ABSTRACT
Objective.
To describe perinatal and neonatal outcomes in newborns exposed to SARS-CoV-2.
Methods.
A systematic review was conducted by searching PubMed Central, LILACS, and Google Scholar using the keywords ‘covid ’ AND ‘newborn’ OR ‘child’ OR ‘infant,’ on 18 March 2020, and again on 17 April 2020. One researcher conducted the search and extracted data on demographics, maternal outcomes, diagnostic tests, imaging, and neonatal outcomes.
Results.
Of 256 publications identified, 20 met inclusion criteria and comprised neonatal outcome data for 222 newborns whose mothers were suspected or confirmed to be SARS-CoV-2 positive perinatally (17 studies) or of newborns referred to hospital with infection/pneumonia (3 studies). Most (12 studies) were case-series reports; all were from China, except three (Australia, Iran, and Spain). Of the 222 newborns, 13 were reported as positive for SARS-CoV-2; most of the studies reported no or mild symptoms and no adverse perinatal outcomes. Two papers among those from newborns who tested positive reported moderate or severe clinical characteristics. Five studies using data on umbilical cord blood, placenta, and/or amniotic fluid reported no positive results. Nine studies reported radiographic imaging, including 5 with images of pneumonia, increased lung marking, thickened texture, or high-density nodular shadow. Minor, non-specific changes in biochemical variables were reported. Studies that tested breast milk reported negative SARS-CoV-2 results.
Conclusions.
Given the paucity of studies at this time, vertical transmission cannot be confirmed or denied. Current literature does not support abstaining from breastfeeding nor separating mothers and newborns. Further evidence and data collection networks, particularly in the Americas, are needed for establishing definitive guidelines and recommendations.
Keywords: Coronavirus infection; virus diseases; pandemics; SARS virus; congenital, hereditary, and neonatal diseases and abnormalities; infectious disease transmission, vertical
RESUMEN
Objetivo.
Describir los resultados perinatales y neonatales de los recién nacidos expuestos al SARS-CoV-2.
Métodos.
Se realizó una revisión sistemática con búsqueda bibliográfica en PubMed Central, LILACS, y Google Scholar usando las palabras clave ‘covid’ Y ‘newborn’ O ‘child’ O ‘infant’, el 18 de marzo de 2020, y de nuevo el 17 de abril de 2020. Un investigador llevó a cabo la búsqueda y extrajo datos sobre demografía, resultados maternos, pruebas de diagnóstico, imágenes y resultados neonatales.
Resultados.
De las 256 publicaciones identificadas, 20 cumplieron los criterios de inclusión y comprendían datos de resultados neonatales de 222 recién nacidos cuyas madres eran casos sospechosos o positivos confirmados de SARS-CoV-2 en el período perinatal (17 estudios) o bien recién nacidos internados en el hospital con infección/neumonía (3 estudios). La mayoría (12 estudios) eran informes de series de casos; todos procedían de China, excepto tres (de Australia, España e Irán). De los 222 recién nacidos, 13 tenían resultados positivos para SARS-CoV-2; en la mayoría de los estudios se informó que los recién nacidos eran asintomáticos o tenían síntomas leves y que no se habían producido resultados perinatales adversos. Entre los estudios con recién nacidos positivos, en dos se informaron características clínicas moderadas o graves. En cinco estudios se analizó la sangre del cordón umbilical, la placenta o el líquido amniótico y no se informaron resultados positivos. En nueve estudios se reportaron imágenes radiográficas, entre ellos cinco con imágenes de neumonía, aumento de la trama pulmonar, textura engrosada u opacidades nodulares de alta densidad. Se informaron alteraciones menores e inespecíficas de los parámetros bioquímicos. En los estudios en que se analizó la leche materna se informaron resultados negativos para el SARS-CoV-2.
Conclusiones.
Dada la escasez de estudios, en este momento no es posible confirmar ni descartar la transmisión vertical. La bibliografía actual no apoya la abstención de la lactancia materna ni la separación de los recién nacidos de sus madres. Se necesitan más evidencia y redes de recolección de datos, en particular en la Región de las América, para establecer directrices y recomendaciones definitivas.
Palabras clave: Infecciones por coronavirus, virosis, pandemias, virus del SRAS, enfermedades y anomalías neonatales congénitas y hereditarias, transmisión vertical de enfermedad infecciosa
RESUMO
Objetivo.
Descrever os resultados perinatais e neonatais dos recém-nascidos expostos à SARS-CoV-2.
Métodos.
Uma revisão sistemática com pesquisa bibliográfica em PubMed Central, LILACS e Google Scholar foi realizada utilizando as palavras-chave ‘covid’ E (‘newborn’ OU ‘child’ OU ‘infant’) em 18 de março de 2020, e novamente em 17 de abril de 2020 por um pesquisador. Foram analisados dados sobre demografia, resultados maternos, testes de diagnóstico, técnicas de imagem e resultados neonatais.
Resultados.
Das 256 publicações identificadas, 20 preenchiam os critérios de inclusão e incluíam dados de resultados neonatais de 222 recém-nascidos cujas mães eram suspeitas ou positivas para a SARS-CoV-2 no período perinatal (17 estudos) ou recém-nascidos internados no hospital com infecção/pneumonia (3 estudos). A maioria (12 estudos) eram relatos de séries de casos; todos, exceto três (Austrália, Irão e Espanha), eram provenientes da China. Dos 222 recém-nascidos, 13 eram positivos para SARS-CoV-2; a maioria dos estudos relatou que os recém-nascidos eram assintomáticos ou tinham sintomas leves e que não foram observados resultados perinatais adversos. Entre os estudos com recém-nascidos positivos, dois descreviam características clínicas moderadas ou graves. O sangue do cordão umbilical, a placenta ou o líquido amniótico foram analisados em cinco estudos, não tendo sido relatados resultados positivos. Imagens radiográficas foram descritas em nove estudos, incluindo cinco com imagens de pneumonia, aumento da trama pulmonar, espessamento da textura ou opacidades nodulares de alta densidade. Foram relatadas alterações menores e não específicas dos parâmetros bioquímicos. Estudos que analisaram leite materno mostraram resultados negativos para SARS-CoV-2.
Conclusões.
Dada a escassez de estudos, neste momento a transmissão vertical não pode ser confirmada ou excluída. A literatura atual não apoia a abstenção da amamentação ou a separação dos recém-nascidos das suas mães. São necessárias mais provas e mais dados, especialmente na Região das Américas, para estabelecer orientações e recomendações definitivas.
