Abstract
Alcohol is the most widely used addictive substance. Severe alcohol abuse is diagnosed as “alcohol use disorder” (AUD). A common and harmful drinking pattern is binge drinking that elevates a person’s blood alcohol concentration to ≥ 0.08%. Such drinking may be an early indicator of AUD. Opioid misuse and dependence have become worldwide crises. Patterned consumption of various opioids can develop into opioid use disorder (OUD). An intertwined epidemic exists between opioid abuse, alcohol addiction, and binge drinking. Currently, studies on the interaction of AUD and OUD are limited and the underlying mechanisms linking these disorders remains unclear. We reviewed studies on AUD and OUD and utilized Ingenuity Pathway Analysis (IPA) to identify mechanisms of AUD and OUD interaction and potential gene targets for therapeutic agents. According to IPA Canonical Pathways Analysis, Gamma-aminobutyric Acid (GABA) Receptor Signaling, Neuroinflammation Signaling Pathway, Opioid Signaling Pathway and Dopamine-DARPP32 Feedback in cAMP Signaling are potential contributors to the interaction of AUD and OUD.
Keywords: alcohol abuse, alcohol use disorder, ingenuity pathway analysis, opioid dependence, opioid use disorder
1. Introduction
1.1. Binge drinking and alcohol use disorders
Alcohol is the most widely used addictive substance in the world. The effects of drinking depend on the volume consumed, the concentration of ethanol by volume, and the drinking pattern. [1–4]. Alcohol consumption volume is the most commonly used measurement in alcohol research. The overall volmne of alcohol consumed was found to be related to various alcohol-related disease, such as alcohol dependence, unipolar major depression and cirrhosis of the liver [5]. Based on the US Natural Alcohol Survey, Relative Risk (RR) of Mortality in heavy drinkers with ≥ 8 drinks on average was 70% higher than non-heavy drinkers [4]. Drinking patterns are defined in terms of frequency of drinking. One especially harmful pattern is binge drinking, which is defined by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) as drinking that brings a person’s blood alcohol concentration (BAC) to ≥ 0.08 g % after consumption of 5 or more drinks for men or 4 or more drinks for women in about 2 hours [6]. Binge drinking is the most common and deadly pattern of excessive alcohol use in the United States [7–9], being associated with health risks such as alcohol poisoning, alcohol dependence, cancer, and other chronic diseases [10].
Alcohol abuse that becomes severe is called “alcohol use disorder” (AUD) [11]. This chronic relapsing brain disease is characterized by compulsive alcohol use, loss of control over alcohol intake, and a negative emotional state when not drinking [11]. According to the NIAAA, 16 million people in the United States have AUD, of which approximately twice as many are men (9.8 million vs. 5.3 million) [11]. The disorder includes alcohol abuse and alcohol dependence. Alcohol abuse is a pattern of regular alcohol intake that prevents performance of daily tasks. A person with alcohol abuse problems tends to drink too much even in situation where such drinking is physically dangerous to them. Alcohol dependence is a classic addiction: the inability to quit drinking. Binge drinking is part of both disorders.
1.2. Opioid use disorders
Opioids are natural or synthetic chemicals that interact with opioid receptors on the cell surface and reduce feelings of pain. Opioids include the illegal drug heroin, synthetic opioids such as fentanyl, and pain relievers available legally by prescription, such as morphine, oxycodone, hydrocodone, and codeine [12].
Opioids exert their effects by binding to the one or all three (mu, delta, kappa) opioid receptors in an agonistic, antagonistic fashion [13]. Prescription opioids are meant to be used to treat acute pain (such as when recovering from injury or surgery), chronic pain, active-phase cancer treatment, palliative care, and end-of-life care. Many people rely on prescription opioids, under the care of a physician, to help manage their conditions [14]. Opioids also create significant positive reinforcement, increasing the odds that people will continue using them despite negative consequences, leading to abuse or addiction [13]. Patterned consumption causes opioid use disorder (OUD), which if not interrupted is a lifelong disorder with serious consequences such as significant social and physical functional impairment [15, 16], disability, and death [17]. Even if a patient is weaned from the drug, the risk of relapse is high; and patients may well manifest all the features of addiction. OUD involves opioid addiction and opioid dependence. Opioid addiction is characterized by a pathologically compulsive urge to use opioid drugs [18]. Opioid dependence results from taking opioids over a long period of time, such that patients show physical and psychological symptoms of withdrawal when stop taking the drug [19]. Opioid misuse and dependence have increased rapidly worldwide. In 2017, more than 70,200 Americans died from drug overdoses, mostly of opioids, a two-fold annual increase in a decade [20].
1.3. Comorbidity of AUD and OUD
During the last decade, intertwined epidemics of alcohol use and opioid use have emerged [21]. Among 3.1 million patients with OUD and related conditions, AUD is diagnosed 8.4 times more frequently than in patients without OUD, and binge drinking is 5.0 times more common in patients with OUD. According to the report on alcohol and drug use from the Michigan Department of Health and Human Services, among youths in 2011, 28.0% of binge drinkers reported using painkillers (including oxycontin, codeine, and Percocet), whereas only 7.0% of nondrinkers and 15.6% of non-binge drinkers took opioids [22].
There are bi-directional interaction between Alcohol Use Disorder (AUD) and Opioid Use Disorder (OUD). AUD has been found to be associated with increased likelihood of misusing prescription drugs [23, 24]. OUD including opioid misuse may increase the risk of co-occurring AUD [23, 25]. Besides, Comorbid OUD and AUD interfere with medication adherence and carry high morbidity and mortality rates [26]. Clinical and preclinical studies have confirmed interactions of AUD and OUD. For example, Kao analyzed the medical records of 4,143 patients using intravenous patient-control opioid analgesia after abdominal surgery and reported that frequent alcohol drinking was associated with greater opioid demand for control of pain and decreased nausea and vomiting [27]. In preclinical studies, cross-tolerance between alcohol and opioid abuse has been proved [28]. Campbell and colleagues reported that acute administration of alcohol to animals can produce a moderate anti-nociceptive effect through interaction in the central nervous system (CNS) [29].
The mechanism underlying the interaction of AUD and OUD is still a puzzle. In this project, we used a bioinformatics tool to explore the genetic basis and molecular mechanism underlying the correlation between alcohol, and opioid use. Understanding the genetic bases of AUD and OUD will encourage integration of genetic studies into the process of drug administration, as well as improve tailoring patients’ medication according to their medical histories and genetic risk factors [30].
1.4. Ingenuity pathway analysis to predict potential gene network
Ingenuity Pathway Analysis (IPA) is a software application based on computational algorithms that analyzes the functional connectivity of the genes from information in the comprehensive, manually curated Ingenuity Knowledge Base (Qiagen Bioinformatics, Redwood City, CA). IPA helps identify connections between genotypes, phenotypes, diseases, drugs, and drug trials to pinpoint disease-causing mutations, new biomarkers, or the right treatment for a patient [31]. IPA and other bioinformatics tools have been used in basic and clinical studies, in planning and justifying new research, in identifying elements of current studies and unknown questions, systematic analysis of meta-data, and more.
2. Methods
We first conducted a literature search in PubMed, and Science Direct using the terms “alcohol use disorder,” “binge drinking,” “alcohol abuse,” “opioid use disorder,” and “opioid abuse.” Few studies report on direct interaction between AUD and OUD, and only 7 relevant papers were identified and reviewed.
With the goal of identifying genes and pathways important to AUD and OUD, a comprehensive gene network analysis was conducted using IPA. The genes involved in AUD or OUD were identified using the search bar tool in IPA based on IPA Knowledgebase: The gene set related to AUD were identified using the defined terms representing AUD in IPA “alcohol abuse,” “alcoholism,” “alcohol use disorder,” [11]; and the gene set related to OUD were identified using the terms “opioid-related disorders”, “opioid use disorders”, “opioid dependence”, “opioid addiction”, which represented OUD [14]. The genes reported to be associated with any of these conditions were made evident by overlapping the two sets of genes in My Pathways tool. Relations between the genes and defined the diseases were mapped based on 87 studies from IPA Knowledgebase supporting the correlation of the genes with the diseases. The 87 literatures and their relevance to these genes related to AUD and OUD were reviewed and tabulated in Tables 1–6.
Table 1:
Genes reported related to Alcohol Use Disorder (AUD).
