Abstract
Context/Objective: Since life expectancy of persons with spinal cord injury (SCI) has improved, it is relevant to know whether this group is able to maintain functional abilities many years after onset of SCI. Objectives of this study were (1) to examine associations between time since injury (TSI) and functional independence in persons with long-standing SCI and (2) to explore associations between functional independence and level of injury, comorbidities, mental health, waist circumference and secondary health conditions (SHCs).
Design: TSI-stratified cross-sectional study. Strata were 10–19, 20–29 and 30+ years.
Setting: Community.
Participants: 226 persons with long-standing SCI. Inclusion criteria: motor complete SCI; age at injury 18–35 years; TSI ≥ 10 years; current age 28–65 years; wheelchair dependency.
Interventions: Not applicable.
Outcome measures: The Spinal Cord Independence Measure III (SCIM) was administered by a trained research assistant. Level of injury, comorbidities, mental health, waist circumference and SHCs were assessed by a rehabilitation physician.
Results: Mean TSI was 23.6 (SD 9.1) years. No significant differences in SCIM scores were found between TSI strata. SCIM scores were lower for persons with tetraplegia, autonomic dysreflexia, hypotension, more than four SHCs and a high waist circumference. In linear regression analyses, TSI nor age was associated with the SCIM total score. Only level of injury (β = –0.7; P < .001) and waist circumference (β = –0.1; P = .042) were independent determinants (explained variance 55%).
Conclusion: We found no association between TSI and functional independence in persons with long-standing motor complete SCI. This study confirms the possible effect of overweight on functional independence.
Keywords: Spinal cord injuries, Activities of daily living, Complications, Long-term
Introduction
A primary goal of rehabilitation after spinal cord injury (SCI) is to attain an optimal level of functional independence. Since life expectancy of persons with SCI has considerably improved, it is relevant to know whether this group is able to maintain their functional abilities as they age.
Aging after SCI has been suggested to be determined by both growing age and time since injury (TSI).1 Although it is often assumed that functional independence in this patient group decreases with aging, the literature on this topic shows conflicting results. Several cross-sectional studies did not find clues for a decline in functioning with a longer TSI or growing age.2–6 Further, Whiteneck et al. found a decrease in mobility and physical independence with increasing age, but not with TSI.7 However, several studies found that part of their participants had experienced a decline of functioning in the years preceding the study.8–10 Amsters et al.11 reported participants experienced an increase in functioning in the first 10 years after SCI but a decrease in functioning after the first 10 years.
Little longitudinal data on long-term functioning with SCI has been published to date. In one longitudinal study, no clear trend in functional independence with increasing age or duration of injury was found, although the participants reported a decline in their functioning.2 Two other longitudinal studies reported a decrease in functioning with increasing age12 or TSI,12,13 although this only applied to higher functioning individuals in one of these studies.13 In another longitudinal study, Functional Independence Measure (FIM) scores slightly increased when measured at 1, 5, 10 and 20 years after injury, although this was not tested for significance.14
In contrast to these conflicting results on TSI and age, the level of SCI demonstrates a strong influence on long-term functioning.3,4,6 Further, secondary health conditions (SHCs) are common in persons aging with SCI,7,10,15 which may contribute to functional limitations. Little information has however been published on this subject.3,16
In most studies on long-term functioning, functional independence was measured using the FIM. The use of the more recently developed Spinal Cord Independence Measure III (SCIM III) is increasing. The SCIM III shows more responsiveness than the FIM to functional changes in sphincter management and mobility indoors and outdoors.17 The SCIM III has been recommended as the primary outcome measure to assess functional recovery in SCI.18 However, we found only one study in which this measure was used to describe long-term functioning.6 Another study used the self-report version of the SCIM III, but only reported dichotomized scores of single mobility items.16
Since information on SCIM scores in persons with long-standing SCI is sparse and it is relevant to know whether this group is able to maintain functional abilities many years after the onset of SCI, the present study was performed to gain insight into long-term functioning measured with the SCIM III of persons with a motor complete SCI in the Netherlands. The primary objective was to examine associations between TSI and functional independence. We hypothesized that persons with a longer TSI would function less independently. The secondary objective was to explore associations between functional independence and level of injury, comorbidities, mental health, waist circumference and SHCs.
