greater efficiency and specificity [20]. NE was known to be aberrantly overexpressed in breast cancer [21]. We found that the expression of NE in metastatic tumors in the brain was significantly greater than that in the normal brain (Figure S1, Supporting Information).
Based on this finding, we designed and synthesized two block NE-responsive copolymers consisting of hydrophilic PEG, hydrophobic PCL, and NE-cleavable peptide RLQLKL, PEG-NECP-PCL and PEG-PCL-NECP-PEG. Control non-responsive polymer, PEG-PCL, was synthesized through ring-opening polymerization without the peptide (Figure 2a), and the molecular weights of PEG-PCL was 10.6 kDa. PEG-NECP-PCL was synthesized using a two-step reaction. In the first step, PEGylated peptide, PEG-NECP-NH2, was obtained by reacting maleimide-terminated PEG with cysteine terminated peptide, RLQLKL-C (lysine in the peptide was acetylated). The PEGylated peptide was then conjugated to carboxy-terminated PCL that was pre-activated by N,N′-carbonyldiimidazole (CDI) (Figure 2b). PEG-PCL-NECP-PEG was obtained by reacting CDI-activated PEG-PCL with PEG-NECP-NH2 (Figure 2c). Analysis by 1H nuclear magnetic resonance (NMR) found that PEG-NECP-PCL and PEG-PCL-NECP-PEG had molecular weights of 11.9 kDa and 15.5 kDa, respectively (Figure S2, Supporting Information).
Figure 2.
Scheme of chemical synthesis of PEG-PCL (a), PEG-NECP-PCL (b), and PEG-PCL-NECP-PEG (c). Lysine in RLQLKL was acetylated during synthesis. NECP was synthesized with cycstine at the C terminal.
2.2. Synthesize and characterization of NE-responsive NPs
NE-responsive micellular NPs were synthesized using PEG-NECP-PCL and PEG-PCL-NECP-PEG through the standard nanoprecipitation process [16a]. Control non-responsive NPs were synthesized using PEG-PCL. Analysis by dynamic light scattering (DLS) found that sizes of the micellular NPs were narrowly dirstributed with polydispersity index (PDI) values ranging from 0.10 to 0.13 (Figure S3a, Supporting Information). To characterize their responsiveness, the NPs were incubated in PBS with 100 nM NE and monitored for change of size and morphology. We found that control PEG-PCL NPs were stable in the solution (Figure 3a, Figure S3b, Supporting Information). In contrast, sizes of both PEG-NECP-PCL NPs and PEG-PCL-NECP-PEG NPs started to change within 10 minutes after incubation with NE (Figure S3c,d, Supporting Information). After 24-hours incubation, the size PEG-NECP-PCL NPs expanded from 103 nm to 1,141 nm (Figure 3b), and the size of PEG-PCL-NECP-PEG NPs shrank from 212.1 nm to 72.7 nm (Figure 3c). These size changes were induced by NE, as both NPs were stable in PBS without NE (Figure 3b,c). The changes of NP size were further confirmed by transmission electron microscopy (TEM) (Figure 3d). To simplify the nomenclature, we designated PEG-NECP-PCL NPs and PEG-PCL-NECP-PEG NPs as expandable NPs and shrinkable NPs, respectively.
Figure 3.
Synthesis and characterization of NE-responsive NPs to BCBMs. a-c) DLS analysis of the changes of hydrodynamic diameter of NPsCtr (a), NPsExp (b), and NPsShr (c) in PBS with or without NE (100 nM). d) TEM analysis of the changes of morphology of the indicated NPs in PBS with NE. Scale bar: 500 nm. e-f) Representative images (e) and semi-quantification (f) of NPs in BCBMs-bearing mice received the indicated treatment.
We characterized both expandable NPs, or NPsExp, and shrinkable NPs, or NPsShr, for drug delivery to the brain. Non-responsive PEG-PCL NPs, or NPsCtr, were included as a control. NPs were synthesized with encapsulation of IR780, a near-infrared fluorescence dye that allows for non-invasive imaging in live animals, and intravenously administered to mice bearing 231BR BCBMs that were engineered to express both GFP and luciferase. Twenty-four hours later, the mice were imaged using IVIS. Afterwards, the mice were euthanized, and their brains were isolated and imaged ex vivo. We found that the mice in all the experimental groups had comparable tumor loads, and both NE-responsive NPs accumulated in the brain in efficiency significantly greater that nonresponsive NPs (Figure 3e). Among them, shrinkable NPs demonstrated the greatest efficiency. Based on the fluorescence intensity, the accumulation of shrinkable NPs in the tumor region was 10.6- and 3.5- fold greater than that of control NPs and expandable NPs, respectively (Figure 3f). Due to their greater delivery efficiency, shrinkable NPs were used for the remainder of the study.