| Gene symbol | Finding | Citations |
|---|---|---|
| ABAT, ALDH5A1 | Valproic acid, an inhibitor of ABAT and ALDH5A1, is being used in Phase IV clinical trials to create lorazepam and valproate drugs for the prevention of alcohol use disorder | [89] |
| CACNA2D1, CACNA2D2 | Pregabalin, a binder of human CACNA2D1 and CNA2D2 proteins, is in Phase III as a treatment for AUD | [90] |
| GABRA1, GABRA3, GABRA4, GABRA5 | Pregnenolone, an inhibitor of human GABRA1, GABRA3, GABRA4, and GABRA5 proteins, is in Phase IV as a treatment for alcohol use disorder | [91] |
| Diazepam, a modulator of human GABRA1, GABRA3, GABRA4, and GABRA5 proteins, is in Phase IV with baclofen as a treatment for alcohol use disorder | [92] | |
| Lorazepam, a modulator of human GABRA1, GABRA3, GABRA4, and GABRA5 proteins, is in Phase IV with valproate [valproic acid] to prevent alcohol use disorder | [93–94] | |
| Lorazepam, a modulator of human GABRA1, GABRA3, GABRA4, and GABRA5 proteins, is in Phase IV with carbamazepine as a treatment for alcoholism | [94–95] | |
| GABRB2 | Lorazepam, a modulator of human GABRB2 proteins, is in Phase IV with valproate [valproic acid] to prevent alcohol use disorder | [93–94] |
| Midazolam, a modulator of human GABRB2 proteins, is in Phase IV as supportive care for alcoholism | [96] | |
| Propofol, a modulator of human GABRB2 protein, is in Phase IV as supportive care for alcoholism | [96] | |
| GABRG1, GABRG2 | Chlordiazepoxide, a modulator of human GABRG1 and GABRG2 proteins, is in Phase III as a treatment for alcoholism | [97] |
| Lorazepam, a modulator of human GABRA6, GABRB1, GABRB2, GABRB3, GABRE, GABRG1, GABRG2, GABRG3, and GABRP proteins, is in Phase IV with valproate [valproic acid] to prevent alcohol use disorder | [93–94] | |
| Lorazepam, a modulator of human GABRG1 and GABRG2 proteins, is in Phase IV with carbamazepine as a treatment for alcoholism | [94–95] | |
| Midazolam, a modulator of human GABRG1 and GABRG2, proteins, is in Phase IV as supportive care for alcoholism | [96] | |
| GABRA6, GABRB1, GABRB2, GABRB3, GABRE, GABRG3, GABRP | Lorazepam, a modulator of human GABRA6, GABRB1, GABRB2, GABRB3, GABRE, GABRG1, GABRG2, GABRG3, and GABRP proteins, is in Phase IV with valproate [valproic acid] to prevent alcohol use disorder | [93–94] |
| Lorazepam, a modulator of human GABRA6, GABRB1, GABRB2, GABRB3, GABRE, GABRG1, GABRG2, GABRG3, and GABRP proteins, is in Phase IV with carbamazepine as a treatment for alcoholism | [94–95] | |
| Midazolam, a modulator of human GABRA6, GABRB1, GABRB2, GABRB3, GABRE, GABRG1, GABRG2, GABRG3, and GABRP proteins, is in Phase IV as supportive care for alcoholism | [96] | |
| GAD2 | Valproic acid, an inhibitor of human GAD2 protein, is in Phase II/III as a treatment for alcoholism | [98] |
| GRIN1, GRIN2B, GRIN2A, GRIN2C, GRIN2D, GRIN3A, GRIN3B | Memantine, an antagonist of human GRIN1, GRIN2A, GRIN2B, GRIN2C, GRIN2D, GRIN3A, GRIN3B proteins, is a potential treatment for alcoholism | [99] |
| OPRD1, OPRM1, OPRK1, SIGMAR1 | Naltrexone, an antagonist of human OPRD1, OPRM1, OPRK1, and SIGMAR1 protein, is in Phase III as a component of a treatment for AUD and alcohol abuse | [100] |
| Naltrexone, an antagonist of human OPRD1, OPRM1, OPRK1, and SIGMAR1 proteins, has been approved as a treatment for alcoholism | [94, 101] | |
| SLC6A2, SLC6A3 | Methylphenidate, an inhibitor of human SLC6A2 and SLC6A3 proteins, is in Phase IV as a treatment for alcoholism | [102] |
| Bupropion, an inhibitor of human SLC6A2 and SLC6A3 proteins, is in Phase IV as a treatment for alcoholism | [103] | |
| SLC6A4 | Bupropion, an inhibitor of human SLC6A4 protein, is in Phase IV as a treatment for alcoholism | [103] |
| Mutant human SERT [SLC6A4] gene (unspecified DNA mutation) is associated with alcoholism | [104] | |
| Sertraline, an inhibitor of human SLC6A4 protein, is in Phase IV as a treatment for alcoholism | [105–106] | |
| Escitalopram, an inhibitor of human SLC6A4 protein, is in Phase IV with Ebixa [memantine] as a treatment for alcoholism | [107] |
Table 6:
Genes reported related to opioid addiction.
| Gene symbol | Finding | Citations |
|---|---|---|
| ADRA1A | The SNP substitution (rs486179) mutation in human ADRA1 leads to heroin addiction in humans | [136] |
| DRD1 | Mutant human DRD1 (SNP substitution [rs5326]) is associated with heroin addiction in humans (p=0.01) | [136] |
| GABRB3 | Mutant human GABRB3 (SNP substitution [rs7165224]) is associated with heroin addiction in humans (p=0.01). | [136] |
| GAD 2 | Mutant human GAD2 (SNP substitution [rs8190646]) is associated with heroin addiction in humans (p=0.01). | [136] |
| GRIN2A | Mutant human GRIN2A is associated with heroin addiction in humans | [136] |
| GRIN3A, HTR3A | Methadone, an antagonist of human GRIN3A, HTR3A protein, and mu-opioid receptor, has been approved as a treatment for opioid addiction | [140] |
| OPRD1, OPRM1, OPRK1 | Methadone, an agonist of human OPRD1 protein, has been approved as a treatment for opioid addiction | [140] |
To analyze the functional connectivity of the genes, the gene set was imported into IPA for canonical pathway analysis through accessing pathway enrichment based on the associations documented in the IPA database. Canonical pathways were scored by calculating a ratio of the number of genes that map to the pathway. The pathways were scored on the basis of the negative base10 logarithm of the p value obtained using Fisher’s exact test (−log10 [p value]) [32–34].
3. Results
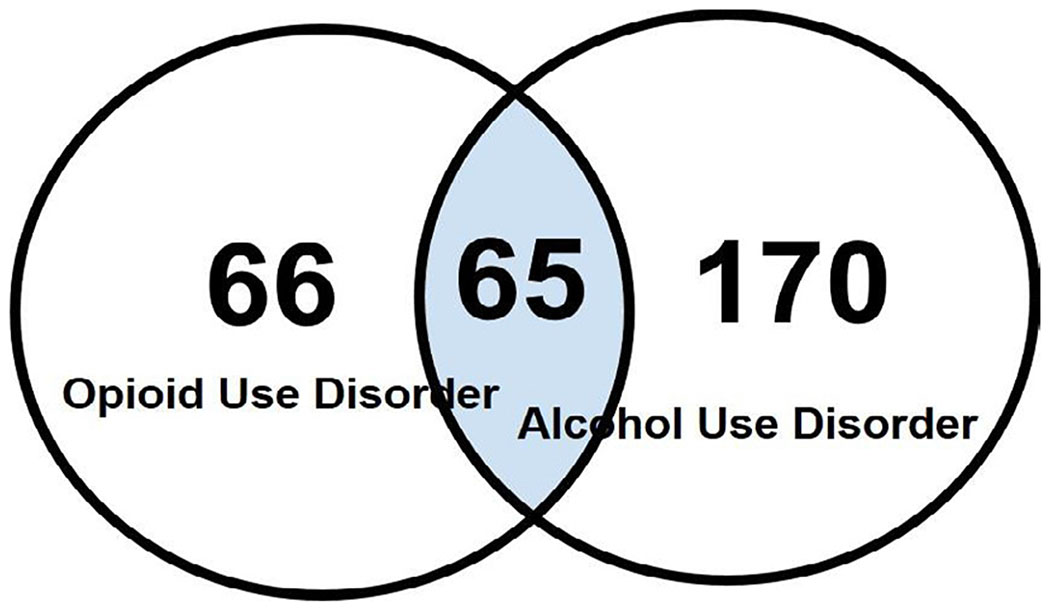
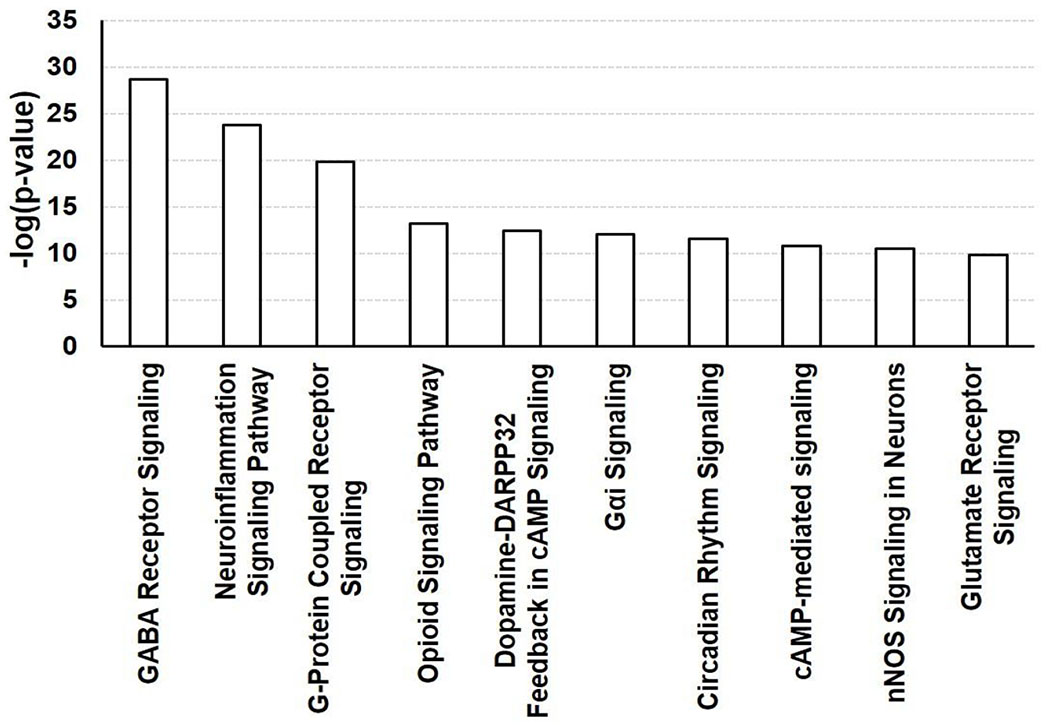
Among the 235 genes related to AUD and the 131 genes related OUD, there were 65 genes related to both disorders. The relations of the 65 genes to AUD (defined tenns “AUD” (Table 1) “alcohol abuse” (Table 2) “alcohol dependence” (Table 3), and “OUD: (defined tenns “OUD” (Table 4), “opioid dependence” (Table 5) and “opioid addiction” (Table 6) were reviewed. The doubly active genes were analyzed further using IPA core analysis, predicting pathways possibly involved in the interaction. Highly enriched canonical pathways were evaluated by p values from Fisher’s exact test. The top ten canonical pathways were Gamma-aminobutyric Acid (GABA) Receptor Signaling, Neuroinflammation Signaling, Opioid Signaling, G-protein Coupled Receptor Signalling, Dopamine-DARPP32 Feedback in cAMP Signaling, Gαi Signalling, Circadian Rhythm Signalling, cAMP-mediated signalling, nNOS Signaling in Neurons, Glutamate Receptor Signalling.
