Abstract
Compound-specific carbon isotope analysis (δ13C) of meteoritic organic compounds can be used to elucidate the abiotic chemical reactions involved in their synthesis. The soluble organic content of the Murchison carbonaceous chondrite has been extensively investigated over the years, with a focus on the origins of amino acids and the potential role of Strecker-cyanohydrin synthesis in the early solar system. Previous δ13C investigations have targeted α-amino acid and α-hydroxy acid Strecker products and reactant HCN; however, δ13C values for meteoritic aldehydes and ketones (Strecker precursors) have not yet been reported. As such, the distribution of aldehydes and ketones in the cosmos and their role in prebiotic reactions have not been fully investigated. Here, we have applied an optimized O-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine (PFBHA) derivatization procedure to the extraction, identification and δ13C analysis of carbonyl compounds in the Murchison meteorite. A suite of aldehydes and ketones, dominated by acetaldehyde, propionaldehyde and acetone, were detected in the sample. δ13C values, ranging from −10.0‰ to +66.4‰, were more 13C-depleted than would be expected for aldehydes and ketones derived from the interstellar medium, based on interstellar 12C/13C ratios. These relatively 13C-depleted values suggest that chemical processes taking place in asteroid parent bodies (e.g. oxidation of the IOM) may provide a secondary source of aldehydes and ketones in the solar system. Comparisons between δ13C compositions of meteoritic aldehydes and ketones and other organic compound classes were used to evaluate potential structural relationships and associated reactions, including Strecker synthesis and alteration-driven chemical pathways.
1. INTRODUCTION
Carbonaceous chondrite meteorites are some of the most primitive materials in our solar system, and their organic inventory contains a wealth of information about the chemistry of the presolar cloud, the protoplanetary disk, and the subsequent chemical processes that took place on and within parent body asteroids. Investigating the origins of organic compounds in meteorites may provide insights into the chemical processes that led to the origin of life on Earth, as well as the potential for life to exist elsewhere in our solar system. The Murchison meteorite, a CM type 2 meteorite that fell in southeastern Australia in 1969, is one of the most widely studied carbonaceous chondrites due to the large mass of sample material available, the relatively pristine nature of the meteorite and the structural diversity of its organic content. The findings from ~50 years of research on the chemistry of organic compounds of the Murchison meteorite have built a valuable reference data set for all other carbonaceous chondrites. The soluble organic inventory of the Murchison meteorite includes amino acids, carboxylic acids, polycyclic aromatic hydrocarbons (PAHs), amines, polyols, aldehydes, ketones, and many more compounds (see reviews and references therein: Botta and Bada, 2002; Pizzarello et al. 2006; Sephton, 2002, 2014).
Aldehydes and ketones (collectively referred to as “carbonyl compounds”) may play an important role as precursors to many biologically relevant organic compounds in the solar system. One of the most widely studied chemical reactions involving aldehyde and ketone precursors is Strecker-cyanohydrin synthesis, a proposed source of α-amino acids and α-hydroxy acids in meteorites (Scheme 1; Peltzer and Bada, 1978). The α-amino acid products of Strecker synthesis are of particular astrobiological interest as they are monomers of proteins and enzymes in living systems and their delivery by meteorites may hold clues to the origins of prebiotic organic compounds on the early Earth (Kvenvolden et al., 1970). For this reason, the distribution and stable isotopic compositions of amino acids in meteorites have been extensively analyzed to elucidate their synthetic pathways (e.g., Strecker synthesis (Peltzer et al., 1984), Michael addition (Miller et al., 1957), carbon dioxide (CO2) addition to amines (Hudson et al., 2009), and reductive amination of keto acids (Huber and Wächtershäuser, 2003)). Despite the potential significance of Strecker synthesis and the relevance of aldehydes and ketones in prebiotic organic chemistry, the stable carbon isotopic compositions (δ13C) of aldehydes and ketones in meteorites have not yet been measured. Thus, the synthetic relationships between meteoritic aldehydes, ketones and amino acids have not been fully investigated, representing a significant gap in the literature.
Scheme 1.
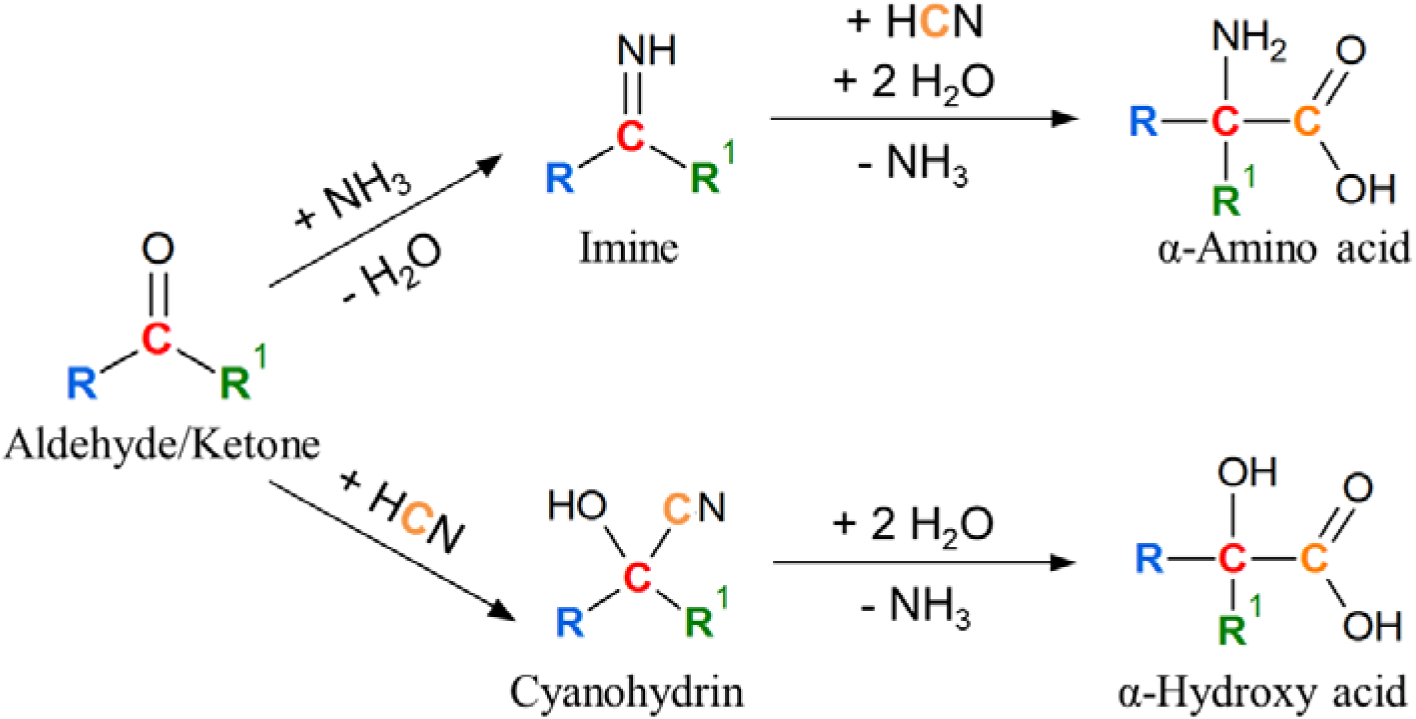
The Strecker-cyanohydrin synthesis reaction (illustration adapted from Elsila et al., 2016), yielding α-amino acids in the presence of NH3 and α-hydroxy acids in the absence of NH3 (Peltzer et al., 1984). Colors are used to trace the path of different carbon atoms from precursor to products.
In addition to the Strecker reaction, aldehydes and ketones are potentially structurally related to several other prebiotic organic compounds (Figure 1), though some of these reactions have yet to be evaluated under astrochemically-relevant conditions. Carbonyl compounds and alcohols may be interconverted via oxidation/hydrogenation reactions (Bisschop et al., 2007). Aldehydes can be further oxidized to produce carboxylic acids (Corey et al., 1968), the chemical building blocks of biological membranes (Deamer et al., 2002). Aldehydes and ketones may also be converted to amines via reductive amination (Burk et al., 1994; Abdel-Magid et al., 1996), and these amines may be subsequently converted to amino acids via CO2 addition from photochemical and/or ion-irradiation reactions (Holtom et al., 2005; Hudson et al., 2009; Bossa et al., 2009; Lee et al., 2009). The simplest aldehyde, formaldehyde, is thought to play a particularly important role in the synthesis of prebiotic organic compounds (Cleaves, 2008). In addition to the reactions summarized above, formaldehyde has been proposed as a precursor to meteoritic sugars, sugar- alcohols and insoluble organic matter (IOM) via formose reactions (Cooper et al., 2001; Cody et al., 2011; Meinert et al., 2016).
Figure 1.
