Abstract
Pulmonary complications are the most common clinical manifestations of coronavirus disease (COVID-19). From recent clinical observation, two phenotypes have emerged: a low elastance or L-type and a high elastance or H-type. Clinical presentation, pathophysiology, pulmonary mechanics, radiological and ultrasound findings of these two phenotypes are different. Consequently, the therapeutic approach also varies between the two. We propose a management algorithm that combines the respiratory rate and oxygenation index with bedside lung ultrasound examination and monitoring that could help determine earlier the requirement for intubation and other surveillance of COVID-19 patients with respiratory failure.
Electronic supplementary material
The online version of this article (10.1007/s12630-020-01704-6) contains supplementary material, which is available to authorized users.
Keywords: COVID-19, lung ultrasound, respiratory failure, respiratory rate, and oxygenation index
Résumé
Les complications pulmonaires du coronavirus (COVID-19) constituent ses manifestations cliniques les plus fréquentes. De récentes observations cliniques ont fait émerger deux phénotypes : le phénotype à élastance faible ou type L (low), et le phénotype à élastance élevée, ou type H (high). La présentation clinique, la physiopathologie, les mécanismes pulmonaires, ainsi que les observations radiologiques et échographiques de ces deux différents phénotypes sont différents. L’approche thérapeutique variera par conséquent selon le phénotype des patients atteints de COVID-19 souffrant d’insuffisance respiratoire.
The coronavirus disease (COVID-19) first emerged in December 2019 in Wuhan, Hubei Province, China. The infection, caused by severe acute respiratory syndrome coronavirus 2 quickly propagated, eventually leading to the pandemic now affecting most countries around the world.1 The viral pneumonia caused by COVID-19 was initially reported in China to be associated with a mortality rate of 1.4%.2 Higher mortality rates have been reported in other countries.3,4 Initial reports on the pulmonary manifestation of COVID-19 described abnormalities observed on computed tomography (CT) in up to 86.2% of patients who presented for acute care and in 94.6% of those with severe disease.2,5 These manifestations consist of ground-glass opacities, local and bilateral patchy shadowing, and interstitial abnormalities.5 Abnormalities were also observed on chest radiography, such as bilateral peripheral consolidation and ground-glass opacities,6 as well with the use of lung ultrasound.7-10
Two phenotypes for one pathophysiology
Two phenotypes have recently been suggested to describe the clinical features of respiratory failure in COVID-19—a low elastance (L-type) phenotype and a high elastance (H-type) phenotype.11-13 As the elastance is the inverse of the compliance, the L-type indicates high compliance, and the H-type the opposite. The phenotype’s differing presentations are based on viral load timing, comorbidities, and likely genetic determinants of the response to hypoxemia.13 The L-type is the most common phenotype (> 50%).13 Those patients present with hypoxemia and hypocapnia but without dyspnea. They have normal respiratory system mechanics, hence the reported term by several emergency physicians of “happy hypoxic COVID” because, despite significant oxygen desaturation, the patient remains alert and able to talk. While some may keep a normal respiratory drive, others may exhibit a significantly increased respiratory drive, which could induce a patient self-inflicted lung injury (P-SILI).14-18 Hypoxemia in the L-type phenotype is thought to result from a ventilation-perfusion mismatch and loss of hypoxic vasoconstriction leading to shunt fraction up to 50%.11 As reported in the acute respiratory system (ARDS), the severity seems to correlate with the difference between the end-tidal carbon dioxide and the arterial partial pressure of carbon dioxide (PaCO2), which is an indirect measure of dead-space.19 The CT and chest radiographs reveal well-aerated compartments, patchy disease and ground-glass opacities with some peripheral involvement.20
The high elastance (H-type) is more consistent with a classical picture of ARDS from non-COVID-19 settings. Hypoxemia in those patients is a result of shunting through the consolidated regions of the lung, which is more important than in the L-type. As those clinical manifestations are different, the treatment will vary according to the predominant pattern. Notably, a transition from L-type to H-type may evolve because of progression of COVID-19 pneumonia, injurious mechanical ventilation, and/or P-SILI.14-18 Regression from an H-type to L-type or gradual normalization has also been observed with CT during recovery (Fig. 1).20,21
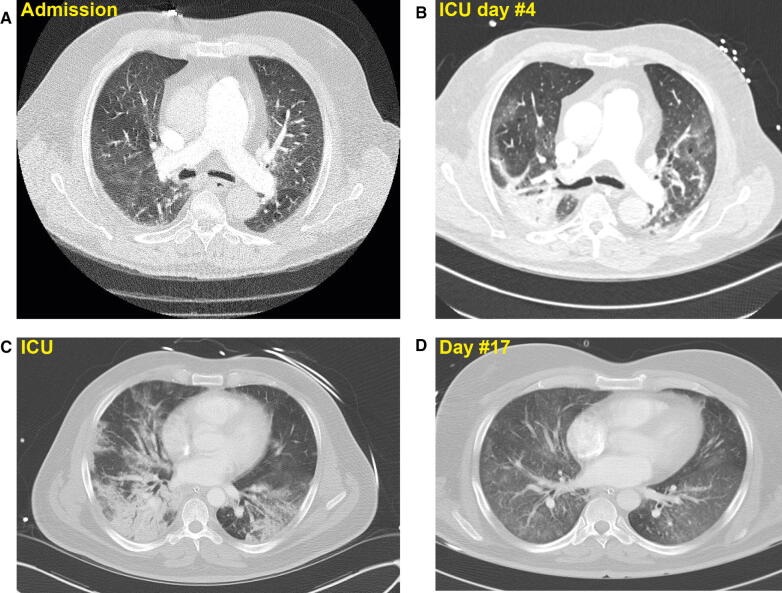
Fig. 1.
