Abstract
Context/objective: To determine the prevalence of deep vein thrombosis (DVT) detected through routine duplex screening and factors associated with DVT in spinal cord injury (SCI) patients on admission to rehabilitation.
Design: Retrospective chart review of medical records.
Setting: Acute inpatient rehabilitation.
Participants: One hundred and eighty-nine individuals admitted to rehabilitation within 2 weeks of initial traumatic SCI who underwent routine surveillance with duplex scan for DVT.
Interventions: Duplex scan of lower extremities.
Outcome measures: The dependent variable was positive duplex screening for either any DVT (distal and/or proximal) or proximal DVT.
Results: Of the 189 patients, 31 patients (16.4%) had a positive scan for any (proximal and/or distal) DVT, with 9 (4.8%) positive for a proximal DVT and 22 (11.6%) positive for isolated distal DVT. Of those with isolated distal DVT, 31.8% later developed propagation with either proximal DVTs or pulmonary embolism (mean = 22 days). Factors significantly associated with positive duplex scans for any (proximal and/or distal) DVT include more severe neurological injury (AIS A, B or C versus AIS D: χ2 = 7.1791, df = 1, P = 0.007) and older age (age ≥50 years old: χ2 = 14.9410, df = 1, P = 0.000).
Conclusion: In acute traumatic SCI, older age and more severe neurological impairment (AIS A, B, and C) are independent risk factors for positive duplex screening for any (proximal and/or distal) DVT detected on rehabilitation admission. Individuals with an acute distal DVT have a high likelihood for future thrombus progression. Routine surveillance for these patients may be warranted.
Keywords: Deep vein thrombosis, Spinal cord injury
Introduction
Individuals with spinal cord injury (SCI) demonstrate the highest rates of deep vein thrombosis (DVT) among trauma patients with prior studies reporting DVT rates ranging from 12 to 100%.1–4 The wide range in prevalence can be attributed to a host of factors including screening method, time from injury, and presence of chemoprophylaxis.4 Patients with acute SCI are at increased risk of thromboembolic disorders including DVT and pulmonary embolism (PE), due to the combined presence of Virchow’s risk factors: stasis, a hypercoagulable state, and intimal injury.5 Thrombosis can occur as early as 72 h after initial injury with a peak between 7 and 10 days.4 Regarding DVT risk stratification, the presence of “SCI with neurological deficit” automatically places a patient in the highest-risk patient category.6 PE has its greatest incidence within the first month and is the third leading cause of death after initial SCI.7,8 Additional complications following DVT include late DVT reoccurrence, post-thrombotic syndrome with prolonged edema, and pressure injuries.9,10
In 1999, clinical practice guidelines (CPG) published by the Consortium for Spinal Cord Medicine (CSCM) did not include an official recommendation regarding duplex screening for SCI patients on entry to rehabilitation.11 However, four retrospective studies showed a high rate of asymptomatic DVT on routine duplex scans performed in SCI patients on admission to a rehabilitation center.12–15 Powell et al. reported that 11.6% of SCI patients had a newly diagnosed DVT detected by duplex scan at admission to acute rehabilitation with only 38.6% receiving preadmission chemoprophylaxis.14 Kaydan et al. reported that 8.7% of patients with traumatic SCI were found to have DVT on admission.13 Additionally, in a subsequent report, Kaydan et al. demonstrated that 6.5% of traumatic SCI exhibited positive duplex scans on admission to rehabilitation and that surveillance with duplex ultrasound was a cost-effective tool for detecting DVT.12 Do et al. reported the occurrence of asymptomatic DVT on rehabilitation admission through routine duplex screening as 27.6%, however, those receiving chemoprophylaxis were excluded from this study.15
While several studies have examined possible risk factors associated with venous thromboembolism, no definitive agreement has been reached. For instance, Green et al. found older age, malignancy, flaccid paralysis and obesity as risk factors for venous thromboembolism.16 Clements et al. found statistically significant associations between DVT occurrence and completeness of motor paralysis, male sex, and pelvic or lower limb fractures in SCI during initial hospitalization.17 Chemoprophylaxis against DVT significantly decreased the risk of a positive duplex scan on rehabilitation admission.14
The updated CPG published (2016) by the CSCM recommend specifically that persons with SCI should not be routinely screened with duplex ultrasound for clinically asymptomatic DVT during their admission to rehabilitation.18 Reasons for this included that duplex ultrasound in asymptomatic patients is considered neither sensitive nor specific.19,20 Moreover, non-diagnostic essential deep veins was reported in trauma patients with rates from 10 to 41%.21,22 The clinical significance of asymptomatic DVT is also unknown.6 Lastly, there is evidence that routine duplex scans may fail to reduce the occurrence of symptomatic DVT or PE.23
While the updated CPG raise valid arguments against routine screening with duplex ultrasound, clinically asymptomatic DVT is challenging to diagnose in the SCI patient population. Many patients with SCI experience abnormal or absent sensation that limits the accuracy of clinical assessment of calf tenderness, calf pain, and pain on diagnostic maneuvers, such as Homan’s sign, associated with DVT.12 Additionally, clinical symptoms such as leg edema are not specific for DVT.24,25 Aito et al. reported that 65% of detected DVTs through duplex scan, regardless of ASIA Impairment Scale (AIS) classification, did not show any prior clinical evidence.26
This exploratory study was undertaken to review the prevalence of DVT found by duplex screening on rehabilitation admission in our acute traumatic SCI population prior to publication of the new CPG to specifically identify who might be at greatest risk and therefore benefit from receiving routine screening. Better understanding of factors that increase risk of thromboembolism could influence future clinical practice guidelines. Specifically, we wanted to assess if age, severity of injury, and type of chemoprophylaxis were consistent predictors to continue to consider performing routine duplex scan on rehabilitation admission. Our hypotheses were that motor complete injuries (AIS A and B), as well as those not receiving low molecular heparin (LMWH) would be at greatest risk for DVT development, and as such may warrant routine screening even if they were apparently asymptomatic.