2.3. . Engineering NPs for targeted drug delivery to BCBMs
In seeking target molecules for preferential delivery to BCBMs, we focused on CXCR4, a chemokine receptor that is highly expressed in BCBMs and plays a critical role in metastasis [16c]. Analysis by Western Blot and immunostaining confirmed that CXCR4 was highly expressed in metastatic tumors but not in the region without tumors in the brain (Figure 4a,b). The preferential expression of CXCR4 in tumors provides a viable target for targeted delivery of NPs. Accordingly, we synthesized maleimide-terminated PEG-PCL-NECP-PEG, or Mal-PEG-PCL-NECP-PEG, which were obtained through the same synthesis procedures as that for PEG-PCL-NECP-PEG, except that MAL-PEG-OH was used to initiate the ring-opening polymerization of ε-caprolactone (Figure S4, Supporting Information). After the nanoprecipitation procedure, Mal-PEG-PCL-NECP-PEG NPs were incubated with AMD3100, which was pre-thiolated according to our recently published procedure [16a]. We characterized and compared the NPs with and without AMD3100 in mice that bear comparable tumor loads (Figure 4c, upper panel), and found that conjugation of AMD3100 significantly enhanced the accumulation of NPs in tumors by 11.9-fold (Figure 4c,d).
Figure 4.
Synthesize and characterization of AMD3100-conjugated NPs for targeted drug delivery to BCBMs. a) Western Blot analysis of the expression of CXCR4 in the brains with and without tumor. b) Representative images of the expression of CXCR4 in the region with (GFP) and without tumor. Scale bar: 20μm. c-d) Representative images (c) and semi-quantification (d) of NPs in BCBMs-bearing mice received the indicated treatment.
2.4. Enhancing drug delivery to the brain through autocatalysis
We recently demonstrated that encapsulation of lexiscan autocatalytically enhanced the delivery of NPs to the brain [9, 18]. To test if this approach works for the NPs designed in this study, we synthesized lexiscan-encapsulated, AMD3100- conjugated, shrinkable NPs, or LANPs. The resulting LANPs encapsulated lexiscan at 0.7% by weight, were stable in serum-containing medium, and bear a zeta potential of +2.7 mV (Figure S5, Supporting Information). We evaluated LANPs in BCBMs-bearing mice. To achieve autocatalysis, mice were primed with LANPs without IR780 daily for two consecutive days and then treated with IR780-loaded NPs at day 3. We found that, in line with the autocatalysis mechanism [9, 18], while delivery of LANPs without priming did not significantly increase the delivery efficiency, delivery with priming enhanced the efficiency by 2.1-fold (Figure 5a,b). Ex vivo imaging of major organs isolated from the primed mice showed that, based on quantification of IR780 fluorescence, the amount of LANPs accumulated in metastatic tumors in the brain was 2.5 times greater than that in the liver, the peripheral organ enriched the most NPs (Figure S6a,b, Supporting Information). The lexiscan-mediated autocatalytic effect was confirmed by Evans-blue leakage assay, which found that the priming significantly improved the penetration of Evans blue into intracranial tumors (Figure S6c,d, Supporting Information). We further characterized the accumulation of LANPs in the brain with and without tumors using confocal microscopy. LANPs were synthesized with encapsulation of rhodamine B (RhoB) and intravenously administered to pre-primed tumor-bearing mice. After 24 hours, the mice were euthanized. The brains were isolated, sliced and stained for DAPI and CXCR4. Confocal microscopy analysis identified a significant amount of NPs in tumors, where CXCR4 was highly expressed, but not in the regions without tumor (Figure 5c).
Figure 5.
Characterization of LANPs for targeted drug delivery to BCBMs. a-b) Representative images (a) and semi-quantification (b) of NPs in tumor-bearing mice received the indicated treatment. Priming was performed daily for two consecutive days with LANPs without IR780. c) Representative images of RhoB-loaded NPs (red) in the regions with and without tumor (GFP) or CXCR4 (magenta). Scale bar: 10μm.
