Abstract
Infectious laryngotracheitis (ILT) is a highly contagious upper respiratory tract disease of chicken caused by a Gallid herpesvirus 1 (GaHV-1) belonging to the genus Iltovirus, and subfamily Alphaherpesvirinae within Herpesviridae family. The disease is characterized by conjunctivitis, sinusitis, oculo-nasal discharge, respiratory distress, bloody mucus, swollen orbital sinuses, high morbidity, considerable mortality and decreased egg production. It is well established in highly dense poultry producing areas of the world due to characteristic latency and carrier status of the virus. Co-infections with other respiratory pathogens and environmental factors adversely affect the respiratory system and prolong the course of the disease. Latently infected chickens are the primary source of ILT virus (ILTV) outbreaks irrespective of vaccination. Apart from conventional diagnostic methods including isolation and identification of ILTV, serological detection, advanced biotechnological tools such as PCR, quantitative real-time PCR, next generation sequencing, and others are being used in accurate diagnosis and epidemiological studies of ILTV. Vaccination is followed with the use of conventional vaccines including modified live attenuated ILTV vaccines, and advanced recombinant vector vaccines expressing different ILTV glycoproteins, but still these candidates frequently fail to reduce challenge virus shedding. Some herbal components have proved to be beneficial in reducing the severity of the clinical disease. The present review discusses ILT with respect to its current status, virus characteristics, epidemiology, transmission, pathobiology, and advances in diagnosis, vaccination and control strategies to counter this important disease of poultry.
Keywords: poultry, chicken, Infectious Laryngotracheitis virus, ILT, epidemiology, pathobiology, diagnosis, vaccine, control, review
1. Introduction
Poultry farming is one of the rapidly developing sectors, which plays an important role in the global food security. The consequence of globalization, climate change and rapidly expanding poultry population results in the emergence of several diseases. Among the emerging diseases, infectious laryngotracheitis (ILT) is a highly contagious upper respiratory tract disease of chicken and has been regarded as a major concern for poultry health and welfare (Bagust et al. 2000). Although chickens are considered to be the primary target host (Bagust 1986), natural disease has been reported in peafowls and pheasants (Crawshaw and Boycott 1982; Hanson and Bagust 1991). Other species, including closely related Galliformes are refractory to infection, and birds such as crows, ducks, pigeons, sparrows and starlings seem to be resistant (Guy and Garcia 2008). This disease causes production losses due to increased morbidity, moderate mortality, decreased weight gain, reduced egg production and expenses spent on vaccination, biosecurity measures and therapy to counteract secondary infection by other avian pathogens (Guy and Bagust 2003; Guy and Garcia 2008; Jones 2010; Garcia et al. 2014). In chickens, two main forms of ILT have been described under field conditions which include the severe acute or epizootic form characterized by significant respiratory distress, sneezing, expectoration of blood-mixed mucus, severe haemorrhagic tracheitis and conjunctivitis accompanied by high mortality reaching up to 70% (ranging from 5 to 70%) and a milder form characterized by mild to moderate catarrhal tracheitis, sinusitis, conjunctivitis, relatively low morbidity and occasional mortality which usually range between 0.1 and 2% (Ou and Giambrone 2012). Chicken embryo origin (CEO) and tissue culture origin (TCO) vaccines developed during 1960s have been extensively used for controlling ILT outbreaks worldwide. In the meantime, both the vaccines had the tendency to revert to virulence following bird to bird passages. It is believed that most of the outbreaks are caused by CEO vaccine isolates that persist in long-lived bird operations and spill-over into poultry populations (Blacker et al. 2011). The recombinant/mutant vaccines, which are considered to be safer alternatives, have limited practical applicability because they fail to stop complete viral shedding and existence of antibodies against vectors can neutralise the vaccines. Increased incidence of the disease is due to more concrete factors such as increase in poultry production density, decrease in downtime of production sites, poor biosecurity, and poor vaccination methods. Vaccine virus reactivation and shedding has been reported from several parts in commercial layers (Thilakarathne et al. 2020). Hence, serious attention must be given to control the ILT in poultry-dense areas not only to prevent the economic loss but also to enhance the poultry welfare and health.
The present review focuses on the comprehensive overview of the ILT with respect to its etiology, epidemiology, transmission, pathobiology, advances in diagnosis and vaccines, and appropriate prevention and control strategies.
2. Etiology
2.1. The virus
ILT is caused by the infectious laryngotracheitis virus, also known as Gallid herpesvirus 1 (GaHV-1), which belongs to the genus Iltovirus, subfamily Alphaherpesvirinae of the family Herpesviridae (Davison et al. 2009). The genome of ILTV contains a 150-155 kb linear double-stranded DNA encoding a unique long (UL), unique short (US) and two inverted repeat (IR) sequences (Figure 1) (McGeoch et al. 2000; Morales Ruiz et al. 2018). A fully assembled complete genome sequence of ILTV comprises 148 kb nucleotides, with a G + C content of 48.2% (Lee et al. 2011). The virions of ILTV under electron microscopy appear as typical herpes virions consisting of a DNA core within an icosahedral capsid which is surrounded by a tegument layer, and outer envelope glycoproteins (Roizman and Pellett 2001). The size of the viral capsid is about 100 nm in diameter, and the complete viral particle size is within the range of 200 to 350 nm (Granzow et al. 2001). The ILTV genome consists of 80 open reading frames (ORFs); out of which 65 are located in the UL region, 9 in the US region and 6 in the IR region (McGeoch et al. 2000; Thureen and Keeler 2006; Lee et al. 2011). Among 80 ORFs, sixty-three ORFs display homologies to Herpes Simplex Virus-1 (HSV-1) genome with respect to position and structure of the deduced translation products. The envelope contains glycoproteins namely gB, gC, gD, gE, gG, gH, gI, gJ, gK, gL and gM, which are encoded by highly conserved ORFs viz. UL27, UL44, US6, US8, US4, UL22, US7, US5, UL53, UL1 and UL10, respectively (Piccirillo et al. 2016). The viral glycoproteins are important for ILTV replication and eliciting humoral and cell-mediated immune responses in the host (Roizman and Pellett 2001). There are two clusters of Iltovirus specific genes, one is located between UL45 and UL22 which encodes five ORFs (ORF A-E). The second cluster of Iltovirus specific genes is located between UL-1 and ICP4 and code for UL-0 and UL-1 (Fuchs and Mettenleiter 1996). The other differing features in ILTV genome are absence of an UL16 or its homologue (Roizman and Knipe 2001), localization of UL47 between the US3 and US4 genes within the US region instead of being located within the UL region and internal inversion of a conserved gene cluster within the UL region (McGeoch et al. 1988; Wild et al. 1996). Two regions designated as UL0 and UL (-1), specific to ILTV genome, show noticeable similarities in the deduced amino acid sequences, suggesting a duplication event during virus evolution (Thureen and Keeler 2006). Deletion of UL (-1) gene of ILTV and replacing with the gene encoding green fluorescent protein (GFP) and major immediate promoter element of cytomegalovirus resulted in defective ILTV which was unable to propagate in permissive cells. Thus, the UL (-1) gene has an important role in ILTV replication (Nadimpalli et al. 2017). Like other alphaherpesviruses, the ILTV genome contains three origins of DNA replication, an OriL positioned within the UL region, and two copies of OriS located within the internal repeat (IR) and terminal repeat (TR) regions (Lee et al. 2011). The ORFs vary in their characteristics from other alphaherpesviruses (McGeoch et al. 2006). The tegument proteins help in the transportation of capsid into the cytoplasm and further to the nucleus (Kelly et al. 2009).
Figure 1.
Structure of ILT virus.
Recent advances in molecular techniques enabled rapid identification of genetic variations with precision. Next generation sequencing platforms such as hybrid next generation sequencing (h-NGS) has been found to be useful to identify mutations in genes related to high and low virulence. Garcia et al. (2013) determined the genomic sequences of low and high passage vaccine strains of ILTV, CEO and TCO by h-NGS.
Virus replication and recombination are near inseparable and hence diverse progeny of recombinant ILT viruses emerge out upon co-infection in natural animal host. Based on TaqMan SNP genotyping assay, 11 SNPs within genes UL (-1), US5, US6, US7, US8, US9 and two SNPs in UL43 and UL47 genes were identified confirming high rate of recombination (Loncoman et al. 2017). ILTV, irrespective of either attenuated strain or wild type, upon infecting the target host, replicate, gain or regain virulence to cause disease, and establishes latent infection. Genome level comparison of field strains of ILTV from different countries with commercially used vaccine strains showed that there were only few amino acids in the field strain similar to vaccine strains. This denotes that field strains might have originated from vaccine strain (Garcia and Spatz 2014).
2.2. Viral replication
The replication of ILTV occurs during the first week of infection (Bagust 1986; Williams et al. 1992). Conjunctiva and tracheal mucosa are the major sites of ILTV replication leading to inflammation, serous or mucoid discharge, and respiratory distress (Coppo et al. 2013; Coppo et al. 2013). As ILTV first interacts with the cells lining the nasal cavity, conjunctival mucosa and harderian glands, these tissues play a pivotal role in early virus replication and dictate the fate of infection (Beltrán et al. 2017). Within respiratory system, the epithelial cells that lines larynx and trachea are always affected, while respiratory sinuses, air sacs and lung tissues may or may not be affected (Hanson and Bagust 1991). ILTV can invade the basement membrane of tracheal and conjunctival mucosa in a time dependant manner which promotes virus spread (Reddy et al. 2014). The virus has the ability to establish latency in the trigeminal ganglion during the lytic phase of infection. The ILTV gets reactivated once carrier birds are subjected to stressors such as vaccination, shifting, and during onset of lay. In addition, the ILTV has been detected in other organs, such as the brain, tongue, thymus, lung, heart, proventriculus, pancreas, duodenum, small intestine, large intestine, cecum, cecal tonsils, liver, spleen, kidney, and bursa (Zhao et al. 2013; Wang et al. 2013). These findings raised speculations that the ILTV undergoes systemic replication. Both the vaccine and virulent strains of ILTV could replicate in embryonated chicken neural stem cell; however, cytopathic effects (CPE) such as cell rounding, syncytium formation and cell detachment have been reported in cells infected with vaccine strains of ILTV, but not in cells infected with field virulent strains (Shahsavandi et al. 2017). Increasing numbers of viral nucleic acid in the host cell during virus replication results in accumulation of more viral DNA, subsequently that is incorporated into newly formed viral particles located inside the host nucleus. This leads to development of basophilic intra nuclear inclusion bodies, which can be detected as early as 12 hours of post infection (Reynolds et al. 1968). In the natural host, the replication rate and transmission efficiency has been found to be greater for CEO than TCO strains, and hence the CEO revertant causes a more severe respiratory disease and higher mortality than those caused by TCO revertant (García 2016).
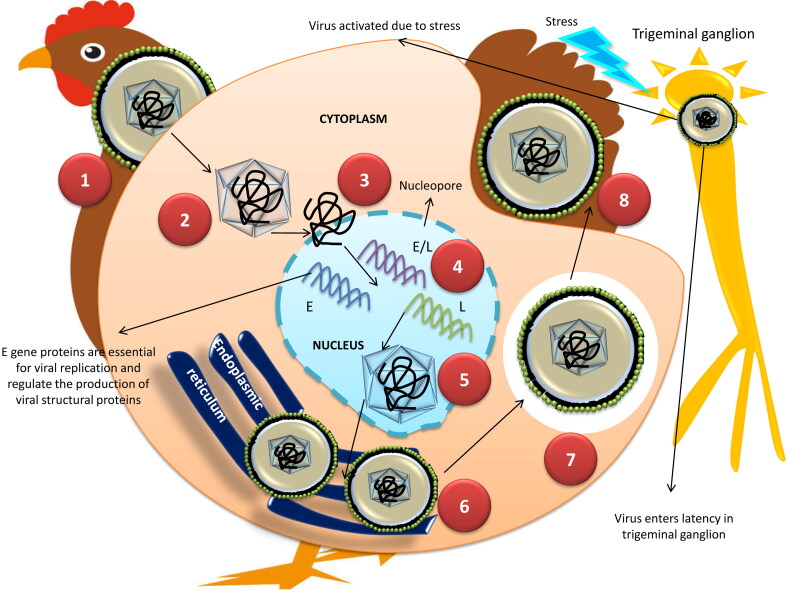
The replication mechanism of ILTV seems similar to other alphaherpesviruses such as HSV-1 (Figure 2). Envelope glycoproteins mainly gC, rather than gB, gD, gH, and gL are assumed to mediate the attachment with host cell receptors (Kingsley et al. 1994; Kingsley and Keeler 1999) and helps in the fusion of the viral envelope to the host cell membrane. The entry of ILTV is heparin sulphate independent, unlike HSV-1 (Kingsley and Keeler 1999). After attachment, the tegument and nucleocapsid get transported into the cytoplasm and the viral DNA released from the nucleocapsid enter into the nucleus through nuclear pores (Trus et al. 2004; Cardone et al. 2007). The highly regulated transcription and replication of ILTV DNA occur within the nucleus by utilizing the host cell machinery (Prideaux et al. 1992; Guo et al. 1993). Three classes of genes, namely immediate early (α), early (β), and late (γ) are expressed during the viral transcription and translation process (Honess and Roizman 1974). The non-structural protein products of α genes play a key role in the expression of β genes between 4 to 16 hrs post-infection (Prideaux et al. 1992). The β gene proteins are critical for viral replication and regulate the production of viral structural proteins encoded by late γ genes. The transcription of γ genes takes place 32 hrs post-infection. Nearly 70 virus-coded proteins regulate the viral DNA replication, which includes several enzymes and DNA binding proteins. In the nucleus, the ILTV DNA replication occurs by a rolling circle mechanism with the formation of concatemer, which is cleaved into monomeric units and packaged into preformed nucleocapsids. The formation of viral capsid and packaging of DNA is completed at the end of the viral replication process in the nucleus of the host cell. The nucleocapsids containing DNA acquire an envelope while budding out from the inner lamellae of the nuclear membrane. Subsequently, the virions are transported into the lumen of the endoplasmic reticulum to acquire second envelope and further accumulate within the cytoplasmic vacuoles (Guo et al. 1993). The virions in the cytoplasm associates with the tegument proteins, and get re-enveloped in the trans-Golgi region during second phase of budding. These virions mature in the cytoplasm and are released by either exocytosis or cell lysis (Guo et al. 1993; Mettenleiter 2002).
