ABSTRACT
Toxoplasma gondii (T. gondii), as an opportunistic neurotropic parasite of the Apicomplexa family, was firstly described in 1908. As attention-deficit hyperactivity disorder (ADHD) is one of the most common neuropsychiatric disorders in children and adolescents and often persists into adulthood, the purpose of this systematic review and meta-analysis was to investigate the relationship between T. gondii infection and ADHD.The data were systematically collected from seven electronic databases up to May 1st 2019 with no language restriction. This study was registered at the International Prospective Register of Systematic Reviews (PROSPERO; code: CRD42020149353). Odds ratios (ORs) and 95% confidence intervals (CI) were estimated using a random effects model. Seven studies involving five cross-sectional and two case-control studies were included in this meta-analysis.Results indicated that there was a statistically non-significant association between exposure to T. gondii infection and increased risk of ADHD based on the detection of immunoglobulin G (IgG) antibody (2.02 [95% CI: 0.97-4.20]; I2=58.7%). However, obtained results of Egger’s tests for anti-T. gondii IgG antibody showed publication bias (P=0.014).Sensitivity analysis revealed stable results for the association between anti-T. gondii IgG antibody with ADHD.Given the small number of studies in this field and the obtained results, it cannot be conclusively stated that T. gondii is a risk factor for ADHD.It is important to have reliable information about the relationship between T. gondii and ADHD around the world; as it may lead to better insight to elucidate the possible association of toxoplasmosis and the pathogenesis of ADHD.
KEYWORDS: Toxoplasma gondii, toxoplasmosis, attention-deficit hyperactivity disorder, ADHD
1. Introduction
Toxoplasma gondii (T. gondii), as a neurotropic apicomplexan parasite, affects approximately 30% of the world population and almost all species of warm-blooded animals. This parasite has congenital and acquired forms and cysts of the parasite can settle in various organs of the body, including the brain, heart, as well as lungs, and the initial establishment of cysts in children leads to the dysfunction of the central nervous system (CNS) [1,2]. Ingestion of sporulated oocysts detected on vegetables or in water and soil and eating raw or uncooked meat of infected animals are the methods of transmission of the parasite to humans. In addition, human-to-human transmission has been reported in association with blood transfusion, hematopoietic stem cell transplantation, and transplacental exposure [3–7]. Toxoplasmosis has a wide range of clinical symptoms, including asymptomatic cases to fetal death in congenital infection and severe and potentially fatal encephalitis in immunocompromised patients, such as AIDS patients, organ transplant patients, and patients receiving chemotherapy [8,9]. Neurons are the primary target of T. gondii, and the parasite interacts directly with the CNS [10].
Attention-deficit hyperactivity disorder (ADHD), as one of the most common psychiatric disorders, is a chronic condition with the onset before 12 years of age. Patients with this disorder have symptoms, such as inattention, hyperactivity, impulsive disruptive behavior, impaired concentration, and motor restlessness that impair both academic performance and interpersonal relationships [11,12]. In addition, ADHD affects about 3-7% of school-age children [13]. Genetic and environmental risk factors can be effective in causing ADHD [14]. Stress [15], obesity [16], inappropriate diet [17], smoking [18], as well as exposure to fetal alcohol, and infections in mothers [19,20] are among the factors that have been mentioned in various studies.
T. gondii is one of the reasons for mental disorders [21]. Feigin et al. in 2017 performed a systematic analysis of the global burden of mental diseases. Findings of the aforementioned study showed that neurological disorders were ranked as the cause of 10.2% of global disability-adjusted life year and 16.8% of global mortalities [22]. In recent years, several studies have investigated the role of T. gondii infection as a potential risk factor for psychiatric and neurological disorders [21,23]. Attention disorders are associated with minimal brain damage and many disturbances in neurotransmitter levels, particularly disturbances in the level of dopamine [24]. T. gondii cysts by settling and damaging the brain, as well as performing changes in dopaminergic systems and neurotransmitters, may be involved in the severity of ADHD [24]. It has been shown that functional impairments in the dopaminergic system can lead to neurologic and psychiatric disorders, such as ADHD [24]. In addition, there may be other disorders in subjects with ADHD, such as schizophrenia, that may be observed in children and adolescents with ADHD in adulthood. Risk of developing schizophrenia was 4.3 times higher in these patients than that in the control group [25]. Autism spectrum disorder (ASD) is also another mental disorder that may be associated with ADHD because the symptoms of ADHD are often noticed in people with ASD [26]. Few studies have surveyed the association of T. gondii infection with ADHD, and there are discrepancies in the obtained results.
1.1. Objectives
The present systematic review and meta-analysis on the association between T. gondii infection and ADHD aimed to investigate this knowledge gap.
2. Methods
2.1. Study design and protocol registration
To ensure scientific rigor, the literature was systematically reviewed according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses [27]. The study protocol was registered on PROSPERO as an international prospective registry of systematic reviews with the registration number of CRD42020149353.