Methods
Design
Data for this study was derived from the research program ‘Active LifestyLe Rehabilitation Interventions in aging Spinal Cord injury (ALLRISC)’, a TSI-stratified cross-sectional study performed in eight rehabilitation centers in the Netherlands. TSI strata were 10–19, 20–29 and 30 years or more after the onset of SCI.19
Study population
Inclusion criteria of ALLRISC were: traumatic or non-traumatic SCI, age at injury between 18 and 35 years, TSI at least 10 years, current age between 28 and 65 years and using a wheelchair (hand-rim propelled wheelchair or electric wheelchair), at least for longer distances (>500 m). Persons were excluded when their mastery of the Dutch language was insufficient.19,20 The age inclusion criteria were applied to limit the confounding effects of age-related comorbidities and thereby to be better able to study the long-term consequences of SCI.16,17
For the current study, participants with a motor complete SCI were selected and persons with incomplete data on SCIM III scores or lesion characteristics were excluded.
Procedure
Eligible persons were identified in databases from all eight Dutch rehabilitation centers specialized in SCI rehabilitation. Participants were asked to complete a self-report questionnaire and invited for a one-day visit to the rehabilitation center. This visit included an extensive medical assessment with a structured interview and a physical examination by an SCI rehabilitation physician, and an oral interview by a trained research assistant.
The study protocol was approved by the Medical Ethics Committee of the University Medical Center Utrecht. All subjects signed an informed consent form prior to participation.
Instruments
Functional independence
Functional independence was measured with the SCIM III, which was administered by the research assistant. The SCIM III consists of three subscales with a total score range of 0–100: ‘Self-care’ including six items (range 0–20), ‘Respiration and sphincter management’ including four items (range 0–40) and ‘Mobility’ including nine items (range 0–40). The items are weighted in terms of their assumed clinical relevance.17,18,21 The SCIM III showed good reliability and validity.17,22,23
Demographics
The self-report questionnaire included information on age, sex, marital status and level of education. For statistical analysis level of education was dichotomized in ‘high level of education’ (at least a college degree) and ‘low level of education’.
Injury characteristics
The patients were neurologically examined by the rehabilitation physician according to the International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI).24 American Spinal Injury Association (ASIA) Impairment Scale (AIS) A and B were considered motor complete. The lowest intact motor level was used to describe the level of injury. Levels of injury C1–C8 were defined as tetraplegia, levels below C8 as paraplegia.
Mental health
Mental health was measured with the Mental Health Inventory-5 (MHI-5), which was part of the self- report questionnaire, and consists of five questions to screen on depression and anxiety over the last four weeks.25 A sum-score was calculated and converted to a 0–100 scale. The MHI-5 scores were dichotomized to ‘mental health problems’ (<60) and ‘no mental health problems’ (≥60).26
Waist circumference
Waist circumference was measured by the rehabilitation physician in supine position.
Secondary health conditions
As part of the structured interview, the rehabilitation physician asked whether participants had suffered from hypotension, pneumonia, autonomic dysreflexia (AD), pressure ulcers, problematic spasticity, urinary tract infections (UTI), musculoskeletal pain and neuropathic pain in the last 3 months. Also the presence of neurogenic heterotopic ossification (NHO) was recorded. The SHCs were defined as follows:
AD: a sudden reaction of the autonomic nervous system triggered by a stimulus below the level of the injury, causing an increase in blood pressure accompanied by other symptoms as pallor, piloerections, cold extremities and profuse sweating below the level of the injury, and severe headaches, flushing of the skin, bradycardia and nasal congestion above the level of injury.
Pneumonia: a lower respiratory tract infection treated with antibiotics.
Pressure ulcers: category I, II, III or IV pressure ulcers according to the classification of European Pressure Ulcer Advisory Panel (EPUAP).27
Problematic spasticity: spasticity interfering moderately or extensively with activities in daily life.
Hypotension: a decrease in blood pressure that was assessed according to symptoms as light-headedness or fainting.
UTI: a urinary tract infection treated with antibiotics, and the presence of one or more of the following symptoms: fever, increased spasticity, discomfort or pain during urination, the onset of urinary incontinence, malaise, AD, mucus or gritty particles in the urine or cloudy urine with increased odor.
NHO: the presence of bone tissue in the soft tissue surrounding paralyzed joints, confirmed by radiological examination.
Neuropathic pain: at- or below-level pain, originating from spinal cord ischemia, syringomyelia or trauma.28
Musculoskeletal pain: nociceptive pain originating from muscle, bone or joint trauma or overuse.29
SHC sum-score
A sum-score of the number of SHCs (0–9) was calculated to obtain insight into the effect of having multiple SHCs in relation to functional independence.