Figure 2.
Replication of ILT virus. 1. Attachment 2.Tegument and nucleocapsid get transported into the cytoplasm 3. Viral DNA released from the nucleocapsid enter into the nucleus through nuclear pores 4. Three classes of genes, namely early (E), early/late (E/L) and late (L) are expressed during the viral transcription and translation process based on the levels of expression. 5. Nucleocapsids containing DNA acquire an envelope while budding out from the inner lamellae of the nuclear membrane 6. Virions are transported into the lumen of the endoplasmic reticulum to acquire second envelope and further accumulate within the cytoplasmic vacuoles. 7,8. The vacuoles containing the virions are released out by exocytosis or cell lysis.
In vitro studies demonstrated that development of progeny virus particle occurs 8 to 12 hrs post-infection and reaches the highest concentration within 24 to 30 hrs post-infection (Davison et al. 1989). After successful replication, the establishment of latency takes place 7-10 days post-infection (Bagust and Johnson 1995). The IR flanking sequences get expressed during latent infections known as latency-associated-transcripts (LATs) are up regulated and maintained until the virus gets reactivated to cause the next episode of cytolytic infection (Bagust 1986). Other uncommon features often observed during ILTV replication are formation of tubular structures and large vacuoles containing virions in the infected cytoplasm (Fuchs et al. 2007).
2.3. Antigenicity
Although ILTV strains seem to be antigenically similar based on various assays like immunofluorescence test, virus-neutralization and cross-protection studies (Cover and Benton 1958; Shibley et al. 1962), the difference in virulence has been demonstrated in chicken embryos and in cell culture (Pulsford and Stokes 1953; Jordan 1958; Izuchi and Hasegawa 1982; Russell and Turner 1983). The envelop glycoproteins of ILTV seem to be the potent immunogenic protein capable of stimulating humoral as well as cell mediated immune responses in chicken (York and Fahey 1990). The antigens of ILTV include glycoproteins such as gB, gC, gD, gE, gG, gH, gI, gJ, gK, gL and gM, and are reported to play a crucial role in virus entry and replication (Goraya et al. 2017). Among envelop glycoproteins, glycoprotein G (gG) is identified to facilitate virus entry (Tran et al. 2000), cell-to-cell spread (Nakamichi et al. 2002), and functions as a broad-spectrum viral chemokine binding protein (vCKBP). The gG binds to chemokines of the subfamily C, CC and CXC, and hence prevent the interaction between chemokines and their receptors. It also blocks binding of chemokine to glycosaminoglycans, which is necessary for in vivo chemokine activity (Bryant et al. 2003). The vCKBP of ILTV (gG), during early stages of infection, induces innate immune responses by recruiting particular subsets of immune cells (Devlin et al. 2010).
2.4. Physico-chemical properties
Given the enveloped nature of the virus, the infectivity of ILTV is greatly modulated by organic solvents such as chloroform, ether and oxidizing agents like H2O2 (Fitzgerald and Hanson 1963; Neighbor et al. 1994). The sensitivity of ILTV to the temperature differs greatly between its strains. In respiratory exudates and chicken carcasses, the virus can remain infective for 10 days to 3 months at a temperature range of 13-23 °C. The survivability of the virus can be extended for several months when stored at 4 °C in enrichment media like nutrient and glycerol broth. Previous studies revealed the loss of infectivity of ILTV by heating at 55 °C for 15 minutes or 38 °C for 48 hrs while some strains are resistant to heat (Meulemans and Halen 1978). In deep litter, the ILTV survives for 3-20 days at 11-24.5 °C, in the droppings of battery cages for 3 days at 11–19.5 °C and at least for 3 weeks in buried carcasses. The studies demonstrate that the viability of the virus in litter reduces while applying heat at 38 °C for 24 hrs or composting (Giambrone et al. 2008). The virus gets readily destroyed (<1 min) by common disinfectants like 3% cresol, 5% phenol or a 1% sodium hydroxide solution (Meulemans and Halen 1978), however the presence of organic matter affects the efficiency of disinfectants (Ruano et al. 2001).
2.5. Host
ILTV has got a narrow host range in contrast to other members of alphaherpesviruses. The main natural host of ILTV is chicken, however, the infections are also reported in peacocks, pheasants, turkeys and guinea fowl (Crawshaw and Boycott 1982; Bautista 2003). Though ducks are refractory to ILT infection, they can act as carriers (Yamada et al. 1980). Other domestic and feral birds such as quail, guinea fowl, pigeons, starlings, sparrows, crows, and doves appear to be resistant to the disease (Beach 1931; Brandly and Bushnell 1934).
2.6. Transmission
Infected birds shed the virus in their respiratory secretions for 10 days post-infection. ILTV enters into the host through the respiratory tract, ocular and to a lesser extent through oral routes (Figure 3) (Hitchner et al. 1977; Robertson and Egerton 1981; Bagust 1986; Williams et al. 1992). Direct bird-to-bird transmission is rampant in comparison to contact with latently infected or carrier birds. Mixing of vaccinated and naive chickens is important with respect to direct transmission. Neither vertical transmission nor transmission of virus through the egg shell has been demonstrated. No typical viremia during ILTV infection occurs, although spread of the virus to non-respiratory sites has been attributed to infected leucocytes (Chang et al. 1973; Oldoni et al. 2009). Carrier birds that have survived from previous outbreaks also act as a source of infection to the naive birds. The infected birds readily transmit the disease through the oral secretion as compared to clinically recovered birds or latent carriers (Hughes et al. 1987). The virus usually gets introduced into a flock by direct contact with respiratory exudates or indirect/mechanical transmission of contaminated equipment, litter, feed bags, feathers, vehicles, dust, footwear, clothes, and movement of people (Dobson 1935; Beaudette 1937; Mallinson et al. 1981; Zellen et al. 1984). Recent studies demonstrated that ILTV can persist in the biofilm of drinking water lines and spread to susceptible birds (Ou et al. 2011). Darkling beetles and mealworms also act as a source of infection to the birds and the live virus has been demonstrated in darkling beetles even 42 days after the disease outbreak (Ou and Giambrone 2012). Dogs and cats retrieving dead bird carcasses from affected poultry houses also spread the infection (Kingsbury and Jungherr 1958). Wind-borne transmission of ILTV has been demonstrated between commercial poultry operations (Johnson et al. 2005).
Figure 3.
Transmission pattern of ILT virus.
3. Epidemiology
The disease was first reported in 1925 in the USA (May and Thittsler 1925) and subsequently in Australia, the UK, and Europe (Cover 1996). Veterinarians initially referred to the disease as avian diphtheria, however, the name ILT was adopted in the year 1931 by the special committee of poultry diseases of American Veterinary Medical Association (Guy and Garcia 2008). ILT was the first poultry viral disease for which vaccine was employed based on the cloacal administration (Gibbs 1934). Presently, ILT has been reported in most of the countries worldwide and remains an important disease. The outbreaks are reported in the USA (Dormitorio et al. 2013), Canada (Ojkic et al. 2006), Brazil (Parra et al. 2015), Europe (Neff et al. 2008), Australia (Agnew-Crumpton et al. 2016), China (Zhuang et al. 2014), Egypt (Magouz et al. 2018) and South Asia (Gowthaman et al. 2016). During the period of 2000-2013, the disease had been reported at least in 100 countries (Menendez et al. 2014). Recently, ILTV was confirmed by molecular techniques in Al-Diwaniyah province, Iraq which was the first report from the country (Alaraji et al. 2019). In 2018, three outbreaks of ILT were reported in Windhoek, Namibia causing huge mortality in commercial layers and broilers (Molini et al. 2019). The trend toward high flock density, shorter production cycles, raising of multi-age and multipurpose chicken within same geographical area, and improper vaccination and breach in the biosecurity have contributed to the increased ILT outbreaks across the world (Garcia et al. 2013; Blakey et al. 2019).
ILT remains a serious threat and negatively impacts the poultry industry worldwide since its report in the mid-1920s. Birds of all ages starting from eight days to four years of age (Kingsbury and Jungherr 1958; Jordan 1966; Linares et al. 1994) are susceptible to ILTV infection; however, birds over three weeks of age are reported to be highly susceptible (Dufour-Zavala 2008). High intense poultry rearing, mixing of the different type of birds in the same geographical area and a breach in biosecurity often lead to outbreaks of ILT in many parts of the world. The morbidity and mortality vary depending on the virulence of circulating field strains of ILTV (Devlin et al. 2006; Oldoni et al. 2009), viral load and concurrent infections with other respiratory pathogens (Guy and Garcia 2008). Concomitant respiratory diseases such as Mycoplasma gallisepticum, Mycoplasma synoviae, infectious coryza, other immunosuppressive diseases such as mycotoxicosis, Chicken anaemia virus, Reticuloendotheliosis virus and Marek’s disease virus-induced immunosuppression, possibly exacerbates the impact of ILT in the field (Zavala 2011). Sporadic cases of ILTV may occur in inadequately vaccinated flocks either due to errors in the application of its vaccines or due to biosecurity failures. In multi-aged layer farms, inadequately vaccinated flocks may get exposed to ILTV during the introduction of younger vaccinated flocks into the farm (Hidalgo 2003). The severe epizootic form is characterized by a rapid spread with a high morbidity (90–100%) or variable mortality ranging from 5 to 70% (average of 10-20%) (Hinshaw et al. 1931; Seddon and Hart 1935). The mild epizootic form is characterized by low morbidity (<5%) to very low mortality (0.1-2%) (Raggi et al. 1961). The vaccine and field strains of ILTV evolve as virulent in high dense poultry rearing areas due to the existence of continuous reservoir, subsequently, the same reverent viruses get established in the field and cause outbreaks (Guy et al. 1990; Kotiw et al. 1995). High-density poultry-producing regions often experience huge economic loss with an overall mortality reaching up to 70% (Bagust et al. 2000). It has been reported that areas previously housed infected flocks probably experience more outbreaks than the farms with no history of ILT (Zellen et al. 1984).
The disease has been reported from several Asian (China, Georgia, India, Japan, Lebanon, Myanmar, Philippines, Sabah, Sarawak, Taiwan, Iraq and Uzbekistan), African (Cameroon, Uganda, Namibia, Egypt, Nigeria), North American (Canada, Delaware, Georgia, Mexico, Maryland, New Brunswick, North Carolina, Ontario, Pennsylvania and Virginia), Central American and Caribbean (Costa Rica, Trinidad and Tobago), South American (Argentina, Brazil, Chile, Peru, Suriname and Uruguay), European (Austria, Belgium, Denmark, Germany, Italy, Moldova, Norway, the Netherlands, Poland, Sweden, Switzerland and UK) and Oceania countries (Australia, Cook Islands, French Polynesia, Guam, Kiribati and New Zealand (Hidalgo 2003; Chacón and Ferreira 2009; OIE 2014; Magouz et al. 2018; Alaraji et al. 2019; Molini et al. 2019). Very recently, it was reported that recombinant ILT virus and CEO vaccine-like virus are causing outbreaks in Eygpt (Bayoumi et al. 2020).
Like other herpes viruses, ILTV can establish latency in the trigeminal ganglion of the central nervous system after 7 days of acute infection (Hughes et al. 1991; Williams et al. 1992). The virus gets reactivated under the stress conditions during shifting, onset of laying and mixing of flocks (Hughes et al. 1989). In general, inapparent, sporadic reactivations with productive replication in the tracheal epithelium lead to virus shedding and transmission of infection to susceptible birds (Bagust and Johnson 1995). Earlier studies demonstrated that detection of long-term tracheal carriers (approximately 2%) among convalescent birds recovered from acute ILT infection play a major role in the establishment of latency (Hanson and Hanson 1984). Recent experimental studies revealed sustained detection of ILTV genome in the Harderian gland, trachea, lung and kidney up to 28 days post-infection (Roy et al. 2015). Backyard poultry flocks also act as an important source of infection for commercial poultry flocks because of viral latency (Ojkic et al. 2006; Kirkpatrick et al. 2006; Neff et al. 2008). Research also reveals a high seroprevalence of ILTV (72%) in non-vaccinated flocks suggesting the role of backyard poultry in its epidemiology (Hernandez-Divers et al. 2008).
Latent infected birds are usually identified by tracheal organ culture and detection of ILTV DNA in the trigeminal ganglion by PCR (Bagust 1986).
4. Pathogenesis
The natural portal of entry of ILTV is respiratory and ocular routes. The initial replication takes place in the epithelium of the conjunctiva, respiratory sinuses, larynx and upper respiratory tract to a greater extent (Guy and Bagust 2003). At the primary virus replication sites, the virus titre peaks between 4 and 6 days post-infection, and the virus can be detected in the latency sites Trigeminal ganglion (TRG) from two of cytolytic infections onwards (Bagust 1986; Kirkpatrick et al. 2006; Oldoni et al. 2009). The active cytolytic infection of ILTV results in severe damage to tracheal and conjunctival epithelial lining leading to haemorrhages and other clinicopathological manifestations in birds (Bang and Bang 1967; Tully 1995; Guy and Bagust 2003). Subsequently, the ILTV disseminates to the underlying lamina propria of the tracheal epithelium after invading through the basement membrane with the help of up-regulated cellular proteases (Glorieux et al. 2009; Steukers et al. 2012, Reddy et al. 2014) and reaches to the liver, caecal tonsils and cloaca (Bagust 1986; Oldoni et al. 2009). However, the mechanism of dissemination is not clear. The highest viral titers have been detected in tracheal tissues during 4 to 6 days post-infection and remain in tracheal secretions between 6 to 10 days post-infection (Purcell and McFerran 1969; Hitchner et al. 1977; Robertson and Egerton 1981; Bagust 1986).
