Abstract
Objective: To identify early predictors and develop reliable, validated prediction models for development of problematic spasticity after traumatic spinal cord injury (SCI).
Design: Prospective cohort study of the Rick Hansen Spinal Cord Injury Registry (RHSCIR), retrospective review of inpatient medical charts.
Setting: Quaternary trauma center, rehabilitation center, community settings.
Participants: Individuals with traumatic SCI between March 1, 2005, and March 31, 2014, prospectively enrolled in the Vancouver site RHSCIR.
Interventions: None.
Main Outcome Measure: Spasticity limiting function or requiring treatment (problematic spasticity) on the Spinal Cord Injury Health Questionnaire.
Results: In 350 patients, variables documented during hospitalization that predicted the development of problematic spasticity up to 5 years post-injury included: initial Glasgow Coma Scale; age at time of injury; admission to rehabilitation center; community discharge anti-spasticity medication prescription, neurological status, Penn Spasm Frequency Scale, and pain interference with quality of life, sleep, activities; greater change in AIS motor scores between admission and discharge. The predictive models had area under the receiver operating characteristic curve of 0.80 (95% CI 0.75, 0.85) in the development set (N = 244) and 0.84 (95% CI 0.74, 0.92) in the validation set (N = 106) for spasticity limiting function and 0.81 (95% CI 0.76, 0.85) in the development set and 0.85 (95% CI 0.77, 0.92) in the validation set for spasticity requiring treatment.
Conclusions: Our prediction models provide an early prognosis of risk of developing problematic spasticity after traumatic SCI, which can be used to improve clinical spasticity management and assist research (e.g. risk stratification in interventional trials).
Keywords: Spinal cord injury, Spasticity, Observational study
Introduction
Spasticity, a sensori-motor disorder characterized by intermittent or sustained involuntary muscle activation,1 is a highly prevalent medical condition that develops in as many as 71% of individuals with traumatic spinal cord injury (SCI).2,3 The prevalence of “problematic spasticity”, defined by spasticity requiring treatment or causing limitation in function, was identified in a large prospective study of individuals with traumatic SCI to be as high as 41% up to 5 years post-injury, despite current management strategies.3 In a large cross-sectional study,4 spasticity was the fourth most prevalent cause for hospitalization in people with SCI in the previous 12 months.
A goal in treating individuals with SCI is to maximize their function and quality of life by minimizing the effect of secondary complications like spasticity. Being able to predict those who are most likely to develop problematic spasticity can help clinicians appropriately allocate limited resources and researchers focus on testing targeted prevention strategies. To date, there have been no large prospective studies that identify early predictors and create validated predictive models of development of problematic spasticity in individuals with SCI.
The first objective of this study was to identify characteristics of patients, measured during their hospitalization following a traumatic SCI, that are most predictive of problematic spasticity in community follow-up. Variables collected at hospital admission, during the course of hospitalization and on community discharge were analyzed. The second objective was to determine whether results could be used to create a validated predictive model that would accurately predict the development of problematic spasticity in the community.
Methods
Study design
This was a prospective cohort study that utilized patients enrolled at the Vancouver site of the national Rick Hansen SCI Registry (RHSCIR). RHSCIR is a prospective observational database of patients admitted with traumatic SCI to major trauma and rehabilitation centers across Canada.5 Medication data and selected injury characteristics were confirmed retrospectively using inpatient medical charts from the acute care and rehabilitation hospitals in Vancouver General Hospital, GF Strong Rehabilitation Centre. Ethics approval was obtained from the local Institutional Review Board.
Eligibility criteria
All patients 16 years and over admitted between 2005 and 2014 with traumatic SCI and enrolled into RHSCIR were eligible. Patients were excluded if they died prior to discharge or did not have spasticity data available (see Fig. 1).
Figure 1.
Study flowchart.
RHSCIR and inpatient medical charts review
In this study, socio-demographic factors, injury characteristics, admission and discharge dates, neurological variables, pain and spasticity questionnaire results were abstracted from the RHSCIR datasets. Medication use during hospital admission was collected retrospectively from chart review separately from RHSCIR data abstraction.
Outcome measures
Spasticity at 1, 2, and 5 years post-injury was determined using the Spinal Cord Injury Health Questionnaire (SCI-HQ) (see Appendix A). Our primary outcomes of interest that determined development of “problematic spasticity” were whether patients selected yes or no to: (1) receiving treatment for spasticity or (2) experiencing limitation in activity due to spasticity in the SCI-HQ.
Predictive variables
Many variables are collected for RHSCIR, some of which have been shown in the literature to potentially be predictive of or associated with spasticity (e.g. neurological level and AIS grade, motor score;6,7 pain;8 age, sex and medication use7). Variables included in the analysis were selected a priori (Table 1). We chose to include Gabapentin use in the month following SCI as a possible predictor of spasticity development due to the recent reports in the literature demonstrating that early use of anticonvulsants, in particular, gabapentinoids such as Gabapentin, may improve motor recovery post SCI.9,10 Variables that were analyzed in addition to demographics and neurological status at admission and community discharge included the first Glasgow Coma Scale (GCS) taken at the scene of the accident (GCS Field) and at the admitting hospital (GCS Facility); GCS between 13 and 15 was categorized as mild, 9–12 was moderate and 3–8, severe. The presence of spasticity within two weeks of community discharge was determined using Part 1 of the Penn Spasm Frequency Scale (PSFS), a self-report measure of spasticity-related muscle spasms (see Appendix A).11,12 Patient-rated pain interference with sleep, normal activities and quality of life was captured using a 0–10 Numeric Rating Scale (NRS, 0 = no interference, 10 = the most interference imaginable; levels categorized as mild if NRS 1–3, moderate if NRS 4–6, and severe if NRS 7–10) at community discharge.13 Patients were considered to be on anti-spasticity medication at community discharge if they had one or more of: (1) an oral medication prescribed for the purpose of spasticity management (e.g. Baclofen, Tizanidine, Diazepam, Clonazepam, or Dantrolene); (2) a botulinum toxin injection for spasticity within the last 90 days14,15 or (3) a phenol injection for spasticity within the last 6 months.16 The neurological data were collected by trained clinicians. The change in AIS motor score was calculated by subtracting the initial (within 72 hours of admission) motor score from the final (community discharge) motor score.