A) Computed tomography scan in a 68-yr-old coronavirus disease (COVID-19) patient with progressive dyspnea and oxygen saturation of 86% on room air. A L-type phenotypic pattern is shown. Lung ultrasound revealed a small right pleural effusion, several anterior subpleural consolidations, and B-lines in the posterior region. While in the intensive care unit, the patient experienced fever and progressive respiratory deterioration requiring intubation after 24 hr. The peripheral pulse oximetry remained at 87% despite a fraction of inspired oxygen (FiO2) at 100%, positive end-expiratory pressure titration of 10 cmH2O, and inhaled nitric oxide of 10 ppm. Blood gas revealed a pH of 7.37, partial pressure of carbon dioxide (PCO2) of 43.4 mmHg, and oxygen partial pressure (PaO2) of 63.8 mmHg. After 15 min of prone positioning, the SaO2 increased to 95% and on the following blood gas assessment, the PaO2/ FiO2 ratio was 268 with a compliance of 53 mL·cmH2O. B) A repeat CT scan in the same patient was done on day 4 to rule-out a pulmonary embolism. A transition to the H-type is observed. C) Transition in a 49-yr-old man recovering from COVID-19 respiratory failure. His phenotype changed from an H-type to an (D) L-type (courtesy of Dr. Emmanuel Charbonney and Dr. Lawrence Leroux)
Proposed management algorithm
Figure 2 outlines a proposed management algorithm for a rational approach to ventilatory support of COVID-19 patients with acute respiratory failure. Although it will need careful prospective clinical evaluation, it can be considered an “outside the box” perspective in this new clinical frontier. Its purpose is to help clarify the importance in ventilation strategies, which will be very different for the two ARDS phenotypes seen in COVID-19.
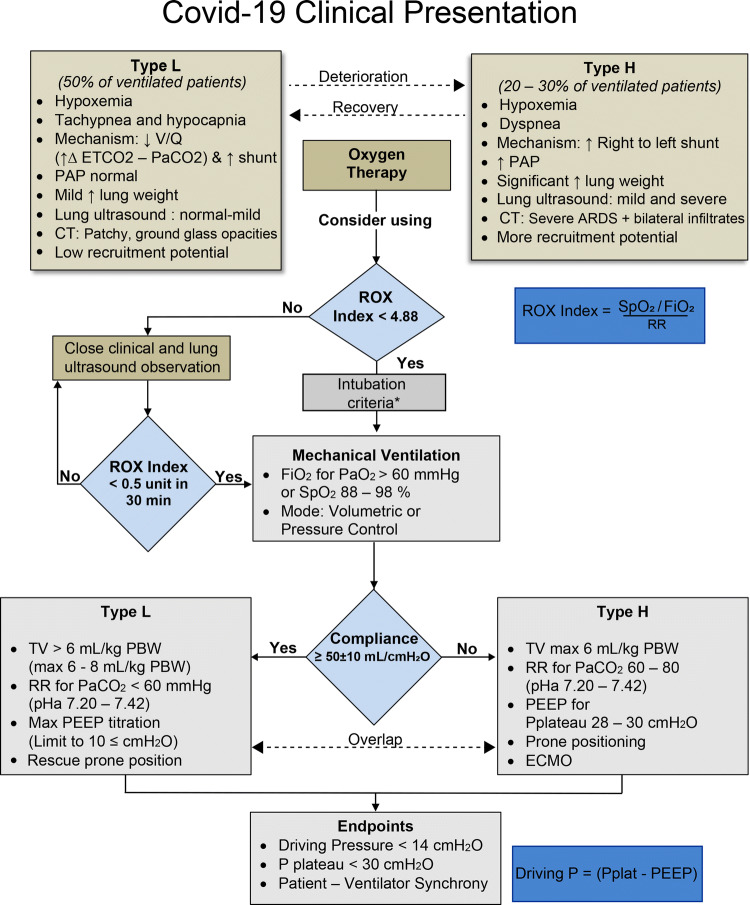
Fig. 2.