Table 2:
Genes reported related to alcohol abuse.
| Gene symbol | Finding | Citations |
|---|---|---|
| ADRA1A, ADRA1B, ADRA1D, ADRA2A, | Quetiapine and risperidone, both inhibitors of ADRA1A, ADRA1B, ADRA1D, and ADRA2A proteins in humans, are being use in Phase IV clinical trials for the treatment of alcohol abuse | [108–110] |
| Aripiprazole, an inhibitor of ADRA1A, ADRA1B, ADRA1D, and ADRA2A proteins in humans, is being use in a Phase III clinical trial for alcohol abuse treatment | [111] | |
| ADRA2B, ADRA2C | Quetiapine and risperidone, both inhibitors of ADRA1A, ADRA1B, ADRA1D, ADRA2A, ADRA2B, and ADRA2C proteins in humans, are being used in Phase IV clinical trials for the treatment of alcohol abuse | [108–110] |
| CA1, CA3, CA5A, CA5B, CA6, CA9 | Topiramate, an inhibitor of human CA1 protein, is in Phase IV as a part of the combination drug quetiapine and topiramate as a treatment for alcohol abuse. It also is being used alone in a Phase IV clinical trial as treatment for alcohol dependence | [110, 112–113] |
| CA2, CA4 | Trokendi XR [topiramate], an inhibitor of human carbonic anhydrase isozyme II [CA2] protein, is in Phase IV as a part of the combination drug quetiapine and topiramate as a treatment for alcohol abuse and alcohol use disorder | [110,113] |
| DRD1 | Quetiapine, an antagonist of human DRD1 protein, is in Phase IV in combination with topiramate as a treatment for alcohol abuse | [113] |
| SEROQUEL [quetiapine], a blocker of human DRD1 protein, is in Phase IV as a treatment for alcohol abuse | [114] | |
| DRD2 | SEROQUEL XR [quetiapine], a blocker of DRD2 protein, is in Phase IV in combination with topiramate as a treatment for alcohol abuse | [113] |
| Quetiapine fumarate [quetiapine], an antagonist of human DRD2 protein, is in Phase IV as a treatment for alcohol abuse | [108] | |
| Risperidone, an antagonist of human DRD2 protein, is in Phase IV as a treatment for alcohol abuse | [109] | |
| Aripiprazole, an agonist of DRD2 protein, is in Phase III as a treatment for alcohol abuse | [111] | |
| DRD3, DRD4 | Aripiprazole, an agonist of DRD3, DRD4 proteins, is in Phase III as a treatment for alcohol abuse | [111] |
| GABRA 1 | Topiramate, a modulator of human GABRA1 protein, is in Phase IV as treatment for alcohol abuse | [114] |
| Acamprosate, a modulator of human GABRA1 protein, is in Phase IV as a part of the combination drug acamprosate and escitalopram as components of a treatment for alcohol abuse | [113] | |
| GRIN1, GRIN2B, GRIN2A, GRIN2C, GRIN2D, GRIN3A, GRIN3B | Ketamine, an antagonist of human GRIN1, GRIN2B, GRIN2A, GRIN2C, GRIN2D, GRIN3A, and GRIN3B proteins, is in Phase III as a treatment for alcohol abuse | [115] |
| HRH1, HTR2, HTR2B, HTR2C | Risperidone and quetiapine, antagonists of human HRH1, HTR2, HTR2B, and HTR2C proteins, is in Phase IV as a treatment for alcohol abuse | [94, 108–109] |
| HTR1A, HTR2A, HTR2C | Quetiapine, an antagonist of human HTR1A, HTR2A, and HTR2C proteins, is in Phase III/IV as a treatment for alcohol abuse | [94, 111] |
| Aripiprazole, an antagonist of human HTR1A, HTR2A, and HTR2C proteins, is in Phase III/IV as a treatment for alcohol abuse | [111] | |
| OPRD1, OPRM1, OPRK1, SIGMAR1 | Naltrexone, an antagonist of human OPRD1, OPRM1, OPRK1, and SIGMAR1 proteins, is in Phase III as a component of a treatment for alcohol abuse | [100–101] |
Table 3:
Genes reported related to alcohol dependence.
| Gene symbol | Finding | Citations |
|---|---|---|
| CA1, CA3, CA5A, CA5B, CA6, CA9 | Topiramate, an inhibitor of human CA1 protein, is in Phase IV as a treatment for alcohol dependence, along and as a part of the combination drug quetiapine and topiramate | [113, 116–118] |
| Topiramate, an inhibitor of human CA6 protein, is in Phase III as a part of the combination drug for cognitive behavioral therapy | [119] | |
| GABRA1, GABRA3, GABRA4, GABRA5, GABRA6, GABRB2, GABRB3, GABRE, GABRG1, GABRG3, GABRP | Olanzapine, an antagonist of human GABRA1, GABRA3, GABRA4, GABRA5, GABRA6, GABRB2, GABRB3, GABRE, GABRG1, GABRG3, and GABRP proteins, is in Phase III as a treatment for alcohol dependence | [120] |
| GABRB1 | Gamma hydroxybutyric acid [4-hydroxybutanoic acid], an agonist of human GABRB1 protein, is in Phase IV as a treatment for alcohol dependence | [121] |
| Olanzapine, an antagonist of human GABRB1 protein, is in Phase III as a treatment for alcohol dependence | [120] | |
| GABRG2 | Flumazenil, an antagonist of human GABRG2 protein, is in Phase II/III as a part of the combination drug flumazenil and gabapentin as a treatment for alcohol dependence | [122] |
| Olanzapine, an antagonist of human GABRG2 protein, is in Phase III as a treatment for alcohol dependence | [120] | |
| GAD2 | Valproic acid, an inhibitor of human GAD2 protein, is in Phase IV in combination with lorazepam to prevent alcohol dependence | [123] |
| Divalproex(valproic acid), an inhibitor of human GAD2 protein, is in Phase III in combination with lithium and quetiapine fumarate as a treatment for alcohol dependence | [124] | |
| OPRM1 | Mutant human MOR [OPRM1] gene (c.118 A>G) is associated with alcohol dependence in humans (p=0.0074). | [125] |
| SLC6A4 | Escitalopram, an inhibitor of human SLC6A4 protein, is in Phase IV in combination with acamprosate as components of a treatment for alcohol dependence | [114] |
| Fluoxetine, an inhibitor of human SLC6A4 protein, is in Phase IV in combination with naltrexone as a treatment for alcohol dependence | [126] | |
| Brisdelle [paroxetine], an inhibitor of human serotonin transporter [SLC6A4] protein, is in Phase IV as a treatment for alcohol dependence | [127] |
Table 4:
Genes reported related to Opioid Use Disorder (OUD).
| Gene symbol | Finding | Citations |
|---|---|---|
| ADRA2A | Lofexidine and dexmedetomidine, agonists of human ADRA2A protein, are in Phase III as treatments for opioid-related disorders. Lofexidine is especially used in research on opiate dependence treatment | [128] |
| ADRA2B, ADRA2C | Dexmedetomidine, an agonist of human ADRA2B and ADRA2C proteins, is in Phase III as a treatment for opioid-related disorders | [129] |
| CA1, CA2, CA3, CA4, CA5A, CA5B, CA6, CA7, CA9 | Acetazolamide, an inhibitor of human CA1, CA2, and CA3 proteins, is in Phase IV to prevent opioid-related disorders | [130] |
| SLC6A2, SLC6A4 | Tramadol, an inhibitor of human SLC6A2 protein, is in Phase III as a treatment for opioid-related disorders | [131] |
Table 5:
Genes reported related to opioid dependence.
| Gene symbol | Finding | Citations |
|---|---|---|
| ABAT, ALDH5A1 | Valproic acid, an inhibitor of the ABAT protein and ALDH5A1 protein, is in Phase IV to treat opiate dependence | [93] |
| ADRA1A, ADRA1B, ADRA1D, ADRA2A, ADRA2B, ADRA2C | Paliperidone, an antagonist of ADRA1A, ADRA1B, ADRA1D, ADRA2A, ADRA2B, and ADRA2C proteins, is in Phase III to treat heroin dependence | [132] |
| CACNA2D1, CACNA2D2 | Gabapentin, an inhibitor of human CACNA2D1 and CACNA2D2 proteins, is in Phase IV as a treatment for opioid dependence | [133] |
| DRD2 | Buspirone, an agonist of human DRD2 protein, is in Phase IV as a treatment for heroin dependence | [103] |
| DRD2, DRD3, DRD4 | Paliperidone, an antagonist of human DRD2, DRD3, and DRD4 proteins, is in Phase III as a treatment for heroin dependence | [132] |
| GABRA1, GABRA3, GABRA4, GABRA5, GABRA6, GABRB1, GABRB2, GABRE, GABRG1, GABRG2, GABRG3, GABRP | Isoniazid, an inhibitor of human GABRA1, GABRA3, GABRA4, GABRA5, GABRA6, GABRB1, GABRB2, GABRE, GABRG1, GABRG2, GABRG3, and GABRP proteins, is in Phase IV in combination with naltrexone as a treatment for opioid dependence | [134–135] |
| GABRB3 | Isoniazid, an inhibitor of human GABRB3 protein, is in Phase IV in combination with naltrexone as a treatment for opioid dependence | [134–135] |
| Mutant human GABRB3 (SNP substitution [rs7165224]) is associated with heroin addiction in humans (p=0.01). | [136] | |
| GAD2 | Valproic acid, an inhibitor of human GAD2 protein, is in Phase IV in combination with buprenorphine as a treatment for opiate dependence | [89] |
| GRIN1, GRIN2B, GRIN2A, GRIN2C, GRIN2D, GRIN3A, GRIN3B | Ketamine, an antagonist of human GRIN1, GRIN2B, GRIN2A, GRIN2C, GRIN2D, GRIN3A, and GRIN3B proteins, is in Phase IV as a treatment for opiate dependence | [137] |
| Memantine, an antagonist of human GRIN1, GRIN2A, GRIN2B, GRIN2C, GRIN2D, GRIN3A, and GRIN3B proteins, is in Phase II/III as a treatment for opioid dependence | [138] | |
| Memantine, an antagonist of human GRIN1, GRIN2A, GRIN2B, GRIN2C, GRIN2D, GRIN3A, and GRIN3B proteins, is being tested in combination with Vivitrol [naltrexone] as a treatment for opioid dependence | [139] | |