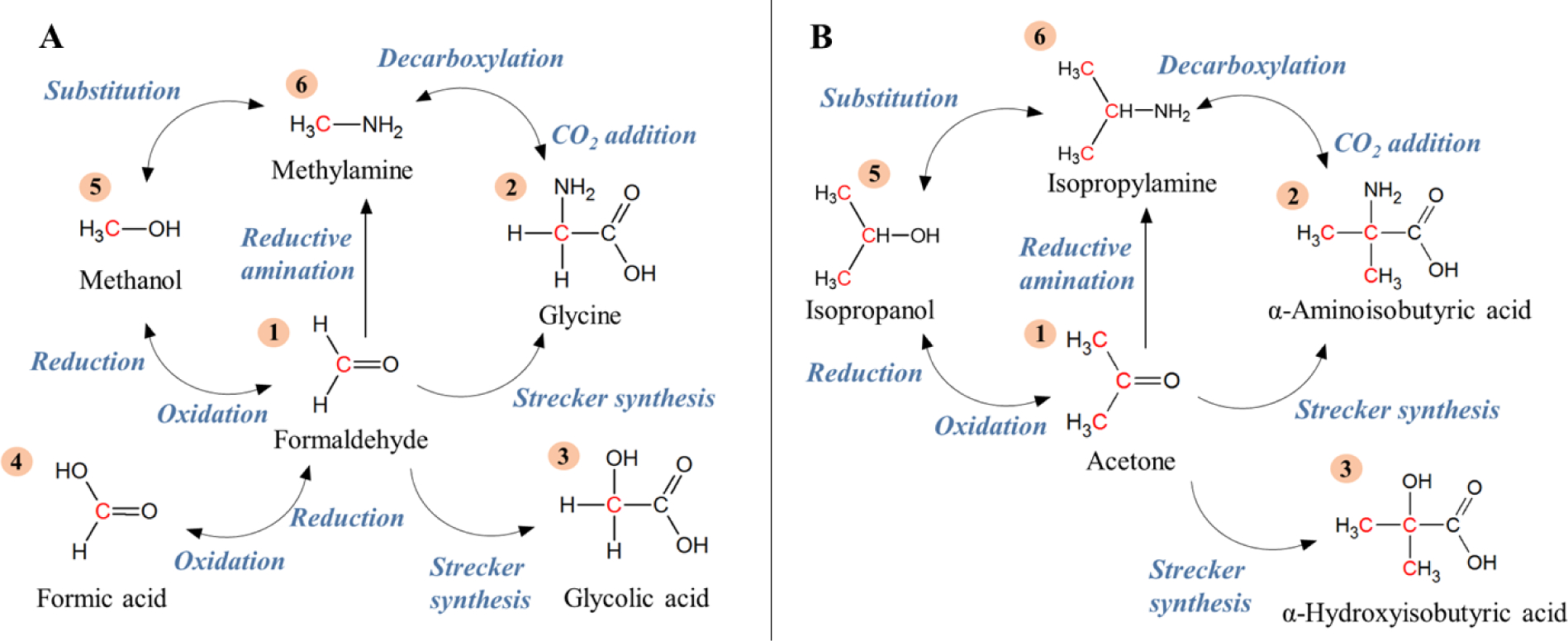
Schematic of potential synthetic relationships between carbonyl compounds(1) and other structurally-related organic compounds. Illustrated are the amino acid(2) and hydroxy acid(3) products of Strecker synthesis, carboxylic acids(4) produced via oxidation of aldehydes, alcohol(5) precursors to aldehydes/ketones, and amines(6) formed through reductive amination of aldehydes/ketones, decarboxylation of amino acids, and nucleophilic substitution of alcohols. A. The simplest aldehyde (formaldehyde) and structurally-related organics. B. The simplest ketone (acetone) and structurally-related organics.
Formaldehyde is ubiquitous in the interstellar medium (ISM) and comets (Irvine, 1999; Cleaves, 2008; Mumma and Charnley, 2011). Proposed mechanisms for the formation of interstellar formaldehyde include successive hydrogenation of carbon monoxide (CO) on ice/grain surfaces (Tielens and Whittet, 1997; Watanabe and Kouchi, 2002; Awad et al., 2005), gas-phase reactions (Shalabiea and Greenberg, 1994), and UV photolysis of H2O-CO ices (Allamandola et al., 1988; Schutte et al., 1996). Higher molecular weight carbonyl compounds in the interstellar medium may have been synthesized by further addition of carbon from CO (Charnley et al., 2004) and then become incorporated into comets and asteroids during early solar system formation (Botta and Bada, 2002).
Aldehydes and ketones have been previously investigated in Murchison meteorite samples via colorimetric analysis, gas chromatography (GC), and gas chromatography-mass spectrometry (GC-MS) analysis of water extracts and headspace gas (Jungclaus et al., 1976) and by applying the 1998 Environmental Protection Agency (EPA) O-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine (PFBHA) derivatization method (EPA Method #556 (1998); Pizzarello and Holmes (2009); Monroe and Pizzarello (2011); Pizzarello et al., 2012)). Derivatization of volatile aldehydes and ketones produces less polar, more thermally stable oxime derivatives that are more amenable to GC-MS analysis. Furthermore, derivatization of the carbonyl compounds allows for chromatographic separation of these species from alcohols and other unknown compounds which would otherwise coelute (Jungclaus et al., 1976). The PFBHA-derivatization method (Scheme 2) is commonly used for the analysis of carbonyl compounds in environmental and biological samples and it has been modified in the literature for various suites of terrestrial compounds (e.g. Spaulding and Charles, 2002; Rodigast et al., 2015; see Table S1); however, this procedure has not previously been optimized specifically for the analysis of carbonyl compounds extracted from carbonaceous chondrites. Method optimization is particularly important for maximizing yields for isotopic analyses when working with limited quantities of extraterrestrial samples of low organic content. The previous reports of aldehydes and ketones in Murchison identified similar suites of compounds, dominated by low molecular weight acetaldehyde, formaldehyde and acetone; however, the explanation of their origin was limited by the absence of stable isotopic measurements. Compound-specific δ13C analysis of meteoritic aldehydes and ketones is a key next step for understanding the origins of these carbonyl compounds in the solar system.
Scheme 2.
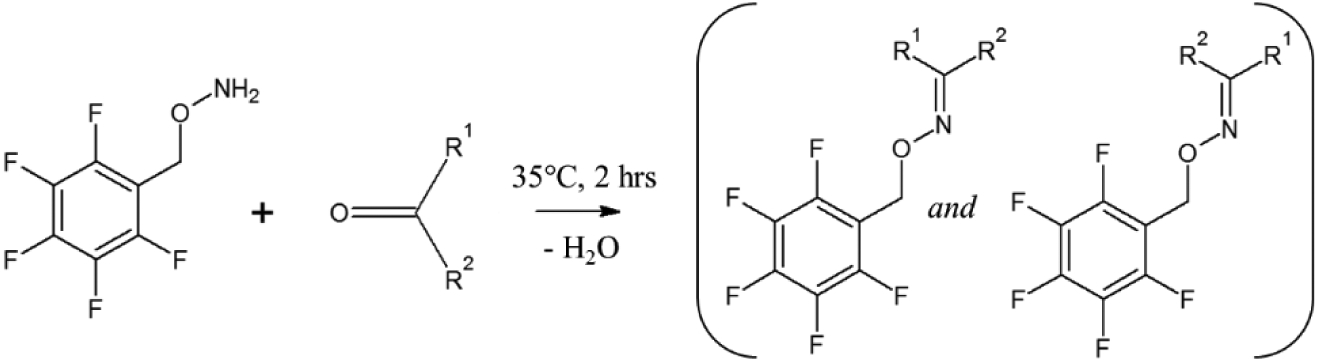
Derivatization of carbonyl compounds. PFBHA reacts with an aldehydes or ketones to produce the (E)-and (Z)-PFBHA derivatives.
The objectives of the present study were: (a) to optimize the EPA PFBHA derivatization method specifically for the analysis of aldehydes and ketones in astromaterials in order to maximize both yields and reproducibility for the detection of low molecular weight aldehydes and ketones in samples with organic contents in nmol/g of meteorite range; (b) to identify, quantify, and, for the first time, measure the compound-specific δ13C values of aldehydes and ketones in the Murchison meteorite; and (c) to compare the measured δ13C values of indigenous aldehydes and ketones with previously measured δ13C values of other meteoritic organics including amino acids, hydroxy acids, HCN, amines, carboxylic acids, and IOM in Murchison, in order to investigate potential synthetic relationships between these compound classes.
2. MATERIALS AND METHODS
2.1. Chemicals and reagents
Standards and reagents were purchased from Sigma Aldrich and Fisher Scientific. Five aldehydes and five ketones previously identified in carbonaceous chondrites (Jungclaus et al., 1976; Yabuta et al., 2007; Pizzarello and Holmes, 2009; Monroe and Pizzarello, 2011; Pizzarello et al., 2012) were used for the method development experiments and as the standards: formaldehyde, acetaldehyde, propionaldehyde, butyraldehyde, benzaldehyde, acetone, 2-butanone, 2-pentanone, 2-hexanone, and acetophenone. Ultrapure water (Millipore Direct Q3 UV, 18.2 MΩ, 3 ppb total organic carbon; hereafter referred to as “water”), HPLC grade dichloromethane (DCM), double-distilled 6 M HCl, and O-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine hydrochloride of ≥ 99.0% purity were used. All glassware and tools were wrapped in aluminum foil and heated at 450°C for a minimum of 6 hours before use. All vials were capped with PTFE-lined lids.
2.2. Murchison sample extraction
An interior chip of the Murchison carbonaceous chondrite (CM2, USNM 54512; extracted mass: 0.5075 g) was provided by the Smithsonian National Museum of Natural History, Washington, D.C. The sample was powdered using a porcelain mortar and pestle and extracted with 1 mL of water in a flame-sealed glass ampule at 100°C for 24 hours. After extraction, the sample was centrifuged, and the supernatant was transferred to a glass vial. The residual meteorite solid was rinsed three more times using 0.5 mL of water for each rinse. The aqueous supernatant and rinses were combined into one fraction and filtered through quartz wool to remove any remaining solid material from the solution. The quartz wool filter was rinsed two more times using 0.5 mL of water for each rinse. The entire extraction procedure was carried out in parallel with a procedural solvent blank and a serpentine mineral analogue blank (0.5013 g; powdered and combusted at 500°C for 16 hours before extraction).
2.3. Derivatization of aldehydes and ketones
Carbonyl compounds were derivatized using an optimized EPA Method #556 for PFBHA derivatization (see Supplementary Information), as follows: 1 mL of 0.2 mg/mL PFBHA solution was added to the 3.5 mL of combined water extract and rinses. The solution was agitated for 5 minutes and then left to react for 24 hours at room temperature to allow the derivatization reaction to go to completion. The reaction was quenched by adding 100 μL of 0.4 M HCl solution. Two mL of dichloromethane were then added to the solution to extract the derivatized carbonyl compounds. The resulting mixture was agitated for 5 minutes and then left undisturbed for 30 minutes to allow the dichloromethane and water layers to settle. The dichloromethane layer was separated from the aqueous layer and brought through an acid-wash step (3 mL of 0.4 M HCl). The extraction of the aqueous layer was repeated with another 2 mL of dichloromethane and the isolated dichloromethane layer was washed with 3 mL of 0.4 M HCl. The 2 × 2 mL dichloromethane extracts containing the PFBHA derivatives were combined, the volume was reduced to 200 μL under a stream of nitrogen, and the resulting concentrated solution was analyzed by gas chromatography coupled to mass spectrometry and isotope ratio mass spectrometry (GC-MS/IRMS; section 2.4).