Methods
A retrospective chart review was conducted of all SCI admissions to our acute rehabilitation facility between January 1, 2011 and December 31, 2016. Inclusion criteria included admission within two-weeks of initial traumatic SCI, duplex screening of lower extremities performed within 72 h of rehabilitation admission, and successful completion of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) exam with an AIS classification of A through D. The protocol at this institution before 2016 had been to obtain routine duplex scans on admission to screen for DVT.
Exclusion criteria included non-traumatic SCI, inability to complete ISNCSCI exam, and known DVT/PE diagnosed in acute care transferred to rehabilitation on therapeutic anticoagulation. Duplex studies that reported technical difficulties in full visualization of deep veins were also excluded.
Data collected included patient demographics, neurological level of injury, AIS, results of duplex scans, location of DVTs in positive duplex scans, and presence of and type of chemoprophylaxis on the admission medication list to rehabilitation. DVTs located in the popliteal vein or more proximal veins were categorized as proximal DVT, while DVTs found in the calf veins were classified as distal DVT. The term “any DVT” referred to a positive duplex scan for proximal and/or distal DVT. For those with duplex scans positive for isolated distal DVTs, results of subsequent duplex scans, if performed, were also reviewed. Forms of chemoprophylaxis included LMWH, unfractionated heparin (UH), warfarin, fondaparinux, apixaban, dabigatran, rivaroxaban, and prasugrel. Data on co-morbidities was also collected regarding history of malignancy, history of lower extremity fracture diagnosed in acute care, presence of inferior vena cava (IVC) filter, and recent DVT or PE diagnosed in the acute care hospital that had not been treated with therapeutic anticoagulation.
Study sample
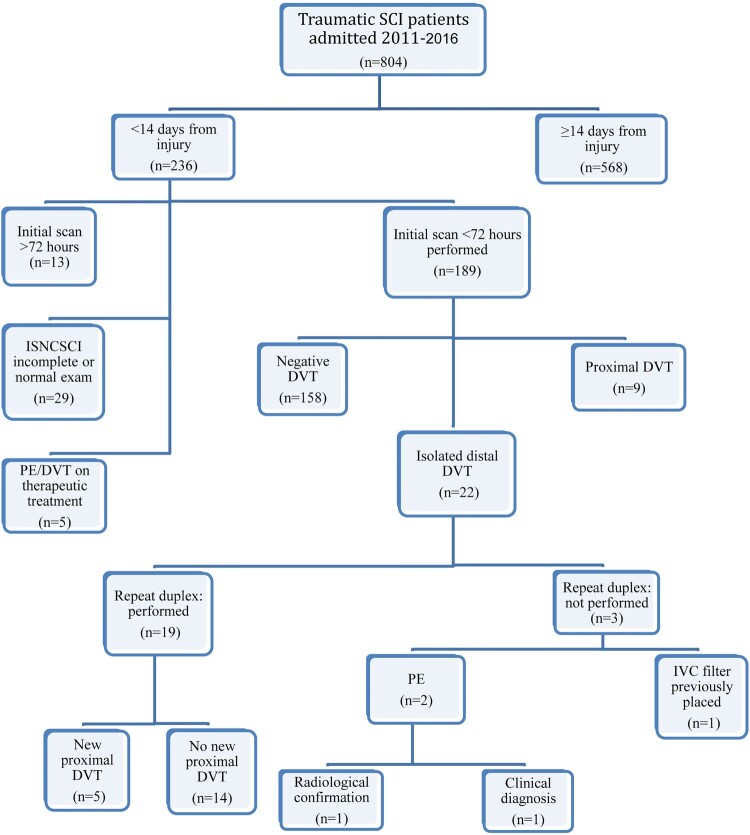
Of the 804 patients with traumatic SCI who were admitted for rehabilitation during the time period of the study, 236 patients met inclusion criteria. In total, 47 subjects were excluded for the following reasons: absence of duplex scan within 72 h of admission (n = 13), ISNCSCI exam was incomplete or normal (n = 29), or known DVT or PE at the time of admission and was already on therapeutic anticoagulation (n = 5). Subsequently, 189 people met the criteria for inclusion in the analysis (see Figure 1).