Palavras-chave: Infecções por coronavirus; viroses; pandemias; vírus da SARS; doenças e anormalidades congênitas, hereditárias e neonatais; transmissão vertical de doença infecciosa
The human coronaviruses—MERS-CoV, SARS-CoV, and SARS-CoV-2—have been the cause of serious infections, including the Middle East Respiratory Syndrome (MERS), the Severe Acute Respiratory Syndrome (SARS), and Coronavirus Infectious Disease 2019 (COVID-19), respectively (1). The latter is responsible for an outbreak that began in Wuhan City, China, in December 2019. The World Health Organization (WHO) declared it a Public Health Emergency of International Concern on 30 January 2020 (2), and subsequently, a pandemic on 11 March 2020 (3). As of 26 March 2020, the number of confirmed cases of COVID-19 reported to the WHO had topped 2 million worldwide (4).
SARS-CoV-2 is a novel virus requiring a rapid response from health services, while ongoing, critical scientific evidence is being gathered and ascertained. Although the primary focus has been on vulnerable groups, particularly the elderly and individuals with underlying medical conditions, it is possible that pregnant women and newborns are also at higher risk. To date, there have been limited case-series and case reports regarding COVID-19 during pregnancy, possible maternal-fetal transmission, and newborn and infant infection. COVID-19 in newborns has been described as mild disease (5). However, there is concern about the infection’s implications in newborns, both in terms of impact, as well as appropriate care. Understanding the issues related to perinatal concerns is critical when developing recommendations for these population groups.
This review aims to consolidate the currently available scientific evidence describing perinatal and neonatal outcomes in newborns exposed to SARS-CoV-2 in order to guide prevention of COVID-19 in newborns and manage the care of mothers and newborns.
MATERIALS AND METHODS
A systematic reviewed was conducted on 18 March 2020, and updated on 17 April 2020, by searching for the keywords ‘covid’ AND ‘newborn’ OR ‘child’ OR ‘infant’ on Google Scholar (Google Inc., Mountain View, California, United States), LILACS (Latin American and Caribbean Center on Health Sciences Information, PAHO/WHO, São Paulo, Brazil), and PubMed Central (U.S. National Library of Medicine, Bethesda, Maryland, United States). The search results included primary case reports, case series, and randomized controlled trials of pregnant women and newborns and infants affected by COVID-19. No date or language restrictions were applied. Additional relevant studies were accessed by manual searches of reference lists.
Due to time constraints, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were considered, but not entirely adhered to and one researcher conducted the search, reviewed the full texts, and extracted data on demographics, maternal outcomes, diagnostic tests, imaging, perinatal and neonatal outcomes, and neonatal diagnostic tests.
RESULTS
The initial search identified 256 publications. Following a review of the abstracts, 201 were excluded because they were letters, recommendations, or reviews that did not analyze primary data. A full-text review of the remaining 55 articles excluded an additional 35 because they were either written in Chinese, did not provide a full text version, did not include data on newborns, or were not based on primary data. The remaining 20 studies were deemed to warrant a full-text review (Figure 1). Data was consolidated into the following categories: infection confirmation, clinical characterization, laboratory, imaging features, and care provided to newborns. Table 1 describes the papers selected and the perinatal and neonatal outcomes they report.
FIGURE 1. Systematic review of literature investigating newborns exposed to SARS-CoV-2, available up to 17 April 2020.
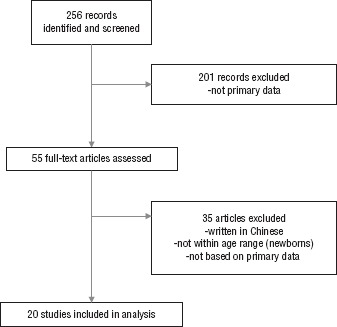
Source: Prepared by the authors from the study results.
TABLE 1. Summary of findings in studies of newborns exposed to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), available from Google Scholar, LILACS, and PubMed Central on 18 March 2020, updated 17 April 2020.
Study (Ref.) |
Design |
Population |
Diagnosis of COVID-19 |
Clinical remarks |
Imaging |
Laboratory |
Care |
Length of stay/perinatal outcome |
|---|---|---|---|---|---|---|---|---|
Wang S et al. (6) |
Case report |
One pregnant woman and one newborn (male) |
Newborn: pharyngeal swab positive at 36 h after birth. Cord blood and placenta specimens: negative. Breast milk: negative. |
Full-term newborn (40 w). Meconium-stained liquor. Birthweight: 3205 g. Apgar scores at 1 and 5 minutes: 8 and 9. Mild clinical manifestations in mother and newborn. |
Newborn chest x-ray thickened lung texture with no abnormalities (day 4). Unilateral upper-right lobe high-density nodular shadow reported on days 6, 12, and 17. |
Lymphopenia, deranged liver function tests and elevated creatine kinase level. |
C-section delivery. Mother wearing an N-95 mask. Baby isolated after birth, transferred to neonatology department. Breastfeeding not recommended. |
18 days |
Li Y et al. (7) |
Case report |
One pregnant woman and one newborn (male) |
Newborn: oropharyngeal swab, blood, feces, and urine, negative at 7 different times. Mother: sputum positive; serum, urine, feces, amniotic fluid, umbilical cord blood, placenta, and breast milk, negative. |
Preterm (35 w) without complications. |
NA |
NA |
Negative pressure operating room and Personal Protective Equipment was used. |
16 days |
Kamali Aghdam M et al. (8) |
Case report |
One newborn (male), admitted to neonatal ward |
Pharyngeal swab tested positive for SARS CoV-2, negative for influenza; blood, urine, and stool culture, negative. |
At admission, fever (38.2 °C axillar) and mottling. No cough, runny nose, or gastrointestinal symptoms. On examination, newborn completely alert, with tachycardia (heart rate of 170/min), tachypnoea (respiratory rate, 66/min), and mild subcostal retraction, O2 saturation 93% (without oxygen). |
Chest x-ray: normal. |
Routine blood test and arterial blood gases within normal values. |
Newborn transferred to the Neonatal Intensive Care Unit and isolated. Treatment: fluid therapy, oxygen therapy, antibiotic therapy (vancomycin, amikacin, and oseltamivir). |
6 days |
Alonso Díaz C et al. (9) |
Case report |
One pregnant woman and one newborn (female) |
Nasopharyngeal swab: negative for SARS CoV-2 on day 6, but positive on days 8 and 12 after birth. |
Emergency Caesarean section due to maternal preeclampsia. Newborn: low birth weight; Apgar score of 7 at 1 min, 9 at 5 min. |
Chest x-ray: faint opacity in frosted glass, predominantly unilateral and perihilar. |
No particular observation |