COVID-19 respiratory failure management. Following clinical and ultrasound examination to determine the L-type (low elastance) or H-type (high elastance) pattern, oxygen therapy is initiated. There can be progression or regression from one type to the other. The respiratory rate and oxygenation (ROX) index can be used, calculated, and monitored along with lung ultrasound to help in the process of deciding if and when to intubate. Mechanical ventilation settings include the tidal volume (TV), the respiratory rate (RR), and the degree of positive end-expiratory pressure (PEEP), which will vary according to the L or H phenotype. * Indicates that the decision to intubate is based on oxygenation and ventilation failure or compromised airway patency before initiating mechanical ventilation. ARDS = acute respiratory distress syndrome; CT = computed tomography; ECMO = extracorporeal membrane oxygenation; ETCO2 = end-tidal carbon dioxide; FiO2 = inspired oxygen; P = pressure; PaO2 = oxygen partial pressure; PAP = pulmonary artery pressure; PBW = predicted body weight; Pplat = plateau pressure; ROX index; SpO2 = pulse oxygen saturation; V/Q = ventilation perfusion. Adapted in part from Gattinoni et al. 11, 13 and Marini et al. 27
Ventilation strategies for different phenotypes
We propose that the algorithm be initiated when oxygen therapy is needed to correct symptomatic hypoxemia in COVID-19 patients with acute respiratory failure—e.g., when hypoxemia occurs that is refractory to oxygen therapy with a high-flow nasal cannula (HFNC) with an increased fraction of inspired oxygen (FiO2). Traditionally, the response to oxygen therapy is assessed with the oxygen partial pressure (PaO2)/FiO2 ratio22 or alternatively, when using peripheral pulse oximetry (SpO2), the SpO2/FiO2.23 Even though these ratios are well documented, they do not take into account the underlying respiratory workload. A PaO2/FiO2 ≤ 150 mmHg is considered a severe hypoxemic state and mandates close clinical supervision and intervention. The medical community is now supporting the use of HFNC as a clinically accepted adjunct approach to treating hypoxemia in COVID-19 patients.24 The literature from HFNC technology proposes a novel index, validated as a prognostic outcome of short-term HFNC therapy. This respiratory rate and oxygenation (ROX) index is based on the traditional PaO2/FiO2 ratio, but is then coupled with the respiratory rate.23,25 For practical bedside use, PaO2 is substituted for SpO2, so the ROX index is defined as the ([SpO2/FiO2]/respiratory rate). It can be calculated using an online tool (https://qxmd.com/calculate/calculator_724/rox-index-to-predict-risk-of-intubation).
As an example, a patient with an SpO2 of 80%, an FiO2 of 0.8, and an respiratory rate of 35/min ([80/0.8]/35) will have a ROX index of 2.8. As the respiratory condition deteriorates, the ROX index decreases.
Roca et al. have suggested various ROX index cut-off values that are associated with the need for intubation.23 Values below 2.85, 3.47, and 3.85 are the cut-off values used at two, six, and twelve hour, respectively. No predictive index is perfect, and a gray zone obviously exists between 3.85 and 4.88, in which it is difficult to firmly conclude what the optimal management should be. However, monitoring the ROX index over time, with emphasis from the 12th hour onwards, suggests that if the ROX index is ≥ 4.88, then the patient has a high chance of avoiding intubation. If the ROX index is < 3.85, then the risk of HFNC failure is high. In the initial study of the ROX index23 among patients in this zone, the ROX index could be repeated one or two hours later. If the score has increased, the patient should be considered with a higher likelihood of avoiding intubation. If it has decreased, then intubation has a higher likelihood to be necessary. If the score is unchanged, then reassessment should be performed after one or two more hours. The ROX index has recently been implemented by one of the authors (N.P.) and is being considered, along with advice from other experts in mechanical ventilation, by the French Ministry of Health, who have arbitrarily decided to re-assess the ROX index every 30 min.26 They have chosen a change in the ROX index of 0.5 to monitor the rapid onset of symptoms in the average COVID-19 patient compared with the usual ARDS patient. Therefore, a reduction of the ROX index by 0.5 from baseline over 30 min is considered significant and may suggest imminent need for intubation.23,25,26 Indeed, such a strategy obviously requires a prospective evaluation.
Role of lung ultrasound monitoring
Following the initiation of mechanical ventilation, it becomes important to discriminate between type-L vs type-H ARDS phenotypes for proper ventilator strategies as proposed by Marini et al. 27 This is where lung ultrasound can be useful.
The sensitivity and specificity of lung ultrasound findings for COVID-19 remains to be determined. Nevertheless, based on several case series,7,8,28-30 editorials/commentaries,9,10,31-35 a recent narrative review,36 and the authors own experience, four basic ultrasound patterns can be observed: 1) normal pattern (A lines and < 3 B-lines); 2) mild disease: ≥ 3 B-lines with some of them being confluent (waterfall or beam like)34 (Fig. 3A) and thickened pleura suggestive of an L-type; 3) broken pleural lines (Fig. 3B) with B-lines; and 4) severe disease with subpleural consolidation (Fig. 3C,3D) and a typical ARDS picture.
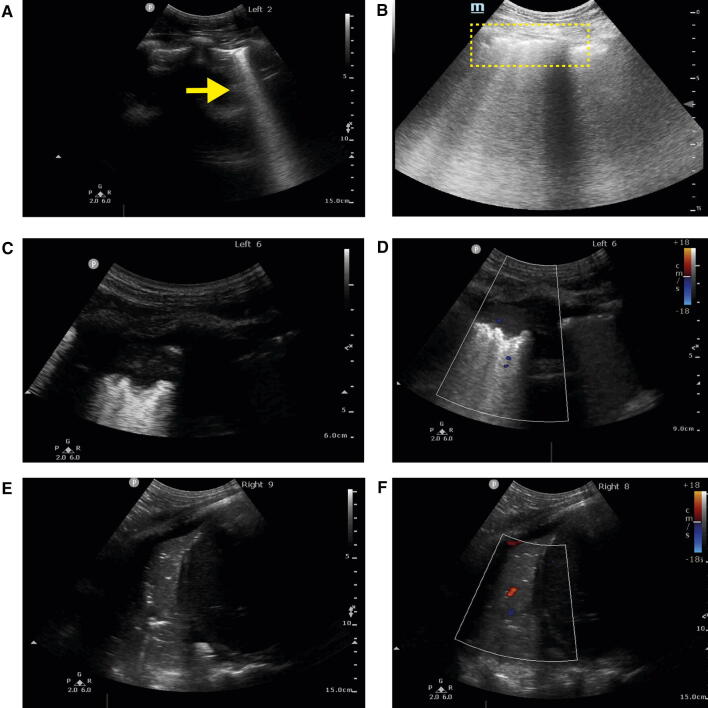
Fig. 3.