| GRIN3A, HTR3A | Methadone, an antagonist of human GRIN3A and HTR3A proteins and mu-opioid receptor, has been approved as a treatment for opioid dependence | [140] |
| HRH1 | Mirtazapine, a blocker of human HRH1, HTR2A, HTR2B, and HTR2C proteins, is in Phase III as a treatment for heroin dependence | [141] |
| Paliperidone, an antagonist of human HRH1, HTR2A, and HTR2C proteins, is in Phase III as a treatment for heroin dependence | [142] | |
| HTR1A | Buspirone, an agonist of human HTR1A proteins, is in Phase IV as a treatment for heroin dependence | [103] |
| HTR2A, HTR2C | Mirtazapine, a blocker of human HRH1, HTR2, HTR2A, HTR2B, and HTR2C proteins, is in Phase III as a treatment for heroin dependence | [141] |
| Paliperidone, an antagonist of human HRH1, HTR2A, and HTR2C proteins, is in Phase III as a treatment for heroin dependence | [142] | |
| Buspirone, an agonist of human HTR1A, HTR2A, HTR2B, and HTR2C proteins, is in Phase IV as a treatment for heroin dependence | [103] | |
| HTR2B, | Mirtazapine, a blocker of human HRH1, HTR2, HTR2A, HTR2B, and HTR2C proteins, is in Phase III as a treatment for heroin dependence | [141] |
| Buspirone, an agonist of human HTR1A, HTR2A, HTR2B, and HTR2C proteins, is in Phase IV as a treatment for heroin dependence | [103] | |
| OPRM1 | Mutant human OPRM1 (c.118A>G [germline] (rs1799971)) is observed to carry susceptibility to opioid dependence type 1 in humans | [55] |
| Naloxone, an antagonist of human OPRM1 protein, has been approved in the combination Cassipa [buprenorphine/naloxone] (maintenance) as a treatment for opioid dependence | ||
| Naltrexone, an antagonist of human OPRM1 protein, is in Phase IV as a treatment for opioid dependence | [56, 143] | |
| Buprenorphine, antagonist of OPRM1 protein, and naloxone in combination are in Phase IV as a treatment for opioid dependence. | [144] | |
| Hydromorphone, an agonist of human OPRM1 protein, is in Phase III as a component of a treatment for opioid dependence | [145–146] | |
| Buprenorphine, an antagonist of OPRM1 protein, and naloxone in combination are in Phase III as a treatment for heroin dependence | [147–155] | |
| OPRD1, OPRK1 | Naloxone, an antagonist of human OPRD1 and OPRK1 proteins, has been approved as a part of the combination drug Cassipa [buprenorphine/naloxone] (maintenance) as a treatment for opioid dependence | [147] |
| Naltrexone, an antagonist of human OPRD1 and OPRK1 proteins, is in Phase IV as a treatment for opioid dependence | [56, 143] | |
| Buprenorphine, an agonist of human OPRD1 and antagonist of OPRK1 proteins, and naloxone in combination are in Phase IV as a treatment for opioid dependence | [144] | |
| Hydromorphone, an agonist of human OPRD1 and OPRK1 proteins, is in Phase III as a component of a treatment for opioid dependence | [145–146] | |
| Buprenorphine, agonist of human OPRD1 and antagonist of OPRK1 protein, and naloxone and a combination are in Phase III as a treatment for heroin dependence | [147–155] | |
| SIGMAR1 | Naloxone, an antagonist of human SIGMAR1 protein, has been approved as a part of the combination drug Cassipa [buprenorphine/naloxone] (maintenance) as a treatment for opioid dependence | [147] |
| Naltrexone, an antagonist of human SIGMAR1 protein, is in Phase IV as a treatment for opioid dependence | [56, 143] | |
| Hydromorphone, an agonist of human SIGMAR1 protein, is in Phase III as a component of a treatment for opioid dependence | [145–146] | |
| SLC6A2, SLC6A3, SLC6A4 | Bupropion, an inhibitor of human SLC6A2, SLC6A3, and SLC6A4 proteins, is in Phase III as a component of a treatment for opioid dependence | [156] |
4. Discussion
During the last decade, intertwined epidemics of opioid abuse, binge drinking, and alcohol addiction have emerged [21]. The co-existence of these three medical processes could have led to death associated with their comorbidity [26]. Unfortunately, only a few studies have explored the interaction between and among these disorders, and the mechanisms underlying it remain unclear. In this review, we investigated the connection between AUD and OUD, utilizing IPA software application.
Our analysis found that 65 genes are associated with both AUD and OUD, a finding supported by 87 literatures retrieved from IPA Knowledgebase, including clinical studies utilizing corresponding inhibitors (Figure 1 and Table 1). Canonical pathway analysis of these genes predicted pathways likely contributing to the interaction (Figures 2 and 3). Although some of the inhibitors used in the reported clinical studies act on a specific family of genes, which may result in redundant correlations. Canonical Pathways Analysis can help identify relevant genes efficiently. Among the Top 10 pathways predicted to contribute to the interaction of AUD and OUD, the role of Gamma-Aminobutyric Acid (GABA) Receptor Signaling, Nemoinflammation Signaling, Opioid Signalling and Glutamate Receptor Signalling were supported by studies; involvement of Dopamine-DARPP32 Feedback in cAmp Signaling, Circadian Rhythm Signalling, nNOS Signaling in Neurons were understudied; G-protein Coupled Receptor Signalling, Gαi Signalling and cAMP-mediated signalling referred to a general group of pathways highlighting involvement of G-protein, G-α subunit or cAMP, respectively. Thus current findings on the involvement in AUD and OUD of file seven pathways (GABA Receptor Signaling, Neuroinflammation Signaling, Opioid Signalling and Glutamate Receptor Signalling, Dopamine-DARPP32 Feedback in cAMP signaling. Circadian Rhythm Signalling, nNOS Signaling in Neurons) were highlighted (Figure 4).
Figure 1:
Genes that might contribute to interaction of alcohol abuse/AUD and OUD, as identified using IPA.
Figure 2:
Canonical pathway analysis of the genes related to AUD and OUD, as analyzed using IPA core analysis. The gene set was imported into IPA for canonical pathway analysis. Such pathways were scored by analyzing the ratio of the number of genes that map to the pathway. The pathways are presented according to the negative base10 logarithm of the p value obtained using the Fisher exact test.
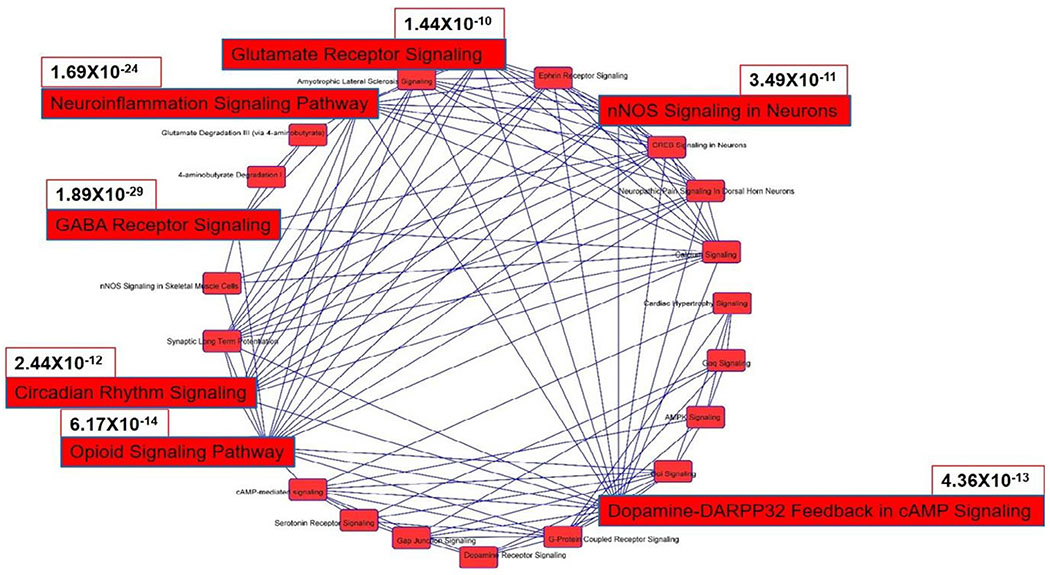
Figure 3:
Overlapping of the canonical pathways predicted to contribute to interaction of AUD and OUD. Interactions between predicted pathways were analysed based on overlap of the genes in the canonical pathways. Seven top pathways are highlighted: Gamma-aminobutyric Acid (GABA) Receptor Signaling, Neuroinflammation Signaling, Opioid Signaling, dopamine-DARPP32 feedback in cAMP signaling, Circadian Rhythm Signalling, nNOS Signaling in Neurons and Glutamate Receptor Signalling. The P values of the 7 pathways are labelled beside the pathway.
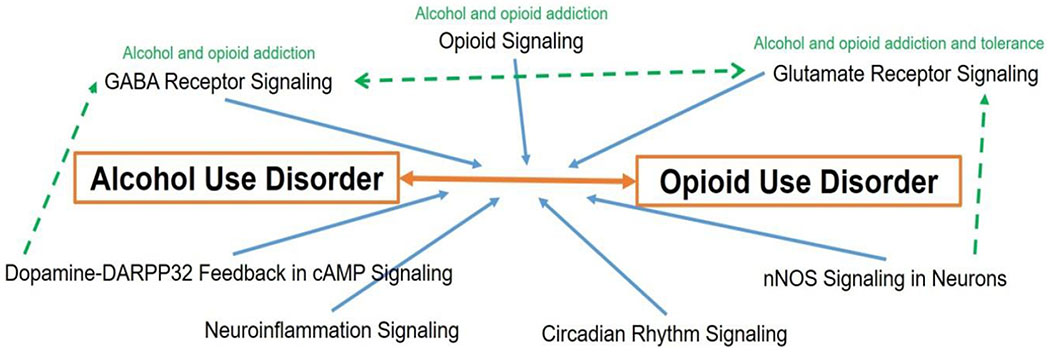
Figure 4:
Predicted contribution of signalling pathways to interaction of AUD and OUD. Orange arrow refers to the interaction of AUD and OUD. Blue arrow refers to predicted involvement of the pathways in the interaction of AUD and OUD. Green dash arrows and green notes refer to correlations between pathways and disorder symptoms supported by literatures.
Gamma-aminobutyric Acid (GABA) Receptor Signaling, Neuroinflammation Signaling, Opioid Signaling, dopamine-DARPP32 feedback in cAMP signaling. Circadian Rhythm Signalling, nNOS Signaling in Neurons and Glutamate Receptor Signalling are predicted to be involved in AUD and OUD interaction in IPA. Literatures reported that GABA Receptor Signalling and Opioid Signalling have been reported to be related to alcohol and opioid addiction; Glutamate Receptor Signalling has been reported to be related to alcohol and opioid addiction and tolerance. It is found that Dopamine-DARPP32 feedback in cAMP signalling cross-talks with GABA Receptor Signaling and that nNOS Signaling in Neurons has cross-talk with Glutamate Receptor Signalling. In addition, cross-talk and regulation between GABA Receptor Signaling and Glutamate Receptor Signalling have been reported. Involvement of Neuroinflammation Signaling and Circadian Rhythm Signalling is unclear.
Alcohol exerts effects on the reward system through both the opioid and dopaminergic systems [35]. GABA Receptor Signaling is involved in both alcohol and opioid addiction [36–38]. Specifically, opioids inhibit GABA-mediated neurotransmission [39], and alcohol exerts its rewarding action through GABA receptors [40, 41]. The GABA receptor agonist baclofen, which inhibits ethanol consumption, decreases alcohol-induced craving and withdrawal symptoms [42–44]. Moreover, a recent study reported that alcohol mediates non-canonical GABA synthesis through aldehyde dehydrogenase 1a1 (ALDH1a1) in midbrain dopaminergic neurons [45].