Figure 1.
Description of the inclusion process of patients in this retrospective study. This figure also describes results of initial duplex imaging and subsequent surveillance.
Statistics
Chi-square analysis was utilized to determine statistically significant differences in occurrence of both proximal and any (proximal and/or distal) DVT as detected by duplex scan and several key risk factors. Participants were grouped by level of injury, severity of neurological injury, presence of chemoprophylaxis, type of chemoprophylaxis, age, and other co-morbidities mentioned previously which were examined independently. Fisher exact test (1-tailed) was used for groups including <5 subjects as necessary.
Results
The average age was 51.2 years (SD = 20.2; range: 16–85) and 74.1% male, which are similar to prior sample populations.14,15 Overall, 16.4% (31 of 189) of our subjects had a positive initial duplex scan on admission for any (proximal and/or distal) DVT; 9 of 31 (29.0%) (4.8% of total population) had proximal DVT and 22 of 31 (71.0%) had isolated distal DVT. Six of the nine subjects with proximal DVT also had distal DVT. The frequency of distal DVT versus proximal DVT is summarized in Table 1. Individuals classified as motor complete (AIS A, B) did not show a significant difference in either any DVT (χ2 = 1.2348, df = 1, P = 0.266) or proximal DVT (χ = 0.000, df = 1, P = 1.000) when compared to motor incomplete (AIS C, D) injuries. Upon further exploration of severity of injury, persons classified as AIS A, B or C showed a statistically significant higher frequency of any (proximal and/or distal) DVT compared to those with AIS D (χ2 = 7.1791, df = 1, P = 0.007), suggesting that people with more severe neurological impairment are disproportionately at risk for DVT. However, Fisher exact test showed no significant difference in proximal DVT with respect to severity of neurological injury (AIS A, B, C versus AIS D, P = 0.151).
Table 1. This table describes the demographics, clinical characteristics, duplex scan results, and chemoprophylaxis for individuals admitted to inpatient rehabilitation with acute traumatic SCI.
| Category | n | Negative duplex | Positive duplex | Isolated distal DVT only as a % of all DVTs | Proximal DVT as a % of all DVTs | Any (proximal and/or distal) DVT χ2, P value | Proximal DVT χ2,P value |
|---|---|---|---|---|---|---|---|
| Total | 189 | 158 (83.6%) | 31 (16.4%) | 22 | 9 | ||
| AIS classification | |||||||
| AIS A | 40 (21.2%) | 31 (77.5%) | 9 (22.5%) | 6 (66.7%) | 3 (33.3%) | ||
| AIS B | 23 (12.2%) | 19 (82.6%) | 4 (17.4%) | 4 (100%) | 0 (0%) | ||
| AIS C | 42 (22.2%) | 31 (73.8%) | 11 (26.2%) | 7 (63.6%) | 4 (36.4%) | ||
| AIS D | 84 (44.4%) | 77 (91.7%) | 7 (8.3%) | 5 (71.4%) | 2 (28.6%) | ||
| Sex | χ2 = 0.8338, df = 1, P = 0.361 | χ2 = 0.2700, df = 1, P = 0.603 | |||||
| Male | 140 (74.1%) | 115 (82.1%) | 25 (17.9%) | 19 (76.0%) | 6 (24.0%) | ||
| Female | 49 (25.9%) | 43 (87.8%) | 6 (12.2%) | 3 (50.0%) | 3 (50.0%) | ||
| Age | χ2 = 14.9410, df = 1, P = 0.000* | χ2 = 1.8900, df = 1, P = 0.169 | |||||
| <50 years of age | 84 (44.4%) | 80 (95.2%) | 4 (4.8%) | 2 (50.0%) | 2 (50.0%) | ||
| ≥50 years of age | 105 (55.5%) | 78 (74.3%) | 27 (25.7%) | 20 (74.1%) | 7 (25.9%) | ||
| Level of injury | χ2 = 0.9353, df = 1, P = 0.333 | χ2 = 0.4233, df = 1, P = 0.4233 | |||||
| C1–C8 | 124 (65.6%) | 106 (85.5%) | 18 (14.5%) | 13 (72.2%) | 5 (27.8%) | ||
| T1 and below | 65 (34.4%) | 52 (80%) | 13 (20.0%) | 9 (69.2%) | 4 (30.8%) | ||
| Co-morbidities | |||||||
| Presence of IVC filter | 29 (15.3%) | 22 (75.9%) | 7 (24.1%) | 5 (71.4%) | 2 (28.6%) | χ2 = 1.4951, df = 1, P = 0.221 | χ2 = 0.3442, df = 1, P = 0.557 |
| History of malignancy | 23 (12.2%) | 17 (73.9%) | 6 (26.1%) | 6 (100.0%) | 0 (0%) | χ2=1.7913, df = 1, P = 0.181 | χ2=1.3093, df = 1, P = 0.253 |
| History of DVT or PE in the acute care | 8 (4.2%) | 3 (37.5%) | 5 (62.5%) | 4 (80.0%) | 1 (20.0%) | ||
| History of lower extremity fracture since injury | 10 (5.3%) | 9 (90.0%) | 1 (10.0%) | 0 (0.0%) | 1 (100.0%) | χ2 = 0.3156, df = 1, P = 0.574 | χ2 = 0.6388, df = 1, P = 0.424 |
| Chemoprophylaxis | |||||||
| No chemoprophylaxis | 49 (25.9%) | 41 (83.7%) | 8 (16.3%) | 7 (87.5%) | 1 (12.5%) | ||
| LMWH | 67 (35.4%) | 59 (88.1%) | 8 (11.9%) | 7 (87.5%) | 1 (12.5%) | ||
| UH | 67 (35.4%) | 56 (83.6%) | 11 (16.4%) | 6 (54.5%) | 5 (45.5%) | ||
| Other anticoagulant** | 6 (3.2%) | 2 (33.3%) | 4 (66.7%) | 2 (50.0%) | 2 (50.0%) |
* statistically significant P < 0.05.