Due to the mother’s clinical situation, separated. Newborn transferred to the neonatal unit for immediate respiratory distress (a continuous nasal pressure device without supplemental oxygen). Respiratory assistance withdrawn at 2 h5s after birth, normal physical examination at 9 h through time of reporting case. |
NA |
Dong L et al. (10) |
Case report |
One pregnant woman positive for COVID-19 and one newborn (female) |
IgM and IgG levels higher than normal (>10 AU/ml) (day of birth and 14 days later). Mother: nasopharyngeal swab positive for SARS CoV-2; vaginal secretion and breastmilk, negative by PCR. |
No clinical symptoms reported. |
Chest x-ray: normal |
White blood cells count, neutrophil count, aspartate aminotransferase, total bilirubin, creatine kinase, and lactate dehydrogenase elevated values. |
Newborn quarantined in the Intensive Care Unit (transferred to a children’s hospital later). |
NA |
Lowe B et al. (11) |
Case report |
One mother positive for COVID-19 and one newborn |
Neonatal COVID-19 negative at 24 h after birth. No follow up deemed necessary given newborn remained asymptomatic. |
Full-term newborn (40+3 w). No neonatal resuscitation was required. |
NA |
NA |
Uncomplicated vaginal birth. Staff wore full personal protective equipment, including N-95 masks. Mother wore a N-95 mask during second stage. No maternal-neonatal separation; the newborn and parents transferred to isolation room on maternity ward postnatally. Strict viral precautions (hand washing and use of masks). Newborn was breastfed throughout. No further neonatal follow-up testing was done given the clinical conditions. |
Day 4, with follow-up from telehealth public health fever clinic and home visits by midwifery team. |
Chen H et al. (12) |
Case series |
Nine pregnant women and nine newborns |
Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six patients tested for SARS-CoV-2: all negative. |
Four newborns 36 - 37 w; six ≥ 37 w of gestation. All delivered by Caesarean section. All nine live births had a 1-min Apgar score of 8–9 and a 5-min Apgar score of 9–10. |
NA |
One newborn had a mild increase in myocardial enzymes on the day of birth (myoglobin 170.8 ng/mL and creatine kinase-myocardial band 8.5ng/mL), but no clinical symptoms. |
NA |
NA |
Zhang L et al. (13) |
Case series |
16 pregnant women positive for COVID-19 and 45 negative, and 10 newborns |
Newborns tested negative for SARS CoV-2. (only abstract; full text in Chinese) |
There were no significant differences in fetal distress, meconium-stained amniotic fluid, preterm birth, and neonatal asphyxia between the two comparison groups (all P > 0.05). |
NA |
NA |
NA |
NA |
Xia W et al. (14) |
Case series |
20 pediatric patients positive for COVID-19 (including three newborns) |
Three newborns tested positive. All positive cases confirmed by pharyngeal swab COVID-19 nucleic acid test (inclusion criteria). |
Fever (over 37.3 °C) in 12/20 (60%), cough in 13/20 (65%), diarrhea in 3/20 (15%), nasal discharge in 3/20 (15%), sore throat in 1/20 (5%), vomiting in 2/20 (10%), tachypnea in 2/20 (10%), and fatigue in 1/20 (5%). Most of the children did not have positive pulmonary signs, moist rales were found in three cases (3/20, 15%), retraction signs in 1/20 (5%), and cyanosis 1/20 (5%). |
Early stage: 6 patients unilateral pulmonary lesions (6/20, 30%), 10 bilateral pulmonary lesions (10/20, 50%), and 3 neonates and 1 child showed no abnormality on chest CT (4/20, 20%). Consolidation with surrounding halo sign was observed in 10 patients (10/20, 50%), ground-glass opacities observed in 12 patients (12/20, 60%). Advanced stage: scope of the lesion expanded and density increased. Recovery stage: lesions completely absorbed in two cases (2/20, 10%), consolidations turned into ground-glass opacities and gradually decreased in three cases (3/20, 15%), and residual fiber strip remained in three cases (3/20, 15%). |
White blood cell count normal in 14 cases (14/20, 70%), decreased in 4 cases (4/20, 20%), and increased in 2 cases (2/20, 10%); % lymphocyte decreased in 7 cases (7/20, 35%) and increased in 3 cases (3/20, 15%); alanine aminotransferase increased in 5 cases (5/20, 25%); creatine kinase-MB increased in 15 cases (15/20, 75%); C-reactive protein (CRP) increased in 9 cases (9/20, 45%); and procalcitonin (PCT) increased in 16 cases (16/20, 80%). Eight patients coinfected with other pathogens (8/20, 40%), including influenza A and B, mycoplasma, respiratory syncytial virus, and cytomegalovirus. Also, 4 cases had abnormal electrocardiogram events. Two patients had history of atrial septal defect surgery and one had epilepsy as a sequela of previous viral encephalitis. |
NA |
12.9 days (range 8 - 20) |
Liu W et al. (Ref. 15) |
Case series |
Three pregnant women positive for COVID-19 (infected in third trimester) and three negative newborns |
Oropharyngeal swabs, plasma, whole blood, urine and faeces tested negative for SARS-CoV-2 RT-PCR. |
Healthy newborns were delivered. One neonate presented chronic fetal distress in utero, chorioamnionitis and Meconium Stained Amniotic Fluid (MSAF). |
NA |
Abnormalities in aspartate aminotransferase levels, leucocytes and neutrophiles |
After delivery, the infants were immediately moved to a sealed incubator with a separate air exchange system in the neonatal ward and received initial care from health care personnel completely protected according to the current personal protection equipment guidelines. Efforts were made during delivery to minimize and exposure to maternal blood and neonate washing was conducted in neonate ward. The infant was not breast fed. |
7 - 11 days |
Zhu H et al. (16) |
Case series |
Nine mothers positive for COVID-19 and 10 newborns (8 males, 2 females; one set of twins) |
Pharyngeal swab specimens collected from 9 of the 10 neonates (1 to 9 days after birth) tested negative for SARS CoV-2. No results reported in one case. |
Six preterm births. Symptoms: reported shortness of breath (n=6), fever (n=2), thrombocytopenia accompanied by abnormal liver function (n=2), rapid heart rate (n=1), vomiting (n=1), and pneumothorax (n=1). Six newborns had a Pediatric Critical Illness Score < 90. |
Chest x-ray: abnormalities in 7 neonates at admission, described as infections (n=4), neonatal respiratory distress syndrome (n=2), and pneumothorax (n=1). |
Two newborns developed thrombocytopenia complicated with abnormal liver function. |
Seven newborns delivered by cesarean-section and three by vaginal delivery. |
Five newborns discharged; one neonatal death (multiorgan failure, preterm) and four newborns remained in hospital (3 preterm, 1 small for gestational age) |
Zhang Z-J et al. (17) |
Case series |
Four newborns hospitalized, positive for SARS-CoV-2 (3 males, 1 female) |
Nasopharyngeal swabs in 2 newborns and anal swabs for 2 newborns: positive for SARS CoV-2; confirmed at 30 h, 17 days, 5 days, 5 days . Mothers: all positive for SARS-CoV-2. |
Two newborn babies had fever, 1 had shortness of breath, 1 had cough, and 1 had no noticeable symptoms. Disease onset occurred in hospital for 2 newborns (in isolation) and at home for 2 newborns. |