2.2. Search strategy
A systematic literature search was conducted using electronic databases, including PubMed, ScienceDirect, Scopus, ProQuest, Web of Science, EMBASE, and Google Scholar. The literature search was carried out on the prevalence of T. gondii antibodies among the patients with ADHD regardless of the publication date and language of the articles. Two researchers independently conducted the database search in May 2019 (Figure 1). The key search terms used in combinations were ‘Toxoplasma’ OR ‘toxoplasmosis’ AND ‘attention-deficit hyperactivity disorder’ OR ‘ADHD’ AND ‘prevalence’ OR ‘seroprevalence’.
Figure 1.

Flow diagram of the study design process. The PRISMA flow diagram of the search strategy, study selection, and data management procedure of T. gondii infection and ADHD.
2.3. Inclusion and exclusion criteria
The inclusion criteria were 1) studies published until May 1st 2019, 2) full-text manuscripts in any language regarding the relationship between toxoplasmosis and ADHD with no limit on the year of publication or study design. Furthermore, the exclusion criteria were 1) review articles, 2) summary of studies presented at congresses and conferences, 3) dissertations; as well as 4) articles with ambiguous data despite attempts to contact the authors.
2.4. Study selection and data extraction
After merging the search results into different databases, duplicate articles were automatically deleted in EndNote software (version X9). Then, the process of removing duplicate articles was completed with a manual second revision. Two independent reviewers screened the titles and abstracts for potential studies. In the next step, relevant articles were selected for full-text download. Finally, extracted data pertaining to the name of the first author, year of publication, place of study, design of study, eligibility criteria, characteristics of the study groups (age and sex), laboratory method, number of seropositive cases and controls, and odds ratio (OR). The data were extracted from the texts or tables or estimated according to the figures when necessary. To obtain more detailed information, the authors of the two articles were contacted through e-mail [28,29].
2.5. Quality assessment
The strengthening the reporting of observational studies in epidemiology (STROBE) is a 22-item checklist thatused in articles with three main study designs of epidemiology, including cohort, case-control, and cross-sectional studies. Out of 22 items, 18 items were common in all three study designs and four cases were specific in cohort, case-control or cross-sectional studies. Title and abstract, introduction, methods, discussion, and funding of the included articles are evaluated by this checklist [30]. In the present study, the articles were evaluated based on the STROBE assessment (i.e., low quality: <15.5, moderate quality: 15.5–29.5, and high quality: 30.0–44.0). Quality score of different eligible studies is represented in the supplementary Table 1.
2.6. Statistical analysis
The present meta-analysis was carried out using STATA software (version 14). The random effects model was used to determine the OR by forest plot with 95% confidence interval (CI) [12]. I2 index (<25%: low; 25-50%: medium, and >50%: high) was employed to assess the heterogeneity among studies [31]. The across-study bias (i.e., small study effects) was assessed using Egger’s test [32]. To calculate the sensitivity analysis, each single article was removed, and its effect on the overall study outcome was determined. The ‘trim and fill’ method was used to calculate unbiased estimates. In addition, a subgroup analysis was performed based on gender and type of study.
3. Results
Full texts of 14 articles were reviewed after the removal of duplicates and initial screening. Among these publications, three articles were reviews and three articles were non-related articles (Figure 1). Finally, eight studies included six cross-sectional and two case–control studies in the present systematic review. A cross-sectional study was excluded due to the lack of measurement of antibodies against T. gondii [29]. The main characteristics of the included studies are shown in Tables 1 and 2. These studies were conducted from 2016 to 2018. The included studies were performed in four countries (i.e., Iran [33,34], Turkey [24], Egypt [35,36], and Czech Republic [28,29,37]).
Table 1.
Characteristics of the included studies to investigate the association between T. gondii infection and ADHD.