**other anticoagulant: warfarin, fondaparinux, apixaban, dabigatran, rivaroxaban, or prasugrel.
AIS, ASIA Impairment Scale; DVT, deep vein thrombosis; PE, pulmonary embolism; χ2, chi-square; LMWH, low molecular weight heparin; UH, unfractionated heparin; IVC, inferior vena cava; df, degree of freedom.
When grouping patients based on age (<50 versus ≥50 years), DVT was more likely to be observed on admission duplex scan among persons ≥50 years with 25.7% having DVT compared to 4.8% of those < 50 years (χ2 = 14.9410, df = 1, P = 0.000). However, no significant association was found between proximal DVT occurrence and age (positive duplex scan: <50 years: n = 2 (2.4%), ≥50 years: n = 7 (6.7%); χ2 = 1.8900, df = 1, P = 0.169) (see Table 1).
At rehabilitation, 67 subjects were receiving LMWH (positive duplex scan for any DVT: n = 8 (11.9%); proximal DVT: n = 1 (1.5%)), 67 were receiving UH (positive duplex scan for any DVT: n = 11 (16.4%); proximal DVT: n = 5 (7.5%)), and 6 were receiving an alternative anticoagulant which was either warfarin, fondaparinux, apixaban, dabigatran, rivaroxaban, or prasugrel (positive duplex scan for any DVT: n = 4 (66.7%); proximal DVT: n = 2 (33.3%)). In total, 140 patients were transferred to rehabilitation with chemoprophylaxis. This included 36 of 40 (90.0%) with AIS A, 14 of 23 (60.9%), 35 of 42 (83.3%), and 55 of 84 (65.5%) with AIS B, C, and D, respectively. A statistically significant association was noted between AIS level and use of chemoprophylaxis on admission to rehabilitation (Fisher’s exact test P = 0.005). Of the patients transferred to rehabilitation without chemoprophylaxis (n = 49), 16.3% (n = 8) had a positive duplex scan for DVT with 4 persons classified as AIS A and 9, 7, and 29 persons classified as AIS B, C, and D, respectively. Individuals placed on LMWH demonstrated fewer proximal DVT and any DVT compared to those placed on UH, although no statistically significant difference was found (proximal: χ2 = 2.7917, df = 1, P = 0.095; any: χ2 = 0.5519, df = 1, P = 0.458). When comparing LMWH versus alternative anticoagulants, admission on an alternative anticoagulant was a statistically significant predictor of a positive result for any (proximal and/or distal) DVT (χ2 = 12.0069, df = 1, P = 0.001) and even proximal DVT (χ2 = 14.1676, df = 1, P = 0.000) on duplex scan.
When subgrouping patients based on AIS, we found that the difference in rates of DVT in patients with AIS A on LMWH (positive duplex: 2 of 20) compared to those on either another anticoagulant or off of chemoprophylaxis (positive duplex: 7 of 20) (any DVT: χ2 = 3.5842, df = 1, P = 0.058) approached statistical significance. Similarly, patients with AIS A on LMWH showed zero proximal DVT compared to those off of LMWH (proximal DVT: 3 of 20 (15.0%)) (χ2 = 3.2432, df = 1, P = 0.072). There was no trend noted through Fisher exact test in rates of any (proximal and/or distal) DVT or proximal DVT between those on LMWH versus off of LMWH in patients classified as AIS B (any DVT: χ2 = 0.4943, df = 1, P = 0.482), AIS C (any DVT: χ2 = 0.7402, df = 1, P = 0.390, proximal DVT: χ2=0.3215, df = 1, P = 0.571), or AIS D (any DVT: χ2 = 0.6586, df = 1, P = 0.417, proximal DVT: χ2 = 0.7725, df = 1, P = 0.379).