CT scans were performed in 3 newborns. All showed increased lung marking. |
NA |
Supportive treatment was provided for all four newborns. None required intensive unit care/mechanical ventilation or had any severe complications. Three newborns were deemed recovered after 2 consecutive negative nucleic acid tests (separated by ≥ 24 h). Three babies were separated from mothers right after being born and were not breastfed; one neonate had not been separated from mother and was breastfed for 16 days until symptom onset. |
Time between dates of admission/symptoms and diagnosis was 0-2 day. The hospital stay was 16, 23, and 30 days, respectively. |
Chen Y et al. (18) |
Case series |
Four newborns with mothers positive for COVID-19 (3 males, 1 female) |
Three of the four newborns were negative for COVID-19 using a throat swab specimen in RT-PCR 72 h after birth; one newborn’s parents did not provide consent for testing. |
One was > 37 weeks’ gestation, with birthweight > 3000 g; two were healthy; two had rashes after birth (with different shape); one newborn presented edema, mother had cholecystitis. |
NA |
NA |
Newborns were isolated from their mothers immediately after birth and received formula feeding. |
NA |
Chen S et al. (19) |
Case series |
Five pregnant women positive for COVID-19 and five newborns |
Oral swabs from the five newborns tested negative; umbilical cord blood and amniotic fluid were not tested. |
Two newborns delivered by Caesarean section and three by vaginal delivery. Adequate birthweight and Apgar scores in all the cases. No newborns showed signs of perinatal COVID-19 infection. |
NA |
NA |
Patients were advised to stop breastfeeding and empirically given oseltamivir and azithromycin indicated. |
NA |
Zeng H et al. (20) |
Case series |
Six pregnant women positive for COVID-19 positive and six newborns |
Throat swabs and blood samples from the 6 newborns tested negative for SARS CoV-2. Five infants had IgG levels higher than normal values (>10 AU/ml) and 2 of them had also high IgM levels (>10 AU/ml) |
All 6 infants had Apgar score >8 at 1 minute and >9 at 5 minutes. |
NA |
NA |
Six newborns delivered by cesarean-section. Deliveries attended in negative pressure isolation rooms. Mothers wore masks and staff wore personal protective equipment. Infants were isolated from mothers after delivery. |
NA |
Yang H et al. (21) |
Case series |
55 pregnant women with suspected COVID-19 and 57 newborns (two sets of twins) |
20 newborns were tested and all were negative for SARS-CoV-2. |
One newborn of pregnant woman positive for COVID-19 had a fever up to 37.7 °C for 1 day after birth. Three newborns (two of which were premature) had neonatal respiratory distress syndrome after birth. |
NA |
NA |
57 newborns (2 sets of twins) were transferred to the isolation suite of neonatal intensive care unit after birth. All were followed up by telephone. |
NA |
Liu W et al. (22) |
Case series |
19 pregnant women with confirmed SARS-CoV-2 and 19 newborns (13 male, 6 female) |
All 19 newborns were negative for SARS CoV-2. Ten breast milk samples tested for SARS-CoV-2 RT-PCR were negative. SARS-CoV-2 RT-PCR test results in throat swab, gastric fluid right after birth, urine, and feces of all newborns were negative, except one positive SARS-CoV-2 RT-PCR in throat swab once that repeated testing showed was a false positive. Consistently, the virus was undetectable in amniotic fluid and umbilical cord blood. |
Gestational age 38.6 + 1.5 weeks, mean birth weight 3293 + 425 g; Apgar scores of 8 and 9 at 1 and 5 min, respectively. No fetal distress found. |
No evidence of COVID 19. Chest x-ray: 17 showed normal results and two showed increased lung marking. |
NA |
Delivery occurred in an isolated operating room. Eighteen pregnant women delivered by cesarean section and one by vaginal delivery. Delivery occurred in an isolation room. Newborns were immediately separated from their mothers. All newborns were isolated in Neonatal Intensive Care Unit for at least 14 days. |
NA |
Khan S et al. (23) |
Case series |
17 pregnant women positive for COVID-19 and 17 newborns |
Cord blood and neonatal throat swab samples were collected immediately after delivery. All newborns were negative for SARS-CoV-2. |
Three of 17 newborns were preterm; birthweight ranging 2300 g - 3750 g. Apgar scores for 16 newborns were 9 - 10. Only two newborns were suspected for COVID-19; five were reported with neonatal pneumonia. |
NA |
NA |
All deliveries were by cesarean section |
No fetal or neonatal deaths |
Li N et al. (24) |
Case-control study |
Four groups: A. pregnant women with suspected COVID-19 (n = 16; 17 newborns); B. pregnant women positive for COVID-19 (n= 18; 19 newborns); C. pregnant women without pneumonia during hospital stay in 2020 (n = 121); and D. pregnant women without pneumonia during hospital stay in 2019 (n = 121) |
Oropharyngeal swab samples from three newborns delivered by cesarean section (group A) at 4 and 14 days after birth were negative for SARS-CoV-2. |
Two singletons were born prematurely. Prevalence of prematurity was similar (23.5% and 21.1%) among group A and B and significantly higher than controls (C: 5.8% and D: 5.0%). Low birth weight more often among groups A and B (17.6% and 10.5%) than control groups (2.5%). No significant differences in key neonatal indicators between groups. Of three newborns with intrauterine fetal distress, two were from COVID-19 confirmed mothers, one also had sinus tachycardia. One case of fetal distress from group B, but no other comorbidity. |
NA |
NA |
All COVID-19 positive mothers were immediately moved to isolation wards after delivery. Newborns were cared for by family members. |
No severe neonatal asphyxia or deaths. |
Zeng L. et al. (25) |
Cohort study |
33 newborns with mothers positive for COVID-19 (17 female, 16 male) |
Three of 33 newborns tested positive. Nasopharyngeal and anal swabs were positive for SARS CoV-2 on days 2 and 4 after birth. |
Three newborns presented pneumonia; two presented fever; and one (preterm) presented asphyxia at birth, with respiratory distress syndrome, shortness of breath, cyanosis, and feeding intolerance |
Chest x-ray: pneumonia in three cases |
One newborn showed elevated procalcitonin; one newborn had leukocytosis, lymphocytopenia, and an elevated creatine kinase-MB fraction; one newborn had leukocytosis, thrombocytopenia, coagulopathy, and suspected sepsis, with an Enterobacter agglomerates-positive blood culture. |
Newborns were referred to the Neonatal Intensive Care Unit. One newborn required noninvasive ventilation, caffeine, and antibiotics. |
Vital signs stabilized at 7 days after birth. |
w: weeks; COVID-19: coronavirus disease 2019; CT: computerized tomography scan; NA: not available
Source: Prepared by the authors from the study results.
