Abstract
Objective: The objective of the present work was to determine the prognostic validity of the trunk control test for walking and independence in individuals with SCI.
Design: A cohort, prospective study was carried out in all individuals with sub-acute SCI.
Setting: All inpatients at the Mexico City based National Rehabilitation Institute (INR).
Participants: Ninety individuals with a clinical diagnosis of sub-acute SCI, American Spinal Injury Association Impairment Scale (AIS) A-D, and that have not participated in a rehabilitation program were included. Thirty-five individuals had good initial trunk control and the remaining 55 had poor trunk control. All individuals participated in a standard rehabilitation program subsequently.
Interventions: N/A
Outcome Measures: The trunk control test was performed at baseline. At 1, 3, 6, 9 and 12 months after the first evaluation, walking and independence were assessed.
Results: Survival Analysis revealed that 62.5% and 100% individuals with good trunk control at baseline assessment were respectively walking and independent in ADL at 12 months and 14% and 48% individuals with poor trunk control were walking and independent in ADL. Cox regression analysis revealed that individuals with good trunk control were 4.6 times more likely to walk independently at 12 months and 2.9 times more likely to be independent in activities of daily living.
Conclusion: The present study revealed that the trunk control test is useful for providing a prognosis of independence and walking at 1 year in individuals with SCI, independently of the neurologic level and the severity of the injury.
Keywords: Spinal cord injuries, Trunk control, Prognosis
Introduction
The damage resulting from a spinal cord injury (SCI) affects conduction of sensory and motor signals across the site of lesion, as well as the autonomic nervous system.1,2 Worldwide, the incidence of SCI is calculated at between 768 473 and 790 695 cases per year.3 The economic impact of SCI in developed countries, the high rate of mortality in developing countries, and the etiology of SCI reveal the significance of this pathology.4,5
Injury to the ascending and descending pathways of the spinal cord triggers an alteration in the posture control system.6 It is known that efficient control of posture is of utmost importance for standing and walking,7 as well as for providing support for voluntary movements.8,9 Alterations in postural control depend on the degree and level of the injury. Therefore, individuals with complete, thoracic, or cervical injuries have poor trunk control, consequently, many of their movements are limited in terms of carrying out daily tasks.10,11 Thus, part of the objectives of rehabilitation in individuals with SCI is improvement in trunk control, in order to achieve independence in daily tasks and, in specific cases, to be able to walk.9–13
In fact, the ability to walk is of critical interest, for the patient and his or her family, as well as for planning the objectives of personalized rehabilitation treatment.14,15 Diverse studies have been developed with the intent to arrive at a prognosis of walking in SCI. The most recent of these and that with the highest methodological quality is that of Van Middendorp and collaborators.16 This is a multicenter cohort study in which a clinical prediction rule was established based on age and certain neurological variables to determine the probability of walking in individuals with acute traumatic SCI. These authors demonstrated that age, combined with strength in quadriceps, soleus and gastrocnemius muscles and light touch of dermatomes L3 and S1, are criteria that can discriminate among those who will walk independently and those who will walk with aids, or those who will not walk. Their results are in agreement with those of prior studies that have determined that the evaluation of muscular strength in the lower limbs is useful for predicting walking in individuals with acute SCI.17,18 The main limitation of these studies in clinical practice is that they are based on assessments conducted in the first 72 hours after the injury, which is not always possible in rehabilitation services. In addition, they are not useful for establishing short-term rehabilitation goals in the first weeks after injury, or for predicting functionality. This brings out the need for a scale that has a prognostic ability regardless of the time of application (arrival to rehabilitation service) and that can help to establish treatment goals through time.
On the other hand, trunk control is directly related to the ability to walk, and its predictive validity has been proven in other nervous-system pathologies. Notably in stroke the usefulness of trunk control clinical scales for establishing the prognosis of walking has been demonstrated.19–22 Furthermore, it has been proven that trunk control scales are helpful to predict independence in ADL in children23 and adults21,22 with neurological disabilities.
To our knowledge, there are no trunk control scales to date that assess individuals with SCI and are predictive of independence and walking.