The virus replication leads to up-regulation of genes related to cell growth and proliferation. The infected cells produce cytokines and other inflammatory mediators leading to immune responses such as elevated body temperature, intensive edema, and infiltration of lymphocytes (Purcell 1971a; Guy and Garcia 2008). Scattering of CD4+ and CD8+ cells, as well as clustering of B lymphocytes in the mucosa, were detected in ILTV infection (Devlin et al. 2010). At this stage, the outcome of infection is influenced by the type of inflammatory cells and the ability to establish adaptive immune response. ILTV establishes latency in the trigeminal ganglion corresponding to the induction of effective adaptive immunity following the lytic phase of an infection (Williams et al. 1992). Reactivation of ILTV from latency is mediated by thymidine kinase and infected-cell polypeptide 4 (ICP4) (Johnson et al. 1995; Schnitzlein et al. 1995; Han et al. 2002).
5. The disease
5.1. Clinical signs
The incubation period of ILTV varies between 6 and 14 days (Kernohan 1931; Seddon and Hart 1935). Previous experimental studies showed that ILTV shedding started 2 days post-infection and 4 days before the appearance of clinical signs (Davison et al. 1989). The clinical course of ILT varies from 11 days to 6 weeks depending on the form of the disease (McMullin 2004). The clinical signs are characterized by a sudden increase in average daily mortality in the affected flock (Aziz 2010). The severity of the disease is influenced by the virulence of the virus, stress conditions, co-infections with other pathogens, immune status of the flock and age of the birds (Gowthaman et al. 2016). The infection is characterized by peracute, acute and chronic forms of ILT.
5.1.1. Peracute form
It is characterized by sudden onset of rapid spread and high mortality which may exceed 50% (OIE 2014). The affected birds become lethargic, often exhibit moderate-to-severe conjunctivitis with swollen eyelids and increased lacrimation. Sometimes death may occur in birds with good body condition before the appearance of any clinical signs (Preis et al. 2013). The clinical signs (Figure 4) are characterized by dyspnea and gasping with an extension of the head and neck. Coughing, rattling, and gurgling also noticed when the birds try to expel the clotted blood and debris from the obstructed trachea (Guy et al. 1990; Blakey et al. 2019). The clotted blood is also found in cages, feed turfs, walls and floor of the poultry houses. The affected birds usually die within 3 days (Cover 1996).
Figure 4.
Different clinico-pathological manifestations of ILTV infection: a. Acutely infected bird shows severe gasping. b. Oculo-nasal discharges in early stages of infection. c. Facial swelling and persistent oophoria in sub-acute to chronic stage of ILTV infection. d. Dried bloody exudates on the commissure of the mouth. e. Fibrino-haemorrhagic exudates in the lumen of the trachea. f. Blood clots in the lumen of the trachea in acute form of ILT. g. Pseudomembrane formation in chronic form of ILT. h. Diffuse hemorrhagic inflammation of trachea lading to accumulation/obstruction of tracheal lumen with fibrino-haemorrhagic and necrotic tissue debris.
5.1.2. Acute form
Characteristic dyspnea is commonly noticed in the acute form of ILT, but the onset is not sudden or severe as seen in peracute form. Initially, the affected birds become inactive and exhibit anorexia (Guy and Bagust 2003). The internal core temperature increases between 4 and 6 days post-infection, and the total leukocyte count shows mild to marked lymphopenia and heterophilia (Chang et al. 1997). Tracheal obstruction with clotted blood and exudates results in a long drawn out gasps with open-mouthed breathing, high-pitched squawk and moist rales (Kernohan 1931; Jordan 1958). The affected birds may also show purulent conjunctivitis with frothy exudates in the inner canthus of the eye, sinusitis and nasal discharge (Beach 1926). The morbidity may reach 100% and the mortality varies from 10 to 30%, which may last up to 15 days. Varying level of egg production is noticed in layer flocks, some flocks may experience the complete cessation of egg production, which may recover to the normal level in due course of time (Lohr 1977; Creelan et al. 2006).
5.1.3. Chronic form
The mild or chronic ILT resembles with other respiratory infections characterized by unthriftiness, coughing, moist rales, head shaking, squinting eyes, swelling of the infraorbital sinuses (almond-shaped eyes), drop in egg production (up to 10%), and reduced body weight (Hinshaw et al. 1931; Ou et al. 2012). The morbidity may go up to 5% and mortality usually restricted <2% (Bagust et al. 2000).
5.2. Gross lesions
The gross lesions are usually restricted to sinuses and upper respiratory tract and vary with the severity of the disease (Seifried 1931; Gough et al. 1977). The gross lesions in peracute form consist of mucoid rhinitis and haemorrhagic tracheitis with blood clots (Guy and Bagust 2003; Barhoom and Dalab 2012). Yellow caseous exudates (cheesy plug) also observed in primary bronchi when the lesions extend deeply (OIE 2014). In the acute form, yellow caseous diphtheritic membranes adherent to the larynx and mucosa of the upper trachea with or without haemorrhages are commonly noticed (Gowthaman et al. 2014). The membrane also forms obstructive plugs in the larynx and syrinx regions leading to suffocation and death. Excess mucous with or without diphtheritic exudates may be observed in the tracheal lumen in the chronic or mild form of ILT (Linares et al. 1994). A pseudomembrane formation with fibrino-necrotic exudates adhering to the upper respiratory tract can also be noticed (Russell and Turner 1983; Russell 1983). Apart from tracheal involvement, conjunctivitis is characterized by edema and congestion with increased ocular discharge (Hinshaw et al. 1931; Kirkpatrick et al. 2006). The inflammatory response in nares is characterized by heterophilic exudates (Gowthaman et al. 2014). The involvement of lungs and air sacs are rare. However, congestion of the lungs and thickening of air sacs with caseous exudates in the lumen are occasionally seen (Aziz 2010). In concurrently infected cases, lesions such as muco-fibrino acute rhinitis and sinusitis, occlusion of paranasal sinuses by caseous exudate, facial swelling, and muco-fibrino tracheitis have been observed (Couto et al. 2015). Recently, a solitary case of severe erosive esophagitis and pharyngitis accompanied with epithelial degeneration, necrosis, and syncytia formation with intranuclear inclusion bodies has been reported as an atypical ILT (Sary et al. 2017).
5.3. Microscopic lesions
The microscopic lesions are restricted to the conjunctiva, sinuses, trachea, and lungs (Linares et al. 1994). In conjunctiva, they consist of early hyperemia, swelling, infiltration of inflammatory cells, followed by epithelial damage. This further leads to sloughing of conjunctival epithelium with an accumulation of inflammatory exudates primarily containing red and white blood cells and fibrinocellular debris (Figure 4) (Aziz 2010). The initial microscopic changes in trachea include infiltration of inflammatory cells. The infected epithelial cells undergo hyperplastic changes followed by lymphocytic and histiocytic infiltrations in the mucosa and sub-mucosa as the disease advances (Russell 1983). Subsequently, the tracheal epithelial cells undergo necrosis with diffuse denudation that results in protrusion and rupture of blood vessels of lamina propria into tracheal lumen leading to severe laryngitis and tracheitis (Sary et al. 2017). Intranuclear basophilic or eosinophilic inclusion bodies surrounded by a halo are usually seen during initial stages of infection (1–5 days) and disappear later due to necrosis and denudation of epithelial cells (Seifried 1931; Guy et al. 1992; Vanderkop 1993). During this stage, the lumen of the trachea contains varying amount of exudates with fibrin, inflammatory cells, red blood cells, epithelial debris and syncytial cells with or without intranuclear inclusion bodies (Hayashi et al. 1985). Regeneration starts six-days after infection with the proliferation of the remaining basal cells in birds that survive the acute phase (Bagust et al. 2000). Subacute hyperplastic tracheitis characterized by proliferation of several layers of regenerating, undifferentiated, non-ciliated epithelial cells lining the mucosa and mucous glands become evident during the healing stage. The histopathological changes in primary and secondary bronchi are characterized by epithelial degeneration and denudation with infiltration of mononuclear cells (Preis et al. 2013).The syncytial cells with the intranuclear inclusion bodies may also be seen in the lesions (Purcell 1971b; Timurkaan et al. 2003). Gross and histopathology of lesions of ILTV are depicted in Figure 5.
Figure 5.
Gross and Histopathology: (a) Severely congested and hemorrhagic trachea collected from field ILT outbreaks; (b) Cross section of trachea showing intraluminal accumulation of necrotic debris mixed with fibrino-heterophilic exudates (H&E, 4X); (c) Section of trachea showing denudation of mucosal layer, mucosal hemorrhages amidst marked fibrinous exudation (H&E, 10X); (d) Sloughed of tracheal mucosa showing a severe hemorrhages and large multinucleated syncytia (circle) (H&E, 20X); Higher magnification of syncytia (arrow) showing presence of intranuclear eosinophilic inclusion bodies (star marks) (H&E, 100X).
6. Diagnosis
Infectious laryngotracheitis in chicken can be tentatively diagnosed based on the clinical signs such as conjunctivitis, gasping, open mouth or extended head respiration, expectoration of bloody mucous, dyspnoea, and finding lesions including catarrhal to hemorrhagic tracheitis, fibrinopurulent to caseous exudates or cheesy or caseous plugs in the larynx and trachea on necropsy. The suspected cases are subjected to laboratory diagnosis by conventional and molecular diagnostic tests. The conventional methods include histopathology, virus isolation by embryonated chicken eggs and cell culture, immunofluorescence (IF), immunoperoxidase (IP) assay, and serology (Burnet 1934; Wilks and Kogan 1979; Hughes and Jones 1988; Guy et al. 1992; Godoy et al. 2013). Detection of syncytial cells and intranuclear inclusion bodies in the trachea, eyelid, and lung tissues using histopathology is routinely practiced (Humberd et al. 2002; Timurkaan et al. 2003; Srinivasan et al. 2012).
The preferred clinical samples for isolation of ILTV are conjunctiva, larynx, trachea, lung and their exudates. Among the different clinical materials, lungs, tracheal scrapings, and exudates from trachea are ideal for virus isolation (Tripathy and Garcia 1998). The ILTV is usually isolated and propagated in 9-11 days-old embryonated chicken eggs through chorioallantoic membrane (CAM) inoculation. Opaque plaques can be observed in ILTV infected CAM as early as 48 h post-inoculation and embryo death occurs between 2 and 8 days post-infection. The embryonic survival time increases with subsequent additional egg passages leading to effective replication of the virus to significant titers (Garcia and Riblet 2001).
The number of primary avian cell cultures, including chicken embryo liver (CEL), chicken embryo lung, chicken embryo kidney (CEK), and chicken kidney (CK) cell cultures are commonly used for ILTV isolation (Chang et al. 1977; Meulemans and Halen 1978; McNulty et al. 1985; Hughes and Jones 1988; Schnitzlein et al. 1995). The cell culture method is more economical and rapid than egg inoculation. The sensitivity of the isolation and virus yield are influenced by the type of cell cultures. The CEL is found to be the most sensitive for ILTV isolation followed by CK. The CEK, chicken embryo lung, chicken embryo fibroblast, Vero, and quail cells were found less sensitive to ILTV infection (Hughes and Jones 1988; Garcia et al. 2014). In addition, Leghorn male hepatoma cells were also used to propagate the virus in research laboratories (Schnitzlein et al. 1995). The cytopathic effects of ILTV infection are characterized by the swelling of cells, chromatin displacement, rounding of the nucleoli and syncytia formation. Intranuclear inclusions are detected as early as 12 hrs post-infection, however, the formation of multinucleated giant cells may be observed 24 hrs post-infection in avian leukocyte cultures (Hinshaw et al. 1931; Chang et al. 1977). The plaque size and morphology are influenced by the strains of ILTV (Srinivasan and Malick 1977; Hughes and Jones 1988). CEK cells infected with ILTV reveal presence of large cytoplasmic vesicles, which become basophilic mass as the cells degenerate (Reynolds et al. 1968). Macrophage culture is equally susceptible to ILTV. However, the viral replication is limited (Calnek et al. 1986). Other cell lines from heterologous hosts such as QT35 or IQ1A from quail-origin, and Vero cells permit limited replication but with a very low virus titre even after several passages. Other culture systems routinely used are tracheal organ culture (TOC) and conjunctival organ cultures (COC) obtained from chicken embryos or day old chicks. However, these TOC and COC are utilized to study the host pathogen interaction (Bagust 1986; Jones and Hennion 2008; Reemers et al. 2009).
Apart from virus isolation, the IF, IP, and immunohistochemistry (IHC) can be used to detect the ILTV in tracheal tissues and smears (Ide 1978). The sensitivity of IHC is reported more superior than IF (Hitchner et al. 1977). The distribution of ILTV antigen within different tissues of respiratory tract is highly variable and highest IHC positivity has always been found in trachea than any other organs (Yavuz et al. 2018).
Agar gel immune diffusion (AGID) technique using ILTV hyperimmune serum is commonly used to differentiate it from a diphtheritic form of fowlpox (Fukui et al. 2016). However, the sensitivity was lower when compared with other serological techniques like virus neutralization test (Devlin et al. 2011), indirect immunofluorescence test and ELISA (Jordan and Chubb 1962; Godoy et al. 2013). Antigen-capture ELISA (AC-ELISA) using ILTV monoclonal antibodies is applied for rapid and more accurate detection of ILTV than AGID, IF or virus neutralization (York and Fahey 1990). As a gold standard, ELISA is preferred for the detection of antibodies from the field sample. Recently, glycoprotein D (gD) based ELISA has been developed where two immunogenic regions were identified and synthesized. This synthetic peptide was used in the developed ELISA which showed sensitivity of 96.9% and specificity of 87.5% (Kumar et al. 2019).
Although the conventional methods are cost-effective and widely applied in diagnostic laboratories, these methods have some limitations like low sensitivity, labor-intensive and time-consuming. Several molecular-based techniques such as PCR, real-time PCR, nested PCR, restriction fragment length polymorphism (RFLP), in situ hybridization have been applied to detect the ILTV because of its high sensitivity, accuracy, rapidity, reproducibility, and simplicity (Nielsen et al. 1998; Vögtlin et al. 1999; Humberd et al. 2002; Creelan et al. 2006; Callison et al. 2007; Mahmoudian et al. 2011; Zhao et al. 2013). Both the probe based and dye based real-time PCR assays and nested real time PCR are found to be highly sensitive as these assays can detect as low as 19 to 1° copies of virus in biological samples (Zhao et al. 2013; Davidson et al. 2015; Santander Parra et al. 2018). Although these molecular methods have significant diagnostic value, they do not discriminate between viable and non-viable virions (Menendez et al. 2014). Hence, positive results need to be carefully interpreted and carryover contamination should be ruled out.