COVID-19 lung ultrasound findings. (A) B-lines (arrow); (B) irregular and broken pleural lines with multiple B-lines (dotted region); (C) peripheral or subpleural consolidation with (D) minimal colour Doppler signal. (E) Larger zone of consolidation in right lower posterior base with air bronchograms and (F) reduced perfusion using colour Doppler. (Courtesy of Dr. Stéphan Langevin and Dr. Caroline Gebhard) (Videos 3A, 3B, 3C, 3D, 3E, and 3F available as Electronic Supplementary Material)
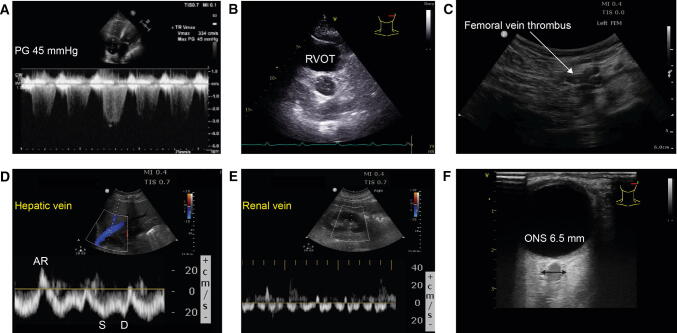
The fourth pattern is more suggestive of an H-type. In H-type, ultrasound examination will often reveal some L-type features, such as loss of aeration and overlapping areas of peripheral or subpleural consolidation (Fig. 3C-F and Fig. 4), but pathological lesions will mostly be seen in the posterior region of the lung.7,30 Management and triage would be different in patients with more extensive disease. Nevertheless, it is possible that the extent of any abnormal lung ultrasound pattern might be more important than the actual pattern or type of pathology itself. A large number of abnormal regions would correlate with the severity of lung inflammation. Pleural effusions have been uncommonly observed7,32 and were present in only 4.7% of patients in a series by Lomoro et al. 28 and in 10% of patients in a separate report by Xing et al.30 The reduction or absence of a vascular Doppler signal in the areas of peripheral or subpleural consolidation (Fig. 1D,1F) is common in COVID-197 and could represent peripheral segmental lung infarction through a microangiopathy, as described recently by autopsies from the United States.37 Coronavirus disease could be associated with an increased incidence of thrombotic complications such as pulmonary embolism.38-41
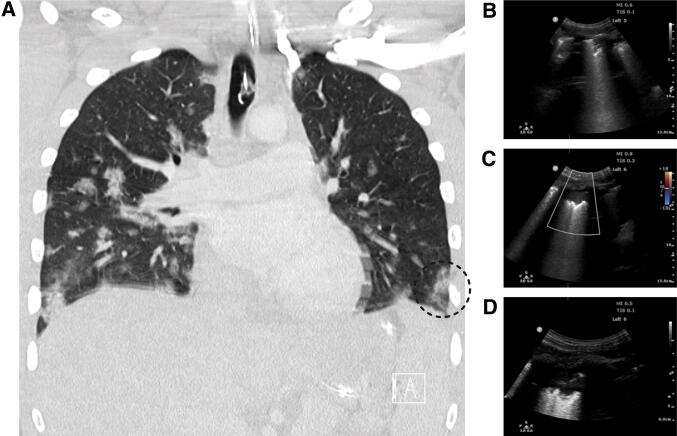
Fig. 4.
Computed tomographic and lung ultrasound correlation in a COVID-19 patient with subpleural consolidation. (Courtesy of Dr. Stéphan Langevin and Mr. Jacques Cadorette) (Video 4 available as Electronic Supplementary Material)
Standardization of lung ultrasound examination using a 14-region approach in COVID-19 has been recently proposed by Soldati et al.33 This would allow both diagnostic and daily monitoring of the pulmonary lesions.42 A simpler approach using six zones has also been recently proposed.43 The relevance of serial lung ultrasound has previously been reported in a small series of patients with ARDS42 unrelated to the current COVID-19 pandemic but the various scoring systems would need comparing and validating in COVID-19 patients. In that study, the non-resolution of pulmonary lesions was associated with worse outcome.42
As with any patients in the intensive care unit, COVID-19 patients can also develop other pulmonary complications. These include superimposed bacterial pneumonia, cardiogenic pulmonary edema related to myocardial dysfunction, right ventricular dysfunction and pulmonary hypertension due to pulmonary embolism, pleural effusions, and pneumothoraces.44,45 As suggested by Volpicelli et al., the bilateral patchy distribution of multiform clusters, alternating with “spared areas” is typical of the disease. Any other ultrasound signs should be considered at intermediate probability and should lead to further testing.34
Bedside ultrasound could be useful in detecting associated pulmonary complications or non-pulmonary complications such as cardiac dysfunction that can occur in such patients.46 Combining heart, lung, deep veins examination41 and whole-body ultrasound47,48 can identify the mechanism,49 risk factors,50 and life-threatening conditions in patients with respiratory symptoms (Fig. 5).51 Lung ultrasound can also impact clinical decision-making in patients with acute respiratory failure52,53 and provide comprehensive monitoring of regional lung aeration changes that could be used to predict response to prone positioning with improved right ventricular function (Figs 6 and 7)54-56 or higher positive end-expiratory pressure strategy.57 Newer modalities such as strain and speckle tracking could eventually be used to detect early abnormal peripheral pulmonary stress.58,59 The authors strongly advocate ultrasound as the key to rapid repeated progress imaging (rather than CT, which is more expensive and has logistical difficulties) and hence a decision to intubate would clearly be influenced by a rapidly deteriorating set of ultrasound images.