Sex, level of injury, history of malignancy, IVC filter, and recent lower extremity fracture were not significantly associated with occurrence of any (proximal and/or distal) or proximal DVT based on duplex scan findings (see Table 1).
Of the 22 individuals found with isolated distal DVT, 19 individuals underwent follow up duplex scans for further surveillance. Repeat duplex scans showed 5 of 19 (26.3%) developed new proximal DVT. Chemoprophylaxis for these five individuals included: LMWH (n = 1), UH (n = 1), no chemoprophylaxis (n = 3). Two of three remaining individuals with isolated distal DVT, who were both admitted on LMWH, were transferred out for acute respiratory distress: one was diagnosed with PE radiologically, while the other died of a presumed PE before confirmation testing was performed. Thus, 31.8% of individuals with isolated distal DVT eventually developed thrombus propagation to either proximal DVT or PE (average = 22 days, SD = 32.3). The last individual who did not undergo further duplex surveillance had an IVC filter placed prior to admission to acute rehabilitation (see Figure 1).
Discussion
The 2016 CPG recommend SCI patients not be routinely screened with duplex scans for clinically inapparent DVT on admission to rehabilitation.18 Our findings suggest that some persons, who are at increased risk for DVT, may benefit from duplex screening and further study would be beneficial.
In total, 16.4% (31 of 189) of admissions who underwent a routine duplex scan were found with any (proximal and/or distal) DVT, which falls within previous documented reports. The rate of positive duplex scans for any (proximal and/or distal) DVT is higher than previously reported by Powell et al. (11.6%) which may possibly be due to shorter average time between injury and rehabilitation admission in our study.14 In our acute traumatic SCI population, almost one-third of individuals with a positive initial duplex scan were identified with proximal DVT. Surveillance with duplex scan successfully identified 4.8% of our population that should receive therapeutic anticoagulation for proximal DVT.28 By placing these patients on appropriate pharmacological treatment, PE, a secondary complication with significant morbidity and mortality, could be potentially avoided. In one review, Hiusman et al., reported duplex scans in those clinically suspected of having an acute proximal DVT with high sensitivity (94%) and specificity (98%).27 For individuals found with isolated distal DVT, 20 of 22 underwent further duplex surveillance or imaging for PE detection and almost one-third later developed thrombus propagation with either proximal DVT or PE. In individuals with isolated distal DVT provoked by a transient risk factor, such as acute traumatic SCI, who are at high risk for bleeding, CHEST guidelines recommend serial imaging to evaluate for thrombus extension and to initiate anticoagulation if thrombus extension occurs.28 Compared with proximal DVT evaluation, duplex scans for distal DVT demonstrate lower sensitivity (70%) and high risk of false-positive findings.27 While the accuracy of duplex scans for distal DVT should be considered if making pharmacological changes to management, additional duplex scans are a non-invasive and easy to perform test. Our findings confirm that continued surveillance to monitor for DVT progression is important to identify those who may need long-term anticoagulation in the future.
Prior literature reports immobility as a risk factor for venous thromboembolism.5 We found that more neurologically impaired patients showed a statistically significant increased likelihood of positive duplex scan for any (proximal and/or distal) DVT on routine screening. Moreover, these individuals with more neurological impairment also demonstrated more than twice the rate of positive duplex scans for proximal DVTs compared to AIS D patients. Persons with AIS A, B, and C injuries are considered functionally complete versus those with AIS D injuries.29 Indeed, individuals classified as AIS D are more likely to be ambulating soon after injury.30,31 While our initial hypothesis predicted that motor complete injuries would be associated with DVT occurrence, our findings suggest that the level of mobility or degree of motor sparing determined through the ISNCSCI and the AIS may serve as a risk factor for any DVT.
Additionally, our results found that older age is also associated with increased risk of positive duplex scan for any (proximal and/or distal) DVT on rehabilitation admission. While 27 of 105 (25.7%) of those ≥50 years were found to have a DVT, only 4 of 84 (4.8%) of those <50 years old had positive scans; a rate of DVT more than 5× higher in the older age group. In trauma patients, prior meta-analysis found increased age is a risk factor for venous thromboembolism, although the specific age that this occurs is debatable.32 One prior study had identified older age (≥50 years) was a strong predictor for venous thromboembolism in individuals with acute SCI who received chemoprophylaxis.20 Our findings demonstrated increased risk of DVT in acute traumatic SCI who are 50 years of age and older.