Glutamate is the most abundant excitatory neurotransmitter in the mammalian nervous system, and acts via glutamate receptors. Both alcohol and mu-opioid are well-known to affect glutamate receptor function. Alcohol is known to inhibit glutamate, particularly at the N-methyl-d-aspartate (NMDA) glutamate receptor [46–48]. And NMDA glutamate receptors associate with mu-opioid and could contribute to opioid tolerance [49–51]. On the other hand, as the excitatory and inhibitory neurotransmitter systems, glutamate signalling and GABA signalling cross-talked and coordinated to maintain brain function [52, 53]. Thus Glutamate Receptor Signalling could be involved in interaction of AUD and OUD directly and indirectly via GABA Receptor Signalling.
Opioid receptor genes have been studied frequently in relation to both alcohol and opioid addiction [54–58]. It is well known that opioid receptors, especially the mu-opioid receptor (MOR), play an important role in the rewarding properties of ethanol [59, 60]. MOR antagonists are mainstays of the treatment of alcohol withdrawal syndrome, as well as OUD [61, 62]. The opioid antagonist naltrexone reduces the desire to drink and the amount of alcohol consumed by alcohol-dependent individuals, implying that it would be an effective treatment for alcohol-dependent subjects [63, 64]. MOR-encoding gene OPRM1 polymorphisms are found to be associated with alcoholism treatment and risk [65]. Adolescents with the A/G or G/G genotypes of A118G are three times more likely to have an AUD than are those with the A/A genotype [66, 67]. The link between Oprm1 polymorphism and alcohol dependence may be explained by different physiological responses to alcohol by persons with the various genotypes. People with the 118G variant have a stronger association between their desire to drink and subsequent drinking than people lacking the variant, an effect attenuated by naltrexone [68]. It is less clear how excessive ethanol consumption affects opioid receptors and downstream signaling. He and Whistler reported that chronic ethanol drinking alters the ability of MOR to endocytose in response to opioid peptides and consequently promotes tolerance to the effects of opioids [69]. And cross-tolerance between alcohol intake and morphine treatment have been proved [28, 69].
Perhaps surprisingly, the inflammation and immune response also is associated with the rewards of alcohol and opioid addiction [34]. Neuroinflammation in the CNS is shared by the response to chronic pain and opioids and contributes to pain sensitization and negative affect [70]. Recent evidence confirms that glial activation and upregulation of inflammatory mediators in the CNS play pivotal roles in neuropathic pain and opioid tolerance. Blocking pro-inflammatory glial activation might block the elevation of dopamine induced by opioid receptor activity. Hutchinson and associates found evidence that Toll-like receptors (TLRs), a class of innate immune receptors, interact with opioids and glial cells, contributing to opioid reward behaviors [71, 72]. Chang et al. [73] created binge exposure to ethanol-enhanced morphine anti-nociception in B6 mice, confirming the role of inflammation [73].
Cyclic AMP-regulated phosphoprotein (DARPP-32) is a key actor of this integration in the GABAergic medium-size spiny neurons, particularly in response to dopamine [74, 75]. Increased cAMP activates cAMP-dependent protein kinase (PKA). Once active, PKA phosphorylates DARPP32 and induces phosphorylation cascades and the dopamine neurotransmission system. DARPP32 act by activating calcium-dependent proteins such as calmodulin and calcineurin [74, 76]. A key role of GABA-mediated dopamine signaling in alcohol and opioid action has been well studied, yet the direct role of dopamine-DARPP32 feedback is unclear.
Circadian rhythm is a roughly 24-hour periodicity in the physiological processes in various organisms, which is endogenously maintained by a series of molecular mechanisms. The circadian rhythm, however, can be moderated by external environment, such as light and temperature. Limited studies have reported effects of alcohol and opioid use on circadian rhythm. Baird et al reported that circadian rhythm of body temperature changed following alcohol treatment [77, 78]. Binge drinking and chronic alcohol use can disrupt circadian rhythms and induce the addictive behaviour associated with AUD [79, 80]. The circadian rhythm is also reported to be affected by acute morphine treatment in rat [81], yet the role of circadian rhythm in OUD remained unclear.
Nitric oxide (NO) is formed endogenously by a family of enzymes known as NO synthases (NOS) Neuronal nitric oxide synthase (nNOS) is a family of enzymes producing Nitric oxide (NO), expressed in specific neurons of the Central Nervous System (CNS) [82]. nNOS is stimulated by free cytosolic Ca2+ through interaction with CALM and Calcineurin. In the brain, NO synthesis is regulated through stimulation of NMDAR by glutamate [83, 84]. NOS expression increased in alcoholic brain [85, 86], and had a protective effect against alcohol toxicity [87] Meanwhile, morphine treatment is known to produce nNOS in a Ca2+-dependent manner [88]. Direct effect of nNOS in interaction of AUD and OUD is unknown.
5. Conclusion
In summary, this review studied AUD and OUD, and predicts potential targets in AUD and OUD interaction based on a bioinformatics tool, IPA. Multiple pathways could contribute to the interaction. According to IPA Canonical Pathways Analysis, GABA Receptor Signaling, Neuroinflammation Signaling Pathway, Opioid Signaling Pathway and Dopamine-DARPP32 Feedback in cAMP Signaling are potential pathways contributing to interaction of AUD and OUD. Our review provides a possible strategy for studying disease interaction and mining drug targets.
7. Acknowledgment
We thank Jarad M Sneed for his assistance on this project. We would also like to thank Seton Hall University, The Office of the Provost and The Office of the Dean, College of Arts and Sciences for their support.
6. Funding
This research is part of the S.H.U. OMICs Course-based Research Laboratory Program, funded by Seton Hall University (SHU) The Office of the Provost and The Office of the Dean, College of Arts and Sciences. JC, TC, and SLC are supported by the SHU Biological Sciences Research Fund. This research was also partially funded by NIH grant awards AA024984, AA025964, AA026071, DA043448 and DA046258 to SLC.
Footnotes
8. Conflict of interest
The authors declare that there is no conflict of interest.
References
- [1].Mukamal KJ, Conigrave KM, Mittleman MA, Camargo CA, Stampfer MJ and Willett WC, et al. , Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men, N Engl J Med, 348 (2003), 109–118. [DOI] [PubMed] [Google Scholar]
- [2].Murray RP, Connett JE, Tyas SL, Bond R, Ekuma O and Silversides CK, et al. , Alcohol volume, drinking pattern, and cardiovascular disease morbidity and mortality: is there a U-shaped function?, Am J Epidemiol,, 155 (2002), 242–248. [DOI] [PubMed] [Google Scholar]
- [3].Shield KD, Parry C and Rehm J, Chronic diseases and conditions related to alcohol use, Alcohol Res, 35 (2014), 155–173. [PMC free article] [PubMed] [Google Scholar]
- [4].Rehm J, Greenfield TK and Rogers JD, Average volume of alcohol consumption, patterns of drinking, and all-cause mortality: results from the US National Alcohol Survey, Am J Epidemiol,153 (2001), 64–71. [DOI] [PubMed] [Google Scholar]
- [5].Rehm J, Room R, Graham K, Monteiro M, Gmel G and Sempos CT, The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview, Addiction, 98 (2003), 1209–1228. [DOI] [PubMed] [Google Scholar]
- [6].N National Institute on Alcohol Abuse and Alcoholism (NIAAA): NIAAA council approves definition of binge drinking. NIAAA Newsletter, (2004). Available from: https://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf
- [7].Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE and Brewer RD, 2010 National and state costs of excessive alcohol consumption, Am J Prev Med, 49 (2015), e73–e79. [DOI] [PubMed] [Google Scholar]
- [8].Esser ΜB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC and Naimi TS, prevalence of alcohol dependence among us adult drinkers, 2009-2011, Prev Chronic Dis, 20 (2014) E206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stahre M, Roeber J, Kanny D, Brewer RD and Zhang X, Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States, Prev Chronic Dis, , 11 (2014), E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Centers for Disease Control and Prevention: Fact Sheets - Binge Drinking, (2019). Available from: https://www.cdc.gov/alcohol/fact-sheets/binge-drinking.htm
- [11].National Institute on Alcohol Abuse and Alcoholism: Alcohol Use Disorder, (2015), https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-use-disorders
- [12].Centers for Disease Control and Prevention: Opioid Overdose, (2019). https://www.cdc.gov/drugoverdose/opioids/terms.html
- [13].Pathan H and Williams J, Basic opioid pharmacology: an update, Br J Pain, 6 (2012), 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].American Psychiatric Association: Opioid Use Disorder, (2018), Available from: https://www.psychiatry.org/patients-families/addiction/opioid-use-disorder/opioid-use-disorder
- [15].Dorahy M, The diagnostic and statistical manual of mental disorders, (5th edn) (DSM-5; ), (2014). [Google Scholar]
- [16].Bawor M, Dennis B, MacKillop J and Samaan Z, Opioid Use Disorder, integrating psychological and pharmacological treatments for addictive disorders, An Evidence-Based Guide, (2017) 124. [Google Scholar]
- [17].American Psychiatric Association, Diagnostic and statistical manual of mental disorders (5th edn.), Arlington, VA: American Psychiatric Publishing, (2013). [Google Scholar]
- [18].American Psychiatric Association: Opioid Use Disorder, (2016). Available from https://www.asam.org/docs/default-source/advocacy/opioid-addiction-disease-facts-figures.pdf
- [19].NIH National Library of Medicine: Opioid Addiction, (2019). Available from https://ghr.nlm.nih.gov/condition/opioid-addiction
- [20].National Institute of Drug Abuse: Overdose Death Rates, (2019). Available from https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates
- [21].Miller JW, Gfroerer JC, Brewer RD, Naimi TS, Mokdad A and Giles WH, Prevalence of adult binge drinking: A comparison of two national surveys, Am J Prev Med, 27 (2004), 197–204. [DOI] [PubMed] [Google Scholar]
- [22].Michigan Department of Health and Human Services: Alcohol/Substance Abuse Epidemiology Program. (2015), Available from https://www.michigan.gov/mdhhs/0,5885,7-339-71548_54783_54784_74886-247299,00.html
- [23].Witkiewitz K, Votaw VR, Vowles KE and Kranzler HR, Opioid misuse as a predictor of alcohol treatment outcomes in the combine study: mediation by medication adherence, Alcohol Clin Exp Res, 42 (2018), 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hughes MR Williams RN. Lipari J. Bose E. P. Copello and Kroutil LA, Prescription Drug Use and Misuse in the United States: results from the 2015 National Survey on Drug Use and Health, (2015). Available from https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR2-2015/NSDUH-FFR2-2015.htm.