Comorbidities
Comorbidities were assessed by the rehabilitation physician in the structured interview. The presence was established according to the Charlson Comorbidity Index.30
Statistical analysis
Cronbach’s alpha and Skewness of the total SCIM III score and subscale scores were calculated to examine their internal consistency and normality. Internal consistency was excellent for the total SCIM III score (0.91) and Self-care (0.93), good for Mobility (0.83), but questionable for Respiration and sphincter management (0.66). The Skewness was 0.73 for the total SCIM III and 0.51–0.62 for the subscale scores, showing no strong deviations from the normal distribution. Descriptive statistics of demographics, injury characteristics, SCIM III scores, SHCs and comorbidities were calculated for the total group as well as for the three TSI strata. Analysis of variance (ANOVA) and the Chi-square test were used to determine differences between the three TSI strata. A Pearson’s correlation was calculated between TSI and the total SCIM III score.
Possible determinants of the total SCIM III scores were tested using t-tests. For these analyses, age was dichotomized to <55 and ≥55 years, comorbidities to ‘no’ and ‘one or more’, waist circumference to ‘high’ (male ≥ 102; female ≥ 88) and ‘normal’ (male < 102; female < 88) and the SHC sum-score in <4 or ≥4.
Furthermore, to obtain more insight into the influence of level of injury on SCIM III scores we calculated a bar chart with SCIM III scores per single level of injury.
Finally, linear hierarchical regression analyses were performed to identify independent determinants of the total SCIM III score. In these analyses, the dichotomized SHC sum-score was used instead of the separate SHCs to minimize the number of potential determinants. Demographics (age and sex) and level of injury were analyzed in the first step of the model, while all other determinants with a P < 0.1 in bivariate analyses were added to the model in the second step.
Missing data were dealt with using pairwise deletion. All analyses were performed using the SPSS statistical software program (SPSS 21.0 for windows, IBM; Armonck NY).
Results
Descriptives
Between November 2011 and February 2014, 566 persons were invited of whom 282 participated in ALLRISC. For the purpose of this study, 54 persons were excluded because they had a motor incomplete injury, one person because of missing injury characteristics and one because of missing SCIM data, leaving a total of 226 participants. Characteristics of participants are displayed in Table 1. The mean age was 48.0 years (SD 8.9; median 47.3; range 29.1–66.5). The mean TSI was 23.6 years (SD 9.1; 21.5; range 10–47).
Table 1. Participant characteristics.
| Characteristic | % |
|---|---|
| Demographics | |
| Sex (male) | 76.1 |
| Marital status (partner) (n = 214) | 36.9 |
| Level of education (high) (n = 215) | 40.5 |
| Injury characteristics | |
| Cause of injury (traumatic) | 93.8 |
| Level of injury (tetraplegia) | 37.6 |
| Comorbidities (1 or more) (n = 225) | 45.3 |
| High waist circumference (m ≥ 102;f ≥ 88) (n = 208) | 44.2 |
| Mental health problems (MHI-5 < 60) (n = 212) | 22.3 |
| Secondary health conditions | |
| Musculoskeletal pain | 61.5 |
| Neuropathic pain | 45.1 |
| Problematic spasticity | 41.2 |
| Urinary tract infections | 35.0 |
| Pressure ulcers | 33.6 |
| Neurogenic heterotopic ossification | 27.9 |
| Hypotension | 20.4 |
| Autonomic dysreflexia | 19.9 |
| Pneumonia (n = 217) | 6.0 |
m, male; f, female; MHI-5, Mental Health Index.
Differences between time since injury strata
The mean ages of persons in the three TSI strata (10–19 years, 20–29 years and 30+ years) were, respectively, 40.4 (SD 5.0), 48.5 (SD 5.6), and 58.1 (SD 5.3) years (F = 203.0, P < .001).
The three TSI-groups were similar with regard to the level of injury, comorbidities and most SHCs. Only the presence of problematic spasticity (respectively 52.3%, 39.5% and 27.4%, P < .01) and AD (respectively 30.7%, 13.2% and 12.9%, P < 0.01) was lower for persons with a longer TSI. In the group with the shortest TSI the fewest people had a high waist circumference, respectively, 33.7%, 52.2% and 50%, P < .05.
No significant differences between the TSI strata were found for the total score and sub-scores of the SCIM III (Table 2). Furthermore, no correlation was found between TSI as a continuous variable and the total SCIM III score (R = –0.02, P = .175). However, SCIM III scores for persons with paraplegia decreased with a longer TSI (R = –0.20, P < .05), in contrast to persons with tetraplegia (R = –0.04, P = .737).