2.4. Identification, quantification and compound-specific δ13C analysis of aldehydes and ketones
The analysis of derivatized carbonyl compounds was performed using GC-MS/IRMS, which provides compound-specific identification and stable carbon isotopic ratios in parallel (Elsila et al., 2012; Aponte et al. 2014). The GC separation was accomplished using a Thermo Trace GC equipped with a 5 m base-deactivated fused silica guard column (Restek, 0.25 mm ID) and three 30 m length × 0.25 mm I.D. × 0.5 μm film thickness Rxi-5ms capillary columns (Restek) connected using Press-Tight® connectors (Restek). The oven program was set as follows: initial temperature was 40 °C, ramped at 10 °C/min to 160 °C, ramped at 5 °C/min to 190 °C, ramped at 10 °C/min to 290 °C and held for 7 min. The carrier gas used was UHP helium (5.0 grade) at 2.6 mL/min flow rate. Triplicate injections of PFBHA derivatives were made in splitless mode in aliquots of 1 μL; splitless mode was used to maximize sensitivity and minimize potential isotopic fractionation during injection. The mass spectrum was used to identify and quantify the meteoritic carbonyl compounds by comparison to reference standards and application of calibration curves. Five-point external calibration curves were prepared for each individual isomer for ten carbonyl standards (Section 2.1). These calibration curves were obtained immediately prior to the Murchison sample analysis to limit temporal variations of response factors, in lieu of introducing internal standards which could result in chromatographic coelutions. Selected ion mass-to-charge ratio (m/z = 181.0) was used to identify and quantify compounds (see Table 1 for compound identifications). Concentrations were calculated using quadratic equations (average R2 = 0.995) derived from the calibration curves of each individual isomer. Approximately 10% of the sample eluting from the GC column was directed into a Thermo DSQII electron-impact quadrupole mass spectrometer (ion source set at 200 °C and 70 eV). The remaining 90% of each eluting compound was directed through a Thermo GC-C III interface for oxidation of the compounds to carbon dioxide; the carbon stable isotopic measurement was then made on a Thermo MAT 253 IRMS. The δ13C values of the eluting compounds were obtained after injection of three pulses of precalibrated CO2 (δ13C = −24.23‰ VPDB) into the IRMS and computation using Thermo Isodat 2.5 software. In order to correct for the isotopic contribution from carbon added by the derivatization reagent, δ13C values were also determined for both derivatized carbonyl standards and underivatized carbonyl standards. The underivatized standards were analyzed on a Costech ECS 4010 combustion elemental analyzer (EA) connected to the IRMS. The final δ13C values of the meteoritic aldehydes and ketones (carbonyls) were calculated using equation 1 (derived from equation 1 of Docherty et al., 2001).
| (1) |
where ncarbonyl = number of carbon atoms in underivatized carbonyl and nd = number of carbons added by derivatizing reagent. The precision (standard deviation) of the δ13C values was obtained using equation 2(Docherty et al., 2001).
Table 1.
Concentrations (nmol/g) and δ13C values of aldehydes and ketones identified in the Murchison meteorite.
| Abundance (nmol/g) | ||||
|---|---|---|---|---|
| Peak label | Compound | This studya (± S.D.b) | Pizzarello and Holmes (2009) | δ13C (‰) |
| Aldehydes | ||||
| 1 | Formaldehydec | 19.2 ± 2.8 | 10.0 | +66.4 ± 3.2 |
| 2 | (E)-Acetaldehydec | 273.4 ± 48.7 | 24.0 | +25.7 ± 0.9 |
| 3 | (Z)-Acetaldehyde | +27.0 ± 0.9 | ||
| 5 | (E)-Propionaldehydec | 149.8 ± 22.8 | 23.5 | n.d. |
| 6 | (Z)-Propionaldehyde | +41.8 ± 1.3 | ||
| 9 | (E)-Butyraldehydec | 42.2 ± 6.6 | 32.2 | +20.3 ± 1.3 |
| 10 | (Z)-Butyraldehyde | n.d | ||
| 16 | (E)-Benzaldehyde | n.d. | 7.6 | n.d. |
| 17 | (Z)-Benzaldehyde | n.d. | ||
| Ketones | ||||
| 4 | Acetone | 87.5 ± 15.4 | 47.3 | +11.8 ± 1.5 |
| 7 | (E)-2-Butanone | 36.0 ± 5.7 | 6.9 | n.d. |
| 8 | (Z)-2-Butanonec | −10.0 ± 1.2 | ||
| 11 | (E)-2-Pentanone | 12.3 ± 1.8 | 1.5 | n.d. |
| 12 | (Z)-2-Pentanone | n.d. | ||
| 13 | (E)-2-Hexanone | 3.5 ± 0.9 | 25.0 | n.d. |
| 14 | (Z)-2-Hexanone | n.d. | ||
| 15 | (E)-Acetophenone | 1.9 ± 0.6 | n.d. | n.d. |
| 18 | (Z)-Acetophenone | n.d. | ||
| Total abundance | 626.2 ± 105.3 | 200 | ||
Abundances represent the sun of the two (E) and (Z) isomers, where applicable.
S.D. – Standard deviation of three sequential injections.
Partially co-eluting with an unidentified compound
n.d. – not determined
| (2) |
3. RESULTS
3.1. Identification, quantification and compound-specific δ13C analysis of aldehydes and ketones in the Murchison meteorite
A suite of aldehydes and ketones were identified and quantified in the Murchison meteorite and the δ13C values were measured for those compounds present in sufficient abundance. Table 1 lists the concentrations and δ13C values for the measurable aldehydes and ketones in the Murchison sample. Figure 2 illustrates the selected ion (m/z = 181.0) GC-MS chromatogram of representative injections from the derivatized Murchison meteorite extract, the procedural blanks, and a mixture of derivatized aldehydes and ketone standards. Two chromatographic peaks are observed for most compounds, as (E)- and (Z)-isomers (two molecules with the same molecular formula but different stereometric configurations) are produced during derivatization for those carbonyl compounds with asymmetrical chemical structures. Abundances are reported as sums of the (E)- and (Z)- isomers, whereas the δ13C values for the two isomers are reported individually. The elution order of the (E)- and (Z)-isomer peaks reported here is based on peak identities from previous studies that have used similar GC columns (EPA Method #556, 1998; Cancho et al., 2001). The Murchison sample was dominated by the low molecular weight aldehyde acetaldehyde (273.4 nmol/g), followed by propionaldehyde (149.8 nmol/g), acetone (87.5 nmol/g), and formaldehyde and higher molecular weight carbonyl compounds at lower abundances. Formaldehyde exhibited the most 13C-enriched carbon isotope composition (+66.4‰). The aldehyde δ13C values (+20.3 to +66.4‰) were more 13C-enriched in comparison to the ketone δ13C values (–10.0‰ and +11.8‰). The δ13C values for the acetaldehyde (E)- and (Z)-isomers fell within one standard deviation of one another. A trace amount of formaldehyde was observed in the procedural blank (0.5 nmol) and in the combusted serpentine blank (0.9 nmol), and trace amounts of acetaldehyde (2.1 nmol) and acetone (0.7 nmol) were observed in the combusted serpentine blank. The Murchison formaldehyde, acetaldehyde and acetone abundances were corrected using these serpentine blank measurements (Table 1).
Figure 2.
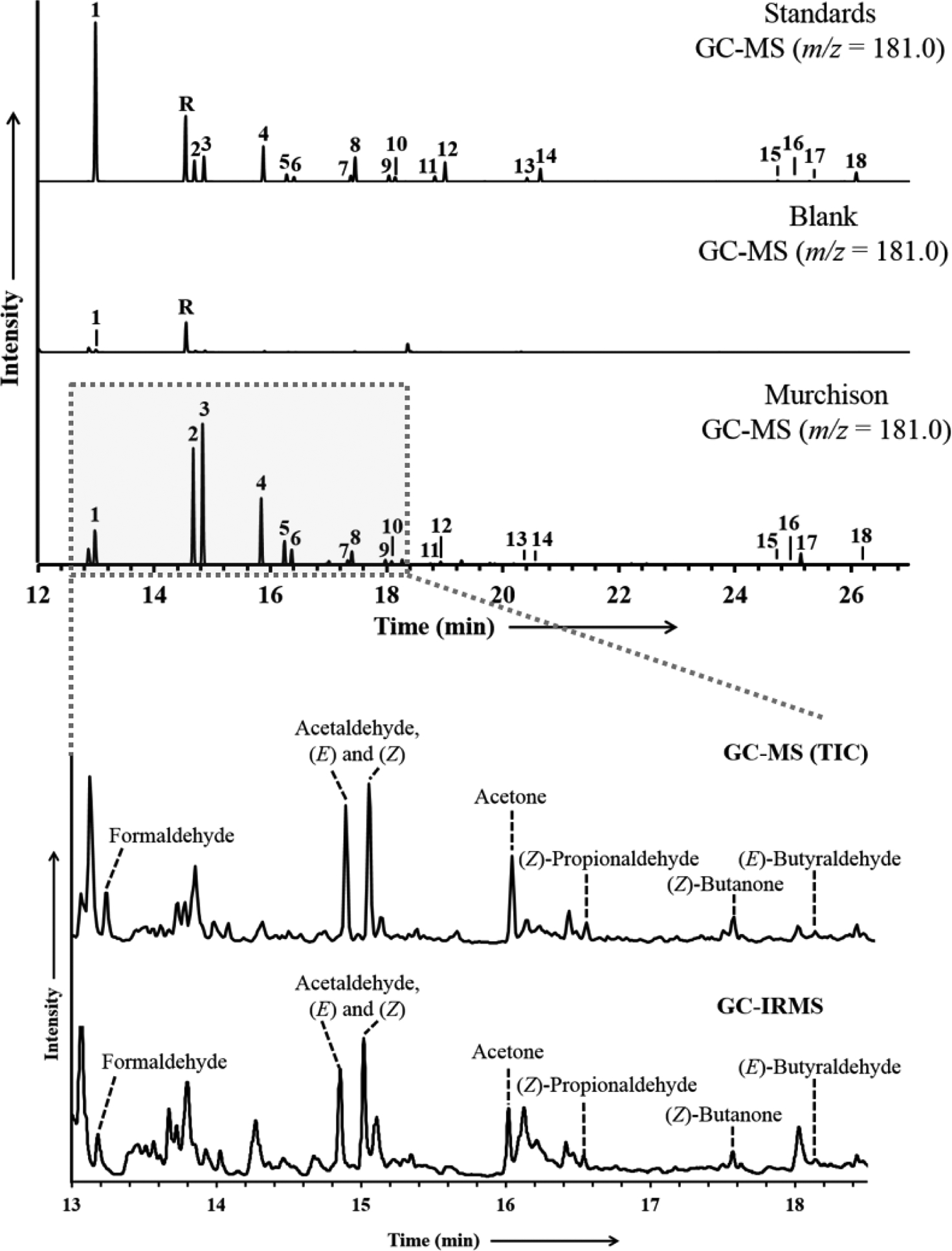
GC-MS chromatograms of derivatized aldehyde and ketone standards, the serpentine mineral analogue blank, and the Murchison meteorite extract, and a GC-IRMS chromatogram of carbonyl compounds in the Murchison sample measured for δ13C. GC-MS peaks in the upper three chromatograms represent PFBHA derivatives, containing the characteristic ion of m/z= 181.0. The lower GC-MS chromatogram is a total ion chromatogram (TIC). The GC-IRMS chromatogram shows the m/z= 44 (12CO2 peak) measured during carbon compound-specific isotope analysis. Peak R represents unreacted PFBHA derivatization reagent. The identities of the labeled peaks are presented in Table 1.
