Abstract
Live imaging is a valuable approach for investigating cell biology questions. The Drosophila larva is particularly suited for in vivo live imaging because the larval body wall and most internal organs are transparent. However, continuous live imaging of intact Drosophila larvae for longer than 30 min is challenging because it is difficult to noninvasively immobilize larvae for a long time. Here we present a larval mounting method called LarvaSPA that allows for continuous imaging of live Drosophila larvae with high temporal and spatial resolution for longer than 10 h. This method involves partially attaching larvae to a coverslip using a UV-reactive glue and additionally restraining larval movement using a polydimethylsiloxane (PDMS) block. This method is compatible with larvae at developmental stages from second instar to wandering third instar. We demonstrate applications of this method in studying the dynamic processes of Drosophila somatosensory neurons, including dendrite growth and injury-induced dendrite degeneration. This method can also be applied to study many other cellular processes that happen near the larval body wall.
Keywords: long-term time-lapse imaging, live imaging, in vivo imaging, LarvaSPA, confocal microscopy, Drosophila larva, body wall, dendritic arborization, da neurons, neurodegeneration, neurodevelopment, cell biology
SUMMARY:
This protocol describes a method for mounting Drosophila larvae to achieve longer than 10 h of uninterrupted time-lapse imaging in intact live animals. This method can be used to image many biological processes close to the larval body wall.
INTRODUCTION:
Time-lapse live imaging is a powerful method for studying dynamic cellular processes. The spatial and temporal information provided by time-lapse movies can reveal important details for answering cell biology questions. The Drosophila larva has been a popular in vivo model for investigations using live imaging because its transparent body wall allows for noninvasive imaging of internal structures1,2. In addition, numerous genetic tools are available in Drosophila to fluorescently label anatomical structures and macromolecules3. However, long-term time-lapse imaging of Drosophila larvae is challenging. Unlike stationary early embryos or pupae, Drosophila larvae move constantly, necessitating immobilization for live imaging. Effective ways of immobilizing live Drosophila larvae include mounting in halocarbon oil with chloroform4, anesthetizing using isoflurane or Dichlorvos solution5, and compressing between the coverslip and the microscope slide6. Although some of these methods have been used for microscopy, none of them is effective for long-term live imaging. Other methods were developed for imaging body wall neurons in crawling larvae using conventional confocal microscopy or light-sheet microscopy7-9. However, these methods are not ideal for monitoring cellular dynamics due to the movement of the larvae.
New methods have been developed to achieve long-term time-lapse imaging of Drosophila larvae. Using a polydimethylsiloxane (PDMS) “larva chip”, Drosophila larvae can be effectively immobilized through vacuum-generated suction in a specialized microchamber without anesthetization. However, this method does not offer high temporal resolution for cell biology studies and it has strict limitations on animal size10. Another method using an anesthetization device achieved live imaging of Drosophila larvae at multiple time points and has been applied to study neuromuscular junctions11-16. However, this method also does not allow for continuous imaging for longer than 30 min and requires using desflurane repeatedly, which can inhibit neural activity and affect the biological process studied17,18. Recently, a new method that combines microfluidic device and cryoanesthesia has been used to immobilize larvae of various sizes for short periods of time (minutes)19. However, this method requires specialized devices such as a cooling system and longer periods of immobilization require repeated cooling of the larvae.
Here we present a versatile method of immobilizing Drosophila larvae that is compatible with uninterrupted time-lapse imaging for longer than 10 h. This method, which we call “Larva Stabilization by Partial Attachment” (LarvaSPA), involves adhering the larval cuticle to a coverslip for imaging in a custom-built imaging chamber. This protocol describes how to make the imaging chamber and how to mount larvae at a variety of developmental stages. In the LarvaSPA method, the desired body segments are affixed to the coverslip using a UV-reactive glue. A PDMS cuboid additionally applies pressure to the larvae, preventing escape. The air and moisture in the imaging chamber ensure the survival of the partially immobilized larvae during imaging. Advantages of LarvaSPA over other techniques include the following: (1) It is the first method that allows for continuous live imaging of intact Drosophila larvae for hours with high temporal and spatial resolution; (2) The method has fewer limitations on larval size; (3) The imaging chamber and PDMS cuboids can be manufactured at a minimal cost and are reusable.
In addition to describing the larval mounting method, we provide several examples of its application for studying dendrite development and dendrite degeneration of Drosophila dendritic arborization (da) neurons.
PROTOCOL:
1. Making the imaging chamber
1.1 The metal frame can be constructed from an aluminum block in a typical machine shop. The specifications of the frame are illustrated in Figure 1A.