Table 2. SCIM III scores of TSI strata.
| SCIM scale | Total group (n = 226) | TSI 10–19 years (n = 88) |
TSI 20–29 years (n = 76) |
TSI ≥ 30 years (n = 62) |
||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ANOVA F | P | |
| SCIM self-care | 12.9 (5.8) | 12.8 (5.6) | 12.9 (6.4) | 12.9 (5.4) | 0.0 | .989 |
| SCIM respiration and sphincter management | 27.7 (7.8) | 27.3 (7.9) | 28.3 (7.6) | 27.7 (7.9) | 0.3 | .728 |
| SCIM mobility | 14.1 (6.1) | 14.5 (6.1) | 14.2 (6.5) | 13.6 (5.5) | 0.4 | .649 |
| SCIM total | 54.9 (18.5) | 54.6 (18.5) | 55.7 (19.3) | 54.2 (17.7) | 0.1 | .888 |
SCIM, Spinal Cord Independence Measure; TSI, time since injury.
Other determinants of the SCIM III
As the internal consistency of the total SCIM III score was excellent and analyses per subscale did not reveal substantial information, analyses of determinants of the SCIM III were performed with the total SCIM III score. Further, these analyses were not performed for separate TSI strata but for all participants, regardless of TSI. SCIM III scores were significantly lower for persons with a tetraplegia, AD, hypotension, less than four SHCs and a high waist circumference. No significant difference was found between the two age categories (Table 3).
Table 3. Determinants of the SCIM III.
| Determinant | N | SCIM total Mean (SD) | T | P |
|---|---|---|---|---|
| Demographics | ||||
| Age | ||||
| <55 years | 175 | 54.6 (18.9) | 0.4 | .662 |
| ≥55 years | 51 | 55.8 (17.1) | ||
| Sex | ||||
| Male | 172 | 54.4 (18.8) | 0.6 | .562 |
| Female | 54 | 56.1 (17.5) | ||
| Education | ||||
| High | 87 | 55.6 (19.3) | –0.5 | .623 |
| Low | 128 | 54.3 (17.9) | ||
| Marital status | ||||
| Partner | 135 | 52.2 (19.1) | 1.6 | .100 |
| No partner | 79 | 56.5 (18.0) | ||
| Injury characteristics | ||||
| Cause of injury | ||||
| Non-traumatic | 14 | 51.8 (19.6) | 0.6 | .522 |
| Traumatic | 212 | 55.1 (18.4) | ||
| Level of injury | ||||
| Paraplegia | 141 | 65.3 (7.8) | –13.4 | <.001 |
| Tetraplegia | 85 | 37.5 (18.1) | ||
| Comorbidities | ||||
| No | 123 | 53.4 (19.6) | 1.5 | .144 |
| One or more | 102 | 56.9 (16.7) | ||
| Waist circumference | ||||
| m < 102; f < 88 | 116 | 59.5 (16.4) | –3.4 | .001 |
| m ≥ 102; f ≥ 88 | 92 | 51.0 (18.9) | ||
| Mental Health | ||||
| MHI-5 ≥ 60 | 186 | 55.7 (18.1) | –1.6 | .110 |
| MHI-5 < 60 | 26 | 49.5 (20.3) | ||
| SHCs | ||||
| Musculoskeletal pain | ||||
| No | 87 | 53.1 (19.7) | 1.1 | .280 |
| Yes | 139 | 55.9 (17.6) | ||
| Neuropathic pain | ||||
| No | 124 | 55.7 (17.7) | –0.8 | .427 |
| Yes | 102 | 53.8 (19.4) | ||
| Problematic spasticity | ||||
| No | 133 | 56.2 (17.9) | –1.3 | .206 |
| Yes | 93 | 53.0 (19.2) | ||
| Urinary tract infections | ||||
| No | 147 | 55.4 (18.3) | –0.6 | .560 |
| Yes | 79 | 53.9 (18.8) | ||
| Pressure ulcers | ||||
| No | 150 | 56.1 (17.8) | –1.5 | .140 |
| Yes | 76 | 52.3 (19.5) | ||
| NHO | ||||
| No | 163 | 54.7 (18.5) | 0.2 | .828 |
| Yes | 63 | 55.3 (18.5) | ||
| Hypotension | ||||
| No | 180 | 56.3 (17.9) | –2.3 | .024 |
| Yes | 46 | 49.4 (19.8) | ||
| AD | ||||
| No | 181 | 56.6 (18.0) | –2.9 | .004 |
| Yes | 45 | 47.8 (18.9) | ||
| Pneumonia | ||||
| No | 204 | 55.0 (18.3) | –1.4 | .171 |
| Yes | 13 | 47.7 (22.2) | ||
| SHC sum-score (0–9) | ||||
| <4 | 141 | 56.8 (17.9) | –2.5 | .014 |
| ≥4 | 76 | 50.3 (19.2) | ||
SCIM, Spinal Cord Independence Measure; SHC, secondary health condition; NHO, neurogenic heterotopic ossification; AD, autonomic dysreflexia, m, male; f, female; MHI-5, Mental Health Index.