Table 1. Patient characteristics for potential spasticity predictors.
| Category | Characteristic | Level | Overall (N = 350) |
|---|---|---|---|
| Admission | |||
| Demographics | Sex (%) | Female | 82 (23.4) |
| Male | 268 (76.6) | ||
| Age (years) (median [IQR]) | 43.0 [27.0, 57.0] | ||
| Age (years) (%) | 16–30 | 113 (32.3) | |
| 31–45 | 83 (23.7) | ||
| 46–60 | 92 (26.3) | ||
| 61–75 | 52 (14.9) | ||
| 76+ | 10 (2.9) | ||
| Household Income (%) | ≥ 20K | 210 (60.0) | |
| < 20K | 39 (11.1) | ||
| Unknown | 101 (28.9) | ||
| Education (%) | No Diploma or G.E.D | 77 (22.0) | |
| Diploma | 272 (77.7) | ||
| Unknown | 1 (0.3) | ||
| Living arrangement (%) | Alone | 84 (24.0) | |
| Not alone | 263 (75.1) | ||
| Unknown | 3 (0.9) | ||
| Trauma | GCS facility (%) | Mild | 277 (79.1) |
| Moderate | 6 (1.7) | ||
| Severe | 35 (10.0) | ||
| Unknown | 32 (9.1) | ||
| GCS field (%) | Mild | 263 (75.1) | |
| Moderate | 14 (4.0) | ||
| Severe | 31 (8.9) | ||
| Unknown | 42 (12.0) | ||
| Medical History | Cannabis use at Admission (%) | No | 304 (86.9) |
| Yes | 46 (13.1) | ||
| Comorbidities (%) | None | 261 (74.6) | |
| At least one | 88 (25.1) | ||
| Missing | 1 (0.3) | ||
| Discharge | |||
| Neurology | Motor neuro level (%) | C1–C8 | 200 (57.1) |
| T1–T12 | 94 (26.9) | ||
| L1–S5 | 39 (11.1) | ||
| C1–T12 + L1–S5 | 1 (0.3) | ||
| Unknown | 16 (4.6) | ||
| AIS (%) | A | 109 (31.1) | |
| B | 48 (13.7) | ||
| C | 38 (10.9) | ||
| D | 147 (42.0) | ||
| E | 3 (0.9) | ||
| Unknown | 5 (1.4) | ||
| AIS motor score (points) (%) | Unknown | 13 (3.7) | |
| Known | 337 (96.3) | ||
| AIS motor score (points) (median [IQR]) | 52.0 [39.0, 83.0] | ||
| AIS UEMS (points) (%) | Unknown | 7 (2.0) | |
| Known | 343 (98.0) | ||
| AIS UEMS (points) (median [IQR]) | 44.0 [28.0, 50.0] | ||
| AIS LEMS (points) (%) | Unknown | 8 (2.3) | |
| Known | 342 (97.7) | ||
| AIS LEMS (points) (median [IQR]) | 12.0 [0.0, 41.0] | ||
| Change in AIS motor score (points) (%) | Unknown | 36 (10.3) | |
| Known | 314 (89.7) | ||
| Change in AIS motor score (points) (median [IQR]) | 7.0 [0.0, 23.0] | ||
| Neurological level (%) | C1–C8 | 206 (58.9) | |
| T1–T12 | 94 (26.9) | ||
| L1–S5 | 42 (12.0) | ||
| C1–T12 + L1–S5 | 2 (0.6) | ||
| Unknown | 6 (1.7) | ||
| Course of care | Length, inpatient stay (days) (median [IQR]) | 132.0 [76.2, 212.0] | |
| Gabapentin, within 1 month (%) | No | 110 (31.4) | |
| Yes | 196 (56.0) | ||
| Unknown | 44 (12.6) | ||
| Operation (%) | No | 55 (15.7) | |
| Yes | 295 (84.3) | ||
| Rehab (%) | No | 47 (13.4) | |
| Yes | 303 (86.6) | ||
| Length, rehab (days) (median [IQR]) | 85.5 [48.0, 147.0] | ||
| Pain | Pain, activity (%) | None | 85 (24.3) |
| Mild | 94 (26.9) | ||
| Moderate | 95 (27.1) | ||
| Severe | 53 (15.1) | ||
| Unknown | 23 (6.6) | ||
| Pain, QOL (%) | None | 86 (24.6) | |
| Mild | 97 (27.7) | ||
| Moderate | 83 (23.7) | ||
| Severe | 60 (17.1) | ||
| Unknown | 24 (6.9) | ||
| Pain, sleep (%) | None | 111 (31.7) | |
| Mild | 99 (28.3) | ||
| Moderate | 69 (19.7) | ||
| Severe | 50 (14.3) | ||
| Unknown | 21 (6.0) | ||
| Spasticity | PSFS (%) | 0 | 98 (28.0) |
| 1 | 108 (30.9) | ||
| 2 | 56 (16.0) | ||
| 3 | 57 (16.3) | ||
| 4 | 18 (5.1) | ||
| Unknown | 13 (3.7) | ||
| Discharge anti-spasticity medication (%) | No | 231 (66.0) | |
| Yes | 119 (34.0) | ||
UEMS, upper extremity motor score; LEMS, lower extremity motor score.
Statistical analysis
The objective of the analysis was to identify the patient characteristics that are most predictive of spasticity in community follow-up. To identify important predictors, we fit elastic net logistic regression models,17 which “select” characteristics associated with the outcome, problematic spasticity in community follow-up. We utilized Variable Importance Probabilities to measure the importance of characteristics, where the importance of a characteristic is measured by the number of times it is “selected” in the models fit in 1,000 bootstrap samples.18 Characteristics that are important will be selected most often by the models when re-sampling the data. When fitting the models, the elastic net tuning parameter was chosen by tenfold cross-validation using area under the receiver operating characteristic (ROC) curve as the prediction criterion.
To account for the drop-off in response during community follow-up, inverse-probability weights of response during community follow-up were estimated. Patients that were less likely to have community follow-up data were upweighted compared to patients that were more likely to have responded during community follow-up.
To identify the most important characteristics, the data were split into two sets: a development set, consisting of 70% of the full dataset, and a validation set, consisting of the remaining 30% of the observations. The development sets were analyzed to identify clinical characteristics that predict each outcome (objective 1). Then, using the most important variables identified in the development data, final predictive models and nomograms were created and the predictive performance of the final models was assessed in the validation set (objective 2). The proportion of development versus validation set was determined a priori. To estimate the direction of the effect of each clinical characteristic on the outcomes, odds ratios and associated confidence intervals (CIs) for each characteristic and outcome were calculated in the development sets. Most important variables were determined a priori to be those with Variable Importance Probabilities greater than or equal to 75%.