Fig. 5.
Examples of various COVID-19 complications detected using bedside ultrasound. Pulmonary hypertension with a trans-tricuspid pressure gradient (PG) of 45 mmHg in a patient developing right ventricular failure. B) Transthoracic short-axis aortic valve view showing a dilated right ventricular outflow tract (RVOT) of 35 mm and pulmonary artery in a patient with pulmonary embolism. C) Deep venous thrombosis of the femoral vein. D) Abnormal hepatic venous flow (HVF) Doppler velocity suggestive of right ventricular diastolic dysfunction.71 E) Renal venous congestion pattern-II associated with right ventricular dysfunction.71,72 F) Enlarged optic nerve sheath (ONS)73,74 in a patient with severe encephalopathy and extra-pyramidal signs. (Courtesy of Dr. Caroline Gebhard and Stéphan Langevin.). AR = atrial reversal HVF; D = diastolic HVF; S = systolic HVF. (Video 5B, 5C available as Electronic Supplementary Material.)
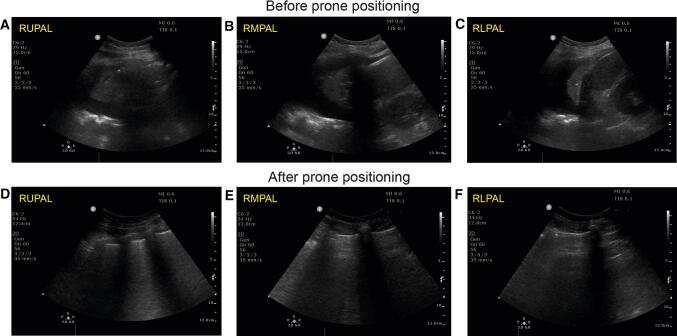
Fig. 6.
Lung ultrasound examination in a COVID-19 patient with severe hypoxia. before (A–C) and after (D–F) prone positioning. Note the significant changes with loss of pleural fluid and consolidation with increased aeration. Oxygen requirement were significantly reduced from 100% inspired oxygen to 60% for an adequate oxygen saturation after proning. RLPAL = right lower posterior axillary line; RMPAL = right middle posterior axillary line; RUPAL = right upper posterior axillary line. (Courtesy of Dr. Stéphan Langevin.) (Videos 6A, 6B, 6C, 6D, 6E, and 6F available as Electronic Supplementary Material.)
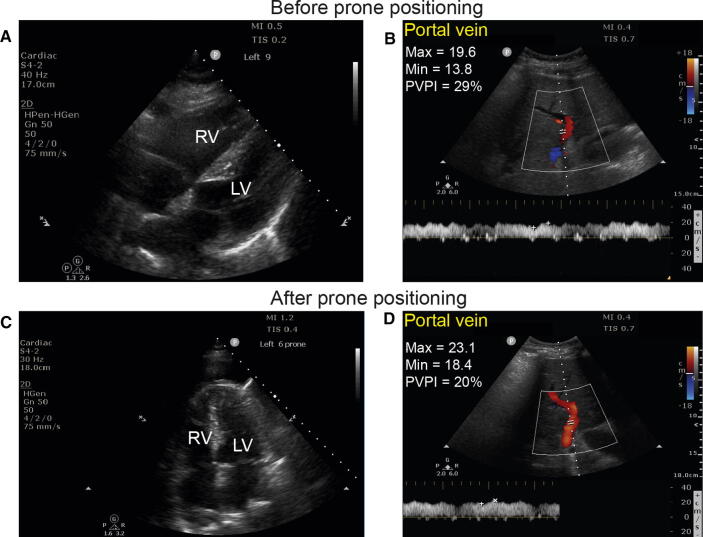
Fig. 7.
Cardiac examination and portal vein interrogation before (A–B) and after (C–D) prone positioning. Note the reduction in the size of the right ventricle (RV) in relation to the left ventricle (LV) and the improved portal velocities and reduction in the portal vein pulsatility index (PVPI). (Courtesy of Dr. Stéphan Langevin.) (Videos 7A and 7C available as Electronic Supplementary Material.)
Repeated ultrasound imaging over time can allow for accurate determination of the progression of lung pathology and can strongly influence a decision to institute early mechanical ventilation. Such repeated ultrasound imaging would be logistically convenient at the bedside without transporting the patient to the radiology department and would not involve any radiation dosing. Nevertheless, although lung ultrasound outperforms chest radiograph,60,61 it cannot be as comprehensive as CT since any lung pathology not involving the surface of the lung (and therefore covered by some aerated lung) cannot be detected by ultrasound. Lung ultrasound can be performed with a wide range of ultrasound machines, including handheld devices.62 The advantage of the handheld devices is that they can be fully enclosed in a plastic sheath, thereby reducing the risk of equipment contamination and spread of infection between patients. The performance and interpretation of lung ultrasound is much simpler than other imaging modalities such as transthoracic echocardiography as the images are easy to acquire and there are a limited number of patterns to interpret.63,64 Education can be scaled to meet the COVID-19 crisis through online courses (e.g., https://www.iteachu.com/courses/covid-19-lung-ultrasound/) simulation, and peer-to-peer mentoring.