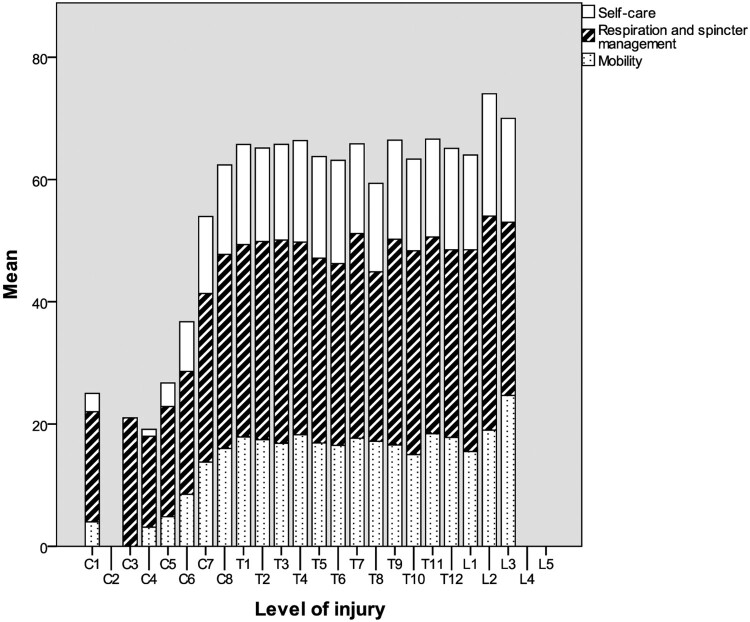
Figure 1 shows the SCIM III scores per level of injury. There was one person with a C1 level of injury. This person had a zone of partial preservation (right sensory C3, left sensory T3, right motor C7, left motor C7). SCIM III scores of persons with C4–T1 level of injury increased with each lower level of injury. We found more or less similar scores for participants with levels of injury across the range T1–L1 (Fig. 1).
Figure 1.
SCIM III scores per level of injury.
Regression analysis
The results of the linear hierarchical regression analyses are displayed in Table 4. The adjusted R2 of the first step of the analyses (including sex, age and level of injury) was 0.534. The R2 change of the second step in the regression analyses was small (0.011), indicating the variables added in the second step (waist circumference and SHC sum-score) had little added value in explaining long-term functioning. Level of injury was the strongest independent determinant of long-term functioning (R2 0.469). Waist circumference was the only other independent, but weak, determinant (R2 0.010).
Table 4. Linear hierarchical regression analyses (final model) of determinants of the SCIM III.
| Determinant | B (SE) | β | P | Uniquely explained variance (%) |
|---|---|---|---|---|
| Level of injury (tetraplegia) | –27.7 (1.9) | –0.7 | <.001 | 49.6 |
| Age (older) | –0.2 (0.1) | –0.1 | .098 | 0.6 |
| Sex (female) | –3.0 (2.1) | –0.1 | .153 | 0.5 |
| SHC sum-score (≥4) | –1.6 (1.9) | –0.04 | .391 | 0.2 |
| Waist circumference (m ≥ 102; f ≥ 88) | –3.7 (1.8) | –0.1 | .042 | 1.0 |
Note: Adjusted R2 0.545 (F = 48.6, p < .001).
SHC, secondary health condition; m, male; f, female.
Discussion
In this cross-sectional study, we found no differences in functional independence measured with the SCIM III between strata of TSI (10–19, 20–29, 30+ years) in persons under 65 years of age with a motor complete SCI in the Netherlands. The level of injury was the strongest determinant, and neither TSI nor age was associated with long-term functioning. Waist circumference was another weak, but independent determinant.
Our study is amongst the first studies to have used the SCIM III to evaluate long-term functional independence.6 The results are thus mainly discussed in the light of studies that have used other outcome measures, mostly the FIM. Due to methodological differences with other studies, a comparison of the results must be interpreted with caution. Furthermore, factors related to the healthcare systems, e.g. the degree of initial rehabilitation or follow-up, add to the difficulties of comparing studies on (long-term) functioning.