Among different molecular techniques, PCR and quantitative real-time PCR (qRT-PCR) are the widely used and preferred molecular assays for confirmation and quantification of viral load in biological samples due to their higher diagnostic sensitivity and accuracy (Guy et al. 1992; Williams et al. 1992; Scholz et al. 1994; Abbas and Andreasen 1996; Creelan et al. 2006; Kirkpatrick et al. 2006; Fuchs et al. 2007; Chacón and Ferreira 2009; Zhao et al. 2013; OIE 2014; Roy et al. 2015; Santander Parra et al. 2018). Earlier, the wild and vaccine strains of ILTV were differentiated based on restriction length polymorphism (RFLP) profiles (Leib et al. 1986; Andreasen et al. 1990; Keeler et al. 1993; Kotiw et al. 1995; Oldoni and García 2007; Craig et al. 2017). Recently, Fakhri et al. (2019) developed high-resolution melting (HRM) analysis to classify ILTV strains and detect ILTV recombination events during field outbreaks. The recent advances in molecular sequencing technologies enabled rapid identification of genetic variations with high precision. Next generation sequencing (NGS) platforms such as hybrid next generation sequencing (h-NGS) are found to be useful to identify mutations in genes related to high and low virulence. Genomic sequences of low and high passaged CEO and TCO ILTV strains were determined by h-NGS wherein both the CEO and TCO strains expressed variable mutations upon passages in the target host. The common genes mutated in these two strains were ORFC, UL27, UL28, UL39, and the virulent ILTV strains isolated in USA showed frequent Thr644 mutation within UL27 gene. Although the genes responsible for reversion to virulence are not very clear, the gene segment US10 has been identified as one of the potential virulence factors for TCO revertant. Similarly, the gene UL41 had been found to be responsible for robust gain in virulence of CEO strains (Garcia et al. 2013). MinIon sequencing was also used as diagnostic tool in USA to genotype the different ILTV isolates. Full genome (n = 27) of ILTV were analyzed and it was identified to have 9 genotypes which can be grouped into 5 genotypes based on single allele assay using MinIon (Spatz et al. 2019).
Recently, a TaqMan single nucleotide polymorphism genotyping (TaqMan-SNP) assay has been developed to study the ILTV recombination in the natural host. Based on this assay, 11 SNPs within genes UL (-1), US5, US6, US7, US8, US9 and two SNPs in UL43 and UL47 genes were identified, and 67% of the progeny ILT viruses were found to be recombinant (Loncoman et al. 2017).
7. Differential diagnosis
The other respiratory diseases exhibiting similar clinical disease must be differentiated from ILT. The diphtheritic lesions induced by ILT spread over the whole length of trachea and resemble lesions induced by the fowlpox virus (Tripathy and Reed 2013). Tracheal lesions in mild or low virulent form of ILTV is similar to that of lesions caused by other respiratory pathogens such as avian influenza virus, Newcastle disease virus, infectious bronchitis virus and fowl adenovirus (Davidson et al. 2015).
7.1. Differentiation of field isolates and vaccine strains
Differentiation of field and vaccine strains of ILTV is complicated because of its high antigenic and genetic resemblance (Guy and Garcia 2008). Several methods including chicken embryo virulence test (Izuchi and Hasegawa 1982), restriction endonuclease analysis (Keller et al. 1992), and DNA hybridization assays (Kotiw et al. 1995) have been attempted to differentiate the wild-type and vaccine strains of ILTV. Later, these methods have been replaced by PCR-RFLP of multiple genes and genome regions including ICP4, TK, gE, gG, ORFB-TK, and ICP18.5 to UL43 genes (Neff et al. 2008; Moreno et al. 2010). A recent approach using sequence analysis of ICP4 gene was successfully used to differentiate vaccine and wild-type strains of ILTV (Chacón and Ferreira 2009).
8. Vaccination
Good biosecurity practices combined with vaccination are the practical methods to control ILTV in the absence of any effective treatment. Nevertheless, ILTV was the first major poultry disease for which an effective vaccine was introduced (Gibbs 1934). However, the disease remains an important issue in the poultry-dense areas (Couto et al. 2015; Chacón et al. 2015; Yan et al. 2016; Gowthaman et al. 2016). The modified live attenuated ILTV vaccines including CEO and TCO have been used for several decades. Chicken embryo origin live attenuated vaccines were the first commercially used vaccine which were introduced on the market during 1950s and start of 1960s (García and Zavala 2019). The protective efficacy of CEO vaccines is better when compared to TCO vaccines (Andreasen et al. 1989). These vaccines are used for prevention as well as during the phase of an outbreak to control virus spread and shorten its duration (Bagust et al. 2000).
Preventive vaccination of ILTV is given at 6 to 8 weeks of age, followed by the booster at 12 to 15 weeks for layers and breeders (Gingerich and Carver 2006). These vaccines elicit the immune response by causing infection in the trachea without producing disease. The highest protective immunity is attained from 15 to 20 weeks post-vaccination, which may last over a year (Neff et al. 2008) and no interference has been reported between ILT and other vaccines if the vaccine interval is more than 2 weeks (Aston et al. 2019). ILTV vaccination is not suggested for broilers because of its economical concern (Giambrone et al. 2008). The route of vaccine administration has always been critical to ensure protection and avoid adverse vaccine reactions. The eye drop method is considered comparatively safer and gives more protection than mass application methods like drinking water and spray administration. A superior ILTV vaccine must contain a titer of >102 plaque-forming units/ml to induce adequate immunity when delivered by routes other than the oral route (Raggi and Lee 1965). Apart from the effectiveness, CEO and TCO vaccines have undesirable properties of reversal to the virulent form following bird to bird passages leading to vaccinal laryngotracheitis in the field (Dufour-Zavala 2008; Chacón et al. 2015). In some occasions, vaccination leads to the creation of latent carrier birds, which act as a source of infection to unvaccinated flocks (Bagust 1986). These latent viruses are reactivated, leading to intermittent shedding of ILTV when the birds are subjected to stress conditions like onset of lay, transport, vaccination, etc. causing further spread of disease to the susceptible birds (Guy et al. 1990; Hughes et al. 1991). Extensive use of live attenuated vaccines resulted in new outbreaks of ILT in many parts of the world. Previous experimental studies suggested exacerbated prolonged ILT infections following poor mass CEO vaccination (García 2016). Another study showed that CEO vaccinated birds had better protection even at 35 weeks of age when compared with TCO or HVT-LT (Palomino-Tapia et al. 2019).
A study of ILTV outbreaks in different geographical regions of USA revealed that 75% of the ILTV isolated from the field were resulting from CEO vaccine strains (Garcia and Riblet 2001). Recent studies revealed that spontaneous natural recombination between attenuated vaccines in the field leads to the emergence of novel virulent variants of ILTV (Lee et al. 2012; Agnew-Crumpton et al. 2016). Very recently, whole genome analysis of an ILTV isolate in Australia revealed that recombination is a continuous process leading to virulent virus. The isolate was suggested to be a recombinant of vaccine strain and another recombinant virus (Sabir et al. 2020). To overcome the limitations and biosafety concerns of conventional vaccines, recombinant vaccines such as FPV vector vaccine expressing glycoprotein B and UL32 genes of ILTV(McGeoch et al. 2006), two HVT vector vaccines, one containing ILTV glycoproteins I and D, and another containing ILTV glycoprotein B (Esaki et al. 2013), LaSota strain of Newcastle diseases virus (NDV) that expresses ILTV glycoproteins (Kanabagatte Basavarajappa et al. 2014; Zhao et al. 2014), modified very virulent Marek’s disease virus (vvMDV) that express ILTV glycoproteins (Gimeno et al. 2015) and recombinant vaccines expressing different ILTV glycoproteins including gB, gC, gD, gG, gI, gJ, TK, UL0, UL32, and UL47 (Vagnozzi et al. 2012; Coppo et al. 2013; Yu et al. 2017) were introduced and evaluated. NDV vector expressing gD of ILTV protected birds against both ILT and ND. Similarly the construct was stable and safe even after 8 chicken egg passages (Yu et al. 2020). The F gene of NDV, gD and gI genes of ILTV double recombinant HVT vector vaccine ((HVT-NDV-ILT) showed 97%, 94% and 97% protection against velogenic NDV (GB Texas), ILTV (LT 96-3) and Marek’s disease virus (GA 5) strains, respectively (Gergen et al. 2019). The advantages of these recombinant vaccines are lack of transmission, the absence of reversion to a virulent form, and lack of latency (Johnson et al. 2010; Coppo et al. 2013; Coppo et al. 2013; Zhao et al. 2014). Utilizing a bacterial artificial chromosome (BAC), genes encoding glycoprotein B (gB) or glycoprotein J (gJ) of ILTV were introduced into meq gene deleted very virulent MDV (vvMDV) to create the BACDMEQ-gB and BACDMEQ- gJ recombinant strains, and the resulted BACDMEQ-gB recombinant had conferred immunity after subcutaneous vaccination at day one after hatch which was comparable to commercial ILT-HVT vectored vaccine (Gimeno et al. 2015; García and Zavala 2019). A study was conducted using the recombinant herpesvirus of turkey based ILT vaccine rHVT-LT and CEO ILT vaccine to know the effect of combined vaccine, to know the effect of rHVT-LT on CEO vaccine and protective efficacy of the vaccine. Results showed that birds primed with rHVT-LT followed by booster with CEO showed reduction in replication of CEO virus and protection was good in combined vaccine compared with rHVT-LT alone (Maekawa et al. 2019). Another study showed that in ovo vaccination of rHVT-LT did not stop the challenge virus spread to naive birds (Maekawa et al. 2019). Eye drop vaccination of CEO based ILT vaccine showed that conjunctiva-associated lymphoid tissues (CALT) and Harderian gland (HG) had a strong role in development of immunity against ILTV (Beltrán et al. 2017). Virus like particles (VLPs) carrying glycoproteins B (gB) or G (gG) had been recently developed and were studied by administration through in ovo and intra muscular route. VLP-gG in ovo vaccination produced antibody response and there was no side effect due to in ovo vaccination. Hence in ovo vaccination using VLPs can be a promising option for control of ILT (Schädler et al. 2019).
These vaccines are administered in ovo at day 18th of embryonating period or subcutaneous route during one day of age. Although the recombinant vaccines have the advantages over conventional vaccines, they fail to give sterile immunity (Johnson et al. 2010; Vagnozzi et al. 2012), moreover protection against ILT was severely affected when ILT and IBD products were inserted into separate HVT vectors (Dunn et al. 2018), because of competition for replication. Hence, it is necessary to develop duel insert vaccines to overcome this disadvantage. Studies have been attempted to develop new ILTV vaccines using deletion of genes such as TK (Han et al. 2002), UL0 (Veits et al. 2003), gJ (Fuchs et al. 2005), gG (Devlin et al. 2006), UL47 (Helferich et al. 2007) and gC (Pavlova et al. 2010). Most recently, Ali et al. (2019) analysed nine epitopes as promising vaccine candidate against ILTV which included 3 B cell epitopes (190KKLP193, 386YSSTHVRS393, and 317KESV320) and six T cell epitopes which comprised three MHC-I binding epitopes (118YVFNVTLYY126, 335VSYKNSYHF343, and 622YLLYEDYTF630) and three MHC-II binding epitopes (301FLTDEQFTI309, 277FLEIANYQV285, and 743IASFLSNPF751). Though these findings are promising, it needs further studies before commercially introducing it into poultry industry. As on today HVT and fowl pox vector based recombinant ILT vaccines are available in the market. The novel approaches that are independent of the immune system of the host, including a high level of biosecurity, exploration of host genetic resistance and further improvement of novel vaccines are needed to control ILTV outbreaks.
9. Control and eradication
ILT remains a significant disease in all intensive poultry producing regions of the world. The eradication of ILTV requires an implementation of coordinated control programme with the cooperative effort of government agencies, laboratories, poultry producers, poultry health companies, and veterinarians (Dufour-Zavala 2008). The control measures should be focused on timely diagnosis, implementation of strict biosecurity, cleaning, and disinfection, application of geographic information system (GIS) technology, vaccination, and communication between poultry farmers and control agencies (Mallinson et al. 1981; Guy and Garcia 2008). There are few reports regarding the use of herbal drugs for the treatment of ILTV. At higher concentration, Yinhuangerchen, a Chinese herbal mixture reduced the level of ILTV in tissues and also developed mucosal immunity, in birds treated with Yinhuangerchen mixture after 72 hours post-infection (Zhang et al. 2018). Cheng et al. (2011) found that the Huangqi Maxingshigan decoction, containing five herbal medicines (Almond, Gypsum fibrosum, Herba ephedrae, Radix astragali, Radix glycytthizae) provided an antioxidant defense in the process of anti-ILT. Additionally, it can enhance mucosal immunity through induction of sIgA production.
An effective biosecurity plan includes site quarantine and hygiene, restriction of movement of potentially contaminated workers, equipment, feed, vehicles, and birds. Proper disinfectant and litter decontamination should be taken into consideration. Preventive measures should also focus on the control of feral birds, rodents, dogs and cats accessing the barns (Volkova et al. 2012). The dead birds should be properly removed and disposed of safely. Proper cleaning and disinfection of poultry houses should be carried out and the downtime should be extended between subsequent batches. The backyard and fancy chicken flocks should be closely monitored and included in the eradication plan since they may act as reservoirs of ILTV (Mallinson et al. 1981). Further, the virus spread and length of an outbreak can be reduced by therapeutic vaccination. The maximum level of diversity in the ILTV progenies was associated with increased frequency of recombination in the ‘hot-spot’ regions of the virus genome (Loncoman et al. 2017).