The 20 selected studies comprise neonatal outcomes for 222 newborns whose mothers were suspected or confirmed to be SARS-CoV-2 positive perinatally (6, 7, 9 –16, 18 – 25), or of newborns referred to hospital with infection or pneumonia (8, 14, 17). The articles were composed of case reports (6 – 11), case-series reports (12 – 23), a case-control study (24), and a cohort study (25). Cases reported were mostly from China, except for one report from Iran (8), one from Spain (9), and one from Australia (11). They referred mostly to pregnant women and their newborns, except for 3 case reports of only newborns.
Six studies (6, 8, 9, 17, 22, 25) examined cases of newborns who tested positive for SARS CoV-2 by reverse transcription polymerase chain reaction (RT-PCR) performed on samples taken 36 hr – 17 days after birth (10 newborns in all). One newborn was initially reported to be positive for COVID-19, but upon a second testing of the same sample, the authors changed the result to a false positive (22). Two additional papers (10, 20) reported newborns who tested negative for SARS CoV-2 by PCR but showed high levels of IgM and IgG. Dong and colleagues (10) reported a newborn who tested negative for SARS CoV-2, with IgM and IgG values > 10 AU/ml on the day of birth and 14 days later. Zeng and colleagues (20) reported a series of 6 newborns who were PCR negative for SARS CoV-2, with 5 having IgG values > 10 AU/ml and 2 also having IgM > 10AU/ml.
Five of the 20 studies (6, 7, 12, 22, 23) reported data on umbilical cord blood, placenta, and/or amniotic fluid, all with no positive results.
Most of the reports informed no or mild perinatal outcomes and clinical characteristics linked to COVID 19 (Table 1). Two papers among those from newborns who tested positive reported moderate or severe clinical characteristics. One reported a newborn with transient respiratory distress, low birth weight and Apgar score of 7 and 9 at 1 and 5 minutes, respectively (9). Another study (25) reported 3 newborns with pneumonia, 2 of whom presented fever and one (preterm) presented asphyxia at birth and respiratory distress syndrome.
Five reports among those in which newborns tested negative informed about newborns presenting moderate clinical conditions. Liu and colleagues (15) reported one newborn with chronic fetal distress in utero, chorioamnionitis and meconium stained amniotic fluid. Zhu and colleagues (16) reported 6 preterm births, out of 10 newborns included, who showed shortness of breath (n=6), fever (n=2) and Pediatric Critical Illness Score (PCIS) of less than 90. One case series (21) reported 3 cases with neonatal respiratory distress syndrome after birth, among which 2 were preterm babies. Kahn and colleagues (23) reported 5 neonates with pneumonia. Li and colleagues (24) reported significantly higher prevalence of preterm birth and low birth-weight among newborns from suspected or confirmed COVID-19 mothers and pregnant women with non-COVID-19 pneumonia, but no significant differences in key neonatal indicators between groups. The same series reported 3 newborns with intrauterine fetal distress, two of them from COVID-19 confirmed mothers and no other comorbidity. No severe neonatal asphyxia or deaths were reported.
In the report by Xia and colleagues (14), the inclusion criterion was children testing positive for SARS-CoV-2; patients ranged in age from 1 day –14 years 7 months and data were not disaggregated by age. Symptoms most frequently mentioned were fever (> 37.3 °C) in 12 of 20 cases (60%) and cough in 13 (65%). One neonatal death was reported (multiorgan failure, preterm) in a non-positive SARS-CoV-2 newborn (16).
Nine articles (6, 8–10, 14, 16, 17, 22, 25) reported information on imaging in newborns. Five out of 6 papers reporting SARS-CoV-2 positive newborns referred radiographic images of pneumonia, increased lung marking, thickened texture, or high-density nodular shadow.
A few studies (6, 10, 12, 14, 15, 16, 25) described non-specific changes in the biochemical variables as non-specific. However, there were some reports of abnormal liver function (6, 10, 14 – 16).
Five of the studies (6, 7, 10, 12, 22) tested for SARS CoV-2 in breast milk and all were negative, but not all newborns were breastfed. Five studies (6, 15, 18, 19, 20) recommended abstaining from breastfeeding, while Lowe and colleagues (11) reported that breastfeeding should be allowed. In this report, both parents tested positive and the newborn negative for SARS-CoV-2; breastfeeding was allowed and no maternal-neonatal separation was indicated. Strict viral precautions of hand washing and use of surgical masks around the baby were observed and no further neonatal follow-up testing was done given the symptom-free clinical condition. One newborn was reported to be breastfeed until onset of symptoms (17).
DISCUSSION
There is only limited data on the impact of the current COVID-19 outbreak on women affected during pregnancy, their newborns, and the pediatric population. However, the reports available and analyzed by this review show similar results.