Other management modalities and limitations
Other important elements in deciding whether to intubate is the work of breathing.27 This can also be monitored clinically by observing the use of accessory muscles or by measuring or estimating changes in pleural pressure.13 Once mechanical ventilation is initiated, tidal volume, respiratory rate, positive-pressure ventilation, prone positioning and veno-venous extracorporeal membrane oxygenation, sedation, and neuromuscular blockade will be adjusted differently depending on the phenotype and in accordance to ARDS management guidelines.65-68
Finally, there are still several unanswered questions and uncertainties regarding COVID-19 respiratory infections and the role of bedside ultrasound. We are still on an ascending learning curve regarding this new medical condition. Our proposed algorithm, which reflects the current understanding of COVID-19 respiratory failure, is likely to evolve as new information becomes available. The Table summarizes key questions on respiratory complications and management in COVID-19 patients with the use of lung ultrasound.
Table.
Undetermined issues in the respiratory complications and use of lung ultrasound in COVID-19 patients
| 1) What are the sensitivity and specificity of the ultrasound findings in determining the severity and type of lung disease phenotypes? |
| 2) What is the sensitivity and specificity of the ROX index in predicting requirement for intubation and how does it correlate with ultrasound findings? |
| 3) What is the mechanism of hypoxia in the early phase or L-type phenotype? |
| 4) What are the performances of the various lung ultrasound scoring systems? |
| 5) What are the role of the new modalities such as strain and speckle tracking in detecting early abnormal peripheral pulmonary stress? |
| 6) What are the case-based or population-based outcomes related to specific lung ultrasound findings? |
| 7) Can we determine response to prone positioning with lung ultrasound? |
| 8) Does the routine use of lung ultrasound improve the care of COVID-19 patients? |
| 9) What is the outcome of a different lung strategy with or without lung ultrasound for the L-type and H-type phenotypes? |
COVID-19 = coronavirus disease; ROX = respiratory rate and oxygenation
Our understanding of COVID-19 and its effect on the respiratory system is rapidly evolving. Biochemical pathways involving cardiovascular issues have recently been described by Liu et al. 46 This novel knowledge might contribute to understanding more about the two suggested phenotypes described by Gattinoni et al.11,13 It might also be used to confirm or challenge the aforementioned phenotypes categorization.69,70 Bedside lung ultrasound can help identify severity of lung involvement and response to therapy, as part of evaluation for ventilation as well as during the recovery and weaning process. With the implementation of this emerging information, clinical management may be somewhat distinct from that of other evidence-based interventions. As a result, clinical trials will be required to determine the best ventilation strategies for the different lung-involvement phenotypes seen in hypoxic COVID-19 patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Author contributions
André Y. Denault, Stéphane Delisle, Stéphan Langevin, and Paul Ouellet contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. David Canty, Alistair Royse, Colin Royse, Ximena Cid Serra, Caroline E. Gebhard, Etienne J. Couture, Martin Girard, Yiorgos Alexandros Cavayas, and Nicolas Peschanski contributed to study conception and design, and interpretation of data.
Disclosure
Dr. Denault is on the speakers bureau for CAE Healthcare (2010), Masimo (2017) and received an equipment grant from Edwards (2019).
Funding statement
Supported by the Richard I. Kaufman Endowment Fund in Anesthesia and Critical Care and the Montreal Heart Institute Foundation. The funding body had no role in the design of study and collection, analysis, and interpretation of data and in writing of the manuscript.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC COVID-19 Response Team. Geographic differences in COVID-19 cases, deaths, and incidence - United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 465-71. [DOI] [PMC free article] [PubMed]
- 4.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 5.Chen R, Chen J, Meng QT. Chest computed tomography images of early coronavirus disease (COVID-19) Can J Anesth. 2020 doi: 10.1007/s12630-020-01625-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong HY, Lam HY, Fong AH, et al. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. 2019 doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Wang S, Liu Y, et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19). Research Square Preprint. Available from URL: https://www.researchgate.net/publication/340198841_A_preliminary_study_on_the_ultrasonic_manifestations_of_peripulmonary_lesions_of_non-critical_novel_coronavirus_pneumonia_COVID-19/fulltext/5e7ce451a6fdcc139c08b101/340198841_A_preliminary_study_on_the_ultrasonic_manifestations_of_peripulmonary_lesions_of_non-critical_novel_coronavirus_pneumonia_COVID-19.pdf?origin=publication_detail (accessed May 2020).