- [25].Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H and Jung J, et al. , Nonmedical prescription opioid use and dsm-5 nonmedical prescription opioid use disorder in the United States, J Clin Psychiatry, 77 (2016), 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Saxon J, The Unmet challenges of co-occurring alcohol and opioid use, Alcohol Clin Exp Res, 42 (2018), 1406–1407. [DOI] [PubMed] [Google Scholar]
- [27].Kao SC, Tsai HI, Cheng CW, Lin TW, Chen CC and Lin CS, The association between frequent alcohol drinking and opioid consumption after abdominal surgery: A retrospective analysis, PloS One, 12 (2017), e0171275–e0171275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Le D, Khanna JM, Kalant H and LeBlanc AE, Cross-tolerance between ethanol and morphine, Adv Exp Med Biol, , 132(1980), 771–777. [DOI] [PubMed] [Google Scholar]
- [29].Campbell VC, Taylor RE and Tizabi Y, Effects of selective opioid receptor antagonists on alcohol-induced and nicotine-induced antinociception, Alcohol Clin Exp Res, 31 (2007),1435–1440. [DOI] [PubMed] [Google Scholar]
- [30].Grossman D, Cohen MJ, Manley GT and Butte AJ, Altering physiological networks using drugs: steps towards personalized physiology, BMC medical genomics, 6 Suppl (2013), S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Qiagen: The QIAGEN Knowledge Base, (2016). Available from http://resources.qiagenbioinformatics.com/flyers-and-brochures/QIAGEN_Knowledge_Base.pdf [Google Scholar]
- [32].Yu F, Shen XY, Fan L and Yu ZC, Genome-wide analysis of genetic variations assisted by Ingenuity Pathway Analysis to comprehensively investigate potential genetic targets associated with the progression of hepatocellular carcinoma, Eur Rev Med Pharmacol Sci, 18(2014), 2102–2108. [PubMed] [Google Scholar]
- [33].Zhang-James Y, Femandez-Castillo N, Hess JL, Malki K, Glatt SJ and Cormand B, et al. , An integrated analysis of genes and functional pathways for aggression in human and rodent models, Mol Psychiatry, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Reyes-Gibby CC, Yuan C, Wang J, Yeung SC and Shete S, Gene network analysis shows immune-signaling and ERK1/2 as novel genetic markers for multiple addiction phenotypes: alcohol, smoking and opioid addiction, BMC Syst Biol, 9 (2015), 25–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Costardi JV, Nampo RA, Silva GL, Ribeiro MA, Stella HJ and Stella MB, et al. , A review on alcohol: from the central action mechanism to chemical dependency, Rev Assoc Med Bras, 61 (2015), 381–387. [DOI] [PubMed] [Google Scholar]
- [36].Nickols ΗH and Conn PJ, Development of allosteric modulators of GPCRs for treatment of CNS disorders, Neurobiol Dis, 61 (2014), 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kumar K, Sharma S, Kumar P and Deshmukh R, Therapeutic potential of GABA(B) receptor ligands in drug addiction, anxiety, depression and other CNS disorders, Pharmacol Biochem Behav, 110(2013), 174–184. [DOI] [PubMed] [Google Scholar]
- [38].Blendy JA and Maldonado R, Genetic analysis of drug addiction: the role of cAMP response element binding protein, J Mol Med (Berl), 76 (1998), 104–110. [DOI] [PubMed] [Google Scholar]
- [39].Vaughan CW, Ingram SL, Connor MA and Christie MJ, How opioids inhibit GABA-mediated neurotransmission, Nature, 390(1997), 611–614. [DOI] [PubMed] [Google Scholar]
- [40].Foster KL, McKay PF, Seyoum R, Milboume D, Yin W and Sarma PV, et al. , GABAA and opioid receptors of the central nucleus of the amygdala selectively regulate ethanol-maintained behaviors, Neuropsychopharmacology: Official publication of the American College of Neuropsychopharmacology, 29 (2014), 269–284. [DOI] [PubMed] [Google Scholar]
- [41].Tabakoff B and Hoffman PL. The neurobiology of alcohol consumption and alcoholism: an integrative history, Pharmacol Biochem Behav, 113 (2013), 20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Colombo G, Agabio R, Carai MA, Lobina C, Pani M and Reali R, et al. , Ability of baclofen in reducing alcohol intake and withdrawal severity: I—Preclinical evidence, Alcohol Clin Exp Res, 24 (2000), 58–66. [PubMed] [Google Scholar]
- [43].Colombo G, Addolorato G, Agabio R, Carai MA, Pibiri F and Serra S, et al. , Role of GABA(B) receptor in alcohol dependence: reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics, Neurotox Res,, 6 (2004), 403–414. [DOI] [PubMed] [Google Scholar]
- [44].Maccioni P and Colombo G, Role of the GABA (B) receptor in alcohol-seeking and drinking behavior, Alcohol, 43 (2009), 555–558. [DOI] [PubMed] [Google Scholar]
- [45].Kim JI, Ganesan S, Luo SX, Wu YW, Park E and Huang EJ, et al. , Aldehyde dehydrogenase lal mediates a GABA synthesis pathway in midbrain dopaminergic neurons, Science, 350 (2015), 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Moykkynen T and Korpi ER, Acute effects of ethanol on glutamate receptors, Basic Clin Pharmacol Toxicol, 111 (2012), 4–13. [DOI] [PubMed] [Google Scholar]
- [47].Nagy J, Alcohol related changes in regulation of NMDA receptor functions, Curr Neuropharmacol, 6 (2008), 39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chandrasekar R, Alcohol and NMDA receptor: current research and future direction, Front Mol Neurosci, 6 (2013), 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Garzon J, Rodriguez-Munoz M and Sanchez-Blazquez R, Direct association of Mu-opioid and NMDA glutamate receptors supports their cross-regulation: molecular implications for opioid tolerance, Curr Drug Abuse Rev, 5 (2012), 199–226. [DOI] [PubMed] [Google Scholar]
- [50].Rodríguez-Muñoz M, Sánchez-Blázquez P, Vicente-Sánchez A, Berrocoso E and Garzón J, The mu-opioid receptor and the NMDA receptor associate in PAG neurons: implications in pain control, Neuropsychopharmacology, 37 (2012), 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chartoff EH and Connery HS, It’s MORe exciting than mu: crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system, Front Pharmacol, 5 (2014), 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kantamneni S, Cross-talk and regulation between glutamate and GABAB receptors, Front Cell Neurosci, 9 (2015), 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Popov V, Semenov V, Amakhin D and Vesselkin N, Interaction of Glutamate Receptors and GABA Neurons in the Central Nervous System, Neurosci Behavior Physiol, 47 (2017), 923–929. [Google Scholar]
- [54].Beer B, Erb R, Pavlic M, Ulmer H, Giacomuzzi S and Riemer Y, et al. , Association of polymorphisms in pharmacogenetic candidate genes (OPRD1, GAL, ABCB1, OPRM1) with opioid dependence in European population: a case-control study, PloS one, 8 (2013), e75359–e75359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Compton P, Geschwind DH and Alarcon M, Association between human mu-opioid receptor gene polymorphism, pain tolerance, and opioid addiction, Am J Med Genet B Neuropsychiatr Genet, 121b (2003), 76–82. [DOI] [PubMed] [Google Scholar]
- [56].Drugbank: Naltrexone, (2005). Available from https://www.drugbank.ca/drugs/DB00704
- [57].Kumar D, Chakraborty J and Das S, Epistatic effects between variants of kappa-opioid receptor gene andA118G of mu-opioid receptor gene increase susceptibility to addiction in Indian population, Prog Neuropsychopharmacol Biol Psychiatry, 36 (2012), 225–230. [DOI] [PubMed] [Google Scholar]
- [58].Deb, Chakraborty J, Gangopadhyay PK, Choudhury SR and Das S, Single-nucleotide polymorphism (A118G) in exon 1 of OPRM1 gene causes alteration in downstream signaling by mu-opioid receptor and may contribute to the genetic risk for addiction, J Neurochem, 112(2010), 486–496. [DOI] [PubMed] [Google Scholar]
- [59].Herz, Endogenous opioid systems and alcohol addiction, Psychopharmacology, 129 (1997), 99–111. [DOI] [PubMed] [Google Scholar]
- [60].Sanchis-Segura, Grisel JE, Olive JE, Ghozland S, Koob GF and Roberts AJ, et al. , Role of the endogenous opioid system on the neuropsychopharmacological effects of ethanol: new insights about an old question, Alcohol Clin Exp Res, 29 (2005), 1522–1527. [DOI] [PubMed] [Google Scholar]
- [61].Jarvis P, Holtyn AF, Subramaniam S, Tompkins DA, Oga EA and Bigelow GE, et al. , Extended-release injectable naltrexone for opioid use disorder: a systematic review, Addiction, 113(2018), 1188–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Le Roux, Tang Y and Drexler K, Alcohol and opioid use disorder in older adults: neglected and treatable illnesses, Curr Psychiatry Rep, 18(2016), 87. [DOI] [PubMed] [Google Scholar]
- [63].O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R and Kreek MJ, Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis, Psychopharmacology 160 (2002), 19–29. [DOI] [PubMed] [Google Scholar]
- [64].Volpicelli JR, Alterman AI, Hayashida M and O’Brien CP, Naltrexone in the treatment of alcohol dependence, Arch Gen Psychiatry, 49 (1992), 876–880. [DOI] [PubMed] [Google Scholar]
- [65].Crist RC and Berrettini WH, Pharmacogenetics of OPRM1, Pharmacol Biochem Behav, 5 (2014), 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Miranda R, Ray L, Justus A, Meyerson LA, Knopik VS and McGeary J, et al. , Initial evidence of an association between OPRM1 and adolescent alcohol misuse, Alcohol Clin Exp Res, 34(2010), 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Miranda R Jr, Reynolds E, Ray L, Justus A, Knopik VS and McGeary J, et al. , Preliminary evidence for a gene-environment interaction in predicting alcohol use disorders in adolescents, Alcohol Clin Exp Res, 37 (2013), 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kranzler HR, Armeli S, Covault J and Tennen H, Variation in OPRM1 Moderates the Effect of Desire to Drink on Subsequent Drinking and it’s Attenuation by Naltrexone Treatment, Addict Biol, 18 (2013), 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].He L and Whistler JL, Chronic Ethanol Consumption in Rats Produces Opioid Antinociceptive Tolerance through Inhibition of Mu Opioid Receptor Endocytosis, PloS One, 6 (2011), el9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Cahill CM and Taylor AM, Neuroinflammation-a co-occurring phenomenon linking chronic pain and opioid dependence, Curr Opin Behav Sci,, 13 (2017), 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS and Thomas J, et al. , Opioid activation of toll-like receptor 4 contributes to drug reinforcement, J Neurosci, 32 (2012), 111 87–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bland ST, Hutchinson MR, Maier SF, Watkins LR and Johnson KW, The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release, Brain Behav Immun, 23(2009), 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chang SL, Huang W, Han H and Sariyer IK, Binge-Like Exposure to Ethanol Enhances Morphine’s Anti-nociception in B6Mice, Front Psychiatry, 9 (2019), 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Fernandez R Schiappa JA. Girault and Le Novere N, DARPP-32 is a robust integrator of dopamine and glutamate signals, PLoS Comput Biol, 2 (2006,), el76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Girault JA and Greengard P, The Neurobiology of Dopamine Signaling, Arch Neurol, 61 (2004), 641–644. [DOI] [PubMed] [Google Scholar]