Our finding that persons with a longer TSI did not function less independent compared to persons with more recent SCI is more or less in line with the inconsistent results of previous longitudinal studies2,12,13 and also with several other cross-sectional studies that did not find a decline.2–6 However, studies that measured the patient’s perception of change in functioning found a decline in experienced functioning in a part of the participants.8–10 Although this may be explained by the subjective measurement of functioning, the SCIM III may not be sensitive enough to detect small functional changes, since the effort needed to perform an activity is not evaluated. Further investigation is necessary to examine whether other measures, e.g., the recently developed Spinal Cord Injury-Functional Index would be more sensitive to detect changes in functional independence in persons with long-standing SCI.31
In contrast to our overall results on long-term functioning, we found a negative correlation between the SCIM III scores and TSI for persons with a paraplegia. This seems to match the results of a study performed with the FIM, in which a decline of functioning was described only for higher functioning individuals.13 The difference in SCIM score between the first and last TSI stratum for persons with a paraplegia we found was however so small that it does not seem to be clinically relevant.32 Altogether, more longitudinal data is needed for more solid conclusions on long-term functioning.
The level of injury has been described as strongest determinant of long-term functional independence.3,4,6 The SCIM III showed sensitivity to detect differences in functioning across cervical levels of injury but not across thoracic level of injury. Due to the absence of key muscles in the thoracic area (Th2–L1), little functional changes would also be expected according to the level of injury within this range. Functional changes on the mobility subscale would be likely across levels L2–S1. As our inclusion criteria included wheelchair dependency and only a few persons with a low level of injury were included, we were not able to analyze this.
Waist circumference was the only other independent determinant of long-term functioning. The role of body composition in long-term mobility has been suggested previously.16 As an increased waist circumference is a modifiable determinant, this finding is relevant to clinical practice, although the association we found was only weak.
Study limitations
As a result of the cross-sectional study design, no conclusions can be drawn on the true effects of aging. A longitudinal study design would be necessary to obtain more insight into these true effects of aging.12
A limitation concerning the representativeness of the study sample concerned absent information regarding the comparability between participants and non-participants. There may have been a survivor effect within our study, meaning that healthier subjects may have been more available or willing to participate.33 However, we found similar SCI characteristics and SHCs in the three TSI strata, which does not indicate a survivor effect in those living with SCI for more than 10 years.
The inclusion of only motor complete injuries rendered more valid information on SCIM III scores per injury level but restricts generalization of the results to all people living with SCI.
As a result of our inclusion criteria, all participants were injured as young adults and maximum 65 years of age at the time of the study. Therefore, our study is less suited to study the influence of age and age at injury on long-term functional independence. Age at injury has been previously described to be a determinant of long-term functioning; the older the age at the time of injury, the greater the influence of aging on disability.34 Further, our study population may have been too young to detect a relation between age and functional independence.
The SCIM III subscale ‘Respiration and sphincter management’ contains information on two common SHCs, namely urinary and fecal incontinence. As spurious relations were expected between these SHCs and the SCIM subscale, we could not analyze these SHCs as a possible determinant of functional independence.
Conclusions
Our study suggests that persons under 65 years of age with a motor complete SCI in the Netherlands seem to be able to maintain their level of functioning for quite a long period. However, longitudinal studies are necessary to reveal the effects of aging on functional independence. Our study further confirms the association between waist circumference and functional independence, and thereby the importance of a healthy lifestyle to prevent an increase in weight.
Abbreviations
- AD:
Autonomic dysreflexia;
- ALLRISC:
Active LifestyLe Rehabilitation Interventions in aging Spinal Cord injury;
- ANOVA:
analysis of variance;
- ASIA:
American Spinal Injury Association;
- AIS:
ASIA Impairment Scale;
- EPUAP:
European Pressure Ulcer Advisory Panel;
- FIM:
Functional Independence Measure;
- ISNCSCI:
International Standards for the Neurological Classification of Spinal Cord Injury;
- MHI-5:
Mental Health Inventory-5;
- NHO:
neurogenic heterotopic ossification;
- SCI:
spinal cord injury;
- SCIM:
Spinal Cord Independence Measure;
- SHC:
secondary health condition;
- TSI:
time since injury;
- UTI:
urinary tract infection
Disclaimer statements
Contributors None.
Funding This study is part of the Dutch ALLRISC research program and is supported financially by ZonMw Rehabilitation program and Fonds NutsOhra [grant number 89000006].
Declaration of interest None.
Conflict of interest The authors declare no conflict of interest.