4. DISCUSSION
4.1. Method optimization for PFBHA derivatization and analysis of aldehydes and ketones in astromaterials
4.1.1. Modifications to the EPA Method #556
We have optimized the EPA Method #556 (Scheme 2), used previously for the analysis of the Murchison meteorite (Pizzarello and Holmes, 2009), with an aim to maximize yields and reproducibility for a set of ten aldehyde and ketone standards previously identified in carbonaceous chondrites (Section 2.1). The EPA Method #556 has been similarly optimized for atmospherically relevant dialdehydes, diketones, and unsaturated and aromatic aldehydes (Rodigast et al., 2015). Our method optimization work, though focused on relatively lower molecular weight target analytes, produced results that are consistent with those obtained by Rodigast et al. (2015). Derivatization times reported in the literature vary widely, from 10 minutes to 96 hours at room temperature (see Table S1, adapted from Rodigast et al., 2015). While most aldehydes are expected to derivatize completely within a few hours, ketones are known to require much longer derivatization times and are generally given 24 hours for the reaction to reach completion (Kobayashi et al., 1980; Glaze et al., 1989; Yamada and Somiya, 1989). In the present study, we tested four derivatization times (2, 8, 16 and 24 hours) to confirm the influence of reaction time on our suite of standards (Figure 3a). While the total yields of the lower molecular weight aldehydes appear to plateau within 2 hours, the ketones required at least 16 hours in order to reach their highest relative yields, as indicated by the similar (within error) GC-MS peak areas for the 16-hour and 24-hour samples. As such, we have chosen a 24-hour derivatization time to maximize our yields and reproducibility. The EPA Method #556 recommends using hexane to extract the carbonyl derivatives from aqueous solution; however, dichloromethane is also commonly used in the literature for this method (e.g. Spaulding and Charles, 2002; de Marcellus et al., 2015; Rodigast et al., 2015; see Table S1). Thus, we compared the extraction efficiencies of dichloromethane and hexane for our suite of derivatized standards (Figure 3b). The use of dichloromethane resulted in relatively higher yields and greater reproducibility for all our compounds of interest and was, therefore, selected as the extraction solvent for this study.
Figure 3. A.
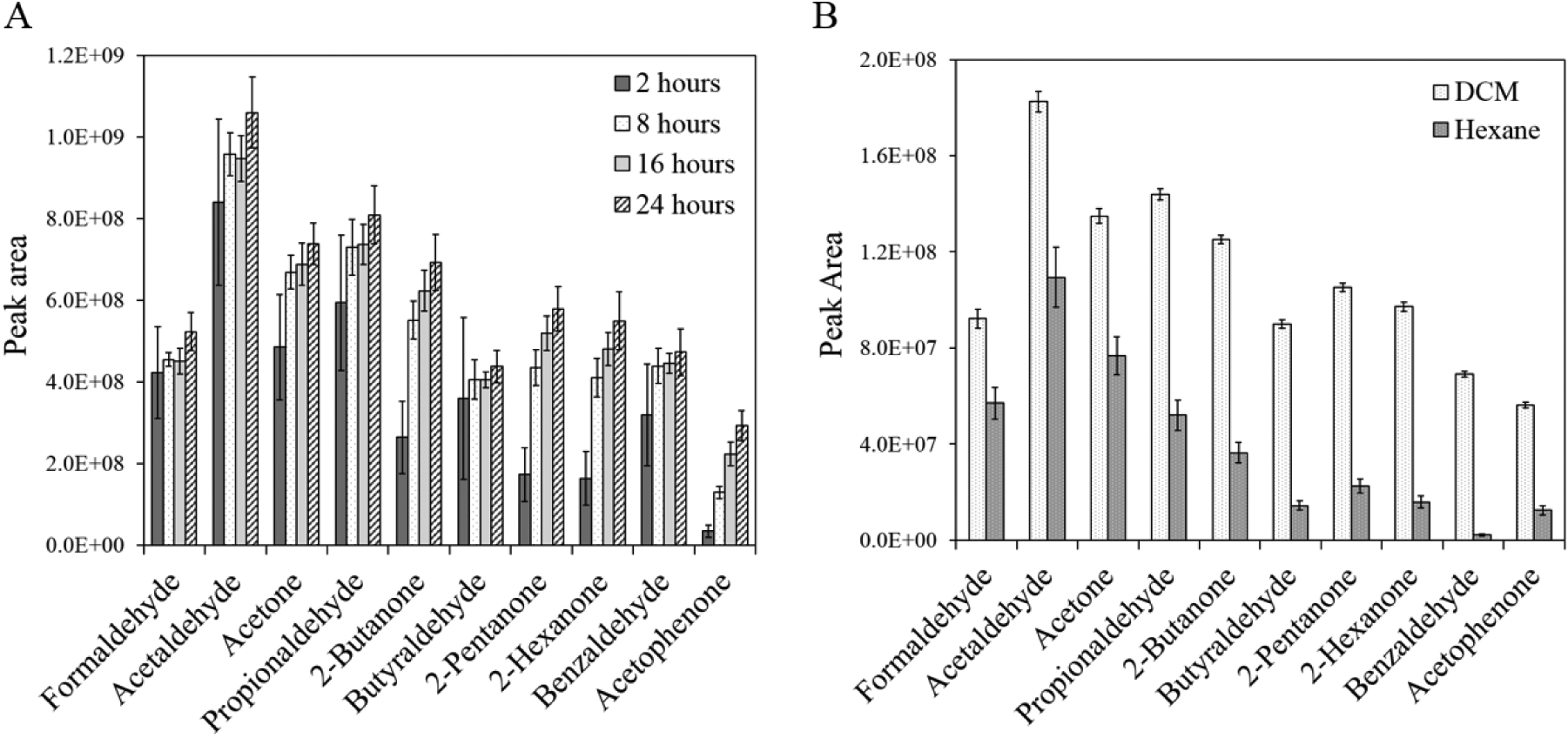
Total yields (GC-MS peak areas) of aldehyde and ketone PFBHA derivatives from 2-, 8-, 16- and 24-hour derivatization reactions (standard abundances: 1 mL of 50 μg/mL solution). Each individual bar represents 3 replicate samples and error bars represent one standard deviation from the mean. All PFBHA derivatives were isolated using dichloromethane (DCM) as the extraction solvent. B. Extraction efficiency of DCM vs. hexane for aldehyde and ketone PFBHA derivatives. Each bar represents the average of 12 replicate samples and the error bars represent one standard deviation from the mean. All derivatization reactions were carried out for 24 hours.
Other changes to the method included: (1) using a lower concentration of PFBHA solution (0.2 mg/mL), as the expected yields of aldehydes and ketones in astromaterials are much lower than terrestrial environmental samples and excess PFBHA reagent in solution can result in less effective extraction of the derivatives into the organic phase (Rodigast et al., 2015); (2) replacing H2SO4 with HCl, as HCl can be double-distilled for higher purity; (3) carrying out a 30 minute extraction time, to allow for a complete extraction of the target analytes, as recommended by Rodigast et al. (2015); and (4) storing samples in solution, at low temperature (4°C), to avoid decomposition of the PFBHA derivatives (see Section 4.1.2).