The inverse-probability weighted logistic elastic net models were fit using the glmnet package in R.19 Odds ratios and associated inverse-probability weighted confidence intervals (CIs) were calculated using the survey package in R.20 Nomograms from the final predictive models were constructed using the rms package21 and weighted areas under the curve and associated bootstrap CIs were calculated using the Weighted ROC package in R.22
Results
The cohort used in the final data analysis was N = 350 (see Fig. 1). Patient characteristics at admission and discharge are reported in Table 1. Mean age was 46 ± 19 years. Mean time from injury to community discharge was 108 ± 95 days (range 1–728 days). Stratification of neurological levels of injury was based on previous work by this group.3 The most common mechanisms of injury were falls (N = 120), transport (N = 114), sports (N = 80), and assault (N = 14). Of the 350 patients included in the cohort, 335 had GCS reported. Of the 335, 101 (30.1%) had a documented GCS Field < 15. Of the total cohort, 142 (40.6%) patients were treated with Botox, Phenol or an oral anti-spasticity medication during their inpatient stay. An increase in AIS motor score from admission to community discharge occurred in 226 patients. In these patients, 127 (56.2%) had problematic spasticity in community follow-up, while 60 (68.2%) of the 88 patients whose AIS motor score did not improve had problematic spasticity in community follow-up. There were 36 patients on whom change in motor score was unknown. Patients with AIS A injuries at discharge were less likely to have had a motor score improvement compared to AIS B, C or D injuries (Holm adjusted P values for multiple comparisons P = 0.048 for A compared to B, P < 0.001 for A compared to C and D from Fisher’s exact test). Patients with T1–T12 neurological levels at discharge were less likely to have experienced motor score improvement compared to patients with C1–C8 or L1–S5 neurological levels at discharge (adjusted P < 0.001 for both comparisons). The same was true for T1–T12 motor score at discharge compared to C1–C8 or L1–S5 (adjusted P < 0.001 for both comparisons).
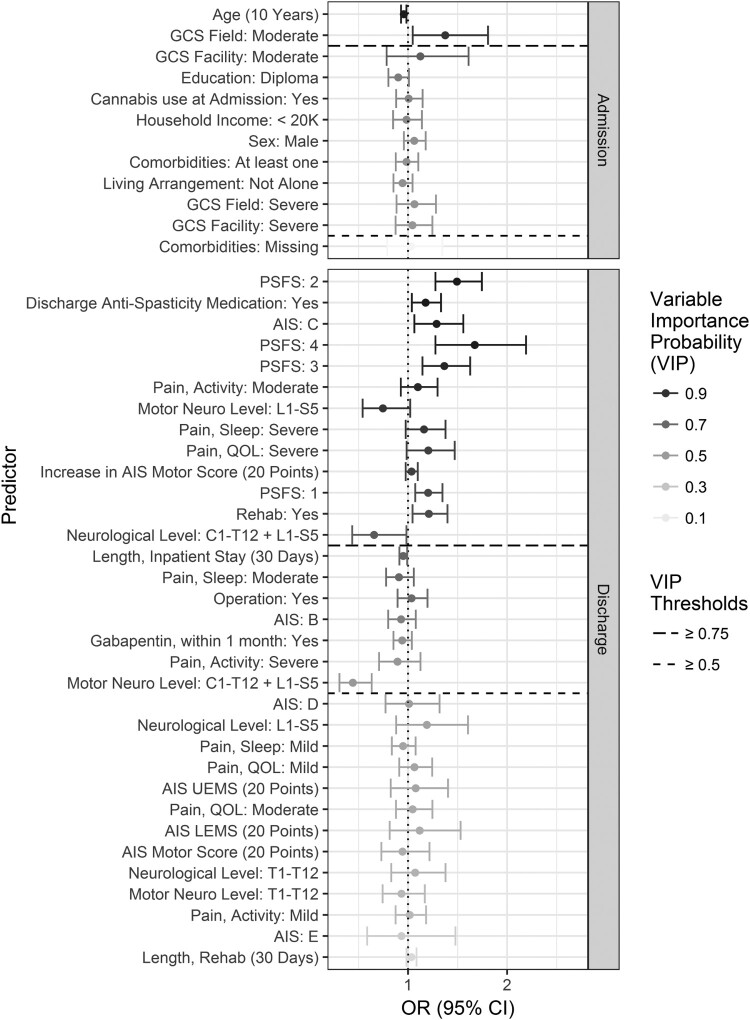
The estimated odds ratios and Variable Importance Probabilities in the development data sets are displayed in Figs 2 and 3 with detailed data provided in Appendix B.
Figure 2.
Estimated odds ratios and variable importance probabilities (VIP) in the development data set for spasticity requiring treatment on community follow-up.
Figure 3.
Estimated odds ratios and variable importance probabilities (VIP) in the development data set for spasticity limiting function on community follow-up.
Predictors for developing spasticity requiring treatment
N = 7 variables were identified as the most predictive (Variable Importance Probabilities greater than or equal to 75%) for either increasing or decreasing the risk of developing spasticity requiring treatment on community follow-up up to 5 years post-injury. Predictors on hospital admission and during the course of hospitalization that increased the risk were identified as: (1) moderate GCS Field (OR 1.38, 95% CI 1.05, 1.81) and (2) admission to the rehabilitation center from acute care (OR 1.21, 95% CI 1.05, 1.40). Predictors at community discharge that increased the risk included: (3) prescription of spasticity medication(s) (OR 1.18, 95% CI 1.04, 1.33); (4) moderate pain during activities (OR 1.10, CI 95% 0.93, 1.30); (5) PSFS 1 (OR 1.20, 95% CI 1.07, 1.35) or 4 (OR 1.67, CI 95% 1.28, 2.19). Predictors at community discharge that decreased the risk were (6) neurological level of L1–S5 and (7) motor level of L1–S5. Variables that almost reached the cut off for statistical significance for increasing risk of spasticity included GCS Field severe (Variable Importance Probability 68%), increase in AIS motor score equal to or greater than 20 (Variable Importance Probability 74%) and PSFS 2 or 3 (Variable Importance Probability 66% and 68%, respectively). See Appendix B for detailed data on odds ratios and CIs for each variable analyzed.
Predictors for spasticity limiting function
N = 12 variables were identified as the most predictive for either increasing or decreasing the risk of developing spasticity limiting function on community follow-up. A variable known at hospital admission that increased the risk was (1) GCS Field moderate. A variable at hospital admission that decreased the risk was (2) older age; for each 10-year increase in patient age at time of injury, the probability of developing spasticity limiting function at community follow-up decreased by 4% (OR 0.96, 95% CI 0.93, 0.98). A predictor during the course of hospitalization that increases the risk was (3) admission to the rehabilitation center (OR 1.21, 95% CI 1.05, 1.40). Characteristics known at community discharge that predicted an increased risk were (4) PSFS 1, 2, 3 or 4; (5) prescription of spasticity medication(s) at discharge; (6) increase in AIS motor score equal to or greater than 20; (7) AIS grade C; (8) severe pain affecting quality of life; (9) severe pain affecting sleep; and (10) moderate pain during activities. Characteristics known at community discharge that predicted a decreased risk were (11) neurological level C1–T12 + L1–S5 (where one patient has two levels of injury, one resulting in neurological level within C1–T12 and the other resulting in a neurological level within L1–S5), and (12) motor level L1–L5.