10. Conclusions and future prospects
Rapid expansion of poultry population has led to increased outbreaks of ILT in many poultry producing regions of the world particularly in countries with high poultry density. Being a Gallid herpesvirus 1, ILTV possesses all the common features of other herpes viruses such as latency and carrier status. Since its first detection in 1925 in USA, ILTV became well established in poultry populations where CEO origin vaccine has been intensively used as a part of control programme. Like other herpesviruses, ILTV undergoes latency in trigeminal ganglion and get reactivated whenever the birds undergo stress leading to increased shedding and environmental spread which makes eradication of ILTV difficult. The darkling beetles acts as an important carrier of ILTV in poultry environments. Further, secondary infections increase the severity of the clinical disease and economic losses. Extensive research have resulted in increased understanding of herpesvirus transmission, pathogenesis and control. This knowledge helps to reduce the impact of ILTV in poultry industry in near future.
Virus isolation, serological techniques and histopathology have been commonly used to diagnose the disease. Modern diagnostic techniques such as PCR, PCR-RFLP, Real time PCR, and NGS have been commonly applied to understand the epidemiology of ILT outbreaks. Increased use of CEO vaccines without much biosecurity leads to outbreaks and persistence of vaccinal ILT worldwide. Recombinant vaccines have been developed by expressing ILTV surface glycoproteins in vectors such as HVT, NDV and Fowl pox virus as an alternative control strategy. Though vectored vaccines show some protection, they are not fully successful in controlling ILT outbreaks. Hence, more sophisticated vaccines need to be developed for ILTV by using advanced biotechnological tools including reverse genetics, recombinant DNA technology with use of novel adjuvants and exploiting advanced delivery methods by overcoming the disadvantages of commercially available vaccines. Besides vaccination, reducing stress conditions, adapting strict biosecurity measures and implementing appropriate pest control programmes are important in ILTV control programme to be made more effective. Of note, certain herbal extracts have been found promising in reducing the disease severity.
ILT remains a significant threat to the poultry industry worldwide. Improved understanding of the virus biology, epidemiology, and pathogenesis along with strict biosecurity may help to control the disease outbreaks. The coordinated plan including rapid diagnosis, implementing strict biosecurity, the vaccination programme, use of GIS technology, proper cleaning, disinfection and heating of poultry houses and increased communication between government and industry will be the most effective approach in controlling ILTV.
Acknowledgment
The poultry research in the laboratory of first author is currently supported by the BBSRC, UK(BB/P025749/1). Other authors acknowledge their University and Institute.
Disclosure statement
No potential conflict of interest was reported by the authors.
Authors’ contributions
All the authors substantially contributed to the conception, design, analysis and interpretation of data, checking and approving final version of the manuscript, and agree to be accountable for its content.
References
- Abbas F, Andreasen JR.. 1996. Comparison of diagnostic tests for infectious laryngotracheitis. Avian Dis. 40(2):290–295. [PubMed] [Google Scholar]
- Agnew-Crumpton R, Vaz PK, Devlin JM, O'Rourke D, Blacker-Smith HP, Konsak-Ilievski B, Hartley CA, Noormohammadi AH.. 2016. Spread of the newly emerging infectious laryngotracheitis viruses in Australia. Infect Genet E. 43:67–73. [DOI] [PubMed] [Google Scholar]
- Alaraji F, Hammadi H, Abed AA, Khudhair YI.. 2019. Molecular detection and phylogenetic tree of infectious laryngotracheitis virus in layers in Al-Diwaniyah province, Iraq. Vet World. 12(4):605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SA, Almofti YA, Abd-Elrahman KA.. 2019. Immunoinformatics approach for multiepitopes vaccine prediction against Glycoprotein B of Avian infectious laryngotracheitis virus. Adv Bioinforma. 2019:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen JR, Glisson JR, Goodwin MA, Resurreccion RS, Villegas P, Brown J.. 1989. Studies of infectious laryngotracheitis vaccines: immunity in layers. Avian Dis. 33(3):524–530. [PubMed] [Google Scholar]
- Andreasen JR, Glisson JR, Villegas P.. 1990. Differentiation of vaccine strains and Georgia field isolates of infectious laryngotracheitis virus by their restriction endonuclease fragment patterns. Avian Dis. 34(3):646–656. [PubMed] [Google Scholar]
- Aston EJ, Jordan BJ, Williams SM, García M, Jackwood MW.. 2019. Effect of pullet vaccination on development and longevity of immunity. Viruses. 11(2):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz T. 2010. Infectious Laryngotracheitis (ILT) targets broilers. World Poult. 25:17–18. [Google Scholar]
- Bagust TJ. 1986. Laryngotracheitis (Gallid-1) herpesvirus infection in the chicken. 4. Latency establishment by wild and vaccine strains of ILT virus. Avian Pathol. 15:581–595. [DOI] [PubMed] [Google Scholar]
- Bagust TJ, Johnson MA.. 1995. Avian infectious laryngotracheitis: virus-host interactions in relation to prospects for eradication. Avian Pathol. 24(3):373–391. [DOI] [PubMed] [Google Scholar]
- Bagust T, Jones R, Guy JS.. 2000. Avian infectious laryngotracheitis. Rev Sci Tech Oie. 19(2):483–492. [DOI] [PubMed] [Google Scholar]
- Bang BG, Bang FB.. 1967. Laryngotracheitis virus in chickens. A model for study of acute nonfatal desquamating rhinitis. J Exp Med. 125(3):409–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhoom S, Dalab A.. 2012. Molecular diagnosis of explosive outbreak of Infectious Laryngotracheitis (ILT) by polymerase chain reaction in Palestine. Proc Elev Vet Sci Conf. :104–109. [Google Scholar]
- Bautista D. 2003. Isolation of infectious laryngotracheitis vírus (ILTV) from peafowls and chickens with a history of respiratory diseases. Proceeding 140th AVMA Annu Conv 2003 Colo Springs Colo USA; p. 24. [Google Scholar]
- Bayoumi M, El-Saied M, Amer H, Bastami M, Sakr EE, El-Mahdy M.. 2020. Molecular characterization and genetic diversity of the infectious laryngotracheitis virus strains circulating in Egypt during the outbreaks of 2018 and 2019. Arch Virol. 165(3):661–670. [DOI] [PubMed] [Google Scholar]
- Beach J. 1926. Infectious bronchitis of fowls. J Am Vet Med Assoc. 68:570–580. [Google Scholar]
- Beach J. 1931. A filterable virus, the cause of infectious laryngotracheitis of chickens. J Exp Med. 54(6):809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudette F. 1937. Infectious laryngotracheitis. Poult Sci. 16(2):103–105. [Google Scholar]
- Beltrán G, Williams SM, Zavala G, Guy JS, García M.. 2017. The route of inoculation dictates the replication patterns of the infectious laryngotracheitis virus (ILTV) pathogenic strain and chicken embryo origin (CEO) vaccine. Avian Pathol. 46(6):585–593. [DOI] [PubMed] [Google Scholar]
- Blacker HP, Kirkpatrick NC, Rubite A, O'Rourke D, Noormohammadi AH.. 2011. Epidemiology of recent outbreaks of infectious laryngotracheitis in poultry in Australia. Aust Vet J. 89(3):89–94. [DOI] [PubMed] [Google Scholar]
- Blakey J, Stoute S, Crossley B, Mete A.. 2019. Retrospective analysis of infectious laryngotracheitis in backyard chicken flocks in California, 2007-2017, and determination of strain origin by partial ICP4 sequencing. J VET Diagn Invest. 31(3):350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandly C, Bushnell L.. 1934. A report of some investigations of infectious laryngotracheitis. Poult Sci. 13(4):212–217. [Google Scholar]
- Bryant NA, Davis-Poynter N, Vanderplasschen A, Alcami A.. 2003. Glycoprotein G isoforms from some alphaherpesviruses function as broad-spectrum chemokine binding proteins. Embo J. 22(4):833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet F. 1934. The propagation of the virus of infectious laryngotracheitis on the CAM of the developing egg. Br J Exp Pathol. 15:52–55. [Google Scholar]
- Callison SA, Riblet SM, Oldoni I, Sun S, Zavala G, Williams S, Resurreccion RS, Spackman E, García M.. 2007. Development and validation of a real-time Taqman PCR assay for the detection and quantitation of infectious laryngotracheitis virus in poultry. J Virol Methods. 139(1):31–38. [DOI] [PubMed] [Google Scholar]
- Calnek BW, Fahey KJ, Bagust TJ.. 1986. In vitro infection studies with infectious laryngotracheitis virus. Avian Dis. 30(2):327–336. [PubMed] [Google Scholar]
- Cardone G, Winkler DC, Trus BL, Cheng N, Heuser JE, Newcomb WW, Brown JC, Steven AC.. 2007. Visualization of the herpes simplex virus portal in situ by cryo-electron tomography. Virology. 361(2):426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacón JL, Ferreira A.. 2009. Differentiation of field isolates and vaccine strains of infectious laryngotracheitis virus by DNA sequencing. Vaccine. 27(48):6731–6738. [DOI] [PubMed] [Google Scholar]
- Chacón JL, Núñez LFN, Vejarano MP, Parra SHS, Astolfi-Ferreira CS, Ferreira A.. 2015. Persistence and spreading of field and vaccine strains of infectious laryngotracheitis virus (ILTV) in vaccinated and unvaccinated geographic regions, in Brazil. Trop Anim Health Prod. 47(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- Chang PW, Jasty V, Fry D, Yates VJ.. 1973. Replication of a cell-culture-modified infectious laryngotracheitis virus in experimentally infected chickens. Avian Dis. 17(4):683–689. [PubMed] [Google Scholar]
- Chang PC, Lee YL, Shien JH, Shieh HK.. 1997. Rapid differentiation of vaccine strains and field isolates of infectious laryngotracheitis virus by restriction fragment length polymorphism of PCR products. J Virol Methods. 66(2):179–186. [DOI] [PubMed] [Google Scholar]
- Chang PW, Sculco F, Yates VJ.. 1977. An in vivo and in vitro study of infectious laryngotracheitis virus in chicken leukocytes. Avian Dis. 21(4):492–500. [PubMed] [Google Scholar]
- Cheng J, Li Q, Shi W, Zhong X.. 2011. Effects of Huangqi Maxingshigan decoction on infectious laryngotracheitis in chickens. Ital J Anim Sci. 10:179–186. [Google Scholar]
- Coppo MJC, Hartley CA, Devlin JM.. 2013. Immune responses to infectious laryngotracheitis virus. Dev Comp Immunol. 41(3):454–462. [DOI] [PubMed] [Google Scholar]
- Coppo MJC, Noormohammadi AH, Browning GF, Devlin JM.. 2013. Challenges and recent advancements in infectious laryngotracheitis virus vaccines. Avian Pathol. 42(3):195–205. [DOI] [PubMed] [Google Scholar]
- Couto RdM, Preis IS, Braga JFV, Brasil BSAF, Drummond MG, Martins NRdS, Ecco R.. 2015. Molecular characterization of infectious laryngotracheitis virus in naturally infected egg layer chickens in a multi-age flock in Brazil. Arch Virol. 160(1):241–252. [DOI] [PubMed] [Google Scholar]
- Cover MS. 1996. The early history of infectious laryngotracheitis. Avian Dis. 40(3):494–500. [PubMed] [Google Scholar]
- Cover MS, Benton W.. 1958. The biological variation of infectious laryngotracheitis virus. Avian Dis. 2(4):375–383. [Google Scholar]
- Craig MI, Rojas MF, van der Ploeg CA, Olivera V, Vagnozzi AE, Perez AM, König GA.. 2017. Molecular characterization and cluster analysis of field isolates of Avian Infectious Laryngotracheitis Virus from Argentina. Front Vet Sci. 4:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawshaw GJ, Boycott BR.. 1982. Infectious laryngotracheitis in peafowl and pheasants. Avian Dis. 26(2):397–401. [PubMed] [Google Scholar]