There is still no evidence supporting vertical transmission of COVID-19. Only 6 studies (6, 8, 9, 14, 17, 25) reported COVID-19-positive newborns (confirmed within 36 hours – 17 days after delivery) and those found only 13 newborns positive of 222 exposed to SARS-CoV-2. Seventeen studies included results from suspected or confirmed pregnant women and their newborns at the time of birth (8, 14 and 17 included only newborns readmitted testing positive for SARS-CoV-2). From these studies, two cases reports (6, 9) and two case series reports (22, 25) reported newborns tested positive at 36 hours (6), 8 days (9), 2 and 4 days (25) after birth; one newborn reported by Liu and colleagues (22) was classified as false positive. Two papers (10, 20) that reported elevated values of IgM and IgG were not consistent enough to support in-utero transmission; they lacked confirmatory results from maternal or newborn samples, e.g., amniotic fluid or infant’s PCR. Even when one study (10) may contribute to suspecting mother-to-child transmission, the evidence is not conclusive (26). Some newborns were positive for COVID-19 in spite of the reported use of preventive measures during and after delivery (6, 8, 9, 22, 25), but even in these cases there was no evidence supporting vertical transmission.
The Chinese Expert Consensus on the Perinatal and Neonatal Management for the Prevention and Control of the 2019 Novel Coronavirus Infection (27) has recommended that symptomatic pregnant women be isolated in the intensive care unit (or critical care unit) in a negative pressure room, with oxygen supplementation and lateral decubitus position, regardless of respiratory status; also, vaginal birth should be protected, according to obstetric indications and the woman’s preferences. Likewise, the WHO is recommending (28) that caesarean section be undertaken only when medically justified, based on gestational age, severity of maternal condition, and fetal viability and well-being.
Delayed cord clamping, skin-to-skin contact, and initiation of breastfeeding are also causing concern during this pandemic, as they impact health and early child development, as well as comprehensive care. The WHO (28) recommends that infants born to mothers with suspected, probable or confirmed COVID-19 infection should be fed according to standard infant feeding guidelines, while applying necessary precautions for infection prevention control. The results of this review do not discourage delayed cord clamping when the newborn’s clinical condition would allow it. The WHO states that whenever a mother is seriously ill due to COVID-19, or when other complications prevent her from caring for and/or breastfeeding her baby, she should be encouraged to safely express breast milk and offer it her baby (28).
The determination of whether or not separate a mother with known or suspected COVID-19 and her infant should be made on a case-by-case basis using shared decision-making between the mother and the clinical team. Some reports in this review show that isolation and non-promotion of breastfeeding have been implemented, according to the recommendations of China’s experts (27). Routine separation of mother and baby is not promoted, however, by the Royal College of Obstetricians & Gynecologists (30), which provides guidance on individualized care based on a systematic review (31) of COVID-19 in pregnancy and delivery. In one case included in this review (11) no infection in a newborn of a COVID-19-positive mother was shown despite unrestricted attachment and breastfeeding along with implementation of strict prevention measures and support from the health system. As seen, widely differing guidelines are currently available, but consistent evidence is lacking.
This review provides additional evidence related to newborn care that can contribute to developing guidelines and recommendations. The knowledge gap regarding mother and newborn separation needs to be filled. According to the current evidence, it seems that skin-to-skin contact and breastfeeding can be recommended, but it is critical to screen pregnant women, implement prevention and control measures, and closely monitor newborns at risk of COVID-19. Solid evidence is needed to develop discharge instructions for newborns born to mothers with COVID-19 or newborns with COVID-19 themselves in terms of their vaccines, and postnatal follow-up, particularly for newborns with risk conditions as extreme premature babies.
This review has some limitations. All the studies included were case reports or low-quality series, case-control, or cohort studies. The outcomes, designs, and data reported varied and were not fully comparable. A full and exhaustive search of all medical literature would have demanded more time and staff than currently available.
To date, evidence on mother-to-newborn transmission is not consistent, given the paucity of studies on COVID-19 and pregnant women. The current literature does not support a recommendation to abstain from breastfeeding—based on a lack of evidence regarding the presence of the virus in breast milk. Likewise, there is not enough evidence to recommend separating mothers and their newborns. It is crucial to screen pregnant women, to implement infection prevention and control measures, and to provide close monitoring of neonates at risk of COVID-19.
The research studies analyzed here are mostly from China. Data collection and communication of the cases is particularly important for countries in the Americas where evidence is lacking. The dynamic of the pandemic urges not only an adequate response, but the strengthening and coordination of efforts to collect and report data. In the context of a pandemic, when health services are saturated and the movement of the population is greatly restricted, it is essential to have evidence on which to base guidelines and recommendations. The dynamic of the pandemic urges not only an adequate response, but the strengthening and coordination of international data collection networks to provide evidence for consistent and accurate COVID-19 guidelines and recommendations.
Author contributions.
All authors conceived the original idea and contributed to the analysis and interpretation of the results. All authors reviewed and approved the final version.
Disclaimer.
Authors hold sole responsibility for the views expressed in the manuscript, which may not necessarily reflect the opinion or policy of the RPSP/PAJPH and/or PAHO.