Predictive models for problematic spasticity in community follow-up
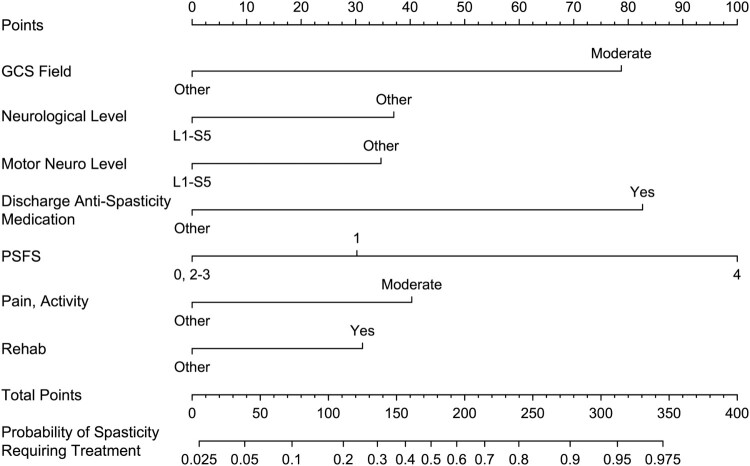
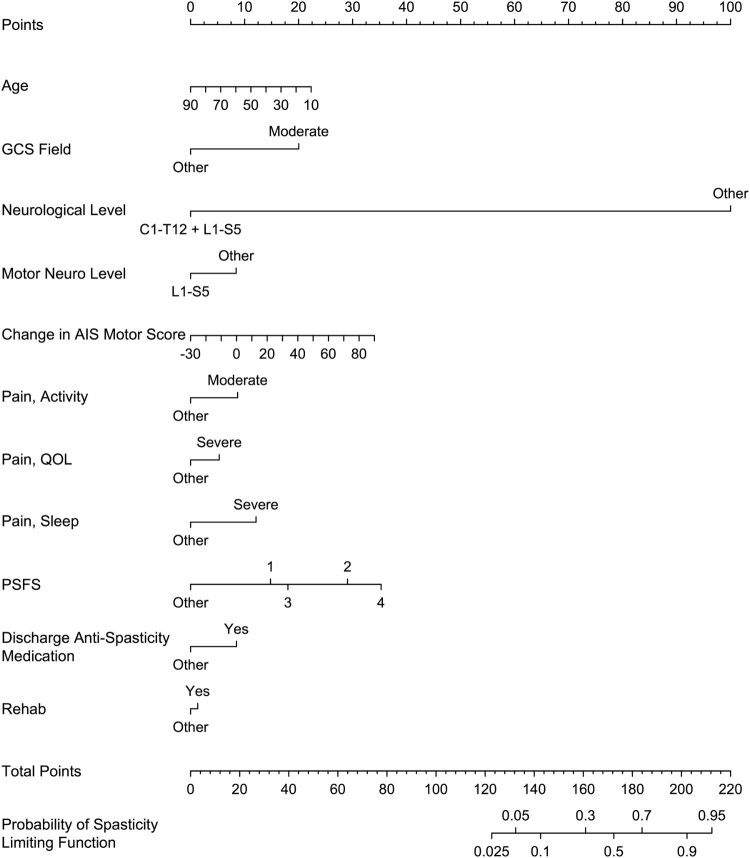
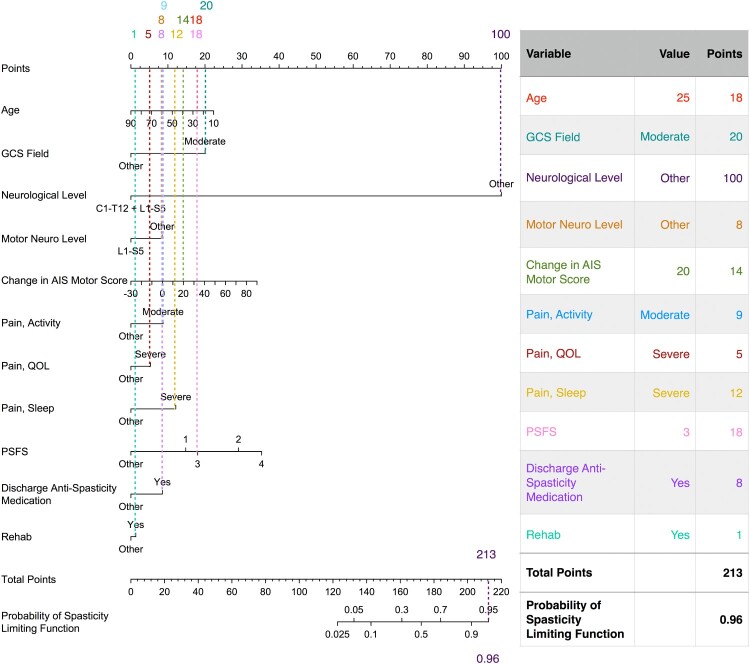
Nomograms from the final predictive models are presented in Figs 4 and 5. The final predictive models had an area under the ROC curve for predictors of spasticity requiring treatment of 0.80 (95% CI 0.76, 0.85) in the development set (N = 244) and 0.85 (95% CI 0.77, 0.92) in the validation set (N = 106), and for predictors of spasticity limiting function of 0.81 (95% CI 0.75, 0.85) in the development set and 0.84 (95% CI 0.74, 0.92) in the validation set. An example of how to use the nomogram for estimating the risk of spasticity limiting function is provided in Fig. 6.
Figure 4.
Nomogram for the predictive model of spasticity requiring treatment on community follow-up.
Figure 5.
Nomogram for the predictive model of spasticity limiting function on community follow-up.
Figure 6.
Example use of nomogram for spasticity limiting function on community follow-up. An example case is provided for a hypothetical individual who acquires a traumatic SCI with values provided for the most important variables (e.g. 25 years old at time of injury, first GCS of at scene of accident = 10, etc).
Discussion
This is the first large prospective cohort study to identify early predictors of the long term (up to 5 years post-injury) development of problematic spasticity in individuals with traumatic SCI. This is also the first study to create models that predict the development of problematic spasticity in the community with excellent discrimination on validation analysis. In summary, variables documented during hospitalization included: at time of injury age and first documented Glasgow Coma Scale; whether admitted to rehabilitation center prior to community discharge or discharged direct from acute care hospital to community (e.g. home, long-term care facility); whether at time of discharge to community the person was on anti-spasticity medication; at time of community discharge neurological status (i.e. neurological level, motor level, AIS grade and change in AIS motor score), score on the Penn Spasm Frequency Scale, and self-reported pain interference with activities, sleep and quality of life.