In previous work,24 a clinical trunk control test was proposed, and its reliability and validity for assessing patients with a SCI of any type and of any neurological level were demonstrated. In fact, the test has a high inter- and intra- observer reliability (0.99 and 0.98, respectively). In addition, adequate content, construct, and criteria validities were determined. Regarding criteria validity, the test possesses 98% sensitivity and 92.2% specificity for discrimination in individuals with adequate or inadequate trunk control.24 Two of the principal objectives of the use of a clinical test comprise discrimination and prediction. The discriminative capacity of a test is important to ensure its ability to differentiate between groups of patients and to identify the capacities of the patients. The discriminative capacity of the clinical trunk control test for individuals with SCI has been demonstrated.24 On the other hand, a predictive measure is used to predict a result or prognosis. Predicting a result in an early phase allows the clinician to establish a therapeutic program, set goals, facilitate a discharge plan, and anticipate the need to eliminate architectural barriers. However, the prognostic validity of the clinical trunk control test in individuals with SCI has not been demonstrated. Thus, the objective of the present work is to determine whether the clinical trunk control test in individuals with SCI possesses a prognostic value at 1, 3, 6, 9 and 12 months of the first evaluation in terms of walking indoors for <10 feet and independence in all indoor and outdoor activities of daily living assessed by the Spinal Cord Independence Measure-III(SCIM-III).
Methods
Study design
This was an observational, comparative, longitudinal, and prospective (cohort) study.
Description of the population
Participants were recruited from patients of the Spinal Cord Injury Service, Neurological Rehabilitation Division, at the National Institute of Rehabilitation (INR) in Mexico City, who were hospitalized in the subacute stage (1 week to 3 months post-injury) who had not participated in a rehabilitation program.
Inclusion criteria
We included male and female patients, older than 18 years, who had a clinical diagnosis of a SCI of any American Spinal Injury Association Impairment Scale (AIS) grade and neurological level of injury (NLI) with traumatic etiology, and less than 3 months of duration, who had not received treatment in a rehabilitation program, and who signed letter of informed consent.
Elimination criterion
Participants who did not present for assessment after the test was performed were eliminated from the study.
Exclusion criteria
We excluded individuals with another neurological diagnosis, visual, hearing or mental impairment, or an orthopedic, metabolic, or cardiovascular condition that could impede test performance.
Procedure
As part of the customary care procedure, the participants were admitted for inpatient intensive rehabilitation treatment. Medical personnel trained in the evaluation and management of SCI obtained the individuals’ clinical and demographic variables.
Independent variables
Demographic variables included age and sex. Clinical variables included: SCI severity determined by AIS category,18 NLI defined as most caudal segment of the cord with intact sensation and antigravity muscle function strength,18 and duration of injury defined as time in days from the SCI at the moment of applying the questionnaire for the descriptive analysis, and dichotomized in <1 month or >1 month to carry out Cox regression analysis.
Additionally, the trunk control test was performed once the participants were able to move without restrictions (orthopedic, cardiovascular or metabolic). The test was performed in the physical therapy area. Participants were seated on a 50 cm in height mattress with an area of 2 × 2.5m, with feet on a supporting surface, knees flexed at 90°, without trunk support, and hands resting on thighs. Briefly, the test is divided into three domains: static control, dynamic control, and dynamic control with upper-limb activities (for more details see supplementary material). Score ranged between 0 and 24 points. A cut-off point to separate individuals with adequate and inadequate trunk control of 13 points was established in a previous study.24
Outcome measures
The SCIM-III was used to assess walking and independence at 1, 3, 6, 9 and 12 months. As suggested by Van Middendorp,16 we used the SCIM-III item 12 (ability to walk < 10 feet) to assess walking function. To distinguish between individuals who could walk indoors independently and those who could not, a cutoff SCIM indoor mobility score was applied; scores 0–3 were grouped and defined as unable to walk or dependent on assistance while walking and scores 4–8 were grouped and defined as able to walk independently.16
The seventeen SCIM-III items for independence were assessed. These SCIM-III items range from total assistance to independently with adaptive devices, to independent without adaptive devices. A cut-off point for each item was set in each item to distinguish between individuals who performed the item independently (with or without adaptive devices or special settings) and those who required assistance (Table 1).
Table 1. Description of the study variables and their measurement scales.