ORCID
Marcel W. M. Post http://orcid.org/0000-0002-2205-9404
References
- 1.Menter RR, Whiteneck GG, Charlifue SW, Gerhart K, Solnick SJ, Brooks CA, et al. Impairment, disability, handicap and medical expenses of persons aging with spinal cord injury. Paraplegia 1991;29(9):613–9. [DOI] [PubMed] [Google Scholar]
- 2.Charlifue SW, Weitzenkamp DA, Whiteneck GG.. Longitudinal outcomes in spinal cord injury: aging, secondary conditions, and well-being. Arch Phys Med Rehabil 1999;80(11):1429–34. doi: 10.1016/S0003-9993(99)90254-X [DOI] [PubMed] [Google Scholar]
- 3.Daverat P, Petit H, Kemoun G, Dartigues JF, Barat M.. The long term outcome in 149 patients with spinal cord injury. Paraplegia 1995;33(11):665–8. [DOI] [PubMed] [Google Scholar]
- 4.Pentland W, McColl MA, Rosenthal C.. The effect of aging and duration of disability on long term health outcomes following spinal cord injury. Paraplegia 1995;33(7):367–73. [DOI] [PubMed] [Google Scholar]
- 5.Fuhrer MJ, Rintala DH, Hart KA, Clearman R, Young ME.. Relationship of life satisfaction to impairment, disability, and handicap among persons with spinal cord injury living in the community. Arch Phys Med Rehabil 1992;73(6):552–7. [PubMed] [Google Scholar]
- 6.Jorgensen S, Iwarsson S, Lexell J.. Secondary health conditions, activity limitations, and life satisfaction in older adults with long-term spinal cord injury. PM R 2017;9(4):356–366. doi: 10.1016/j.pmrj.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 7.Whiteneck GG, Charlifue SW, Frankel HL, Fraser MH, Gardner BP, Gerhart KA, et al. Mortality, morbidity, and psychosocial outcomes of persons spinal cord injured more than 20 years ago. Paraplegia 1992;30(9):617–30. [DOI] [PubMed] [Google Scholar]
- 8.Thompson L. Functional changes in persons aging with spinal cord injury. Assist Technol 1999;11(2):123–9. doi: 10.1080/10400435.1999.10131996 [DOI] [PubMed] [Google Scholar]
- 9.Liem NR, McColl MA, King W, Smith KM.. Aging with a spinal cord injury: factors associated with the need for more help with activities of daily living. Arch Phys Med Rehabil 2004;85(10):1567–77. doi: 10.1016/j.apmr.2003.12.038 [DOI] [PubMed] [Google Scholar]
- 10.Gerhart KA, Bergstrom E, Charlifue SW, Menter RR, Whiteneck GG.. Long-term spinal cord injury: functional changes over time. Arch Phys Med Rehabil 1993;74(10):1030–4. doi: 10.1016/0003-9993(93)90057-H [DOI] [PubMed] [Google Scholar]
- 11.Amsters DI, Pershouse KJ, Price GL, Kendall MB.. Long duration spinal cord injury: perceptions of functional change over time. Disabil Rehabil 2005;27(9):489–97. doi: 10.1080/09638280400018478 [DOI] [PubMed] [Google Scholar]
- 12.Weitzenkamp DA, Jones RH, Whiteneck GG, Young DA.. Ageing with spinal cord injury: cross-sectional and longitudinal effects. Spinal Cord 2001;39(6):301–9. doi: 10.1038/sj.sc.3101146 [DOI] [PubMed] [Google Scholar]
- 13.Pershouse KJ, Barker RN, Kendall MB, Buettner PG, Kuipers P, Schuurs SB, et al. Investigating changes in quality of life and function along the lifespan for people with spinal cord injury. Arch Phys Med Rehabil 2012;93(3):413–9. doi: 10.1016/j.apmr.2011.10.014 [DOI] [PubMed] [Google Scholar]
- 14.Cohen JT, Marino RJ, Sacco P, Terrin N.. Association between the functional independence measure following spinal cord injury and long-term outcomes. Spinal Cord 2012;50(10):728–33. doi: 10.1038/sc.2012.50 [DOI] [PubMed] [Google Scholar]
- 15.Hitzig SL, Tonack M, Campbell KA, McGillivray CF, Boschen KA, Richards K, et al. Secondary health complications in an aging Canadian spinal cord injury sample. Am J Phys Med Rehabil 2008;87(7):545–55. doi: 10.1097/PHM.0b013e31817c16d6 [DOI] [PubMed] [Google Scholar]
- 16.Hinrichs T, Lay V, Arnet U, Eriks-Hoogland I, Koch HG, Rantanen T, et al. Age-related variation in mobility independence among wheelchair users with spinal cord injury: a cross-sectional study. J Spinal Cord Med 2016;39(2):180–9. doi: 10.1179/2045772315Y.0000000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itzkovich M, Gelernter I, Biering-Sorensen F, Weeks C, Laramee MT, Craven BC, et al. The Spinal Cord Independence Measure (SCIM) version III: reliability and validity in a multi-center international study. Disabil Rehabil 2007;29(24):1926–33. doi: 10.1080/09638280601046302 [DOI] [PubMed] [Google Scholar]