Neurological predictors like more severe GCS and sparing of lower motor nerves are consistent with what is expected pathophysiologically based on spasticity occurring as a result of injury to the central nervous system. The odds ratios in the tables are conditional on all other variables, therefore results suggest that for a given AIS or neurological level at discharge, a greater improvement in motor score was associated with increased odds of problematic spasticity in community follow-up. Among patients with motor score improvement, patients with AIS C, problematic spasticity at discharge and those who had rehab were most likely to have problematic spasticity in community follow-up. The finding of a greater change in AIS motor score being predictive of increased risk of developing problematic spasticity is consistent with the findings by a study by Dvorak et al.6 who studied individuals with traumatic central cord syndrome. Not only was a greater change in AIS motor score associated with increased risk of spasticity requiring medication for treatment (in other words, problematic spasticity) it was also associated with decreased quality of life (as measured by the SF-36) and functioning (as measured by the Functional Independence Measure). This was a very interesting finding, as one might expect that greater AIS motor recovery would be associated with improved quality of life and functioning. However, it appears that problematic spasticity can prevent the individual from optimizing use of available motor strength, presumably due to the spasticity-related intermittent or sustained involuntary muscle contractions. These results are further supported by a study by our group demonstrating that individuals with AIS C (incomplete motor and sensory SCI) had the highest prevalence of problematic spasticity on community discharge.3 A comprehensive review on the pathophysiological changes following SCI that contribute to development of spasticity23 describes that while motor neuron adaptation to loss of supraspinal input can occur following SCI, changes can be maladaptive resulting in uncontrolled muscle activation (e.g. spasticity) thus impairing function, which generally requires controlled or purposeful muscle activation. Therefore, it stands to reason that motor recovery can be associated with an increase in both adaptive (controlled) and maladaptive (uncontrolled) muscle activation, and that it is important to identify those who are at greatest risk for experiencing maladaptive motor recovery so as to target preventative treatment strategies that will optimize function and, ultimately, quality of life.
Increased age at time of injury being “protective” of developing spasticity was an interesting finding. A large longitudinal study (N = 1790) of individuals with chronic traumatic SCI also identified age as negatively associated with self-reported spasticity severity.24 Further research is needed to ascertain why this may be the case.
Admission to the rehabilitation center as compared to being discharged to the community directly from the acute care hospital was associated with an increased risk of developing problematic spasticity in the community. This is probably as a result of the more severe, complex injuries requiring additional rehabilitation compared to those that have minimal deficits. All individuals with a history of SCI would benefit from appropriate community follow tailored to their needs; results of this study highlight the importance of ongoing long-term community follow-up for those who require inpatient rehabilitation post-injury.
Pain has been associated with spasticity in SCI, with a large cross-sectional study (N = 1579) demonstrating that individuals with SCI and spasticity had a 2.38 increased odds of reporting a chronic pain problem compared individuals with SCI who did not experience spasticity.25 The interaction between pain and spasticity is complex, as spasticity can cause pain, and pain can trigger spasticity in a bidirectional relationship. Findings from this study highlight the importance of including questions regarding pain when asking a patient regarding their spasticity history.
Results from this study suggest that the PSFS could be a valuable tool for assessment of spasticity in SCI. Not only is the presence of muscle spasms on the PSFS a predictor of problematic spasticity, it has low burden of use as it can be quickly administered at the time of community discharge. Spasticity is difficult to assess solely with objective clinical measures; the additional use of self-report measures is thought to more accurately capture the experience of spasticity in people with SCI.26,27
Anti-spasticity medication prescription at discharge being a predictor for spasticity limiting function in the community may just mean that the affected individuals had more spasticity and thus needed more medications. However, this finding could be concerning, as clinicians hope that appropriately managed spasticity during hospitalization using interventions such as medications will decrease the risk of spasticity impairing function in the long term. This finding highlights the need for ongoing follow-up of these individuals in the long term as well as the need for further research on how to best treat spasticity in the SCI population.
Predictive modeling of development of problematic spasticity
In validation analysis of prediction models, area under the ROC curve values ≥ 0.8 are considered excellent and values ≥ 0.7 are acceptable.28 The predictive models created in this study provide excellent discrimination with lower boundaries of the CI crossing into acceptable discrimination. In order to utilize the nomograms developed, at the time of community discharge the clinician will need to: (1) review the chart for GCS at the scene of the accident, age at time of injury, admission to rehabilitation, and prescription of anti-spasticity medication at time of discharge; (2) Administer the NRS pain interference for activities, sleep and quality of life as well as the PSFS questionnaires; (3) Perform a detailed neurological examination to ascertain discharge neurological and motor level (which is considered part of standard clinical care at our local acute care and rehabilitation centers), and determine the change in AIS motor score between admission and discharge. An important role of the clinician is to be a steward of resources in settings that often have limited funding. Therefore, it is important to be able to determine those who are higher risk for developing significant secondary medical complications such as problematic spasticity so as to allocate resources (such as referral to a spasticity clinic and regular long-term follow-up with an SCI medicine trained clinician) to those who are most likely to benefit.
Study limitations
Strengths of our study include the prospectively collected data in a large population, the availability of detailed information about patients’ initial neurological impairments assessed by trained clinicians, and a validation population of the derived predictive models. Spasticity was assessed using self-report questionnaires; clinical data such as the Modified Ashworth Scale were not collected. There is a reasonable correlation between the physical findings of spasticity on clinical exam and patient-reported spasm severity scores according to the PSFS.29 Also, it has been hypothesized that a 1-time clinical exam is a weak reflection of general spasticity, and self-report measures may better capture the overall burden of spasticity. Therefore, using self-report questionnaires to determine the presence of spasticity is considered a strength of this study. Potential limitations include that the primary outcome measure was self-reported; results from this study are limited to the SCI population that received their post SCI acute treatment and rehabilitation at the RHSCIR centers involved in the study. We acknowledge that one of the limitations of this study is that we utilized the PSFS and medication usage at discharge, but that the length of stay varied greatly in our patient cohort. Differences in practice and the time between admission to community discharge between centers may affect outcomes. Therefore, an external validation study is needed to assess the generalizability of these prediction models.30
Conclusion
Improving ability to provide an early prognosis on the development of problematic spasticity in the long term allows clinicians to better allocate limited resources, ensure appropriate follow-up and educate patients regarding expected outcomes. Results of the study suggest that there is an opportunity to enhance early rehabilitation efforts especially in those who fit the predictive model for problematic spasticity. These efforts include preventative strategies such as patient education on avoidance of spasticity triggers, and physical therapies such as a stretching program, the standing frame, and electrical therapy for life long management of spasticity. Validated prediction models also assist research (e.g. risk stratification in interventional trials). Further research is needed to confirm the predictive ability of these nomograms in other SCI populations. If demonstrated to be robust, these predictive models can greatly assist clinical care and research in spasticity management following SCI.