REFERENCES
- 1.Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]; 1. Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–23. 10.1002/jmv.25681 [DOI] [PMC free article] [PubMed]
- 2.World Health Organization Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) Available at: https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov); 2. World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). Available at: https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)
- 3.World Health Organization WHO Director-General’s opening remarks at the media briefing on COVID-19. 2020. Mar 11, Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.; 3. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 4.World Health Organization Coronavirus disease (COVID-19) Pandemic: Public advice and Country technical guidance. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.; 4. World Health Organization. Coronavirus disease (COVID-19) Pandemic: Public advice and Country technical guidance. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 5.Lu Q, Shi Y. 2020. Mar 01, Coronavirus disease (COVID-19) and neonate: What neonatologist need to know. J Med Virol. [Epub ahead of print] [DOI] [PMC free article] [PubMed]; 5. Lu Q, Shi Y. Coronavirus disease (COVID-19) and neonate: What neonatologist need to know. J Med Virol. 2020, Mar 1. doi: 10.1002/jmv.25740. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 6.Wang S, Guo L, Chen L, Liu W, Cao Y, Zhang J, Feng L. 2020. Mar 12, A case report of neonatal COVID-19 infection in China. Clin Infect Dis. ciaa225. [Epub ahead of print] [DOI] [PMC free article] [PubMed]; 6. Wang S, Guo L, Chen L, Liu W, Cao Y, Zhang J, Feng L. A case report of neonatal COVID-19 infection in China. Clin Infect Dis. 2020 Mar 12. pii: ciaa225. doi: 10.1093/cid/ciaa225. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 7.Li Y, Zhao R, Zheng S, Chen X, Wang J, Sheng X, Zhou J, Cai H, Fang Q, Yu F, Fan J, Xu K, Chen Y, Sheng J. Lack of Vertical Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, China. Emerg Infect Dis. 2020;26(6) doi: 10.3201/eid2606.200287. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]; 7. Li Y, Zhao R, Zheng S, Chen X, Wang J, Sheng X, Zhou J, Cai H, Fang Q, Yu F, Fan J, Xu K, Chen Y, Sheng J. Lack of Vertical Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, China. Emerg Infect Dis. 2020;26(6). doi: 10.3201/eid2606.200287. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 8.Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect Dis (Lond) 2020 Apr 01;:1–3. doi: 10.1080/23744235.2020.1747634. [DOI] [PMC free article] [PubMed] [Google Scholar]; 8. Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect Dis (Lond). 2020 Apr 1:1-3. [DOI] [PMC free article] [PubMed]
- 9.Alonso Díaz C, López Maestro M, Moral Pumarega MT, Flores Antón B, Pallás Alonso C. First case of neonatal infection due to SARS-CoV-2 in Spain. An Pediatr (Barc) 2020 Mar 31;:pii. doi: 10.1016/j.anpedi.2020.03.002. S1695-4033(20)30130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; 9. Alonso Díaz C, López Maestro M, Moral Pumarega MT, Flores Antón B, Pallás Alonso C. First case of neonatal infection due to SARS-CoV-2 in Spain. An Pediatr (Barc). 2020 Mar 31. pii: S1695-4033(20)30130-2. [DOI] [PMC free article] [PubMed]
- 10.Dong L, Tian J, He S, Zhu C, Wang J, Liu C, Yang J. 2020. Mar 26, Possible Vertical Transmission of SARS-CoV-2 from an Infected Mother to Her Newborn. JAMA. [DOI] [PMC free article] [PubMed]; 10. Dong L, Tian J, He S, Zhu C, Wang J, Liu C, Yang J. Possible Vertical Transmission of SARS-CoV-2 from an Infected Mother to Her Newborn. JAMA. 2020 Mar 26. doi:10.1001/jama.2020.4621 [DOI] [PMC free article] [PubMed]
- 11.Lowe B, Bopp B. 2020. Apr 15, COVID-19 vaginal delivery – a case report. Aust N Z J Obstet Gynaecol. [Epub ahead of print] [DOI] [PMC free article] [PubMed]; 11. Lowe B and Bopp B. COVID-19 vaginal delivery – a case report. Aust N Z J Obstet Gynaecol. 2020 Apr 15. doi: 10.1111/ajo.13173. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 12.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. Epub 2020 Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]; 12. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809-15. doi: 10.1016/S0140-6736(20)30360-3. Epub 2020 Feb 12. [DOI] [PMC free article] [PubMed]
- 13.Zhang L, Jiang Y, Wei M, Cheng BH, Zhou XC, Li J, Tian JH, Dong L, Hu RH. [Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province] Zhonghua Fu Chan Ke Za Zhi. 2020 Mar 07;55(0):E009. doi: 10.3760/cma.j.cn112141-20200218-00111. [Epub ahead of print] [Article in Chinese; Abstract in English] [DOI] [PubMed] [Google Scholar]; 13. Zhang L, Jiang Y, Wei M, Cheng BH, Zhou XC, Li J, Tian JH, Dong L, Hu RH. [Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province]. Zhonghua Fu Chan Ke Za Zhi. 2020 Mar 7;55(0):E009. doi: 10.3760/cma.j.cn112141-20200218-00111. [Epub ahead of print] [Article in Chinese; Abstract in English] [DOI] [PubMed]
- 14.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. 2020. Mar 05, Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol. [Epub ahead of print] [DOI] [PMC free article] [PubMed]; 14. Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 15.Liu W, Wang Q, Zhang Q, Chen L, Chen J, Zhang B, et al. Coronavirus disease 2019 (COVID-19) during pregnancy: a case series. Preprints. 2020. [Accessed 28 February 2020]. Available from: https://www.preprints.org/manuscript/202002.0373/v1.; 15. Liu W, Wang Q, Zhang Q, Chen L, Chen J, Zhang B, et al. Coronavirus disease 2019 (COVID-19) during pregnancy: a case series. Preprints 2020;2020020373. Available from: https://www.preprints.org/manuscript/202002.0373/v1. Accessed 28 February 2020.