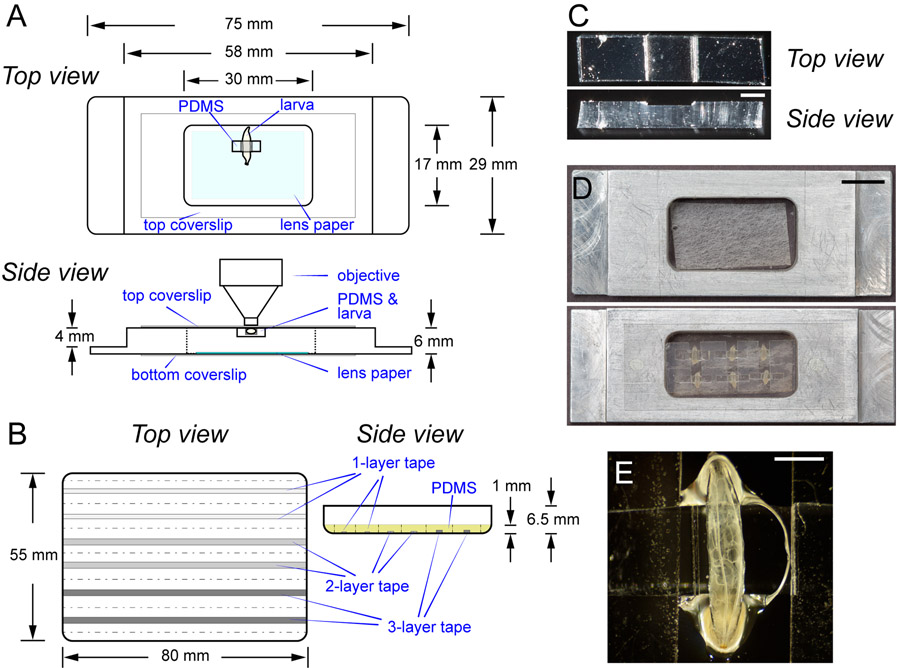
Figure 1: The imaging chamber for LarvaSPA mounting.
(A) Diagrams of the imaging chamber with detailed specifications, showing both the top view and the side view. The light blue shading in the top view indicates the moistened lens paper. The chamber is sealed by a top coverslip and a bottom coverslip. The position of a mounted larva is illustrated. (B) Diagrams of the PDMS mold (the top view) and the mold after filling with the PDMS mixture (side view). Strips in grey indicate two 1-layer, two 2-layer, and two 3-layer tape strips, respectively. The yellow shading in the side view indicates the PDMS mixture in the mold. The dotted lines indicate where to cut the cured PDMS. The side view of the diagram is not drawn to scale. (C) Photographs showing a top view and a side view of a PDMS cuboid. Scale bar = 1 mm. (D) Photographs showing an imaging chamber before mounting, and an imaging chamber with six immobilized late third instar Drosophila larvae mounted dorsal side up. Scale bar = 10 mm. (E) A closer view of a larva in (D). Scale bar = 1 mm.
1.2 To construct the imaging chamber, seal the bottom of the metal frame using a long coverslip (22 mm x 50 mm) and UV glue (Figure 1A). Cure the UV glue using a hand-held UV lamp.
2. Making PDMS cuboids
2.1 Prepare the mold for PDMS cuboids.
2.1.1 Attach layers of packaging tape to the inner surface of a rectangular (80 mm x 55 mm) Petri dish or round cell culture plate. Use one layer (0.063 mm thick) for second instar larvae, 2 layers (0.126 mm thick) for early third instar larvae, or three layers (0.189 mm thick) for late third instar larvae (Figure 1B).
2.1.2 Cut the tape into strips of specific width with a razor blade: 1.5 mm for the 1-layer tape, and 2 mm for 2-layer or 3-layer tape. The width and thickness of the strip will determine the size of the larvae that the final PDMS cuboid can hold. Leave at least a 5 mm space between the two strips. Remove the tape layers covering the space (Figure 1B).
2.1.3 Remove dust from the inner surface of the plate using sticky tape. The mold is ready for use.
2.2 Prepare the PDMS mix.
2.2.1 Mix 7 g of PDMS base and 0.7 g of curing agent (10:1 ratio) thoroughly in a small container.
2.2.2 Place the container in a vacuum desiccator for at least 15 min to remove air from the mixture.
2.2.3 Slowly pour about 5.5 g of PDMS mixture onto the mold to reach a 1–2 mm thickness (Figure 1B).
2.2.4 Place the PDMS mixture in the vacuum desiccator again for at least 15 min to remove remaining air bubbles from the mixture. Break the last few bubbles with a pipette tip.