- 18.Anderson K, Aito S, Atkins M, Biering-Sorensen F, Charlifue S, Curt A, et al. Functional recovery measures for spinal cord injury: an evidence-based review for clinical practice and research. J Spinal Cord Med 2008;31(2):133–44. doi: 10.1080/10790268.2008.11760704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Woude LH, de Groot S, Postema K, Bussmann JB, Janssen TW, Post MW.. Active LifestyLe Rehabilitation interventions in aging spinal cord injury (ALLRISC): a multicentre research program. Disabil Rehabil 2013;35(13):1097–103. doi: 10.3109/09638288.2012.718407 [DOI] [PubMed] [Google Scholar]
- 20.Adriaansen JJ, van Asbeck FW, Lindeman E, van der Woude LH, de Groot S, Post MW.. Secondary health conditions in persons with a spinal cord injury for at least 10 years: design of a comprehensive long-term cross-sectional study. Disabil Rehabil 2013;35(13):1104–10. doi: 10.3109/09638288.2012.712196 [DOI] [PubMed] [Google Scholar]
- 21.Catz A, Itzkovich M, Agranov E, Ring H, Tamir A.. SCIM--spinal cord independence measure: a new disability scale for patients with spinal cord lesions. Spinal Cord 1997;35(12):850–6. doi: 10.1038/sj.sc.3100504 [DOI] [PubMed] [Google Scholar]
- 22.Bluvshtein V, Front L, Itzkovich M, Aidinoff E, Gelernter I, Hart J, et al. SCIM III is reliable and valid in a separate analysis for traumatic spinal cord lesions. Spinal Cord 2011;49(2):292–6. doi: 10.1038/sc.2010.111 [DOI] [PubMed] [Google Scholar]
- 23.Almeida C, Coelho JN, Riberto M.. Applicability, validation and reproducibility of the Spinal Cord Independence Measure version III (SCIM III) in patients with non-traumatic spinal cord lesions. Disabil Rehabil 2016;38(22):2229–34. doi: 10.3109/09638288.2015.1129454 [DOI] [PubMed] [Google Scholar]
- 24.Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, et al. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med 2011;34(6):547–54. doi: 10.1179/107902611X13186000420242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forchheimer M, McAweeney M, Tate DG.. Use of the SF-36 among persons with spinal cord injury. Am J Phys Med Rehabil 2004;83(5):390–5. doi: 10.1097/01.PHM.0000124441.78275.C9 [DOI] [PubMed] [Google Scholar]
- 26.Perenboom R OK, van Herten L, Hoeymans N, Bijl R.. TNO-report PG/VGZ/99.067. Life-expectancy in good mental health: establishing cut-off points for the MHI-5 and GHQ-12. TNO-report PG/VGZ/99067, Leiden; 2000. (in Dutch).
- 27.European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel . Prevention and treatment of pressure ulcers: clinical practice guideline. Washington DC: National Pressure Ulcer Advisory Panel; 2009. [Google Scholar]
- 28.Burchiel KJ, Hsu FP.. Pain and spasticity after spinal cord injury: mechanisms and treatment. Spine (Phila Pa 1976) 2001;26(24 Suppl):S146–60. doi: 10.1097/00007632-200112151-00024 [DOI] [PubMed] [Google Scholar]
- 29.Burchiel KJ, Burns AS.. Summary statement: pain, spasticity, and bladder and sexual function after spinal cord injury. Spine (Phila Pa 1976) 2001;26(24 Suppl):S161. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 31.Jette AM, Tulsky DS, Ni P, Kisala PA, Slavin MD, Dijkers MP, et al. Development and initial evaluation of the spinal cord injury-functional index. Arch Phys Med Rehabil 2012;93(10):1733–50. doi: 10.1016/j.apmr.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 32.Corallo V, Torre M, Ferrara G, Guerra F, Nicosia G, Romanelli E, et al. What do spinal cord injury patients think of their improvement? A distribution and anchor based study of the minimal clinically important difference of the Spinal Cord Independence Measure III (SCIM III). Eur J Phys Rehabil Med 2017;53(4):508–515. [DOI] [PubMed] [Google Scholar]
- 33.Krause JS, Bozard JL.. Natural course of life changes after spinal cord injury: a 35-year longitudinal study. Spinal Cord 2012;50(3):227–31. doi: 10.1038/sc.2011.106 [DOI] [PubMed] [Google Scholar]
- 34.Rodakowski J, Skidmore ER, Anderson SJ, Begley A, Jensen MP, Buhule OD, et al. Additive effect of age on disability for individuals with spinal cord injuries. Arch Phys Med Rehabil 2014;95(6):1076–82. doi: 10.1016/j.apmr.2014.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]