| Independent variables | Operational definition | Unit/Values | |
|---|---|---|---|
| Age | |||
| Sex | |||
| Duration of injury | Days for the descriptive analysis Dichotomized in 0 = less than a month and 1 = one month or morefor the regression analysis |
||
| SCI severity | AIS Category, with ISNCSCI modifications18 | A, B, C, D, E | |
| SCI NLI | AIS Category, with ISNCSCI modifications18 | C, HT, LT, L, S | |
| Clinicaltrunk control test | Maximal score achieved in the trunk control test24 | Score 0 to 24 points 0–12: Poor trunk control 13–24: Goodtrunk control |
|
| Depression | Score obtained on the Beck inventory25 | 0–16: no depression >16: depression |
|
| Dependent variables |
Operational definition |
Unit/Values |
|
| Walking |
Score obtained on the item 12 of the SCIM-III26,27 |
0–3: unable to walk 4–8:able to walk independently |
|
| Independence | Score obtained in the SCIM-III26,27 | Dependent | Independent |
| Item 17 Items 10, 11, 16 Items 1, 4, 15 Item 8 Item 9 Item 2 Items 3, 12, 13, 14 Item 7 Item 6 Item 5 |
0 0–1 0–1 0–2 0–2 0–3 0–3 0–5 0–6 0–8 |
1 2 2–3 4–5 4–6 4–6 4–8 8–10 9–15 10 |
|
SCI, spinal cord injury; ASIA, American Spinal Injury Association; AIS, ASIA Impairment Scale; ISNCSCI, International Standards for Classification of the Spinal Cord Injury; AIS, ASIA Impairment Scale; NLI, neurologic level of injury; C, cervical; HT, high thoracic; LT, low thoracic; L, lumbar; S, sacral; SCIM-III, Spinal Cord Independence Measure-III.
Statistical analysis
Sample size
The Epidat 4 software program (Santiago de Compostela, A Coruña Spain) was used for calculating the sample size. The following preliminary results were considered.
Walking
In a preliminary analysis, a total of 67% of individuals with good trunk control (initial score on the trunk control test of 13 or more) presented walking at 1 year, while 21.6% of the individuals with poor trunk control presented walking in this period. With these data, 18 patients with good trunk control and 18 patients with poor trunk control were required for 80% study power, with a probability of an alpha error of less than 0.05.
Independence
In a preliminary analysis, a total of 89% and 46% of good and poor trunk control individuals, respectively, achieved independence at 1 year. With these data, 18 individuals with good trunk control and 18 individuals with poor trunk control were required for 80% study power, with a probability of an alpha error of less than 0.05.
Considering the possibility of a 20% reduction in participants due to attrition, we decided to recruit 22 patients in each group, for a total of 44 patients.
Statistical analysis was performed using of the SPSS/PC ver. 20 statistical software program. The results were considered statistically significant for P < 0.05.
Descriptive statistics were performed with frequencies for qualitative variables and with mean and standard deviation (SD) for quantitative variables.
The prognostic value of the trunk control test for walking and independence (as dependent variables of the result) was evaluated through application of the survival analysis. The survival analysis examined the survival of “not walking” and “no independence”. In this way, using the survival function estimator with the Kaplan-Meier method, the probabilities of walking and independence at 1, 3, 6, 9 and 12 months were obtained.28 Differences between curves were evaluated by log-rank test.
Afterward, to estimate the probability of walking and independence according to the baseline score of the trunk control test, controlling for possible confounders (patient age and sex, type and level of injury, depression, duration of injury), the Cox proportional risk analysis was conducted to derive hazard ratios adjusted in multivariate models by using the exponential of the regression coefficients.29
The decision to use survival analysis was based on the importance of time in the establishment of rehabilitation goals. In fact, the short, medium and long term goals of the programs in spinal cord injury rehabilitation vary through time, notably the first year.
Table 1 describes the analyzed variables.
Results
Descriptive analysis
Ninety patients were recruited, among whom35 had a good trunk control (≥13 test score) and 55 had a poor trunk control (<13 test score).
The average age of the patients was 32.2 years (range, 18–73 years). In the group with good trunk control, the average age was 32.1years (SD. 12.7) and in the group with poor trunk control, 32.2 years (SD, 12.9 years). There was no statistically significant difference between the groups.
The average time since injury was 23 days (range, 1–82 days). In the group with good trunk control, the average duration of injury was 21.75 days (SD, 5.12 days), while in the group with poor trunk control, it was 24.89 days (SD, 5.86 days). There was no statistically significant difference between the groups.
Most participants were men (71.1%), with a similar distribution when analyzed by group (62.5% in group with the good trunk control and 78% in the group with poor trunk control).
Regarding the SCI, most patients presented with high cervical injuries (37.8%) followed by low thoracic (25.6%), high thoracic (20%), low cervical (13.3%), and lumbar(3.3%) injuries. Most participants presented with complete injuries (AIS A, 52.2%), followed by AIS C (18.9%), AIS D (17.8%), and AIS B (11.1%). In the case of SCI severity, there were significant differences with regards to trunk control between AIS groups. (Table 2). This was expected, because during validation of the trunk control test, a strong association was found between the scoring on this test and these variables.