2.2.5 Cure the PDMS on a flat surface in a heat incubator at 65 °C for 2 h.
2.2.6 Use a razor blade to loosen the cured PDMS along the edge of the mold and detach it from the mold. Store the PDMS between two pieces of large sticky tape at room temperature.
2.2.7 For early and late third instar larvae, cut the PDMS into 8 mm x 2 mm x 1 mm cuboids (along the dotted lines in Figure 1B) by positioning the groove created by the tape strip (step 2.1.2) at the center of the long side of the cuboid (Figure 1B,C). For second instar larvae, cut the cuboid to 8 mm x 1 mm x 1 mm.
3. Mounting larvae for long-term time-lapse imaging
3.1 Prepare the top coverslip for mounting.
3.1.1 Choose six PDMS cuboids with grooves matching the sizes of the larvae. Follow the recommended groove and size of PDMS based on steps 2.1.1, 2.1.2, and 2.2.7.
3.1.2 Remove dust from the surface of the PDMS with sticky tape.
3.1.3 Attach four pieces of double-sided tape (12 mm x 5 mm) on a long coverslip (22 mm x 50 mm) for fixing PDMS cuboids later. The spaces between the two pieces of double-sided tape should be the same as the width of the PDMS groove.
3.1.4 Apply a small drop (~1.2 μL) of UV glue into the groove of each PDMS cuboid and add six small drops of UV glue into the space between the double-sided tape on the coverslip.
3.2 Prepare the larvae for mounting.
3.2.1 Using a pair of forceps, clean the larvae in water to remove food from the body surface.
3.2.2 Place clean larvae on a small piece of moistened tissue paper in a small (35 mm) Petri dish without a lid. Place the small Petri dish into a large (60 mm) Petri dish containing a piece of dry tissue paper. In a chemical hood, apply 8–12 drops (160–240 μL) of isoflurane onto the dry tissue paper using a plastic transferring pipette and close the lid of the large Petri dish.
3.2.3 Wait 2–3 min while monitoring the larvae. Take out the larvae from the large Petri dish once their mouth hooks stop moving.
3.3 Mount the animals.
3.3.1 To image structures on the dorsal side of the animal, place the immobilized larvae onto the UV glue between the double-sided tape on the coverslip with the dorsal cuticle facing the coverslip.
3.3.2 Cover each larva with a PDMS block and fit the trunk of the larva into the groove of the PDMS. Leave the head and the tail of the larva outside the PDMS groove. Avoid blocking the spiracles of the larva by the glue.
3.3.3 Press down on the ends of the PDMS block onto the double-sided tape without applying force on the groove. Gently pull on the tail of the larva to flatten the cuticle under the PDMS.
3.3.4 Cure the UV glue for 4 min using a hand-held UV lamp at the high setting (at about 0.07 mW/mm2).
CAUTION: Protect eyes with safety glasses while using the UV lamp.
3.3.5 Flip the coverslip upside down and repeat step 3.3.4.
3.3.6 Moisten a small piece of lens paper (15 mm x 30 mm) with 20 μL–30 μL of water. Place the moistened lens paper at the bottom of the imaging chamber (Figure 1A,D).
3.3.7 Place the coverslip on the chamber so that the larvae are facing the inside of the chamber. Adhere both ends of the coverslip to the metal surface using UV glue (Figure 1A,D). The dorsal side of the larvae is ready for imaging under confocal microscope (Figure 1E).
4. Imaging
4.1 Image larvae with an appropriate microscope. All results shown in this protocol (Figure 2, Figure 3, Video S2, Video S3, and Video S4) were acquired using a confocal system with a 40x (1.30 NA) oil objective.