2.5. LANPs for systemic delivery of chemotherapy for BCBMs treatment
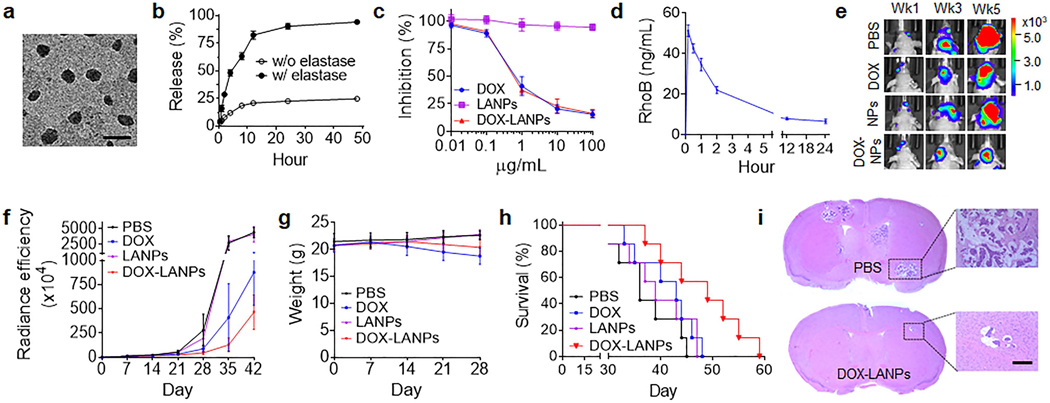
DOX is one of the most commonly used chemotherapy drugs for treatment of breast cancer. However, due to its limited ability to penetrate the BBB [22], DOX is not used for clinical management of BCBMs. We assessed LANPs for systemic delivery of DOX to the brain for BCBMs treatment. LANPs were synthesized with encapsulation of DOX. The resulting DOX-loaded LANPs, or DOX-LANPs, contained DOX at 6.2% by weight. Analysis by TEM showed that encapsulation of DOX did not alter the shape and size of LANPs (Figure 6a). In the presence of NE, LANPs released DOX by over 90% in 48 hours. In contrast, only 24.8% of DOX was released without the presence of NE (Figure 6b). We evaluated the cytotoxicity of DOX-LANPs on 231BR cells. Cells were treated with DOX-LANPs, free DOX, or blank LANPs. Three days later, the growth of cell proliferation was determined by the standard MTT assay. We found that free DOX and DOX-LANPs had comparable inhibitory effects on cell proliferation, while blank LANPs exhibited limited toxicity (Figure 6c). We determined the pharmacokinetics (PK) of LANPs in mice. RhoB-loaded LANPs were synthesized and intravenously administrated to into mice. The blood was collected at various time points. The concentration of RhoB in the plasma was determined and plotted versus time (Figure 6d). Analysis of the PK data found that the half-life of LANPs in the circulatory system was 20.1 hours.
Figure 6.
Characterization of DOX-LANPs for systemic treatment of BCBMs. (a) A representative SEM image of DOX-LANPs. Scale bar: 500 nm. (b) Controlled release of DOX in PBS with and without NE (100 nM). (c) Cytotoxicity of blank LANPs, free DOX and DOX-LANPs on 231BR cells. (d) Plasma concentrations of RhoB verse time in mice after intravenous administration of RhoB-loaded LANPs. (e-f) Representative images (e) and semi-quantification (f) of luciferase signals in tumors imaged by IVIS in mice received the indicated treatment. (g) Change in body weight with time in mice received the indicated treatments. (h) Kaplan-Meier survival curves of tumor-bearing mice received the indicated treatments (n=7). (i) H&E staining of the brains isolated from mice received the indicated treatments. Scare bar: 100 μm.
We assessed DOX-LANPs for BCBMs treatment. BCBMs-bearing mice were established and treated with DOX-LANPs at a dose equivalent to 3 mg DOX per kg. Control mice received treatment of PBS, or the same amount of free DOX or blank LANPs. Treatments were performed three days a week for four weeks. The mice were monitored for tumor growth based on luciferase imaging, weight, and survival. We found that tumors in mice treated with PBS or blank LANPs grew rapidly. In contrast, treatments with free DOX and DOX-LANPs inhibited tumor growth. Compared to free DOX, DOX-LANPs demonstrated a greater inhibitory effect (Figure 6e,f). Despite its inhibitory effect on tumor growth, treatment with DOX significantly reduced mouse weight (Figure 6g). Histological analysis revealed that this side effect was caused by significant myocardial injury, a well-known dose-limiting factor for in vivo used of DOX [23]; in contrast, DOX-LANPs did not induce detectable myocardial toxicity nor damage to normal organs (Figure S7, Supporting Information). Kaplan-Meier survival analysis found that treatment with DOX-LANPs significantly enhanced the survival of BCBMs-bearing mice (p < 0.05). The median survival in the group receiving treatment of DOX-LANPs was 49 days, compared to 36 days and 43 days for the group treated with PBS and free DOX, respectively (Figure 6h). Treatment with the same drug-loaded NPs without targeting molecule AMD31000, DOX-loaded lexiscan-containing shrinkable NPs or DOX-LNPsShr, prolonged the survival of tumor-bearing mice to 44 days, which are shorter than those by DOX-LANPs but significantly longer than those by PBS, suggesting that the size-shrinkable strategy designed in this study is effective for BCBM treatment (Figure S8, Supporting Information). A separate cohort of mice were euthanized at 35 days after treatment. The brains were harvested and subjected to histological analysis. H&E staining and Ki67 staining showed that, compared to the control PBS group, the group treated with DOX-LANPs had significantly less tumor lesions, which were also smaller in size (Figure 6i). TUNEL staining found that tumors in the group treated with DOX-LANPs but not in the control group were undergone significant apoptosis (Figure S9, Supporting Information).