In terms of chemoprophylaxis, only 35.4% of individuals in our study were transferred on LMWH, 38.6% were on another form of chemoprophylaxis and 25.9% were transferred off of pharmacological prophylaxis. Currently, the CPG (2016) and others recommend LMWH for DVT prophylaxis in the acute-care phase of SCI once there is no evidence of active bleeding.18,33 Subgroup analysis by AIS showed that patients with AIS A on LMWH had clinically lower rates of DVT compared to individuals on either another form of chemoprophylaxis or off of chemoprophylaxis. However, we did not find a statistically significant difference between the overall rate of positive duplex scans for any (proximal and/or distal) or proximal DVT between patients admitted on LMWH versus UH. While both a recently published CPG by AOSpine34 as well as a review by Liu et al.2 did not find a significant difference in thromboembolism occurrence between patients on LMWH and UH, the most recent CSCM-CPG18 does not recommend the use of low-dose UH in the prevention of thromboembolism. These findings suggest that further study is needed to determine whether LMWH offers additional protection against developing proximal DVTs regardless of AIS and against developing any (proximal and/or distal) DVT in individuals with complete injuries. Additionally, in our study six individuals were discharged from acute care to rehabilitation on other anticoagulation. Regarding these six persons, four had atrial fibrillation, one had chronic DVTs, and no reason could be determined based on chart review for the last person.
Of the subjects transferred to rehabilitation without chemoprophylaxis, 59.2% (29 of 49) were classified as AIS D and 16.3% (8 of 49) had an initial positive duplex scan for any (proximal and/or distal) DVT. We found a statistically significant association (P = 0.005) between AIS level and use of chemoprophylaxis on admission. Our study showed 34.5% of individuals with AIS D were transferred without chemoprophylaxis (3 of 29 with positive duplex scan (10.3%)). Our overall rate of positive DVT found by routine duplex scan is lower than that previously reported (27.6%) in individuals without chemoprophylaxis.15 The reason for our lower incidence rate may be since more than half of the subjects who did not receive chemoprophylaxis were categorized as AIS D in our study, while only 20% were classified as AIS D in the population studied by Do et al.15 Despite prior recommendations that patients with acute injury, regardless of AIS, should receive pharmacological prophylaxis against thromboembolism once active bleeding has resolved, more than 25% of patients in our study were transferred without any form of chemoprophylaxis.18,33 Although individuals with AIS D show a significantly lower rate of DVT than those with more severe neurological injuries, they are still at increased risk for development of DVT and should receive appropriate chemoprophylaxis. Continued education regarding the importance of chemoprophylaxis in the acute period once hemostasis is achieved is needed.
We did not find a significant association regarding sex, level of injury (paraplegia versus tetraplegia), presence of lower limb fractures, history of malignancy, or IVC filter with a positive admission duplex scan for any (proximal and/or distal) DVT or proximal DVT. However, our lack of significant findings may be secondary to an insufficient sample size. Prior studies demonstrated male sex and lower limb/pelvic fracture were significantly associated with DVT.17
Power for this analysis was estimated post-hoc, given that the study design was a retrospective chart review of existing data and the occurrence of DVT was already known. We ran a series of power calculations for estimating group differences in two independent proportions assuming unequal groups. Using the probabilities of DVT for the overall sample (0.16), for people given LMWH at admission (0.12) and people 50 years and older (0.25), we estimated that group sizes of 20, 15, and 30, respectively were needed to obtain 80% power. That is, the numbers observed in this investigation were adequate to estimate 2-group differences using Chi-Square analysis and in instances of smaller subgroups, the more conservative Fisher’s exact test. However, the sample size precludes these risk factors in a multivariate framework using logistic regression. Additionally, while we did have an adequate sample size to attain a statistically reliable difference in any DVT by LMWH, our sample size is likely underpowered to detect further group differences by DVT.
Limitations of this study include that the duration of chemoprophylaxis in the acute care center prior to transfer was not determined. In trauma patients, delaying chemoprophylaxis beyond 96 h triples the risk of thromboembolic disorders.35 The positive rate of duplex scans in patients on chemoprophylaxis would be affected by how soon chemoprophylaxis was started post injury. In addition, we are unaware if patients who had duplex scans were symptomatic or asymptomatic for DVT.
Conclusion
In persons with acute traumatic SCI at admission to rehabilitation within two weeks of injury, age (≥50 years) and more severe neurological impairment (AIS A, B, and C) are independent risk factors for a positive duplex scan for any (proximal and/or distal) lower extremity DVT. There was no statistically significant association between proximal DVT and severity of neurological injury or age. However, individuals with isolated distal DVTs are at high risk for the development of additional thrombus and would benefit from serial imaging because of the high rate of propagation. Further study is needed to duplicate our findings and to assess further other risk factors for positive duplex screening on rehabilitation admission.
Disclaimer statements
Contributors None.
Funding This study was supported in part by a grant from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR grant number 90SI5026) as well as a SCI Clinical Educational funding grant from the Craig H. Neilsen Foundation.
Conflicts of interest We have no conflicts of interest to declare at this time.