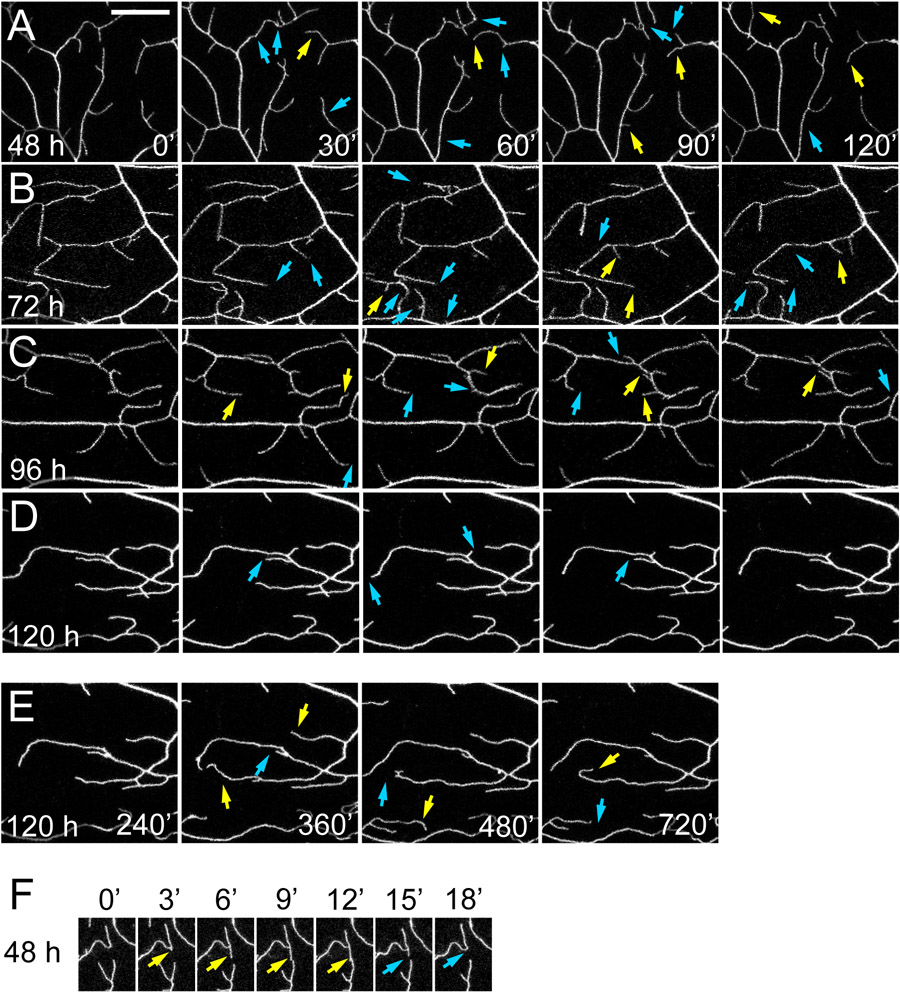
Figure 2: Time-lapse imaging of dendrite dynamics.
(A-F) Selected frames from time-lapse movies of class IV da neuron dendrites at specific time points after the start of imaging. Images of the larvae were taken 48 h AEL (A and F), 72 h AEL (B), 96 h AEL (C), and 120 h AEL (D). The neurons are labeled by ppk>CD4-tdTom (A, D, E, and F) or ppk >CD4-tdTom (B and C). Blue arrows indicate dendrite retractions compared to the previous time point. Yellow arrows indicate dendrite extensions compared to the previous time point. (E) Dendrite morphology at the end of 12 h of imaging. (F) Consecutive frames with 3 min intervals in a movie illustrating how a dendrite branch in a second instar larva extended (yellow arrows) and subsequently retracted (blue arrows) after it made contact with another dendrite.
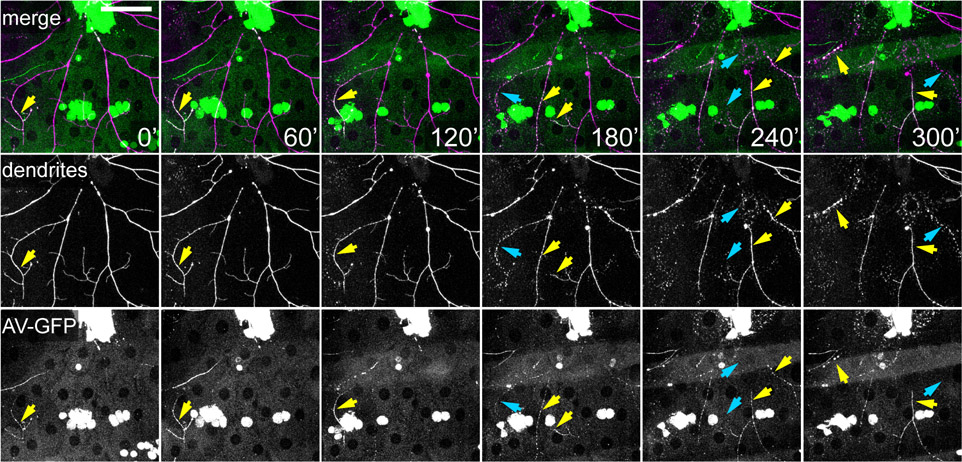
Figure 3: Time-lapse imaging of dendrite degeneration and exposure of an eat-me signal.