4.1.2. Stability of the PFBHA derivatives
The stability of the aldehyde and ketone derivatives during sample work-up and long-term storage is particularly important when working with astromaterials, as sample abundances are generally small and limited, and degradation of the compounds may induce stable isotopic and enantiomeric fractionations. Our experiments revealed a notable drawback of the PFBHA derivatization method; the PFBHA derivatives appear to degrade when the sample solutions are evaporated to dryness, and the decomposition reaction appears to be accelerated when the samples are stored dry at room temperature, as opposed to in low temperature conditions (4°C) (see Figure S1). A decomposition product, identified by the NIST Mass Spectral Library as PFBHA, was observed in the GC-MS chromatograms of dried samples (see Figure S2), suggesting that the aldehyde and ketone derivatives decomposed back to their original constituents (i.e., PFBHA and volatile aldehydes and ketones) during or after the solvent evaporation step. This decomposition reaction, involving hydrolysis of the C-N bond (Kalia and Raines, 2008), could be taking place as a result of a small amount of residual acid in the vials, which becomes difficult to remove as the sample is concentrated. As a preventative measure, we stored the PFBHA derivatives in solution, at low temperatures (<4°C). Several studies report using amber vials and bottles for derivatizing and storing aldehyde and ketone samples, implying that the PFBHA derivatives may be light-sensitive (Glaze et al., 1989; Spaulding and Charles, 2002; Hudson et al., 2007; Serrano et al., 2013). We have ruled out photolysis as the cause of the decomposition reaction observed here by limiting sample exposure to light. The decomposition of the derivatives in dried samples persisted despite carrying out all derivatization reactions, clean-up steps, and storage in amber vials.
4.2. Distribution and abundances of aldehydes and ketones in Murchison
The total yield of aldehydes and ketones reported here is substantially higher (3-fold larger) than total abundances reported for Murchison by previous studies (Table 1; Jungclaus et al., 1976, Pizzarello and Holmes, 2009), likely resulting from increased extraction efficiencies and derivatization yields. This variation between studies may also be partially attributable to sample heterogeneity, as has been reported for other compound classes (Cronin and Pizzarello, 1983; Krishnamurthy et al., 1992; Pizzarello et al., 2003; Aponte et al., 2014). Considering the ubiquitous presence of formaldehyde in the ISM, the abundance of formaldehyde in the Murchison meteorite (19.2 nmol/g) is lower than expected. A relatively low abundance of formaldehyde in Murchison was similarly observed by Pizzarello and Holmes (2009) and Jungclaus et al. (1976) and may be partially attributable to its low boiling point (–19°C), as the Murchison meteorite samples have been stored long-term in room temperature conditions, potentially allowing volatile compounds to be lost. Its low abundance relative to other carbonyl species in Murchison may also be a result of its high reactivity and involvement in abiotic synthetic reactions inside the parent body, including for example the formose reaction; it is possible that formaldehyde originally contained in Murchison may have converted into more complex molecules, including insoluble macromolecules, during secondary processing on the asteroid parent body (Cooper et al., 2001; Cody et al., 2011; Meinert et al., 2016).
4.3. Potential origins for the aldehydes and ketones in Murchison
4.3.1. Isotopic interpretation of Murchison aldehyde and ketone δ13C compositions
The positive δ13C values measured here for individual aldehydes and ketones in Murchison (+11.8‰ to +66.4‰) are attributed to an extraterrestrial origin, as terrestrial aldehydes and ketones generally exhibit negative δ13C values (–28.3 to −17.0‰; Goldstein and Shaw, 2003). The negative δ13C value (–10.0‰) measured for 2-butanone in Murchison is not unusual for meteoritic organic compounds (Sephton and Gilmour, 2001) and does not necessarily indicate terrestrial input; it may reflect a distinct abiotic origin for this type of compound in the parent body asteroid.
In order to make direct comparisons between δ13C values of different meteoritic compound classes, some assumptions need to be made regarding the original isotopic compositions of the compounds, the alteration history of the asteroid parent body and the associated chemical reactions (Aponte et al., 2017). The carbon isotope compositions of early solar system aldehydes and ketones prior to their incorporation into the asteroid parent body are assumed to have been highly 13C-enriched (δ13C > +117‰), based on recent 12C/13C measurements for interstellar formaldehyde (δ13C = 117–4933‰, 12C/13C = 15–80; Wirstrӧm et al., 2012) and the average 12C/13C measurement for nearby molecular clouds (δ13C = 117–392‰, 12C/13C = 64–80; Henkel et al., 1980, 1982). If the aldehydes and ketones detected in the Murchison meteorite are relict, unreacted Strecker synthesis precursors, we would expect them to be relatively enriched in 13C compared to their corresponding α-amino acid products. This prediction assumes that the Strecker synthesis reaction involves significant kinetic isotope fractionations (i.e. that isotopically lighter (12C-enriched) molecules react faster than isotopically heavier (13C-enriched) molecules at a rate that significantly affects the relative δ13C values of the products and reactants). This prediction also assumes that the amino acid products have not been isotopically fractionated via subsequent chemical reactions during thermal/aqueous alteration (i.e. that the pool of amino acid products has not been shifted towards a relatively 13C-enriched composition due to the preferential reaction of isotopically lighter amino acids). The isotopic composition of the unreacted carbonyl pool, which would gradually shift towards a more 13C-enriched composition as isotopically light carbonyl molecules are preferentially consumed depends on what proportion of the aldehydes and ketones have been converted into amino acids or vice versa, which remains unknown. Lastly, it is assumed that any variation observed between studies primarily reflects differences in methodologies and that the Murchison meteorite does not exhibit large heterogeneity between subsamples.
The δ13C measurements for the aldehydes and ketones in Murchison are less 13C-enriched than would be expected for a primordial pool of unreacted Strecker synthesis precursors, assuming that early solar system aldehydes and ketones incorporated into the Murchison parent body asteroid exhibited highly 13C-enriched isotopic signatures and that this signature either remained unaltered until our analyses or was amplified as Strecker synthesis shifts the reactant pool towards a more 13C-enriched composition. The discrepancy between the 13C-isotopic signatures for the carbonyl compounds we found in Murchison and the 13C-enriched values expected from interstellar observations may indicate that all of the early solar system aldehydes and ketones in the asteroid parent body were consumed early on, either via Strecker amino acid synthesis or through alternate chemical reactions, and were later resynthesized from reactions occurring through aqueous and thermal processing inside the asteroid parent body. This is a reasonable possibility as aldehydes and ketones are highly reactive species and are likely to polymerize and react with other chemical species fairly readily in asteroidal conditions (Cleaves, 2008; Cody et al. 2011; Kebukawa and Cody, 2015; Kebukawa et al., 2017). Our observations suggest that the aldehydes and ketones identified in this study were synthesized at a later stage, potentially during aqueous alteration reactions after accretion of the parent body asteroid. A secondary generation of aldehydes and ketones on asteroid parent bodies may imply that these compounds are more readily available in the solar system than previously thought.
4.3.2. Chemical oxidation of insoluble organic matter as a potential source of carbonyl compounds in Murchison
Low-temperature aqueous alteration of IOM has been proposed as a potential source of water-soluble organic compounds in carbonaceous chondrites (Cody and Alexander, 2005). The oxidation of IOM in aqueous solution has been shown to convert aliphatic carbon to CO moieties, in addition to organic acids and CO2 (Cody and Alexander, 2005) and this mechanism may partially explain the contrasting isotopic signatures for the carbonyl compounds in Murchison and those seen in interstellar environments. The more labile, aliphatic moieties of the IOM are expected to be relatively 13C-enriched compared to the aromatic portions (Kerridge et al., 1987), as the aromatic components of the IOM are considered to reflect a longer history of chemical processing in the cold ISM and/or during early solar system formation, resulting in progressive 13C-depletion over time. Consistent with this theory, the carbonyl δ13C compositions reported here (–10.0 to +66.4‰; Figure 4) are generally more 13C-enriched in comparison to measurements of IOM- derived aromatics in Murchison (–25‰ to −1‰; Figure 4; Sephton et al., 1998; Sephton and Gilmour, 2001). The Murchison ketone δ13C values (–10.0‰ and +11.8‰) are the most 13C- depleted of the carbonyl suite, suggesting that they are derived from a more 13C-depleted source (e.g. IOM) in comparison to the aldehydes. IOM-derived CO moieties are discussed as primarily representing ketone compounds, rather than aldehydes, based on their solid-state 1H and 13C Nuclear Magnetic Resonance (NMR) spectra (Cody and Alexander, 2005). Likewise, pyrolysis of IOM from the Murchison meteorite has been shown to release several aromatic ketones (Remusat et al., 2005).
Figure 4.