Table 2. Clinical Characteristics of the Groups.
| Total | Good trunk control | Poor trunk control | P | |
|---|---|---|---|---|
| Age (mean, SD) | 32.2 (12.8) | 32.1 (12.7) | 32.2 (12.9) | 0.952* |
| Sex | ||||
| Men | 71.1% | 62.5% | 78% | 0.084’ |
| Women | 28.9% | 37.5% | 22% | |
| Duration of injury (mean, SD) | 29.4 (22.9) | 25.4 (20.5) | 32.3 (24.4) | 0.176* |
| AIS | ||||
| A | 52.2% | 37.5% | 64% | |
| B | 11.1% | 10% | 12% | 0.001’ |
| C | 18.9% | 15% | 22% | |
| D | 17.8% | 37.5% | 1% | |
| NLI | ||||
| HC | 37.8% | 54% | 17.5% | 0.001’ |
| LC | 13.3% | 16% | 10% | |
| HT | 20% | 16% | 25% | |
| LT | 25.6% | 14% | 40% | |
| L | 3.3% | 0% | 7.5% | |
| Depression | 13.6% | 16.6% | 10% | 0.271 |
*T Test, ‘Chi square.
ASIA, American Spinal Injury Association; AIS, ASIA Impairment Scale; NLI, neurologic level of injury; C, cervical; HT, high thoracic; LT, low thoracic; L, lumbar.
Survival analysis
Walking
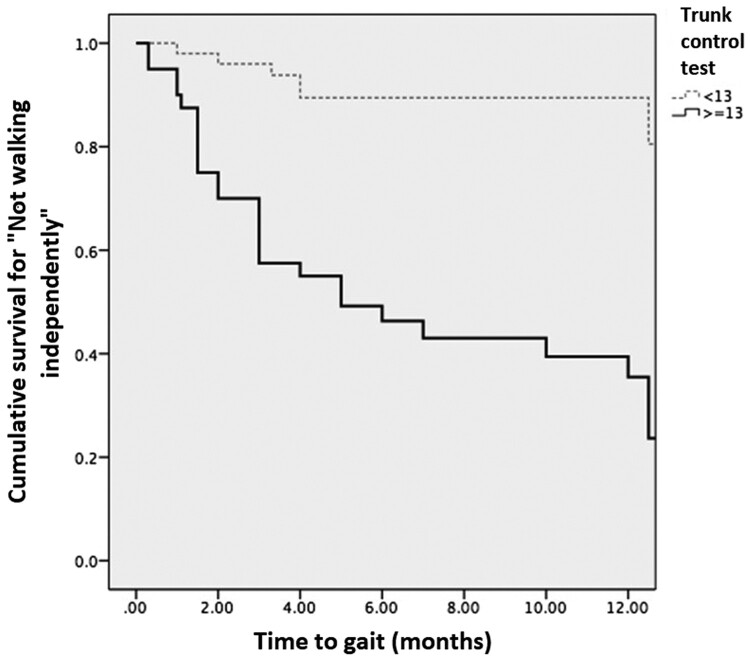
As may be observed in Figure 1, the probability of walking if a participant had good trunk control was greater that than those with poor trunk control. In effect, 62.5% of individuals with good initial trunk control achieved walking at 1 year in comparison with 14% of individuals who initially had poor trunk control. The average time of walking in the good trunk control group was 6.73 (SD, 0.94) months, and in the poor trunk control group, the average time was 12.25 months (SD, 0.53). A statistically significant difference between the curves was found by log-rank test (P = 0.001).
Figure 1.
Kaplan-Meier curve for gait. The light, dotted line represents individuals with poor trunk control and the thick one represents individuals with good trunk control at baseline. Since the censed event is gait, the lines show the cumulative survival “free of gait”. The two lines separate from the first month and at the end of the study, the minority of individuals with good trunk control at baseline did not walk, or, in other words, the majority of them achieved walking. In contrast, the majority of individuals in the poor control group did not walk.
Achieving walking relative to the baseline score in the trunk control test, and control for possible confounders, a Cox proportional risk analysis was carried out. The ordinal variables were factorized by creation of dummy variables, and the AIS A injury was used as a reference. As shown in Table 3, the probability of walking at 1 year with an AIS B injury was three times greater than with an AISA injury (P = 0.145), while for AIS C, the probability was 8.29 times greater than with an AIS A (P = 0.001), and for AIS D, this was 56.1 times greater than with an AIS A (P = 0.001). With regard to NLI, the cervical level was employed as a reference. With the latter, it was found that the probability of achieving gait in 1 year in individuals with a lumbar level injury was 5 times greater than with a cervical injury (P = 0.029). Finally, in an independent manner, patients with good trunk control had a 4.59 times greater probability of walking within 1 year than those with poor trunk control (P = 0.001). This shows that the AIS grade of D is the most important predictor of walking at 1 year.