Abbreviations
- SCI
spinal cord injury
- RHSCIR
Rick Hansen Spinal Cord Injury Registry
- AIS
American Spinal Injury Association Impairment Scale
- PSFS
Penn Spasm Frequency Scale
- SCI-HQ
Spinal Cord Injury Health Questionnaire
- GCS
Glasgow Coma Scale
- NRS
Numeric Rating Scale
- ROC
receiver operating characteristic
- CI
confidence interval
Appendix A. Spinal Cord Injury Health Questionnaire
The Spinal Cord Injury Health Questionnaire (SCI-HQ) is a self-reported questionnaire that assesses the presence of health conditions in community follow-up at 1, 2 and 5 years’ post-injury. Below are the questions asked:
In the expanded RHSCIR dataset the following is encoded: presence of spasticity (yes/no), ongoing treatment for spasticity (yes/no) and functional limitation of activities (yes/no). Treatment for spasticity includes physical therapy, anti-spasticity medication and/or surgery.
Penn Spasm Frequency Scale
The Penn Spasm Frequency Scale (PSFS) is a self-reported questionnaire used to assess spasticity-related muscle spasms. It is composed of 2 Parts. Part 1 is the portion of the self-report measure with items on 5-point scale assessing spasm frequency over the last 7 days.
Part 1: Spasm Frequency
0 = No spasm
1 = Mild spasms induced by stimulation
2 = Infrequent full spasms occurring less than once per hour
3 = Spasms occurring more than once per hour
4 = Spasms occurring more than ten times per hour
Appendix B
Table A1. Detailed data for spasticity requiring treatment in community follow-up.
| Category | Characteristic | Level | OR (95% CI) | Variable ImportanceProbability |
|---|---|---|---|---|
| Admission | ||||
| Demographics | Gender | Female | 1 | – |
| Male | 1.06 (0.96, 1.18) | 0.436 | ||
| Age (10 years) | 0.96 (0.93, 0.98) | 0.371 | ||
| Living arrangement | Alone | 1 | – | |
| Not alone | 0.94 (0.85, 1.05) | 0.363 | ||
| Household income | >= 20K | 1 | – | |
| < 20K | 0.98 (0.85, 1.14) | 0.345 | ||
| Education | No Diploma or G.E.D | 1 | – | |
| Diploma | 0.90 (0.80, 1.01) | 0.375 | ||
| Trauma | GCS facility | Mild | 1 | – |
| Moderate | 1.12 (0.78, 1.61) | 0.425 | ||
| Severe | 1.04 (0.87, 1.25) | 0.443 | ||
| GCS field | Mild | 1 | – | |
| Moderate | 1.38 (1.05, 1.81) | 0.798 | ||
| Severe | 1.06 (0.88, 1.28) | 0.68 | ||
| Medical History | Comorbidities | None | 1 | – |
| At least one | 0.98 (0.88, 1.10) | 0.359 | ||
| Missing | 1.03 (0.79, 1.34) | 0.131 | ||
| Cannabis use at admission | No | 1 | – | |
| Yes | 1.00 (0.88, 1.15) | 0.357 | ||
| Discharge | ||||
| Neurology | AIS | A | 1 | – |
| B | 0.93 (0.80, 1.08) | 0.372 | ||
| C | 1.29 (1.06, 1.56) | 0.626 | ||
| D | 1.01 (0.77, 1.32) | 0.187 | ||
| E | 0.93 (0.59, 1.48) | 0.367 | ||
| AIS motor score (20 points) | 0.94 (0.73, 1.22) | 0.096 | ||
| Increase in AIS motor score (20 points) | 1.04 (0.98, 1.10) | 0.744 | ||
| AIS UEMS (20 points) | 1.08 (0.83, 1.40) | 0.184 | ||
| AIS LEMS (20 points) | 1.12 (0.81, 1.53) | 0.257 | ||
| Neurological level | C1–C8 | 1 | – | |
| T1–T12 | 1.07 (0.83, 1.38) | 0.4 | ||
| L1–S5 | 0.94 (0.88, 1.61) | 0.867 | ||
| C1–T12 + L1–S5 | 0.66 (0.44, 0.98) | 0.374 | ||
| Motor neuro level | C1–C8 | 1 | – | |
| T1–T12 | 0.93 (0.74, 1.17) | 0.252 | ||
| L1–S5 | 0.74 (0.54, 1.02) | 0.824 | ||
| C1–T12 + L1–S5 | 0.44 (0.31, 0.63) | 0.196 | ||
| Course of care | Operation | No | 1 | – |
| Yes | 1.03 (0.89, 1.20) | 0.417 | ||
| Gabapentin, within 1 month | No | 1 | – | |
| Yes | 0.94 (0.85, 1.04) | 0.385 | ||
| Length, inpatient stay (30 days) | 0.95 (0.91, 0.99) | 0.3 | ||
| Rehab | No | 1 | – | |
| Yes | 1.21 (1.05, 1.40) | 0.762 | ||
| Length, rehab (30 days) | 1.03 (0.98, 1.08) | 0.24 | ||
| Pain | Pain, activity | None | 1 | – |
| Mild | 1.02 (0.87, 1.18) | 0.45 | ||
| Moderate | 1.10 (0.93, 1.30) | 0.928 | ||
| Severe | 0.89 (0.71, 1.13) | 0.328 | ||
| Pain, QOL | None | 1 | – | |
| Mild | 1.06 (0.91, 1.24) | 0.481 | ||
| Moderate | 1.05 (0.88, 1.25) | 0.234 | ||
| Severe | 1.20 (0.99, 1.47) | 0.465 | ||
| Pain, sleep | None | 1 | – | |
| Mild | 0.95 (0.84, 1.08) | 0.291 | ||
| Moderate | 0.91 (0.78, 1.06) | 0.458 | ||
| Severe | 1.16 (0.98, 1.38) | 0.296 | ||
| Spasticity | PSFS | 0 | 1 | – |
| 1 | 1.20 (1.07, 1.35) | 0.886 | ||
| 2 | 1.49 (1.28, 1.75) | 0.66 | ||
| 3 | 1.37 (1.15, 1.63) | 0.682 | ||
| 4 | 1.67 (1.28, 2.19) | 0.964 | ||
| Discharge anti-spasticity medication | No | 1 | – | |
| Yes | 1.18 (1.04, 1.33) | 1 | ||
Table A2. Detailed data for spasticity limiting function in community follow-up.