- 16.Zhu H, Wang L, Fang C, Peng S, Zhang L, Chang G, Xia S, Zhou W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]; 16. Zhu H, Wang L, Fang C, Peng S, Zhang L, Chang G, Xia S, Zhou W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr 2020;9(1):51-60. 10.21037/tp.2020.02.06 [DOI] [PMC free article] [PubMed]
- 17.Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shao J, Zhou W. Neonatal Early-Onset Infection With SARS-CoV-2 in 33 Neonates Born to Mothers With COVID-19 in Wuhan, China. JAMA Pediatr. 2020 Mar 26; doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]; 17. Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shao J, Zhou W. Neonatal Early-Onset Infection With SARS-CoV-2 in 33 Neonates Born to Mothers With COVID-19 in Wuhan, China. JAMA Pediatr. 2020 Mar 26. [DOI] [PMC free article] [PubMed]
- 18.Zhang Z-J, Zhang ZJ, Yu XJ, Fu T, Liu Y, Jiang Y, Yang BX, Bi Y. Novel Coronavirus Infection in Newborn Babies Under 28 Days in China. Eur Respir J. 2020 Apr 08;:pii. doi: 10.1183/13993003.00697-2020. 2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]; 18. Zhang Z-J, Zhang ZJ, Yu XJ, Fu T, Liu Y, Jiang Y, Yang BX, Bi Y. Novel Coronavirus Infection in Newborn Babies Under 28 Days in China. Eur Respir J. 2020 Apr 8. pii: 2000697. [DOI] [PMC free article] [PubMed]
- 19.Chen Y, Peng H, Wang L, Zhao Y, Zeng L, Gao H, Liu Y. Infants Born to Mothers with a New Coronavirus (COVID-19) Front Pediatr. 2020;8:104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]; 19. Chen Y, Peng H, Wang L, Zhao Y, Zeng L, Gao H, Liu Y. Infants Born to Mothers with a New Coronavirus (COVID-19). Front Pediatr. 2020;8:104. [DOI] [PMC free article] [PubMed]
- 20.Chen S, Liao E, Cao D, Gao Y, Sun G, Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. 2020 Mar 28; doi: 10.1002/jmv.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]; 20. Chen S, Liao E, Cao D, Gao Y, Sun G, Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. 2020 Mar 28 [DOI] [PMC free article] [PubMed]
- 21.Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang W, Long X. Antibodies in Infants Born to Mothers With COVID-19 Pneumonia. AMA. 2020 Mar 26; doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]; 21. Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang W, Long X. Antibodies in Infants Born to Mothers With COVID-19 Pneumonia. AMA. 2020 Mar 26. [DOI] [PMC free article] [PubMed]
- 22.Yang H, Sun G, Tang F, Peng M, Gao Y, Peng J, Xie H, Zhao Y, Jin Z. Clinical Features and Outcomes of Pregnant Women Suspected of Coronavirus Disease 2019. J Infect. 2020 Apr 12;:pii. doi: 10.1016/j.jinf.2020.04.003. S0163-4453(20)30212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; 22. Yang H, Sun G, Tang F, Peng M, Gao Y, Peng J, Xie H, Zhao Y, Jin Z. Clinical Features and Outcomes of Pregnant Women Suspected of Coronavirus Disease 2019. J Infect. 2020 Apr 12. pii: S0163-4453(20)30212-7. [DOI] [PMC free article] [PubMed]
- 23.Liu W, Wang J, Li W, Zhou Z, Liu S, Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. 2020 Apr 13; doi: 10.1007/s11684-020-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; 23. Liu W, Wang J, Li W, Zhou Z, Liu S, Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. 2020 Apr 13. [DOI] [PMC free article] [PubMed]
- 24.Khan S, Jun L, Nawsherwan, Siddique R, Li Y, Han G, Xue M, Nabi G, Liu J. Association of COVID-19 infection with pregnancy outcomes in healthcare workers and general women. Clin Microbiol Infect. 2020 Apr 08;:pii. doi: 10.1016/j.cmi.2020.03.034. S1198-743X(20)30180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; 24. Khan S, Jun L, Nawsherwan, Siddique R, Li Y, Han G, Xue M, Nabi G, Liu J. Association of COVID-19 infection with pregnancy outcomes in healthcare workers and general women. Clin Microbiol Infect. 2020 Apr 8. pii: S1198-743X(20)30180-4. [DOI] [PMC free article] [PubMed]
- 25.Li N, Han L, Peng M, Lv Y, Ouyang Y, Liu K, Yue L, Li Q, Sun G, Chen L, Yang L. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis. 2020 Mar 30;:pii. doi: 10.1093/cid/ciaa352. ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]; 25. Li N, Han L, Peng M, Lv Y, Ouyang Y, Liu K, Yue L, Li Q, Sun G, Chen L, Yang L. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis. 2020 Mar 30. pii: ciaa352. [DOI] [PMC free article] [PubMed]
- 26.Shah PS, Diambomba Y, Acharya G, Morris SK, Bitnun A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020 Apr 11; doi: 10.1111/aogs.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]; 26. Shah PS, Diambomba Y, Acharya G, Morris SK, Bitnun A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020 Apr 11. [DOI] [PMC free article] [PubMed]
- 27.Wang L, Shi Y, Xiao T, Fu J, Feng X, Mu D, et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition ) Ann Transl Med. 2020;8(3):47. doi: 10.21037/atm.2020.02.202020. [DOI] [PMC free article] [PubMed] [Google Scholar]; 27. Wang L, Shi Y, Xiao T, Fu J, Feng X, Mu D, et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition ). Ann Transl Med. 2020;8(3):47. doi: 10.21037/atm.2020.02.202020 [DOI] [PMC free article] [PubMed]
- 28.World Health Organization . Geneva: WHO; 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. Available from: https://apps.who.int/iris/handle/10665/331446. [Google Scholar]; 28. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. Geneva: WHO; 2020. Available from: https://apps.who.int/iris/handle/10665/331446
- 29.U.S. CDC Interim Considerations for Infection Prevention and Control of Coronavirus Disease 2019 (COVID-19) in Inpatient Obstetric Healthcare Settings. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/inpatient-obstetric-healthcare-guidance.html.; 29. U.S. CDC. Interim Considerations for Infection Prevention and Control of Coronavirus Disease 2019 (COVID-19) in Inpatient Obstetric Healthcare Settings. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/inpatient-obstetric-healthcare-guidance.html
- 30.Royal College of Obstetricians & Gynaecologists . London: RCOG; 2020. Mar 18, Coronavirus (COVID-19) infection in pregnancy. Information for healthcare professionals. Version 3. Available from: https://www.rcog.org.uk/globalassets/documents/guidelines/coronavirus-covid-19-infection-in-pregnancy-v3-20-03-18.pdf. [Google Scholar]; 30. Royal College of Obstetricians & Gynaecologists. Coronavirus (COVID-19) infection in pregnancy. Information for healthcare professionals. Version 3. London: RCOG; March 18 2020. Available from: https://www.rcog.org.uk/globalassets/documents/guidelines/coronavirus-covid-19-infection-in-pregnancy-v3-20-03-18.pdf
- 31.Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020 Mar 17; doi: 10.1002/uog.22014. [DOI] [PubMed] [Google Scholar]; 31. Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020 Mar 17 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lu Q, Shi Y. 2020. Mar 01, Coronavirus disease (COVID-19) and neonate: What neonatologist need to know. J Med Virol. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Wang S, Guo L, Chen L, Liu W, Cao Y, Zhang J, Feng L. 2020. Mar 12, A case report of neonatal COVID-19 infection in China. Clin Infect Dis. ciaa225. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Dong L, Tian J, He S, Zhu C, Wang J, Liu C, Yang J. 2020. Mar 26, Possible Vertical Transmission of SARS-CoV-2 from an Infected Mother to Her Newborn. JAMA. [DOI] [PMC free article] [PubMed]
- Lowe B, Bopp B. 2020. Apr 15, COVID-19 vaginal delivery – a case report. Aust N Z J Obstet Gynaecol. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. 2020. Mar 05, Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol. [Epub ahead of print] [DOI] [PMC free article] [PubMed]