- Creelan JL, Calvert VM, Graham DA, McCullough SJ.. 2006. Rapid detection and characterization from field cases of infectious laryngotracheitis virus by real-time polymerase chain reaction and restriction fragment length polymorphism. Avian Pathol. 35(2):173–179. [DOI] [PubMed] [Google Scholar]
- Davidson I, Raibstein I, Altory A.. 2015. Differential diagnosis of fowlpox and infectious laryngotracheitis viruses in chicken diphtheritic manifestations by mono and duplex real-time polymerase chain reaction. Avian Pathol. 44(1):1–4. [DOI] [PubMed] [Google Scholar]
- Davison AJ, Eberle R, Ehlers B, Hayward GS, McGeoch DJ, Minson AC, Pellett PE, Roizman B, Studdert MJ, Thiry E.. 2009. The order Herpesvirales. Arch Virol. 154(1):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison S, Smith G, Eckroade RJ.. 1989. Laryngotracheitis in chickens: the length of the preinfectious and infectious periods. Avian Dis. 33(1):18–23. [PubMed] [Google Scholar]
- Devlin JM, Browning GF, Hartley CA, Kirkpatrick NC, Mahmoudian A, Noormohammadi AH, Gilkerson JR.. 2006. Glycoprotein G is a virulence factor in infectious laryngotracheitis virus. J Gen Virol. 87(10):2839–2847. [DOI] [PubMed] [Google Scholar]
- Devlin JM, Hartley CA, Gilkerson JR, Coppo MJC, Vaz P, Noormohammadi AH, Wells B, Rubite A, Dhand NK, Browning GF.. 2011. Horizontal transmission dynamics of a glycoprotein G deficient candidate vaccine strain of infectious laryngotracheitis virus and the effect of vaccination on transmission of virulent virus. Vaccine. 29(34):5699–5704. [DOI] [PubMed] [Google Scholar]
- Devlin JM, Viejo-Borbolla A, Browning GF, Noormohammadi AH, Gilkerson JR, Alcami A, Hartley CA.. 2010. Evaluation of immunological responses to a glycoprotein G deficient candidate vaccine strain of infectious laryngotracheitis virus. Vaccine. 28(5):1325–1332. [DOI] [PubMed] [Google Scholar]
- Dobson N. 1935. Infectious laryngotracheitis in poultry. Vet Rec. 15:1467–1471. [Google Scholar]
- Dormitorio TV, Giambrone JJ, Macklin KS.. 2013. Detection and isolation of infectious laryngotracheitis virus on a broiler farm after a disease outbreak. Avian Dis. 57(4):803–807. [DOI] [PubMed] [Google Scholar]
- Dufour-Zavala L. 2008. Epizootiology of infectious laryngotracheitis and presentation of an industry control program. Avian Dis. 52(1):1–7. [DOI] [PubMed] [Google Scholar]
- Dunn JR, Dimitrov KM, Miller PJ, Garcia M, Turner-Alston K, Brown A, Hartman A.. 2018. Evaluation of protective efficacy when combining Turkey herpesvirus-vector vaccines. Avian Dis. 63(1):75–83. [DOI] [PubMed] [Google Scholar]
- Esaki M, Godoy A, Rosenberger JK, Rosenberger SC, Gardin Y, Yasuda A, Dorsey KM.. 2013. Protection and antibody response caused by Turkey herpesvirus vector Newcastle disease vaccine. Avian Dis. 57(4):750–755. [DOI] [PubMed] [Google Scholar]
- Fakhri O, Hartley CA, Devlin JM, Browning GF, Noormohammadi AH, Lee S-W.. 2019. Development and application of high-resolution melting analysis for the classification of infectious laryngotracheitis virus strains and detection of recombinant progeny. Arch Virol. 164(2):427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JE, Hanson LE.. 1963. A comparison of some properties of laryngotracheitis and herpes simplex viruses. Am J Vet Res. 24:1297–1303. [PubMed] [Google Scholar]
- Fuchs W, Mettenleiter TC.. 1996. DNA sequence and transcriptional analysis of the UL1 to UL5 gene cluster of infectious laryngotracheitis virus. J Gen Virol. 77 (9):2221–2229. [DOI] [PubMed] [Google Scholar]
- Fuchs W, Veits J, Helferich D, Granzow H, Teifke JP, Mettenleiter TC.. 2007. Molecular biology of avian infectious laryngotracheitis virus. Vet Res. 38(2):261–279. [DOI] [PubMed] [Google Scholar]
- Fuchs W, Wiesner D, Veits J, Teifke JP, Mettenleiter TC.. 2005. In vitro and in vivo relevance of infectious laryngotracheitis virus gJ proteins that are expressed from spliced and nonspliced mRNAs. JVI. 79(2):705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui D, Nakamura M, Yamaguchi T, Takenaka M, Murakami M, Yanai T, Fukushi H, Yanagida K, Bando G, Matsuno K, et al. 2016. An epizootic of emerging novel avian pox in carrion crows (corvus corone) and large-billed crows (corvus macrorhynchos) in Japan. J Wildl Dis. 52(2):230–241. [DOI] [PubMed] [Google Scholar]
- Garcia M, Riblet S.. 2001. Characterization of infectious laryngotracheitis virus (ILTV) isolates: demonstration of viral subpopulations within vaccine preparations. Avian Dis. 45(3):558–566. [PubMed] [Google Scholar]
- García M, Spatz SJ, Guy JS. 2013. Infectious laryngotracheitis. In: Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL, Nair V. Editors. Diseases of Poultry. Ames, Iowa. Blackwell Publishing; pp. 161–179. [Google Scholar]
- Garcia M, Spatz S.. 2014. Infectious Laryngotracheitis. In: Swayne D, editor. 14th Edition Diseases of Poultry. Ames, IA: State Press; p. 189–209. [Google Scholar]
- Garcia M, Spatz S, Guy JS.. 2014. Infectious Laryngotracheitis. In: Saif YM, Glisson JR, Fadly AM, Mcdougald LR, Nolan LK, Swayne D, editors. Diseases of poultry. Ames, IA: State Press; p. 161–179. [Google Scholar]
- García M. 2016. Current and future vaccines and vaccination strategies against infectious laryngotracheitis (ILT) respiratory disease of poultry. Vet Microbiol. 206:157–162. [DOI] [PubMed] [Google Scholar]
- García M, Zavala G.. 2019. Commercial vaccines and vaccination strategies against infectious Laryngotracheitis: What we have learned and knowledge gaps that remain. Avian Dis. 63(2):325–334. [DOI] [PubMed] [Google Scholar]
- Gergen L, Cook S, Ledesma B, Cress W, Higuchi D, Counts D, Cruz-Coy J, Crouch C, Davis P, Tarpey I, et al. 2019. A double recombinant herpes virus of turkeys for the protection of chickens against Newcastle, infectious laryngotracheitis and Marek’s diseases. Avian Pathol. 48(1):45–56. [DOI] [PubMed] [Google Scholar]
- Giambrone J, Fagbohun O, Macklin K.. 2008. Management practices to reduce infectious laryngotracheitis virus in poultry litter. J Appl Poult Res. 17(1):64–68. [Google Scholar]
- Gibbs C. 1934. Infectious laryngotracheitis vaccination. Mass Agric Exp Stn Bull. 295:1–20. [Google Scholar]
- Gimeno IM, Cortes AL, Faiz NM, Hernandez-Ortiz BA, Guy JS, Hunt HD, Silva RF.. 2015. Evaluation of the protection efficacy of a serotype 1 Marek’s disease virus-vectored bivalent vaccine against infectious Laryngotracheitis and Marek’s Disease. Avian Dis. 59(2):255–262. [DOI] [PubMed] [Google Scholar]
- Gingerich E and Carver DK. 2006. Infectious Laryngotracheitis Virus (ILT) Facts. Pennsylvania. http://agriculture.state.pa.us. Accessed April 25, 2020.
- Glorieux S, Favoreel HW, Meesen G, de Vos W, Van den Broeck W, Nauwynck HJ.. 2009. Different replication characteristics of historical pseudorabies virus strains in porcine respiratory nasal mucosa explants. Vet Microbiol. 136(3–4):341–346. [DOI] [PubMed] [Google Scholar]
- Godoy A, Icard A, Martinez M, Mashchenko A, García M, El-Attrache J.. 2013. Detection of infectious laryngotracheitis virus antibodies by glycoprotein-specific ELISAs in chickens vaccinated with viral vector vaccines. Avian Dis. 57(2s1):432–436. [DOI] [PubMed] [Google Scholar]
- Goraya M, Ali L, Younis I.. 2017. Innate immune responses against avian respiratory viruses. Hosts Viruses. 4:78–87. [Google Scholar]
- Gough AW, Pettit JR, Gagnon A, Weber LJ.. 1977. An outbreak of infectious laryngotracheitis in commercial poultry flocks in Ontario. Can J Comp Med Rev Can Médecine Comparée. 41:146–151. [PMC free article] [PubMed] [Google Scholar]
- Gowthaman V, Koul M, Kumar S.. 2016. Avian infectious laryngotracheitis: A neglected poultry health threat in India. Vaccine. 34(36):4276–4277. [DOI] [PubMed] [Google Scholar]
- Gowthaman V, Singh SD, Dhama K, Barathidasan R, Mathapati BS, Srinivasan P, Saravanan S, Ramakrishnan MA.. 2014. Molecular detection and characterization of infectious laryngotracheitis virus (Gallid herpesvirus-1) from clinical samples of commercial poultry flocks in India. VirusDis. 25(3):345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzow H, Klupp BG, Fuchs W, Veits J, Osterrieder N, Mettenleiter TC.. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J Virol. 75(8):3675–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Scholz E, Turek J, Nodgreen R, Maloney B.. 1993. Assembly pathway of avian infectious laryngotracheitis virus. Am J Vet Res. 54(12):2031–2039. [PubMed] [Google Scholar]
- Guy JS, Bagust T.. 2003. Laryngotracheitis. In: Saif M, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne D, editors. Diseases of Poultry 11th edition. Ames, IA: Iowa State University Press; p. 121–134. [Google Scholar]
- Guy JS, Barnes HJ, Morgan LM.. 1990. Virulence of infectious laryngotracheitis viruses: comparison of modified-live vaccine viruses and North Carolina field isolates. Avian Dis. 34(1):106–113. [PubMed] [Google Scholar]
- Guy JS, Barnes HJ, Smith LG.. 1992. Rapid diagnosis of infectious laryngotracheitis using a monoclonal antibody-based immunoperoxidase procedure. Avian Pathol. 21(1):77–86. [DOI] [PubMed] [Google Scholar]
- Guy JS, Garcia M.. 2008. Laryngotracheitis. In: Saif M, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne D, editors. Diseases of Poultry 12th edition. Ames, IA: Blackwell Publ Prof.; p. 137–152. [Google Scholar]
- Han MG, Kweon CH, Mo IP, Kim SJ.. 2002. Pathogenicity and vaccine efficacy of a thymidine kinase gene deleted infectious laryngotracheitis virus expressing the green fluorescent protein gene. Arch Virol. 147(5):1017–1031. [DOI] [PubMed] [Google Scholar]
- Hanson LE, Bagust TJ. 1991. Infectious laryngotracheitis. In: Calnek BW, editor. Diseases of Poultry 9th edition. Ames, IA: State Univ Press; p. 485–495. [Google Scholar]
- Hanson L, Hanson LE.. 1984. Laryngotracheitis. In: Hofstad MS, editor. Diseases of Poultry 8th edition. Ames, IA: Iowa State Univ Press; pp. 444–451. [Google Scholar]
- Hayashi S, Odagiri Y, Kotani T, Horiuchi T.. 1985. Pathological changes of tracheal mucosa in chickens infected with infectious laryngotracheitis virus. Avian Dis. 29(4):943–950. [PubMed] [Google Scholar]
- Helferich D, Veits J, Teifke JP, Mettenleiter TC, Fuchs W.. 2007. The UL47 gene of avian infectious laryngotracheitis virus is not essential for in vitro replication but is relevant for virulence in chickens. J Gen Virol. 88(3):732–742. [DOI] [PubMed] [Google Scholar]
- Hernandez-Divers SM, Villegas P, Jimenez C, Hernandez-Divers SJ, Garcia M, Riblet SM, Carroll CR, O'Connor BM, Webb JL, Yabsley MJ, et al. 2008. Backyard chicken flocks pose a disease risk for neotropic birds in Costa Rica. Avian Dis. 52(4):558–566. [DOI] [PubMed] [Google Scholar]
- Hidalgo H. 2003. Infectious Laryngotracheitis: A review. Rev Bras Cienc Avic. 5(3):157–168. [Google Scholar]
- Hinshaw W, Jones E, Graybill H.. 1931. A study of mortality and egg production in flocks affected with laryngotracheitis. Poult Sci. 10(7):375–382. [Google Scholar]
- Hitchner SB, Fabricant J, Bagust TJ.. 1977. A fluorescent-antibody study of the pathogenesis of infectious laryngotracheitis. Avian Dis. 21(2):185–194. [PubMed] [Google Scholar]
- Honess RW, Roizman B.. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 14(1):8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CS, Gaskell RM, Jones RC, Bradbury JM, Jordan FT.. 1989. Effects of certain stress factors on the re-excretion of infectious laryngotracheitis virus from latently infected carrier birds. Res Vet Sci. 46(2):274–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CS, Jones RC.. 1988. Comparison of cultural methods for primary isolation of infectious laryngotracheitis virus from field material. Avian Pathol. 17(2):295–303. [DOI] [PubMed] [Google Scholar]