- 8.Peng QY, Wang XT, Zhang LN; Chinese Critical Care Ultrasound Study Group. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med 2020; DOI: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed]
- 9.Poggiali E, Dacrema A, Bastoni D, et al. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology. 2020 doi: 10.1148/radiol.2020200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soldati G, Smargiassi A, Inchingolo R, et al. Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med. 2020 doi: 10.1002/jum.15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020 doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatment for different phenotypes? Intensive Care Med. 2020 doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barach AL, Martin J, Eckman M. Positive pressure respiration and its application to the treatment of acute pulmonary edema. Ann Intern Med. 1938;12:754–795. doi: 10.7326/0003-4819-12-6-754. [DOI] [Google Scholar]
- 15.Mascheroni D, Kolobow T, Fumagalli R, Moretti MP, Chen V, Buckhold D. Acute respiratory failure following pharmacologically induced hyperventilation: an experimental animal study. Intensive Care Med. 1988;15:8–14. doi: 10.1007/BF00255628. [DOI] [PubMed] [Google Scholar]
- 16.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 17.Grieco DL, Menga LS, Eleuteri D, Antonelli M. Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol. 2019;85:1014–1023. doi: 10.23736/S0375-9393.19.13418-9. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida T, Grieco DL, Brochard L, Fujino Y. Patient self-inflicted lung injury and positive end-expiratory pressure for safe spontaneous breathing. Curr Opin Crit Care. 2020;26:59–65. doi: 10.1097/MCC.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 19.Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Dong C, Hu Y, et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020 doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology 2020; DOI: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed]
- 22.Covelli HD, Nessan VJ, Tuttle WK., 3rd Oxygen derived variables in acute respiratory failure. Crit Care Med. 1983;11:646–649. doi: 10.1097/00003246-198308000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Roca O, Messika J, Caralt B, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: the utility of the ROX index. J Crit Care. 2016 doi: 10.1016/j.jcrc.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Cinesi Gomez C, Penuelas Rodriguez O, Lujan Torne M, et al. Clinical consensus recommendations regarding non-invasive respiratory support in the adult patient with acute respiratory failure secondary to SARS-CoV-2 infection. Med Intensiva. 2020 doi: 10.1016/j.medin.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199:1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 26.Ministere des Solidarites et de la Sante Direction Generale de la Sante Centre de Crise Sanitaire. Doctrine d’usage des dispositifs de ventilation et des respirateurs pour les patients COVID-19. Available from URL: https://www.reulian.fr/app/download/24477393/Message_MARS_2020_27_8791_9.pdf (accessed May 2020).
- 27.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020 doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 28.Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020 doi: 10.1016/j.ejro.2020.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu W, Zhang S, Chen B, et al. A clinical study of noninvasive assessment of lung lesions in patients with coronavirus disease-19 (COVID-19) by bedside ultrasound. Ultraschall Med. 2020 doi: 10.1055/a-1154-8795. [DOI] [PubMed] [Google Scholar]
- 30.Xing C, Li Q, Du H, Kang W, Lian J, Yuan L. Lung ultrasound findings in patients with COVID-19 pneumonia. Crit Care. 2020 doi: 10.1186/s13054-020-02876-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore S, Gardiner E. Point of care and intensive care lung ultrasound: a reference guide for practitioners during COVID-19. Radiography. 2020 doi: 10.1016/j.radi.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sofia S, Boccatonda A, Montanari M, et al. Thoracic ultrasound and SARS-COVID-19: a pictorial essay. J Ultrasound. 2020 doi: 10.1007/s40477-020-00458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soldati G, Smargiassi A, Inchingolo R, et al. Proposal for international standardization of the use of lung ultrasound for patients with COVID-19: a simple, quantitative, reproducible method. J Ultrasound Med. 2020 doi: 10.1002/jum.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volpicelli G, Gargani L. Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J. 2020 doi: 10.1186/s13089-020-00171-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vetrugno L, Bove T, Orso D, et al. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography. 2020;37:625–627. doi: 10.1111/echo.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith MJ, Hayward SA, Innes SM, Miller AS. Point-of-care lung ultrasound in patients with COVID-19 - a narrative review. Anaesthesia. 2020 doi: 10.1111/anae.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and cardiac pathology in covid-19: the first autopsy series from New Orleans. medRxiv preprint. 2020; DOI: 10.1101/2020.04.06.20050575. [DOI] [PMC free article] [PubMed]
- 38.Leonard-Lorant I, Delabranche X, Severac F, et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020 doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klok FA, Kruip MJ, van der Meer NJ, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ullah W, Saeed R, Sarwar U, Patel R, Fischman DL. COVID-19 complicated by acute pulmonary embolism and right-sided heart failure. JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tavazzi G, Civardi L, Caneva L, Mongodi S, Mojoli F. Thrombotic events in SARS-CoV-2 patients: an urgent call for ultrasound screening. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mongodi S, Pozzi M, Orlando A, et al. Lung ultrasound for daily monitoring of ARDS patients on extracorporeal membrane oxygenation: preliminary experience. Intensive Care Med. 2018;44:123–124. doi: 10.1007/s00134-017-4941-7. [DOI] [PubMed] [Google Scholar]