- [76].Bastia and Schwarzschild MA DAPPP chocolate: a caffeinated morsel of striatal signaling, Sci STKE, 2003 (2003), Pe2. [DOI] [PubMed] [Google Scholar]
- [77].Wasielewski JA and Holloway FA, Alcohol’s interactions with circadian rhythms, A focus on body temperature, Alcohol Res Health, 25 (2001), 94–100.a [PMC free article] [PubMed] [Google Scholar]
- [78].Baird TJ, Briscoe RJ, Vallett M, Vanecek SA, Holloway FA and Gauvin DV, Phase-response curve for ethanol: alterations in circadian rhythms of temperature and activity in rats, Pharmacol Biochem Behav, 61 (1998), 303–315 [DOI] [PubMed] [Google Scholar]
- [79].Lindberg L Andres-Beck YF. Jia S. Kang and D S. Choi, Purinergic signaling in neuron-astrocyte interactions, circadian rhythms, and alcohol use disorder, Front Physiol, 9 (2018), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Davis BT IV, Voigt RM, Shaikh M, Forsyth CB and Keshavarzian A, Circadian Mechanisms in Alcohol Use Disorder and Tissue Injury, Alcohol Clin Exp Res, 42 (2018), 668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Pačesová D, Volfová B, Červená K, Hejnová L, Novotný J and Bendová Z, Acute morphine affects the rat circadian clock via rhythms of phosphorylated EPK1/2 and GSK3β kinases and Per1 expression in the rat suprachiasmatic nucleus, Br J Pharmacol, 172 (2015), 3638–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zhou L and Zhu DY Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications, Nitric Oxide, 20 (2009), 223–230. [DOI] [PubMed] [Google Scholar]
- [83].Sánchez-Blazquez P, Rodríguez-Muñoz M and Garzon J, Mu-opioid receptors transiently activate the Akt-nNOS pathway to produce sustained potentiation of PKC-mediated NMDAR-CaMKII signaling, PloS One, 5 (2010), ell278–ell278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].IPAM, nNOS signaling in neurons In, Qiagen, (2019). [Google Scholar]
- [85].Gerlach M, Blum-Degen D, Ransmayr G, Leblhuber F, Pedersen V and Riederer P, Expression, but not activity, of neuronal nitric oxide synthase is regionally increased in the alcoholic brain, Alcohol and Alcohol, 36 (2001), 65–69. [DOI] [PubMed] [Google Scholar]
- [86].Situmorang JH, Lin ΗH, Lo H and Lai CC, Role of neuronal nitric oxide synthase (nNOS) at medulla in tachycardia induced by repeated administration of ethanol in conscious rats, J Biomed Sci, 25(2018), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bonthius DJ, Bonthius NE, Li S and Karacay B, The protective effect of neuronal nitric oxide synthase (nNOS) against alcohol toxicity depends upon the NO-cGMP-PKG pathway and NF-kappaB, Neurotoxicology, 29 (2008), 1080–1091. [DOI] [PubMed] [Google Scholar]
- [88].Toda N, Kishioka S, Hatano Y and Toda H. Modulation of Opioid Actions by Nitric Oxide Signaling, Anesthesiology, 110 (2009), 166–181. [DOI] [PubMed] [Google Scholar]
- [89].Sorlandet Hospital HF: Treatment of polydrug-using opiate dependents during withdrawal, (2003). Identifier: NCT00367874, Available from https://clinicaltrials.gov/ct2/show/NCT00367874 [Google Scholar]
- [90].University of Maryland College Park, National Institute on Alcohol Abuse and Alcoholism: Pharmacogenetic treatment with anti-glutaminergic agents for comorbid PTSD & AUD, (2016). Identifier: NCT02884908, https://ClinicalTrials.gov/show/NCT02884908 [Google Scholar]
- [91].University of Texas Southwestern Medical Center: Aripiprazole for bipolar disorder and alcohol use disorder, (2016). Identifier: NCT02918370, https://ClinicalTrials.gov/show/NCT02918370
- [92].Universitair Ziekenhuis Brussel: Baclofen in managing acute alcohol withdrawal, (2016). Identifier: NCT03293017, https://ClinicalTrials.gov/show/NCT03293017
- [93].CAMC Health System: Assessment of valproate on ethanol withdrawal, (2017). Identifier: NCT03235531, Available from https://ClinicalTrials.gov/show/NCT03235531 [Google Scholar]
- [94].Skidmore-Roth L, Mosby’s drug guide for nursing students, Elsevier, (2018). [Google Scholar]
- [95].National Institute on Alcohol Abuse and Alcoholism, Drug therapy for alcohol detoxification, (2000). Available from:http://clinicaltrials.gov/ct2/show/NCT00000441
- [96].Virginia Commonwealth University, Pilot study evaluating stress response and immune function in mechanically ventilated patients with alcohol use disorders treated with propofol or midazolam, (2009). Available from: https://clinicaltrials.gov/ct2/show/NCT00871039 [Google Scholar]
- [97].Hvidovre University Hospital, Outpatient treatment of alcohol withdrawal syndrome, (2006). Identifier: NCTOO136617, Available from: https://clinicaltrials.gov/ct2/show/NCT00136617. [Google Scholar]
- [98].Seattle Institute for Biomedical and Clinical Research, Abbott: Placebo controlled trial of depakote ER in alcohol dependent patients with mood and/or anxiety symptoms, (2004). Identifier: NCT00202514, Available from: https://clinicaltrials.gov/ct2/show/NCT00202514. [Google Scholar]
- [99].National Institute for Health and Welfare Finland, Finnish Foundation for Alcohol Studies: Efficacy study of memantine hydrochloride and escitalopram for the treatment of co-morbid depression and alcoholism, (2005). Identifier: NCT00368862, Available from: https://clinicaltrials.gov/ct2/show/NCT00368862.
- [100].National Institute on Alcohol Abuse and Alcoholism: Medication and counseling for controlled drinking (Project SMART), (2006). Identifier: NCT00006204, Available from https://clinicaltrials.gov/ct2/show/NCT00444418
- [101].DrugBank: Naltrexone, (2005). Available from https://www.drugbank.ca/drugs/DB00704
- [102].National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health Clinical Center, Treatment of patients with alcoholism and attention deficit disorder, (2007). Identifier: NCT00261872, Available from: https://clinicaltrials.gov/ct2/show/NCT00261872
- [103].National Institute on Alcohol Abuse and Alcoholism, Bupropion as a smoking cessation aid in alcoholics, (2002). Identifier: NCT00044434, Available from: https://clinicaltrials.gov/ct2/show/NCT00044434
- [104].Lin Z, Canales JJ, Bjorgvinsson T, Thomsen M, Qu H and et al. , Monoamine transporters: vulnerable and vital doorkeepers, Prog Mol Biol Transl Sci, 98 (2011), 1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].University of Pennsylvania: Sertraline for alcohol dependence and depression, (2000). Identifier: NCT00004554, Available from https://clinicaltrials.gov/ct2/show/NCT00004554
- [106].Uconn Health, National Institute on Alcohol Abuse and Alcoholism: Sertraline pharmacotherapy for alcoholism subtypes, (2004). Identifier: NCT00368550, Available from: https://clinicaltrials.gov/ct2/show/NCT00368550
- [107].Uconn Health, National Institute on Alcohol Abuse and Alcoholism: Sertraline pharmacotherapy for alcoholism subtypes, (2004). Available from https://ClinicalTrials.gov/show/NCT00156715 [Google Scholar]
- [108].Uconn Health, National Institute on Alcohol Abuse and Alcoholism: Sertraline pharmacotherapy for alcoholism subtypes, (2004). Identifier: NCT00368550, Available from: https://clinicaltrials.gov/ct2/show/NCT00368550 [Google Scholar]
- [109].Janssen LP, Dartmouth-HitchcockMedical Center, Risperidone long-acting versus oral risperidone in patients with schizophrenia and alcohol use disorder, (2005). Identifier: NCT00156715, Available from: https://ClinicalTrials.gov/show/NCT00156715 [Google Scholar]
- [110].University of Cincinnati, National Institute on Alcohol Abuse and Alcoholism: Efficacy and tolerability of topiramate in treatment of bipolar mania and alcohol use in adolescents and young adults, (2008). Identifier: NCT00550394, Available from: http://ClinicalTrials.gov/show/NCT00550394
- [111].University of Texas Southwestern Medical Center, Aripiprazole for bipolar disorder and alcohol use disorder, (2016). Identifier: NCT02918370, Available from: https://ClinicalTrials.gov/show/NCT02918370
- [112].Winum JY, Vullo D, Casini A, Montero JL, Scozzafava A and Supuran CT, Carbonic anhydrase inhibitors, inhibition of cytosolic isozymes i and ii and transmembrane, tumor-associated isozyme ix with sulfamates including emate also acting as steroid sulfatase inhibitors, J Med Chem, 46 (2003), 2197–2204. [DOI] [PubMed] [Google Scholar]
- [113].University of California San Francisco, United States Department of Defense, US Department of veterans affairs: topiramate treatment of alcohol use disorders in veterans with post-traumatic stress disorder (ptsd): a pilot controlled trial of augmentation therapy, (2010). Identifier: NCT01087736, Available from: https://ClinicalTrials.gov/show/NCT01087736
- [114].Massachusetts General Hospital, National alliance for research on schizophrenia and depression: a double-blind, placebo-controlled study of acamprosate added to escitalopram and behavioral treatment in major depressive disorder (mdd) with comorbid alcohol abuse/dependence, (2007). Identifier: NCT00452543, Available from: https://clinicaltrials.gov/ct2/show/NCT00452543