| No | First author | Publication year | Place of study | Type of study | Method (s) | Antibodies | Age (mean±SD) | Sex [n (%)] |
|---|---|---|---|---|---|---|---|---|
| 1 | Shehata et al [35] | 2016 | Egypt | Cross sectional | ELISA | IgG, IgM | 16.84 ± 7.021 | – |
| 2 | Afsharpaiman et al [34] | 2016 | Iran | Cross sectional | ELISA | IgG, IgM | P: 8.12 ± 3.25 C: 8.12 ± 2.40 |
P: [F: 16 (33.33), M: 32(66.66)] C: [F: 16 (33.33), M: 32(66.66)] |
| 3 | Flegr & Escudero [37] | 2016 | Czech Republic | Cross sectional | CFT ELISA | IgG, IgM | P:M: 34.0 ± 10.5 F: 36.5 ± 12.3 C: M: 34.8 ± 12.7 F: 32.4 ± 11.0 |
P: [F:22 (66.66), M:11(33.33)] C: [F:872 (71.30), M:351(28.69)] |
| 4 | Flegr & Horacek [28] | 2017 | Czech Republic | Cross sectional | CFT ELISA | IgG, IgM | M: 35.6 ± 12.4 F: 32.9 ± 12.3 |
P:[F:6 (50.00), M:6 (50.00)] C: [F:2859 (73.34), M:1039 (26.65)] |
| 5 | Flegr & Horacek [29] | 2018 | Czech Republic | Cross sectional | – | – | M: 34.8 ± 12.0 F: 30.5 ± 10.9 |
P: [F:44 (55.69), M:35 (44.30)] C: [F:2032 (62.12), M:1239 (37.87)] |
| 6 | Khademvatan et al [33] | 2018 | Iran | Cross-sectional | ELISA | IgG, IgM | P: 7.8 ± 3.15 C: 8.2 ± 2.9 |
P: [F:43 (36.75), M:74 (63.24)] C:[F:25 (30.12), M:58 (69.87)] |
| 7 | El-Beshbishi et al [36] | 2018 | Egypt | Case–control | ELISA | IgG, IgM | ≤5 | P: – C: [F:9 (30.00), M:21 (70.00)] |
| 8 | Akaltun et al [24] | 2018 | Turkey | Case–control | ELISA | IgG, IgM | P: 10.84 C: 10.71 |
P: [F:21 (19.62), M:86 (80.37)] C: [F:50 (43.72), M:57 (53.27)] |
ELISA: enzyme-linked immunosorbent assay, IgG: immunoglobulin G, IgM: immunoglobulin M, P: patient, C: control, F: female, M: male, n: number
Table 2.
Description of data extracted from the included studies in the systematic review and meta-analysis of the association between T. gondii infection and ADHD.
| No | Reference | N | Case: ADHD+ (n) | Control: ADHD- (n) | ADHD+ & T+ (n, %) | ADHD- & T+ (n, %) | OR (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|
| 1 | Shehata et al [35] | 188 | 14 | 174 | 12 (85.7) | 82 (47.12) | 0.73 (1.48–30.97) | p = 0.034 |
| 2 | Afsharpaiman et al [34] | 96 | 48 | 48 | 2 (4.2) | 1 (2.1) | 2.04 (0.18–23.32) | p = 0.92 |
| 3 | Flegr & Escudero [37] | 1256 | 1223 | 33 | 8 (24.24) | 285 (23.30) | 1.05 (0.47–2.36) | p = 0.694 |
| 4 | Flegr & Horacek [28] | 1336 | 12 | 1324 | 5 (41.66) | 361 (27.26) | 2.22 (0.67–7.33) | p = 0.274 |
| 5 | Flegr & Horacek [29] | 3350 | 79 | 3271 | 27 (34.17) | 520 (15.89) | – | p = 0.000 |
| 6 | Khademvatan et al [33] | 200 | 117 | 83 | 21 (17.94) | 20 (24.09) | 0.69 (0.35–1.37) | p > 0.05 |
| 7 | El-Beshbishi et al [36] | 35 | 5 | 30 | 3 (60) | 3 (10) | 13.50 (1.57–115.94) | – |
| 8 | Akaltun et al [24] | 214 | 107 | 107 | 8 (7.47) | 3 (2.8) | 2.80 (0.72–10.86) | p = 0.215 |
N and n: number, CI: confidence interval; ADHD +: individuals with attention-deficit hyperactivity disorder; ADHD -: individuals without attention-deficit hyperactivity disorder; ADHD + & T+: individuals with attention-deficit hyperactivity disorder and Toxoplasma positive; ADHD – & T+: individuals without attention-deficit hyperactivity disorder and Toxoplasma positive; OR: odds ratio
A total number of 3325 participants were entered into the meta-analysis, including 336 patients with ADHD (i.e., 17.55% positive for toxoplasmosis) and 2989 controls (i.e., 25.25% positive for toxoplasmosis). Five studies used enzyme-linked immunosorbent assay (ELISA) to diagnose anti- T. gondii antibodies (i.e., IgG and IgM) [24,33–36]. Diagnosis method of T. gondii has not been mentioned in one study [29]. In addition, both ELISA and complement fixation test were used in two studies [28,37].
The obtained findings indicated that the common OR of anti-T. gondii IgG and IgM antibodies in patients with ADHD were 2.02 (95% CI: 0.97–4.20) and 2.39 (95% CI: 0.68–8.45) in comparison to control groups, respectively (Figures 2 and 3). Results of heterogeneity test among the studies for IgG (I2 = 58.7%; P = 0.024) and IgM (I2 = 10.9%; P = 0.326) antibodies were different. There was a publication bias in studies evaluating anti-T. gondii IgG antibody according to Egger’s test (P = 0.014). In addition, the publication bias is supported by the asymmetric pattern observed in the funnel plot (Figure 4). Also, according to the results of the ‘trim and fill’ method, no change was observed in the results of the meta-analysis based on the random effects models (0.90 [95% CI: 0.41–1.94]; P = 0.789) (Figure 5). Results of subgroup analysis based on gender indicated that the pooled ORs of the risk of toxoplasmosis were (1.46 [95% CI: 0.61–3.53]; I2 = 17.7%) and (1.08 [95% CI: 0.39–3.01]; I2 = 59.2%) in males and females, respectively. Also, the combined OR of the risk of anti-T. gondii IgG antibody in patients with ADHD based on the type of study in cross-sectional and case–control studies were (1.49 [95% CI: 0.71–3.15]; I2 = 54.0%) and (4.88 [95% CI: 1.12–21.31]; I2 = 32.0%), respectively. The results of the meta-regression test showed that the effect of study type on the heterogeneity of studies was not statistically significant (P = 0.231). Findings of sensitivity analysis indicated the stability of the results of the present study (Figure 6).
