Pneumonia is the leading cause of death among children aged 1–59 months worldwide [1]. WHO recommends providing low flow oxygen by nasal cannula in pediatric patients with oxygen saturation < 90% [2]. With the provision of oxygen supplementation, some children still require further respiratory support such as mechanical ventilation due to the failure to maintain oxygen saturation and severe respiratory distress [3, 4]. However, mechanical ventilation may result in ventilator-induced lung injury [5]. As a simple and minimally invasive technique, continuous positive airway pressure (CPAP) has been used in children with severe pneumonia [6, 7], but its effect and safety are uncertain. Therefore, we performed a meta-analysis that compared CPAP with standard therapy or low-flow oxygen to figure out the effects and safety of CPAP.
This meta-analysis was performed according to the PRISMA guidelines [8]. The present meta-analysis was registered at PROSPERO (CRD42020136450).
The literature search was carried out in Pubmed, Cochrane Library, Embase, and Web of Science from inception until February 12, 2020 and the search was restricted to articles published in English. The search terms and keywords were provided in Table S1 in the supplement.
Inclusion criteria: (1) randomized controlled trials (RCTs) and crossover studies; (2) having a comparison group (standard therapy or low-flow oxygen); and (3) involving inpatients with severe pneumonia defined by WHO younger than 18 years. Case reports, observational studies, reviews, comments, conference abstracts were excluded.
The primary outcomes were the intubation rate and mortality. Secondary outcomes included the change in respiratory rate (RR), heart rate (HR), duration of hospital stay, and adverse events.
Data extraction was performed independently by two reviewers. Data extracted included: study design, participants and settings, interventions, outcomes, and adverse effects. Disagreements were solved by consensus.
We summarized the intervention effects by calculating risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes; we also calculated 95% confidence interval for each outcome. Fixed-effects model or random-effects model were used to perform meta-analysis depending on the heterogeneity across studies. I2 statistic were used for the assessment of heterogeneity, with I2 > 50% indicating significant heterogeneity [9]. To explore the potential causes of significant heterogeneity, we performed subgroup analyses for primary outcomes if data were sufficient. The factors including age, sample size, settings, and high-risk conditions were prespecified for subgroup analyses. Sensitivity analysis was conducted by removing crossover trails and those studies without the supervision of physicians respectively. P value < 0.05 was considered statistically significant.
Four studies (three RCTs [10–12], one randomized crossover trial [13]) with a total of 3040 patients satisfied the inclusion criteria and were included in the analysis. Descriptive data for each study are presented in Table 1.
Table 1.
Characteristics of the researches included in the study
| Studies | Design | Age | n | High-risk conditions | Treatment group | Control group | Main outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Device | PEEP | Name | Device | Flow | |||||||
|
Wilson et al. [10] Ghana, rural ED |
RCT | 3 mon–5 y | 69 |
Hypoxemia (0%) Severe malnutrtion (NR) HIV infection (NA) HIV exposure (NA) |
bCPAP | Nasal cannula | 5 cm H2O |
Standard care (1st h) bCPAP (2nd h) |
NR | NR | RR and HR at first 1 h | |
|
Chisti et al. [11] Bangladesh, urban ICU |
RCT | < 5 y | 146 |
Hypoxemia (100%) Severe malnutrition(49%) HIV infection (NA) HIV exposure (NA) |
bCPAP | Nasal prongs | 5–10 cmH2O | Low-flow oxygen | Nasal cannula | 0.5–2 L/min for < 2 y and 2–4 L/min for > 2 y | Treatment failure, intubation, mortality, LOS | |
|
McCollum et al. [12] Malawi, rural General ward |
RCT | 1–59 mon | 644 |
Hypoxemia (89%) Severe malnutrtion (69%) HIV infection (12%) HIV exposure (30%) |
bCPAP | Nasal masks or nasal prongs | 7–8 cmH2O | Low-flow oxygen | Nasal prongs | 0.5 L/min for 30–59 d and 2 L/min for 2–59 mon | Mortality, treatment failure, adverse events | |
|
Wilson et al. [13] Ghana, rural ED |
Crossover trial | 1 mon–5 y | 2181 |
Hypoxemia (0%) Severe malnutrtion (NR) HIV infection (NA) HIV exposure (NA) |
bCPAP | Nasal prongs | 5 cmH2O | Standard medical therapy | NR | NR | Mortality rate, change in RR at 24 h, adverse events | |