Table 3. Probability of walking at 1 year for each study variable.
| Parameter estimate | Standard error | Statistical Significance (p) | Risk Ratio | |
|---|---|---|---|---|
| AIS | 0.001 | |||
| BvsA | −3.95 | 1.105 | 0.001 | 3.001 |
| CvsA | 2.115 | 0.619 | 0.001 | 8.290 |
| DvsA | 4.027 | 0.767 | 0.001 | 56.102 |
| NLI | 0.045 | |||
| CvsHT | 0.535 | 0.565 | 0.344 | 0.642 |
| CvsLT | 0.483 | 0.557 | 0.386 | 1.621 |
| CvsL | 1.633 | 0.746 | 0.029 | 5.117 |
| Age | 0.006 | 0.019 | 0.760 | 1.006 |
| Sex | −0.65 | 0.360 | 0.071 | 0.522 |
| Clinical trunk control test | 1,532 | 0.471 | 0.001 | 4.587 |
| Depression | 0.006 | 433 | 0.843 | 0.918 |
| Duration of injury (<1 month vs >1 month) | −0.457 | 0.595 | 0.443 | 0.633 |
ASIA, American Spinal Injury Association; AIS, ASIA Impairment Scale; NLI, neurologic level of injury; C, cervical; HT, high thoracic; LT, low thoracic; L, lumbar.
Independence
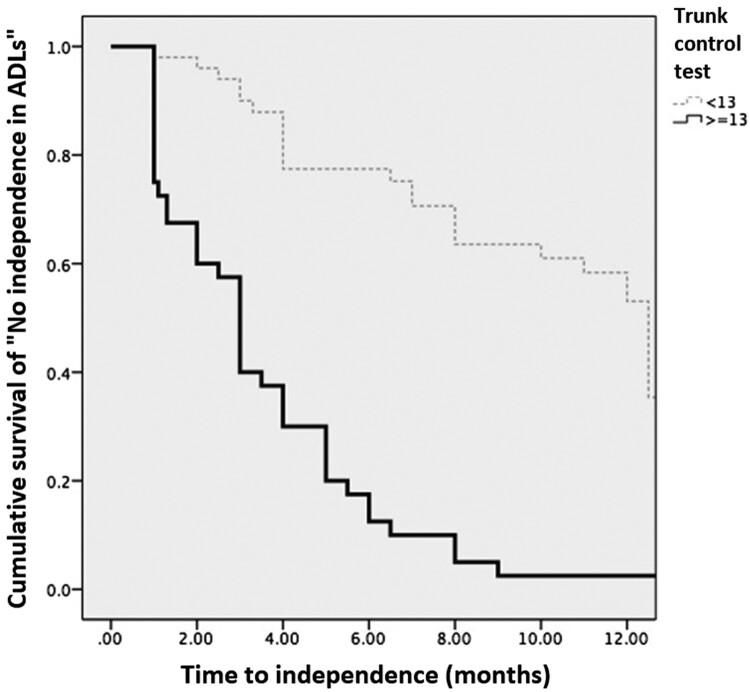
The probability of attaining independence in ADLs was greater in individuals with good trunk control compared to those with poor trunk control, which is depicted in Figure 2. In fact, 100% of individuals with good trunk control on their initial evaluation achieved independence at 1-year follow-up, while only 48% of those with poor trunk control achieved independence in that time period. The average time for achieving independence in the group with good trunk control was 3.37 (SD, 0.44) months, and in the group with poor trunk control, it was 9.48 months (SD, 0.61). The difference between the curves was statistically significant using the log-rank test (P = 0.001).
Figure 2.
Kaplan-Meier curve for independence. The light, dotted line represents individuals with poor trunk control and the thick one represents individuals with good trunk control at baseline. Since the censed event is independence, the lines show the cumulative survival “free of independence”, or dependent in all activities of daily living (ADL). The two lines separate from the first month and at the end of the study, none of the individuals with good trunk control at baseline were dependent on ADL, in other words all of them achieved independence in ADL. In contrast, the majority, but not all, of the individuals in the poor control group achieved independence in ADL.