- Hughes CS, Jones RC, Gaskell RM, Jordan FT, Bradbury JM.. 1987. Demonstration in live chickens of the carrier state in infectious laryngotracheitis. Res Vet Sci. 42(3):407–410. [PubMed] [Google Scholar]
- Hughes CS, Williams RA, Gaskell RM, Jordan FT, Bradbury JM, Bennett M, Jones RC.. 1991. Latency and reactivation of infectious laryngotracheitis vaccine virus. Arch Virol. 121(1–4):213–218. [DOI] [PubMed] [Google Scholar]
- Humberd J, García M, Riblet SM, Resurreccion RS, Brown TP.. 2002. Detection of infectious laryngotracheitis virus in formalin-fixed, paraffin-embedded tissues by nested polymerase chain reaction. Avian Dis. 46(1):64–74.2.0.CO;2] [DOI] [PubMed] [Google Scholar]
- Ide PR. 1978. Sensitivity and specificity of the fluorescent antibody technique for detection of infectious laryngotracheitis virus. Can J Comp Med Rev Can Médecine Comparée. 42:54–62. [PMC free article] [PubMed] [Google Scholar]
- Izuchi T, Hasegawa A.. 1982. Pathogenicity of infectious laryngotracheitis virus as measured by chicken embryo inoculation. Avian Dis. 26(1):18–25. [PubMed] [Google Scholar]
- Johnson Y, Gedamu N, Colby M, Myint M, Steele S, Salem M, Tablante N.. 2005. Wind-borne transmission of infectious laryngotracheitis between commercial poultry operations. Int J Poultry Sci. 4(5):263–267. [Google Scholar]
- Johnson MA, Tyack SG, Prideaux C, Kongsuwan K, Sheppard M.. 1995. Nucleotide sequence of infectious laryngotracheitis virus (gallid herpesvirus 1) ICP4 gene. Virus Res. 35(2):193–204. [DOI] [PubMed] [Google Scholar]
- Johnson DI, Vagnozzi A, Dorea F, Riblet SM, Mundt A, Zavala G, García M.. 2010. Protection against infectious laryngotracheitis by in ovo vaccination with commercially available viral vector recombinant vaccines. Avian Dis. 54(4):1251–1259. [DOI] [PubMed] [Google Scholar]
- Jones RC. 2010. Viral respiratory diseases (ILT, aMPV infections, IB): are they ever under control? Br Poult Sci. 51(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BV, Hennion RM.. 2008. The preparation of chicken tracheal organ cultures for virus isolation, propagation, and titration. Methods Mol Biol Clifton NJ. 454:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan FT. 1958. Some observations of laryngotracheitis. Vet Rec. 70:605–610. [Google Scholar]
- Jordan FT. 1966. A review of the literature on infectious laryngotracheitis. Avian Dis. 10(1):1–26. [Google Scholar]
- Jordan FT, Chubb R.. 1962. The agar gel diffusion technique in the diagnosis of infectious laryngotracheitis (ILT) and its differentiation from fowl pox. Res Vet Sci. 3(3):245–255. [Google Scholar]
- Kanabagatte Basavarajappa M, Kumar S, Khattar SK, Gebreluul GT, Paldurai A, Samal SK.. 2014. A recombinant Newcastle disease virus (NDV) expressing infectious laryngotracheitis virus (ILTV) surface glycoprotein D protects against highly virulent ILTV and NDV challenges in chickens. Vaccine. 32(28):3555–3563. [DOI] [PubMed] [Google Scholar]
- Keeler CL, Hazel JW, Hastings JE, Rosenberger JK.. 1993. Restriction endonuclease analysis of Delmarva field isolates of infectious laryngotracheitis virus. Avian Dis. 37(2):418–426. [PubMed] [Google Scholar]
- Keller LH, Benson CE, Davison S, Eckroade RJ.. 1992. Differences among restriction endonuclease DNA fingerprints of Pennsylvania field isolates, vaccine strains, and challenge strains of infectious laryngotracheitis virus. Avian Dis. 36(3):575–581. [PubMed] [Google Scholar]
- Kelly BJ, Fraefel C, Cunningham AL, Diefenbach RJ.. 2009. Functional roles of the tegument proteins of herpes simplex virus type 1. Virus Res. 145(2):173–186. [DOI] [PubMed] [Google Scholar]
- Kernohan G. 1931. Infectious laryngotracheitis in fowls. J Am Vet Med Assoc. 78:196–202. [Google Scholar]
- Kingsbury F, Jungherr E.. 1958. Indirect transmission of infectious laryngotracheitis in chickens. Avian Dis. 2(1):54–63. [Google Scholar]
- Kingsley DH, Hazel JW, Keeler CL.. 1994. Identification and characterization of the infectious laryngotracheitis virus glycoprotein C gene. Virology. 203(2):336–343. [DOI] [PubMed] [Google Scholar]
- Kingsley DH, Keeler CL.. 1999. Infectious laryngotracheitis virus, an alpha herpesvirus that does not interact with cell surface heparan sulfate. Virology. 256(2):213–219. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick NC, Mahmoudian A, Colson CA, Devlin JM, Noormohammadi AH.. 2006. Relationship between mortality, clinical signs and tracheal pathology in infectious laryngotracheitis. Avian Pathol. 35(6):449–453. [DOI] [PubMed] [Google Scholar]
- Kotiw M, Wilks CR, May JT.. 1995. The effect of serial in vivo passage on the expression of virulence and DNA stability of an infectious laryngotracheitis virus strain of low virulence. Vet Microbiol. 45(1):71–80. [DOI] [PubMed] [Google Scholar]
- Kumar V, Yadav K, Kumar R, Chaudhary N, Kumar S.. 2019. Glycoprotein D peptide-based diagnostic approach for the detection of avian infectious laryngotracheitis antibodies. Avian Pathol. 48(6):602–609. [DOI] [PubMed] [Google Scholar]
- Lee J, Bottje WG, Kong B-W.. 2012. Genome-wide host responses against infectious laryngotracheitis virus vaccine infection in chicken embryo lung cells. BMC Genomics. 13(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-W, Markham PF, Markham JF, Petermann I, Noormohammadi AH, Browning GF, Ficorilli NP, Hartley CA, Devlin JM.. 2011. First complete genome sequence of infectious laryngotracheitis virus. BMC Genomics. 12(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DA, Bradbury JM, Gaskell RM, Hughes CS, Jones RC.. 1986. Restriction endonuclease patterns of some European and American isolates of avian infectious laryngotracheitis virus. Avian Dis. 30(4):835–837. [PubMed] [Google Scholar]
- Linares JA, Bickford AA, Cooper GL, Charlton BR, Woolcock PR.. 1994. An outbreak of infectious laryngotracheitis in California broilers. Avian Dis. 38(1):188–192. [PubMed] [Google Scholar]
- Lohr JE. 1977. Causes of sudden drop in egg production in New Zealand laying flocks. N Z Vet J. 25(4):100–102. [DOI] [PubMed] [Google Scholar]
- Loncoman CA, Hartley CA, Coppo MJC, Vaz PK, Diaz-Méndez A, Browning GF, Lee S-W, Devlin JM.. 2017. Development and application of a TaqMan single nucleotide polymorphism genotyping assay to study infectious laryngotracheitis virus recombination in the natural host. PloS One. 12(3):e0174590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa D, Beltrán G, Riblet SM, García M.. 2019. Protection efficacy of a recombinant herpesvirus of Turkey vaccine against infectious laryngotracheitis virus administered in ovo to broilers at three standardized doses. Avian Dis. 63(2):351–358. [DOI] [PubMed] [Google Scholar]
- Maekawa D, Riblet SM, Newman L, Koopman R, Barbosa T, García M.. 2019. Evaluation of vaccination against infectious laryngotracheitis (ILT) with recombinant herpesvirus of turkey (rHVT-LT) and chicken embryo origin (CEO) vaccines applied alone or in combination. Avian Pathol. 48(6):573–581. [DOI] [PubMed] [Google Scholar]
- Magouz A, Medhat S, A, AsaS, Desouky A. 2018. Detection of infectious laryngotracheitis virus (Gallid herpesvirus-1) from clinically infected chickens in Egypt by different diagnostic methods. Iran J Vet Res. 19:194–201. [PMC free article] [PubMed] [Google Scholar]
- Mahmoudian A, Kirkpatrick NC, Coppo M, Lee S-W, Devlin JM, Markham PF, Browning GF, Noormohammadi AH.. 2011. Development of a SYBR Green quantitative polymerase chain reaction assay for rapid detection and quantification of infectious laryngotracheitis virus. Avian Pathol. 40(3):237–242. [DOI] [PubMed] [Google Scholar]
- Mallinson ET, Miller KF, Murphy CD.. 1981. Cooperative control of infectious laryngotracheitis. Avian Dis. 25(3):723–729. [PubMed] [Google Scholar]
- May H, Thittsler R.. 1925. Tracheo-laryngotracheitis in poultry. J Am Vet Med Assoc. 67:229–231. [Google Scholar]
- McGeoch DJ, Dalrymple MA, Davison AJ, Dolan A, Frame MC, McNab D, Perry LJ, Scott JE, Taylor P.. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 69 (7):1531–1574. [DOI] [PubMed] [Google Scholar]
- McGeoch DJ, Dolan A, Ralph AC.. 2000. Toward a comprehensive phylogeny for mammalian and avian herpesviruses. J Virol. 74(22):10401–10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch DJ, Rixon FJ, Davison AJ.. 2006. Topics in herpesvirus genomics and evolution. Virus Res. 117(1):90–104. [DOI] [PubMed] [Google Scholar]
- McMullin P. 2004. Infectious Laryngotracheitis. Pocket Guide Poult Health Dis. [Google Scholar]
- McNulty M, Allan G, McCracken R.. 1985. Infectious laryngotracheitis in Ireland. Ir Vet J. 39:124–125. [Google Scholar]
- Menendez KR, García M, Spatz S, Tablante NL.. 2014. Molecular epidemiology of infectious laryngotracheitis: a review. Avian Pathol. 43(2):108–117. [DOI] [PubMed] [Google Scholar]
- Mettenleiter TC. 2002. Herpesvirus assembly and egress. JVI. 76(4):1537–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulemans G, Halen P.. 1978. Some physico-chemical and biological properties of a Belgian strain (U 76/1035) of infectious laryngotracheitis virus. Avian Pathol. 7(2):311–315. [DOI] [PubMed] [Google Scholar]
- Molini U, Aikukutu G, Khaiseb S, Kahler B, Van der Westhuizen J, Cattoli G, Dundon WG.. 2019. Investigation of infectious laryngotracheitis outbreaks in Namibia in 2018. Trop Anim Health Prod. 51(7):2105–2108. [DOI] [PubMed] [Google Scholar]
- Morales Ruiz S, Bendezu Eguis J, Montesinos R, Tataje-Lavanda L, Fernández-Díaz M.. 2018. Full-genome sequence of infectious laryngotracheitis virus (Gallid Alphaherpesvirus 1) strain VFAR-043, isolated in Peru.Genome Announc. 6(10): e00078–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A, Piccirillo A, Mondin A, Morandini E, Gavazzi L, Cordioli P.. 2010. Epidemic of infectious laryngotracheitis in Italy: characterization of virus isolates by PCR-restriction fragment length polymorphism and sequence analysis. Avian Dis. 54(4):1172–1177. [DOI] [PubMed] [Google Scholar]
- Nadimpalli M, Lee SW, Devlin JM, Gilkerson JR, Hartley CA.. 2017. Impairment of infectious laryngotracheitis virus replication by deletion of the UL[-1] gene. Arch Virol. 162(6):1541–1548. [DOI] [PubMed] [Google Scholar]
- Nakamichi K, Matsumoto Y, Otsuka H.. 2002. Bovine herpesvirus 1 glycoprotein G is necessary for maintaining cell-to-cell junctional adherence among infected cells. Virology. 294(1):22–30. [DOI] [PubMed] [Google Scholar]
- Neff C, Sudler C, Hoop RK.. 2008. Characterization of western European field isolates and vaccine strains of avian infectious laryngotracheitis virus by restriction fragment length polymorphism and sequence analysis. Avian Dis. 52(2):278–283. [DOI] [PubMed] [Google Scholar]
- Neighbor NK, Newberry LA, Bayyari GR, Skeeles JK, Beasley JN, McNew RW.. 1994. The effect of microaerosolized hydrogen peroxide on bacterial and viral poultry pathogens. Poult Sci. 73(10):1511–1516. [DOI] [PubMed] [Google Scholar]
- Nielsen OL, Handberg KJ, Jørgensen PH.. 1998. In situ hybridization for the detection of infectious laryngotracheitis virus in sections of trachea from experimentally infected chickens. Acta Vet Scand. 39(4):415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE . 2014. Avian infectious laryngotracheitis. Man Diagn Tests Vaccines Terr Anim. Chapter 2.3.3.