- [115].The University of Texas Health Science Center Houston: Trial of the rapid antisuicidal effects of intranasal ketamine in comorbid depression and alcohol abuse, (2018). Identifier: NCT03539887, Available from https://clinicaltrials.gov/ct2/show/NCT03539887 [Google Scholar]
- [116].Chiang Mai University, Topiramate for hospitalized patients with alcoholism: a 12-week study, (2010). Identifier: NCT01135602, Available from: https://clinicaltrials.gov/ct2/show/NCT01135602
- [117].Elizabeth R, Yale University: The use of anticonvulsants for treatment of patients with alcohol dependence and post-traumatic stress disorder, (2016). Identifier: NCT00571246, Available from: https://clinicaltrials.gov/ct2/show/NCT00571246
- [118].University of California San Francisco, United States Department of Defense, San Francisco Veterans Affairs Medical Center, Northern California Institute of Research and Education: A controlled trial of topiramate treatment for alcohol dependence in veterans with PTSD, (2013). Identifier: NCTO1749215, Available from: https://clinicaltrials.gov/ct2/show/NCT01749215
- [119].Bankole J, National institute on drug abuse, University of Virginia: novel pharmacotherapy for dual dependence, (2013). Identifier: NCT00448825, Available from: https://www.clinicaltrials.gov/ct2/show/NCT00448825
- [120].The Mind Research Network, A new pharmacotherapy for alcohol dependence: olanzapine, (2010). Identifier: NCT00746785, Available from: https://clinicaltrials.gov/ct2/show/NCT00746785
- [121].Catholic University of the Sacred Heart, CT Pharmaceutical Industries, Sanremo - Italy, University of Bologna, Medical University of Vienna: Comparative study of gamma-hydroxy butyrate versus oxazepam in the treatment of alcohol withdrawal syndrome, (2002), Identifier: NCT02090504, Available from: https://clinicaltrials.gov/ct2/show/NCT02090504
- [122].Medical University of South Carolina: Prometa protocol for alcohol dependence, (2005). Identifier: NCT00262639, Available from: https://clinicaltrials.gov/ct2/show/NCT00262639
- [123].Sorlandet Hospital HF, Treatment of polydrug-using opiate dependents during withdrawal, (2003). Identifier: NCT00367874, Available from: https://clinicaltrials.gov/ct2/show/NCT00367874
- [124].AstraZeneca, Efficacy and Safety of quetiapine fumarate (SEROQUEL®) in the treatment of alcohol dependency in patients with bipolar disorder, (2006). Identifier: NCT00114686, Available from: https://clinicaltrials.gov/ct2/show/NCT00114686
- [125].Bart G, Kreek MJ, Oh J, LaForge KS, Proudnikov D and Poliak L, Increased attributable risk related to a functional mu-opioid receptor gene polymorphism in association with alcohol dependence in central Sweden, Neuropsychopharmacology, 30 (2005), 417–422. [DOI] [PubMed] [Google Scholar]
- [126].National Institute on Alcohol Abuse and Alcoholism: Drug treatment for depressed alcoholics (Naltrexone/Fluoxetine), (2000). Identifier: NCT00006204, https://clinicaltrials.gov/ct2/show/NCT00006204
- [127].Medical University of South Carolina, National Institute on Alcohol Abuse and Alcoholism: Paroxetine for comorbid social anxiety disorder and alcoholism, (2008). Identifier: NCT00246441, Available from: https://clinicaltrials.gov/ct2/show/NCT00246441 [Google Scholar]
- [128].US Department of Veterans Affairs, National Institute on Drug Abuse, US World Meds LLC, VA Office of Research and Development: Lofexidine for inpatient opiate detox, (2006). Identifier: NCT00235729, Available from: https://ClinicalTrials.gov/show/NCT00235729
- [129].Rennes University Hospital: Postoperative and opioid free anesthesia, (2017). Identifier: NCT03316339, Available from: hhps://ClinicalTrials.gov/show/NCT03316339
- [130].University of Chile: Administration of acetazolamide to prevent remifentanil induced hyperalgesia, (2016). Identifier: NCT02992938, Available from: https://ClinicalTrials.gov/show/NCT02992938
- [131].University of Alabama at Birmingham, Comparison of three opioid detoxification treatment regimens, (2019). Identifier: NCT03678792, Available from: https://clinicaltrials.gov/ct2/show/NCT03678792
- [132].University of Science Malaysia, Yale University: An open-label study of oral paliparidone for the treatment of patients with co-occurring opioid and ATS dependence, (2013). Identifier: NCT02541513, Available from: https://ClinicalTrials.gov/show/NCT02541513
- [133].Brigham and Women’s Hospital, Gabapentin regimens and their effects on opioid consumption, (2018). Identifier: NCT03334903, Available from: https://ClinicalTrials.gov/show/NCT03334903
- [134].Artico M, Corelli F, Massa S, Stefancich G, Avigliano L and Befani O, et al. , Inhibition of copper-dependent amine oxidases by some hydrazides of pyrrol-1-ylbenzoic and pyrrol-1-ylphenylacetic acids, J Med Chem, 31 (1988), 802–806. [DOI] [PubMed] [Google Scholar]
- [135].Yale University, University of Malaya, National Institute on Drug Abuse: Addiction, HIV and tuberculosis in Malaysian criminal justice settings, (2017). Identifier: NCT03089983, Available from: https://ClinicalTrials.gov/show/NCT03089983
- [136].Levran O, Londono D, O’Hara K, Randesi M, Rotrosen J and Casadonte P, et al. , Heroin addiction in African Americans: a hypothesis-driven association study, Genes Brain Behav, 8 (2009), 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Vilnius University, Effects of Ketamine on precipitated opioid withdrawal under general anaesthesia, (2003). Identifier: NCT00300794, Available from: https://clinicaltrials.gov/ct2/show/NCT00300794
- [138].University of Massachusetts, Worcester, National Institute on Drug Abuse: Memantine-enhanced buprenorphine treatment for opioid-dependent young adults, (2009). Identifier: NCT01052662, Available from: hhps://clinicaltrials.gov/ct2/show/NCT01052662
- [139].New York State Psychiatric Institute, National Institute on Drug Abuse: Memantine as a supplement to naltrexone in treating heroin dependence, (2008). Identifier: NCT00476242, Available from: https://clinicaltrials.gov/ct2/show/NCT00476242
- [140].DrugBank: Methadone, (2005), Available from https://www.drugbank.ca/drugs/DB00333
- [141].University of Science Malaysia, Yale University: Mirtazapine as a treatment for co-occurring opioid and ATS dependence in Malaysia, (2013). Identifier: NCT02541526, Available from: hhps://clinicaltrials.gov/ct2/show/NCT02541526
- [142].University of Science Malaysia, Yale University: An open-label study of oral paliparidone for the treatment of patients with co-occurring opioid and ATS dependence, (2013). Identifier: NCT02541500, Available from: https://clinicaltrials.gov/ct2/show/NCT02541500
- [143].Alkermes Inc, A study of vivitrol in the prevention of re-arrest and re-incarceration, (2011). Identifier: NCT01453374, Available from: https://clinicaltrials.gov/ct2/show/NCT01453374
- [144].National Institute on Drug Abuse, New York MDRU: Buprenorphine/Naloxone in the treatment of heroin dependence-14, (1999). Identifier: NCT00015340, Available from: https://clinicaltrials.gov/ct2/show/NCT00015340
- [145].Gharagozlou P, Hashemi E, DeLorey TM, Clark JD and Lameh J, Pharmacological profiles of opioid ligands at kappa opioid receptors, BMC Pharmacol, 6 (2006), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].University of British Columbia, Canadian Institutes of Health Research, Providence Healthcare, Innerchange Charitable Society: Study to assess longer-term opioid medication effectiveness (SALOME), (2011). Identifier: NCT01447212. Available from: https://clinicaltrials.gov/ct2/show/results/NCT01447212
- [147].National Institute on Drug Abuse, New York MDRU, Buprenorphine and naloxone for the treatment of opiate dependence-1, (1996). Identifier: NCT00015171, Available from: https://clinicaltrials.gov/ct2/show/NCT00015171
- [148].University of California Los Angeles, National Institute on Drug Abuse: Buprenorphine/Naloxone versus clonidine for outpatient opiate detoxification – 1, (2001). Identifier: NCT00032968, Available from: https://clinicaltrials.gov/ct2/show/NCT00032968
- [149].University of California Los Angeles, National Institute on Drug Abuse: Buprenorphine/Naloxone versus clonidine for inpatient opiate detoxification – 1, (2001). Identifier: NCT00032955, Available from: https://clinicaltrials.gov/ct2/show/NCT00032955
- [150].National Institute on Drug Abuse, Study comparing liquid and tablet buprenorphine formulations – 5, (2005). Identifier: NCT00000302, Available from: https://clinicaltrials.gov/ct2/show/NCT00000302
- [151].University of Pennsylvania, National Institute on Drug Abuse, St. Petersburg State Pavlov Medical University: Addiction treatment in Russia: Oral vs. Naltrexone Implant, (2006). Identifier: NCT00218426, Available from: https://clinicaltrials.gov/ct2/show/NCT00218426
- [152].New York State Psychiatric Institute, National Institute on Drug Abuse: Behavioral naltrexone therapy for promoting adherence to oral naltrexone vs. extended release injectable depot naltrexone, (2007). Identifier: NCT00577408, Available from: http://clinicaltrials.gov/ct2/show/NCT00577408
- [153].Friends Research Institute Inc, National Institute on Drug Abuse: Buprenorphine for prisoners, (2008). Identifier: NCT00574067, Available from: https://clinicaltrials.gov/ct2/show/NCT00574067
- [154].New York State Psychiatric I, Alkermes I, Buprenorphine stabilization and induction onto vivitrol for heroin-dependent individuals, (2018). Identifier: NCT03711318. Available from: https://clinicaltrials.gov/ct2/show/NCT03711318
- [155].New York University Langone Health, National institute on drug abuse, friend’s research institute inc, university of California los angeles: extended-release naltrexone opioid treatment at jail Re-Entry, (2014). Identifier: NCT01999946, Available from: http://clinicaltrials.gov/ct2/show/NCT01999946
- [156].University of Maryland College Park, Smoking cessation interventions for people living with HIV in Nairobi, Kenya, (2019). Identifier: NCT03342027. Available from: http://clinicaltrials.gov/ct2/show/NCT03342027