Selected frames from a time-lapse movie of degenerating dendrites of a class IV da neuron after laser injury. Dendrites were labeled by ppk>CD4-tdTom. The eat-me signal PS on degenerating dendrites was detected by Annexin-GFP (AV-GFP), which is secreted by the fat body. Yellow arrows point to the branches showing AV-GFP labeling. Blue arrows point to dendrites undergoing fragmentation.
5. Recovery of imaging chamber and PDMS cuboids
5.1 After imaging, remove the oil on the top coverslip using a lens paper. Detach the top coverslip from the metal frame by cutting into the space between the coverslip and the metal frame with a razor blade. The imaging chamber is ready for reuse.
5.2 Detach the PDMS cuboids from the top coverslip with forceps. Roll the PDMS cuboids on sticky tape to remove glue residue and dust. The PDMS cuboids are ready for reuse.
RESULTS:
The larva imaging chamber is constructed by gluing a custom-made metal frame and two coverslips together. The design of the metal frame is specified in Figure 1A. Drosophila larvae inside the chamber are adhered to the top coverslip with the aid of UV glue and PDMS cuboids. The groove on the PDMS cuboid and the double-sided tape the cuboid is attached to create the space to hold the larvae (Figure 1B,C). The PDMS also applies gentle pressure to flatten the larval body wall and physically restrict larval movement. Lastly, a small piece of wet lens paper is placed at the bottom of the chamber to provide moisture. This setup can immobilize Drosophila larvae for longer than 10 h for continuous imaging. Most of the animals are alive after 10 h and can be recovered to grow into the pupal stage. The imaging chamber can accommodate up to nine larvae at once. Figure 1D shows six late third instar larvae mounted in the chamber. The trunks of the larvae are fixed while their heads and tails are free to move (Figure 1E and Video S1). This method has been successfully used to image second instar to wandering third instar larvae with continuous high-resolution imaging for up to 15 h. The chamber is designed for imaging using upright microscopes, but the setup also works for inverted microscopes by simply flipping the chamber.
Here we demonstrate the application of LarvaSPA in studying neuronal dendrite dynamics and dendrite degeneration using class IV da (C4 da) neurons as a model (Figure 2, Figure 3, Videos S2-S4). C4 da neurons are somatosensory nociceptors located on the larval body wall, whose dendrites innervate the larval epidermis1,20-22. C4 da dendrites exhibit highly dynamic growth behaviors throughout the larval development, resulting in complete coverage of the body surface or space-filling23. C4 da neurons have also been successfully used to study dendrite degeneration and regeneration after physical injury24-27.
For imaging dendrite dynamics, larvae ranging from 48 h after egg laying (AEL) at second instar to 120 h AEL at wandering third instar were mounted in the imaging chamber for time-lapse imaging using point-scanning confocal microscopy. Time-lapse movies were taken with a 3 min interval to capture growth behaviors of C4 da dendrites labeled by ppk-CD4-tdTom or UAS-CD4-tdTom driven by ppk-Gal46. Throughout the imaging period (up to 12 h), the high order dendrite branches of C4 da neurons exhibited complex growth behaviors, including extension, retraction, branch formation, and branch elimination (Figure 2A-2F), indicating the health of the neurons. Our movies also captured homotypic dendro-dendrite repulsions in which dendrite tips retracted after contacting other dendrites (Figure 2F). Overall, these results demonstrate that time-lapse imaging using LarvaSPA is effective for studying neurodevelopment.
To image dendrite degeneration, we used a MaiTai laser to sever primary dendrites near C4 da neuronal cell bodies. The larvae were recovered and mounted in the chamber for imaging starting from 1.5 h after injury (AI) (Figure 3, Video S4). The movies recorded key events during dendrite degeneration, including dendrite swelling, dendrite fragmentation, and clearance of dendrite debris. In the same experiment, the larval fat body was also engineered to secrete Annexin V-GFP (AV-GFP), a sensor that labels externalized phosphatidylserine (PS) on the cell surface27. We observed specific labeling of the degenerating dendrites by AV-GFP (Figure 3), suggesting that PS was exposed on the surface of degenerating dendrites to serve as an eat-me signal for subsequent clearance by phagocytosis27.
DISCUSSION:
Here we describe LarvaSPA, a versatile method of mounting live Drosophila larvae for long-term time-lapse imaging. This method does not require recovering or remounting larvae, enabling uninterrupted imaging. It is therefore ideal for tracking biological processes that take hours to complete, such as dendrite degeneration and regeneration. This method can be also used for imaging intracellular calcium dynamics and subcellular events such as microtubule growth. As the larval body wall is stable during the imaging, the spatial and temporal resolution can be adjusted to fit the imaging application at hand (e.g., to track fast subcellular vesicle movement or to monitor slow global changes of neuronal branch patterns). In addition, this method is compatible with larvae at various developmental stages and does not require special equipment. Thus, LarvaSPA can potentially be used by many Drosophila labs to address diverse questions.