Figure 2.

Forest plot diagram of studies showing IgG seropositivity rates of T. gondii. Perpendicular discontinuous line indicates the odds ratio index. The horizontal lines illustrate 95% confidence intervals for odds ratios.
Figure 3.

Forest plot diagram of studies showing IgM seropositivity rates of T. gondii. Perpendicular discontinuous line indicates the odds ratio index. The horizontal lines illustrate 95% confidence intervals for odds ratios.
Figure 4.
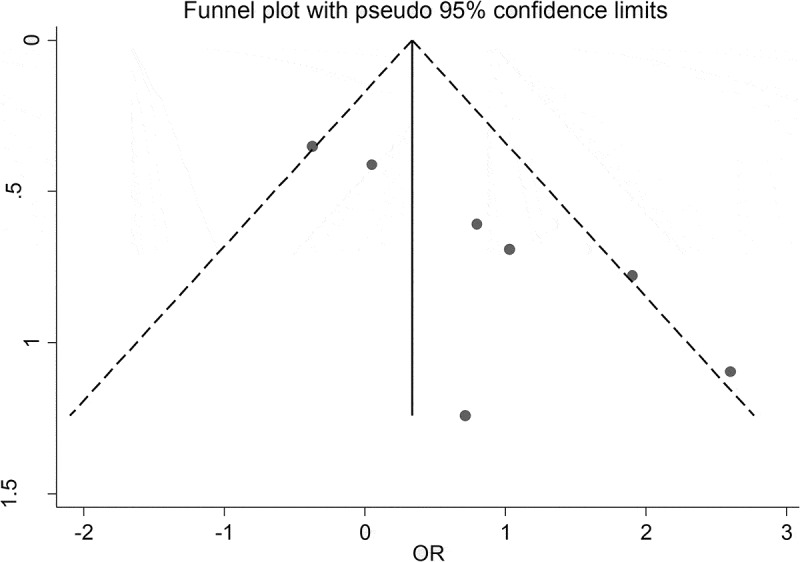
A bias assessment plot from Egger for seroprevalence of T. gondii among patients with ADHD.
Figure 5.
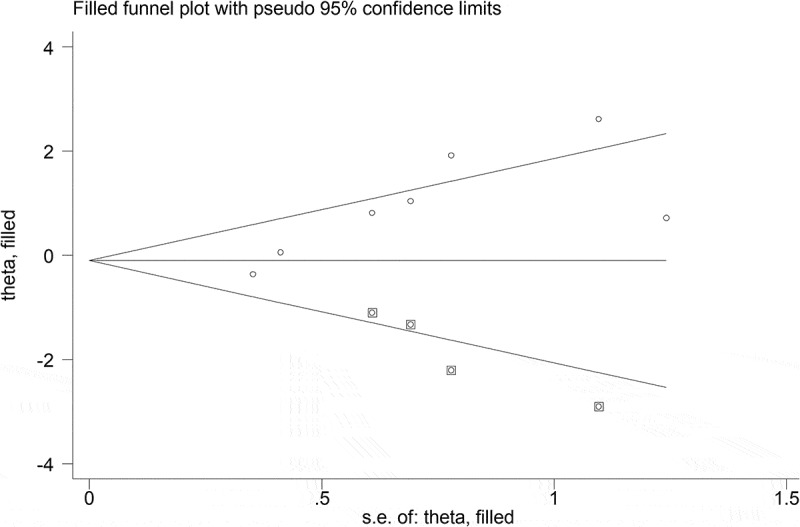
Filled funnel plot with pseudo 95% confidence interval.
Figure 6.

Sensitivity analysis for assessing the effect of each primary study on the total estimates in included studies.
4. Discussion
Obtained results of the present systematic review and meta-analysis showed that the overall ORs of anti-T. gondii IgG and IgM antibodies in patients with ADHD were 2.02 (95% CI: 0.97–4.20) and 2.39 (95% CI: 0.68–8.45), respectively. Based on these results, a relationship between T.gondii infection and ADHD cannot be confirmed. However, given the limited amount of studies and the magnitude of the OR found in our meta-analysis, more research is warranted to shed more light on this hypothesis. In fact, the cysts in the chronic stage of the disease in some tissues, such as the brain, could cause damage [24]. Different types of nerve cells, such as astrocytes, neurons, and microglial cells in the brain, may be invaded by tachyzoites and tissue cysts developed in them, and the destruction of cyst walls may be responsible for behavioral changes [38,39].