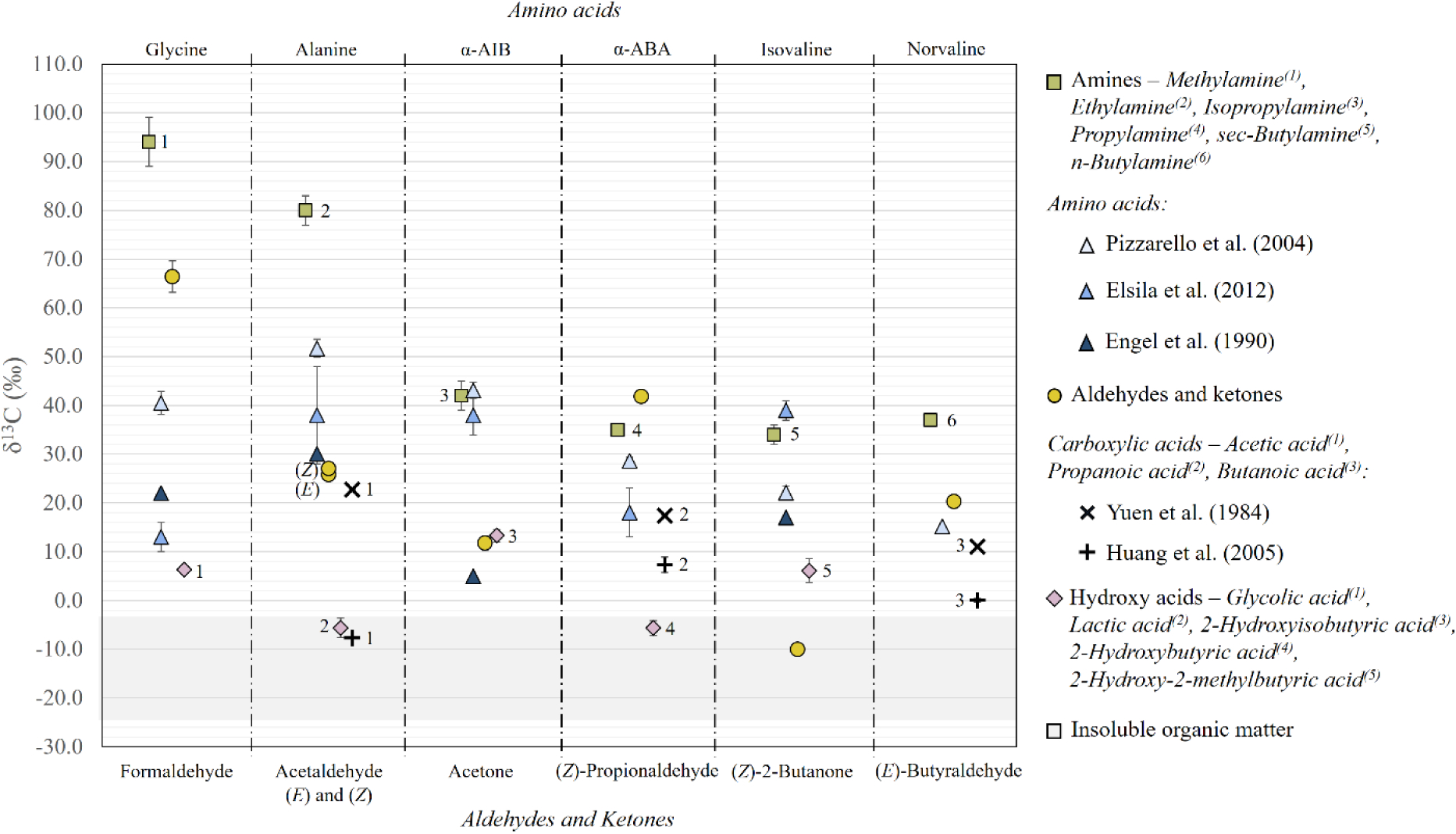
Carbon stable isotope compositions of aldehydes and ketones (circles, this study), their structurally analogous amines (Aponte et al., 2014), amino acids (Engel et al., 1990; Pizzarello et al., 2004; Elsila et al., 2012), monocarboxylic acids (Yuen et al., 1984; Huang et al., 2005), hydroxy acids (Pizzarello et al., 2010) and aromatics derived from macromolecular insoluble organic material (Sephton et al., 1998; Sephton and Gilmour, 2001) in the Murchison meteorite. Individual (E) and (Z) isomers of derivatized aldehydes and ketones are labeled where applicable. Upper indices assigned to individual amines, carboxylic acids and hydroxy acids in the legend denote the corresponding data points for each compound in the figure.
4.3.3. Alteration-driven decomposition of soluble organics
Another possible origin for the carbonyl compounds detected in Murchison may be alteration-driven degradation of other soluble organic compound classes (e.g. amino acids, keto acids) in the parent body asteroid. Similarities or trends observed between the compound-specific carbon isotope compositions of the carbonyl compounds in Murchison and other indigenous organics may be indicative of precursor-product synthetic relationships. While these values and data comparisons are not definitive indicators for specific chemical reactions, due to unknowns regarding the original budget of primordial soluble organic compounds and the effects of aqueous alteration on isotopic compositions (Section 4.3), they do provide an informative basis for elucidating the reaction history of aldehydes and ketones in the asteroid parent body.
4.3.3.1. Strecker-cyanohydrin synthesis precursors and products
The δ13C values of Strecker α-amino acid and α-hydroxy acid products depend on a combination of: (1) the carbon isotope composition of the precursor carbonyl compound, (2) the carbon isotope composition of the reactant HCN, and (3) the kinetic isotope fractionation effects associated with each step of the reaction (Scheme 1; Elsila et al., 2012; Aponte et al., 2017). The α-carbon and the alkyl (R) groups of the amino acid and hydroxy acid products are derived from the precursor aldehyde or ketone, while the carboxyl carbon is derived from HCN (Scheme 1). The relative influence of the aldehyde or ketone δ13C value on the amino acid/hydroxy acid δ13C value depends on the number of carbon atoms contained in, and thus contributed from, the R groups. For example, among the Strecker amino acid products, meteoritic HCN (δ13C = +1.4 to +7.3‰; Pizzarello et al., 2004) will have a substantial influence on the glycine isotopic composition and will play an increasingly lesser role on amino acid δ13C values as the carbonyl R groups increase in chain length. Comparing the δ13C values of carbonyl compounds, amino acids, hydroxy acids and HCN can be used to evaluate a potential Strecker precursor-product relationship between these compounds.
In this study, we focus primarily on isotopic comparisons between aldehydes, ketones and their structurally analogous α-amino acids, due to the large data set of stable isotopic measurements previously attained for amino acids in Murchison (Engel et al., 1990; Pizzarello et al., 2004; Elsila et al., 2012). Strecker amino acid products are expected to be relatively depleted in 13C compared to their aldehyde and ketone precursors (Elsila et al., 2012; Aponte et al. 2014, 2017), unless the chemical reactions do not involve significant carbon isotope fractionations, in which case the δ13C values for the reactants and products would be approximately equal. Amino acids with δ13C values that are relatively enriched in 13C compared to their structurally analogous aldehydes and ketones can be interpreted as having been either isotopically fractionated to some extent via subsequent parent body processing-driven chemical reactions following Strecker synthesis, or as having been derived, at least partially, from a carbon source that does not involve Strecker starting materials.
The carbon isotope compositions of the Murchison aldehydes and ketones (–10.0‰ to +66.4‰) and their structurally-analogous Strecker amino acids (+5.0‰ to +51.7‰) fall within a similar wide range of values (Figure 4). The aldehyde δ13C values are generally more 13C-enriched than their corresponding Strecker products; the formaldehyde, propionaldehyde, and butyraldehyde values are relatively enriched in 13C compared to their structurally analogous amino acids, and all four aldehyde δ13C values are more 13C-enriched compared to the structurally analogous hydroxy acid δ13C values (Figure 4). This relative 13C-enrichment observed for the aldehydes relative to Strecker products suggests that a precursor-product relationship could relate these compound classes in Murchison, as kinetic isotope effects involved in the reaction would produce isotopically light (13C-depleted) amino acid and hydroxy acid products, relative to the carbonyl reactants. In contrast, the ketone δ13C values are 13C-depleted relative to both their structurally-analogous amino acids and hydroxy acids, implying that other factors likely influenced the isotopic compositions of Murchison ketones. We consider the possibility that the aldehydes, ketones and amino acids may be intimately connected, not solely by Strecker synthesis, but also by decomposition of the amino acids during aqueous alteration on the parent body asteroid. An episodic decomposition or dissolution of α-amino acids in between intermittent Strecker synthesis could explain the similar δ13C values amongst the aldehydes, ketones and amino acids, although this is currently only speculative, as the temperatures experienced during aqueous alteration on the Murchison asteroid are arguably insufficient for amino acid degradation. Aqueous solutions of α-amino acids can undergo decomposition at elevated temperatures, yielding aldehyde and ketone products (Schonberg and Moubacher, 1951); however, these reactions generally take place at temperatures > 220°C (Lien and Nawar, 1974) and temperature estimates for aqueous alteration on the Murchison parent body asteroid are much lower, ranging from 0–80°C (Clayton and Mayeda, 1999; Baker et al., 2002; Guo and Eiler, 2007). Whether or not long-duration (several million years; Krot et al., 2015) aqueous alteration, catalyzed by mineral interactions, would be sufficient to induce amino acid degradation at these low temperatures has not been evaluated experimentally; however, laboratory simulations of aqueous alteration in the presence of mineral surfaces at high temperatures (>156°C) have shown that amino acid degradation via decarboxylation and deamination is greatly accelerated under these conditions (McCollom, 2013).
In addition to possible decomposition of water-soluble amino acids, long-duration parent body aqueous alteration could also result in the release of carbonyl compounds from the insoluble organic matter (Cody and Alexander, 2005). The relative depletion in 13C observed for the ketones in Murchison may reflect this secondary source of carbonyl compounds (Section 4.3.2; Figure 4).
4.3.3.2. δ13C comparisons with keto acids, amines, and carboxylic acids
The similar δ13C compositions of aldehydes, ketones and amino acids in Murchison could also indicate that they are all partially derived from a common source of soluble organic carbon. For example, some of the aldehydes, ketones and amino acids could be linked by reactions involving keto acids; aldehydes and ketones could be derived from degradation of keto acids during aqueous alteration, while the amino acids may be synthesized from the α-keto acids via reductive amination (Huber and Wächtershäuser, 2003). For instance, thermal decomposition of pyruvic acid yields acetaldehyde (Taylor, 1987), while reductive amination of pyruvic acid yields its structurally analogous amino acid, alanine (Yanagawa et al., 1982). Keto acids, including pyruvic acid, acetoacetic acid, oxaloacetic acid, and citric acid, have been detected in carbonaceous chondrites (Cooper et al., 2005; Cooper et al., 2011); however, their stable isotopic compositions have not yet been measured, limiting our ability to investigate this theory. Furthermore, these types of chemical reactions have only been described for terrestrial samples and have not yet been evaluated for cosmochemical conditions.
Amines and carboxylic acids are also potential structural relatives of carbonyl compounds as they can be synthesized via reductive amination of aldehydes and ketones and oxidation of aldehydes, respectively (Figure 1). The amine δ13C values reported by Aponte et al. (2014) are generally much more 13C-enriched than the aldehydes and ketones reported here, suggesting that the amines in Murchison were not produced via reductive amination of the aldehydes and ketones identified. Murchison carboxylic acid δ13C values, on the other hand, are 13C-depleted relative to the aldehyde values (Figure 4), consistent with synthesis via either oxidation of the aldehydes (Corey et al., 1968) or oxidation of the macromolecular organic matter in Murchison (Oba and Naraoka, 2006).