Factors affecting the success of the method, including the nature of the PDMS cuboid and the developmental stage of the larvae
The size of the PDMS cuboid and the depth of the groove need to match the size of the larvae. A PDMS cuboid too wide for the larvae can cover the head and the tail and cause hypoxia. However, a narrow PDMS cuboid may not be effective in preventing the larvae from moving. A too deep groove similarly would not generate enough pressure to restrain the movement of the larvae. A too shallow groove would not hold enough glue to adhere the larvae to the coverslip. Based on our experience, the following dimensions of the PDMS and the groove are recommended for various stages of larvae: 8 mm x 1 mm x 1 mm PDMS cuboids with 1.5 mm x 1 mm x 0.063 mm grooves for second instar larvae (~48 h AEL); 8 mm x 2 mm x 1 mm PDMS cuboids with 2 mm x 2 mm x 0.126 mm grooves for early third instar larvae (~72 h AEL); 8 mm x 2 mm x 1 mm PDMS cuboids with 2 mm x 2 mm x 0.189 mm grooves for late third instar larvae (~96 h AEL or older).
Wandering third instar larvae (at or older than 120 h AEL) move less compared to younger ones and require less moisture to survive once mounted in the chamber. They also have a thicker cuticle and can endure more physical stress. Therefore, experiments using wandering third instar larvae have the highest success rate. In our hands, more than 80% of wandering third instar larvae survived and remained immobile 12 h after mounting. To prevent larvae from pupariating during imaging, we recommend mounting the larvae between 96 h to 120 h AEL. Younger third instar larvae have a greater chance of escape or death during imaging. Typically, at least 2 out of 6 young third instar larvae mounted in the same chamber would survive and remain immobile 10 h after mounting. Our experience with second instar larvae is limited and the survival rate is hard to estimate. Nevertheless, we have successfully imaged second instar larvae for 7 h using this method.
Potential concerns of long-term imaging
Although LarvaSPA has been very useful for imaging dendrite growth dynamics and degeneration, there are a few potential caveats to consider. First, in our current method, the larvae are deprived of food for the whole duration of imaging, which may lead to starvation-induced responses in many tissues. We have observed formation of granular structures in epidermal cells around 10 h after mounting, which may result from autophagy. Therefore, the results obtained in the first few hours of imaging should be more physiologically relevant. Results over longer time scales require more cautious interpretation. To minimize this concern, our procedure could potentially be adapted to allow for larval feeding. Second, our method may interfere with the rapid growth of younger larvae due to the physical constraint and the lack of nutrient intake. However, this concern may not apply to older animals. For example, wandering third instar larvae can turn into pupae even after 12 h of continuous imaging, making this method ideal for investigations of early metamorphosis. Third, imaging of deeper structures such as the fat body and the gut presents some challenges. A main reason is that deeper tissues often move during imaging and therefore require motion correction in postprocessing. Additionally, mismatches of refractive indices in the light path can cause spherical aberration when imaging deeper tissues. While oil objectives are appropriate for imaging da neurons because their dendrites are within 15 μm from the body surface, water immersion objectives could be better choices to correct refractive-index mismatches for imaging deeper inside the larvae. Fourth, because C4 da neurons can be activated by UV light28, using UV light during the larval mounting can potentially alter C4 da neuron physiology. Although the light intensity we recommend is far below the levels that robustly activate C4da neurons28, results related to C4da neuronal activity need to be cautiously interpreted. The UV glue has been used for imaging a wide variety of biological samples29-31 and does not cause obvious toxicity to the larvae in our hands. Lastly, one should consider the proper microscope setup for in vivo live imaging. For example, resonance scanners and spinning disc confocal microscopes are better options for long-term live imaging because they cause less phototoxicity and photobleaching; multi-photon microscopy is more effective for imaging deeper internal structures in the larvae; long-term imaging could be prone to sample or focus drifting, which could potentially be corrected by post-imaging processing.
The most common causes of failure for LarvaSPA and the recommended solutions to address them
Larvae move too much or escape: Two common reasons could contribute to this problem. The first is that the PDMS groove may be too shallow or too deep for the larvae. Trying another PDMS cuboid with a different groove depth may solve the problem. The second reason is that there is too much moisture in the chamber, weakening the UV glue. Reducing the volume of water added to the lens paper in the chamber may help immobilize the larvae. In addition, try to image only the segments covered by the PDMS cuboid, because the head and the tail are free to move.