In addition, the genome of T. gondii contains two aromatic amino acid hydroxylases capable of directly affecting the biosynthesis of neurotransmitters, such as dopamine and serotonin [40]. The production of a large amount of dopamine, as the main neurotransmitter associated with ADHD, by parasite increases the destruction of the cyst wall during in vitro neuronal infection. In addition, dopamine activated T. gondii toward tachyzoites and increased parasite proliferation in human fibroblasts and neonatal rat astrocytes [41]. Dopamine excess plays a role in the pathophysiology of schizophrenia, psychosis and the kynurenine pathway induced by intracerebral T. gondii infection [24,42]. However, despite evidence that indicates the possibility of the relationship between T. gondii infection and ADHD; the results did not show a significant relationship.
The results of Egger’s test for anti-T. gondii IgG antibody showed that there was publication bias. Also, the visual inspection of Figure 4 suggests that the observed association can be driven by two studies showing large OR but also large standard errors. It is possible that similar studies showing the opposite effects of toxoplasmosis on the risk of ADHD remained in drawers of their authors.
Results of the present study are in line with the findings of the published systematic reviews and meta-analyses examining the role of T. gondii infection as a potential risk factor for psychiatric disorders, such as depression [43] and Parkinson’s disease [44]. In addition, the results of this study are in contradiction with the results of studies such as obsessive-compulsive disorder [45], Alzheimer’s disease [46], and epilepsy [47]. In the present systematic review, high heterogeneity was observed in the relationship between ADHD and T. gondii infection. The high heterogeneity index indicated the potential variation that can be due to several reasons, including 1) diversity of target groups, 2) selection of the cases and controls in different ways and populations, 3) differences in the age of participants, and 4) differences in the type of included studies in the meta-analysis. However, the results of the meta-regression test showed that the type of study in this meta-analysis had no significant effect on heterogeneity.
The results of subgroup analysis based on gender show that although the CI for the OR between males and females is not statistically significant, the difference between point estimates of the OR related to the association between T. gondii and ADHD in men (1.46) and women (1.08) is considerable. The prevalence of ADHD is more common in men than that in women probably related to high levels of prenatal testosterone [48,49]. Gender differences in the prevalence of ADHD are more frequently pronounced in childhood and become less obvious with increasing age. In females, due to the effect of buffering of their earlier developmental maturity, ADHD is often diagnosed later than in men [50,51]. Among men with latent toxoplasmosis, testosterone levels are higher than in controls [52,53]. Some rodents infected with T. gondii are directed to feline odor due to sexual stimulation caused by the activation of testosterone [53]. The decrease in fear and the attractiveness of male infected rodents may be due to the increased levels of testosterone. The male rats castrated prior to infection did not show the loss of fear [54].
T. gondii affects the expression of approximately 3000 host genes throughout its life cycle. Susceptibility genes for mental disorders, such as ADHD, are highly enriched in the human arm of this interactome. Moreover, the expression of 17.7% of 237 ADHD susceptibility genes is affected by the Toxoplasma infection [55,56]. Furthermore, the primary common emphasis in ADHD was on the calcium-signaling pathway and number of other metabolic pathways, such as tyrosine, tryptophan, and histidine, and the number of recovered genes in this pathway is 44 [55,57]. The calcium-signaling pathway is activated by voltage or receptor-gated ion channels, processes modulating intracellular stores, and phosphatidylinositol signaling system [58]. Calcium channel blockers, calmodulin antagonism, or extracellular calcium reduce cell invasion by parasites [59,60].
Interferon-gamma (IFN-γ) and tumor necrosis factor-α are important for controlling the proliferation of tachyzoites in the acute and chronic stages of infection [61]. The INF-γ induces the release of indoleamine 2,3-dioxygenase (IDO), and this enzyme plays a role in tryptophan catabolism [62]. Since tryptophan is required for the growth of the parasite, the decrease of concentration of this amino acid inhibits the growth of T. gondii [63]. Products of tryptophan catabolism play an important role in the increase of oxidative stress and apoptosis in the brain [64]. The amino acid tryptophan is a precursor for serotonin synthesis. Concentration of IDO increases during the infection of T. gondii, and this increase may decrease serotonin synthesis [64]. Inattention, reduced behavioral inhibition, and increased impulsivity are caused by decreased serotonin [65].
Only a few articles have reviewed the relationship between T. gondii and ADHD. These studies were from the three continents, including Asia (i.e., Turkey [n = 1] and Iran [n = 2]), Europe (Czech and Slovak Republics [n = 3]), as well as Africa (Egypt [n = 2]). Therefore, the lack of information available for the rest of the world is a basic gap. Therefore, further studies should be performed to investigate the influence of age and other socioeconomic factors in its global prevalence, as this may provide insight into associated exposure risks and disease management.
5. Study limitation
There were several limitations in the present study as follows:
A low number of participants in the case group compared to the control group. In some cross-sectional studies, despite having a large sample size, the number of individuals with ADHD was limited, and the lack of significant association of T. gondii infection with ADHD may reflect limited statistical power.