LANPs as a delivery vehicle are potentially safe for intravenous applications. Throughout the animal studies, treatment with LANPs did not induce significant body weight loss (Figure 6g, Figure S8a). Analyses by H&E staining of major organs (Figure S7, Supporting Information), serum aspartate aminotransferase (AST) assay, and alanine aminotransferase (ALT) assay also did not identify obvious tissue damage and liver toxicity by systemic LANP treatment (Figure S10a,b, Supporting Information). LANPs were synthesized with surface conjugation of AMD3100, which is known to have biological activities, such as induction of breast cancer cell apoptosis [24]. We found that treatment of LANPs at 1 mg/ml or AMD3100 equivalent dose of 6.1 nmol/ml did not induce detectable cytotoxicity to 231BR cells (Figure S10c,d, Supporting Information), suggesting that the amount of AMD3100 conjugated as ligands on the surface of LANPs is insufficient to induce significant anti-tumor effects.
3. Discussion
Chemotherapy, which has been a major driving force to improve clinical management of most cancers, plays a limited role in BCBMs treatment, largely because of the lack of approaches to overcoming the BBB [4–5]. To address this challenge, targeted delivery approaches using various tumor targeting ligands, including HA [6], TAT [7], iRGD [8], polysorbate 80 [1b], and chlorotoxin [9], have been explored but have had limited success. Consistently, we found that the simple ligand-mediated delivery approach is insufficient for systemic delivery of NPs to the brain and further improved delivery of NPs to BCBMs could be achieved through integration of intelligent responsiveness and BBB penetration (Figure 3–5). In particular, we found that incorporation of intelligent responsiveness by shrinking NP size drastically improved the delivery (Figure 3e,f). This finding is in line with two previous reports [13], in which NPs responded to tumor microenvironment through disintegration and release of small size NPs and thus use the similar principles as the shrinkable NPs developed in this study. This discovery suggests that engineering NPs capable of shrinking in size in the tumor microenvironment may represent a new direction to improve drug delivery to tumors. We showed that encapsulation of lexiscan further improved delivery of NPs to tumors in the brain, substantiating the potential of application of autocatalysis to improve drug delivery to the brain [9, 18]. We demonstrated that the approach integrated intelligent responsiveness, tumor targeting, and BBB penetration allowed efficient drug delivery to BCBMs, and, as a result, the concentration of NPs was over 2.5 greater in tumors than in peripheral organs (Figure S6, Supporting Information). This degree of enhancement is significant, as a recent study showed that, after intravenous administration, the accumulation of free dugs, including DOX and paclitaxel, in tumor lesions in the brain was less than 15% of that in peripheral tissues [15a]. Finally, we tested the delivery approach for BCBMs treatment and found that systemic delivery of DOX via LANPs effectively inhibited tumor growth, prolonged the survival of tumor-bearing mice, and reduced systemic toxicity (Figure 6).
4. Conclusion
Improved treatment of BCBMs requires delivering therapeutic agents preferentially to tumors with high efficiency. We demonstrate that this could be achieved through autocatalytic delivery of tumor-targeting, size-shrinkable NPs via LANPs. By using DOX as a model drug, we showed that LANPs efficiently delivered DOX to the brain and significantly enhanced the efficacy of DOX for BCBMs treatment. LANPs consists of PEG, PCL, AMD3100 and LEX, all of which have been used in clinic for management of human patients with other diseases, and, thus, might be safe for human use. Due to their high efficiency in penetrating the BBB and their construction from safe materials with minimal toxicity, LANPs have the potential to be translated into clinical applications for improved treatment of patients with BCBMs.
5. Experimental Section
Materials:
NE-cleavable peptide (RLQLKLC) was synthesized by AnaSpec. AMD3100 tetrahydrochloride was purchased from Santa Cruz Biotechnology. Lexiscan was purchased from Toronto Research Chemicals. Methoxy poly (ethylene glycol) maleimide (mPEG-MAL, molecular weight (MW): 5000) and maleimide poly (ethylene glycol) (MAL-PEG-OH, MW: 5000) were purchased from Jenkem Technology. All other chemicals were purchased from Sigma-Aldrich.