References
- 1.Weingarden SI. Deep venous thrombosis in spinal cord injury. Overview of the problem. Chest. 1992;102:636S–9S. [PubMed] [Google Scholar]
- 2.Liu Y, Xu H, Liu F, Lv Z, Kan S, Ning G, et al. Meta-analysis of heparin therapy for preventing venous thromboembolism in acute spinal cord injury. Int J Surg. 2017;43:94–100. doi: 10.1016/j.ijsu.2017.05.066 [DOI] [PubMed] [Google Scholar]
- 3.Todd JW, Frisbie JH, Rossier AB, Adams DF, Als AV, Armenia RJ, et al. Deep venous thrombosis in acute spinal cord injury: a comparison of 125I fibrinogen leg scanning, impedance plethysmography and venography. Paraplegia. 1976;14:50–7. [DOI] [PubMed] [Google Scholar]
- 4.Merli GJ, Crabbe S, Paluzzi RG and Fritz D.. Etiology, incidence, and prevention of deep vein thrombosis in acute spinal cord injury. Arch Phys Med Rehabil. 1993;74:1199–205. [PubMed] [Google Scholar]
- 5.Anderson FA, Jr. and Spencer FA.. Risk factors for venous thromboembolism. Circulation. 2003;107:I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6 [DOI] [PubMed] [Google Scholar]
- 6.Bandle J, Shackford SR, Kahl JE, Sise CB, Calvo RY, Shackford MC, et al. The value of lower-extremity duplex surveillance to detect deep vein thrombosis in trauma patients. J Trauma Acute Care Surg. 2013;74:575–80. doi: 10.1097/TA.0b013e3182789330 [DOI] [PubMed] [Google Scholar]
- 7.DeVivo MJ, Kartus PL, Stover SL, Rutt RD and Fine PR.. Cause of death for patients with spinal cord injuries. Arch Intern Med. 1989;149:1761–6. doi: 10.1001/archinte.1989.00390080043011 [DOI] [PubMed] [Google Scholar]
- 8.DeVivo MJ, Krause JS and Lammertse DP.. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–9. doi: 10.1016/S0003-9993(99)90252-6 [DOI] [PubMed] [Google Scholar]
- 9.DeLisa JA and Kirshblum S.. A review: frustrations and needs in clinical care of spinal cord injury patients. J Spinal Cord Med. 1997;20:384–90. doi: 10.1080/10790268.1997.11719494 [DOI] [PubMed] [Google Scholar]
- 10.Ho P, Lim HY, Chua CC, Sleeman M, Tacey M, Donnan G, et al. Retrospective review on isolated distal deep vein thrombosis (IDDVT) – A benign entity or not? Thromb Res. 2016;142:11– 16. doi: 10.1016/j.thromres.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 11.Consortium for Spinal Cord Medicine . Prevention of venous thromboembolism in individuals with spinal cord injury. 2nd ed. Washington, DC: Paralyzed Veterans of America. 2009. [Google Scholar]
- 12.Kadyan V, Clinchot DM and Colachis SC.. Cost-effectiveness of duplex ultrasound surveillance in spinal cord injury. Am J Phys Med Rehabil. 2004;83:191–7. doi: 10.1097/01.PHM.0000113401.47681.A6 [DOI] [PubMed] [Google Scholar]
- 13.Kadyan V, Clinchot DM, Mitchell GL and Colachis SC.. Surveillance with duplex ultrasound in traumatic spinal cord injury on initial admission to rehabilitation. J Spinal Cord Med. 2003;26:231–5. doi: 10.1080/10790268.2003.11753689 [DOI] [PubMed] [Google Scholar]
- 14.Powell M, Kirshblum S and O'Connor KC.. Duplex ultrasound screening for deep vein thrombosis in spinal cord injured patients at rehabilitation admission. Arch Phys Med Rehabil. 1999;80:1044–6. doi: 10.1016/S0003-9993(99)90058-8 [DOI] [PubMed] [Google Scholar]
- 15.Do JG, Kim du H and Sung DH.. Incidence of deep vein thrombosis after spinal cord injury in Korean patients at acute rehabilitation unit. J Korean Med Sci. 2013;28:1382–7. doi: 10.3346/jkms.2013.28.9.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green D, Hartwig D, Chen D, Soltysik RC and Yarnold PR.. Spinal cord injury risk assessment for thromboembolism (SPIRATE Study). Am J Phys Med Rehabil. 2003;82:950–6. doi: 10.1097/01.PHM.0000098043.88979.BA [DOI] [PubMed] [Google Scholar]
- 17.Clements R, Churilov L, Wahab AL and Ng LC.. Exploratory analysis of factors associated with venous thromboembolism in Victorian acute traumatic spinal cord-injured patients 2010-2013. Spinal Cord. 2017;55:74–8. doi: 10.1038/sc.2016.94 [DOI] [PubMed] [Google Scholar]
- 18.Prevention of Venous Thromboembolism in Individuals with Spinal Cord Injury: Clinical Practice Guidelines for Health Care Providers, 3rd ed. Consortium for Spinal Cord Medicine. Top Spinal Cord Inj Rehabil. 2016;22(3):209–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schellong SM, Beyer J, Kakkar AK, Halbritter K, Eriksson BI, Turpie AG, et al. Ultrasound screening for asymptomatic deep vein thrombosis after major orthopaedic surgery: the VENUS study. J Thromb Haemost. 2007;5:1431–7. doi: 10.1111/j.1538-7836.2007.02570.x [DOI] [PubMed] [Google Scholar]