| Category | Characteristic | Level | OR (95% CI) | Variable Importance Probability |
|---|---|---|---|---|
| Admission | ||||
| Demographics | Gender | Female | 1 | – |
| Male | 1.06 (0.96, 1.18) | 0.558 | ||
| Age (10 years) | 0.96 (0.93, 0.98) | 0.98 | ||
| Living arrangement | Alone | 1 | – | |
| Not alone | 0.94 (0.85, 1.05) | 0.549 | ||
| Household income | ≥ 20K | 1 | – | |
| < 20K | 0.98 (0.85, 1.14) | 0.589 | ||
| Education | No Diploma or G.E.D | 1 | – | |
| Diploma | 0.90 (0.80, 1.01) | 0.633 | ||
| Trauma | GCS facility | Mild | 1 | – |
| Moderate | 1.12 (0.78, 1.61) | 0.741 | ||
| Severe | 1.04 (0.87, 1.25) | 0.525 | ||
| GCS field | Mild | 1 | – | |
| Moderate | 1.38 (1.05, 1.81) | 0.888 | ||
| Severe | 1.06 (0.88, 1.28) | 0.536 | ||
| Medical history | Comorbidities | None | 1 | – |
| At least one | 0.98 (0.88, 1.10) | 0.553 | ||
| Missing | 1.03 (0.79, 1.34) | 0.052 | ||
| Cannabis use at Admission | No | 1 | - | |
| Yes | 1.00 (0.88, 1.15) | 0.6 | ||
| Discharge | ||||
| Neurology | AIS | A | 1 | – |
| B | 0.93 (0.80, 1.08) | 0.622 | ||
| C | 1.29 (1.06, 1.56) | 0.977 | ||
| D | 1.01 (0.77, 1.32) | 0.499 | ||
| E | 0.93 (0.59, 1.48) | 0.256 | ||
| AIS motor score (20 points) | 0.94 (0.73, 1.22) | 0.428 | ||
| Increase in AIS motor score (20 points) | 1.04 (0.98, 1.10) | 0.849 | ||
| AIS UEMS (20 points) | 1.08 (0.83, 1.40) | 0.461 | ||
| AIS LEMS (20 points) | 1.12 (0.81, 1.53) | 0.434 | ||
| Neurological level | C1–C8 | 1 | – | |
| T1–T12 | 1.07 (0.83, 1.38) | 0.402 | ||
| L1–S5 | 1.19 (0.88, 1.61) | 0.475 | ||
| C1–T12 + L1–S5 | 0.66 (0.44, 0.98) | 0.756 | ||
| Motor neuro level | C1–C8 | 1 | – | |
| T1–T12 | 0.93 (0.74, 1.17) | 0.369 | ||
| L1–S5 | 0.74 (0.54, 1.02) | 0.887 | ||
| C1–T12 + L1–S5 | 0.44 (0.31, 0.63) | 0.516 | ||
| Course of care | Operation | No | 1 | – |
| Yes | 1.03 (0.89, 1.20) | 0.698 | ||
| Gabapentin, within 1 month | No | 1 | – | |
| Yes | 0.94 (0.85, 1.04) | 0.55 | ||
| Length, inpatient stay (30 days) | 0.95 (0.91, 0.99) | 0.727 | ||
| Rehab | No | 1 | – | |
| Yes | 1.21 (1.05, 1.40) | 0.818 | ||
| Length, rehab (30 days) | 1.03 (0.98, 1.08) | 0.191 | ||
| Pain | Pain, activity | None | 1 | – |
| Mild | 1.02 (0.87, 1.18) | 0.367 | ||
| Moderate | 1.10 (0.93, 1.30) | 0.937 | ||
| Severe | 0.89 (0.71, 1.13) | 0.525 | ||
| Pain, QOL | None | 1 | – | |
| Mild | 1.06 (0.91, 1.24) | 0.465 | ||
| Moderate | 1.05 (0.88, 1.25) | 0.445 | ||
| Severe | 1.20 (0.99, 1.47) | 0.852 | ||
| Pain, sleep | None | 1 | – | |
| Mild | 0.95 (0.84, 1.08) | 0.467 | ||
| Moderate | 0.91 (0.78, 1.06) | 0.703 | ||
| Severe | 1.16 (0.98, 1.38) | 0.883 | ||
| Spasticity | PSFS | 0 | 1 | – |
| 1 | 1.20 (1.07, 1.35) | 0.825 | ||
| 2 | 1.49 (1.28, 1.75) | 0.998 | ||
| 3 | 1.37 (1.15, 1.63) | 0.944 | ||
| 4 | 1.67 (1.28, 2.19) | 0.977 | ||
| Discharge anti-spasticity medication | No | 1 | – | |
| Yes | 1.18 (1.04, 1.33) | 0.998 | ||
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Acknowledgements
We are grateful to the following individuals for their contribution: Juliet Batke and Allan Aludino of the Vancouver Spine Research Program for database management and data abstraction; Glenys MacIsaac of the Rick Hansen Institute for technical support of data management; and Darby Thompson from Emmes Canada for statistical support and assistance with data analysis.
Disclaimer statements
Contributors None
Funding Dr Kwon is the Canada Research Chairs in Spinal Cord Injury
Declaration of interest Dr Holtz, Dr Kwon and Dr Mills declare no conflict of interest.
Conflicts of interest Ms Szefer is an employee of Emmes Canada. Dr Noonan is an employee of the Rick Hansen Institute.
ORCID
Kaila A. Holtz http://orcid.org/0000-0001-8919-8954
References
- 1.Pandyan AD, Gregoric M, Barnes MP, Wood D, Van Wijck F, et al. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil 2005;27(1–2):2–6. [cited 2015 February 17]. Available from http://www.ncbi.nlm.nih.gov/pubmed/15799140. doi: 10.1080/09638280400014576 [DOI] [PubMed] [Google Scholar]
- 2.Andresen SR, Biering-Sørensen F, Hagen EM, Nielsen JF, Bach FW, Finnerup NB.. Pain, spasticity and quality of life in individuals with traumatic spinal cord injury in Denmark. Spinal Cord 2016;54(11):973–9. doi: 10.1038/sc.2016.46 [DOI] [PubMed] [Google Scholar]
- 3.Holtz KA, Lipson R, Noonan VK, Kwon BK, Mills PB.. Prevalence and effect of problematic spasticity following traumatic spinal cord injury Arch Phys Med Rehabil 2016;98(6):1132–8. doi: 10.1016/j.apmr.2016.09.124 [DOI] [PubMed] [Google Scholar]
- 4.Noreau L, Proulx P, Gagnon L, Drolet M, Laramée MT.. Secondary impairments after spinal cord injury: a population-based study. Am J Phys Med Rehabil 2000;79(6):526–35. [cited 2017 May 11] Available from http://www.ncbi.nlm.nih.gov/pubmed/11083303. doi: 10.1097/00002060-200011000-00009 [DOI] [PubMed] [Google Scholar]
- 5.Noonan VK, Kwon BK, Soril L, Fehlings MG, Hurlbert RJ, Townson A, et al. The Rick Hansen Spinal Cord Injury Registry (RHSCIR): a national patient-registry. Spinal Cord 2012;50(1):22–7. doi: 10.1038/sc.2011.109 [DOI] [PubMed] [Google Scholar]
- 6.Dvorak MF, Fisher CG, Hoekema J, Boyd M, Noonan V, Wing PC, et al. Factors predicting motor recovery and functional outcome after traumatic central cord syndrome: a long-term follow-up. Spine 2005;30(20):2303–11. [cited 2015 January 17] Available from http://www.ncbi.nlm.nih.gov/pubmed/16227894. doi: 10.1097/01.brs.0000182304.35949.11 [DOI] [PubMed] [Google Scholar]