Lack of assessment in terms of different risk factors, such as host factors (age, race, and family history). However, the majority of studies included in meta-analysis have not evaluated these factors. Accordingly, the meta-analysis of these risk factors was not possible to perform.
Lack of adjustments for confounding factors or inadequate matching in the selection of the controls. In the case–control studies, the case and control groups were not adjusted for different factors including age and sex.
Citations with moderate quality. When a study receives a higher score from a quality assessment checklist, it will be of a higher quality and has paid attention to most of the items that are effective in article quality. The studies in this meta-analysis showed moderate quality.
6. Conclusion
As far as the researchers are aware, this was the first systematic review and meta-analysis focused on the relationship between T. gondii infection and ADHD. In this study, patients with ADHD indicated a lower prevalence of T. gondii infection compared to controls. There was no significant relationship between the prevalence rate of T. gondii infection and ADHD. However, it is required to perform further studies to determine the details of the association between T. gondii infection and ADHD and to obtain a more definite answer.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
This article is the approved plan (No. 6376) from the Student Research Committee of Mazandaran University of Medical Sciences, Sari, Iran. The code of ethics of this plan is (IR.MAZUMS.REC.1398.5504).
Author Contributions
AD conceived the idea for this meta-analysis. TN and SS searched the databases for potentially eligible articles based on their titles and abstracts. ZH and TN extracted the data. MM analyzed the data. AD and TN participated in the study design and wrote the manuscript. AD and AA critically reviewed the manuscript. All authors read and approved the final manuscript for publication.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Supplementary Materials
Supplemental data for this article can be accessed here.
References
- [1].Brynska A, Tomaszewicz-Libudzic E, Wolanczyk T.. Obsessive-compulsive disorder and acquired toxoplasmosis in two children. Eur Child Adolesc Psychiatry. 2001;10(3):200–204. [DOI] [PubMed] [Google Scholar]
- [2].Flegr J, Prandota J, Sovickova M, et al. Toxoplasmosis–a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS One. 2014;9(3):e90203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alvarado-Esquivel C, Rascon-Careaga A, Hernandez-Tinoco J, et al. Seroprevalence and associated risk factors for Toxoplasma gondii infection in healthy blood donors: a cross-sectional study in Sonora, Mexico. Biomed Res Int. 2016;2016:9597276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Elnahas A, Gerais AS, Elbashir MI, et al. Toxoplasmosis in pregnant Sudanese women. Saudi Med J. 2003;24(8):868–870. [PubMed] [Google Scholar]
- [5].Hajsoleimani F, Ataeian A, Nourian A, et al. Seroprevalence of Toxoplasma gondii in pregnant women and bioassay of IgM positive cases in Zanjan, northwest of Iran. Iran J Parasitol. 2012;7(2):82–86. [PMC free article] [PubMed] [Google Scholar]
- [6].Hill D, Dubey JP.. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8(10):634–640. [DOI] [PubMed] [Google Scholar]
- [7].Pereira KS, Franco RM, Leal DA. Transmission of toxoplasmosis (Toxoplasma gondii) by foods. Adv Food Nutr Res. 2010;60:1–19. [DOI] [PubMed] [Google Scholar]
- [8].Robert-Gangneux F, Darde ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Luft BJ, Brooks RG, Conley FK, et al. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA. 1984;252(7):913–917. [PubMed] [Google Scholar]
- [10].Cabral CM, Tuladhar S, Dietrich HK, et al. Neurons are the primary target cell for the brain-tropic intracellular parasite Toxoplasma gondii. PLoS Pathog. 2016;12(2):e1005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Afsharpaiman S, Khosravi MH, Mahmoodinejad M, et al. Assessment of Toxoplasma seropositivity in children suffering from anxiety disorders. Iran J Child Neurol. 2017;11(4):32–37. [PMC free article] [PubMed] [Google Scholar]
- [12].American psychiatric association . Diagnostic and statistical manual of mental disorders; autism spectrum disorder. Washington, DC, USA: American psychiatric publishing; 2013. p. 50–59. [Google Scholar]
- [13].Arabgol F, Panaghi L, Hebrani P. Reboxetine versus methylphenidate in treatment of children and adolescents with attention deficit-hyperactivity disorder. Eur Child Adolesc Psychiatry. 2009;18(1):53–59. [DOI] [PubMed] [Google Scholar]