Cell culture:
231BR breast cancer brain metastasis cells were kindly provided by Dr. Patricia Steeg at the NCI. Cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS, Invitrogen), 100 units/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) in a 37 °C incubator containing 5% CO2.
Polymer synthesis and characterization:
PEG-PCL was synthesized through ring-opening polymerization of ε-caprolactone initiated by mPEG-OH using Sn(Oct)2 as a catalyst. mPEG-OH (0.1 g, 0.02 mmol), ε- caprolactone (0.2 g, 1.75 mmol) and Sn(Oct)2 (0.1 wt % of ε-caprolactone) were added to a round-bottom flask, then degassed under vacuum and heated to 140 °C for 6 h. Afterwards, the mixture was cooled to room temperature and dissolved in DCM, precipitated in excess cold ethanol, and collected by centrifugation. The copolymers were dried overnight under vacuum.
For synthesis of PEG-NECP-PCL, mPEG-MAL (25 mg, 0.005 mmol) and peptide RLQLKLC (6.5mg, 0.005 mmol) were dissolved in PBS and reacted for 1 h. The unreacted peptide was removed by ultrafiltration (MWCO 3000). PEGylated peptide, NECP-PEG, was obtained by lyophilization. For synthesis of PCL-CDI, PCL (100 mg, 0.01 mmol) and N,N′-carbonyldiimidazole (CDI) (16 mg, 0.1 mmol) were dissolved in dry dichloromethane (DCM) and reacted at room temperature under nitrogen atmosphere for 6 h. The excess DCM was removed by rotary evaporator. The product was precipitated in excess cold ethanol to remove unreacted CDI. PCL-CDI was dried under vacuum to obtain the final product. For synthesis of PEG-NECP-PCL, PEG- NECP-NH2 (106 mg, 0.02 mmol) and PCL-CDI (40 mg, 0.004 mmol) were dissolved in dimethyl sulfoxide (DMSO) and stirred overnight. The product was precipitated in excess cold ethanol and collected by centrifugation. PEG-NECP-PCL was dried under vacuum.
For synthesis of PEG-PCL-NECP-PEG, PEG-PCL was activated by CDI. PEG-PCL (150 mg, 0.01 mmol) and CDI (16 mg, 0.1 mmol) were dissolved in dry DCM and reacted at room temperature under nitrogen atmosphere for 6 h. The excess DCM was removed by rotary evaporator. The product was precipitated in excess cold ethanol to remove unreacted CDI. PEG-PCL-CDI and then was dried under vacuum to obtain the final product. Next, PEG-NECP-NH2 was synthesized. mPEG-MAL (25 mg, 0.005 mmol) and NH2-NECP-SH (6.5 mg, 0.005 mmol) were dissolved in PBS and reacted for 1 h. The unreacted peptide was removed by ultrafiltration (MWCO 3000). PEG- NECP-NH2 was obtained by lyophilization. Last, PEG-PCL-NECP-PEG was synthesized. PEG-PCL-CDI (60 mg, 0.004 mmol) and PEG- NECP-NH2 (26 mg, 0.004 mmol) were dissolved in DMSO and stirred overnight. The product was precipitated in excess cold ethanol and collected by centrifugation. PEG-PCL-NECP-PEG was dried under vacuum.
Synthesis of NPs:
NPs were synthesized through nanoprecipitation according to our recent report [16a]. Briefly, for synthesis of PEG-PCL and PEG-NECP-PCL NPs, the polymers were dissolved in tetrahydrofuran (THF) and added dropwise into deionized water (THF/water = 1:3) under stirring. The NPs were formed with the evaporation of THF. For synthesis of PEG-PCL-NECP-PEG NPs, the polymer was dissolved in DMSO and added dropwise into deionized water (DMSO/water = 1:10) under stirring. Then, the solution was transferred to a dialysis bag and dialyzed against deionized water. The NPs were formed with the removal of DMSO. For a typical synthesis of LANPs, 5 mg MAL-PEG-PCL-NECP-PEG (0.3 μmol) was co-dissolved with 0.125 mg lexiscan (0.32 μmol) in 200 μL DMSO and added dropwise into 2 mL deionized water (DMSO/water = 1:10) under stirring. Then, the solution was transferred to a dialysis bag and dialyzed against deionized water. The NPs were formed with the removal of DMSO. After standard nanoprecipitation procedures, NPs were obtained with surface display of maleimide groups. AMD3100 was conjugated according to our previously reported method [16a].
Transmission electron microscope (TEM):
A drop of NP suspension was added onto a glow-discharged carbon-coated grid (Electron Microscopy Sciences). After 5 minutes, the samples were negatively stained by applying 10 μL of 1% phosphotungstic acid. After an additional 2 minutes, a filter paper was used to absorb the phosphotungstic acid solution. The grids were left at fume hood until completely dried and then visualized by using a JEOL 1230 transmission electron microscope (JEOL Ltd., Japan) at 100 kV.