- 7.Sköld C, Levi R, Seiger A.. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil 1999;80(12):1548–57. doi: 10.1016/S0003-9993(99)90329-5 [DOI] [PubMed] [Google Scholar]
- 8.Anson CA, Shepherd C.. Incidence of secondary complications in spinal cord injury. Int J Rehabil Res 1996;19(1):55–6. doi: 10.1097/00004356-199603000-00006 [DOI] [PubMed] [Google Scholar]
- 9.Warner FM, Cragg JJ, Jutzeler CR, et al. Early administration of gabapentinoids improves motor recovery after human spinal cord injury. Cell Rep 2017;18(7):1614–8. doi: 10.1016/j.celrep.2017.01.048 [DOI] [PubMed] [Google Scholar]
- 10.Cragg JJ, Haefeli J, Jutzeler CR, Rohrich F, Weidner N, Saur M, et al. Effects of pain and pain management on motor recovery of spinal cord-injured patients. Neurorehabil Neural Repair 2016;30(8):753–61. doi: 10.1177/1545968315624777 [DOI] [PubMed] [Google Scholar]
- 11.Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B, et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med 1989;320(23):1517–21. [cited 2015 March 8]. Available from http://www.ncbi.nlm.nih.gov/pubmed/2657424. doi: 10.1056/NEJM198906083202303 [DOI] [PubMed] [Google Scholar]
- 12.Priebe MM, Sherwood AM, Thornby JI, Kharas NF, Markowski J.. Clinical assessment of spasticity in spinal cord injury: a multidimensional problem. Arch Phys Med Rehabil 1996;77(7):713–6. [cited 2015 February 16]. Available from http://www.ncbi.nlm.nih.gov/pubmed/8670001. doi: 10.1016/S0003-9993(96)90014-3 [DOI] [PubMed] [Google Scholar]
- 13.Krebs EE, Carey TS, Weinberger M.. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med 2007;22(10):1453–8. doi: 10.1007/s11606-007-0321-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward AB. A summary of spasticity management – a treatment algorithm. Eur J Neurol 2002;9(s1):48–52. doi: 10.1046/j.1468-1331.2002.0090s1048.x [DOI] [PubMed] [Google Scholar]
- 15.Tilton AH. Injectable neuromuscular blockade in the treatment of spasticity and movement disorders. J Child Neurol 2003;18(Suppl 1):S50–66. [cited 2015 December 22]. Available from http://www.ncbi.nlm.nih.gov/pubmed/13677571. doi: 10.1177/0883073803018001S0701 [DOI] [PubMed] [Google Scholar]
- 16.Lui J, Sarai M, Mills PB.. Chemodenervation for treatment of limb spasticity following spinal cord injury: a systematic review. Spinal Cord 2015;53(4):252–64. doi: 10.1038/sc.2014.241 [DOI] [PubMed] [Google Scholar]
- 17.Zou H, Hastie T.. Regularization and variable selection via the elastic net. J R Stat Soc Ser B (Statistical Methodol) 2005;67(2):301–20. doi: 10.1111/j.1467-9868.2005.00503.x [DOI] [Google Scholar]
- 18.Bunea F, She Y, Ombao H, Gongvatana A, Devlin K, Cohen R.. Penalized least squares regression methods and applications to neuroimaging. Neuroimage 2011;55(4):1519–27. doi: 10.1016/j.neuroimage.2010.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman J, Hastie T, Tibshirani R.. Regularization paths for generalized linear models via coordinate descent. J Stat Softw 2010;33(1):1–22. [cited 2018 January 16]. Available from http://www.ncbi.nlm.nih.gov/pubmed/20808728. doi: 10.18637/jss.v033.i01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lumley T. Analysis of complex survey samples. J Stat Softw 2004;9(8):1–19. doi: 10.18637/jss.v009.i08 [DOI] [Google Scholar]
- 21.Harrell FE Jr. RMS: Regression Modeling Strategies. R package version 5.1-1. [cited 2018 January 16]. Available from: https://cran.r-project.org/package=rms.
- 22.Toby Dylan Hocking . WeightedROC: Fast, Weighted ROC Curves. R package version 2017.07.12. [cited 2018 January 17]. Available fromhttps://cran.r-project.org/package = WeightedROC.
- 23.D’Amico JM, Condliffe EG, Martins KJB, Bennett DJ, Gorassini MA.. Recovery of neuronal and network excitability after spinal cord injury and implications for spasticity. Front Integr Neurosci 2014;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiPiro ND, Li C, Krause JS.. A longitudinal study of self-reported spasticity among individuals with chronic spinal cord injury. Spinal Cord 2017;56(3):218–25. doi: 10.1038/s41393-017-0031-5 [DOI] [PubMed] [Google Scholar]
- 25.Müller R, Brinkhof MWG, Arnet U, Hinrichs T, Landmann G, Jordan X, et al. Prevalence and associated factors of pain in the Swiss spinal cord injury population. Spinal Cord 2017;55(4):346–54. doi: 10.1038/sc.2016.157 [DOI] [PubMed] [Google Scholar]
- 26.Lechner H, Frotzler A, Eser P.. Relationship between self- and clinically rated spasticity in spinal cord injury. Arch Phys Med Rehabil 2006;87(1):15–19. doi: 10.1016/j.apmr.2005.07.312 [DOI] [PubMed] [Google Scholar]
- 27.Priebe MM, Sherwood AM, Thornby JI, Kharas NF, Markowski J.. Clinical assessment of spasticity in spinal cord injury: a multidimensional problem. Arch Phys Med Rehabil 1996;77(7):713–6. [cited 2014 July 22]. Available from http://www.ncbi.nlm.nih.gov/pubmed/8670001. doi: 10.1016/S0003-9993(96)90014-3 [DOI] [PubMed] [Google Scholar]
- 28.Hosmer DW, Lemeshow S, Sturdivant RX.. Applied Logistic Regression. [cited 2018 January 16]. Available from https://www.wiley.com/en-ca/Applied+Logistic+Regression,+3rd+Edition-p-9780470582473.
- 29.Benz EN, Hornby TG, Bode RK, Scheidt RA, Schmit BD.. A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch Phys Med Rehabil 2005;86(1):52–9. [cited 2014 July 24]. Available from http://www.ncbi.nlm.nih.gov/pubmed/15640989. doi: 10.1016/j.apmr.2004.01.033 [DOI] [PubMed] [Google Scholar]
- 30.Altman DG, Vergouwe Y, Royston P, Moons KGM.. Prognosis and prognostic research: validating a prognostic model. Br Med J 2009;338:b605. doi: 10.1136/bmj.b605 [DOI] [PubMed] [Google Scholar]