The probability of attaining independence in ADLs in 1 year was analyzed relative to AIS category. Using AIS A as a reference, the probability of individuals with AIS C was 2.85 times greater than in those with an AIS A injury (P = 0.005), and with AIS D it was3.91 times greater than with a complete injury (P = 0.002).In relation to the NLI, using cervical level as the reference, the probability of achieving independence was 7.99 times greater with a low lumbar injury (P = 0.004), 3.41 times greater with a low thoracic injury (P = 0.001), and 2.53 times greater with a high thoracic injury (P = 0.014). Finally, individuals with good trunk control had a 2.8 times greater possibility of being independent than those with a poor trunk control score (P = 0.003). This shows that the lumbar level is the most important predictor of independence at one year (Table 4)
Table 4. Probability of being independent at 1 year for each study variable.
| Parameter estimate | Standard error | Statistical significance (p | Risk Ratio | |
|---|---|---|---|---|
| AIS | 0.005 | |||
| BvsA | 0.327 | 0.437 | 0.455 | 1.386 |
| CvsA | 1.048 | 0.374 | 0.005 | 2.853 |
| DvsA | 1.365 | 0.450 | 0.002 | 3.915 |
| NLI | ||||
| CvsHT | 0.930 | 0.380 | 0.014 | 2.534 |
| CvsLT | 1.226 | 0.376 | 0.001 | 3.409 |
| CvsL | 2.078 | 0.730 | 0.004 | 7.992 |
| Age | 0.005 | 0.011 | 0.681 | 1.005 |
| Sex | -−0.060 | 0.295 | 0.839 | 0.942 |
| Clinical trunk control test | 1.048 | 0.348 | 0.003 | 2.851 |
| Depression | −0.586 | 318 | 0.065 | 0.556 |
| Duration of injury (<1 month vs >1 month) | −0.187 | 0.410 | 0.648 | 0.829 |
ASIA, American Spinal Injury Association; AIS, ASIA Impairment Scale; NLI, neurologic level of injury; C, cervical; HT, high thoracic; LT, low thoracic; L, lumbar.
Discussion
In the clinical environment, integral management of persons with a SCI implies that feasible goals are established based on the objective evaluation of each patient.30 Determining the AIS grade of SCI, remaining muscular function, and NLI is essential within this objective evaluation, and their prognostic values have been demonstrated.12,13 In the present work, we demonstrated that the trunk control test for individuals with SCI is also predictive for walking and independence in all indoor and outdoor daily tasks considered in the SCIM-III instrument, independently of injury type and NLI. Our findings support that this test is a useful tool in the evaluation as well as in the establishment of management objectives.
Recovering the ability to walk is one of the main expectations of individuals with SCI, and one of the objectives of health care personnel who care for these persons. As previously mentioned, the need to evaluate the patient in the first 72 hours of the injury limits the prognostic value of the prediction rule established by Van Middendorp and collaborators.16 While it has been reported that in high-income countries, the great majority of patients arrive at the rehabilitation facility within this time period,30 in medium- and low-income countries, this is not common. Thus, determining clinical tests that aid in predicting functional outcomes on arrival at rehabilitation-service facilities is still needed. In the present work, a grade of AIS D was determined to be the principal prognostic factor for recovery of walking, which is in agreement with reports in the literature.14,16 NLI (low lumbar) was the second most important predictive factor for the recovery of walking. However, it was found that the initial score on the trunk control scale aids in predicting this function independently of the AIS grade and the NLI.
While the pathophysiology of alterations in trunk control and the mechanisms of neurological recovery are different in cerebral and spinal cord pathology, improvement in posture control comprises an essential objective for the management of both. Whit regards to stroke, the prognostic value for walking of different variables has been studied. With the latter, it has been shown that improvement in impairments related to deviations in walking, principally trunk balance and, to a lesser degree, pelvic-limb strength, aid in predicting the functional limitations associated with walking.31 Because assessment of balance in individuals with stroke is very useful not only for prognosis, but also for a complete and objective evaluation, the clinical scales that evaluate trunk control form part of the tools recommended as clinical practice guidelines for the management of these patients.20,22,32 It has been found that trunk control is an early predictor of functioning in daily tasks, which implies that early evaluation and the timely initiation of trunk control after stroke should be emphasized.33
In the present work, we demonstrated that persons with acute SCI with good trunk control have a greater probability of achieving the ability to perform daily tasks without the support of another person compared with those who had poor trunk control initially, independently of the AIS grade and NLI. Another interesting finding is that the most important predictive variable in carrying out daily tasks was the NLI below T12, which is congruent with the findings of other authors.34
The main limitation of this study is that the evaluations were not conducted at the same time in all patients. Although most patients were evaluated in the first month of injury, the time for carrying out the test comprised a variable that was unable to be controlled. In fact, individuals with any AIS grade and any NLI were included, therefore some of the tests had to be postponed until the participants were stable. Because the purpose of the study was to achieve greater external validity, practically all of the individuals with SCI were included, irrespective of their clinical characteristics. The trunk control test cannot be performed if the spinal column is unstable, or if the patient has cardiovascular, pulmonary, or orthopedic complications, which warrant postponing the evaluation.