Dynamic light scattering (DLS):
The hydrodynamic size and zeta potential of NPs were measured by DLS. Briefly, 0.1 mg NPs in 1 ml ddH2O water was added to a transparent cuvette, which was subjected to measurement using a Malvern Zetasizer.
Characterization of drug loading and release:
For characterization of drug loading, selected NPs were dissolved in DMSO to release released agents, which were quantified by a BioTek microplate reader for IR780 or DOX and HPLC for lexiscan. For characterization of drug release, DOX-LANPs were placed into a dialysis bags (MWCO 3000) against PBS in the presence or absence of 100 nM NE and immersed into 30 mL PBS in a prepared tube under a predetermined sink condition. The tubes were kept at room temperature with a shaking speed at 100 cycles/min. At selected time intervals from 0.5 to 48 h, 1 mL of solution outside the dialysis bag was taken out and replaced with the same volume of fresh medium. DOX in the sampled solution was quantified by a BioTek microplate reader. The cumulative DOX release was calculated and plotted against time.
Characterization of NE-responsiveness:
To characterize the response to NE, 1 mg/mL NPs were incubated with 100 nM NE at 37 °C. DLS and TEM were used to detect the time-dependent size distribution and morphological change with time.
Characterization of cytotoxicity:
231BR cells were plated in a 96-well cell culture plate at a concentration of 2×103 cells per well and incubated with concentrations of DOX-LANPs ranging from 0.01 to 100 ug/mL. The same amounts of free DOX were added to parallel wells as controls. After 72 hours treatment, the effect of treatments on cell proliferation was determined using the standard thiazolyl diphenyl tetrazolium salt (MTT) assay.
BCBMs mouse model and imaging:
Female athymic NCr-nu/nu mice (5–6 weeks old, Charles River Laboratories), ~20 g each, were given free access to food and water before all experiments. All animal experiments were approved by the Yale University Institutional Animal Care and Utilization Committee. Tumor bearing mice were established through intracardiac injection of 1.75×105 231BR cells, which were engineered for expression of luciferase and GFP, according to our previous report [9]. Imaging of tumors and IR780-loaded NPs were also carried out using IVIS (Xenogen) according to the same procedures described in our previous publication [9]. For imaging LANPs and co-colocalization with CXCR4, tumor-bearing mice were primed with blank LANPs daily for two consecutive days. At day 3, the mice were intravenously administered with RhoB-loaded LANPs. Twenty fours later, the mice were perfused with 1× PBS followed by 4% paraformaldehyde (PFA). The brains were incubated overnight in 4% PFA. Thick sections of 30 μm were obtained using a vibratome (Leica). After blocking with 4% BSA for 30 minutes and washed 3 times, the sections were incubated with CXCR4 (1:200, Novus NB100–74396) for overnight at 4 °C. The sections were incubated with Donkey anti-Rabbit IgG (ab150075, abcam) for 30 min after washed by PBS for 3 times. The samples were mounted and imaged using a confocal microscope (Leica TCS SP8) using lasers at 340 nm (nuclei), 488 nm (GFP), 553 nm (NPs) and 647 nm (CXCR4).
Characterization of elastase activity and expression of CXCR4:
Using the operating microscope (Leica A60), metastatic tumors in the brain were isolated from the tumor-bearing mice. Brain tissues isolated from normal mice were used as controls. Activity of NE in tumor and normal brain tissues were measured by using a SensoLyte® Green Elastase Assay Kit (#AS-72178, AnaSpec, CA). For characterization of CXCR4, Western Blot analysis was performed according to the standard procedures as previously described [25]. Antibodies used in this study included anti-CXCR4 (#NB100–74396, Novus), HRP anti-rabbit IgG (#656120, invitrogen), beta-actin (c643802, BioLegend), and HRP anti-mouse IgG (AP307P, Sigma-Aldrich).
Characterization of NPs in mice:
For characterization of PK, mice received intravenous administration of RhoB-loaded LANPs (n=3). Blood samples were collected at 10min, 30min, 1, 2, 6, 12 and 24 h after NPs injection. The plasma RhoB concentration was quantified based on RhoB fluorescence and plotted with time. For characterization of NPs for BCBMs treatment, tumor-bearing mice were divided into four groups based on tumor loads (n=7) that were determined by IVIS one week after inoculation, which received treatment of PBS, blank LANPs, free DOX (3 mg per kg), and DOX-LANPs (DOX equivalent dose at 3 mg per kg), respectively. Treatment started at day 8 after tumor inoculation and were performed 3 days a week for 4 weeks. The mice were monitored daily for body weight change and survival. Mice exhibiting neurological symptoms or significant weight loss were humanely euthanized. Tumors in the brain was imaged by IVIS weekly. For pathological analysis, a separate cohort of mice were euthanized five weeks after tumoor inoculation (n=3). The brains were harvested, fixed, sectioned and subjected to H&E, Ki67, and TUNEL staining.