Larvae die during imaging: If a larva dies soon after imaging, it is likely that it was exposed to too much anesthetic. Properly anesthetized larvae should wake up after mounting and show motions including mouth hook extension and retraction and dorsal vessel contraction. To solve this problem, less isoflurane could be used to anesthetize larvae. Transfer a larva out of the anesthesia Petri dish immediately after the mouth hook stops moving. If the larva dies about one hour after imaging, it is usually because of dehydration. Remember to place a piece of moistened lens paper in the imaging chamber before sealing. Another common reason of lethality is that the UV glue blocks the spiracles. Try to limit UV glue to the middle segments of the larva and avoid covering the head and the tail. An overly wide PDMS cuboid can also easily cause the UV glue to block the spiracles.
Folds on the body wall interfere with imaging: To avoid generating folds during mounting, it is important to fully immobilize the larvae during the anesthesia step. When a larva is paralyzed, it is easier to straighten and stretch the body. For animals older than 96 h AEL, gently dragging the tails before curing the UV glue can effectively reduce fold formation. Dragging the tails of young larvae is not recommended because their body walls are fragile.
Supplementary Material
Video S1: A third instar larva is immobilized in the notch of a PDMS cuboid.
Video S2: Dendrites extend and retract dynamically in a second instar larva.
Video S3: Dendrites extend and retract dynamically in a wandering third instar larva.
Video S4: Dendrites degenerate and expose phosphatidylserine after laser injury in a third instar larva.
| Name of Material/Equipment | Company | Catalog Number | Comments/Description |
|---|---|---|---|
| 6061 Aluminum bars | McMaster-Carr | 9246K421 | |
| Glass coverslip | Azer Scientific | 1152250 | |
| 3M Scotch Packaging tape | 3M | 1.88"W x 22.2 Yards | |
| Rectangular petri dish | VWR | 25384-322 | |
| Razor blade | Ted Pella, Inc. | 121-20 | |
| SYLGARD® 184 kit (PBMS kit) | Electron Microscopy Sciences | 24236-10 | |
| Vacuum desiccator | Electron Microscopy Sciences | 71232 | |
| 3M double-sided tape | Ted Pella, Inc. | 16093 | |
| UV glue | Norland products | #6106, NOA 61 | Refractive Index 1.56 |
| DUMONT # 3 Forceps | Fisher Scientific | 50-241-34 | |
| Petri dishes (small) | VWR | 10799-192 | |
| Petri dishes (medium) | VWR | 25373-085 | |
| Wipes | Kimberly-Clark | Kimwipes | |
| Isoflurane | Midwest Veterinary Supply | 193.33161.3 | |
| Transferring pipette | Thermo Fisher Scientific | 1371126 | |
| Lens paper | Berkshire | LN90.0406.24 | |
| UV lamp (Workstar 2003) | Maxxeon | MXN02003 | |
| Leica Confocal Microscope | Leica | SP8 equipped with a resonant scanner |
ACKNOWLEDGMENTS:
We thank Lingfeng Tang for establishing an earlier version of the LarvaSPA method; Glenn Swan at Cornell Olin Hall Machine shop for making earlier prototypes of the imaging chamber; Philipp Isermann for constructing metal frames and providing suggestions on making PDMS cuboids; Cornell BRC Imaging facility for access to microscopes (funded by NIH grant S10OD018516); Maria Sapar for critical reading of the manuscript. This work was supported by a Cornell Fellowship awarded to H.J.; a Cornell start-up fund and NIH grants (R01NS099125 and R21OD023824) awarded to C.H. H.J. and C.H. conceived the project and designed the experiments. H.J. conducted the experiments. H.J and C.H. wrote the manuscript.
Footnotes
DISCLOSURES:
The authors declare no competing interests.