5. CONCLUSIONS
The aldehyde and ketone content of the Murchison meteorite and their carbon isotope compositions provide a snapshot of the post-alteration organic chemistry of the parent body asteroid, as well as some important information about the history of the asteroid and the synthetic reactions involved. Our carbon isotope analyses of aldehydes and ketones in Murchison revealed a distinct composition, dominated by acetaldehyde, propionaldehyde, and acetone, with carbon isotopic compositions covering a range of 13C-depleted values (–10.0‰ to +66.4‰), similar to other previously analyzed soluble organics in Murchison. This is in contrast to the formaldehyde-dominated, highly 13C-enriched, carbonyl content of the interstellar medium. This large disparity in composition between the two environments suggests that the organic chemistry and alteration history of the parent body asteroid may play a major role in generating a secondary source of carbonyl compounds in the solar system. Given the long history of the Murchison parent body asteroid and the complexity of its organic content, this compilation of data demonstrates that the aldehydes, ketones, and other compound classes are interconnected by a dynamic series of chemical reactions.
Supplementary Material
ACKNOWLEDGEMENTS
D.N.S. and R.W.H. would like to thank Drs. Matthew Ross and Aaron Skelhorne, MacEwan University, for their assistance with the GC-MS analyses during method optimization. J.C.A. and J.E.E. acknowledge support from NASA’s Emerging Worlds program for this work, as well as from the NASA Astrobiology Institute and the Goddard Center for Astrobiology, and a grant from the Simons Foundation (SCOL award 302497). Funding for this study was also provided by Natural Sciences and Engineering Research Council (NSERC) Grant 261740 to C.D.K.H., and NSERC CGS D3 and Izaak Walton Killam Memorial scholarships to D.N.S. Two anonymous reviewers are thanked for their careful review of this manuscript.
REFERENCES
- Abdel-Magid AF, Carson KG, Harris BD, Maryanoff CA, and Shah RD (1996) Reductive amination of aldehydes and ketones with sodium triacetoxyborohydride. Studies on direct and indirect reductive amination procedures. Journal of Organic Chemistry 61, 3849–3862. [DOI] [PubMed] [Google Scholar]
- Allamandola LJ, Sandford SA, and Valero GJ (1988) Photochemical and thermal evolution of interstellar/precometary ice analogs. Icarus 76, 225–252. [Google Scholar]
- Aponte JC, Dworkin JP, and Elsila JE (2014) Assessing the origins of aliphatic amines in the Murchison meteorite from their compound-specific carbon isotopic ratios and enantiomeric composition. Geochimica et Cosmochimica Acta 141, 331–345. [Google Scholar]
- Aponte JC, Elsila JE, Glavin DP, Milam SN, Charnley SB, and Dworkin JP (2017) Pathways to meteoritic glycine and methylamine. ACS Earth and Space Chemistry, DOI: 10.1021/acsearthspacechem.6b00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad Z, Chigai T, Kimura Y, Shalabiea OM, and Yamamoto T (2005) New rate constants of hydrogenation of CO on H2O-CO ice surfaces. The Astrophysical Journal 626, 262–271. [Google Scholar]
- Baker L, Franchi IA, Wright IP, and Pillinger CT (2002) The oxygen isotopic composition of water from Tagish Lake: Its relationship to low-temperature phases and to other carbonaceous chondrites. Meteoritics & Planetary Science 37, 977–985. [Google Scholar]
- Bisschop SE, Fuchs GW, van Dishoeck EF, and Linnartz H (2007) H-atom bombardment of CO2, HCOOH, and CH3CHO containing ices. Astronomy & Astrophysics 474, 1061–1071. [Google Scholar]
- Bossa J-B Duvernay F, Theulé P, Borget F, d’Hendecourt L, Chiavassa T (2009) Methylammonium methylcarbamate thermal formation in interstellar ice analogs: a glycine salt precursor in protostellar environments. Astronomy & Astrophysics 506, 601–608. [Google Scholar]
- Botta O and Bada JL (2002) Extraterrestrial organic compounds in meteorites. Surveys in Geophysics 23, 411‒467. [Google Scholar]
- Burk MJ, Martinez JP, Feaster JE, and Cosford N (1994) Catalytic asymmetric reductive amination of ketones via highly enantioselective hydrogenation of the C=N double bond. Tetrahedron 50, 4399–4428. [Google Scholar]
- Cancho B, Ventura F and Galceran MT (2001) Determination of aldehydes in drinking water using pentafluorobenzylhydroxylamine derivatization and solid-phase microextraction. Journal of Chromatography 943, 1–13. [DOI] [PubMed] [Google Scholar]
- Charnley SB, Ehrenfreund P, Millar TJ, Boogert ACA, Markwick AJ, Butner HM, Ruiterkamp R, and Rodgers SD (2004) Observational tests for grain chemistry: Posterior Isotopic labelling. Monthly Notices of the Royal Astronomical Society 347, 157–162. [Google Scholar]
- Clayton RN, and Mayeda TK (1999) Oxygen isotope studies in carbonaceous chondrites. Geochimica et Cosmochimica Acta 63, 2089–2104. [Google Scholar]
- Cleaves HJ (2008) The prebiotic geochemistry of formaldehyde. Precambrian Research, 164, 111–118. [Google Scholar]
- Cody GD, and Alexander CMO’D (2005) NMR studies of chemical structural variation of insoluble organic matter from different carbonaceous chondrite groups. Geochimica et Cosmochimica Acta 69, 1085–1097. [Google Scholar]
- Cody GD, Heying E, Alexander CMO’D, Nittler LR, Kilcoyne ALD, Sandford SA, and Stroud RM (2011) Establishing a molecular relationship between chondritic and cometary organic solids. PNAS 108, 19171–19176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G, N., Kimmich N, Belisle W, Sarinana J, Brabham K, and Garrel L (2001) Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 414, 879–883. [DOI] [PubMed] [Google Scholar]
- Cooper G, Dugas A, Byrd A, Chang PM and Washington N (2005) Keto-acids in carbonaceous meteorites. 36th Lunar and Planetary Science Conference, #2381. [Google Scholar]
- Cooper G, Reed C, Nguyen D, Carter M, and Wang Y (2011) Detection and formation scenario of citric acid, pyruvic acid, and other possible metabolism precursors in carbonaceous meteorites. PNAS 108, 14015–14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey EJ, Gilman NW, and Ganem BE (1968) New methods for the oxidation of aldehydes to carboxylic acids and esters. Journal of the American Chemical Society 90, 5616–5617. [Google Scholar]
- Cronin JR and Pizzarello S (1983) Amino acids in meteorites. Advances in Space Research 3, 5–18. [DOI] [PubMed] [Google Scholar]
- Deamer D, Dworkin JP, Sandford SA, Bernstein MP, and Allamandola LJ (2002) The first cell membranes. Astrobiology 2, 371–381. [DOI] [PubMed] [Google Scholar]
- de Marcellus P, Meinert C, Myrgorodska I, Nahon L, Buhse T, Le Sergeant D’Hendecourt L, and Meierhenrich UJ (2015) Aldehydes and sugars from evolved precometary ice analogs: Importance of ices in astrochemical and prebiotic evolution. PNAS 112, 965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty G, Jones V, and Evershed RP (2001) Practical and theoretical considerations in the gas chromatography/combustion/isotope ratio mass spectrometry δ13C analysis of small polyfunctional compounds. Rapid Communications in Mass Spectrometry 15, 730–738. [DOI] [PubMed] [Google Scholar]
- Elsila JE, Charnley SB, Burton AS, Glavin DP, and Dworkin JP (2012) Compound-specific carbon, nitrogen, and hydrogen isotopic ratios for amino acids in CM and CR chondrites and their use in evaluating potential formation pathways. Meteoritics & Planetary Science 47, 1517–1536. [Google Scholar]
- Engel MH, Macko SA, and Silfer JA (1990) Carbon isotope composition of individual amino acids in the Murchison meteorite. Nature 348, 47–49. [DOI] [PubMed] [Google Scholar]
- EPA Method 556: Determination of carbonyl compounds in drinking water by pentafluorobenzyl hydroxylamine derivatization and capillary gas chromatography with electron capture detection (1998) National exposure research laboratory office of research and development US Environmental Protection Agency, Cincinnati, Ohio, 45268. [Google Scholar]
- Glaze WH, Koga M and Cancilla D (1989) Ozonation byproducts. 2. Improvement of an aqueous-phase derivatization method for the detection of formaldehyde and other carbonyl compounds formed by the ozonation of drinking water. Environmental Science & Technology 23, 838–847. [Google Scholar]
- Goldstein AH and Shaw SL (2003) Isotopes of volatile organic compounds: An emerging approach for studying budgets and chemistry. Chemical Reviews 103, 5025–5048. [DOI] [PubMed] [Google Scholar]
- Guo W, and Eiler JM (2007) Temperatures of aqueous alteration and evidence for methane generation on the parent bodies of the CM chondrites. Geochimica et Cosmochimica Acta 71, 5565–5575. [Google Scholar]
- Henkel C, Wilson TL, and Bieging J (1982) Further (12C/13C) ratios from formaldehyde: A variation with distance from the galactic center. Astronomy & Astrophysics 109, 344‒351. [Google Scholar]
- Holtom PD, Bennett CJ, Osamura Y, Mason NJ, and Kaiser RI (2005). A combined experimental and theoretical study on the formation of the amino acid glycine (NH2CH2COOH) and its isomer (CH3NHCOOH) in extraterrestrial ices. The Astrophysical Journal 626, 940. [Google Scholar]
- Huang Y, Wang Y, Alexandre MR, Lee T, Rose-Petruck C, Fuller M, and Pizzarello S (2005) Molecular and compound-specific isotopic characterization of monocarboxylic acids in carbonaceous chondrites. Geochimica et Cosmochimica Acta 69, 1073–1084. [Google Scholar]
- Huang Y, Aponte JC, Zhao J, Tarozo R, and Hallmann C (2015) Hydrogen and carbon isotopic ratios of polycyclic aromatic compounds in two CM2 carbonaceous chondrites and implications for prebiotic synthesis. Earth and Planetary Science Letters 426, 101–108. [Google Scholar]