- 43.Cid X, Canty D, Royse A, et al. Impact of point-of-care ultrasound on the hospital length of stay for internal medicine inpatients with cardiopulmonary diagnosis at admission: study protocol of a randomized controlled trial-the IMFCU-1 (Internal Medicine Focused Clinical Ultrasound) study. Trials. 2020 doi: 10.1186/s13063-019-4003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108:1345–1348. doi: 10.1378/chest.108.5.1345. [DOI] [PubMed] [Google Scholar]
- 45.Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013 doi: 10.1186/cc13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 47.Karabinis A, Fragou M, Karakitsos D. Whole-body ultrasound in the intensive care unit: a new role for an aged technique. J Crit Care. 2010;25:509–513. doi: 10.1016/j.jcrc.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Denault A, Canty D, Azzam M, Amir A, Gebhard CE. Whole body ultrasound in the operating room and the intensive care unit. Korean J Anesthesiol. 2019 doi: 10.4097/kja.19186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sekiguchi H, Schenck LA, Horie R, et al. Critical care ultrasonography differentiates ARDS, pulmonary edema, and other causes in the early course of acute hypoxemic respiratory failure. Chest. 2015;148:912–918. doi: 10.1378/chest.15-0341. [DOI] [PubMed] [Google Scholar]
- 50.Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139:538–542. doi: 10.1378/chest.10-1479. [DOI] [PubMed] [Google Scholar]
- 51.Laursen CB, Sloth E, Lambrechtsen J, et al. Focused sonography of the heart, lungs, and deep veins identifies missed life-threatening conditions in admitted patients with acute respiratory symptoms. Chest. 2013;144:1868–1875. doi: 10.1378/chest.13-0882. [DOI] [PubMed] [Google Scholar]
- 52.Silva S, Biendel C, Ruiz J, et al. Usefulness of cardiothoracic chest ultrasound in the management of acute respiratory failure in critical care practice. Chest. 2013;144:859–865. doi: 10.1378/chest.13-0167. [DOI] [PubMed] [Google Scholar]
- 53.Xirouchaki N, Kondili E, Prinianakis G, Malliotakis P, Georgopoulos D. Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40:57–65. doi: 10.1007/s00134-013-3133-3. [DOI] [PubMed] [Google Scholar]
- 54.Vieillard-Baron A, Charron C, Caille V, Belliard G, Page B, Jardin F. Prone positioning unloads the right ventricle in severe ARDS. Chest. 2007;132:1440–1446. doi: 10.1378/chest.07-1013. [DOI] [PubMed] [Google Scholar]
- 55.Prat G, Guinard S, Bizien N, et al. Can lung ultrasonography predict prone positioning response in acute respiratory distress syndrome patients? J Crit Care. 2016;32:36–41. doi: 10.1016/j.jcrc.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 56.Haddam M, Zieleskiewicz L, Perbet S, et al. Lung ultrasonography for assessment of oxygenation response to prone position ventilation in ARDS. Intensive Care Med. 2016;42:1546–1556. doi: 10.1007/s00134-016-4411-7. [DOI] [PubMed] [Google Scholar]
- 57.Bouhemad B, Brisson H, Le-Guen M, Arbelot C, Lu Q, Rouby JJ. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med. 2011;183:341–347. doi: 10.1164/rccm.201003-0369OC. [DOI] [PubMed] [Google Scholar]
- 58.Rubin JM, Horowitz JC, Sisson TH, Kim K, Ortiz LA, Hamilton JD. Ultrasound strain measurements for evaluating local pulmonary ventilation. Ultrasound Med Biol. 2016;42:2525–2531. doi: 10.1016/j.ultrasmedbio.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duclos G, Bobbia X, Markarian T, et al. Speckle tracking quantification of lung sliding for the diagnosis of pneumothorax: a multicentric observational study. Intensive Care Med. 2019;45:1212–1218. doi: 10.1007/s00134-019-05710-1. [DOI] [PubMed] [Google Scholar]
- 60.Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9–15. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Tierney DM, Huelster JS, Overgaard JD, et al. Comparative performance of pulmonary ultrasound, chest radiograph, and CT among patients with acute respiratory failure. Crit Care Med. 2020;48:151–157. doi: 10.1097/CCM.0000000000004124. [DOI] [PubMed] [Google Scholar]
- 62.Sforza A, Mancusi C, Carlino MV, et al. Diagnostic performance of multi-organ ultrasound with pocket-sized device in the management of acute dyspnea. Cardiovasc Ultrasound. 2017 doi: 10.1186/s12947-017-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.See KC, Ong V, Wong SH, et al. Lung ultrasound training: curriculum implementation and learning trajectory among respiratory therapists. Intensive Care Med. 2016;42:63–71. doi: 10.1007/s00134-015-4102-9. [DOI] [PubMed] [Google Scholar]
- 64.Ford JW, Heiberg J, Brennan AP, et al. A Pilot assessment of 3 point-of-care strategies for diagnosis of perioperative lung pathology. Anesth Analg. 2017;124:734–742. doi: 10.1213/ANE.0000000000001726. [DOI] [PubMed] [Google Scholar]
- 65.Acute Respiratory Distress Syndrome Network; Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301-8. [DOI] [PubMed]
- 66.Malhotra A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med. 2007;357:1113–1120. doi: 10.1056/NEJMct074213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 68.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 69.Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beaubien-Souligny W, Rola P, Haycock K, et al. Quantifying systemic congestion with point-of-care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J. 2020 doi: 10.1186/s13089-020-00163-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beaubien-Souligny W, Denault A, Robillard P, Desjardins G. The role of point-of-care ultrasound monitoring in cardiac surgical patients with acute kidney injury. J Cardiothorac Vasc Anesth. 2019;33:2781–2796. doi: 10.1053/j.jvca.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 73.Robba C, Goffi A, Geeraerts T, et al. Brain ultrasonography: methodology, basic and advanced principles and clinical applications. A narrative review. Intensive Care Med. 2019 doi: 10.1007/s00134-019-05610-4. [DOI] [PubMed] [Google Scholar]
- 74.Palermo J, Bojanowski M, Langevin S, Denault AY. Point-of-care handheld ophthalmic ultrasound in the diagnosis and evaluation of raised intracranial pressure and Terson syndrome: a description of two cases. Can J Anesth. 2020;67:353–359. doi: 10.1007/s12630-019-01531-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.