REFERENCE:
- 1.Grueber WB, Jan LY, Jan YN Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 129 (12), 2867–2878 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Schmid A et al. Activity-dependent site-specific changes of glutamate receptor composition in vivo. Nature Neuroscience. 11 (6), 659–666 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Venken KJ, Simpson JH, Bellen HJ Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron. 72 (2), 202–230 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniele JR, Baqri RM, Kunes S Analysis of axonal trafficking via a novel live-imaging technique reveals distinct hedgehog transport kinetics. Biology Open. 6 (5), 714–721 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Csordas G et al. In vivo immunostaining of hemocyte compartments in Drosophila for live imaging. PLoS One. 9 (6), e98191 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han C, Jan LY, Jan YN Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 108 (23), 9673–9678 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He L et al. Direction Selectivity in Drosophila Proprioceptors Requires the Mechanosensory Channel Tmc. Current Biology. 29 (6), 945–956 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heckscher ES, Lockery SR, Doe CQ Characterization of Drosophila larval crawling at the level of organism, segment, and somatic body wall musculature. The Journal of Neuroscience. 32 (36), 12460–12471 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaadia RD et al. Characterization of Proprioceptive System Dynamics in Behaving Drosophila Larvae Using High-Speed Volumetric Microscopy. Current Biology. 29 (6), 935–944 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra B et al. Using microfluidics chips for live imaging and study of injury responses in Drosophila larvae. Journal of Visualized Experiments. 84, e50998 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andlauer TF, Sigrist SJ In vivo imaging of the Drosophila larval neuromuscular junction. Cold Spring Harbor Protocols. 2012 (4), 481–489 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Andlauer TF, Sigrist SJ Building an imaging chamber for in vivo imaging of Drosophila larvae. Cold Spring Harbor Protocols. 2012 (4), 476–480 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Andlauer TF, Sigrist SJ Quantitative analysis of Drosophila larval neuromuscular junction morphology. Cold Spring Harbor Protocols. 2012 (4), 490–493 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Andlauer TF, Sigrist SJ In vivo imaging of Drosophila larval neuromuscular junctions to study synapse assembly. Cold Spring Harbor Protocols. 2012 (4), 407–413 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y et al. In vivo imaging of intact Drosophila larvae at sub-cellular resolution. Journal of Visualized Experiments. 43, e2249 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuger P, Behrends LB, Mertel S, Sigrist SJ, Rasse TM Live imaging of synapse development and measuring protein dynamics using two-color fluorescence recovery after photo-bleaching at Drosophila synapses. Nature Protocols. 2 (12), 3285–3298 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Sandstrom DJ Isoflurane depresses glutamate release by reducing neuronal excitability at the Drosophila neuromuscular junction. The Journal of Physiology. 558 (Pt 2), 489–502 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandstrom DJ Isoflurane reduces excitability of Drosophila larval motoneurons by activating a hyperpolarizing leak conductance. Anesthesiology. 108 (3), 434–446 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Chaudhury AR et al. On chip cryo-anesthesia of Drosophila larvae for high resolution in vivo imaging applications. Lab on a Chip. 17 (13), 2303–2322 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han C et al. Integrins regulate repulsion-mediated dendritic patterning of drosophila sensory neurons by restricting dendrites in a 2D space. Neuron. 73 (1), 64–78 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim ME, Shrestha BR, Blazeski R, Mason CA, Grueber WB Integrins establish dendrite-substrate relationships that promote dendritic self-avoidance and patterning in drosophila sensory neurons. Neuron. 73 (1), 79–91 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grueber WB et al. Projections of Drosophila multidendritic neurons in the central nervous system: links with peripheral dendrite morphology. Development. 134 (1), 55–64 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Poe AR et al. Dendritic space-filling requires a neuronal type-specific extracellular permissive signal in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 114 (38), E8062–E8071 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han C et al. Epidermal cells are the primary phagocytes in the fragmentation and clearance of degenerating dendrites in Drosophila. Neuron. 81 (3), 544–560 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song Y et al. Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the Akt pathway involving Pten and microRNA bantam. Genes, Development. 26 (14), 1612–1625 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone MC, Albertson RM, Chen L, Rolls MM Dendrite injury triggers DLK-independent regeneration. Cell Reports. 6 (2), 247–253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sapar ML et al. Phosphatidylserine Externalization Results from and Causes Neurite Degeneration in Drosophila. Cell Reports. 24 (9), 2273–2286 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang Y et al. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 468 (7326), 921–926 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desjardins M et al. Awake Mouse Imaging: From Two-Photon Microscopy to Blood Oxygen Level-Dependent Functional Magnetic Resonance Imaging. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 4 (6), 533–542 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C et al. Long-term optical brain imaging in live adult fruit flies. Nature Communications. 9 (1), 872 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Low RJ, Gu Y, Tank DW Cellular resolution optical access to brain regions in fissures: imaging medial prefrontal cortex and grid cells in entorhinal cortex. Proceedings of the National Academy of Sciences of the United States of America. 111 (52), 18739–18744 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1: A third instar larva is immobilized in the notch of a PDMS cuboid.
Video S2: Dendrites extend and retract dynamically in a second instar larva.
Video S3: Dendrites extend and retract dynamically in a wandering third instar larva.
Video S4: Dendrites degenerate and expose phosphatidylserine after laser injury in a third instar larva.