- Huber C, and Wächtershäuser G (2003) Primordial reductive amination revisited. Tetrahedron Letters 44, 1695–1697. [Google Scholar]
- Hudson ED, Okuda K, and Ariya PA (2007) Determination of acetone in seawater using derivatization solid-phase microextraction. Analytical and Bioanalytical Chemistry 388, 1275–1282. [DOI] [PubMed] [Google Scholar]
- Hudson RL, Lewis AS, Moore MH, Dworkin JP, and Martin MP (2009) Enigmatic isovaline: investigating the stability, racemization and formation of a non-biological meteoritic amino acid. ASP Conference Series 420, 157–162. [Google Scholar]
- Irvine (1999) The composition of interstellar molecular clouds. Space Science Reviews 90, 203–218. [DOI] [PubMed] [Google Scholar]
- Jungclaus GA, Yuen GU, Moore CB, Lawless JG (1976) Evidence for the presence of low molecular weight alcohols and carbonyl compounds in the Murchison meteorite. Meteoritics 11, 231–237. [Google Scholar]
- Kalia J and Raines RT (2008) Hydrolytic stability of hydrazones and oximes. Angewandte Chemie 47, 7523–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebukawa Y, and Cody GD (2015) A kinetic study of the formation of organic solids from formaldehyde: Implications for the origin of extraterrestrial organic solids in primitive Solar System objects. Icarus 248, 412–423. [Google Scholar]
- Kebukawa Y, Chan QHS, Tachibana S, Kobayashi K, and Zolensky ME (2017) One-pot synthesis of amino acid precursors with insoluble organic matter in planetesimals with aqueous activity. Science Advances 3, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Tanaka M, and Kawai S (1980) Gas chromatographic determination of low-molecular-weight carbonyl compounds in aqueous solution as their O-(2,3,4,5,6-pentafluorobenzyl) oximes. Journal of Chromatography 187, 413–417. [Google Scholar]
- Krishnamurthy RV, Epstein S, Cronin JR, Pizzarello S, and Yuen GU (1992) Isotopic and molecular analyses of hydrocarbons and monocarboxylic acids of the Murchison meteorite. Geochimica et Cosmochimica Acta 56, 4045–4058. [DOI] [PubMed] [Google Scholar]
- Krot AN, Nagashima K, O’D Alexander CM, Ciesla FJ, Fujiya W, and Bonal L (2015) Sources of water and aqueous activity on the chondrite parent asteroids. In Asteroids IV, Michel P et al. , Eds. University of Arizona Press, Tuscon, 635–660. [Google Scholar]
- Kvenvolden K, Lawless J, Pering K, Peterson E, Flores J, Ponnamperuma C, Kaplan IR, Moore C (1970) Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite. Nature 228, 923–926. [DOI] [PubMed] [Google Scholar]
- Lee C-W, Kim J-K, Moon E-S, Minh YC, and Kang H (2009) Formation of glycine on ultraviolet-irradiated interstellar ice-analog films and implications for interstellar amino acids. The Astrophysical Journal 697, 428–435. [Google Scholar]
- Lien YC and Nawar WW (1974) Thermal degradation of some amino acids. Journal of Food Science 39, 911–913. [Google Scholar]
- McCollom TM (2013) The influence of minerals on decomposition of the n-alkyl-α-amino acid norvaline under hydrothermal conditions. Geochimica et Cosmochimica Acta 104, 330–357. [Google Scholar]
- Meinert C, Myrgorodska I, de Marcellus P, Buhse T, Nahon L, Hoffmann SV, Le Sergeant d’Hendecourt L, Meierhenrich UJ (2016) Ribose and related sugars from ultraviolet irradiation of interstellar ice analogs. Science 352, 208–212. [DOI] [PubMed] [Google Scholar]
- Miller S (1957) The mechanism of synthesis of amino acids by electric discharges. Biochimica et Biophysica Acta 23, 480–489. [DOI] [PubMed] [Google Scholar]
- Monroe AA and Pizzarello S (2011) The soluble organic compounds of the Bells meteorite: Not a unique or unusual composition. Geochimica et Cosmochimica Acta 75, 7585–7595. [Google Scholar]
- Mumma MJ, and Charnley SB (2011) The chemical composition of comets. Annual Review of Astronomy and Astrophysics 49, 471–524. [Google Scholar]
- Oba Y and Naraoka H (2006) Carbon isotopic composition of acetic acid generated by hydrous pyrolysis of macromolecular organic matter from the Murchison meteorite. Meteoritics & Planetary Science 41 (8), 1175–1181. [Google Scholar]
- Peltzer ET and Bada JL (1978) A-Hydroxycarboxylic acids in the Murchison meteorite. Nature 272, 443–444. [Google Scholar]
- Peltzer ET, Bada JL, Schlesinger G, and Miller SL (1984) The chemical conditions on the parent body of the Murchison meteorite: Some conclusions based on amino, hydroxy and dicarboxylic acids. Advances in Space Research 4, 69‒74. [DOI] [PubMed] [Google Scholar]
- Pizzarello S, Zolensky M, and Turk KA (2003) Non racemic isovaline in the Murchison meteorite: chiral distribution and mineral association. Geochimica et Cosmochimica Acta 67, 1589–1595. [Google Scholar]
- Pizzarello S, Huang YS, and Fuller M (2004) The carbon isotopic distribution of Murchison amino acids. Geochimica et Cosmochimica Acta 68, 4963–4969. [Google Scholar]
- Pizzarello S, Cooper GW, Flynn GJ (2006) The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles Meteorites and the Early Solar System II, Lauretta DS and McSween HY, Eds. (University of Arizona Press, 2006), p. 625–650. [Google Scholar]
- Pizzarello S and Holmes W (2009) Nitrogen-containing compounds in two CR2 meteorites: 15N composition, molecular distribution and precursor molecules. Geochimica et Cosmochimica Acta 73, 2150–2162. [Google Scholar]
- Pizzarello S, Wang Y, and Chaban GM (2010) A comparative study of the hydroxy acids from the Murchison, GRA 95229 and LAP 02342 meteorites. Geochimica et Cosmochimica Acta 74, 6206–6217. [Google Scholar]
- Pizzarello S, Schrader DL, Monroe AA, and Lauretta DS (2012) Large enantiomeric excesses in primitive meteorites and the diverse effects of water in cosmochemical evolution. Proceedings of the National Academy of Sciences 109, 11949–11954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remusat L, Derenne S, Robert F, and Knicker H (2005) New pyrolytic and spectroscopic data on Orgueil and Murchison insoluble organic matter: A different origin than soluble? Geochimica et Cosmochimica Acta 69, 3919–3932. [Google Scholar]
- Rodigast M, Mutzel A, Iinuma Y, Haferkorn S, and Herrmann H (2015) Characterisation and optimization of a sample preparation method for the detection and quantification of atmospherically relevant carbonyl compounds in aqueous medium. Atmospheric Measurement Technique 8, 2409–2416. [Google Scholar]
- Schonberg A and Moubacher R (1951) The Strecker degradation of α-amino acids. Chemical Reviews 50, 261–277. [Google Scholar]
- Schutte WA, Gerakines PA, Geballe TR, vanDishoeck EF, and Greenberg JM (1996) Discovery of solid formaldehyde toward the protostar GL 2136: Observations and laboratory simulation. Astronomy & Astrophysics 309, 633–647. [Google Scholar]
- Seaman VY, Charles MJ, and Cahill TM (2006) A sensitive method for the quantification of acrolein and other volatile carbonyls in ambient air. Analytical Chemistry 78, 2405–2412. [DOI] [PubMed] [Google Scholar]
- Sephton MA (2002) Organic compounds in carbonaceous meteorites. Natural Product Reports 19, 292–311. [DOI] [PubMed] [Google Scholar]
- Sephton MA (2014) Organic geochemistry of meteorites Treatise on Geochemistry 2nd Edition, Holland HD and Turekian KK, Eds. (Elsevier, 2014) vo1. 12, p. 1–31. [Google Scholar]
- Serrano M, Silva M, and Gallego M (2013) Development of an environment-friendly microextraction method for the determination of aliphatic and aromatic aldehydes in water. Analytica Chimica Acta 784, 77–84. [DOI] [PubMed] [Google Scholar]
- Shalabiea OM, and Greenberg JM (1994) Two key processes in dust/gas chemical modeling – photoprocessing of grain mantles and explosive desorption. Astronomy & Astrophysics 290, 266–278. [Google Scholar]
- Spaulding RS, and Charles MJ (2002) Comparison of methods for extraction, storage, and silylation of pentafluorobenzyl derivatives of carbonyl compounds and multi-functional carbonyl compounds. Analytical and Bioanalytical Chemistry 372, 808–816. [DOI] [PubMed] [Google Scholar]
- Taylor R (1987) The mechanism of thermal eliminations Part XXIII: [1] The thermal decomposition of pyruvic acid. International Journal of Chemical Kinetics 19, 709–713. [Google Scholar]
- Tielens AGGM and Whittet DCB (1997) Ice in star forming regions Molecules in Astrophysics: Probe and Processes, van Dishoeck EF, Ed. (Springer Science & Business Media; ), 45–60. [Google Scholar]
- Watanabe N and Kouchi A (2002) Efficient formation of formaldehyde and methanol by the addition of hydrogen atoms to CO in H2O-CO ice at 10 K. The Astrophysical Journal 571, 173–176. [Google Scholar]
- Wirstrӧm ES, Charnley SB, Geppert WD, and Persson CM (2012) Observations of carbon isotopic fractionation in interstellar formaldehyde. 43rd Lunar and Planetary Science Conference, #1611. [Google Scholar]
- Yamada H, and Somiya I (1989) The determination of carbonyl compounds in ozonated water by the PFBOA method. Ozone Science & Engineering 11, 127–141. [Google Scholar]
- Yanagawa H, Makino Y, Sato K, Nishizawa M, and Egami F (1982) Novel formation of α- amino acids and their derivatives from oxo acids and ammonia in an aqueous medium. The Journal of Biochemistry 91, 2087–2090. [DOI] [PubMed] [Google Scholar]
- Yuen G, Blair N, Des Marais DJ, and Chang S (1984) Carbon isotope composition of low molecular weight hydrocarbons and monocarboxylic acids from Murchison meteorite. Nature 307, 252–254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