- [14].Dunn GA, Nigg JT, Sullivan EL. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol Biochem Behav. 2019;182:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal anti-inflammatory treatment. Endocrinology. 2014;155(7):2635–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Buss C, Entringer S, Davis EP, et al. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS One. 2012;7(6):e37758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rijlaarsdam J, Cecil CA, Walton E, et al. Prenatal unhealthy diet, insulin-like growth factor 2 gene (IGF2) methylation, and attention deficit hyperactivity disorder symptoms in youth with early-onset conduct problems. J Child Psychol Psychiatry. 2017;58(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Becker K, El-Faddagh M, Schmidt MH, et al. Interaction of dopamine transporter genotype with prenatal smoke exposure on ADHD symptoms. J Pediatr. 2008;152(2):263–269. [DOI] [PubMed] [Google Scholar]
- [19].Thapar A, Cooper M, Eyre O, et al. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry. 2013;54(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Terasaki LS, Schwarz JM. Effects of moderate prenatal alcohol exposure during early gestation in rats on inflammation across the maternal-fetal-immune interface and later-life immune function in the offspring. J Neuroimmune Pharmacol. 2016;11(4):680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sutterland AL, Fond G, Kuin A, et al. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. Acta Neurol Scand. 2015;132(3):161–179. [DOI] [PubMed] [Google Scholar]
- [22].Feigin VL, Abajobir AA, Abate KH, et al. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet Neurol. 2017;16(11):877–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ngoungou EB, Bhalla D, Nzoghe A, et al. Toxoplasmosis and epilepsy–systematic review and meta-analysis. PLoS Negl Trop Dis. 2015;9(2):e0003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Akaltun İ, Kara T, Ayaydın H, et al. The relation between serum Toxoplasma gondii IgG antibody in children and ADHD and its severity. Klinik Psikofarmakol Bülteni. 2018;29(3):326–331. [Google Scholar]
- [25].Dalsgaard S, Mortensen PB, Frydenberg M, et al. Association between attention-deficit hyperactivity disorder in childhood and schizophrenia later in adulthood. Eur Psychiatry. 2014;29(4):259–263. [DOI] [PubMed] [Google Scholar]
- [26].Ames CS, White SJ. Are ADHD traits dissociable from the autistic profile? Links between cognition and behaviour. J Autism Dev Disord. 2011;41(3):357–363. [DOI] [PubMed] [Google Scholar]
- [27].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. [DOI] [PubMed] [Google Scholar]
- [28].Flegr J, Horacek J. Toxoplasma-infected subjects report an obsessive-compulsive disorder diagnosis more often and score higher in obsessive-compulsive inventory. Eur Psychiatry. 2017;40:82–87. [DOI] [PubMed] [Google Scholar]
- [29].Flegr J, Horacek J. Toxoplasmosis, but not borreliosis, is associated with psychiatric disorders and symptoms. Schizophr Res. 2018;197:603–604. [DOI] [PubMed] [Google Scholar]
- [30].Vandenbroucke JP, von Elm E, Altman DG, et al. strengthening the reporting of observational Studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. [DOI] [PubMed] [Google Scholar]
- [31].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- [32].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Khademvatan S, Riahi F, Izadi-Mazidi M, et al. Toxoplasma gondii exposure and the risk of attention deficit hyperactivity disorder in children and adolescents. Pediatr Infect Dis J. 2018;37(11):1097–1100. [DOI] [PubMed] [Google Scholar]
- [34].Afsharpaiman S, Khosravi MH, Faridchehr M, et al. Assessment of Toxoplasma sero-positivity in children suffering from attention deficit hyperactivity disorder. Galen. 2016;5(4):188–193. [Google Scholar]
- [35].Shehata AI, Hassanein FI, Abdul-Ghani R. Seroprevalence of Toxoplasma gondii infection among patients with non-schizophrenic neurodevelopmental disorders in Alexandria, Egypt. Acta Trop. 2016;154:155–159. [DOI] [PubMed] [Google Scholar]
- [36].El-Beshbishi SN, El-Tantawy NL, Elzeky SM, et al. Seroprevalence of Toxoplasma gondii infection in children with central nervous system disorders in Mansoura, Egypt: a case-control study. Trans R Soc Trop Med Hyg. 2018;112(12):555–560. [DOI] [PubMed] [Google Scholar]
- [37].Flegr J, Escudero DQ. Impaired health status and increased incidence of diseases in Toxoplasma-seropositive subjects - an explorative cross-sectional study. Parasitology. 2016;143(14):1974–1989. [DOI] [PubMed] [Google Scholar]
- [38].Cetinkaya Z, Yazar S, Gecici O, et al. Anti-Toxoplasma gondii antibodies in patients with schizophrenia–preliminary findings in a Turkish sample. Schizophr Bull. 2007;33(3):789–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ene L, Marcotte TD, Umlauf A, et al. Latent toxoplasmosis is associated with neurocognitive impairment in young adults with and without chronic HIV infection. J Neuroimmunol. 2016;299:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Henriquez SA, Brett R, Alexander J, et al. Neuropsychiatric disease and Toxoplasma gondii infection. Neuroimmunomodulation. 2009;16(2):122–133. [DOI] [PubMed] [Google Scholar]