- 20.Spinal Cord Injury Thromboprophylaxis Investigators . Prevention of venous thromboembolism in the acute treatment phase after spinal cord injury: a randomized, multicenter trial comparing low-dose heparin plus intermittent pneumatic compression with enoxaparin. J Trauma. 2003;54:1116–24; discussion 25-6. doi: 10.1097/01.TA.0000066385.10596.71 [DOI] [PubMed] [Google Scholar]
- 21.Satiani B, Falcone R, Shook L and Price J.. Screening for major deep vein thrombosis in seriously injured patients: a prospective study. Ann Vasc Surg. 1997;11:626–9. doi: 10.1007/s100169900101 [DOI] [PubMed] [Google Scholar]
- 22.Germing A, Schakrouf M, Lindstaedt M, Grewe P, Meindl R and Mugge A.. Serial compression B-scan and Doppler sonography for the screening of deep venous thrombosis in patients with spinal cord injuries. J Clin Ultrasound. 2010;38:17–20. [DOI] [PubMed] [Google Scholar]
- 23.Haut ER, Noll K, Efron DT, Berenholz SM, Haider A, Cornwell EE, 3rd, et al. Can increased incidence of deep vein thrombosis (DVT) be used as a marker of quality of care in the absence of standardized screening? The potential effect of surveillance bias on reported DVT rates after trauma. J Trauma. 2007;63:1132–5; discussion 5–7. doi: 10.1097/TA.0b013e31814856ad [DOI] [PubMed] [Google Scholar]
- 24.Schwarcz TH, Quick RC, Minion DJ, Kearney PA, Kwolek CJ and Endean ED.. Enoxaparin treatment in high-risk trauma patients limits the utility of surveillance venous duplex scanning. J Vasc Surg. 2001;34:447–52. doi: 10.1067/mva.2001.117146 [DOI] [PubMed] [Google Scholar]
- 25.McKinley WO, Gittler MS, Kirshblum SC, Stiens SA and Groah SL.. Spinal cord injury medicine. 2. Medical complications after spinal cord injury: Identification and management. Arch Phys Med Rehabil. 2002;83:S58–64, S90-8. doi: 10.1053/apmr.2002.32159 [DOI] [PubMed] [Google Scholar]
- 26.Aito S, Pieri A, D'Andrea M, Marcelli F and Cominelli E.. Primary prevention of deep venous thrombosis and pulmonary embolism in acute spinal cord injured patients. Spinal Cord. 2002;40:300–3. doi: 10.1038/sj.sc.3101298 [DOI] [PubMed] [Google Scholar]
- 27.Huisman MV and Klok FA.. Diagnostic management of acute deep vein thrombosis and pulmonary embolism. J Thromb Haemost. 2013;11:412–22. doi: 10.1111/jth.12124 [DOI] [PubMed] [Google Scholar]
- 28.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel report. Chest. 2016;149:315–52. doi: 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 29.Hall KM, Cohen ME, Wright J, Call M and Werner P.. Characteristics of the Functional Independence Measure in traumatic spinal cord injury. Arch Phys Med Rehabil. 1999;80:1471-6. doi: 10.1016/S0003-9993(99)90260-5 [DOI] [PubMed] [Google Scholar]
- 30.Burns SP, Golding DG, Rolle WA, Jr., Graziani V and Ditunno JF, Jr. Recovery of ambulation in motor-incomplete tetraplegia. Arch Phys Med Rehabil. 1997;78:1169–72. doi: 10.1016/S0003-9993(97)90326-9 [DOI] [PubMed] [Google Scholar]
- 31.Kay ED, Deutsch A and Wuermser LA.. Predicting walking at discharge from inpatient rehabilitation after a traumatic spinal cord injury. Arch Phys Med Rehabil. 2007;88:745–50. doi: 10.1016/j.apmr.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 32.Rogers FB, Cipolle MD, Velmahos G, Rozycki G and Luchette FA.. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group. J Trauma. 2002;53:142–64. doi: 10.1097/00005373-200207000-00032 [DOI] [PubMed] [Google Scholar]
- 33.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656 [DOI] [PubMed] [Google Scholar]
- 34.Fehlings MG, Tetreault LA, Aarabi B, Anderson P, Arnold PM, Brodke DS, et al. A clinical practice guideline for the management of patients with acute spinal cord injury: recommendations on the type and timing of anticoagulant thromboprophylaxis. Global Spine J. 2017;7:212S–20S. doi: 10.1177/2192568217702107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nathens AB, McMurray MK, Cuschieri J, Durr EA, Moore EE, Bankey PE, et al. The practice of venous thromboembolism prophylaxis in the major trauma patient. J Trauma. 2007;62:557–62; discussion 62-3. doi: 10.1097/TA.0b013e318031b5f5 [DOI] [PubMed] [Google Scholar]