PEEP positive end expiratory pressure, RCT randomized controlled trial, bCPAP bubble continuous positive airway pressure, NR nor reported, RR respiratory rate, HR heart rate, NA not applicable, ED emergency department, ICU intensive care unit, LOS length of hospital stay
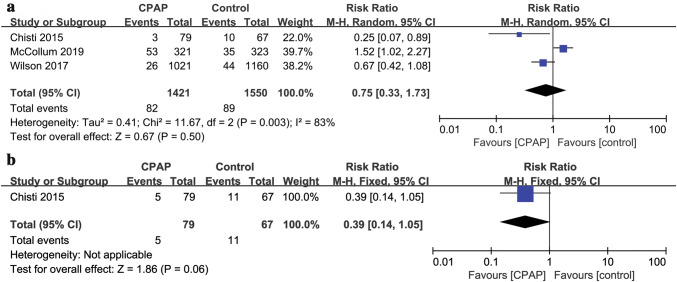
Three studies [11–13] (n = 2971) investigated the mortality. The pooled data suggested that mortality was not significantly reduced in CPAP group with significant heterogeneity (RR: 0.75, 95%CI = 0.33, 1.73; P = 0.50, I2 = 83%) (Fig. 1a). The probability of intubation was reported in only one RCT [11] (n = 146), in which a trend towards reduction in intubation was found in the CPAP group, but the difference was not statistically significant between CPAP and control groups (RR = 0.39, 95%CI 0.14, 1.05, P = 0.06) (Fig. 1b). We conducted subgroup analyses to investigate whether effects of CPAP on mortality and intubation differed according to different age. In the subgroup analysis, no significant differences regarding mortality were found between CPAP and control groups in different age groups (children age < 1 year: RR = 0.66, 95%CI 0.20, 2.15, P = 0.49, I2 = 84%; children age 1–5 years: RR = 0.95, 95%CI 0.53, 1.72, P = 0.88, I2 = 34%) (see Fig. S1a in supplement). Similarly, there was no difference in intubation rate in CPAP group compared with control group (children age < 1 year: RR = 0.41, 95%CI 0.11, 1.44, P = 0.16; children age 1–5 years: RR = 0.32, 95%CI 0.06, 1.73, P = 0.19) (see Fig. S1b in supplement). We did not conduct subgroup analyses based on different settings, sample size, and high-risk conditions because of the limited number of studies. Note that sensitivity analysis by removing the crossover trail or the study without the supervision of physicians seperately did not change the result of mortality (see Fig. S2a, Fig.S2b in supplement).
Fig. 1.
Efficacy of CPAP vs. control on a mortality rate and b intubation rate
Data for change in RR were available from only one RCT [10] (n = 69). The RR was remarkably decreased in the CPAP group after 1 h (MD = − 15.00 95%CI − 21.52, − 8.48, P < 0.00001) (Fig. 2a). HR was observed with no significant change in the same trial [10]; nonetheless, the lack of original data prevented us from a meta-analysis. The pooled data from one RCT [11] (n = 146) suggested there was no significant reduction in the length of hospital stay in CPAP group (MD = 0.29, 95%CI − 0.37, 0.96, P = 0.39) (Fig. 2b). Two studies [12, 13] (n = 2825) reported the incidence of adverse events and most of the adverse events were not serious. Only one child was reported to be suffering from probable pneumothorax and four probable aspiration episodes happened in CPAP group in one study [12]. The pooled data suggested there was no significant difference in adverse events between CPAP and control groups (RR = 3.06, 95%CI 0.38, 24.98, P = 0.30, I2 = 76%) (Fig. 2c).
Fig. 2.
Efficacy of CPAP vs. control on a respiratory rate, b duration of hospital stay, and c incidence of adverse events
The pooled data are contradictory regarding mortality. One crossover trial [13] found no significant difference in mortality between groups, but the study population was relatively low-risk; few children had hypoxemia or were severely malnourished. Another RCT [11] that recruited children with severe pneumonia and hypoxemia showed significantly lower mortality in CPAP group, but the study was done in an intensive care unit, which means higher nurse-to-patient ratios and better medical supervision. Differently, the latest and largest RCT [12] which was done in the general ward without daily physician supervision showed an increase in mortality with the use of CPAP among high-risk (such as HIV, malnutrition, or hypoxemia) children with severe pneumonia. The differences mentioned above may contribute to the inconsistent results. Notably, WHO-defined severe pneumonia is a clinical diagnosis that does not require chest radiographs to confirm the presence of lower respiratory disease. The nonspecific signs and symptoms can be found in children without primary respiratory disorders such as circulatory failure [12, 14], which may cause the recruited participants not to be wholly homogenous in different studies. Due to heterogeneity between the studies and the conflicting results, it is difficult to conclude the effect and safety of CPAP.