Statistical analysis:
All data were collected in triplicate and reported as mean and standard deviation. Comparison between the groups were performed using a t-test. One-way ANOVA was used to analyze multiple comparisons by GraphPad Prism 7.0. P < 0.05 (*), 0.01 (**), 0.001 (***) and 0.0001 (****) were considered significant.
Supplementary Material
Acknowledgements
S. Z. and D.G. contributed equally to this work. This work was supported by NIH Grants NS095817, CA149128, and the State of Connecticut.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interests
The authors declare no conflict of interest.
On page xxx, S. Zhang and co-workers developed and validated a nanotechnology approach for targeted drug delivery to breast cancer brain metastases through synthesis of lexiscan-loaded, AMD3100-conjugated, shrinkable NPs (LANPs). LANPs have the potential to serve as a versatile platform for delivery of therapeutic agents to improve treatment of BCBMs.
Contributor Information
Shenqi Zhang, Department of Neurosurgery, Yale University, New Haven, CT 06511, USA.; Department of Neurosurgery, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, China.
Gang Deng, Department of Neurosurgery, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, China..
Fuyao Liu, Department of Neurosurgery, Yale University, New Haven, CT 06511, USA..
Bin Peng, Department of Neurosurgery, Yale University, New Haven, CT 06511, USA..
Youmei Bao, Department of Neurosurgery, Yale University, New Haven, CT 06511, USA..
Fengyi Du, Department of Neurosurgery, Yale University, New Haven, CT 06511, USA..
Ann T. Chen, Department of Biomedical Engineering, Yale University, New Haven, CT 06511, USA.
Jun Liu, Department of Neurosurgery, Yale University, New Haven, CT 06511, USA..
Zeming Chen, Department of Neurosurgery, Yale University, New Haven, CT 06511, USA..
Junning Ma, Department of Neurosurgery, Yale University, New Haven, CT 06511, USA..
Xiangjun Tang, Department of Neurosurgery, Yale University, New Haven, CT 06511, USA..
Qianxue Chen, Department of Neurosurgery, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, China..
Jiangbing Zhou, Department of Neurosurgery, Yale University, New Haven, CT 06511, USA.; Department of Biomedical Engineering, Yale University, New Haven, CT 06511, USA.
References
- [1].a) Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, Peters S, Arvold ND, Harsh GR, Steeg PS, Chang SD, Nat Rev Dis Primers 2019, 5, 5; [DOI] [PubMed] [Google Scholar]; b) Li J, Cai P, Shalviri A, Henderson JT, He C, Foltz WD, Prasad P, Brodersen PM, Chen Y, DaCosta R, Rauth AM, Wu XY, ACS nano 2014, 8, 9925. [DOI] [PubMed] [Google Scholar]
- [2].Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K, J Clin Oncol 2010, 28, 3271. [DOI] [PubMed] [Google Scholar]
- [3].Fidler IJ, Semin Cancer Biol 2011, 21, 107. [DOI] [PubMed] [Google Scholar]
- [4].Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, Sperduto PW, Vogelbaum MA, Radawski JD, Wang JZ, Gillin MT, Mohideen N, Hahn CA, Chang EL, Practical radiation oncology 2012, 2, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Connell JJ, Chatain G, Cornelissen B, Vallis KA, Hamilton A, Seymour L, Anthony DC, Sibson NR, Journal of the National Cancer Institute 2013, 105, 1634; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Pardridge WM, NeuroRx 2005, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mittapalli RK, Liu XL, Adkins CE, Nounou MI, Bohn KA, Terrell TB, Qhattal HS, Geldenhuys WJ, Palmieri D, Steeg PS, Smith QR, Lockman PR, Mol Cancer Ther 2013, 12, 2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Morshed RA, Muroski ME, Dai Q, Wegscheid ML, Auffinger B, Yu D, Han Y, Zhang L, Wu M, Cheng Y, Lesniak MS, Mol Pharm 2016, 13, 1843. [DOI] [PubMed] [Google Scholar]