- [41].Ihara F, Nishimura M, Muroi Y, et al. Toxoplasma gondii infection in mice impairs long-term fear memory consolidation through dysfunction of the cortex and amygdala. Infect Immun. 2016;84(10):2861–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Strobl JS, Goodwin DG, Rzigalinski BA, et al. Dopamine stimulates propagation of Toxoplasma gondii tachyzoites in human fibroblast and primary neonatal rat astrocyte cell cultures. J Parasitol. 2012;98(6):1296–1299. [DOI] [PubMed] [Google Scholar]
- [43].Nayeri Chegeni T, Sharif M, Sarvi S, et al. Is there any association between Toxoplasma gondii infection and depression? a systematic review and meta-analysis. PloS One. 2019;14(6):e0218524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhou Z, Zhou R, Li K, et al. The association between Toxoplasma gondii infection and risk of Parkinson’s disease: a systematic review and meta-analysis. Biomed Res Int. 2019;2019:8186017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nayeri Chegeni T, Sarvi S, Amouei A, et al. Relationship between toxoplasmosis and obsessive compulsive disorder: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2019;13(4):e0007306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nayeri Chegeni T, Sarvi S, Moosazadeh M, et al. Is Toxoplasma gondii a potential risk factor for Alzheimer’s disease? a systematic review and meta-analysis. Microb Pathog. 2019;137:103751. [DOI] [PubMed] [Google Scholar]
- [47].Sadeghi M, Riahi SM, Mohammadi M, et al. An updated meta-analysis of the association between Toxoplasma gondii infection and risk of epilepsy. Trans R Soc Trop Med Hyg. 2019;113(8):453–462. [DOI] [PubMed] [Google Scholar]
- [48].Davies W. Sex differences in attention deficit hyperactivity disorder: candidate genetic and endocrine mechanisms. Front Neuroendocrinol. 2014;35(3):331–346. [DOI] [PubMed] [Google Scholar]
- [49].James WH. Further evidence that some male-based neurodevelopmental disorders are associated with high intrauterine testosterone concentrations. Dev Med Child Neurol. 2008;50(1):15–18. [DOI] [PubMed] [Google Scholar]
- [50].Nussbaum NLADHD. and female specific concerns: a review of the literature and clinical implications. J Atten Disord. 2012;16(2):87–100. [DOI] [PubMed] [Google Scholar]
- [51].Simon V, Czobor P, Balint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204–211. [DOI] [PubMed] [Google Scholar]
- [52].Flegr J, Lindova J, Kodym P. Sex-dependent toxoplasmosis-associated differences in testosterone concentration in humans. Parasitology. 2008;135(4):427–431. [DOI] [PubMed] [Google Scholar]
- [53].Zghair KH, Al-Qadhi BN, Mahmood SH. The effect of toxoplasmosis on the level of some sex hormones in males blood donors in Baghdad. J Parasit Dis. 2015;39(3):393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lim A, Kumar V, Hari Dass SA, et al. Toxoplasma gondii infection enhances testicular steroidogenesis in rats. Mol Ecol. 2013;22(1):102–110. [DOI] [PubMed] [Google Scholar]
- [55].Carter CJ. Toxoplasmosis and polygenic disease susceptibility genes: extensive Toxoplasma gondii host/pathogen interactome enrichment in nine psychiatric or neurological disorders. J Pathog. 2013;2013:965046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Skallova A, Kodym P, Frynta D, et al. The role of dopamine in Toxoplasma-induced behavioural alterations in mice: an ethological and ethnopharmacological study. Parasitology. 2006;133(Pt 5):525–535. [DOI] [PubMed] [Google Scholar]
- [57].Liao P, Soong TW. CaV1.2 channelopathies: from arrhythmias to autism, bipolar disorder, and immunodeficiency. Pflugers Arch. 2010;460(2):353–359. [DOI] [PubMed] [Google Scholar]
- [58].Deckelbaum RJ, Torrejon C. The omega-3 fatty acid nutritional landscape: health benefits and sources. J Nutr. 2012;142(3):587s–591s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Pezzella N, Bouchot A, Bonhomme A, et al. Involvement of calcium and calmodulin in Toxoplasma gondii tachyzoite invasion. Eur J Cell Biol. 1997;74(1):92–101. [PubMed] [Google Scholar]
- [60].Song HO, Ahn MH, Ryu JS, et al. Influence of calcium ion on host cell invasion and intracellular replication by Toxoplasma gondii. Korean J Parasitol. 2004;42(4):185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Suzuki Y. Host resistance in the brain against Toxoplasma gondii. J Infect Dis. 2002;185(Suppl 1):S58–S65. [DOI] [PubMed] [Google Scholar]
- [62].Tan L, Yu JT, Tan L. The kynurenine pathway in neurodegenerative diseases: mechanistic and therapeutic considerations. J Neurol Sci. 2012;323(1–2):1–8. [DOI] [PubMed] [Google Scholar]
- [63].Cerávolo IP, Chaves ACL, Bonjardim CA, et al. Replication of Toxoplasma gondii, but not Trypanosoma cruzi, is regulated in human fibroblasts activated with gamma interferon: requirement of a functional JAK/STAT pathway. Infect Immun. 1999;67(5):2233–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Schwarcz R, Bruno JP, Muchowski PJ, et al. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13(7):465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Banerjee E, Nandagopal K. Does serotonin deficit mediate susceptibility to ADHD? Neurochem Int. 2015;82:52–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