Limited evidence indicated that CPAP decreased the respiratory rate. There was no significant difference in intubation rate, length of hospital stay, and adverse events between the CPAP and control groups.
In conclusion, data regarding safety is contradictory for the use of CPAP among children with severe pneumonia. Limited evidence suggests CPAP may reduce respiratory rate. Still, there is a lack of evidence to show significant benefit for the use of CPAP for other outcomes. More well-designed RCTs are needed to evaluate which specific patient populations could benefit from CPAP in various settings. Case reports, observational studies, reviews, comments, conference abstracts were excluded.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
ZLW is responsible for the conception, data search, inclusion and exclusion of studies, data extraction, data analysis, and writing the manuscript. YH is responsible for inclusion and exclusion of studies, data extraction, checking the data of the outcomes, and writing the manuscript. ZXL is responsible for the supervision and reviewed the manuscript for important intellectual content. All authors approved the manuscript. ZLW and YH contributed equally to this manuscript.
Funding
No external funding for this manuscript.
Compliance with ethical standards
Ethical approval
Not reqired for this paper.
Conflict of interest
No financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu L, Oza S, Hogan D, Chu Y, Perin J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . Pocket book of hospital care for children: guidelines for the management of common childhood illnesses. 2. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 3.Harris M, Clark J, Coote N, Fletcher P, Harnden A, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66:ii1–ii23. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 4.Kneyber MCJ, de Luca D, Calderini E, Jarreau PH, Javouhey E, et al. Recommendations for mechanical ventilation of critically ill children from the Paediatric Mechanical Ventilation Consensus Conference (PEMVECC) Intensive Care Med. 2017;43:1764–1780. doi: 10.1007/s00134-017-4920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Principi T, Fraser DD, Morrison GC, Farsi SA, Carrelas JF, et al. Complications of mechanical ventilation in the pediatric population. Pediatr Pulmonol. 2011;46:452–457. doi: 10.1002/ppul.21389. [DOI] [PubMed] [Google Scholar]
- 6.Viscusi CD, Pacheco GS. Pediatric emergency noninvasive ventilation. Emerg Med Clin N Am. 2018;36:387–400. doi: 10.1016/j.emc.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Duke T. CPAP: a guide for clinicians in developing countries. Paediatr Int Child Health. 2013;34:3–11. doi: 10.1179/2046905513Y.0000000102. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:1. [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG, Deeks JJ, Abo-Zaid DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res Ed) 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson PT, Morris MC, Biagas KV, Otupiri E, Moresky RT. A randomized clinical trial evaluating nasal continuous positive airway pressure for acute respiratory distress in a developing country. J Pediatr. 2013;162:988–992. doi: 10.1016/j.jpeds.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Chisti MJ, Salam MA, Smith JH, Ahmed T, Pietroni MAC, et al. Bubble continuous positive airway pressure for children with severe pneumonia and hypoxaemia in Bangladesh: an open, randomised controlled trial. Lancet. 2015;386:1057–1065. doi: 10.1016/S0140-6736(15)60249-5. [DOI] [PubMed] [Google Scholar]
- 12.McCollum ED, Mvalo T, Eckerle M, Smith AG, Kondowe D, Makonokaya D, et al. Bubble continuous positive airway pressure for children with high-risk conditions and severe pneumonia in Malawi: an open label, randomised, controlled trial. Lancet Respir. Med. 2019;7:964–74. [DOI] [PMC free article] [PubMed]
- 13.Wilson PT, Baiden F, Brooks JC, Morris MC, Giessler K, et al. Continuous positive airway pressure for children with undifferentiated respiratory distress in Ghana: an open-label, cluster, crossover trial. Lancet Global Health. 2017;5:e615–e623. doi: 10.1016/S2214-109X(17)30145-6. [DOI] [PubMed] [Google Scholar]
- 14.Scott JA, Wonodi C, Moïsi JC, Deloria-Knoll M, DeLuca AN, Karron RA, et al. The definition of pneumonia, the assessment of severity, and clinical standardization in the Pneumonia Etiology Research for Child Health study. Clin Infect Dis. 2012;54:S109–116. doi: 10.1093/cid/cir1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.