- [8].Hamilton AM, Aidoudi-Ahmed S, Sharma S, Kotamraju VR, Foster PJ, Sugahara KN, Ruoslahti E, Rutt BK, J Mol Med (Berl) 2015, 93, 991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Han L, Kong DK, Zheng MQ, Murikinati S, Ma C, Yuan P, Li L, Tian D, Cai Q, Ye C, Holden D, Park JH, Gao X, Thomas JL, Grutzendler J, Carson RE, Huang Y, Piepmeier JM, Zhou J, ACS nano 2016, 10, 4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].a) Belfiore L, Saunders DN, Ranson M, Thurecht KJ, Storm G, Vine KL, Journal of controlled release : official journal of the Controlled Release Society 2018, 277, 1; [DOI] [PubMed] [Google Scholar]; b) Bae YH, Park K, Journal of controlled release 2011, 153, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW, Nature Reviews Materials 2016, 1. [Google Scholar]
- [12].a) Albanese A, Tang PS, Chan WC, Annu Rev Biomed Eng 2012, 14, 1; [DOI] [PubMed] [Google Scholar]; b) Lu Y, Aimetti AA, Langer R, Gu Z, Nature Reviews Materials 2016, 2, 1. [Google Scholar]
- [13].a) Wong C, Stylianopoulos T, Cui JA, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D, Proc Natl Acad Sci U S A 2011, 108, 2426; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li HJ, Du JZ, Du XJ, Xu CF, Sun CY, Wang HX, Cao ZT, Yang XZ, Zhu YH, Nie S, Wang J, Proc Natl Acad Sci U S A 2016, 113, 4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, Kurek R, Vega-Valle E, Feigenbaum L, Halverson D, Vortmeyer AO, Steinberg SM, Aldape K, Steeg PS, Cancer research 2007, 67, 4190; [DOI] [PubMed] [Google Scholar]; b) Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R, Journal of bone and mineral research 2001, 16, 1486. [DOI] [PubMed] [Google Scholar]
- [15].a) Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA, Huang S, Palmieri D, Steeg PS, Smith QR, Clinical cancer research 2010, 16, 5664; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Taskar KS, Rudraraju V, Mittapalli RK, Samala R, Thorsheim HR, Lockman J, Gril B, Hua E, Palmieri D, Polli JW, Castellino S, Rubin SD, Lockman PR, Steeg PS, Smith QR, Pharmaceutical research 2012, 29, 770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].a) Guo X, Deng G, Liu J, Zou P, Du F, Liu F, Chen AT, Hu R, Li M, Zhang S, Tang Z, Han L, Liu J, Sheth KN, Chen Q, Gou X, Zhou J, ACS nano 2018, 12, 8723; [DOI] [PubMed] [Google Scholar]; b) Wang Y, Xie Y, Oupicky D, Curr Pharmacol Rep 2016, 2, 1; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mukherjee D, Zhao J, Am J Cancer Res 2013, 3, 46. [PMC free article] [PubMed] [Google Scholar]
- [17].Carman AJ, Mills JH, Krenz A, Kim DG, Bynoe MS, The Journal of neuroscience 2011, 31, 13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].a) Chen Z, Liu F, Chen Y, Liu J, Wang X, Chen AT, Deng G, Zhang H, Liu J, Hong Z, Zhou J, Adv Funct Mater 2017, 27, 1703036; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Han L, Cai Q, Tian D, Kong DK, Gou X, Chen Z, Strittmatter SM, Wang Z, Sheth KN, Zhou J, Nanomedicine 2016, 12, 1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].a) Yao Q, Kou L, Tu Y, Zhu L, Trends Pharmacol Sci 2018, 39, 766; [DOI] [PubMed] [Google Scholar]; b) Yu X, Gou X, Wu P, Han L, Tian D, Du F, Chen Z, Liu F, Deng G, Chen AT, Ma C, Liu J, Hashmi SM, Guo X, Wang X, Zhao H, Liu X, Zhu X, Sheth KN, Chen Q, Fan L, Zhou J, Advanced Materials 2018, 30, 1705383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Whitney M, Crisp JL, Olson ES, Aguilera TA, Gross LA, Ellies LG, Tsien RY, J Biol Chem 2010, 285, 22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Akizuki M, Fukutomi T, Takasugi M, Takahashi S, Sato T, Harao M, Mizumoto T, Yamashita J, Neoplasia 2007, 9, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Arvanitis CD, Askoxylakis V, Guo Y, Datta M, Kloepper J, Ferraro GB, Bernabeu MO, Fukumura D, McDannold N, Jain RK, Proc Natl Acad Sci U S A 2018, 115, E8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sun C, Zhou L, Gou M, Shi S, Li T, Lang J, Oncol Rep 2016, 35, 3600. [DOI] [PubMed] [Google Scholar]
- [24].Huang MB, Giesler KE, Katzman BM, Prosser AR, Truax V, Liotta DC, Wilson LJ, Bond VC, Oncotarget 2018, 9, 16996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen Y, Gou X, Kong DK, Wang X, Wang J, Chen Z, Huang C, Zhou J, Oncotarget 2015, 6, 32575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.