- Ojkic D, Swinton J, Vallieres M, Martin E, Shapiro J, Sanei B, Binnington B.. 2006. Characterization of field isolates of infectious laryngotracheitis virus from Ontario. Avian Pathol. 35(4):286–292. [DOI] [PubMed] [Google Scholar]
- Oldoni I, García M.. 2007. Characterization of infectious laryngotracheitis virus isolates from the US by polymerase chain reaction and restriction fragment length polymorphism of multiple genome regions. Avian Pathol. 36(2):167–176. [DOI] [PubMed] [Google Scholar]
- Oldoni I, Rodríguez-Avila A, Riblet SM, Zavala G, García M.. 2009. Pathogenicity and growth characteristics of selected infectious laryngotracheitis virus strains from the United States. Avian Pathol. 38(1):47–53. [DOI] [PubMed] [Google Scholar]
- Ou S-C, Giambrone JJ.. 2012. Infectious laryngotracheitis virus in chickens. WJV. 1(5):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou S, Giambrone J, Macklin K.. 2011. Infectious laryngotracheitis vaccine virus detection in water lines and effectiveness of sanitizers for inactivating the virus. J Appl Poult Res. 20(2):223–230. [Google Scholar]
- Ou S, Giambrone JJ, Macklin M.. 2012. Detection of infectious laryngotracheitis virus from darkling beetles and their immature stage (lesser mealworms) by quantitative polymerase chain reaction and virus isolation. J Appl Poult Res. 21(1):33–38. [Google Scholar]
- Palomino-Tapia VA, Zavala G, Cheng S, García M.. 2019. Long-term protection against a virulent field isolate of infectious laryngotracheitis virus induced by inactivated, recombinant, and modified live virus vaccines in commercial layers. Avian Pathol. 48(3):209–220. [DOI] [PubMed] [Google Scholar]
- Parra SHS, Nuñez LF, Astolfi-Ferreira CS, Ferreira A.. 2015. Persistence of the tissue culture origin vaccine for infectious laryngotracheitis virus in commercial chicken flocks in Brazil. Poult Sci. 94(11):2608–2615. [DOI] [PubMed] [Google Scholar]
- Pavlova SP, Veits J, Blohm U, Maresch C, Mettenleiter TC, Fuchs W.. 2010. In vitro and in vivo characterization of glycoprotein C-deleted infectious laryngotracheitis virus. J Gen Virol. 91(4):847–857. [DOI] [PubMed] [Google Scholar]
- Piccirillo A, Lavezzo E, Niero G, Moreno A, Massi P, Franchin E, Toppo S, Salata C, Palù G.. 2016. Full genome sequence-based comparative study of wild-type and vaccine strains of infectious laryngotracheitis virus from Italy. PLoS One. 11(2):e0149529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preis I, Braga J, Couto R, Brasil SA, Martins R, Ecco R.. 2013. Outbreak of infectious laryngotracheitis in large multi-age egg layer chicken flocks in Minas Gerais, Brazil. Pesq Vet Bras. 33(5):591–596. [Google Scholar]
- Prideaux CT, Kongsuwan K, Johnson MA, Sheppard M, Fahey KJ.. 1992. Infectious laryngotracheitis virus growth, DNA replication, and protein synthesis. Arch Virol. 123(1–2):181–192. [DOI] [PubMed] [Google Scholar]
- Pulsford M, Stokes J.. 1953. Infectious laryngotracheitis in South Australia. Australian Vet J. 29(1):8–12. [Google Scholar]
- Purcell DA. 1971. a. The ultrastructural changes produced by infectious laryngotracheitis virus in tracheal epithelium of the fowl. Res Vet Sci. 12(5):455–458. [PubMed] [Google Scholar]
- Purcell DA. 1971. b. Histopathology of infectious laryngotracheitis in fowl infected by an aerosol. J Comp Pathol. 81(3):421–431. [DOI] [PubMed] [Google Scholar]
- Purcell DA, McFerran JB.. 1969. Influence of method of infection on the pathogenesis of infectious laryngotracheitis. J Comp Pathol. 79(3):285–291. [DOI] [PubMed] [Google Scholar]
- Raggi L, Brownell J, Stewart G.. 1961. Effect of infectious laryngotracheitis on egg production and quality. Poult Sci. 40(1):134–140. [Google Scholar]
- Raggi LG, Lee GG.. 1965. Infections laryngotracheitis outbreaks following vaccination. Avian Dis. 9(4):559–565. [PubMed] [Google Scholar]
- Reddy V, Steukers L, Li Y, Fuchs W, Vanderplasschen A, Nauwynck HJ.. 2014. Replication characteristics of infectious laryngotracheitis virus in the respiratory and conjunctival mucosa. Avian Pathol. 43(5):450–457. [DOI] [PubMed] [Google Scholar]
- Reemers SS, Groot Koerkamp MJ, Holstege FC, van Eden W, Vervelde L.. 2009. Cellular host transcriptional responses to influenza A virus in chicken tracheal organ cultures differ from responses in in vivo infected trachea. Vet Immunol Immunopathol. 132(2–4):91–100. [DOI] [PubMed] [Google Scholar]
- Reynolds HA, Watrach AM, Hanson LE.. 1968. Development of the nuclear inclusion bodies of infectious laryngotracheitis. Avian Dis. 12(2):332–347. [PubMed] [Google Scholar]
- Robertson GM, Egerton JR.. 1981. Replication of infectious laryngotracheitis virus in chickens following vaccination. Australian Vet J. 57(3):119–123. [DOI] [PubMed] [Google Scholar]
- Roizman B, Knipe D.. 2001. Herpes simplex viruses and their replication. In: Knipe DM, Howley PM, editors. Fields virology. Philadelphia (PA): Lippincott Williams Wilkins; pp. 2399–2459. [Google Scholar]
- Roizman B, Pellett PE.. 2001. The family Herpesviridae: a brief introduction. In: Knipe M, Howley PM, editors. Fields virology. 4th ed. Philadelphia (PA): Lippincott Williams Wilkins; pp. 2381–2397. [Google Scholar]
- Roy P, Fakhrul Islam AFM, Burgess SK, Hunt PW, McNally J, Walkden-Brown SW.. 2015. Real-time PCR quantification of infectious laryngotracheitis virus in chicken tissues, faeces, isolator-dust and bedding material over 28 days following infection reveals high levels in faeces and dust. J Gen Virol. 96(11):3338–3347. [DOI] [PubMed] [Google Scholar]
- Ruano M, El-Attrache J, Villegas P.. 2001. Efficacy comparisons of disinfectants used by the commercial poultry industry. Avian Dis. 45(4):972–977. [PubMed] [Google Scholar]
- Russell RG. 1983. Respiratory tract lesions from infectious laryngotracheitis virus of low virulence. Vet Pathol. 20(3):360–369. [DOI] [PubMed] [Google Scholar]
- Russell RG, Turner AJ.. 1983. Characterization of infectious laryngotracheitis viruses, antigenic comparison by kinetics of neutralization and immunization studies. Can J Comp Med Rev Can Médecine Comparée. 47:163–171. [PMC free article] [PubMed] [Google Scholar]
- Sabir AJ, Olaogun OM, O'Rourke D, Fakhri O, Coppo MJC, Devlin JM, Konsak-Ilievski B, Noormohammadi AH.. 2020. Full genomic characterisation of an emerging infectious laryngotracheitis virus class 7b from Australia linked to a vaccine strain revealed its identity. Infect Genet E. 78:104067. [DOI] [PubMed] [Google Scholar]
- Santander Parra S, Nunez L, Buim MR, Astolfi-Ferreira CS, Piantino Ferreira AJ.. 2018. Development of a qPCR for the detection of infectious laryngotracheitis virus (ILTV) based on the gE gene. Br Poult Sci. 59(4):402–407. [DOI] [PubMed] [Google Scholar]
- Sary K, Chénier S, Gagnon CA, Shivaprasad HL, Sylvestre D, Boulianne M.. 2017. Esophagitis and pharyngitis associated with avian infectious laryngotracheitis in backyard chickens: Two cases. Avian Dis. 61(2):255–260. [DOI] [PubMed] [Google Scholar]
- Schädler J, Sigrist B, Meier SM, Albini S, Wolfrum N.. 2019. Virus-like particles in a new vaccination approach against infectious laryngotracheitis. J Gen Virol. 100(6):1013–1026. [DOI] [PubMed] [Google Scholar]
- Schnitzlein WM, Winans R, Ellsworth S, Tripathy DN.. 1995. Generation of thymidine kinase-deficient mutants of infectious laryngotracheitis virus. Virology. 209(2):304–314. [DOI] [PubMed] [Google Scholar]
- Scholz E, Porter RE, Guo P.. 1994. Differential diagnosis of infectious laryngotracheitis from other avian respiratory diseases by a simplified PCR procedure. J Virol Methods. 50(1–3):313–321. [DOI] [PubMed] [Google Scholar]
- Seddon H, Hart L.. 1935. The occurrence of infectious laryngotracheitis in fowls in New South Wales. Aust Vet J. 11:11–221. [Google Scholar]
- Seifried O. 1931. Histopathology of infectious laryngotracheitis in chickens. J Exp Med. 54(6):817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahsavandi S, Jamshidi-Navroud Z, Firouzi M, Ebrahimi M.. 2017. Examining responses of chicken embryonic neural stem cell to infectious laryngotracheitis virus infections. Comp Clin Pathol. 26(2):493–498. [Google Scholar]
- Shibley G, Luginbuhl R, Helmboldt C.. 1962. A study of infectious laryngotracheitis virus. I. Comparison of serologic and immunogenic properties. Avian Dis. 6(1):59–71. [Google Scholar]
- Spatz SJ, Garcia M, Riblet S, Ross TA, Volkening JD, Taylor TL, Kim T, Afonso CL.. 2019. MinION sequencing to genotype US strains of infectious laryngotracheitis virus. Avian Pathol. 48(3):255–269. [DOI] [PubMed] [Google Scholar]
- Srinivasan P, Balachandran CG, Murthy T, Saravanan S, Pazhanivel N, Mohan B.. 2012. Pathology of infectious laryngotracheitis in commercial layer chicken. Indian Vet J. 89:75–78. [Google Scholar]
- Srinivasan V, Malick B.. 1977. Studies on multiple inoculations of infectious laryngotracheitis virus in chickens. Indian Vet J. 54:1–5. [Google Scholar]
- Steukers L, Vandekerckhove A, Van den Broeck W, Glorieux S, Nauwynck H.. 2012. Kinetics of BoHV-1 dissemination in an in vitro culture of bovine upper respiratory tract mucosa explants. Inst Lab Anim Res J. 53(1):E43–E54. [DOI] [PubMed] [Google Scholar]
- Thilakarathne DS, Hartley CA, Diaz-Méndez A, Coppo MJC, Devlin JM.. 2020. Development and application of a combined molecular and tissue culture-based approach to detect latent infectious laryngotracheitis virus (ILTV) in chickens. J Virol Methods. 277:113797. [DOI] [PubMed] [Google Scholar]
- Thureen DR, Keeler CL.. 2006. Psittacid herpesvirus 1 and infectious laryngotracheitis virus: Comparative genome sequence analysis of two avian alphaherpesviruses. JVI. 80(16):7863–7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timurkaan N, Yilmaz F, Bulut H, Ozer H, Bolat Y.. 2003. Pathological and immunohistochemical findings in broilers inoculated with a low virulent strain of infectious laryngotracheitis virus. J Vet Sci. 4(2):175–180. [PubMed] [Google Scholar]
- Tran LC, Kissner JM, Westerman LE, Sears AE.. 2000. A herpes simplex virus 1 recombinant lacking the glycoprotein G coding sequences is defective in entry through apical surfaces of polarized epithelial cells in culture and in vivo. Proc Natl Acad Sci U S A. 97(4):1818–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy DN, Garcia M.. 1998. Infectious laryngotracheitis. In: Swayne E, Glisson JR, Jackwood MW, Pearson JE, Reed WM, editors. Lab Man Isol Identif Avian Pathog. 4th ed. Kennett Square, PA: Am Assoc Avian Pathol; p. 94–98. [Google Scholar]
- Tripathy DN, Reed WM.. 2013. Pox. Dis Poultry. 13th ed. Swayne CDE, editor. USA: Wiley-Blackwell; p. 333–349. [Google Scholar]
- Trus BL, Cheng N, Newcomb WW, Homa FL, Brown JC, Steven AC.. 2004. Structure and polymorphism of the UL6 portal protein of herpes simplex virus type 1. JVI. 78(22):12668–12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T. 1995. Avian respiratory diseases: clinical overview. J Avian Med Surg. 9:162–174. [Google Scholar]
- Vagnozzi A, Zavala G, Riblet SM, Mundt A, García M.. 2012. Protection induced by commercially available live-attenuated and recombinant viral vector vaccines against infectious laryngotracheitis virus in broiler chickens. Avian Pathol. 41(1):21–31. [DOI] [PubMed] [Google Scholar]
- Vanderkop MA. 1993. Alberta. Infectious laryngotracheitis in commercial broiler chickens. Can Vet J Rev Vét Can. 34:185. [PMC free article] [PubMed] [Google Scholar]
- Veits J, Lüschow D, Kindermann K, Werner O, Teifke JP, Mettenleiter TC, Fuchs W.. 2003. Deletion of the non-essential UL0 gene of infectious laryngotracheitis (ILT) virus leads to attenuation in chickens, and UL0 mutants expressing influenza virus haemagglutinin (H7) protect against ILT and fowl plague. J Gen Virol. 84(12):3343–3352. [DOI] [PubMed] [Google Scholar]
- Vögtlin A, Bruckner L, Ottiger HP.. 1999. Use of polymerase chain reaction (PCR) for the detection of vaccine contamination by infectious laryngotracheitis virus. Vaccine. 17(20–21):2501–2506. [DOI] [PubMed] [Google Scholar]
- Volkova V, Thornton D, Hubbard SA, Magee D, Cummings T, Luna L, Watson J, Wills R.. 2012. Factors associated with introduction of infectious laryngotracheitis virus on broiler farms during a localized outbreak. Avian Dis. 56(3):521–528. [DOI] [PubMed] [Google Scholar]
- Wang L-G, Ma J, Xue C-Y, Wang W, Guo C, Chen F, Qin J-P, Huang N-H, Bi Y-Z, Cao Y-C.. 2013. Dynamic distribution and tissue tropism of infectious laryngotracheitis virus in experimentally infected chickens. Arch Virol. 158(3):659–666. [DOI] [PubMed] [Google Scholar]
- Wild MA, Cook S, Cochran M.. 1996. A genomic map of infectious laryngotracheitis virus and the sequence and organization of genes present in the unique short and flanking regions. Virus Genes. 12(2):107–116. [DOI] [PubMed] [Google Scholar]
- Wilks CR, Kogan VG.. 1979. An immunofluorescence diagnostic test for avian infectious laryngotracheitis. Australian Vet J. 55(8):385–388. [PubMed] [Google Scholar]
- Williams RA, Bennett M, Bradbury JM, Gaskell RM, Jones RC, Jordan FT.. 1992. Demonstration of sites of latency of infectious laryngotracheitis virus using the polymerase chain reaction. J Gen Virol. 73 (9):2415–2420. [DOI] [PubMed] [Google Scholar]
- Yamada S, Matsuo K, Fukuda T, Uchinuno Y.. 1980. Susceptibility of ducks to the virus of infectious laryngotracheitis. Avian Dis. 24(4):930–938. [PubMed] [Google Scholar]
- Yan Z, Li S, Xie Q, Chen F, Bi Y.. 2016. Characterization of field strains of infectious laryngotracheitis virus in China by restriction fragment length polymorphism and sequence analysis. J Vet Diagn Invest. 28(1):46–49. [DOI] [PubMed] [Google Scholar]
- Yavuz O, Özdemir O, Aras Z, Terzi F.. 2018. Immunohistochemical studies on infectious laryngotracheitis in the respiratory tract lesions in naturally infected laying hens. Kafkas Univ Vet Fak Derg. 24:257–264. [Google Scholar]
- York JJ, Fahey KJ.. 1990. Humoral and cell-mediated immune responses to the glycoproteins of infectious laryngotracheitis herpesvirus. Arch Virol. 115(3–4):289–297. [DOI] [PubMed] [Google Scholar]
- Yu Q, Li Y, Dimitrov K, Afonso CL, Spatz S, Zsak L.. 2020. Genetic stability of a Newcastle disease virus vectored infectious laryngotracheitis virus vaccine after serial passages in chicken embryos. Vaccine. 38(4):925–932. [DOI] [PubMed] [Google Scholar]
- Yu Q, Spatz S, Li Y, Yang J, Zhao W, Zhang Z, Wen G, Garcia M, Zsak L.. 2017. Newcastle disease virus vectored infectious laryngotracheitis vaccines protect commercial broiler chickens in the presence of maternally derived antibodies. Vaccine. 35(5):789–795. [DOI] [PubMed] [Google Scholar]
- Zavala G. 2011. The old and new landscapes of infectious laryngotracheitis. Poult Inf Prof. :1–7. [Google Scholar]
- Zellen GK, Weber LJ, Martin SW.. 1984. Infectious laryngotracheitis in the niagara peninsula: a case control study. Can Vet J Rev Vét Can. 25:75–77. [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Chen J, Wang C, Shi W, Li D.. 2018. The therapeutic effect of Yinhuangerchen mixture on Avian infectious laryngotracheitis. Poult Sci. 97(8):2690–2697. [DOI] [PubMed] [Google Scholar]
- Zhao K, He W, Xie S, Song D, Lu H, Pan W, Zhou P, Liu W, Lu R, Zhou J, et al. 2014. Highly pathogenic fowlpox virus in cutaneously infected chickens, China. Emerging Infect Dis. 20(7):1208–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Kong C, Cui X, Cui H, Shi X, Zhang X, Hu S, Hao L, Wang Y.. 2013. Detection of infectious laryngotracheitis virus by real-time PCR in naturally and experimentally infected chickens. PloS One. 8(6):e67598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Q-Y, Wang S-C, Li J-P, Liu D, Liu S, Jiang W-M, Chen J-M.. 2014. A clinical survey of common avian infectious diseases in China. Avian Dis. 58(2):297–302. [DOI] [PubMed] [Google Scholar]