Conclusions and future perspectives
The trunk control test is a useful tool for evaluating individuals with SCI, as well as for establishing a prognosis for walking and independence in activities of daily living at 1 year.
It will be necessary to evaluate walking and independence in more specific ways, such as with the use of other items of the SCIM-III and the Walking Index for Spinal Cord Injury.
It will be important to investigate a variable that combines AIS and NLI, which would be more relevant with regard to prognosis and guiding clinicians with regards to setting appropriate goals and treatment plan.
In clinical practice, it is important to establish cut-off points on the scale that help to establish specific management objectives, both in the short- and medium-term, such as if and when the individual will be able to dress himself or herself or use a light-weight wheelchair.
Supplementary Material
Acknowledgements
We thank the entire team at the Neurological Rehabilitation Department from the National Institute of Rehabilitation (INR) and the residents as part of the entire health personnel at this National Institute for their support.
Disclaimer statements
Contributors None.
Funding None.
Declaration of interest None.
Conflict of Interest Statement The Authors declare that there is no conflict of interest.
ORCID
Paola C. Fratini-Escobar http://orcid.org/0000-0002-6482-4565
References
- 1.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. , International standards for neurological classification of spinal cord injury (Revised 2011). J Spinal Cord Med 2011;34(6):535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karimi MT. Functional walking ability of paraplegic patients: comparison of functional electrical stimulation versus mechanical orthoses. Eur J Orthop Surg Traumatol 2013;23(6):631–38. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R, Lim J, Mekary R, Rattani A, Dewan MC, Sharif SY, et al. Traumatic spinal injury: global epidemiology and worldwide volume. World Neurosurg 2018;113:e345–63. [DOI] [PubMed] [Google Scholar]
- 4.Of A. Spinal cord injury facts and figures at a glance. J Spinal Cord Med 2014;37(4):479–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee BB, Cripps RA, Fitzharris M, Wing PC.. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord 2014;52(2):110–16. [DOI] [PubMed] [Google Scholar]
- 6.Reft J, Hasan Z.. Trajectories of target reaching arm movements in individuals with spinal cord injury: effect of external trunk support. Spinal Cord 2002;40(4):186–91. [DOI] [PubMed] [Google Scholar]
- 7.Horak FB, Wrisley DM, Frank J.. The Balance Evaluation System Test (BESTest) to differenciate balance deficits. Phys Ther 2009;89:484–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CL, Yeung KT, Bih LI, Wang CH, Chen MI, Chien JC.. The relationship between sitting stability and functional performance in patients with paraplegia. Arch Phys Med Rehabil 2003;84:1276–81. [DOI] [PubMed] [Google Scholar]
- 9.Filed-Fote EC, Ray SS.. Seated reach distance and trunk excursion accurately reflect dynamic postural control in individuals with motor-incomplete spinal cord injury. Spinal Cord 2010;48:745–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, et al. , Retraining the injured spinal cord. J Physiol 2001;533:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curt A, Van Hedel HJA, Klaus D, Dietz V, Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J Neurotrauma 2008;25(6):677–85. [DOI] [PubMed] [Google Scholar]
- 12.Kirshblum SC, Priebe MM, Ho CH, Scelza WM, Chiodo AE, Wuermser LA, Spinal cord injury medicine. 3. Rehabilitation phase after acute spinal cord injury. Arch Phys Med Rehabil 2007;88(3suppl 1):S62–70. [DOI] [PubMed] [Google Scholar]
- 13.Ditunno PL, Patrick M, Stineman M, Ditunno JF.. Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord 2008;46(7):500–06. [DOI] [PubMed] [Google Scholar]
- 14.Marinho AR, Flett HM, Craven C, Ottensmeyer CA, Parsons D, Verrier MC, Walking-related outcomes for individuals with traumatic and non-traumatic spinal cord injury inform physical therapy practice. J Spinal Cord Med 2012;35(5):371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marino RJ, Graves DE.. Metric properties of the ASIA motor score: Subscales improve correlation with functional activities. Arch Phys Med Rehabil 2004;85(11):1804–10. [DOI] [PubMed] [Google Scholar]
- 16.van Middendorp JJ, Hosman AJF, Donders ART, Pouw MH, Ditunno JF, Curt A, et al. , A clinical prediction rule for ambulation outcomes after traumatic spinal cord injury: a longitudinal cohort study. Lancet 2011;377(9770):1004–10. [DOI] [PubMed] [Google Scholar]
- 17.Oleson CV, Burns AS, Ditunno JF, Geisler FH, Coleman WP.. Prognostic value of pinprick preservation in motor complete, sensory incomplete spinal cord injury. Arch Phys Med Rehabil 2005;86(5):988–92. [DOI] [PubMed] [Google Scholar]
- 18.Anderson K, Aito S, Atkins M, Biering-Sørensen F, Charlifue S, Curt A, et al, Functional recovery measures for spinal cord injury: an evidence-based review for clinical practice and research. J Spinal Cord Med 2008;31(2):133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montecchi MG, Muratori A, Lombardi F, Morrone E, Briant K.. Trunk recovery scale: A new tool to measure posture control in patients with severe acquired brain injury. A study of the psychometric properties. Eur J Phys Rehabil Med 2013;49(3):341–51. [PubMed] [Google Scholar]
- 20.Feigin L, Sharon B, Czaczkes B, Rosin AJ.. Sitting equilibrium 2 weeks after a stroke can predict the walking ability after 6 months. Gerontology 1996;42:348–53. [DOI] [PubMed] [Google Scholar]
- 21.Duarte E, Marco E, Muniesa JM, Belmonte R, Diaz P, Tejero M, et al. , Trunk control test as a functional predictor in stroke patients. J Rehabil Med 2002;34(6):267–72. [DOI] [PubMed] [Google Scholar]
- 22.Wang CH, Hsueh IP, Sheu CF, Hsieh CL.. Discriminative, predictive, and evaluative properties of a trunk control measure in patients with stroke. Phys Ther 2005;85(9):887–94. [PubMed] [Google Scholar]
- 23.Butler P, Saavedra S, Sofranac M, Jarvis S, Woollacott M.. Refinement, reliability, and validity of the segmental assessment of trunk control. Pediatr Phys Ther 2010; 22(3):246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinzaños J, Villa AR, Flores AA, Pérez R.. Proposal and validation of a clinical trunk control test in individuals with spinal cord injury. Spinal Cord 2014;52(6):449–54. [DOI] [PubMed] [Google Scholar]
- 25.Dozois DJA, Dobson KS, Ahnberg JL.. A psychometric evaluation of the beck depression inventory-II. Psychol Assess 1998;10(2):83–9. [Google Scholar]
- 26.Catz A, Itzkovich M, Tesio L, Biering-Sorensen F, Weeks C, Laramee MT, et al. A multicenter international study on the spinal cord independence measure, version III: Rasch psychometric validation. Spinal Cord 2007;45:275–91. [DOI] [PubMed] [Google Scholar]
- 27.Zarco-Periñan MJ, Barrera-Chacón MJ, García-Obrero I, Mendez-Ferrer JB, Alarcon LE, Echevarria-Ruiz de Vargas C.. Development of the Spanish version of the spinal cord independence measure version III: cross-cultural adaptation and reliability and validity study. Disabil Rehabil 2014;36(19):1644–51. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P.. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53(282):457–81. [Google Scholar]
- 29.Cox DR, Society S, Methodological SB.. Regression models and life-tables. J R Stat Soc Ser B 1972;34(2):187–220. [Google Scholar]
- 30.Nas K, Yazmalar L, Şah V, Aydın A, Öneş K.. Rehabilitation of spinal cord injuries. World J Orthop 2015;6(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kollen B, Van De Port I, Lindeman E, Twisk J, Kwakkel G.. Predicting improvement in gait after stroke: A longitudinal prospective study. Stroke 2005;36(12):2676–80. [DOI] [PubMed] [Google Scholar]
- 32.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. , Guidelines for adult stroke rehabilitation and recovery. Stroke 2016;47(6):e98–e169. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh CL, Sheu C-F, Hsueh I-P, Wang C-H.. Trunk control as an early predictor of comprehensive activities of daily living function in stroke patients. Stroke 2002;33(11):2626–30. [DOI] [PubMed] [Google Scholar]
- 34.Burns AS, Ditunno JF.. Establishing prognosis and maximizing functional outcomes after spinal cord injury. Spine 2001;26:S137–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.